Abstract
Background: A high intake of trans fatty acids decreases HDL cholesterol and is associated with increased LDL cholesterol, inflammation, diabetes, cancer, and mortality from cardiovascular disease. The relation between trans fat intake and all-cause mortality has not been established.
Objective: The aim of this study was to determine the relation between trans fat intake and all-cause mortality.
Design: We used data from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study—a prospective cohort study of white and black men and women residing in the continental United States. Energy-adjusted trans fat intake was categorized into quintiles, and Cox-regression was used to evaluate the association between trans fat intake and all-cause mortality.
Results: During 7 y of follow-up, there were 1572 deaths in 18,513 participants included in REGARDS. From the first to the fifth quintile of trans fat intake, the mortality rates per 1000 person-years of follow-up (95% CIs) were 12.8 (11.3, 14.5), 14.3 (12.7, 16.2), 14.6 (13.0, 16.5), 19.0 (17.1, 21.1), and 23.6 (21.5, 25.9), respectively. After adjustment for demographic factors, education, and risk factors for mortality, the HRs (95% CIs) for all-cause mortality were 1.00, 1.03 (0.86, 1.23), 0.98 (0.82, 1.17), 1.25 (1.05, 1.48), and 1.24 (1.05, 1.48), respectively (P-trend = 0.004). The population attributable risk due to trans fat intake was 7% (95% CI: 5%, 8%).
Conclusion: Higher trans fat intake is associated with an increased risk of all-cause mortality.
INTRODUCTION
Intake of trans-unsaturated fatty acids (TFAs)5, even at low levels (∼2% of energy), is associated with several adverse outcomes, including dyslipidemia, inflammation, myocardial infarction, and increased cardiovascular mortality (1–5). In addition, there is growing evidence suggesting that increased TFA intake increases the risk of several chronic diseases (eg, diabetes, cancer, and stroke) that contribute greatly to total mortality.
The Nurses’ Health Study showed a 39% increased risk of developing type 2 diabetes mellitus for every 2% increase in energy from TFA (6). In addition, a nested case-control study with an average follow-up of 7 y by Chajès et al (7) showed that increasing concentrations of serum TFA, a marker of TFA intake, was associated with an increased risk of breast cancer in women (OR: 1.75; 95% CI: 1.08, 2.83; P-trend = 0.018). Furthermore, a case-control study in Canada by Hu et al (8) showed that men in the highest quintile of TFA intake had an increased risk of prostate cancer compared with men in the lowest quintile (OR: 1.45; 95% CI: 1.16, 1.81). Moreover, data from the Women's Health Initiative study showed that women in the highest quintile of TFA intake had an increased risk of ischemic stroke compared with women in the lowest quintile of TFA intake (HR: 1.39; 95% CI: 1.08, 1.79) (9). A systematic review by Astorg (10) also indicated that elevated TFA intake is associated with increased risk of colorectal cancer.
Given that cardiovascular diseases, diabetes, cancer, and stroke are leading causes of death in the United States and elsewhere, we hypothesized that TFA intake is associated with increased risk of all-cause mortality. Apart from data from mathematical models (4, 11), we are not aware of any studies that have directly assessed the relation between TFA intake and all-cause mortality. In the current study, we investigated the association between TFA intake and all-cause mortality using data from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study.
SUBJECTS AND METHODS
Details of the REGARDS study design were described elsewhere (12). Briefly, between January 2003 and October 2007, the study recruited 30,239 black and white men and women, aged ≥45 y, from across the continental United States with an oversampling of blacks and persons living in the stroke belt—a region that encompasses 8 states (North Carolina, South Carolina, Georgia, Tennessee, Mississippi, Alabama, Louisiana, and Arkansas) in the southeast with higher stroke mortality than the rest of the United States. Potential participants were contacted via mail and telephone and those who consented were interviewed by telephone to collect demographic and risk factor information (13). Blood samples, anthropometric measurements, and other risk factor evaluations, such as blood pressure, were obtained during in-home visits using standardized protocols. Self-administered questionnaires were left with participants to complete and mail back after the in-home visit. Participants or their proxies were contacted every 6 mo to monitor their vital status and proxies could report deaths by telephone or mail contact. Participants lost to follow-up were searched using the social security death index, and/or the National Death Index. The REGARDS study was approved by the institutional review boards of all participating institutions and participants gave informed consent.
Dietary assessment
Dietary intake was assessed by using a self-administered Block 98 food-frequency questionnaire (www.Nutritionquest.com). Participants were given pictures to help them estimate their portion sizes. This instrument has been extensively validated against multiple food records in several diverse populations in the United States and has been shown to reliably estimate the intake of total fat and specific fats, including SFA, MUFA, and PUFA, and trans fat (14–17). For example, in a study that compared the Block 98 food-frequency questionnaire with the average of two 24-h dietary recalls, the correlation coefficient for trans fat intake was 0.53 (17).
Laboratory analyses
Blood samples were collected after a 10–12-h fast and centrifuged locally within 2 h of collection. Samples were then shipped overnight to a central laboratory at the University of Vermont for further processing and analysis. High-sensitivity C-reactive protein (CRP) was analyzed by particle-enhanced immunonephelometry (N High-Sensitivity CRP; Dade Behring Inc) with an interassay CV of 2.1% to 5.7%. Lipid profiles were measured by using colorimetric reflectance spectrophotometry (18).
Definitions
The outcome was all-cause mortality, whereas energy-adjusted TFA intake categorized into quintiles was the main exposure variable.
