Abstract
17β-estradiol and progesterone exert a number of physiological effects throughout the brain due to interactions with several types of receptors belonging to the traditional family of intracellular hormonal receptors as well as to membrane-bound receptors. In particular, both hormones elicit rapid modifications of neuronal excitability that have been postulated to underlie their effects on synaptic plasticity and learning and memory. Likewise, both hormones have been shown to be neuroprotective under certain conditions, possibly due to the activation of pro-survival pathways and the inhibition of pro-apoptotic cascades. Because of the similarities in their cellular effects, there have been a number of questions raised by numerous observations that progesterone inhibits the effects of estrogen. In this manuscript, we first review the interactions between 17β-estradiol (E2) and progesterone (P4) in synaptic plasticity, and conclude that, while E2 exerts a clear and important role in long-term potentiation of synaptic transmission in hippocampal neurons, the role of P4 is much less clear, and could be accounted by the direct or indirect regulation of GABAA receptors. We then discuss the neuroprotective roles of both hormones, in particular against excitotoxicity. In this case, the neuroprotective effects of these hormones are very similar to those of the neurotrophic factor BDNF. Interestingly, P4 antagonizes the effects of E2, possibly through the regulation of estrogen receptors or of proteins associated with the receptors or interactions with signaling pathways activated by E2. Overall, this review emphasizes the existence of common molecules and pathways that participate in the regulation of both synaptic plasticity and neurodegeneration.
Keywords: estrogen, progesterone, hippocampus, calpain, synaptic plasticity, neurodegeneration
1. Introduction
For many years, the physiological effects of sex steroid hormones on the brain were attributed to circulating hormones that originated in steroidogenic organs such as the gonads and adrenal glands. However, the past few decades have indicated that steroid hormones are in fact synthesized locally in brain (Amateau et al., 2004; Murakami et al., 2006; Remage-Healey et al., 2009), skin (Forstrom, 1980; Mills et al., 2005), and other organs or tissues (Lecain et al., 2003; Schmidt et al., 2008), where they participate in the regulation of a variety of functions. The landmark Women's Health Initiative study brought much attention to the potentially detrimental interactions of estrogens and progestins, showing an increased risk of breast cancer among postmenopausal women who received hormone therapy with combined estrogen plus progestin compared to women receiving estrogen alone (WHI, 2002). Although many factors, such as age, type of progestin, and course of treatment were confounding factors in the study, it did indicate the need for further studies on the biological mechanisms of 17β-estradiol (E2) and progesterone (P4) interactions.
The nervous system is a major target of steroid hormones, and contains specific receptors for hormones secreted from peripheral organs such as the adrenal cortex, testes, and ovaries, and for locally synthesized and released hormones. Sex steroids, such as estrogens, androgens and progestins, exert their effects not only at the genomic level through classical receptors that belong to the superfamily of nuclear receptors (Guerriero, 2009), but also non-genomically by interacting with G protein-coupled receptors (GPCR) and membrane-localized steroid receptors (Hammes and Levin, 2007) (Fig. 1). Thus, the effects of E2 and other hormones on the brain, which require a prolonged latency and last for a long time, are likely due to genomic activation (McEwen and Alves, 1999); on the other hand, the rapid and short-term effects of steroid hormones on electrophysiological properties of neurons with latencies and durations on the scale of milliseconds to minutes are likely due to the stimulation of membrane-associated receptors and the activation of second messenger cascades (Bi et al., 2001; Teyler et al., 1980).
