Abstract
Background
Social interaction can serve as a natural reward that attenuates drug reward in rats; however, it is unknown if age or housing conditions alter the choice between social interaction and drug.
Methods
Individually- and pair-housed adolescent and adult rats were tested using conditioned place preference (CPP) in separate experiments in which: (1) social interaction was conditioned against no social interaction; (2) amphetamine (AMPH; 1 mg/kg, s.c.) was conditioned against saline; or (3) social interaction was conditioned against AMPH.
Results
Social interaction CPP was obtained only in individually-housed adolescents, whereas AMPH CPP was obtained in both individually-housed adolescents and adults; however, the effect of AMPH was not statistically significant in pair-housed adults. When allowed to choose concurrently between compartments paired with either social interaction or AMPH, individually-housed adolescents preferred the compartment paired with social interaction, whereas pair-housed adolescents preferred the compartment paired with AMPH. Regardless of housing condition, adults showed a similar preference for the compartments paired with either social interaction or AMPH.
Conclusions
Although some caution is needed in interpreting cross-experiment comparisons, the overall results suggest that individually-housed adolescents were most sensitive to the rewarding effect of social interaction, and this hypersensitivity to social reward effectively competed with AMPH reward.
Keywords: Conditioned place preference, social interaction, amphetamine, concurrent choice, development, adolescence, differential housing
1. INTRODUCTION
The relationship between social context and drug abuse is complex. During development, peer influences contribute to experimental drug use (Allen et al., 2003; Bahr et al., 2005). In preclinical work, rats exposed to a conspecific treated with ethanol drink more ethanol relative to rats exposed to a conspecific treated with water (Hunt et al., 2001). Rats also self-administer more d-amphetamine (AMPH) when given visual access to a conspecific relative to when they are alone (Gipson et al., 2011). Furthermore, rats self-administer more cocaine in the presence of a conspecific performing the same task (Smith, 2012). Although social influences have been linked to experimental drug use, individuals with substance abuse disorders often decrease social interactions and spend less time with their peers (American Psychiatric Association, 1994). It is not clear to what extent the social isolation precedes or results from substance abuse.
The conditioned place preference (CPP) paradigm can assess how social interactions modulate drug reward. With CPP, animals learn to associate contextual cues with an appetitive stimulus (e.g., food, social interaction, drugs of abuse; see Bardo and Bevins, 2000; Tzschentke, 2007 for reviews on CPP). During conditioning, the previously neutral environmental cues come to act as conditioned stimuli that then elicit approach to the environment previously paired with the appetitive stimulus. Relative to rats that receive either social interaction or marginally rewarding doses of a stimulant drug alone, rats that receive simultaneous social interaction and marginal doses develop greater CPP (Thiel et al., 2008, 2009). Recently, the CPP paradigm has been used to determine the relative rewarding effects of social interaction versus cocaine. For example, when social interaction and cocaine are conditioned against each other in different environments, rats show reduced CPP to both the social- and cocaine-associated environments compared to either social- or cocaine-associated environments conditioned alone (Fritz et al., 2011).
Despite the evidence that a context paired with social interaction can reduce drug-induced CPP (Fritz et al., 2011), that study was limited to adult rats housed in individual cages. Evidence indicates that adolescent rats are more sensitive than adults to the stimulant locomotor effects of cocaine (Badanich et al., 2008; Catlow and Kirstein, 2005; but see Laviola et al., 1995), and adults show greater locomotor activity to AMPH when group-housed compared to individually-housed (Gill et al., 2012; Wang et al., 2010). In addition, social interaction and drug CPP are modulated by age and housing condition. For example, adolescents show social interaction CPP regardless of housing condition, whereas adults show social interaction CPP only when isolated (Douglas et al., 2004). Age differences are also observed with drug-induced CPP, although results are somewhat mixed. Some reports show that, relative to adult rats, adolescents develop cocaine CPP at lower doses (Badanich et al., 2006; Zakharova et al., 2009a; but see Adriani and Laviola, 2003; Campbell et al., 2000) and acquire methamphetamine CPP at a faster rate (Zakharova et al., 2009a). Housing conditions also modulate drug-induced CPP in adults, as cocaine CPP is obtained in group-housed rats, but not in individually-housed rats (Schenk et al., 1986). Similarly, rats housed in an enriched condition with social partners are more sensitive than individually-housed rats to AMPH CPP tested during adulthood (Bowling and Bardo, 1994).
