Abstract
Background: Undernutrition remains a significant problem worldwide, with environmental enteropathy implicated as a contributing factor. An understanding of the pathogenesis and identification of children at risk are critical to the design of more-effective interventions.
Objective: The stool regenerating gene 1β (REG1B) protein, which is a putative measure of intestinal injury and repair, was tested as a noninvasive biomarker of future childhood stunting.
Design: A total of 222 children from Bangladesh and 97 children from Peru, who were from impoverished communities, were followed from birth through 24 mo of age with anthropometric measures obtained every 3 mo. Stool REG1B protein concentrations were obtained by using an REG1B polyclonal-polyclonal ELISA at 3 mo of age. We tested for the ability of REG1B to forecast future anthropometric shortfalls, independent of known predictors of undernutrition of family income and baseline height and weight.
Results: In the Bangladesh cohort of 222 children, higher REG1B concentrations at month 3 were significantly and independently associated with a growth shortfall in a linear regression analysis at months 9, 12, 18, 21, and 24 and, in the Peru cohort, at months 12, 15, 18, 21, and 24. With the use of a mixed model for repeated measurements, higher stool REG1B concentrations at 3 mo were also independently predictive of a lower future length-for-age z score through 24 mo of age (Bangladesh P = 0.006; Peru P = 0.058).
Conclusion: The ability of fecal REG1B to predict growth shortfall in independent cohorts of impoverished children from the developing world offers promise as a malnutrition biomarker and supports a role for environmental enteropathy in the pathogenesis of growth shortfall.
INTRODUCTION
WHO growth standards were developed by using data obtained from infants and young children of different ethnic and cultural groups around the world and can be used as a screening tool to detect children at risk of nutritional problems or disease (1, 2). A child whose height or weight is >2 SDs below the WHO growth standard is considered stunted (low-height-for-age) or underweight (low-weight-for-age), respectively (1, 3).
Despite progress over the years in many countries, undernutrition remains a widespread problem. For example, the 2007 Bangladesh Demographic and Health Survey showed that 43% of children <5 y of age are stunted, 41% are underweight, and 17% are wasted. The survey also showed that 16% of children <5 y of age are severely stunted, 12% are severely underweight, and 3% are severely wasted (4).
Infectious diseases are the leading cause of death in children <5 y of age worldwide (5). Undernutrition increases risk of infections such as pneumonia and diarrhea, which in turn increase risk of additional undernutrition (6). Both frequent infections and inadequate nutrition in early childhood are common causes of childhood stunting, and the negative consequences of stunting can last into adulthood. Stunted children are more likely to grow into stunted adults, and are also more likely to have learning disabilities in adulthood. Undernutrition during early childhood has also been associated with hypertension, insulin resistance, and dyslipidemia during adulthood (7, 8). Fetal intrauterine growth restriction is another common cause of childhood stunting, and maternal stunting is subsequently associated with intrauterine growth restriction and increased perinatal mortality (3).
Fortunately, nutritional interventions early in life (within the first 3 y) can have a significant effect on long-term height and future economic productivity (3). The identification of children at risk of undernutrition, so that interventions can be intensified early, is critical. To this end, noninvasive biomarkers that are easy to perform in a resource-limited setting are needed.
The purpose of this study was to determine whether there is an association between stool regenerating gene 1β (REG1B)4 protein concentrations with subsequent childhood growth deficits. The regenerating gene family has 4 subclasses (I, II, III, and IV), and regenerating gene 1α (REG1A) and REG1B genes are highly homologous (9). Frequent or persistent childhood diarrhea may lead to malnutrition, and REG1A messenger RNA expression has been shown to be increased within inflamed colonic tissue (10–12) whereby REG1 proteins may be involved in tissue repair (13, 14), cell growth, and regeneration (15, 16). Therefore, we hypothesized that higher regenerating gene 1 (REG1) protein concentrations in stool early in life may be a measure of ongoing intestinal injury that may predict future growth deficits. Thus, the rationale for the work was to determine whether REG1B was a potential biomarker and test whether intestinal injury was associated with undernutrition. We evaluated this combined hypothesis by testing a stool REG1B ELISA format in birth cohorts in Bangladesh and Peru.