Covariates
The covariates considered included sex, age, smoking status (never, past, or current smoker), race (black or white), region [stroke buckle (coastal plain of North Carolina, South Carolina, and Georgia), the rest of the stroke belt, or other], alcohol use (never, past, or current drinker), education, waist circumference, level of physical activity, diabetes, ischemic heart disease (IHD), hypertension, stroke, heart failure (HF), chronic kidney disease, and statin use (yes or no). Diabetes was defined as any self-reported use of glucose control medication or a fasting blood glucose concentration >126 mg/dL or nonfasting glucose >200 mg/dL. We defined IHD as any self-report of myocardial infarction/heart attack, coronary angioplasty, or bypass surgery or electrocardiographic evidence of myocardial infarction. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or any self-report of antihypertension medication use. Use of digoxin in the absence of atrial fibrillation was used as a proxy for HF based on the fact that digoxin is only indicated to patients with atrial fibrillation or HF (19). Chronic kidney disease was defined as a glomerular filtration rate <60 mL/min per 1.73 m (estimated using the modification of diet in renal disease equation) (20). Education was modeled in 4 groups (less than high school, high school, some college, or college graduate), whereas physical activity (“How often do you exercise enough to work up a sweat?”) was modeled in 3 categories (none, 1–3 times/wk, or ≥4 times/wk.
Statistical analyses
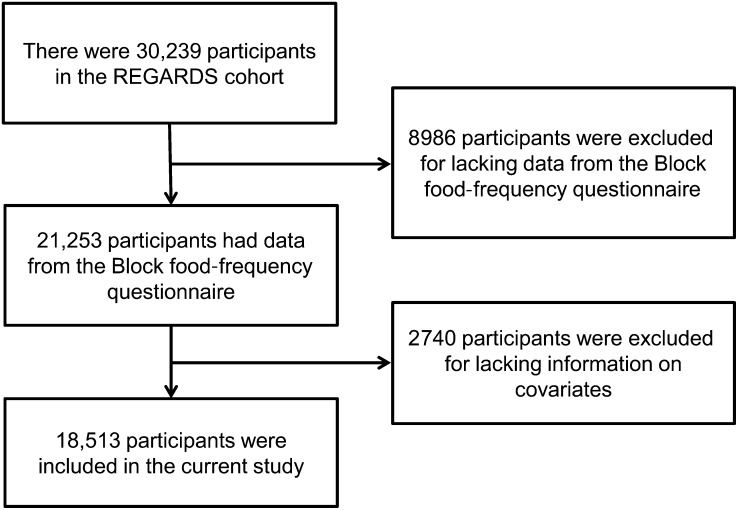
Of the 30,239 participants in the REGARDS cohort, we excluded 8986 who did not have data from the Block food-frequency questionnaire (Figure 1). In addition, we excluded 2740 for missing data on covariates, which left 18,513 participants with complete data for the current analyses. Survival time was defined as the period between in-home visit and death/last follow-up/data freeze (April 2011). Because of high correlations between dietary variables and total energy intake, variables from the food-frequency questionnaire including TFA intake were adjusted for total energy intake by using the residual method (21). The resulting energy-adjusted TFA variable was then distributed into quintiles. We preferred to use quintiles instead of trans fat as a continuous variable because we wanted to assess a dose-response relation, to avoid model misspecification and to mitigate the potential influence of outliers (21).
FIGURE 1.
A flow diagram showing how participants in the current analysis (n = 18,513) were selected from the REGARDS study (n = 30,239). REGARDS, Reasons for Geographic and Racial Differences in Stroke.
We fitted Kaplan-Meier survival curves and used the log-rank test to determine the difference in mortality between individuals in different quintiles of TFA intake. Next, we fitted Cox proportional hazards models to estimate HRs (95% CIs) for all-cause mortality for the different quintiles of TFA before and after the sequential adjustment for various potential confounders as well as total energy intake and energy-adjusted intakes of SFA, MUFA, PUFA, protein, and carbohydrates.
Because TFA intake is associated with a reduction in HDL cholesterol and an increase in LDL cholesterol, CRP (22–26), and all major predictors of mortality, we considered these variables to be potential intermediate variables between TFA and all-cause mortality. We thus conducted additional analyses that further adjusted for lipids (HDL cholesterol, LDL cholesterol, and triglycerides) and variables related to inflammation (CRP, serum albumin, and total white blood cell count). Because serum albumin and white blood cells (WBCs) were not measured in the initial phase of the study (13), the addition of these variables reduced the sample size to 13,070. We refitted the final model without lipids and markers of inflammation (model 3) by using the reduced sample size and additionally refitted the model further by adjusting for HDL cholesterol, LDL cholesterol, triglycerides CRP, serum albumin, and WBCs to determine whether the association between TFA and all-cause mortality was driven in part by these variables. Results are reported as HRs and 95% CIs. Schoenfeld residuals were used to check the proportionality assumption by using the Lowess smoothing technique. In addition to analyses for energy-adjusted TFA as a main effect, we tested for 3 a priori specified interactions between TFA as a continuous variable and sex, race, and PUFA intake. The latter was prompted by recent findings of interactions between TFA and PUFA intake on lipid concentrations (27), in which we found that the relation between TFA and HDL or triglycerides varied according to PUFA intake.
We used logistic regression to obtain risk estimates used to calculate population attributable risk associated with TFA consumption as recommended by Spiegelman and colleagues (28, 29). TFA was categorized in the logistic regression models by using quintiles of energy-adjusted TFA in the same manner as in the Cox regression models. All analyses were done by using SAS version 9.2 (SAS Institute Inc).
