SUMMARY
Calcitonin gene-related peptide (CGRP) is a classic molecular marker of peptidergic primary somatosensory neurons. Despite years of research, it is unknown if these neurons are required to sense pain or other sensory stimuli. Here, we found that genetic ablation of CGRPα-expressing sensory neurons reduced sensitivity to noxious heat, capsaicin and itch (histamine and chloroquine) and impaired thermoregulation but did not impair mechanosensation or β-alanine itch—stimuli associated with nonpeptidergic sensory neurons. Unexpectedly, ablation enhanced behavioral responses to cold stimuli and cold mimetics without altering peripheral nerve responses to cooling. Mechanistically, ablation reduced tonic and evoked activity in postsynaptic spinal neurons associated with TRPV1/heat, while profoundly increasing tonic and evoked activity in spinal neurons associated with TRPM8/cold. Our data reveal that CGRPα sensory neurons encode heat and itch and tonically cross-inhibit cold-responsive spinal neurons. Disruption of this crosstalk unmasks cold hypersensitivity, with mechanistic implications for neuropathic pain and temperature perception.
INTRODUCTION
Somatosensory neurons located in the dorsal root ganglia (DRG) detect distinct stimulus modalities, such as pain, temperature and itch then relay this information to postsynaptic neurons in the dorsal spinal cord (Basbaum et al., 2009; Woolf and Ma, 2007). In the DRG, calcitonin gene-related peptide-immunoreactivity (CGRP-IR) has long served as a molecular marker of peptidergic nociceptive neurons (Basbaum et al., 2009). CGRP-IR actually reflects expression of two peptides (CGRPα and CGRPβ) that are encoded by separate genes (Calca and Calcb), with Calca being expressed at higher levels in DRG neurons (Schutz et al., 2004). Despite decades of research, it is unknown if CGRP-IR DRG neurons are required to sense specific types of thermal, mechanical or chemical stimuli.
To facilitate functional studies of CGRP-IR DRG neurons, we recently targeted an axonal tracer (farnesylated enhanced green fluorescent protein; GFP) and a LoxP-stopped cell ablation construct (human diphtheria toxin receptor; hDTR) to the Calca locus (McCoy et al., 2012). This knock-in mouse faithfully marked the peptidergic subset of DRG neurons as well as other cell types that express Calca. Using the GFP reporter to identify cells, we found that ~50% of all Calca/CGRPα DRG neurons expressed TRPV1 and responded to the TRPV1 agonist capsaicin. Several CGRPα DRG neurons also responded to the pruritogens histamine and chloroquine. In contrast, almost no CGRPα DRG neurons expressed TRPM8 or responded to icilin, a TRPM8 agonist that evokes the sensation of cooling. Less than 10% of all CGRPα-expressing neurons stained positive for isolectin B4-binding (IB4) and few stained positive for Prostatic acid phosphatase (PAP), markers of nonpeptidergic and some peptidergic DRG neurons (Basbaum et al., 2009; Zylka et al., 2008). Taken together, our data suggested that peptidergic CGRP-IR neurons might encode heat and itch, although direct in vivo evidence for this was lacking.
To directly study the importance of CGRP-IR neurons in somatosensation, we took advantage of the LoxP-stopped hDTR that we knocked into the Calca locus. Neurons expressing hDTR can be selectively ablated through intraperitoneal (i.p.) injections of diphtheria toxin (DTX) (Cavanaugh et al., 2009; Saito et al., 2001). Since Calca is expressed in cell types other than DRG neurons, we restricted hDTR expression to DRG neurons by using an Advillin-Cre knock-in mouse, a line that mediates excision of LoxP-flanked sequences in sensory ganglia (Hasegawa et al., 2007; Minett et al., 2012). Here, we provide the first direct evidence that CGRPα DRG neurons are required to sense heat and itch. Unexpectedly, we also found that CGRPα DRG neurons tonically inhibit spinal circuits that transmit cold signals, with ablation of CGRPα DRG neurons unmasking a form of cold hypersensitivity, a symptom that is associated with neuropathic pain.
RESULTS
Selective ablation of CGRPα primary sensory neurons in adult mice
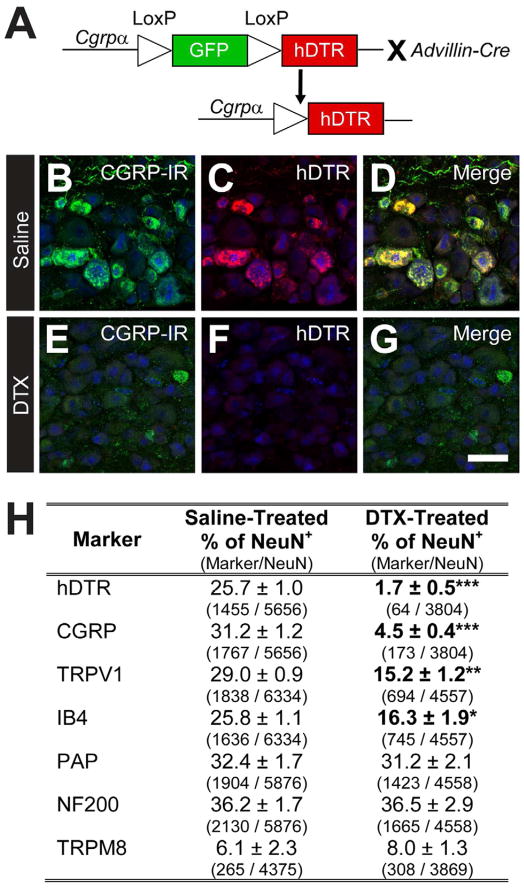
To selectively express hDTR in CGRPα-expressing DRG neurons, we crossed our Cgrpα-GFP knock-in mice Advillin-Cre with knock-in mice (Figure 1A) to generate double heterozygous “CGRPα-DTR+/−” mice. Histochemical studies revealed that hDTR was selectively expressed in CGRP-IR DRG neurons in CGRPα-DTR+/− mice (Figure 1B–D; saline-treated), but was not expressed in other CGRP-IR cell types (data not shown).
Figure 1. Conditional ablation of peptidergic DRG neurons in adult CGRPα-DTR+/− mice.
(A) Advillin-Cre was used to remove floxed GFP and drive selective expression of hDTR in CGRPα-expressing sensory neurons. (B–G) Lumbar DRG from CGRPα-DTR+/− mice treated with saline or DTX were stained with antibodies to (B,E) CGRP, (C,F) hDTR and (B–G) NeuN (blue). Images were acquired by confocal microscopy. Scale bar in (G) is 50 μm. See Figure S1 for additional markers. (H) Sensory neuron marker quantification, as percentage relative to total number of NeuN+ neurons. Cell counts are from representative sections of L3–L6 ganglia (8 representative sections per animal, with n=3 animals per treatment group). The number of neurons expressing each marker is shown in parentheses. All values are means ± SEM. *p<0.05, **p<0.005, ***p<0.0005. (B–H) Tissue was collected 7 days after second saline/DTX injection.
To ablate CGRPα DRG neurons, we injected CGRPα-DTR+/− mice i.p. with 100 μg/kg DTX (two injections, separated by 72 h). Using immunohistochemistry, we observed a near-complete loss of all CGRP-IR and hDTR+ DRG neurons, with neurons defined by expression of NeuN (Figure 1E–G, quantified in Figure 1H). We included neurons expressing low and high levels of CGRP-IR in our counts. There was also a significant reduction in the number of TRPV1+ and IB4+ DRG neurons in DTX-treated animals (Figure 1H, Figure S1), consistent with the known overlap between these markers and CGRP-IR (low and high) in the mouse (Cavanaugh et al., 2011; Zwick et al., 2002; Zylka et al., 2005). Other sensory neuron markers were not affected (Figure 1H, Figure S1). We counted 26,616 and 20,657 NeuN+ DRG neurons in saline- and DTX-treated mice, respectively (n=3 male mice/condition). We also looked more carefully at TRPM8+ neurons, some of which are myelinated (Neurofilament-200+; NF200+) while others are unmyelinated (NF200−) (Cain et al., 2001; Kobayashi et al., 2005). Neither of these subsets was affected in DTX-treated mice (saline-treated: n=255 TRPM8+ cells examined, 39.0 ± 5.0% were NF200+ and 61.0 ± 5.0% were NF200−. DTX-treated: n=253 TRPM8+ cells examined, 39.7 ± 7.8% were NF200+ and 60.3 ± 7.8% were NF200−).