The present experiments sought to further validate the use of the concurrent choice CPP paradigm in which the rewarding value of different reinforcers (social interaction vs. AMPH) are conditioned against one another (Fritz et al., 2011). Although previous research has demonstrated age and housing condition effects in social interaction and drug-induced CPP, studies have not directly measured whether age or housing condition alter social interaction versus AMPH CPP. Thus, the primary goal of the present experiments was to determine if social interaction and AMPH CPP are modulated by age (adolescent vs. adult) and housing condition (individual vs. paired). In Experiment 1, adolescent and adult rats were tested for social interaction reward by receiving access to an unfamiliar sex- and weight-matched partner in one compartment and received no partner in the alternate compartment. In Experiment 2, rats were tested for drug reward by receiving AMPH (1 mg/kg) in a one compartment and saline in the alternate compartment; the dose of AMPH was selected based on previous literature indicating that it produces robust CPP (Bardo, et al., 1995). AMPH-induced locomotor activity was also measured in this experiment to confirm that the AMPH dose selected produced hyperactivity across conditioning sessions. In Experiment 3, rats were tested for concurrent choice between social interaction and AMPH reward by receiving social interaction in one compartment and AMPH in the alternate compartment.
2. METHODS
2.1. Animals
A total of 98 male, Sprague Dawley rats (Harlan Industries, Indianapolis, IN, USA) were used. Rats arrived at either postnatal day (PND) 21 (n = 54) or PND 60 (n = 44). Adolescent and adult rats were housed either individually or in pairs immediately upon delivery to the laboratory. Rats were housed in a temperature- and humidity-controlled colony room that was maintained on a light-dark cycle in which lights were on from 6:00 a.m. to 6:00 p.m. Rats were allowed to acclimate to the colony for 7 days before the start of the experiment. Rats had ad libitum access to food and water in their home cage for the entire experiment. All procedures were in accordance with the 2011 edition of the “Guide for the Care and Use of Laboratory Animals” (National Research Council) and were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
2.2. Apparatus
A 3-compartment chamber (68 × 21 × 21 cm; ENV-013; MED Associates, St. Albans, VT) located inside a sound-attenuating chamber (ENV-020M; MED Associates) was used to measure CPP. The three compartments were separated by sliding guillotine doors. The middle compartment (12 × 21 × 21 cm) had gray walls with a smooth gray PVC floor. The end compartments (28 × 21 × 21 cm) provided distinct contexts, with one compartment having black walls with a stainless steel grid rod floor and the other end compartment having white walls with a stainless steel mesh floor. Recessed trays were located 2 cm below each compartment. A computer controlled the experimental trial using Med-IV software. A series of infrared photobeams (6 beams in the black and white compartments and 3 beams in the gray compartment) were used to detect the rats' presence in a particular compartment and record the amount of time spent in that compartment, as well as to record locomotor activity during conditioning trials.
2.3. Procedure
Rats were tested for CPP using a 10-day procedure. On day 1 (pre-conditioning test), the guillotine doors were opened, and adolescent (PND 28) or adult (PND 67) rats were placed in the center gray compartment and were allowed to explore all three compartments for 15 min. The duration spent in each compartment was recorded. Following the pre-conditioning test, rats went through 8 days of conditioning (days 2–9) in which rats were confined by the guillotine door to either the black or white compartment for 30 min; this trial duration was chosen because it reliably establishes AMPH CPP (Bardo et al., 1995). Although a 10 min trial duration is often used to establish social interaction CPP (Calcagnetti and Schechter, 1992; Douglas et al., 2004; Peartree et al., 2012; Van den Berg et al., 1999), the current experiments used a 30 min trial duration in order to conform to the AMPH CPP procedure. Further, it has been shown that trial duration does not affect social interaction CPP (Thiel et al., 2008).
In Experiment 1, on every other day, each rat (n = 24) was given an injection of saline (s.c) and was placed immediately into either the white or black compartment that contained a weight-, and age-matched male partner. On alternating days, each rat was given saline and placed immediately into the opposite compartment without any partner. In Experiment 2, on every other day, each rat (n = 24) was given an injection of AMPH (1.0 mg/kg, s.c.) and was placed immediately into either the white or black compartment. On alternating days, each rat received saline (s.c.) and was placed immediately into the opposite chamber. In this experiment, locomotor activity also was recorded during each conditioning trial by measuring the total number of photobeam breaks. In Experiment 3, on every other day, each rat (n= 24) was given an injection of saline (s.c.) and was allowed social interaction in the white or black compartment as described in Experiment 1. On alternating days, each rat received AMPH (1.0 mg/kg; s.c.) and was placed immediately into the opposite compartment as described in Experiment 2. For all three experiments, the chamber in which rats received social interaction or AMPH was counterbalanced (i.e., unbiased), and the order in which rats received social interaction or AMPH was counterbalanced within each group. For Experiments 1 and 3, rats received the same partner during each conditioning session. On the post-conditioning test (day 10), each rat was placed in the center gray compartment with the guillotine doors open and was allowed to explore all three compartments for 15 min. The time spent in each compartment was recorded
2.4. Drug
d-Amphetamine sulfate (Sigma, St. Louis, MO) was prepared in sterile 0.9% NaCl (saline) and was injected subcutaneously in a volume of 1 ml/kg. The dose was calculated based on the salt weight.