SUBJECTS AND METHODS
Subjects were from birth cohorts in Dhaka, Bangladesh, or Iquitos, Peru. The populations were not derived via random sampling procedures to derive a sample that was representative of Bangladesh or Peru populations. However, anthropometric indexes were consistent with regional and national indexes. The children were followed longitudinally from birth until ≤24 mo of age, and anthropometric measures were obtained every 3 mo. Stool REG1B concentrations were measured by using a REG1B polyclonal-polyclonal ELISA at 3 mo for the first 222 and 97 children enrolled in the Bengali and Peruvian birth cohorts respectively. The research protocol was reviewed by the Institutional Review Boards of the University of Virginia, International Centre for Diarrhoeal Disease Research, Bangladesh, The Johns Hopkins Bloomberg School of Public Health, and the Asociacion Benefica PRISMA, Lima, Peru.
Weights and lengths of children were assessed by using standard procedures of the WHO (2) by using electronic scales that were precise to 10 g (SECA GmbH & Co) and length boards that were precise to 1 mm (SECA GmbH & Co). Weight and length were taken 2 times, and the mean of 2 measurements were recorded. Weight and length measurements were converted to weight-for-age z scores (WAZs) and length-for-age z scores (LAZs) according to the WHO Multicenter Growth Reference Study child growth standards (2). Underweight was defined as a WAZ less than –2, and stunting was defined as a LAZ less than –2.
ELISA plates were coated with goat polyclonal anti-REG1B at 1 μg/well. Fecal samples were diluted at 1:10,000 before the addition of 100 μL REG1B standards/well, a negative control, or fecal samples to plates. Plates were incubated at 37°C, washed 5 times, and 100 μL 0.2 mg/mL goat anti-REG1B–horseradish peroxidase/well, which was diluted 1:5000, was added. Plates were again incubated at 37°C, washed, and substrate was added before additional incubation at room temperature. After the stop solution was added, plates were read at OD 450/620.
The study involved a longitudinal design with repeated anthropometric measures over a 24-mo period. This design is powerful because it allows for the study of changes within individuals over time; however, a limitation of this design is that the repeated measurements within each child are correlated. Therefore, by focusing on a specific time point, a simple linear regression analysis may assume a greater association than is actually present (17). A mixed-model analysis, in contrast, involves repeated measurements to detect developmental trends across the whole follow-up time. A mixed model incorporates both fixed and random effects and adjusts for within-subject correlation. Therefore, we performed a linear regression analysis and confirmed the results of the linear regression analysis with a mixed model for repeated measurements.
First, a linear regression analysis was performed in a cohort of 222 Bengali children and a cohort of 97 Peruvian children to determine whether there was an association with REG1B concentrations at 3 mo and future anthropometric measures, with adjustment for potential confounding effects of family income, sex, and LAZ, WAZ, or weight-for-height z score (WHZ) at 3 mo or birth weight. A mixed model for repeated measurements was further used to confirm if REG1B concentrations at 3 mo were associated with future anthropometric measures in both Bengali and Peruvian birth cohorts. Statistical significance in this study was set at P < 0.05, and all reported P values were 2 sided. All analyses were performed with SAS software (version 9.3; SAS Institute), and the hier.part package in R statistical software (version 2.15; R Project for Statistical Computing).
RESULTS
We performed studies in birth cohorts of children in both Bangladesh and Peru to test for an association between stool REG1B concentrations and future anthropometric measures. We used 2 cohorts on separate continents to evaluate the consistency of the relation between the biomarker and the anthropometric outcomes in different epidemiologic contexts. Demographic data were similar for the 2 cohorts except that Bengali mothers were more likely to have BMI (in kg/m2) <18.5 (Table 1).
TABLE 1.