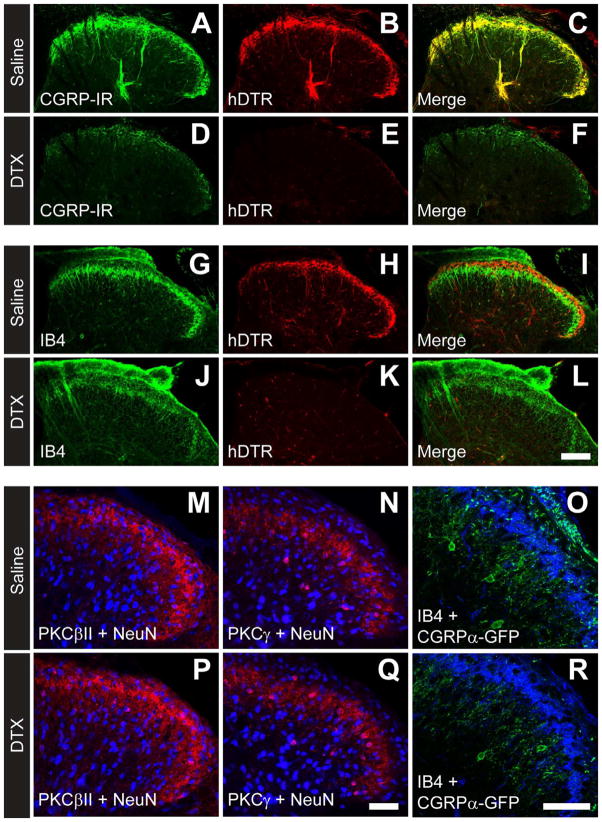
In the spinal cord, the axons of CGRP-IR DRG neurons terminate in lamina I, IIouter and deeper lamina and partially overlap with IB4+ terminals (Zylka et al., 2005). Consistent with this fact, hDTR was co-localized with CGRP-IR in axon terminals (Figure 2A–C) and only partially overlapped with nonpeptidergic IB4+ terminals in saline-treated mice (Figure 2G–I). Following DTX-treatment, virtually all hDTR+ and CGRP-IR terminals were eliminated in the dorsal horn (Figure 2D–F) while IB4+ terminals in lamina II remained (Figure 2J–L). In contrast, DTX-treatment did not eliminate PKCβII+ or PKCγ+ spinal neurons (Mori et al., 1990; Todd, 2010) and did not eliminate CGRPα-GFP+ spinal neurons in the dorsal horn (Figure 2M–R) (McCoy et al., 2012).
Figure 2. Selective ablation of CGRPα primary afferents in dorsal spinal cord.
(AR) Immunostaining in lumbar spinal cord sections from adult CGRPα-DTR+/− mice. (A–L) hDTR marks CGRPα-expressing afferents. (M,P) PKCβII (red), (N,Q) PKCγ (red) and (O,R) CGRPα-GFP (green) mark populations of intrinsic dorsal spinal neurons. (M, N, P, Q) NeuN (blue). (O,R) IB4 (blue). Images were acquired by confocal microscopy. Scale bar in (L) is 100 μm and (Q,R) is 50 μm. All tissue collected 7 days after second saline/DTX injection. Images are representative of n=3 male mice per condition.
Taken together, these data indicate that >90% of all CGRPα DRG neurons and CGRPα afferents in spinal cord were ablated in adult CGRPα-DTR+/− mice. This ablation also eliminated ~50% of all TRPV1+ DRG neurons. TRPV1 is the receptor for capsaicin and can be activated by thermal and nonthermal stimuli (Caterina et al., 1997; Romanovsky et al., 2009). In contrast, our ablation spared PAP+ nonpeptidergic neurons and neurons that express TRPM8, a cold temperature and icilin-sensitive receptor (Bautista et al., 2007; Dhaka et al., 2007; Knowlton et al., 2010).
Ablation of CGRPα terminals in skin reduces peripheral nerve responses to noxious heat but does not alter responses to cold or mechanical stimuli
In the epidermis of skin, CGRP-IR terminals are morphologically distinct from nonpeptidergic terminals, suggesting distinct sensory functions for peptidergic and nonpeptidergic DRG neurons (McCoy et al., 2012; Zylka et al., 2005). To determine if peptidergic endings were missing in DTX-treated CGRPα-DTR+/− mice, we immunostained hindpaw sections with antibodies to CGRP and the pan-nerve fiber marker PGP9.5. We found that DTX-treatment eliminated CGRP-IR terminals from the glabrous skin and hairy skin (epidermis and dermis) and from guard hairs (Figure 3A–F, Figure S2). In contrast, DTX-treatment did not eliminate CGRP-IR−, PGP9.5+ terminals, including terminals in the epidermis, hair follicles and sweat glands (Figure 3A–F, Figure S2, data not shown).
Figure 3. Ablation of CGRP-IR primary afferents in skin causes loss of peripheral nerve responses to noxious heat, while cold and mechanical responses are unaffected.
(A–F) Hindpaw glabrous skin from saline- and DTX-treated CGRPα-DTR+/− adult mice was stained with antibodies to (A,B) CGRP and (C,D) the pan-nerve fiber marker PGP9.5. (E,F) Merged images were stained with the nuclear marker DRAQ5 to facilitate visualization of skin cells. Images were acquired by confocal microscopy. Scale bar in (F) is 100 μm. CGRP-IR afferents were also ablated in hairy skin (Figure S2). Location of (G) laser heat responsive and (H) cold responsive spots in skin innervated by sural nerve; representative of n=3 animals per group. Filled circles=responsive spots. Open circles=non-responsive spots. (I, J) Representative multiunit electrophysiological recordings from the sural nerve before and after stimulation with the laser or cold solution. (K) Quantification of % heat and % cold responsive spots and number of laser-evoked action potentials in sural nerve as well as laser (heat), cold and mechanical threshold in isolated unmyelinated C-fibers. The number of recorded fibers is shown in parentheses. n=3 male mice/condition. Mean ± SEM. ***p<0.0005.
Since ~50% of all TRPV1+ DRG neurons were ablated in DTX-treated CGRPα-DTR+/− mice (Figure 1H), we hypothesized that peripheral nerve responses to noxious heat might be impaired. To test this hypothesis, we utilized a skin-nerve preparation to quantify hot, cold and mechanical responses of isolated C-fibers in the hindpaw of saline- and DTX-treated CGRPα-DTR+/− mice (Koltzenburg et al., 1997; Pribisko and Perl, 2011). We also mapped the distribution of noxious heat and cold receptive fields in this preparation by recording from the entire sural nerve (Figure 3G–K). A near-infrared diode laser was used to control the intensity and location of heat stimulation (Pribisko and Perl, 2011).
In saline-treated mice, laser heat stimulation (using an intensity that is in excess of the threshold of most C-fibers) activated multiple units in all of the spots (Figure 3G,I,K). However, in DTX-treated mice, activity was detected in only 38.1 ± 2.4% of the spots (Figure 3G,I,K). This is a profound reduction, particularly given that a response was scored as positive if as few as one action potential was detected when recording from the entire sural nerve. When averaged over all 40 spots, significantly fewer heat-evoked action potentials were generated over the 2 s stimulation period in DTX-treated mice (Figure 3K). Furthermore, the laser heat threshold to activate isolated C-fibers was ~two-fold higher in DTX-treated mice (Figure 3K). In contrast, there was no statistically significant change in the number of cold responsive spots when recording from the entire sural nerve and no change in the cold threshold of activation in isolated C-fibers between groups (Figure 3H,J,K). There was also no change in the mechanical thresholds of isolated C-fibers between saline- and DTX-treated CGRPα-DTR+/− mice (Figure 3K). Taken together, these data demonstrate that ablation of CGRPα+ afferents causes a profound loss of noxious heat sensitivity in skin with no change in cold or mechanical sensitivity.
Ablation of CGRPα DRG neurons impairs behavioral responses to heat and capsaicin
To determine if this profound physiological loss of heat sensitivity also affected behavioral responses to heat, we tested saline- and DTX-treated CGRPα-DTR+/− mice using multiple heat-related behavioral assays (Table 1). For all of these experiments, we studied mice pre- and post-saline/DTX treatment and separately tested males and females. For the tail immersion assay, we placed the distal third of the tail into hot water (46.5°C or 49°C) then quantified latency to f lick the tail. At both temperatures, there was a significant (~two-fold) increase in the latency to flick in DTX-treated males and females (Table 1). Next, we placed the mice on a hot plate heated to 52°C and measured the latency to flick, lick or shake a hindpaw. DTX-treated mice of both sexes exhibited over a two-fold increase in withdrawal latency (Table 1). Finally, we pharmacologically activated the thermosensor TRPV1 by injecting 0.1 μg/μl capsaicin into the left hindpaw. We found that the DTX-treated male and female mice showed a two-fold reduction in the time spent licking the capsaicin-injected hindpaw. Collectively, these experiments revealed that CGRPα DRG neurons were required to sense and behaviorally respond to noxious heat and capsaicin.
Table 1.