2.5. Statistical Analyses
2.5.1. Locomotor Activity
Locomotor activity in Experiment 2 was analyzed using a mixed factor ANOVA; drug treatment and conditioning trial were within-subject factors, whereas age and housing condition were between-subject factors. These analyses were conducted to determine if age and/or housing condition altered the locomotor stimulant effect of AMPH. Main effects and significant interactions were probed using additional ANOVAs and/or student's t tests when appropriate.
2.5.2. CPP
A preference ratio was calculated by dividing the amount of time spent in the compartment paired with AMPH (Experiment 1) or social interaction (Experiment 2) by the time spent in both the white and black compartments. A preference ratio of 0.5 indicated no preference for either compartment, with ratios above 0.5 designating a preference and ratios below 0.5 designating an aversion. For Experiment 3, a preference ratio was calculated by dividing the amount of time spent in the compartment paired with social interaction by the time spent in both the white and black compartments. A preference ratio of 0.5 indicated no preference for either compartment, with ratios above 0.5 designating a preference for the social-paired compartment and ratios below 0.5 designating a preference for the AMPH-paired compartment
Preference ratios were analyzed with ANOVA, with age and housing as between-subject factors. Significant interactions were probed with student's t tests. One-sample t tests were performed to determine if each preference ratio was significantly different from 0.5. Preference ratios more than 1.5 times the interquartile range were considered statistical outliers and were excluded from data analyses. One rat from Experiment 1 was defined as a statistical outlier and thus was excluded from statistical analyses. All tests were considered significant at p < .05.
3. RESULTS
3.1. Experiment 1: Social-induced CPP
In Experiment 1, a 2 × 2 ANOVA revealed a main effect of age (F(1, 19) = 9.29, p < .01), with overall preference ratios being higher in adolescents than in adults. Although the overall ANOVA found no age × housing interaction, one-sample t tests revealed that individually-housed adolescent rats developed social interaction CPP (t(4) = 9.48, p < .01; Fig. 1, left panel), whereas social interaction CPP was not observed in pair-housed adolescents (Fig. 1, left panel) or in either individually- or pair-housed adults (Fig. 1, right panel).
Figure 1.
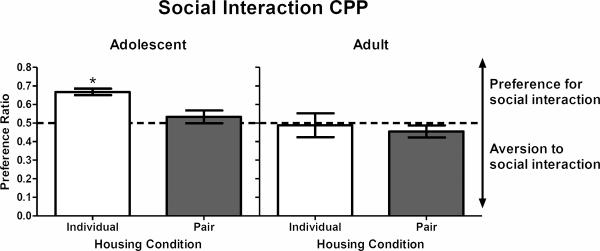
Social interaction CPP for individually- and pair-housed adolescents (left panel) and individually- and pair-housed adults (right panel) in Experiment 1. Bar represents mean (±SEM) preference ratio, with the dashed line indicating an equal preference for the compartment previously paired with social interaction and the compartment previously paired with no social interaction. Values above 0.5 indicate a preference for the social interaction compartment. *p < .05, compared to a preference ratio of 0.5.
3.2. Experiment 2: AMPH-induced CPP and locomotor activity
In Experiment 2, a 2 × 2 ANOVA revealed a main effect of housing (F(1, 20) = 5.97, p < .05), with individually-housed rats showing overall higher preference ratios compared to pair-housed rats. Although the overall ANOVA found no age × housing interaction, one-sample t tests revealed that individually- and pair-housed adolescent rats developed AMPH CPP (t(5) = 5.78, p < .01; t(5) = 5.16, p < .01; Fig. 2, left panel). AMPH CPP also was obtained in individually-housed adults (t(5) = 3.23, p < .05), but not in pair-housed adults (Fig. 2, right panel).
Figure 2.
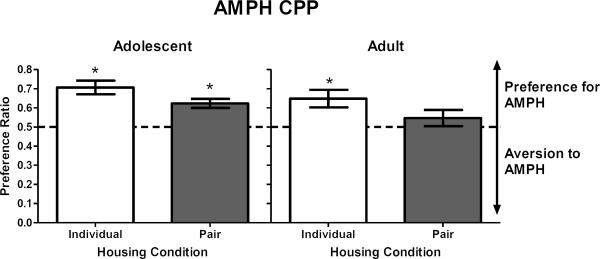
AMPH CPP for individually- and pair-housed adolescents (left panel) and individually-and pair-housed adults (right panel) in Experiment 2. Bar represents mean (±SEM) preference ratio, with the dashed line indicating an equal preference for the compartment previously paired with AMPH and the compartment previously paired with saline. Values above 0.5 indicate a preference for the AMPH compartment. *p < .05, compared to a preference ratio of 0.5.