Demographic information for Bangladesh and Peru cohorts1
| Variables | Bangladesh cohort (n = 222) | Peru cohort (n = 97) |
| M [n (%)] | 114 (51.4) | 50 (51.5) |
| Family monthly income ($) | 82.2 ± 35.1 | 180.8 ± 162.3 |
| Maternal education [n (% with formal education)] | 134 (60.4) | 97 (100) |
| Maternal BMI (kg/m2) | 20.9 ± 2.9 | 25.5 ± 3.5 |
| Mothers with BMI <18.5 kg/m2 [n (%)] | 44 (19.8) | 1 (1.0) |
| Birth weight (kg) | 2.7 ± 0.4 | 3.2 ± 0.4 |
Data are expressed as counts (percentages) for categorical measures and means ± SDs for continuous measures.
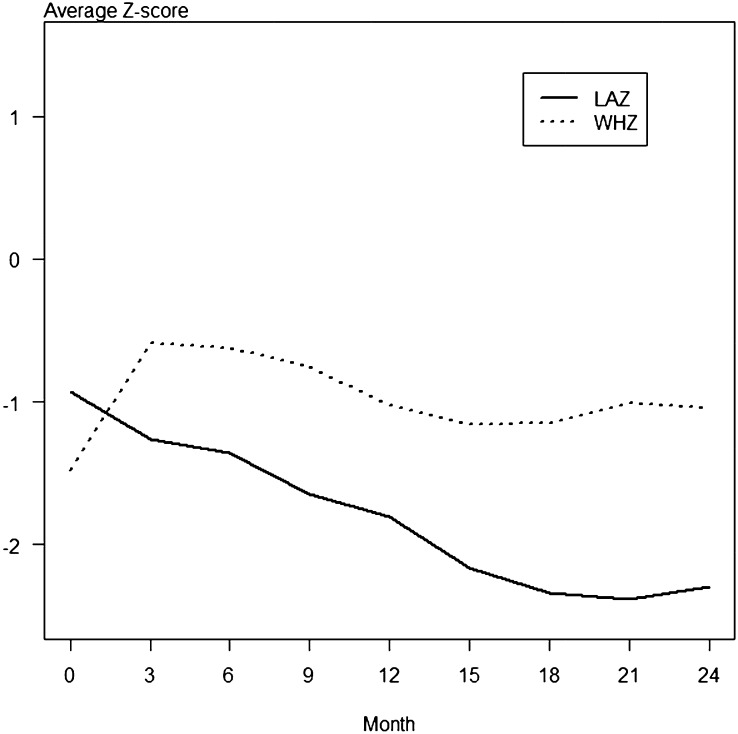
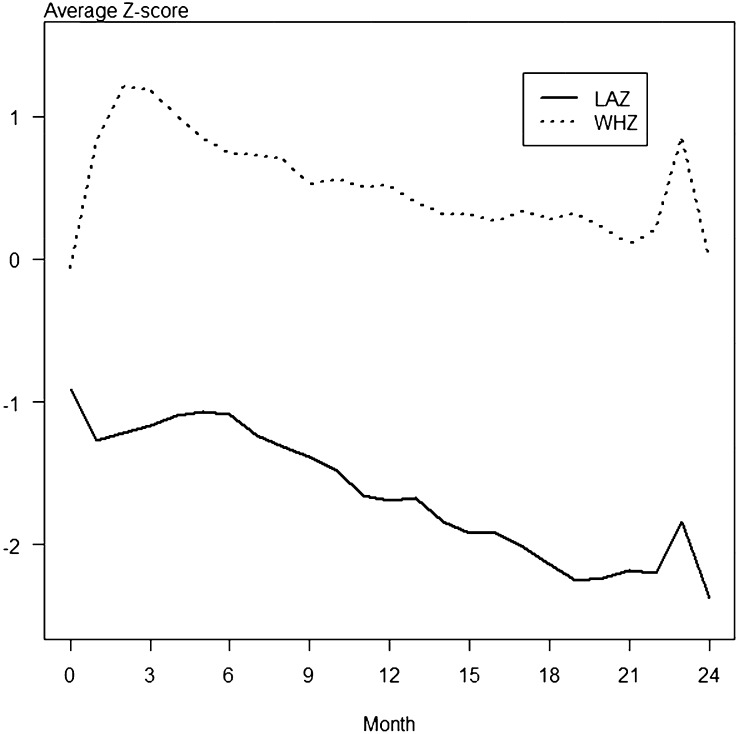
As shown in Figure 1, average LAZs in the Bengali cohort, which consisted of 222 children, were below normal at the initiation of the study and declined over the following 24 mo. WHZs were also below normal throughout the study, although less abnormal than LAZs. As shown in Figure 2, average LAZs in the Peruvian cohort, which consisted of 97 children, were also below normal at the initiation of the study. LAZs were the most abnormal anthropometric measure throughout the study, whereas WHZs stayed near the normal range.