Quantification of heat, mechanical and cold behavioral assays.
| Behavior | Male | Female |
|---|---|---|
| HEAT | ||
| Tail Immersion (46.5°C) | Latency to Flick (s) | |
| Pre-Saline | 16.9 ± 1.3 | 18.4 ± 1.4 |
| Post-Saline | 13.6 ± 1.5 | 16.7 ± 1.6 |
| Pre-DTX | 17.5 ± 1.4 | 17.6 ± 1.7 |
| Post-DTX | 30.0 ± 2.7** | 29.4 ± 3.5* |
|
| ||
| Tail Immersion (49°C) | Latency to Flick (s) | |
| Pre-Saline | 5.3 ± 0.4 | 6.1 ± 0.3 |
| Post-Saline | 4.5 ± 0.4 | 5.8 ± 0.5 |
| Pre-DTX | 5.7 ± 0.4 | 5.8 ± 0.3 |
| Post-DTX | 13.6 ± 2.1** | 17.3 ± 1.7*** |
|
| ||
| Hot Plate (52°C) | Withdrawal Latency (s) | |
| Pre-Saline | 14.6 ± 1.6 | 17.2 ± 1.6 |
| Post-Saline | 15.6 ± 2.0 | 15.0 ± 1.3 |
| Pre-DTX | 14.6 ± 2.1 | 18.0 ± 1.5 |
| Post-DTX | 35.9 ± 3.5*** | 37.1 ± 3.3*** |
|
| ||
| Capsaicin | Time Spent Licking (s) | |
| Saline | 42.3 ± 3.9 | 49.8 ± 1.9 |
| DTX | 18.5 ± 5.4** | 24.7 ± 5.4** |
|
| ||
| Mechanical | ||
| Tail Clip | Latency to Bite Clip (s) | |
| Saline | 5.7 ± 1.5 | 3.7 ± 0.9 |
| DTX | 8.2 ± 2.0 | 4.1 ± 0.4 |
|
| ||
| Cotton Swab | Paw Withdrawal Frequency (%) | |
| Saline | 34.3 ± 7.2 | 56 ± 7.5 |
| DTX | 51.3 ± 10.1 | 49 ± 5.5 |
|
| ||
| COLD | ||
| Acetone | Time Spent Licking (s) | |
| Pre-Saline | 6.4 ± 1.0 | 7.1 ± 0.6 |
| Post-Saline | 4.9 ± 0.7 | 7.6 ± 1.0 |
| Pre-DTX | 6.0 ± 0.9 | 5.8 ± 0.5 |
| Post-DTX | 10.9 ± 2.0* | 13.0 ± 1.5** |
|
| ||
| Tail Immersion (−10°C) | Latency to Flick (s) | |
| Pre-Saline | 48.2 ± 6.1 | 39.6 ± 3.1 |
| Post-Saline | 40.9 ± 5.3 | 37.8 ± 6.9 |
| Pre-DTX | 56.0 ± 6.4 | 40.1 ± 3.7 |
| Post-DTX | 26.3 ± 4.6** | 28.6 ± 4.3* |
|
| ||
| Icilin | Number of Flinches | |
| Saline | 10.9 ± 1.6 | 15 ± 1.2 |
| DTX | 20.3 ± 1.7** | 25 ± 3.4* |
|
| ||
| Cold Plantar Assay | Withdrawal Latency (s) | |
| Pre-Saline | 12.7 ± 0.8 | 10.4 ± 0.8 |
| Post-Saline | 12.4 ± 0.5 | 10.5 ± 0.6 |
| Pre-DTX | 11.3 ± 0.6 | 10.6 ± 0.6 |
| Post-DTX | 8.8 ± 0.4** | 8.4 ± 0.4** |
n=6–26 mice/group,
p<0.05,
p<0.005,
p<0.0005.
Ablation of CGRPα DRG neurons causes a profound loss of heat hypersensitivity
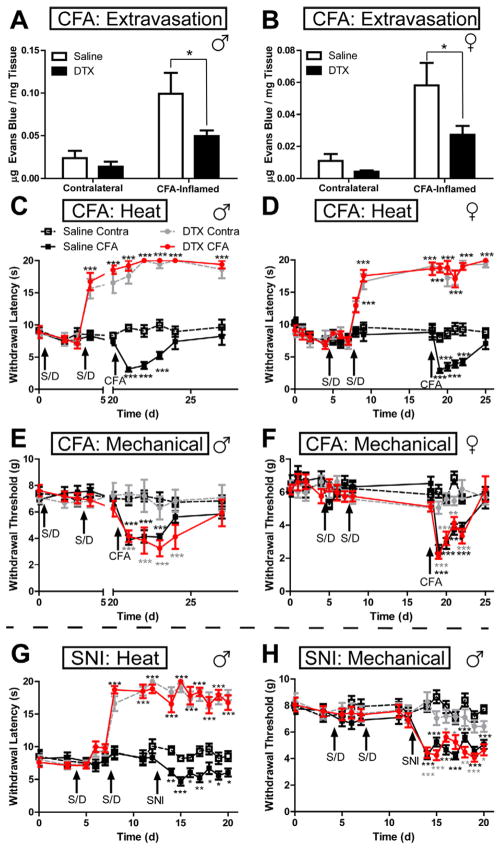
Heat and mechanical hypersensitivity are two common symptoms of inflammatory pain and neuropathic pain (Basbaum et al., 2009). To determine if CGRPα DRG neuron ablation impaired heat and mechanical hypersensitivity, we studied saline- and DTX-treated CGRPα-DTR+/− mice in the complete Freund’s adjuvant (CFA) model of inflammatory pain (Figure 4A–F) and in the spared nerve injury (SNI) model of neuropathic pain (Figure 4G,H). We monitored heat and mechanical sensitivity before, during and after saline/DTX treatment. We also monitored plasma extravasation in the non-inflamed (contralateral) and CFA-inflamed hindpaw with Evans Blue dye. We found that plasma extravasation was increased in both groups of mice following inflammation of the hindpaw; however, plasma extravasation was significantly lower in the inflamed hindpaw of DTX-treated male and female mice (when compared to the inflamed paw of saline-treated mice; Figure 4A,B). This reduction supports a role for peptidergic, CGRP+ afferents in neurogenic inflammation (Basbaum et al., 2009).
Figure 4. Ablation of CGRPα DRG neurons eliminates heat sensitivity and hypersensitivity but does not affect mechanical sensitivity in models of inflammatory pain and neuropathic pain.
(A–F) CFA inflammatory pain model in male and female CGRPα-DTR+/− mice. CFA injected into one hindpaw. Contralateral hindpaw not injected and served as a control. (A,B) Plasma extravasation, (C,D) hindpaw radiant heat sensitivity, (E,F) mechanical sensitivity. Arrows indicate when mice were injected i.p. with saline or DTX (S/D). (G,H) SNI model of neuropathic pain in male mice. (G) Heat sensitivity and (H) mechanical sensitivity. n=10 mice/treatment group. Paired t tests were used to compare responses at each time point between saline- and DTX-treated mice, same paw comparisons. Black asterisks relative to Saline Contra paw; gray asterisks relative to DTX Contra; red asterisks relative to DTX CFA. *p<0.05, **p<0.005, ***p<0.0005.
In both chronic pain models, heat withdrawal latencies increased to the cutoff time (20 s) after the second DTX injection and remained at this level for at least two weeks (Figure 4C,D,G). Moreover, DTX-treated animals showed no sign of heat hyperalgesia following inflammation (CFA) or nerve injury (SNI). In contrast, mechanical sensitivity and hypersensitivity were not impaired in DTX-treated animals in either chronic pain model (Figure 4E,F,H). Likewise, noxious (tail clip) and innocuous (cotton swab) mechanical sensitivity was not impaired in DTX-treated animals (Table 1). Taken together, these behavioral experiments provide direct evidence that CGRPα DRG neurons are required for noxious thermosensation but are not required for noxious or innocuous mechanosensation in vivo.
Ablation of CGRPα DRG neurons impairs histamine- and chloroquine-induced itch but not β-alanine-induced itch
Capsaicin-responsive DRG neurons respond to the pruritogens histamine and chloroquine (Imamachi et al., 2009; Liu et al., 2009; Schmelz et al., 2003; Sikand et al., 2011). Since DTX-treated mice were less sensitive to capsaicin, we hypothesized that responses to pruritogens that act through capsaicin-responsive/TRPV1+ neurons might also be reduced. Indeed, we found that itch responses to histamine and chloroquine (CQ) were reduced by >80% in DTX-treated male and female mice (Figure 5A–D), consistent with the observation that the number of DRG neurons expressing Mrgpra3, the receptor for CQ (Liu et al., 2009), was significantly reduced in DTX-treated mice (Figure S3). In contrast, DTX-treated male and female mice showed normal itch responses to β-alanine, a pruritogen that activates Mrgprd and acts through nonpeptidergic Mrgprd+ neurons (Liu et al., 2012; Rau et al., 2009) (Figure 5E,F). Thus, ablation of CGRPα DRG neurons did not globally impair scratching but instead selectively impaired itch associated with capsaicin/heat-responsive neurons.
Figure 5. Histamine- and chloroquine-dependent itch are reduced in DTX-treated CGRPα-DTR+/− mice, while β-alanine-dependent itch is unaffected.
(A,B) Histamine (10 μg/μl), (C,D) chloroquine (CQ; 4 μg/μl), or (E,F) β-alanine (20 μg/μl) was injected into the nape of the neck in saline- or DTX-treated CGRPα-DTR+/− mice (male and female data plotted separately). Scratching bouts were measured for 30 min in 5 min blocks. (Insets) Total number of scratching bouts. n=10–26 mice/treatment group. T tests were used to compare responses at each time point between saline- and DTX-treated mice. *p<0.05, **p<0.005, ***p<0.0005.