RESULTS
The distribution of important covariates by quintiles of energy-adjusted TFA intake is shown in Table 1. Most of the study participants were women, were white, were current alcohol drinkers, were less likely to be current smokers, and had a history of hypertension. A higher TFA intake was associated with residence in the stroke belt region, male sex, low educational attainment, less physical activity, greater waist circumference, higher WBC count, lower HDL-cholesterol concentration, and a history of IHD or HF. In addition, a higher TFA intake was associated with higher intakes of MUFA, PUFA, SFA, total fat, and total energy and a lower protein intake.
TABLE 1.
Distribution of potential confounders by quintiles of energy-adjusted trans fat intake in the REGARDS study, 2003–20071
| Quintiles of trans fat intake |
||||||
| Variable | 1 (n = 3702) | 2 (n = 3703) | 3 (n = 3703) | 4 (n = 3702) | 5 (n = 3703) | P value2 |
| trans Fat (% of energy) | 1.60 ± 0.393 | 2.30 ± 0.32 | 2.84 ± 0.36 | 3.45 ± 0.43 | 4.68 ± 0.93 | — |
| Age (y) | 64.7 ± 9.0 | 64.6 ± 9.1 | 64.7 ± 9.4 | 64.6 ± 9.2 | 65.7 ± 9.5 | <0.001 |
| Sex (% female) | 72 | 63 | 57 | 48 | 35 | <0.001 |
| Race (% white) | 65 | 65 | 66 | 69 | 68 | <0.001 |
| Region (%) | <0.001 | |||||
| Buckle | 21 | 21 | 22 | 22 | 21 | |
| Belt | 30 | 32 | 34 | 36 | 40 | |
| Other | 49 | 47 | 44 | 42 | 39 | |
| Income (%) | <0.001 | |||||
| <$20,000 | 16 | 14 | 15 | 15 | 16 | |
| $20,000–$34,000 | 23 | 23 | 24 | 24 | 27 | |
| $35,000–$74,000 | 29 | 31 | 32 | 34 | 33 | |
| ≥$75,000 | 20 | 20 | 18 | 17 | 13 | |
| Refused | 12 | 12 | 11 | 10 | 11 | |
| Education (%) | <0.001 | |||||
| <High school | 8 | 8 | 8 | 9 | 12 | |
| High school | 22 | 24 | 25 | 27 | 30 | |
| Some college | 27 | 28 | 29 | 28 | 27 | |
| Graduate | 43 | 40 | 38 | 36 | 31 | |
| Smoking status (%) | <0.001 | |||||
| Never smoker | 47 | 46 | 46 | 45 | 42 | |
| Past smoker | 40 | 41 | 41 | 40 | 44 | |
| Current smoker | 13 | 13 | 13 | 15 | 14 | |
| Alcohol (%) | <0.001 | |||||
| Never drinker | 26 | 26 | 28 | 29 | 32 | |
| Past drinker | 13 | 13 | 16 | 19 | 22 | |
| Current drinker | 61 | 61 | 56 | 52 | 46 | |
| Physical activity (%) | <0.001 | |||||
| None | 28 | 31 | 33 | 34 | 36 | |
| 1–3 times/wk | 37 | 39 | 37 | 37 | 35 | |
| ≥4 times/wk | 35 | 30 | 30 | 29 | 29 | |
| BMI (kg/m2) | 28.4 ± 6.0 | 29.0 ± 6.0 | 29.3 ± 6.1 | 29.4 ± 6.1 | 28.9 ± 5.8 | <0.001 |
| Waist circumference (cm) | 92.0 ± 14.8 | 94.1 ± 15.5 | 95.5 ± 14.8 | 96.9 ± 15.3 | 97.5 ± 14.6 | <0.001 |
| IHD (%) | 15 | 16 | 16 | 19 | 19 | <0.001 |
| Hypertension (%) | 56 | 55 | 58 | 56 | 57 | 0.37 |
| Stroke (%) | 5 | 5 | 5 | 6 | 5 | 0.24 |
| Diabetes (%) | 18 | 18 | 20 | 19 | 20 | 0.23 |
| Heart failure (%) | 11 | 11 | 11 | 13 | 13 | <0.001 |
| CKD (%) | 1 | 1 | 1 | 1 | 2 | <0.001 |
| Statin users (%) | 29 | 32 | 33 | 35 | 34 | <0.001 |
| HDL cholesterol (mg/dL) | 57 ± 18 | 54 ± 16 | 52 ± 16 | 50 ± 15 | 49 ± 15 | <0.001 |
| LDL cholesterol (mg/dL) | 114 ± 35 | 115 ± 34 | 113 ± 35 | 118 ± 34 | 113 ± 34 | 0.01 |
| Serum albumin (g/dL)4 | 4.21 ± 0.33 | 4.19 ± 0.33 | 4.18 ± 0.32 | 4.18 ± 0.32 | 4.16 ± 0.33 | <0.001 |
| WBC count (×109 cells/L)4 | 5.51 (4.54, 6.73)5 | 5.59 (4.64, 6.83) | 5.64 (4.63, 6.86) | 5.72 (4.72, 6.81) | 5.82 (4.76, 7.03) | <0.001 |
| Triglycerides (mg/dL) | 105 (77, 147) | 109 (80, 154) | 111 (82, 157) | 114 (84, 164) | 115 (84, 162) | <0.001 |
| hsCRP (mg/L) | 2.04 (0.89, 4.63) | 2.09 (0.91, 4.87) | 2.11 (0.94, 4.93) | 2.15 (0.97, 4.82) | 2.05 (0.91, 4.63) | 0.15 |