FIGURE 1.
Anthropometric z scores from 0 to 24 mo of age in the Bangladesh cohort. A total of 222 children from a birth cohort in Bangladesh were followed during their first 24 mo of life. Anthropometric measures were obtained every 3 mo. Average LAZs in the Bengali cohort were below normal at the initiation of the study and declined over the next 24 mo. WHZs were also below normal throughout the study, although less abnormal than LAZs. (The 95% CIs were too small to depict in the graph.) LAZ, length-for-age z score; WHZ, weight-for-height z score.
FIGURE 2.
Anthropometric z scores from 0 to 24 mo of age in the Peru cohort. A total of 97 children from a birth cohort in Peru were followed during their first 24 mo of life. Anthropometric measures were obtained every 3 mo. Average LAZs in the Peruvian cohort were below normal at the initiation of the study and declined over the next 24 mo, whereas WHZs stayed near the normal range. (The 95% CIs were too small to depict in the graph.) LAZ, length-for-age z score; WHZ, weight-for-height z score.
Fecal REG1B concentrations were measured when children were 3 mo of age because this was a time at which the WHZ was maximal (Figures 1 and 2). The median ± SD REG1B concentration in stool was 30.8 ± 26.3 and 16.5 ± 17.7 μg/mL for Bangladesh and Peru respectively. After adjustment for sex, a linear regression analysis was performed for LAZs at 3 mo of age, family income, and REG1B concentrations in each cohort. In the Bengali cohort, higher REG1B concentrations at month 3 were significantly and independently associated with a lower LAZ at months 9, 12, 18, 21, and 24 (Table 2). The association of REG1B with future stunting also remained significant when controlled for birth weight instead of the 3-mo LAZ (data not shown). There was not a significant interactive effect of low birth weight (<2.5 kg) and REG1B on repeated LAZs in either Bangladesh or Peru cohorts (data not shown). Higher REG1B concentrations at month 3 were not associated with a diminished WHZ at any month (data not shown).
TABLE 2.
Linear regression analysis modeling future LAZs at 6–24 mo to LAZs at 3 mo, REG1B, and family monthly income for the Bangladesh birth cohort1
| Bangladesh cohort |
|||||||||
| LAZ at 3 mo (every unit) |
REG1B (every 20 μg/mL) |
Family income (every $100) |
|||||||
| Visit | Estimate (SE)2 | Contribution3 | P | Estimate (SE)2 | Contribution3 | P | Estimate (SE)2 | Contribution3 | P |
| % | % | % | |||||||
| Month 6 (n = 220) | 0.62 (0.04) | 54.3 | <0.0001 | −0.07 (0.04) | 0.5 | 0.067 | 0.39 (0.13) | 7.1 | 0.004 |
| Month 9 (n = 218) | 0.57 (0.05) | 43.8 | <0.0001 | −0.13 (0.04) | 1.8 | 0.001 | 0.24 (0.15) | 4.2 | 0.114 |
| Month 12 (n = 220) | 0.57 (0.05) | 42.5 | <0.0001 | −0.11 (0.04) | 1.4 | 0.011 | 0.47 (0.16) | 7.5 | 0.003 |
| Month 15 (n = 216) | 0.57 (0.05) | 37.6 | <0.0001 | −0.09 (0.05) | 0.9 | 0.064 | 0.60 (0.18) | 8.2 | 0.001 |
| Month 18 (n = 214) | 0.55 (0.05) | 38.6 | <0.0001 | −0.10 (0.05) | 1.2 | 0.023 | 0.51 (0.17) | 7.9 | 0.002 |
| Month 21 (n = 214) | 0.54 (0.05) | 38.8 | <0.0001 | −0.12 (0.04) | 1.9 | 0.007 | 0.66 (0.16) | 10.9 | <0.0001 |
| Month 24 (n = 215) | 0.55 (0.05) | 35.2 | <0.0001 | −0.16 (0.05) | 2.6 | 0.001 | 0.68 (0.17) | 10.4 | <0.0001 |
LAZ, length-for-age z score; REG1B, regenerating gene 1β.