Ablation of CGRPα DRG neurons unexpectedly enhanced sensitivity to cold stimuli and impaired thermoregulation
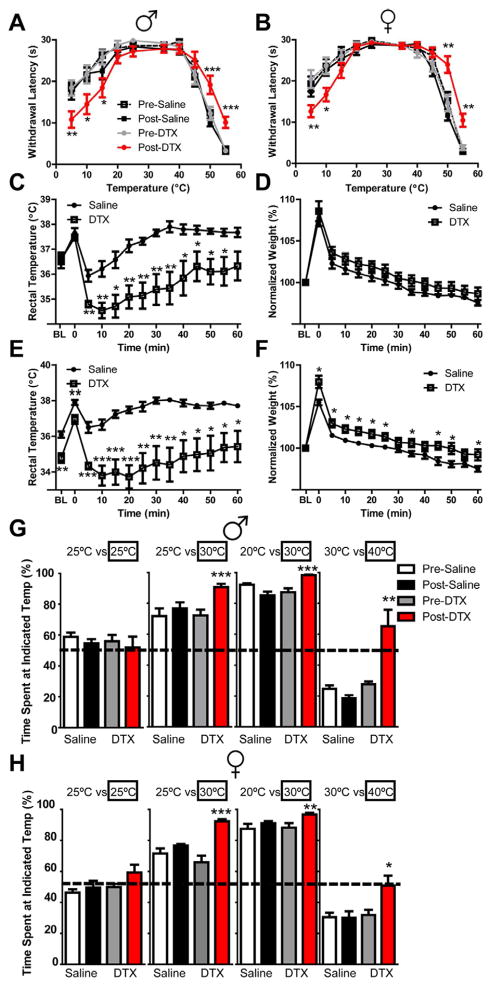
Considering that ablation of CGRPα DRG neurons did not affect cold-evoked activity in peripheral nerves or the number of TRPM8+ neurons, we hypothesized that behavioral responses to cold temperature would not be altered following CGRPα neuron ablation. However, we found that DTX-treated mice (male and female) were significantly more sensitive to numerous cold and cold-mimetic stimuli, including acetone-evoked evaporative cooling of the hindpaw, tail immersion at −10°C, injection of 2.4 μg/μl icilin into the hindpaw (this concentration of icilin evokes behavioral responses that are TRPM8-dependent (Knowlton et al., 2010)) and the cold plantar assay (Brenner et al., 2012) (Table 1). In addition, we immobilized saline- and DTX-treated mice on a metal plate that could be set at temperatures ranging from very cold to noxious hot, then quantified hindpaw withdrawal latency (Gentry et al., 2010). Remarkably, DTX-treated mice withdrew their hindpaws significantly faster at 5°C and 10°C (Figure 6A,B), indicating enhanced sensitivity to cold. Conversely, at temperatures at or above 45°C, DTX-treated mice took significantly longer to withdraw their hindpaws (Figure 6A,B), consistent with our data above showing reduced heat sensitivity following ablation. No differences were observed at any temperature between groups prior to saline/DTX treatment (Figure 6A,B). As an additional control, we found that DTX-treatment did not affect heat or cold sensory responses in wild-type mice or body weight (Figure S4), consistent with other studies (Cavanaugh et al., 2009).
Figure 6. Mice lacking CGRPα sensory neurons are more sensitive to cold, show impaired thermoregulation following evaporative cold challenge and prefer warmer temperatures.
(A,B) Sensitivity to temperatures ranging from very cold to noxious hot were measured using a hindpaw withdrawal assay in (A) male and (B) female CGRPα-DTR+/− mice, pre- and post-saline/DTX treatment. Cutoff time was 30 s. n=10 mice/group. (C–F) The water repulsion assay was performed on saline- and DTX-treated CGRPα-DTR+/− male mice (C,D) 3 days and (E,F) 6 days after the second saline/DTX injection. (C,E) Rectal (deep) body temperature and (D,F) body weight was monitored before (BL) and following brief (2 min) immersion of mice in 37°C water. Means ± SEM. n=7–8 mice/group. Weights were normalized in (D,F) because DTX-treated mice weigh significantly less than saline-treated mice (D: 23.4 ± 1.8 g saline-treated verses 18.3 ± 1.2 g DTX-treated. F: 23.5 ± 1.7 g saline-treated verses 16.9 ± 0.9 g DTX-treated). T tests were used to compare responses at each time point between saline- and DTX-treated mice. (G,H) Two-choice temperature preference assay in (G) male and (H) female CGRPα-DTR+/− mice, pre- and post-saline/DTX treatment. Floor temperatures were maintained at 25°C/25°C, 25°C/30°C, 20°C/30°C, or 30°C/40°C, and time spent on each side was measu red for 10 min. n=10 mice/group. (A,B,G,H) T tests were used to compare responses at each time point between pre-saline- and pre-DTX-treated mice and between post-saline and post-DTX-treated mice. *p<0.05, **p<0.005, ***p<0.0005.
We noticed that the fur of DTX-treated mice appeared disheveled and piloerected, suggesting the mice might feel cold at room temperature and/or that there was a problem with their fur (note, DTX-treated mice showed no visible shivering). Thus, to examine thermoregulation and fur barrier function, we briefly immersed (2 min) saline- and DTX-treated mice in warm water then measured their ability to thermoregulate and to repel water (Figure 6C–F). Immersion causes whole body warming, as evidenced by increased core body temperature, followed by whole body evaporative cooling when removed from the warm water. While there were no differences between the groups prior to immersion or when warmed, immediately after removal from the warm water, the core body temperature of DTX-treated mice dropped significantly lower than that of saline-treated mice and took longer to recover (Figure 6C,E, on days 3 and 6 post saline/DTX treatment). Moreover, on day 6, core body temperature at baseline was significantly lower in DTX-treated mice when compared to saline-treated controls (Figure 6E). These data collectively indicate that CGRPα DRG neurons play a critical role in thermoregulatory mechanisms following whole-body cooling but not warming.
In the same assay, DTX-treated mice repelled water to the same extent as saline-treated mice 3 days post saline/DTX treatment (Figure 6D) but retained significantly more water weight on day 6 (Figure 6F), suggesting a moderate impairment of fur barrier function. This impairment might be due to loss of CGRP-IR guard hair innervation (Figure S2). Guard hairs add a water repellent, oily sheen to the coat of furry mammals. And, CGRP-IR primary afferents fire in response to guard hair displacement (Lawson et al., 2002; Woodbury et al., 2001).
Given that DTX-treated mice had enhanced responses to multiple cold stimuli and had difficulty warming themselves when cooled, we hypothesized that DTX-treated mice might prefer a warmer environment over a relatively cooler environment. To test this possibility, we monitored the amount of time saline- and DTX-treated mice spent on two surfaces set at equivalent (25°C vs. 25°C) or different (25°C vs. 30°C; 20°C vs. 30°C; 30°C vs. 40°C) temperatures. The mice demons trated no preference when the two surface temperatures were equivalent, as expected (Figure 6G,H). However, when surface temperatures differed, DTX-treated mice spent significantly more time on the warmer surfaces (Figure 6G,H). This behavior was remarkably consistent between male and female mice and suggests that DTX-treated mice prefer warmer temperatures (or show enhanced avoidance of cooler temperatures).
CGRPα/heat-sensing neurons tonically cross-inhibit cold-responsive spinal neurons
Since ablation of CGRPα DRG neurons enhanced behavioral sensitivity to cold but did not alter peripheral nerve responses to cold, this suggested that CGRPα DRG neuron ablation might instead alter central processing of temperature signals, at postsynaptic targets in the spinal cord. To assess central alterations in function, we measured baseline and agonist-evoked spontaneous excitatory postsynaptic current (EPSC) frequency in spinal cord slices from saline- and DTX-treated CGRPα-DTX+/− mice. We used capsaicin to activate TRPV1/heat-sensing afferents and icilin to activate TRPM8/cold-sensing afferents. These agonists are known to increase EPSC frequency in spinal neurons that are postsynaptic to TRPV1 and TRPM8 DRG neurons, respectively (Yang et al., 1998; Zheng et al., 2010). Responsive and non-responsive spinal neurons were further classified into four physiologically distinct subgroups (Grudt and Perl, 2002). We found that capsaicin (10 μM; pressure ejected using a picospritzer for 10 s) caused a two-fold increase in EPSC frequency in a subset of spinal neurons from saline- and DTX-treated mice (Figure 7A,C,E; Table S1). However, the tonic (baseline) and evoked EPSC frequency of capsaicin-responsive neurons was significantly lower in DTX-treated mice than in saline-treated animals (lower by 41.4% at baseline, 44.7% evoked; Figure 7E). This reduction in capsaicin-responsiveness at the spinal level was consistent with our observation that DTX-treated mice had ~50% fewer TRPV1+ DRG neurons (Figure 1H) and were ~50% less responsive to capsaicin injection (Table 1). In addition, significantly fewer total neurons responded to capsaicin in slices from DTX-treated mice (Table S1). Intriguingly, there were no capsaicin-responsive transient neurons in DTX-treated mice (Table S1), consistent with the fact that capsaicin-responsive primary afferents are monosynaptically connected to transient neurons (Zheng et al., 2010).