Table 1 shows locomotor activity following AMPH or saline during each conditioning trial for adolescents and adults in Experiment 2. The overall ANOVA revealed a significant treatment × trial × age interaction (F(3, 60) = 3.48, p < .05). To explore this interaction, separate ANOVAs were conducted to determine if AMPH or saline differentially altered locomotor activity in adolescent and adult rats. Neither age group showed changes in activity with repeated saline trials; however; there was a trial × age interaction (F(3, 66) = 4.42, p < .01) following AMPH treatment. Adolescents showed increased activity on the first AMPH conditioning trial (t(22) = 2.37, p < .05; student's t test), whereas adults did not. Across repeated trials, linear trend analyses revealed that adults had a significant increase in activity with AMPH (F(1, 46) = 32.14, p < .001), whereas adolescent rats did not. However, adolescents had greater locomotor activity following AMPH relative to saline on conditioning trials 2–4 [trial 2: t(11) = 2.57, p < .05; trial 3: t(11) = 2.66, p < .05; trial 4: t(11) = 3.19, p < .01]. Similarly, adults showed enhanced locomotor activity following AMPH relative to saline on conditioning trials 2–4 [trial 2: t(11) = 3.27; p < .01; trial 3: t(11) = 8.09; p < .01; trial 4: t(11) = 5.45; p < .01]. There was no significant main effect or interaction involving the housing factor (results not shown).
Table 1.
Locomotor activity (mean photobeam breaks ± SEM) during conditioning trials for Experiment 2.
| Adolescent AMPH | Adolescent Saline | Adult AMPH | Adult Saline | |
|---|---|---|---|---|
| Conditioning Trial 1 | 26.8 (± 2.3)# | 20.7 (± 1.4) | 20.6 (± 1.0) | 20.7 (± 2.2) |
| Conditioning Trial 2 | 27.7 (± 2.1)* | 20.2 (± 1.7) | 24.1 (± 1.0)* | 19.1 (±2.0) |
| Conditioning Trial 3 | 28.2 (± 2.2)* | 19.8 (± 1.6) | 28.4 (± 1.4)* | 18.3 (± 1.3) |
| Conditioning Trial 4 | 30.5 (± 2.9)* | 18.1 (± 1.6) | 29.8 (± 1.4)* | 19.76 (± 1.6) |
p < .05, relative to saline.
p < .05, relative to adults treated with AMPH.
Note: data from the individually- and pair-housed animals have been combined into one group.
3.3. Experiment 3: Social Interaction vs. AMPH CPP
In Experiment 3, a 2 × 2 ANOVA revealed a main effect of housing (F(1, 20) = 4.84, p < .05), as well as an age × housing interaction (F(1, 20) = 21.63, p < .001). Housing condition significantly affected CPP in adolescents (t(10) = 5.01, p < .01), but not adults. Individually-housed adolescents spent more time in the social interaction compartment compared to pair-housed adolescents (t(5) = 2.69, p < .05). Conversely, pair-housed adolescents spent more time in the AMPH compartment relative to individually-housed adolescents (t(5) = −5.99, p < .01; Fig. 3, left panel). Adults did not develop either social interaction or AMPH CPP (Fig. 3, right panel). Furthermore, preference ratios were significantly different between adolescents and adults, with individually-housed adolescents showing increased preference for social interaction relative to adults (t(10) = −3.53, p < .01; Fig 3) and pair-housed adolescents showing increased preference for the AMPH relative to adults (t(10) = 3.03, p < .05; Fig 3).
Figure 3.
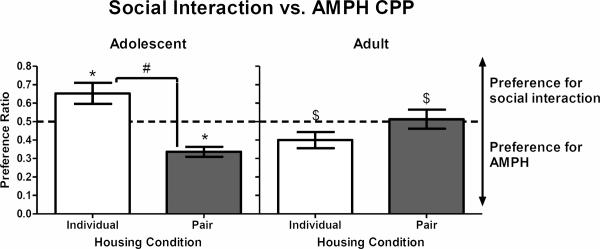
Social interaction vs. AMPH CPP for individually- and pair-housed adolescents (left panel) and individually- and pair-housed adults (right panel) during concurrent choice in Experiment 3. Bar represents mean (±SEM) preference ratio, with the dashed line indicating an equal preference for the compartment previously paired with social interaction and the compartment previously paired with AMPH. A preference ratio greater than 0.5 indicates a preference for the social interaction compartment, whereas a preference ratio lower than 0.5 indicates a preference for the AMPH compartment. *p < .05, compared to a preference ratio of 0.5. #p < .05, compared to pair-housed adolescent rats. $p < .05, compared to adolescents in the same housing condition.