Estimated effect of LAZ at 3 mo, REG1B, and family income on future LAZ expressed as the magnitude change in future LAZ for every unit increment in LAZ at 3 mo, 20-μg/mL increment in REG1B, and $100 increment in family income. SE values are for the corresponding covariate effect.
Independent contribution of a covariate expressed as the percentage of the explained variation in future LAZ.
Within the Peruvian cohort, higher REG1B concentrations at month 3 were significantly associated with decreased LAZs at 12, 15, 18, 21, and 24 mo (Table 3). REG1B concentrations at month 3 were not associated with subsequent WAZ or WHZ measures (data not shown).
TABLE 3.
Linear regression analysis modeling future LAZ at 6–24 mo to LAZ at 3 mo, REG1B, and family monthly income for the Peru birth cohort1
| Peru cohort |
|||||||||
| LAZ at 3 mo (every unit) |
REG1B (every 20 μg/mL) |
Family income (every $100) |
|||||||
| Visit | Estimate (SE)2 | Contribution3 | P | Estimate (SE)2 | Contribution3 | P | Estimate (SE)2 | Contribution3 | P |
| % | % | % | |||||||
| Month 6 (n = 97) | 0.68 (0.07) | 48.4 | <0.0001 | −0.11 (0.08) | 2.3 | 0.153 | 0.02 (0.04) | 0.02 | 0.558 |
| Month 9 (n = 97) | 0.65 (0.07) | 46.4 | <0.0001 | −0.07 (0.08) | 1.5 | 0.375 | 0.03 (0.04) | <0.01 | 0.406 |
| Month 12 (n = 88) | 0.55 (0.08) | 32.7 | <0.0001 | −0.19 (0.08) | 4.4 | 0.025 | 0.10 (0.05) | 1.4 | 0.029 |
| Month 15 (n = 84) | 0.55 (0.07) | 36.5 | <0.0001 | −0.20 (0.07) | 7.4 | 0.009 | 0.06 (0.04) | 0.3 | 0.147 |
| Month 18 (n = 74) | 0.53 (0.08) | 34.4 | <0.0001 | −0.31 (0.09) | 12.5 | 0.0006 | 0.07 (0.04) | 0.2 | 0.079 |
| Month 21 (n = 49) | 0.46 (0.13) | 20.2 | 0.0009 | −0.30 (0.01) | 13.5 | 0.010 | 0.07 (0.06) | 0.8 | 0.239 |
| Month 24 (n = 37) | 0.41 (0.15) | 21.3 | 0.0107 | −0.42 (0.18) | 28.4 | 0.025 | 0.23 (0.19) | 3.4 | 0.244 |
LAZ, length-for-age z score; REG1B, regenerating gene 1β.
Estimated effect of LAZ at 3 mo, REG1B, and family income on future LAZ expressed as the magnitude change in future LAZ for every unit increment in LAZ at 3 mo, 20-μg/mL increment in REG1B, and $100 increment in family income. Data in parentheses are SEs for the corresponding covariate effect.
Independent contribution of a covariate expressed as the percentage of the explained variation in future LAZ.