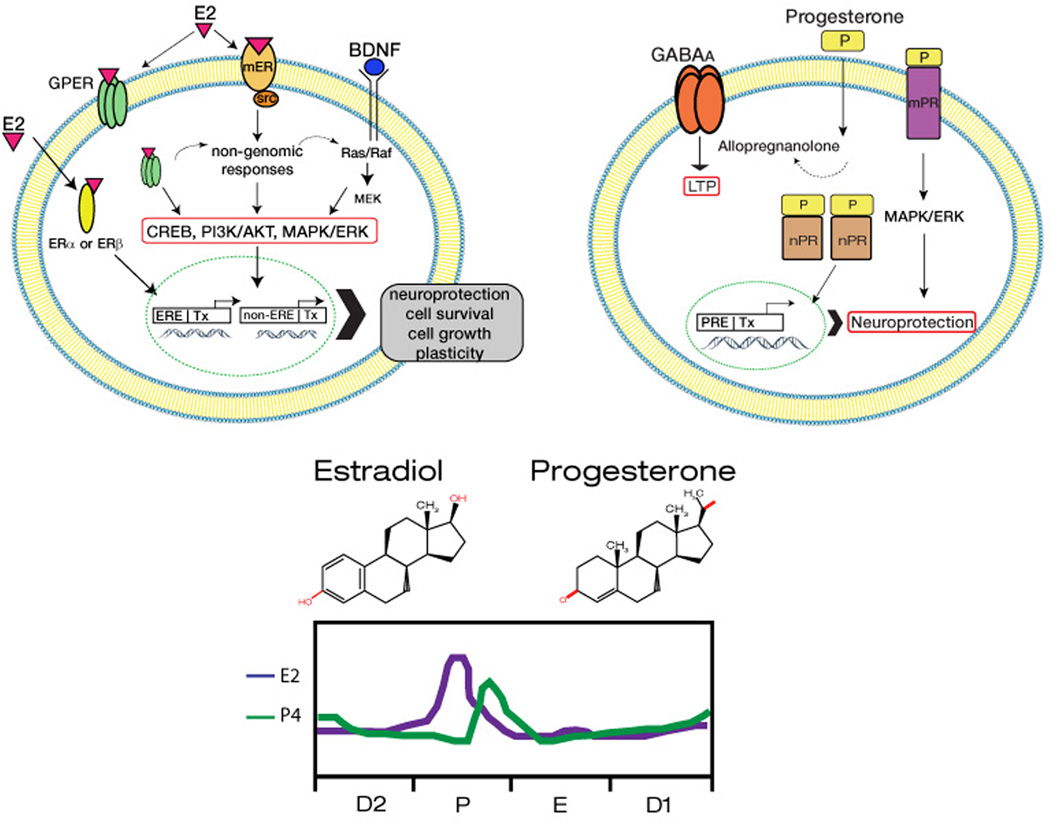
Figure 1. Schematic representation of the pathways activated by E2 and P4 in neurons.
E2 is proposed to activate three types of receptors, i) membrane-bound receptors (ERm) resulting in activation of the src tyrosine kinase and leadong to MAPK/ERK activation. ii) the traditional cytoplasmic receptors, which in conjunction with the steroid receptor coactivator-1 (SRC-1) triggers transcriptional responses, and iii) the GPER receptor, which is localized either in membrane or in the cytoplasm; the functions of this receptor ar still being unraveled and it appears to play a significant role in neuroprotection. P4 is shown to activate i) a membrane bound receptor (mPR) as well as ii) traditional cytoplamc receptors that trigger transcription. Through metabolites such as allopregnanolone, P4 may also activate GABAA receptors, which induce LTP. Also shown are the structures of E2 and P4 along with the corresponding fluctuations in their plasma levels across the rat estrus cycle.
Considerable progress has been made in understanding of the molecular events involved in both synaptic plasticity and neurodegeneration. Interestingly, many cellular processes participating in synaptic plasticity also play a critical role in neurodegeneration. Historically, the role of the synapse was limited to simply relaying information from one neuron to another in a fixed manner. Over the past half century, a seminal development in neurobiology has been the realization that synapses are in fact extremely plastic – and that this plasticity may be the basis for learning and memory. Synaptic plasticity, or the ability of synapses to modify their functional strength in an activity-dependent manner, also includes the ability of neuronal circuits to change as a result of certain patterns of neuronal activity, and is prominent in forebrain structures, including the hippocampal formation. In the late 1940’s, Hebb postulated that synaptic plasticity was an essential mechanism used by the brain to encode experience and practice, a concept that has since led to the notion of hebbian synapses (Berlucchi and Buchtel, 2009). Since the discovery by Bliss and Lomo (Bliss and Lomo, 1973) of a long-lasting enhancement of synaptic transmission at hippocampal synapses resulting from a brief period of high frequency stimulation of the inputs, long-term potentiation (LTP) of synaptic transmission has been proposed to be a major form of synaptic plasticity that could represent a cellular model of memory trace formation in brain (Baudry and Lynch, 2001; Bear and Malenka, 1994; Bliss and Collingridge, 1993). While the molecular and synaptic mechanisms underlying LTP have been studied extensively, there has been some reluctance to accept the idea that LTP plays a critical role in behavioral learning and memory (Shors and Matzel, 1997), although more recent results strongly support it (see (Lee and Silva, 2009), for a recent discussion of this issue). In particular, studies of its mechanisms have revealed the existence of a number of processes that undoubtedly play critical roles in memory formation (Bi and Poo, 2001; Fanselow and Poulos, 2005; Lynch et al., 2008). In area CA1 of hippocampus where it has been extensively studied, LTP requires N-methyl-d-aspartate (NMDA) receptor activation for induction, and an increase in the number of α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionate (AMPA) receptors for expression and maintenance (Baudry et al., 2011; Lisman, 2003; Malenka and Nicoll, 1999; Stanton, 1996). On the other hand, hippocampal long-term depression, LTD, is a form of activity-dependent synaptic plasticity where activation of presynaptic inputs generally at low frequency results in a decrease in responsiveness of activated neurons. It requires activation of NMDA and metabotropic glutamate receptors, and is due to internalization of AMPA receptors (Malenka and Bear, 2004; Stanton, 1996); however, its relationship to learning and memory remains unclear.