| SBP (mm Hg)4 | 125.96 ± 16.02 | 125.87 ± 16.49 | 126.85 ± 16.33 | 126.94 ± 15.64 | 127.70 ± 16.19 | <0.001 |
| DBP (mm Hg)4 | 75.74 ± 9.65 | 75.67 ± 9.40 | 76.05 ± 9.40 | 76.35 ± 9.47 | 76.59 ± 9.35 | <0.001 |
| Total energy (kcal/d) | 1656 ± 630 | 1624 ± 659 | 1674 ± 688 | 1745 ± 741 | 1835 ± 786 | <0.001 |
| Protein (g/d)6 | 58.7 ± 14.8 | 59.1 ± 13.9 | 58.2 ± 13.2 | 57.7 ± 12.8 | 55.2 ± 12.2 | <0.001 |
| Carbohydrates (g/d)6 | 188 ± 47 | 187 ± 40 | 190 ± 38 | 192 ± 35 | 197 ± 34 | <0.001 |
| Total fat (g/d)6 | 58.8 ± 17.2 | 63.4 ± 14.7 | 65.7 ± 13.7 | 68.8 ± 13.0 | 72.1 ± 12.9 | <0.001 |
| MUFA (g/d)6 | 22.7 ± 8.0 | 23.9 ± 6.5 | 24.7 ± 5.9 | 26.0 ± 5.6 | 27.6 ± 5.5 | <0.001 |
| PUFA (g/d)6 | 14.9 ± 5.6 | 16.4 ± 5.3 | 17.1 ± 5.2 | 17.8 ± 5.0 | 18.6 ± 5.1 | <0.001 |
| SFA (g/d)6 | 16.5 ± 5.4 | 18.2 ± 5.0 | 19.0 ± 4.7 | 19.9 ± 4.5 | 20.7 ± 4.3 | <0.001 |
| trans Fat (g/d)6 | 2.8 ± 0.6 | 4.0 ± 0.3 | 5.0 ± 0.3 | 6.2 ± 0.4 | 8.6 ± 1.6 | <0.001 |
There were 30,239 participants in the REGARDS study; 8986 were excluded for missing data from the Block 98 food-frequency questionnaires and 2740 for missing data on covariates, which left 18,513 participants for the current analysis. CKD, chronic kidney disease; DBP, diastolic blood pressure; hsCRP, high-sensitivity C-reactive protein; IHD, ischemic heart disease; REGARDS, Reasons for Geographic and Racial Differences in Stroke; SBP, systolic blood pressure; WBC, white blood cell.
Differences between quintiles of trans fat were analyzed by using the chi-square test for categorical variables and 1-factor ANOVA or the Kruskal-Wallis test for continuous variables
Mean ± SD (all such values).
Missing values: 4861 for serum albumin, 5442 for WBC count, 7 for SBP, and 8 for DBP.
Median; 25th and 75th quartiles in parentheses (all such values).
Adjusted for total energy intake.
During 7 y of follow-up (93,147 person years), 1572 of the 18,513 study participants died. The mortality rate per 1000 person-years of follow-up (95% CI) was 12.8 (11.3, 14.5), 14.3 (12.7, 16.2), 14.6 (13.0, 16.5), 19.0 (17.1, 21.1), and 23.6 (21.5, 25.9) for the first through the fifth quintile of TFA intake, respectively.
Kaplan-Meier and Cox regression analyses
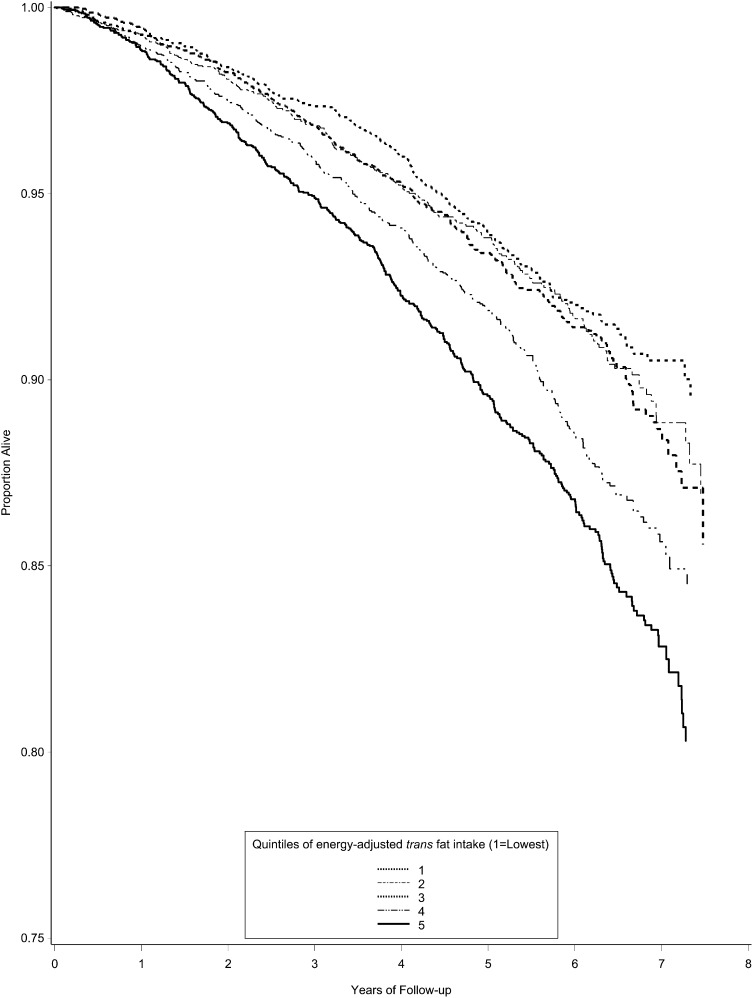
Kaplan-Meier curves for survival across quintiles of TFA intake are shown in Figure 2, which show a lower survival in the top quintiles of TFA intake (log rank, P < 0.001). The HRs of death by quintiles of energy-adjusted TFA intake are shown in Table 2. All models satisfied the proportionality assumption. In the unadjusted model, individuals in the highest 2 quintiles had an increased risk of all-cause mortality for the fourth (HR: 1.48; 95% CI: 1.26, 1.75) and fifth (HR: 1.83; 95% CI: 1.57, 2.15) quintiles. This association remained significant after adjustment for age, sex, smoking status, race, region, alcohol use, education, waist circumference, level of physical activity, diabetes, IHD, hypertension, stroke, probable HF, chronic kidney disease, use of statins, total energy intake, and energy-adjusted intakes of SFA, MUFA, PUFA, and proteins for the fourth quintile (HR: 1.25; 95% CI: 1.05, 1.48) and fifth quintile (HR: 1.24; 95% CI: 1.05, 1.48). In all models, a significant trend of increasing risk of all-cause mortality with increasing quintiles of TFA intake was observed (P < 0.05). No significant interactions were found between TFA and sex (P = 0.36), race (P = 0.77), or PUFA intake (P = 0.34).