A mixed model for repeated measurements was further used to verify if stool REG1B concentrations at 3 mo of age within both cohorts were independently associated with future anthropometric measures. In both Bengali and Peruvian cohorts, higher stool REG1B concentrations at 3 mo were associated with lower future LAZ measures through 24 mo of age (P = 0.006 and P = 0.059, respectively) (Tables 4 and 5). We also evaluated the predictive power of REG1B on the probability of stunting at 12 mo (LAZ less than −2) in the logistic regression. We showed that REG1B was significant (P = 0.0174) after adjustment for sex, LAZ at 3 mo, and monthly family income. The AUC was 0.83, which indicated a reasonably good discrimination.
TABLE 4.
Mixed-model analysis for repeated LAZ and WAZ from 6 to 24 mo by regressing on sex, monthly family income, REG1B polyclonal-polyclonal ELISA, and LAZ and WAZ at 3 mo of age for the Bangladesh birth cohort1
| Responses/outcomes and variables | Values | P |
| LAZ | ||
| Month | −0.058 ± 0.003 | <0.0001 |
| Male sex | −0.022 ± 0.095 | 0.8165 |
| Family income (every $100) | 0.412 ± 0.134 | 0.0021 |
| REG1B (every 20 μg/mL) | −0.100 ± 0.036 | 0.0061 |
| LAZ at 3 mo (every unit) | 0.581 ± 0.040 | <0.0001 |
| WAZ | ||
| Month | −0.034 ± 0.003 | <0.0001 |
| Male sex | −0.045 ± 0.088 | 0.606 |
| Family income (every $1000) | 0.207 ± 0.123 | 0.0918 |
| REG1B (every 20 μg/mL) | −0.017 ± 0.034 | 0.6119 |
| WAZ at 3 mo (every unit) | 0.714 ± 0.040 | <0.0001 |
All values are estimated effects ± SEs. LAZ, length-for-age z score; REG1B, regenerating gene 1β WAZ, weight-for-age z score.
TABLE 5.
Mixed-model analysis for repeated LAZ and WAZ from 6 to 24 mo by regressing on sex, monthly family income, REG1B polyclonal-polyclonal ELISA, and LAZ and WAZ at 3 mo of age for the Peru birth cohort1
| Responses/outcomes and variables | Values | P |
| LAZ | ||
| Month | −0.065 ± 0.004 | <0.0001 |
| Male sex | −0.128 ± 0.120 | 0.2838 |
| Family income (every $100) | 0.045 ± 0.037 | 0.2212 |
| REG1B (every 20 μg/mL) | −0.129 ± 0.068 | 0.0588 |
| LAZ at 3 mo (every unit) | 0.634 ± 0.065 | <0.0001 |
| WAZ | ||
| Month | −0.041 ± 0.004 | <0.0001 |
| Male sex | −0.113 ± 0.113 | 0.3211 |
| Family income (every $100) | −0.022 ± 0.034 | 0.5173 |
| REG1B (every 20 μg/mL) | −0.088 ± 0.064 | 0.1714 |
| WAZ at 3 mo (every unit) | 0.882 ± 0.058 | <0.0001 |
All values are estimated effects ± SEs. LAZ, length-for-age z score; REG1B, regenerating gene 1β WAZ, weight-for-age z score.
DISCUSSION
Childhood undernutrition continues to be prevalent in developing countries. In 2005, an estimated 32% of children <5 of age in the developing world were stunted, 20% were underweight, and 10% were wasted. Although a child's low weight-for-age may indicate wasting or stunting, stunting is the most common and the result of a chronic restriction of a child's growth potential (18). Rapid, noninvasive biomarkers that are able to predict the subsequent development of undernutrition are needed so that early interventions can be implemented and optimized.
This study tested a REG1B polyclonal-polyclonal ELISA to assess associations with REG1B concentrations in stool and future childhood stunting, low weight, or wasting. REG1 proteins may be important markers for increased risk of undernutrition because they have a role in gut health. REG1 proteins are involved with cell differentiation and proliferation within the gastrointestinal tract (19). They are also increased with intestinal inflammation, including during active inflammatory bowel disease, pseudomembranous colitis (20), and amebiasis (21).