The neuropathology underlying diseases that affect learning, memory, and cognition, such as Alzheimer’s disease (AD), is characterized by progressive synaptic dysfunction and neuronal degeneration. Neurodegeneration can therefore be viewed as a maladaptive form of synaptic plasticity, as it can result from an over-activation of the same processes that lead to physiological synaptic plasticity. As mentioned above, synaptic plasticity requires a brief activation of NMDA and other types of glutamate receptors. However, prolonging the activation of certain glutamate receptors results in excitotoxicity. Thus, activation of the same pathways that result in physiological modifications of synaptic transmission could also lead to first dysfunction of synaptic transmission and ultimately to neuronal damage. Interestingly, both E2 and P4 have been shown to regulate synaptic plasticity and neurodegeneration, and in the following we will discuss the mechanisms underlying these effects as well as the ways these two steroid hormones interact to modify their regulatory effects.
E2 and P4 are thought to impart their biological functions via activation of specific estrogen (ERs) and progesterone receptors (PRs) (Fig. 1). ERs have been localized throughout the cortex, limbic system and hippocampus, in neurons, astrocytes and dendritic processes (Milner et al., 2005; Simerly et al., 1990; Su et al., 2001). Similarly, PR isoforms are widely expressed in various brain regions, including hypothalamus, cortex, cerebellum, and in pyramidal neurons of the hippocampus (Hirata et al., 1992). Moreover, numerous studies suggest distinct functional patterns between the classical estrogen receptors, ERα and ERβ. For instance, fertility was impaired in mice lacking the ERα gene, but not in ERβ KO mice (Shughrue et al., 1997). While ERα receptors play a major role in peripheral disorders, as in breast cancer (Ali and Coombes, 2000), accumulating evidence has emphasized the role of ERβ in cognition and behavior. In particular, activity-dependent regulation of dendritic spine cytoskeleton, LTP induction, and memory formation has been shown to be dependent on ERβ activation (Kramar et al., 2009; Liu et al., 2008). In vivo results have also shown that ERβ KO animals had impaired performance in object recognition and placement tasks, even when given E2 supplements (Walf et al., 2008). Recently, the use of a selective ERβ agonist, WAY-200070, revealed that ERβ activation mediates the effects of E2 on memory in a hippocampus-dependent task, as it mimics E2 effects in a spatial memory task. Furthermore, activation of ERβ by this agonist led to increases in dendritic spine density, dendritic branching, synaptic proteins and LTP (Kramar et al., 2009; Liu et al., 2008). Although compelling, this evidence leaves room for interpretation, as the efficacy and specificity of this agonist for ERβ and other putative ERs are not completely known. Given that a number of studies exploring estrogenic effects are performed in ovariectomized (OVX) animals, it is also important to understand these effects on cycling intact animals, as cycling changes in E2 and P4 are likely to play important roles in their central effects (Fig. 1). In a recent study, wild-type animals in the proestrous phase had improved performance in object recognition and T-maze tasks, as well as reduced anxiety-like behavior in the plus maze and mirror chamber tasks when compared to ERβ KO mice. Because ER genotype did not seem to play a role in circulating hormone levels across the estrous cycle (Walf et al., 2009), these results implicate an important role of ERβ in mediating E2’s influences on cognition and affective behaviors. Interestingly, a previous paper had found that anxiolytic-like behavior in cycling animals correlated with elevated circulating and hippocampal P4 concentrations (Frye et al., 2000). Thus, understanding the relationship between P4 and ERβ can help elucidate some of the mechanisms underlying hormonal regulation of cognitive and behavioral functions. Recently, selective agonists for ERs have been investigated in a rodent model of Alzheimer’s disease (AD), in which treatment with propyl pyrazole triol (PPT), an ERβ agonist, but not diarylpropionitrile (DPN), an ERα agonist, in OVX 3xTg-AD mice reduced accumulation of β-amyloid protein and improved hippocampus-dependent performance (Carroll and Pike, 2008). However, the distribution and expression pattern of ERs in 3xTg-AD mice have not yet been elucidated, making it difficult to interpret these results. A shortcoming prevalent in all these studies is that they failed to investigate the effects of progesterone (P4). Notably, both E2 and P4 are depleted as a consequence of menopause in women and in OVX experimental animal paradigms. In fact, normal or high E2 levels characterize the perimenopausal period, while a decline in P4 preceding estrogenic decline may result in perimenopausal symptoms (Prior, 1998). Furthermore ERβ knockout mice have deficits in ovulation (Krege et al., 1998), signifying low levels of P4 in these animals. Thankfully, the field of P4 and of its effects in the CNS is slowly emerging.