FIGURE 2.
Proportion of living participants by quintiles of energy-adjusted trans fat intake in the Reasons for Geographic and Racial Differences in Stroke study (recruited from all across the continental United States from January 2003 to October 2007). Of 30,239 participants, 8986 were excluded for missing data from the Block 98 food-frequency questionnaires and 2740 for missing data on covariates, which left 18,513 participants for the current analysis. Differences in survival by quintiles of trans fat intake were analyzed by using the Kaplan-Meier method. The log-rank P value was <0.001.
TABLE 2.
The association between quintiles of trans fat intake and all-cause mortality in the REGARDS study, 2003–20071
| Quintiles of trans fat intake |
||||||
| 1 (n = 3702) | 2 (n = 3703) | 3 (n = 3703) | 4 (n = 3702) | 5 (n = 3703) | P-trend | |
| No. of deaths | 238 | 266 | 272 | 354 | 442 | |
| Person-years | 18,627 | 18,566 | 18,579 | 18,656 | 18,720 | |
| Mortality/1000 person-years | 12.8 (11.3, 14.5)2 | 14.3 (12.7, 16.2) | 14.6 (13.0, 16.5) | 19.0 (17.1, 21.1) | 23.6 (21.5, 25.9) | |
| Model3 | ||||||
| 1 | 1.00 | 1.12 (0.94, 1.34) | 1.15 (0.97, 1.37) | 1.48 (1.26, 1.75) | 1.83 (1.57, 2.15) | <0.001 |
| 2 | 1.00 | 1.04 (0.87, 1.24) | 1.04 (0.87, 1.24) | 1.28 (1.08, 1.51) | 1.36 (1.15, 1.59) | <0.001 |
| 3 | 1.00 | 1.03 (0.86, 1.23) | 0.98 (0.82, 1.17) | 1.25 (1.05, 1.48) | 1.24 (1.05, 1.48) | 0.004 |
There were 30,239 participants in the REGARDS study; 8986 were excluded for missing data from the Block food-frequency questionnaires and 2740 for missing data on covariates, which left 18,513 participants for the current analysis. Cox regression was used to test the association between trans fat and all-cause mortality. REGARDS, Reasons for Geographic and Racial Differences in Stroke.
HR; 95% CI in parentheses (all such values).
Model 1: unadjusted. Model 2: adjusted for sex, age, and smoking status. Model 3: adjusted as for model 2 plus race, region, alcohol use, education, waist circumference, level of physical activity, diabetes, coronary heart disease, hypertension, stroke, heart failure, chronic kidney disease, statin use, total energy intake, and energy-adjusted intakes of SFA, MUFA, PUFA, proteins, and carbohydrates.
In analyses that additionally adjusted for HDL cholesterol, LDL cholesterol, triglycerides, and CRP, the HR (95% CI) for all-cause mortality for the first through fifth quintiles of TFA intake were 1.00, 1.02 (0.85, 1.21), 0.98 (0.82, 1.17), 1.25 (1.05, 1.49), and 1.25 (1.05, 1.49), respectively (P-trend = 0.002). In analyses that excluded people without data on inflammatory markers (n = 13,070), the HRs (95% CIs) for the first through fifth quintiles of TFA intake were 1.00, 1.20 (0.95, 1.51), 1.06 (0.83, 1.34), 1.26 (1.00, 1.60), and 1.33 (1.04, 1.69), respectively (P-trend = 0.103) for the model without serum lipids and inflammatory markers and 1.00, 1.16 (0.92, 1.47), 1.01 (0.80, 1.29), 1.25 (0.98, 1.58), and 1.30 (1.02, 1.65), respectively (P-trend = 0.095) for the model with lipids and inflammatory markers.
Population attributable risk estimates
The population attributable risk was 0.21 (95% CI: 0.18, 0.23) in the unadjusted model, whereas it was 0.07 (95% CI: 0.05, 0.08) in the fully adjusted model.
DISCUSSION
A higher intake of TFA was associated with an increased risk of all-cause mortality in the REGARDS cohort. Compared with the first quintile, the risk of increased mortality was 25% (95% CI: 5%, 48%) in the fourth quintile and 24% (95% CI: 5%, 48%) in the fifth quintile. A dose-response relation with a significant trend in mortality was observed across quintiles of TFA intake (P < 0.05). Contrary to studies on TFA and cardiovascular mortality, the association between TFA and all-cause mortality observed in this study was only significant at higher intakes of TFA (3.45% of energy compared with ∼2% of energy in cardiovascular disease mortality studies) (2).