4. DISCUSSION
There were three main findings in the current experiments. First, social interaction CPP was obtained only in individually-housed adolescents. Second, AMPH CPP was observed in both adolescents and adults, although AMPH CPP was not statistically significant among pair-housed adults. Third, when given access to a compartment paired with either social interaction or AMPH, individually-housed adolescents preferred the social interaction compartment, whereas pair-housed adolescents preferred the AMPH compartment; adults in both housing conditions showed no preference for either compartment. Taken together, these results indicate that individually-housed adolescents were most sensitive to social reward and that conditioned social reward among these rats reduced the conditioned rewarding effect of AMPH.
Individually-housed adolescent rats preferred the compartment previously paired with social interaction, which is consistent with previous reports (Calcagnetti and Schechter, 1992; Douglas et al., 2004; Peartree et al., 2012; Thiel et al., 2008; Trezza et al., 2009; Van den Berg et al., 1999). Although previous studies have reported social interaction CPP in individually-housed adult rats (Douglas et al., 2004; Fritz et al., 2011; Van den Berg et al., 1999), individually-housed adults did not develop social interaction CPP in the current study. One discrepancy between the current experiment and previous studies is the duration of each conditioning trial. Whereas previous reports used relatively short conditioning trials (10–15 min), rats in the current study received 30-min conditioning trials. Douglas et al. (2004) reported that social behavior decreases after the first 10 min. Thus, adults in the current experiment may have spent a majority of latter portions of each conditioning trial engaging in non-social behavior, such as resting or grooming. Furthermore, whereas adolescents show increased play fighting, adults tend to engage in social investigation (Douglas et al., 2004). These non-play behaviors may have become associated with the environmental context, thus inhibiting the establishment of social interaction CPP using the current methods. Although one study found that trial duration does not affect social interaction CPP in adolescents (Thiel et al., 2008), trial duration may be a factor in the establishment of social interaction CPP in adults. Overall, these results further demonstrate that adolescent rats are more sensitive to social reward relative to adults (Douglas et al., 2004).
Pair-housed adolescents and adults did not show a preference for the compartment paired with social interaction, which corroborates previous studies showing that individual housing increases motivation for social reward during adolescence (Douglas et al., 2004; Trezza et al., 2009). These findings parallel previous results showing that other motivational factors such as hunger or thirst induced by food or water restriction in the home cage also alters CPP in an environment previously paired with those natural rewards (Perks and Clifton, 1997). The most likely explanation for the current results is that social interaction within the home cage is rewarding, thus reducing the relative rewarding effect of the social interaction used to establish CPP. Further research is needed to determine if the effect of this alternative reward within the home cage is specific to social-induced CPP in male adolescents or whether it generalizes to females, as well as perhaps generalizing to CPP induced by other natural rewards such as food or water.
Regarding AMPH CPP, adolescents developed CPP to the compartment paired with AMPH (1.0 mg/kg), which is in agreement with previous results demonstrating CPP in adolescent rats using nicotine (Thiel et al., 2009) or cocaine (Zakharova et al., 2009b). Although AMPH CPP did not differ between individually- and pair-housed adolescents, housing condition altered AMPH CPP in adults. Whereas individually-housed adults developed AMPH CPP, pair-housed adults did not. These latter results contrast with several studies reporting AMPH CPP in group-housed adult rats using doses of AMPH similar to that used in the current report (Leone and Di Chiara, 1987; Rademacher et al., 2006; Spyraki et al., 1982). Moreover, other previous reports show that group-housed adult rats may be more sensitive to CPP induced by either cocaine (Schenk et al., 1986) or heroin (Schenk et al., 1983). Interestingly, however, Schenk et al. (1986) found that differential housing does not alter AMPH CPP, although that study used doses of AMPH (≤ 0.5 mg/kg) lower than that used in the current study (1 mg/kg). Thus, a limitation of the current study is the use of a single dose to assess AMPH CPP in differentially housed adolescents and adults. In addition, other parametric manipulations, such as the duration of the conditioning trial, number of trials and sex, may be the subject of future investigations.