P4 exerts its biological functions via the two classical PR isoforms, PRA and PRB (Fig. 1). As with ERs, these PRs have distinct physiological functions both in vivo and in vitro. Although the classical E2 and P4 receptors have been well characterized, there is also evidence for membrane-associated E2 and P4 receptors (Toran-Allerand et al., 2002; Guennoun et al., 2008), complicating the interpretation of the observed effects of female sex steroids on the brain. Interactions between E2 and P4 have been documented in brain, as E2 can stimulate PR expression in the CA1 region of hippocampus and in the medial preoptic nucleus, suggesting possible effects of E2 on gene expression via P4 receptors (Alves et al., 2000; Chung et al., 2006). Recent studies have also documented that ovarian E2 can stimulate neuronal P4 synthesis via a rapid, membrane-associated ERα-initiated mechanism in astrocyte cultures (Micevych and Sinchak, 2008). Although the mechanisms of E2 and P4 interactions are not fully elucidated, their understanding may provide new insight for developing potential therapeutic treatments for a variety of neurodegenerative diseases, including AD and Parkinson’s disease. This review is therefore directed at discussing the potential mechanisms underlying interactions between E2 and P4 in both synaptic plasticity (limited to LTP) and neurodegeneration. In particular, we will develop the idea that E2 and P4 interact at the level of common molecules and pathways that participate in the regulation of both synaptic plasticity and neurodegeneration.
2. P4/E2 interactions in LTP
2.1. E2 effect on LTP
Earlier studies suggested that estrogen therapy in postmenopausal women decreases the probability of developing AD and slows the progress of the disease (Henderson, 1997). However, more recent studies have led to the notion that there exists a critical window for the estrogen treatment to be beneficial in postmenopausal women (Henderson and Brinton, 2010; Zhang et al., 2011). It has thus been suggested that E2 could act both as a cognitive enhancer and a neuroprotective agent. For over three decades, electrophysiological studies have found that E2 regulates synaptic plasticity within the nervous system. In a pioneering study, female rats treated with E2 in proestrus, the time of the estrous cycle when E2 levels are at their highest levels, exhibited decreased hippocampal seizure thresholds (Terasawa and Timiras, 1968). The magnitude of long-term potentiation (LTP) was found to be maximal in hippocampal slices prepared from female rats in the afternoon of proestrus (Warren et al., 1995), and hippocampal LTP induction was facilitated in OVX rats treated with E2, as compared to untreated OVX rats (Cordoba-Montoya and Carrer, 1997). Initial studies by Teyler and colleagues (1980) using in vitro hippocampal slices showed that gonadal steroids rapidly modulated neuronal excitability. In the initial experiments, field excitatory postsynaptic potentials (fEPSPs) recorded from area CA1 of hippocampal slices from male and female rats were monitored before and after the addition of E2 (100 pM) to the slice incubation medium. In male rats, E2 produced a rapid (<10 min) enhancement of fEPSPs evoked by stimulation of the Schaffer collateral pathway, providing the first evidence that physiological concentrations of E2 directly and rapidly enhanced glutamatergic synaptic transmission in hippocampus (Teyler et al., 1980). Since then many laboratories have reported that E2 rapidly increased basal synaptic responses in field CA1 of hippocampal slices (Bi et al., 2000; Foy et al., 1999; Kramar et al., 2009; Liu et al., 2008). Several laboratories have also showed that E2 increased LTP magnitude in acute hippocampal slices (Foy et al., 1999; Ito et al., 1999; Teyler et al., 1980). In addition, E2 increased both AMPA and NMDA receptor-mediated synaptic responses in hippocampal neurons (Foy et al., 1999; Gu and Moss, 1998; Moss and Gu, 1999). These studies strongly suggest that estrogenic actions on synaptic plasticity occur via the action of non-genomic responses.