To our knowledge, this was the first study to demonstrate a direct association between TFA intake and all-cause mortality. Our findings are in line with those from the Seven Countries ecologic study (3), in which Kromhout et al showed a strong positive correlation between the average intake of the trans fatty acid elaidic acid and the 25-y mortality from IHD (r = 0.78, P < 0.001).
Our results partly corroborate results from mathematical models that suggested that a high TFA intake is associated with an increase in all-cause mortality. For example, a study conducted by Danaei et al (11) combined knowledge on risk factors with data from previous studies as well as disease-specific mortality records and estimated that, in 2005, 82,000 deaths were attributed to high dietary TFA in the United States. An earlier estimate by Willet and Ascherio (4) indicated that, every year, >30,000 deaths could be attributed to TFA intake (assuming 2% energy from TFA) in the United States. On the basis of our results, we estimated that 7% (95% CI: 5%, 8%) of deaths due to any cause would be avoided if the REGARDS study participants reduced their TFA energy intake to <1.6% (the intake in our first quintile). Using the 2005 reported US total mortality (2,448,017 deaths) and our estimated population attributable risk, we estimated that 24,480 deaths per year could be avoided by reducing the TFA intake to <1.6%—an intake still higher than the current guideline of <1%.
The adverse effects of TFA intake on cardiovascular outcomes are thought to be mediated mainly through lipids and inflammation (2). A higher TFA intake is associated with increases in TNF-α, monocyte chemotactic protein-1, IL-6, and CRP (22–24). Elevated inflammation is an independent risk factor for cardiovascular disease (24, 30) and all-cause mortality (13, 31–34). In addition, an elevated TFA intake is associated with endothelial dysfunction (5, 25), decreased activity of serum paraoxonase (35), higher cholesterol ester transfer protein activity (36), elevated serum LDL cholesterol (26), decreased LDL cholesterol particle size (37), decreased serum HDL cholesterol (24, 25), and impaired postprandial activity of tissue plasminogen activator (2). All of these are associated with adverse cardiovascular outcomes and other chronic conditions.
The proinflammatory effects of TFA may also be the link between TFA and cancer (38). Chronic inflammation is a critical component in cancer pathogenesis (39). Reactive oxygen and nitrogen species produced by inflammatory cells are thought to induce DNA damage, which ultimately results in neoplastic transformation of the affected cells (38, 39). People with chronic inflammatory conditions have a higher risk of cancer than do those without these conditions. For example, people with chronic Helicobacter pylori infection have a higher risk of stomach cancer (40) and people with chronic ulcerative colitis or Crohn disease have a higher risk of colon cancer (38). Indeed, long-term use of nonsteroidal antiinflammatory drugs is associated with a 40–50% lower risk of colon cancer (41, 42).
Inflammation may also mediate the association between TFA intake and stroke (9, 43). Atherosclerosis, one of the most prevalent causes of stroke (44), is widely regarded as an inflammatory process (45). Increased TFA intake is associated with atherogenic dyslipidemia involving elevated LDL cholesterol, decreased LDL particle size, and a reduction of HDL cholesterol (37, 46). In addition, an elevated TFA intake is associated with an increased incidence of diabetes (6)—an independent risk factor for stroke (44). Despite the aforementioned studies, analyses that adjusted for lipids did not substantially alter the HR for all-cause mortality. We observed a small attenuation in the HRs after adjustment for inflammation, but there was a concomitant reduction in the sample size from 18,513 to 13,070. Thus, the small changes in the HRs may have been due to a change in the sample size.
We acknowledge many limitations of this analysis. First, TFA and other dietary intake data were self-reported and were thus subject to measurement error. However, the Block food-frequency questionnaire used in this study has been validated in many studies in the United States and yielded consistent TFA intake estimates (14–17). In addition, TFA intake measured in this cohort showed associations with lipids, especially HDL cholesterol, that are consistent with those from other studies. Second, because the study used baseline dietary data to predict future mortality, the implicit assumption was that an individual's TFA intake remained constant over the follow-up period. As in Canada, where Health Canada called for a voluntary reduction in the amount of TFA in foods (47), TFA intakes in the United States are likely to have decreased over the years following legislation to reduce the amount of TFA in foods (48). Thus, the associations observed in our study may be imprecise. On the other hand, our approach of using intakes at baseline has been used by many studies and has apparently provided accurate data (5, 15, 16). Third, because of the lack of data on TFA isomers in the REGARDS cohort, we were not able to compare the effects of TFA from ruminant sources with those from partial hydrogenation of cooking oils. However, whereas some studies have suggested that TFA from ruminant sources may be cardioprotective, a recent meta-analysis showed that both ruminant- and industry-derived TFA have similar effects on the cardiovascular system (49). Finally, adjudicated data on all individual causes of death are not yet available in the REGARDS cohort. This precluded analyses to determine the relation between TFA intake and individual causes of death. This will be the subject of subsequent analyses.
The current study also had many strengths. First, it was based on a large sample (n = 18,513) of black and white men and women from the continental United States. Second, there was a large number of deaths during the 7-y follow-up period (n = 1572), which provided ample statistical power. Third, deaths were confirmed by using validated methods. Finally, this study included various energy-adjusted macronutrient variables (eg, PUFAs and carbohydrates) in the models because of their known correlations with trans fat (50) and various major causes of death. Although this was likely to have reduced the potential for spurious findings, residual confounding cannot be ruled out.