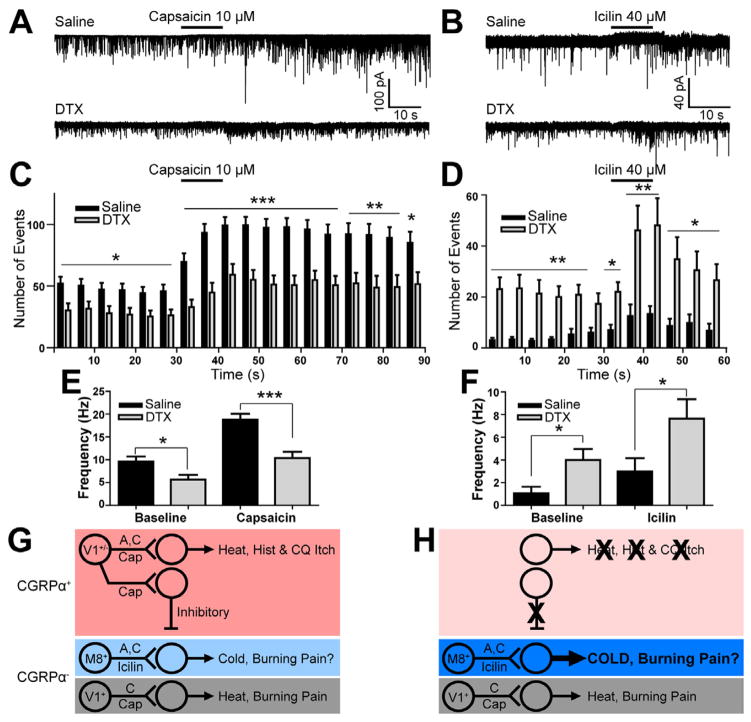
Figure 7. Tonic and evoked activity in icilin (cold)-responsive spinal neurons is enhanced after ablating CGRPα DRG neurons.
(A–F) Patch recordings of lamina II spinal neurons from saline-treated and DTX-treated CGRPα-DTR+/− mice (4 weeks old). Sagittal lumbar spinal cord slices harvested 3 days after last saline/DTX treatment. Picospritzer filled with drugs at the indicated concentrations then pressure ejected near the recorded neuron. (A) Example recordings, (C) EPSC event histogram and (E) EPSC frequency in capsaicin-responsive spinal neurons. (B) Example recordings, (D) EPSC event histogram (F) EPSC frequency in icilin-responsive spinal neurons. Number of neurons studied and their physiological classification are shown in Table S1. T test relative to saline-treated; *p<0.05, **p<0.005, ***p<0.0005. (G) Somatosensory circuits before and (H) after ablation suggests central disinhibition mechanism for enhanced cold sensitivity. CGRP-IR neurons are myelinated (A-fibers) or unmyelinated (C-fibers) (Lawson et al., 2002) and ~50% express the capsaicin receptor TRPV1 (Cavanaugh et al., 2011; McCoy et al., 2012). As found in the present study, CGRPα neurons are selectively required to sense heat, capsaicin and some pruritogens (histamine=hist and CQ) and, via an interneuron, tonically inhibit cold-responsive postsynaptic spinal neurons. The existence of this inhibitory interneuron that monosynaptically connects capsaicin-responsive spinal neurons with icilin-responsive spinal neurons was demonstrated by paired recordings (Zheng et al., 2010). Following CGRPα neuron ablation, tonic and evoked excitatory activity in cold-sensitive spinal neurons is enhanced. Ablation also enhanced cold and cold-mimetic sensitivity at the behavioral level. CGRPα neurons do not express TRPM8 and very few (2%) respond to icilin (McCoy et al., 2012). Cold/TRPM8+ neurons are either myelinated or unmyelinated (Cain et al., 2001; Kobayashi et al., 2005), and both subsets are present in CGRPα-neuron ablated mice. Activity in unmyelinated TRPM8+ afferents likely colors the perception of hot and cold temperatures with a “burning” sensation (Campero et al., 2009). TRPV1+ neurons are primarily unmyelinated (Lawson et al., 2008).
We then pressure ejected icilin (40 μM) onto slices from saline- and DTX-treated mice to identify TRPM8/cold-responsive spinal neurons. We found that the total number of spinal neurons that responded to icilin did not differ between saline- and DTX-treated mice (approximately 14% of all lamina II neurons responded in both groups; Table S1). However, icilin-responsive spinal neurons from DTX-treated mice had significantly higher tonic and evoked EPSC activity (270% increase at baseline, 157% increase evoked, relative to saline-treated mice) (Figure 7B,D,F). Collectively, these experiments suggest that CGRPα DRG neurons tonically cross-inhibit TRPM8/cold-sensing circuits at the spinal level (see Figure 7G,H for mechanism).
DISCUSSION
CGRP-IR has long served as a classic molecular marker of peptidergic nociceptive neurons. However, whether CGRP-IR DRG neurons were actually required to sense painful stimuli was never directly tested. Using a genetically precise ablation strategy, we found that CGRPα DRG neurons are required to sense noxious heat, capsaicin and some pruritogens. In contrast, CGRPα DRG neurons were not required to sense cold, innocuous or noxious mechanical or β-alanine itch stimuli. Our findings were remarkably consistent across a large number of mice, sexes and across multiple behavioral assays, conclusively revealing that CGRPα DRG neurons contribute directly to noxious heat and itch sensations. CGRPα neurons are clearly not the only DRG neurons that sense these stimuli, as some noxious heat, capsaicin and itch responses remained after ablation. This remaining sensitivity likely originates from TRPV1+/CGRPα− (nonpeptidergic) neurons that were not ablated. Indeed, ablation of TRPV1+ DRG neurons or spinal afferents completely eliminated responses to capsaicin, heat and some pruritogens (Cavanaugh et al., 2009; Karai et al., 2004; Mishra and Hoon, 2010; Mishra et al., 2011).
Using a physiological preparation, Lawson and colleagues found that many CGRP-IR neurons responded to noxious heat and mechanical stimuli, classifying CGRP-IR neurons as polymodal (Lawson et al., 2002). In contrast, our physiological and behavioral data indicate CGRPα DRG neurons are required to sense noxious heat but are not required to detect innocuous or noxious mechanical stimuli. However, our data do not exclude a redundant role for CGRPα DRG neurons in mechanosensation or for sensing forms of mechanical stimuli that we did not test, such as pleasurable touch or pressure. This discrepancy between Lawson’s study and our present study suggests that physiology alone may not be sufficient to define the function of somatosensory neurons. Indeed, using a different physiological preparation, Rau and colleagues found that Mrgprd-expressing sensory neurons were polymodal and could be activated by noxious heat and mechanical stimuli (Rau et al., 2009); however, when these neurons were ablated, only mechanosensory behaviors were impaired (Cavanaugh et al., 2009).
We previously found that <10% of all CGRPα-expressing DRG neurons (defined by expression of a knocked-in GFP reporter) were IB4+ (McCoy et al., 2012). However, in our present study, the number of IB4+ neurons was reduced by 36% following CGRPα DRG neuron ablation (from 25.8 to 16.3%; Figure 1H). This suggests that there may be greater overlap between IB4 and CGRPα than our previous histochemical studies indicated. Alternatively, quantification of markers relative to NeuN (in representative sections as done in this study) may not estimate how many cells were lost following ablation as accurately as counting the total number of marker-positive neurons in a specific ganglia (such as L4). Although these potential discrepancies in IB4 and CGRPα overlap should be noted, based on the maintenance of an independent additional marker for nonpeptidergic neurons (PAP) and the ablation of the majority of CGRPα-expressing neurons (Figure 1H), our conclusions related to the function of CGRPα DRG neuron remain well-founded.
CGRPα neuron ablation unmasks heat inhibition of cold at the spinal level
Unexpectedly, we found that behavioral responses to cold temperatures and cold-mimetics were enhanced when CGRPα DRG neurons were ablated. This enhancement in cold sensitivity was not due to an increase in the number of TRPM8+ DRG neurons, an increase in the number of cold receptive fields or to a change in C-fiber cold threshold (which also excluded peripheral sensitization of C-fibers to low temperature). Furthermore, since physiological responses to cold were not altered peripherally after ablating CGRPα DRG neurons, it is unlikely that any other cold-sensing channel, including TRPA1 (Story et al., 2003), was more active peripherally. Since cold signals were processed normally in the periphery in DTX-treated mice, this suggested that enhanced cold sensitivity might instead be due to alterations in central processing of cold signals.