Regardless of housing condition, relative to adults, adolescents were more sensitive to the acute locomotor stimulant effects of AMPH, an effect consistent with previous research showing enhanced locomotor activity following acute cocaine treatment in adolescents relative to adults (Badanich et al., 2008; Catlow and Kirstein, 2005; but see Laviola et al., 1995). Although adolescents and adults showed similar increases in locomotor activity following repeated AMPH conditioning trials in the current study, the increase in activity was greater in adults relative to adolescents (44% vs. 13% increase from trial 1 to trial 4). Thus, adults were more sensitive to the locomotor sensitizing effects of AMPH compared to adolescents. These results agree with previous studies showing locomotor sensitization in adult, but not adolescent, male rats following repeated AMPH (Laviola et al., 2001; Mathews and McCormick, 2007), cocaine (Collins and Izenwasser, 2002; but see Laviola et al., 1995), and methamphetamine (Zakharova et al., 2009a). Some caution is needed when interpreting the current results because the attenuated locomotor sensitization observed in adolescents could reflect a ceiling effect. In any case, however, neither the acute nor repeated stimulant effects of AMPH were modulated by housing condition in the current study. This finding corroborates a previous study showing that individual- and group-housed rats display similar locomotor activity following acute and repeated AMPH (Gill et al., 2012), thus suggesting that housing condition has less of an influence on AMPH-induced locomotor activity than on AMPH CPP.
Although not directly measured in the current study, age-related differences have been observed in dopaminergic pathways involved in AMPH-induced locomotor activity. Adolescent rats have higher dopamine D1 receptor densities relative to adult rats (Dalton and Zavitsanou, 2010), and activation of these receptors increases locomotor activity (David et al., 2004). The increased density of dopamine D1 receptors in adolescent rats may account for the observed increased locomotor activity following acute AMPH administration. In contrast, alterations in dopamine D2/D3 receptors may be involved in AMPH-induced locomotor sensitization observed in adults, but not in adolescents. Consistent with this possibility, D2/D3 receptor activation in adults attenuates AMPH-induced locomotor activity (Thorn et al., 1997) and repeated AMPH down-regulates D2/D3 receptors (Chiang et al., 2003; Ginovart et al., 1999; Robertson, 1986). In addition, repeated cocaine increases striatal dopamine transporter density in adults, but not adolescents (Collins and Izenwasser, 2002). However, it is unknown if repeated AMPH alters dopamine transporter or receptor levels differentially in adolescents and adults. Future work is needed to address the neuroadaptations that occur following acute and repeated AMPH treatment in adolescent and adult animals.
When social interaction and AMPH were conditioned against one another and rats then were given a concurrent choice between the two contexts, only individually-housed adolescents showed a preference for the social interaction compartment. Importantly, preference for the social interaction compartment was negated by pair-housing during adolescence, likely due to a reduction in the relative rewarding effect of the social context in the CPP apparatus by the social interaction reward provided in the home cage. In adult rats, there also was some evidence that access to a social-paired compartment decreased AMPH CPP, but only among individually-housed animals. That is, although individually-housed adults showed AMPH CPP in Experiment 2, no significant CPP was obtained during the concurrent choice between social interaction and AMPH compartments in Experiment 3. This result was unexpected because social interaction CPP was not observed in individually-housed adults in Experiment 1. However, some caution is needed in interpreting these effects because they represent cross-experiment comparisons. Nonetheless, social interaction has been shown previously to attenuate cocaine CPP in adult rats using a concurrent choice procedure (Fritz et al., 2011). Thus, these results suggest that the protective effects of social interaction on AMPH reward may be dependent on housing condition in both adolescents and adults.
In conclusion, these results further validate the use of the concurrent choice CPP paradigm (Fritz et al., 2011) to examine the relative rewarding value of two distinct appetitive stimuli (i.e., social interaction vs. drug). Furthermore, this procedure can be used to determine the factors that modulate choice between such stimuli, such as age and housing condition in the current study. Considering that adolescence is a period marked by increased drug abuse vulnerability (Spear, 2000), the current results indicate that individually-housed male adolescent rats show an especially strong propensity to find social interaction to be rewarding and that this social reward is effective as an alternate to AMPH reward. However, using the current procedures, the protective effect of social reward was reduced by pair-housing adolescents, as well as by testing animals during adulthood. Thus, future studies examining the protective effects of social reward on drug abuse vulnerability requires attention to the role of different housing conditions across the lifespan.
Acknowledgements
The authors would like to thank Mr. Travis McCuddy, Mrs. Emily Denehy, and Mrs. Kristin Howell for technical assistance. We also thank Drs. Gerald Zernig and Janet Neisewander for discussions on social CPP.
Role of Funding Source This research was funded by NIH grants P50 DA05312, R01 DA012964, and T32 DA016176. The NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors All authors have contributed to and approved the final manuscript. Joshua Beckmann, Andrew Meyer, and Justin Yates designed the experiments. Justin Yates conducted the experiments, analyzed the data, and prepared the manuscript. Michael Bardo, as PI on the project, participated in study design and manuscript preparation.