In conclusion, an increased intake of TFAs is associated with an increased risk of all-cause mortality independent of known risk factors. Contrary to studies on TFA intake and cardiovascular mortality, the association between TFA intake and all-cause mortality was significant only at higher intakes of TFA (≥3.5% of energy in this study compared with ∼2% in cardiovascular disease mortality studies), which suggests that TFA may be more potent at increasing the risk of cardiovascular disease than of other causes of death. More studies are needed to further evaluate the contribution of TFA to death from all causes.
Acknowledgments
We thank the investigators and staff of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
The authors’ responsibilities were as follows—EKK: conceived the idea and designed the analysis plan; JNK, BCH, CJR, YC, TAM, and PL: conducted the literature searches; PDM: conducted the data analysis; JNK: drafted the manuscript; and JNK, BCH, VJH, EKK, PDM, SEJ, CJR, YC, TAM, PL, and MC: interpreted the results, edited various drafts of the manuscript, and approved the final manuscript. The authors declared no conflicts of interest. Amgen had no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation or approval of the manuscript.
Footnotes
Abbreviations used: CRP, C-reactive protein; HF, heart failure; IHD, ischemic heart disease; REGARDS, Reasons for Geographic and Racial Differences in Stroke; TFA, trans-unsaturated fatty acids; WBC, white blood cell.
REFERENCES
- 1.Smit LA, Katan MB, Wanders AJ, Basu S, Brouwer IA. A high intake of trans fatty acids has little effect on markers of inflammation and oxidative stress in humans. J Nutr 2011;141:1673–8. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med 2006;354:1601–13. [DOI] [PubMed] [Google Scholar]
- 3.Kromhout D, Menotti A, Bloemberg B, Aravanis C, Blackburn H, Buzina R, Dontas AS, Fidanza F, Giampaoli S, Jansen A, et al. Dietary saturated and trans fatty acids and cholesterol and 25-year mortality from coronary heart disease: the Seven Countries Study. Prev Med 1995;24:308–15. [DOI] [PubMed] [Google Scholar]
- 4.Willett WC, Ascherio A. Trans fatty acids: are the effects only marginal? Am J Public Health 1994;84:722–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez-Garcia E, Schulze MB, Meigs JB, Manson JE, Rifai N, Stampfer MJ, Willett WC, Hu FB. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J Nutr 2005;135:562–6. [DOI] [PubMed] [Google Scholar]
- 6.Salmerón J, Hu FB, Manson JE, Stampfer MJ, Colditz GA, Rimm EB, Willett WC. Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutr 2001;73:1019–26. [DOI] [PubMed] [Google Scholar]
- 7.Chajès V, Thiébaut ACM, Rotival M, Gauthier E, Maillard V, Boutron-Ruault M-C, Joulin V, Lenoir GM, Clavel-Chapelon F. Association between serum trans-monounsaturated fatty acids and breast cancer risk in the E3N-EPIC study. Am J Epidemiol 2008;167:1312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu J, La Vecchia C, Gibbons L, Negri E, Mery L. Nutrients and risk of prostate cancer. Nutr Cancer 2010;62:710–8. [DOI] [PubMed] [Google Scholar]
- 9.Yaemsiri S, Sen S, Tinker L, Rosamond W, Wassertheil-Smoller S, He K. Trans fat, aspirin, and ischemic stroke in postmenopausal women. Ann Neurol 2012;72:704–15.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Astorg P. Dietary fatty acids and colorectal and prostate cancers: epidemiological studies. Bull Cancer 2005;92:670–84. [PubMed] [Google Scholar]
- 11.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med 2009;6:e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005;25(3):135–43. [DOI] [PubMed]
- 13.Kabagambe EK, Judd SE, Howard VJ, Zakai NA, Jenny NS, Hsieh M, Warnock DG, Cushman M. Inflammation biomarkers and risk of all-cause mortality in the Reasons for Geographic and Racial Differences in Stroke cohort. Am J Epidemiol 2011;174:284–92. [DOI] [PMC free article] [PubMed]
- 14.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol 2001;154:1089–99. [DOI] [PubMed] [Google Scholar]
- 15.Mares-Perlman JA, Klein BE, Klein R, Ritter LL, Fisher MR, Freudenheim JL. A diet history questionnaire ranks nutrient intakes in middle-aged and older men and women similarly to multiple food records. J Nutr 1993;123:489–501. [DOI] [PubMed] [Google Scholar]
- 16.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol 1990;43:1327–35. [DOI] [PubMed] [Google Scholar]
- 17.Boucher B, Cotterchio M, Kreiger N, Nadalin V, Block T, Block G. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr 2006;9(1):84–93. [DOI] [PubMed]
- 18.Cushman M, McClure LA, Howard VJ, Jenny NS, Lakoski SG, Howard G. Implications of increased C-reactive protein for cardiovascular risk stratification in black and white men and women in the US. Clin Chem 2009;55:1627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pullicino PM, McClure LA, Wadley VG, Ahmed A, Howard VJ, Howard G, Safford MM. Blood pressure and stroke in heart failure in the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Stroke 2009;40:3706–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy RE, Howard G, Go RC, Rothwell PM, Tiwari HK, Feng R, McClure LA, Prineas RJ, Banerjee A, Arnett DK. Association between family risk of stroke and myocardial infarction with prevalent risk factors and coexisting diseases. Stroke 2012;43:974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65(suppl):1220S–31S. [DOI] [PubMed]
- 22.Mozaffarian D, Rimm EB, King IB, Lawler RL, McDonald GB, Levy WC. trans Fatty acids and systemic inflammation in heart failure. Am J Clin Nutr 2004;80:1521–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mozaffarian D, Pischon T, Hankinson SE, Rifai N, Joshipura K, Willett WC, Rimm EB. Dietary intake of trans fatty acids and systemic inflammation in women. Am J Clin Nutr 2004;79:606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baer DJ, Judd JT, Clevidence BA, Tracy RP. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: a randomized crossover study. Am J Clin Nutr 2004;79:969–73. [DOI] [PubMed] [Google Scholar]
- 25.de Roos NM, Bots ML, Katan MB. Replacement of dietary saturated fatty acids by trans fatty acids lowers serum HDL cholesterol and impairs endothelial function in healthy men and women. Arterioscler Thromb Vasc Biol 2001;21:1233–7. [DOI] [PubMed] [Google Scholar]
- 26.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 2003;77:1146–55. [DOI] [PubMed] [Google Scholar]
- 27.Kabagambe EK, Ordovas JM, Hopkins PN, Tsai MY, Arnett DK. The relation between erythrocyte trans fat and triglyceride, VLDL- and HDL-cholesterol concentrations depends on polyunsaturated fat. PLoS ONE 2012;7:e47430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hertzmark E, Wand H, Spiegelman D. The SAS PAR macro. Available from: http://www.hsph.harvard.edu/faculty/donna-spiegelman/files/par_documentation-_march_2012.pdf (cited 20 June 2012).