We evaluated stool REG1B concentrations in 2 birth cohorts of children in Bangladesh and Peru to identify a possible association between higher REG1B concentrations and lower future anthropometric measures. A linear regression analysis showed that higher stool REG1B concentrations at 3 mo were significantly associated with future LAZs at months 9, 12, 15, 18, 21, and 24 in the cohort of 222 Bengali children and marginally significant at month 6 (Table 1). In the Peruvian cohort, which consisted of 97 children, a linear regression analysis revealed that higher REG1B concentrations at 3 mo were also significantly associated with future stunting at months 12, 15, 18, 21, and 24 (Table 2). The linear regression analysis was confirmed with a mixed model for repeated measurements. When family income was included in the mixed model, the coefficient that corresponded to REG1B and the P value hardly changed, which indicated that income was independent of REG1B in the prediction of a future growth shortfall.
There were several potential limitations of this study. Sex effect, family income, and baseline weight and length were adjusted; however, there may have been residual confounding unknowingly included in the analysis. In addition the polyclonal anti-REG1B antibody may have cross-reacted with the highly similar REG1A protein.
In conclusion, associations with higher REG1B stool protein concentrations at 3 mo of age and future stunting were shown within a cohort of 222 Bengali children by using both linear regression analysis and a mixed model for repeated measures. A linear regression analysis in a smaller cohort of 97 children in Peru similarly showed an association with higher REG1B stool protein concentrations and future stunting. This association with stunting was independent of the LAZ at 3 mo of age. Larger studies with both REG1A and REG1B ELISAs should be performed in additional birth cohorts to verify this preliminary association and attempt to clarify the individual roles of these REG1 proteins. The goal is to predict children at risk of malnutrition so that early interventions can be made.
However, the significance of this work is not only in the identification of a potential biomarker to help predict linear growth retardation but also that the biomarker measures intestinal epithelial health. The association of REG1 with growth implicates gut health and barrier function in the pathogenesis of malnutrition.
Acknowledgments
We thank the children and parents for their participation in the study.
The authors’ responsibilities were as follows—KMP: directed the research and led the writing of the manuscript; JB and RE: developed the REG1B ELISA; ZY, FN, PSK, and JZM: conducted the data analysis; MPO and MNK: directed the Peru site, RH: directed the Bangladesh site; and WAP: conceived the idea and coordinated the work. None of the authors reported any conflicts of interest.
Footnotes
Abbreviations used: LAZ, length-for-age z score; REG1, regenerating gene 1; REG1A, regenerating gene 1α REG1B, regenerating gene 1β WAZ, weight-for-age z score; WHZ, weight-for-height z score.
REFERENCES
- 1.World Health Organization. Global database on child growth and malnutrition. Available from: http://www.who.int/nutgrowthdb/about/introduction/en/index2.html (cited 10 April 2012).
- 2.World Health Organization. The WHO Multicentre Growth Reference Study (MGRS). Available from: http://www.who.int/childgrowth/mgrs/en/ (cited 10 April 2012).