At the first central relay for CGRPα afferents—the dorsal spinal cord—we found that tonic and evoked activity in capsaicin-responsive spinal neurons was reduced by ~50% in DTX-treated mice. Reduced activity in capsaicin-responsive spinal neurons was paralleled by a five-fold increase in tonic and evoked activity in icilin/cold-responsive spinal neurons. These data suggest that CGRPα afferents (50% of which are TRPV1+) tonically cross-inhibit icilin/cold-responsive spinal neurons, with cross-inhibition mediated through capsaicin-responsive interneurons. Indeed, a subset of capsaicin-responsive spinal neurons monosynaptically inhibit icilin-responsive spinal neurons (Zheng et al., 2010), highlighting a direct line of communication between these modality-selective circuits at the spinal level. Ablation of CGRPα/heat neurons removes this tonic inhibition, causing central disinhibition and hypersensitivity/allodynia to cold stimuli.
Our findings do not support the pattern theory of somatosensation, which is based on the idea that different frequencies and firing patterns in sensory neurons encode different sensory experiences, such as heat and cold (Ma, 2010). Instead, our findings suggest that tonic activity in a modality-selective class of neurons—TRPM8 neurons—is directly responsible for driving enhanced cold sensitivity when a different class of neurons—CGRPα DRG neurons—is ablated. It should be possible to test this prediction in future studies with TRPM8 antagonists or Trpm8−/− mice, especially given that tonic activity in the majority of cold-sensitive C-fibers is reduced when Trpm8 is deleted (Bautista et al., 2007). For example, deletion of Trpm8 may rescue some of the enhanced cold and thermoregulatory phenotypes in CGRPα DRG neuron-ablated mice.
Our findings also do not entirely support the labeled-line theory of somatosensation (Ma, 2010), as this would imply that CGRPα/heat and TRPM8/cold pathways remain segregated and do not interact (anatomically or functionally) in the periphery, spinal cord or the brain. If these modality-selective circuits remain segregated from one another, this raises the question of how else could CGRPα DRG neuron ablation simultaneously enhance activity in cold-responsive spinal neurons and reduce activity in capsaicin/heat-responsive neurons? This might occur, for example, if our genetic ablation were leaky and eliminated additional classes of neurons that synapse directly or indirectly with these heat- and cold-responsive spinal neurons. However, we found no evidence that any classes of spinal neurons were missing in DTX-treated animals, including CGRPα-GFP spinal neurons (Figure 2M–R, Table S1). In fact, given that the floxed GFP reporter (Figure 1A) was expressed (and hence not excised) in these spinal neurons (Figure 2O,R), this further confirms the specificity of Advillin-Cre for sensory ganglia over spinal cord. We can also rule out the possibility that phenotypes were due to developmental compensation or to a broader loss of CGRPα-lineage neurons since we ablated CGRPα neurons in adult mice.
Ultimately, our study supports a fundamental role for crosstalk in shaping modality-selective somatosensory responses, consistent with previous studies (Craig and Bushnell, 1994; Fruhstorfer, 1984; Lagerstrom et al., 2010; Liu et al., 2010; Ochoa and Yarnitsky, 1994; Proudfoot et al., 2006; Ross et al., 2010; Wahren et al., 1989; Yarnitsky and Ochoa, 1990; Yosipovitch et al., 2007), and with studies showing that spinal neurons are extensively interconnected through cross-excitation and cross-inhibition (Kato et al., 2009; Labrakakis et al., 2009; Prescott and Ratté, 2012; Todd, 2010; Zheng et al., 2010). Moreover, our study provides direct in vivo support for the population coding model of somatosensation (Ma, 2010)—a model that integrates modality-selective labeled-lines with the pattern hypothesis. Indeed, our data suggest that modality-selective pathways can communicate with one another yet still preserve their molecular and modality-specific identity.
Implications for somatosensory responses and temperature perception
Intriguingly, humans also report enhanced sensory responses to cold and enhanced cold perception under experimental and pathological conditions. For example, selective block of myelinated A-fibers induces a form of cold allodynia, causing stimuli originally perceived as cool to become icy cold, stinging or burning hot (Fruhstorfer, 1984; Wahren et al., 1989; Yarnitsky and Ochoa, 1990). The molecular identity of the myelinated fibers that were blocked in these studies was not determined. Similarly, in the triple cold syndrome, neuropathic pain patients describe paradoxical burning hot sensations in response to cool temperature stimuli (Ochoa and Yarnitsky, 1994). A population of unmyelinated afferents in humans, termed Type 2 C-afferents (C2 afferents), is sensitive to warming, cooling and TRPM8 agonists (Campero et al., 2009). C2 afferents are hypothesized to convey sensations of burning pain and unpleasantness when not inhibited by myelinated afferents (Campero et al., 2009). Since some CGRPα+ DRG neurons are myelinated (Lawson et al., 2002) and virtually all CGRPα DRG neurons were ablated in our mice, CGRPα neuron ablation could model and provide mechanistic insights into these enhanced cold sensory conditions in humans.
Additionally, our findings could provide new insights into why TRPV1 antagonists cause hyperthermia—a major side-effect. While less well-appreciated, three different TRPV1 antagonists reproducibly caused all patients to “feel cold” and shiver before the onset of hyperthermia (6 mg dose of ABT-102; K. Schaffler et al, 13th World Congress on Pain, Montreal, Quebec, 2010) (Gavva et al., 2008; Krarup et al., 2011). Hyperthermia is associated with a reduction in nonthermal tonic activation of TRPV1 (Romanovsky et al., 2009), but why patients initially report enhanced cold perception is unclear. Perhaps analogous to what we found when CGRPα DRG neurons were ablated, TRPV1 antagonists also reduce tonic excitatory activity in capsaicin-responsive spinal neurons (Shoudai et al., 2010). Thus, TRPV1 antagonists might enhance tonic activity in icilin/cold-responsive spinal neurons and enhance behavioral sensitivity to cold (although enhanced behavioral sensitivity to cold may require inactivating not just TRPV1+ neurons but also CGRPα+/TRPV1− neurons). Furthermore, if TRPV1 antagonists enhance activity in cold-responsive spinal circuits in humans, this could simultaneously trigger shivering, the percept of “feeling cold” and homeostatic mechanisms that warm the body, ultimately producing hyperthermia.
While TRPV1 antagonists cause hyperthermia in rodents, CGRPα DRG neuron-ablated mice showed neither hyperthermia nor hypothermia at baseline (Figure 6C). This difference could be due to the fact that it takes several days for phenotypes to develop following the first DTX injection (for example, see Figure 4). In contrast, TRPV1 antagonists have a rapid onset. Moreover, ablation caused the permanent loss of neurons, which could produce phenotypes that are more typical of sustained TRPV1 antagonism. For example, the hyperthermic response to TRPV1 antagonists eventually dissipates when these antagonists are administered over longer periods of time (Romanovsky et al., 2009).
Lastly, we noticed that DTX-treated CGRPα-DTR+/− mice gradually lost weight over the course of our experiments (using DTX from two different vendors; Figure 6, Figure S5). This appears to be an on-target effect because weight loss did not occur when wild-type mice were treated with DTX (Figure S4). This then raises the question of why DTX-treated CGRPα-DTR+/− mice lost weight. Given that these mice showed enhanced sensitivity to cold, greater tonic activity in cold-responsive spinal neurons and preferred warmer temperatures over cooler temperatures, one possibility is that DTX-treated mice tonically “feel” cold and are in a cold-challenged physiological state at room temperature. In such a state, animals metabolize brown fat and other body tissues to generate energy and heat (Romanovsky et al., 2009). Ultimately, additional studies will be needed to determine if CGRPα DRG neurons regulate energy and fat metabolism in a manner similar to TRPV1 neurons (Motter and Ahern, 2008; Romanovsky et al., 2009).
EXPERIMENTAL PROCEDURES
Animals
All procedures involving vertebrate animals were approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill. Cgrpα-GFP−/− female mice (McCoy et al., 2012) (available from MMRRC:36773) were crossed with male Advillin-Cre−/− mice (Hasegawa et al., 2007) to generate double heterozygous CGRPα+/−; Advillin-Cre+/− (CGRPα-DTR+/−) mice. Heterozygous offspring were used for all experiments and have one functional Calca allele. All mice were backcrossed to C57BL/6 mice for at least 8 generations. Mice were raised on a 12 h:12 h light:dark cycle, were fed DietGel 76A (#72-03-502, ClearH2O) and water ad libitum, and were tested during the light phase. Estrous cycle was not monitored in females. Diphtheria toxin (DTX; List Biological Laboratories, Product #150) was dissolved in sterile 0.9% saline and stored at −20°C in aliquots that were th awed once. DTX from List Biological was used for all experiments except those shown in Figure S5. DTX from Sigma (D0564) was used in Figure S5, to demonstrate that behavioral and thermoregulatory phenotypes were reproducible with DTX from a different vendor. For all experiments, the experimenter was blind to which group received saline or DTX injections. Mice were acclimated to the testing room, equipment and experimenter 1–3 days before behavioral testing (see Supplemental Experimental Procedures for detailed description of each behavioral assay). Tissues were processed for histology as described previously (McCoy et al., 2012), and see Supplemental Experimental Procedures for further details.