Conflict of Interest All authors declare that they have no conflicts of interest.
References
- Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: Two behavioral features of adolescence in mice. Behav. Neurosci. 2003;117:695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- Allen M, Donohue WA, Griffin A, Ryan D, Mitchell Turner MM. Comparing the influence of parents and peers on the choice to use drugs. Crim. Justice Behav. 2003;30:163–186. [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; Washington, DC: 1994. DSM-IV. [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur. J. Pharmacol. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Maldonado AM, Kirstein CL. Early adolescents show enhanced acute cocaine-induced locomotor activity in comparison to late adolescent and adult rats. Dev. Psychobiol. 2008;50:127–133. doi: 10.1002/dev.20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr SJ, Hoffmann JP, Yang X. Parental and peer influence on the risk of adolescent drug use. J. Prim. Prev. 2005;26:529–551. doi: 10.1007/s10935-005-0014-8. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: What does it add to our preclinical understanding of drug reward? Psychopharmacol. (Berl.) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci. Biobehav. Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Bowling SL, Bardo MT. Locomotor and rewarding effects of amphetamine in enriched, social, and isolate reared rats. Pharmacol. Biochem. Behav. 1994;48:459–464. doi: 10.1016/0091-3057(94)90553-3. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol. Behav. 1992;51:667–672. doi: 10.1016/0031-9384(92)90101-7. [DOI] [PubMed] [Google Scholar]
- Campbell JO, Wood RD, Spear LP. Cocaine and morphine-induced place conditioning in adolescent and adult rats. Physiol. Behav. 2000;68:487–493. doi: 10.1016/s0031-9384(99)00225-5. [DOI] [PubMed] [Google Scholar]
- Catlow BJ, Kirstein CL. Heightened cocaine-induced locomotor activity in adolescent compared to adult female rats. J. Psychopharmacology. 2005;19:443–447. doi: 10.1177/0269881105056518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang Y-C, Chen P-C, Chen J-C. D3 dopamine receptors are down-regulated in amphetamine sensitized rats and their putative antagonists modulate the locomotor sensitization to amphetamine. Brain Res. 2003;972:159–167. doi: 10.1016/s0006-8993(03)02522-8. [DOI] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Cocaine differentially alters behavior and neurochemistry in periadolescent versus adult rats. Dev. Brain Res. 2002;138:27–34. doi: 10.1016/s0165-3806(02)00471-6. [DOI] [PubMed] [Google Scholar]
- Dalton VS, Zavitsanou K. Differential treatment regimen-related effects of cannabinoids on D1 and D2 receptors in adolescent and adult rat brain. J. Chem. Neuroanat. 2010;40:272–280. doi: 10.1016/j.jchemneu.2010.07.005. [DOI] [PubMed] [Google Scholar]
- David HN, Sissaoui K, Abraini JH. Modulation of the locomotor responses induced by D1-like and D2-like dopamine receptor agonists and D-amphetamine by NMDA and non-NMDA glutamate receptor agonists and antagonists in the core of the rat nucleus accumbens. Neuropharmacol. 2004;46:179–191. doi: 10.1016/j.neuropharm.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: Impact of social versus isolate housing of subjects and partners. Dev. Psychobiol. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Fritz M, El Rawas R, Salti A, Klement S, Bardo MT, Kemmler G, Dechant G, Saria A, Zernig G. Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addict. Biol. 2011;16:273–284. doi: 10.1111/j.1369-1600.2010.00285.x. [DOI] [PubMed] [Google Scholar]
- Gill MJ, Arnold JC, Cain ME. Impact of mGluR5 during amphetamine-induced hyperactivity and conditioned hyperactivity in differentially reared rats. Psychopharmacol. (Berl.) 2012;221:227–237. doi: 10.1007/s00213-011-2565-0. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Farde L, Halldin C, Swahn CG. Changes in striatal D2-receptor density following chronic treatment with amphetamine as assessed with PET in nonhuman primates. Synapse. 1999;31:154–162. doi: 10.1002/(SICI)1098-2396(199902)31:2<154::AID-SYN9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Yates JR, Beckmann JS, Marusich JA, Zentall TR, Bardo MT. Social facilitation of d-amphetamine self-administration in rats. Exp. Clin. Psychopharmacology. 2011;19:409–419. doi: 10.1037/a0024682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PS, Holloway JL, Scordalakes EM. Social interaction with an intoxicated sibling can result in increased intake of ethanol by periadolescent rats. Dev. Psychobiol. 2001;38:101–109. doi: 10.1002/1098-2302(200103)38:2<101::aid-dev1002>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Laviola G, Pascucci T, Pieretti S. Striatal dopamine sensitization to D-amphetamine in periadolescent but not adult rats. Pharmacol. Biochem. Behav. 2001;68:115–124. doi: 10.1016/s0091-3057(00)00430-5. [DOI] [PubMed] [Google Scholar]
- Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. J. Pharmacol. Exp. Ther. 1995;275:345–357. [PubMed] [Google Scholar]
- Leone P, Di Chiara G. Blockade of D-1 receptors by SCH 23390 antagonizes morphine and amphetamine-induced place preference conditioning. Eur. J. Pharmacol. 1987;135:251–254. doi: 10.1016/0014-2999(87)90621-2. [DOI] [PubMed] [Google Scholar]
- Mathews IZ, McCormick CM. Female and male rats in late adolescence differ from adults in amphetamine-induced locomotor activity, but not in conditioned place preference for amphetamine. Behav. Pharmacol. 2007;18:641–650. doi: 10.1097/FBP.0b013e3282effbf5. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the Care and Use of Laboratory Animals. National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- Peartree NA, Hood LE, Thiel KJ, Sanabria F, Pentkowski NS, Neisewander JL. Limited physical contact through a mesh barrier is sufficient for social reward-conditioned place preference in adolescent male rats. Physiol. Behav. 2012;105:749–756. doi: 10.1016/j.physbeh.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perks SM, Clifton PG. Reinforcer revaluation and conditioned place preference. Physiol. Behav. 1997;61:1–5. doi: 10.1016/s0031-9384(96)00243-0. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Kovacs B, Shen F, Napier TC, Meredith GE. The neural substrates of amphetamine conditioned place preference: implications for the formation of conditioned stimulus-reward associations. Eur. J. Neurosci. 2006;24:2089–2097. doi: 10.1111/j.1460-9568.2006.05066.x. [DOI] [PubMed] [Google Scholar]
- Robertson HA. Cerebral decortication reverses the effect of amphetamine on striatal D2 dopamine binding site density. Neurosci. Lett. 1986;72:325–329. doi: 10.1016/0304-3940(86)90535-5. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hunt T, Colle L, Amit Z. Isolation versus grouped housing in rats: differential effects of low doses of heroin in the place preference paradigm. Life Sci. 1983;32:1129–1134. doi: 10.1016/0024-3205(83)90118-2. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hunt T, Malovechko R, Robertson A, Klukowski G, Amit Z. Differential effects of isolation housing on the conditioned place preference produced by cocaine and amphetamine. Pharmacol. Biochem. Behav. 1986;24:1793–1796. doi: 10.1016/0091-3057(86)90523-x. [DOI] [PubMed] [Google Scholar]
- Smith MA. Peer influences on drug self-administration: social facilitation and social inhibition of cocaine intake in male rats. Psychopharmacol. (Berl.) 2012;224:81–90. doi: 10.1007/s00213-012-2737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Fibiger HC, Phillips AG. Dopaminergic substrates of amphetamine-induced place preference conditioning. Brain Res. 1982;293:185–193. doi: 10.1016/0006-8993(82)90685-0. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: a model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008;96:202–212. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacol. (Berl.) 2009;204:391–402. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn L, Ashmeade TE, Storey VJ, Routledge C, Reavill C. Evidence to suggest that agonist modulation of hyperlocomotion is via post-synaptic dopamine D2 or D3 receptors. Neuropharmacol. 1997;36:787–792. doi: 10.1016/s0028-3908(97)00033-6. [DOI] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Vanderschuren LJMJ. Conditioned place preference induced by social play behavior: parametrics, extinction, reinstatement and disruption by methylphenidate. Eur. Neuropsychopharmacology. 2009;19:659–669. doi: 10.1016/j.euroneuro.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict. Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Van den Berg CL, Pijlman FTA, Koning HAM, Diergaarde L, Van Ree JM, Spruijt BM. Isolation changes the incentive value of sucrose and social behaviour in juvenile and adult rats. Behav. Brain Res. 1999;106:133–142. doi: 10.1016/s0166-4328(99)00099-6. [DOI] [PubMed] [Google Scholar]
- Wang Y-C, Wang C-C, Lee C-C, Huang ACW. Effects of single and group housing conditions and alterations in social and physical contexts on amphetamine-induced behavioral sensitization in rats. Neurosci. Lett. 2010;486:34–37. doi: 10.1016/j.neulet.2010.09.039. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav. Brain Res. 2009a;198:45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Miller J, Unterwald E, Wade D, Izenwasser S. Social and physical environment alter cocaine conditioned place preference and dopaminergic markers in adolescent male rats. Neurosci. 2009b;163:890–897. doi: 10.1016/j.neuroscience.2009.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]