- 29.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control 2007;18:571–9. [DOI] [PubMed] [Google Scholar]
- 30.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342:836–43. [DOI] [PubMed] [Google Scholar]
- 31.Ye J, Kiage JN, Arnett DK, Bartolucci AA, Kabagambe EK. Short-term effect of fenofibrate on C-reactive protein: a meta-analysis of randomized controlled trials. Diabetol Metab Syndr 2011;3:24. [DOI] [PMC free article] [PubMed]
- 32.Jenny NS, Yanez ND, Psaty BM, Kuller LH, Hirsch CH, Tracy RP. Inflammation biomarkers and near-term death in older men. Am J Epidemiol 2007;165:684–95. [DOI] [PubMed] [Google Scholar]
- 33.Amemiya N, Ogawa T, Otsuka K, Ando Y, Nitta K. Comparison of serum albumin, serum C-reactive protein, and pulse wave velocity as predictors of the 4-year mortality of chronic hemodialysis patients. J Atheroscler Thromb 2011;18:1071–9. [DOI] [PubMed] [Google Scholar]
- 34.Xie Q, Zhou Y, Xu Z, Yang Y, Kuang D, You H, Ma S, Hao C, Gu Y, Lin S, et al. The ratio of CRP to prealbumin levels predict mortality in patients with hospital-acquired acute kidney injury. BMC Nephrol 2011;12:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Roos NM, Schouten EG, Scheek LM, van Tol A, Katan MB. Replacement of dietary saturated fat with trans fat reduces serum paraoxonase activity in healthy men and women. Metabolism 2002;51:1534–7. [DOI] [PubMed] [Google Scholar]
- 36.Abbey M, Nestel PJ. Plasma cholesteryl ester transfer protein activity is increased when trans-elaidic acid is substituted for cis-oleic acid in the diet. Atherosclerosis 1994;106(1):99–107. [DOI] [PubMed]
- 37.Mauger JF, Lichtenstein AH, Ausman LM, Jalbert SM, Jauhiainen M, Ehnholm C, Lamarche B. Effect of different forms of dietary hydrogenated fats on LDL particle size. Am J Clin Nutr 2003;78:370–5. [DOI] [PubMed] [Google Scholar]
- 38.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park) 2002;16(2):217ndash26, 29; discussion 30–2. [PubMed]
- 40.Ernst PB, Gold BD. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol 2000;54:615–40. [DOI] [PubMed] [Google Scholar]
- 41.Baron JA, Sandler RS. Nonsteroidal anti-inflammatory drugs and cancer prevention. Annu Rev Med 2000;51:511–23. [DOI] [PubMed] [Google Scholar]
- 42.García-Rodríguez LA, Huerta-Alvarez C. Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Epidemiology 2001;12:88–93. [DOI] [PubMed] [Google Scholar]
- 43.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation 1998;98:731–3. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, Creager MA, Culebras A, Eckel RH, Hart RG, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:517–84. [DOI] [PubMed] [Google Scholar]
- 45.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 1999;340:115–26. [DOI] [PubMed] [Google Scholar]
- 46.Ascherio A, Katan MB, Zock PL, Stampfer MJ, Willett WC. Trans fatty acids and coronary heart disease. N Engl J Med 1999;340:1994–8. [DOI] [PubMed] [Google Scholar]
- 47.Ratnayake WM, L'Abbe MR, Farnworth S, Dumais L, Gagnon C, Lampi B, Casey V, Mohottalage D, Rondeau I, Underhill L, et al. Trans fatty acids: current contents in Canadian foods and estimated intake levels for the Canadian population. J AOAC Int 2009;92:1258–76. [PubMed] [Google Scholar]
- 48.F DA. Food labeling: trans fatty acids in nutrition labeling, nutrient content claims, and health claims. Final rule. Fed Regist 2003;68:41433–506. [PubMed]
- 49.Brouwer IA, Wanders AJ, Katan MB. Effect of animal and industrial trans fatty acids on HDL and LDL cholesterol levels in humans—a quantitative review. PLoS ONE 2010;5:e9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kris-Etherton PM, Lefevre M, Mensink RP, Petersen B, Fleming J, Flickinger BD. Trans fatty acid intakes and food sources in the U.S. population: NHANES 1999-2002. Lipids 2012;47:931–40. [DOI] [PMC free article] [PubMed] [Google Scholar]