- 3.Dewey KG, Begum K. Long-term consequences of stunting in early life. Matern Child Nutr 2011;7(suppl 3):5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institute of Population Research and Training (NIPORT), Mitra and Associates, and Macro International. Bangladesh Demographic and Health Survey 2007. Dhaka, Bangladesh; Calverton, MD: National Institute of Population Research and Training, Mitra and Associates, and Macro International, 2009. [Google Scholar]
- 5.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulski R, et al. Global, regional, and national causes of child mortality in 2008: a systemic analysis. Lancet 2010;375:1969–87. [DOI] [PubMed] [Google Scholar]
- 6.Schlaudecker EP, Steinhoff MC, Moore SR. Interactions of diarrhea, pneumonia, and malnutrition in childhood: recent evidence from developing countries. Curr Opin Infect Dis 2011;24:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein AD, Wang M, Ramirez-Zea M, Flores R, Grajeda R, Melgar P, Ramakrishnan U, Martorell R. Exposure to a nutrition supplementation intervention in early childhood and risk factors for cardiovascular disease in adulthood: evidence from Guatemala. Am J Epidemiol 2006;164:1160–70. [DOI] [PubMed] [Google Scholar]
- 8.Sawaya AL, Sesso R, Florêncio TM, Fernandes MT, Martins PA. Association between chronic undernutrition and hypertension. Matern Child Nutr 2005;1:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez D, Figarella C, Marchand-Pinatel S, Bruneau N, Guy-Crotte O. Preferential expression of reg I beta gene in human adult pancreas. Biochem Biophys Res Commun 2001;284:729–37. [DOI] [PubMed] [Google Scholar]
- 10.Shinozaki S, Nakamura T, Limura M, Kato Y, Lizuka B, Kobayashi M, Hayashi N. Upregulation of reg 1alpha and GW112 in the epithelium of inflamed colonic mucosa. Gut 2001;48:623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sekikawa A, Fukui H, Fujii S, Nanakin A, Kanda N, Uenoyama Y, Sawabu T, Hisatsune H, Kusaka T, Ueno S, et al. Possible role of REG 1alpha protein in ulcerative colitis and colitic cancer. Gut 2005;54:1437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sekikawa A, Fukui H, Suzuki K, Karibe T, Fujii S, Ichikawa K, Tomita S, Imura J, Shiratori K, Chiba T, et al. Involvement of the IL-22/REG I alpha axis in ulcerative colitis. Lab Invest 2010;90:496–505. [DOI] [PubMed] [Google Scholar]
- 13.Kawanami C, Fukui H, Kinoshita Y, Nakata H, Asahara M, Matsushima Y, Kishi K, Chiba T. Regenerating gene expression in normal gastric mucosa and indomethacin-induced mucosal lesions of the rat. J Gastroenterol 1997;32:12–8. [DOI] [PubMed] [Google Scholar]
- 14.Fukuhara H, Kadowaki Y, Ose T, Monowar A, Imaoka H, Ishihara S, Takasawa S, Kinoshita Y. In vivo evidence for the role of RegI in gastric regeneration: transgenic overexpression of RegI accelerates the healing of experimental gastric ulcers. Lab Invest 2010;90:556–65. [DOI] [PubMed] [Google Scholar]
- 15.Gross DJ, Weiss L, Reibstein I, van den Brand J, Okamoto H, Clark A, Slavin S. Amelioration of diabetes in nonobese diabetic mice with advanced disease by linomide-induced immunoregulation combined with Reg protein treatment. Endocrinology 1998;139:2369–74. [DOI] [PubMed] [Google Scholar]
- 16.Fukui H, Kinoshita Y, Maekawa T, Okada A, Waki S, Hassan S, Okamoto H, Chiba T. Regenerating gene protein may mediate gastric mucosal proliferation induced by hypergastrinemia in rats. Gastroenterology 1998;115:1483–93. [DOI] [PubMed] [Google Scholar]
- 17.Burton P, Gurrin L, Sly P. Extending the simple linear regression model to account for correlated responses: An introduction to generalized estimating equations and multi-level mixed modelling. Stat Med 1998;17:1261–91. [DOI] [PubMed] [Google Scholar]
- 18.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition study group. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371:243–60. [DOI] [PubMed] [Google Scholar]
- 19.Miyaoka Y, Kadowaki Y, Ishihara S, Ose T, Fukuhara H, Kazumori H, Takasawa S, Okamoto H, Chiba T, Kinoshita Y. Transgenic overexpression of Reg protein caused gastric cell proliferation and differentiation along parietal cell and chief cell lineages. Oncogene 2004;23:3572–9. [DOI] [PubMed] [Google Scholar]
- 20.Granlund A, Beisvag V, Torp SH, Flatberg A, Kleveland PM, Ostvik AE, Waldum HL, Sandvik AK. Activation of REG family proteins in colitis. Scand J Gastroenterol 2011;46:1316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson KM, Guo X, Elkahloun AG, Mondal D, Bardhan PK, Sugawara A, Duggal P, Haque R, Petri WA., Jr The expression of REG1A and REG1B is increased during acute amebic colitis. Parasitol Int 2011;60:296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]