Skin-nerve preparation, single-unit and whole-nerve recordings
Animals were anesthetized to areflexia with i.p. ketamine (100 mg/kg) and xylazine (10 mg/kg). The sural nerve was dissected free from the sciatic notch to its distal cutaneous termination in the lateral hindpaw. Skin was placed dermal (corium) side up into an organ bath and superfused with temperature- and pH-adjusted (32°C; 7.4), oxygenated, synthetic interstitial fluid (SIF; in mM: 123 NaCl, 3.5 KCl, 0.7 MgSO4, 2.0 CaCl2, 9.5 sodium gluconate, 1.7 NaH2PO4, 5.5 glucose, 7.5 sucrose, and 10 HEPES) as described (Pribisko and Perl, 2011).
For single-unit experiments, the desheathed sural nerve was teased into fine filaments on a mirrored stage. Filaments were suspended onto a gold recording electrode and isolated in mineral oil. Cutaneous receptive fields of C-fibers (conduction velocity ≤1 m/s) were identified through electrical stimulation with a search electrode (modified 0.25 mm, 5 MΩ, epoxy-insulated tungsten electrode, A-M Systems, Inc. Sequim, WA). Thresholds were established by applying ascending, incremental mechanical (hand-held Semmes Weinstein filaments, von Frey Aesthesiometer, Stoelting, Wood Dale, IL), heat (980 nm, 7.5 W, continuous wave diode laser, Lass/DLD-7-NM3, LASMED, Mountain View, CA), and cold (perfusion of 20, 15, 10 and 5°C SIF into the ring reservoir applied to the rece ptive field) stimulation. Extracellular recordings were filtered, amplified and digitized (World Precision Instruments, Sarasota, FL; Digidata 1440A data system, Molecular Devices, Sunnyvale, CA). Significant group differences in mechanical (kPa) and thermal (mA or °C) thresholds between groups were derived from Mann-Whitney’s U- or Student’s t-hypothesis testing.
After completion of single-unit recordings, a survey was conducted of the thermal sensitivity (heat or cold) of the entire skin preparation, similar to what has been done by others (Banik and Brennan, 2008). The proximal end of the sural nerve, trimmed of teased filaments, was placed in its entirety on the recording electrode. Mechanical sensitivity of the cutaneous distribution of the sural nerve was confirmed by blunt glass probe stimulation. For heat sensitivity, the total receptive area was divided into forty contiguous, non-overlapping 5 mm2 incident areas. Each area was exposed to laser energy of 2,100 mA (laser driver current) for 2 s. This stimulus elevates skin temperature to 50.3°C (centrally beneath laser beam ) and 43.6°C (adjacent) and is below the threshold for a thermal burn but in excess of the threshold of most C-fibers in C57BL/6 mice (Pribisko and Perl, 2011). This stimulus also exceeds the temperature threshold of TRPV1 (Caterina et al., 1997). An incident area was rated as sensitive to heat if action potentials were observed (Spike2, Cambridge Electronic Design, Cambridge, UK). For cold sensitivity, the total receptive area was divided into ten contiguous, non-overlapping 25 mm2 receptive areas. Each area was perfused with 5°C SIF over 2 s, with a small cylinder used to con fine perfusate to small regions of the hindpaw. A receptive area was rated as sensitive to cold if one or more action potentials were observed.
Two tests were performed to validate this whole nerve recording method. In the first test, the trunk of the whole nerve was positioned over the recording electrode which included a previously isolated laser heat-sensitive C fiber. The receptive field of the C fiber was re-stimulated with the laser and action potentials with the same shape and response rate of the single fiber were observed in the integrated multi-fiber responses. Second, to confirm that multiple classes of fibers were present and responsive in the whole nerve, compound action potentials were recorded from saline and DTX-treated mice. A suction stimulus electrode was placed proximal to the divergence of the distal sural nerve before entry into the dermis. All components (Aβ, Aδ, C) of the compound action potentials were detected in these recordings.
Spinal cord slice electrophysiology
Sagittal mouse spinal cord slices were prepared from saline- and DTX-treated CGRPα-DTR+/− mice as previously reported (Wang and Zylka, 2009). Spinal cord slices were superfused with artificial cerebrospinal fluid (ACSF; in mM: 125 NaCl, 2.5 KCl, 2.5 CaCl2, 1.5 MgCl2, 1.25 NaH2PO4, 25 NaHCO3, 25 glucose at pH 7.4.) at room temperature in a recording chamber mounted on a Nikon FN1 microscope and lamina II neurons were visualized under infrared-differential interference contrast illumination. Patch-clamp recordings were performed using an Axon Instruments Multi-clamp 700B amplifier, Digidata 1400 and pClamp software for data acquisition. Electrodes were pulled from borosilicate glass with a Sutter P-2000 electrode puller to a tip resistances of 3.0–7.0 MΩ, and filled with electrode solution (contained in mM: 126 K-Gluconate, 10 NaCl, 1 MgCl2, 0.5 EGTA, 2 MgATP, 0.1 NaGTP with pH adjusted to 7.3 with KOH, and osmolarity adjusted to 287 mOsM with sucrose). Spontaneous EPSCs were recorded in voltage clamp mode with a holding potential of −70 mV, approximately equal to the reversal potential for Cl−. Baseline EPSC frequency was recorded for 30 s, after which capsaicin (10 μM) or icilin (40 μM) was applied to the area adjacent to the neuron with a Picospritzer III (Parker Automation) for 10 s at 10 psi.
Following the assessment of EPSC frequency, the cells were held in current clamp mode while current steps were presented to the neurons in order to characterize the neurons based on firing properties, as described (Grudt and Perl, 2002). Neurons that showed a delayed discharge of action potentials after the current step were characterized as delayed. Neurons that fired one or a few action potentials only were characterized as transient. Neurons that regularly fired action potentials throughout the duration of the current step were characterized as tonic. Neurons that did not fire action potentials, fired irregular patters of action potentials or were lost before switching to current clamp were classified as other.
Supplementary Material
HIGHLIGHTS.
CGRPα DRG neurons are required to sense heat and some pruritogens
Cold sensation is enhanced after ablating CGRPα neurons in adult mice
CGRPα afferents tonically cross-inhibit cold/icilin-responsive spinal neurons
CGRPα primary sensory neurons contribute to thermoregulation and cold defense
Acknowledgments
We thank JrGang Cheng at the University of North Carolina BAC Core for generating the CGRPα targeting arms, Megumi Aita for performing in situ hybridization, Fan Wang at Duke University for providing Advillin-Cre mice, Edward Perl for allowing us to use his skin-nerve electrophysiology rig, Sarah Shoemaker for mouse colony management, Brendan Fitzpatrick for performing surgeries, Kenji Kohno for providing hDTR (pTRECK1) plasmid and Masatoshi Takeichi for providing TRPM8 antibody. This work was supported by grants to M.J.Z. from The Searle Scholars Program, The Klingenstein Foundation, The Rita Allen Foundation and NINDS (R01NS060725, R01NS067688). The BAC Core, Confocal Imaging Core and In situ Hybridization Core are funded by grants from NINDS (P30NS045892) and NICHD (P30HD03110).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banik RK, Brennan TJ. Sensitization of primary afferents to mechanical and heat stimuli after incision in a novel in vitro mouse glabrous skin-nerve preparation. Pain. 2008;138:380–391. doi: 10.1016/j.pain.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Brenner DS, Golden JP, Gereau RWt. A novel behavioral assay for measuring cold sensation in mice. PLoS One. 2012;7:e39765. doi: 10.1371/journal.pone.0039765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain DM, Khasabov SG, Simone DA. Response properties of mechanoreceptors and nociceptors in mouse glabrous skin: an in vivo study. J Neurophysiol. 2001;85:1561–1574. doi: 10.1152/jn.2001.85.4.1561. [DOI] [PubMed] [Google Scholar]
- Campero M, Baumann TK, Bostock H, Ochoa JL. Human cutaneous C fibres activated by cooling, heating and menthol. J Physiol. 2009;587:5633–5652. doi: 10.1113/jphysiol.2009.176040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Chesler AT, Braz JM, Shah NM, Julius D, Basbaum AI. Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J Neurosci. 2011;31:10119–10127. doi: 10.1523/JNEUROSCI.1299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD, Bushnell MC. The thermal grill illusion: unmasking the burn of cold pain. Science. 1994;265:252–255. doi: 10.1126/science.8023144. [DOI] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Fruhstorfer H. Thermal sensibility changes during ischemic nerve block. Pain. 1984;20:355–361. doi: 10.1016/0304-3959(84)90112-X. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, Alvarez F, Bak A, Darling M, Gore A, et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008;136:202–210. doi: 10.1016/j.pain.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Gentry C, Stoakley N, Andersson DA, Bevan S. The roles of iPLA2, TRPM8 and TRPA1 in chemically induced cold hypersensitivity. Mol Pain. 2010;6:4. doi: 10.1186/1744-8069-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol. 2002;540:189–207. doi: 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H, Abbott S, Han BX, Qi Y, Wang F. Analyzing somatosensory axon projections with the sensory neuron-specific Advillin gene. J Neurosci. 2007;27:14404–14414. doi: 10.1523/JNEUROSCI.4908-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, Wellisch OM, Neubert JK, Olah Z, Iadarola MJ. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest. 2004;113:1344–1352. doi: 10.1172/JCI20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato G, Kawasaki Y, Koga K, Uta D, Kosugi M, Yasaka T, Yoshimura M, Ji RR, Strassman AM. Organization of intralaminar and translaminar neuronal connectivity in the superficial spinal dorsal horn. J Neurosci. 2009;29:5088–5099. doi: 10.1523/JNEUROSCI.6175-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton WM, Bifolck-Fisher A, Bautista DM, McKemy DD. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain. 2010;150:340–350. doi: 10.1016/j.pain.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol. 1997;78:1841–1850. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- Krarup AL, Ny L, Astrand M, Bajor A, Hvid-Jensen F, Hansen MB, Simren M, Funch-Jensen P, Drewes AM. Randomised clinical trial: the efficacy of a transient receptor potential vanilloid 1 antagonist AZD1386 in human oesophageal pain. Aliment Pharmacol Ther. 2011;33:1113–1122. doi: 10.1111/j.1365-2036.2011.04629.x. [DOI] [PubMed] [Google Scholar]
- Labrakakis C, Lorenzo LE, Bories C, Ribeiro-da-Silva A, De Koninck Y. Inhibitory coupling between inhibitory interneurons in the spinal cord dorsal horn. Mol Pain. 2009;5:24. doi: 10.1186/1744-8069-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerstrom MC, Rogoz K, Abrahamsen B, Persson E, Reinius B, Nordenankar K, Olund C, Smith C, Mendez JA, Chen ZF, et al. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68:529–542. doi: 10.1016/j.neuron.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson JJ, McIlwrath SL, Woodbury CJ, Davis BM, Koerber HR. TRPV1 unlike TRPV2 is restricted to a subset of mechanically insensitive cutaneous nociceptors responding to heat. J Pain. 2008;9:298–308. doi: 10.1016/j.jpain.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN, Crepps B, Perl ER. Calcitonin gene-related peptide immunoreactivity and afferent receptive properties of dorsal root ganglion neurones in guinea-pigs. J Physiol. 2002;540:989–1002. doi: 10.1113/jphysiol.2001.013086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, Sun S, Lamotte RH, Dong X. Mechanisms of Itch Evoked by beta-Alanine. J Neurosci. 2012;32:14532–14537. doi: 10.1523/JNEUROSCI.3509-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Abdel Samad O, Zhang L, Duan B, Tong Q, Lopes C, Ji RR, Lowell BB, Ma Q. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010;68:543–556. doi: 10.1016/j.neuron.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. Labeled lines meet and talk: population coding of somatic sensations. J Clin Invest. 2010;120:3773–3778. doi: 10.1172/JCI43426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy ES, Taylor-Blake B, Zylka MJ. CGRPalpha-Expressing Sensory Neurons Respond to Stimuli that Evoke Sensations of Pain and Itch. PLoS One. 2012;7:e36355. doi: 10.1371/journal.pone.0036355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minett MS, Nassar MA, Clark AK, Passmore G, Dickenson AH, Wang F, Malcangio M, Wood JN. Distinct Nav1.7-dependent pain sensations require different sets of sensory and sympathetic neurons. Nat Commun. 2012;3:791. doi: 10.1038/ncomms1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Hoon MA. Ablation of TrpV1 neurons reveals their selective role in thermal pain sensation. Mol Cell Neurosci. 2010;43:157–163. doi: 10.1016/j.mcn.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA. TRPV1-lineage neurons are required for thermal sensation. EMBO J. 2011;30:582–593. doi: 10.1038/emboj.2010.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Kose A, Tsujino T, Tanaka C. Immunocytochemical localization of protein kinase C subspecies in the rat spinal cord: light and electron microscopic study. J Comp Neurol. 1990;299:167–177. doi: 10.1002/cne.902990204. [DOI] [PubMed] [Google Scholar]
- Motter AL, Ahern GP. TRPV1-null mice are protected from diet-induced obesity. FEBS Lett. 2008;582:2257–2262. doi: 10.1016/j.febslet.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa JL, Yarnitsky D. The triple cold syndrome. Cold hyperalgesia, cold hypoaesthesia and cold skin in peripheral nerve disease. Brain. 1994;117(Pt 1):185–197. doi: 10.1093/brain/117.1.185. [DOI] [PubMed] [Google Scholar]
- Prescott SA, Ratté S. Pain processing by spinal microcircuits: afferent combinatorics. Curr Opin Neurobiol. 2012;22:631–639. doi: 10.1016/j.conb.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribisko AL, Perl ER. Use of a near-infrared diode laser to activate mouse cutaneous nociceptors in vitro. J Neurosci Methods. 2011;194:235–241. doi: 10.1016/j.jneumeth.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Proudfoot CJ, Garry EM, Cottrell DF, Rosie R, Anderson H, Robertson DC, Fleetwood-Walker SM, Mitchell R. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr Biol. 2006;16:1591–1605. doi: 10.1016/j.cub.2006.07.061. [DOI] [PubMed] [Google Scholar]
- Rau KK, McIlwrath SL, Wang H, Lawson JJ, Jankowski MP, Zylka MJ, Anderson DJ, Koerber HR. Mrgprd enhances excitability in specific populations of cutaneous murine polymodal nociceptors. J Neurosci. 2009;29:8612–8619. doi: 10.1523/JNEUROSCI.1057-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, Nakamura K, Burmeister JJ, Nucci TB. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev. 2009;61:228–261. doi: 10.1124/pr.109.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, Mekada E, Kimata Y, Tsuru A, Kohno K. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol. 2001;19:746–750. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, Handwerker HO. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89:2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- Schutz B, Mauer D, Salmon AM, Changeux JP, Zimmer A. Analysis of the cellular expression pattern of beta-CGRP in alpha-CGRP-deficient mice. J Comp Neurol. 2004;476:32–43. doi: 10.1002/cne.20211. [DOI] [PubMed] [Google Scholar]
- Shoudai K, Peters JH, McDougall SJ, Fawley JA, Andresen MC. Thermally active TRPV1 tonically drives central spontaneous glutamate release. J Neurosci. 2010;30:14470–14475. doi: 10.1523/JNEUROSCI.2557-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikand P, Shimada SG, Green BG, LaMotte RH. Sensory responses to injection and punctate application of capsaicin and histamine to the skin. Pain. 2011;152:2485–2494. doi: 10.1016/j.pain.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren LK, Torebjork E, Jorum E. Central suppression of cold-induced C fibre pain by myelinated fibre input. Pain. 1989;38:313–319. doi: 10.1016/0304-3959(89)90218-2. [DOI] [PubMed] [Google Scholar]
- Wang H, Zylka MJ. Mrgprd-expressing polymodal nociceptive neurons innervate most known classes of substantia gelatinosa neurons. J Neurosci. 2009;29:13202–13209. doi: 10.1523/JNEUROSCI.3248-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury CJ, Ritter AM, Koerber HR. Central anatomy of individual rapidly adapting low-threshold mechanoreceptors innervating the “hairy” skin of newborn mice: early maturation of hair follicle afferents. J Comp Neurol. 2001;436:304–323. [PubMed] [Google Scholar]
- Woolf CJ, Ma Q. Nociceptors--noxious stimulus detectors. Neuron. 2007;55:353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Yang K, Kumamoto E, Furue H, Yoshimura M. Capsaicin facilitates excitatory but not inhibitory synaptic transmission in substantia gelatinosa of the rat spinal cord. Neurosci Lett. 1998;255:135–138. doi: 10.1016/s0304-3940(98)00730-7. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D, Ochoa JL. Release of cold-induced burning pain by block of cold-specific afferent input. Brain. 1990;113(Pt 4):893–902. doi: 10.1093/brain/113.4.893. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Duque MI, Fast K, Dawn AG, Coghill RC. Scratching and noxious heat stimuli inhibit itch in humans: a psychophysical study. Br J Dermatol. 2007;156:629–634. doi: 10.1111/j.1365-2133.2006.07711.x. [DOI] [PubMed] [Google Scholar]
- Zheng J, Lu Y, Perl ER. Inhibitory neurones of the spinal substantia gelatinosa mediate interaction of signals from primary afferents. J Physiol. 2010;588:2065–2075. doi: 10.1113/jphysiol.2010.188052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick M, Davis BM, Woodbury CJ, Burkett JN, Koerber HR, Simpson JF, Albers KM. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci. 2002;22:4057–4065. doi: 10.1523/JNEUROSCI.22-10-04057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Sowa NA, Taylor-Blake B, Twomey MA, Herrala A, Voikar V, Vihko P. Prostatic acid phosphatase is an ectonucleotidase and suppresses pain by generating adenosine. Neuron. 2008;60:111–122. doi: 10.1016/j.neuron.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.