2.2. Signaling pathways mediating E2 effects on LTP
Several studies have since provided detailed information regarding the mechanisms underlying the multiple effects of E2 on glutamatergic synaptic transmission and LTP. In particular, the effects of E2 on NMDA receptor-mediated synaptic currents were blocked by an inhibitor of src tyrosine kinase, as well as by PD98059, a MAPK inhibitor (Bi et al., 2000). NMDA receptors are a major substrate for Src tyrosine kinase, and Src-mediated tyrosine phosphorylation of NR2 subunits has been shown to increase NMDA receptor-mediated responses (Lancaster and Rogers, 1998; Lu et al., 1999; Thomas and Brugge, 1997; Yu et al., 1997; Zheng et al., 1998). Thus, the E2 effect on LTP is likely caused by the activation of Src and the resulting tyrosine phosphorylation of NR2 subunits. E2-mediated enhancement of the degree of LTP as a result of the activation of Src/MAPK pathway also fits well with several data indicating that this pathway is important for LTP (Atkins et al., 1998; Lisman and Fallon, 1999; Orban et al., 1999). Mice with deletion of another member of the Src kinase family, fyn, have LTP and learning impairment, whereas MAPK inhibitors impair spatial learning (Blum et al., 1999). However, it is important to note that the inhibitor of Src tyrosine kinase, PP2, did not affect LTP and that Src knockout mice have normal LTP (Grant et al., 1992). Therefore, different tyrosine kinases might play different roles in regulating NMDA receptors and LTP characteristics.
2.3. E2-mediated cytoskeletal regulation and LTP
Recently, several studies have shown that E2 rapidly regulates dendritic spine cytoskeleton, and that this effect is likely to be responsible for E2-mediated regulation of synaptic transmission and LTP. In particular, E2 has been shown to activate a cellular cascade that consists of calpain-2 and LIM kinase resulting in cofilin phosphorylation, increased actin polymerization and increased number of synaptic AMPA receptors (Kramar et al., 2009; Sanchez et al., 2009; Zadran et al., 2009) (Fig. 2). Calpains belong to a large family of calcium-dependent cysteine proteases and two isoforms, calpain-1 (a.k.a. µ-calpain) and calpain-2 (a.k.a. m-calpain) are ubiquitously distributed in mammalian brain (Goll et al., 2003). Both isoforms require calcium for activation, with calpain-1 requiring micromolar concentrations of calcium and calpain-2 millimolar concentrations (Croall and DeMartino, 1991). We previously proposed the hypothesis that calpain played a critical role in LTP of synaptic transmission elicited by high frequency stimulation of glutamatergic synapses and in learning and memory by participating in the modification of synaptic structure and function (Baudry and Lynch, 2001; Lynch and Baudry, 1984). As studies conducted in calpain-1 knock-out mice failed to provide evidence for LTP or learning and memory deficits in these mice, we suggested that calpain-2 was critically involved in synaptic plasticity (Grammer et al., 2005). However, the seemingly non-physiological levels of calcium required for calpain-2 activation necessitated the existence of another mechanism for its activation. Recent studies in fibroblasts have demonstrated that calpain-2 activation by epidermal growth factor (EGF) was mediated by extracellular signal-regulated kinase (ERK) phosphorylation of calpain-2 at Ser50 (Glading et al., 2004). Utilizing a FRET-based assay to monitor calpain activation, we recently reported that both EGF and BDNF activated calpain-2 in neurons as a result of MAPK-mediated serine phosphorylation (Zadran et al., 2010). E2 was also shown to rapidly (within minutes) activate calpain-2 in primary cultured neurons in a MAPK-dependent but calcium-independent manner. E2-induced calpain-2 activation resulted in actin polymerization and increases in levels of membrane AMPA receptor subunit GluR1 and synaptic responses in acute hippocampal slices (Zadran et al., 2009).
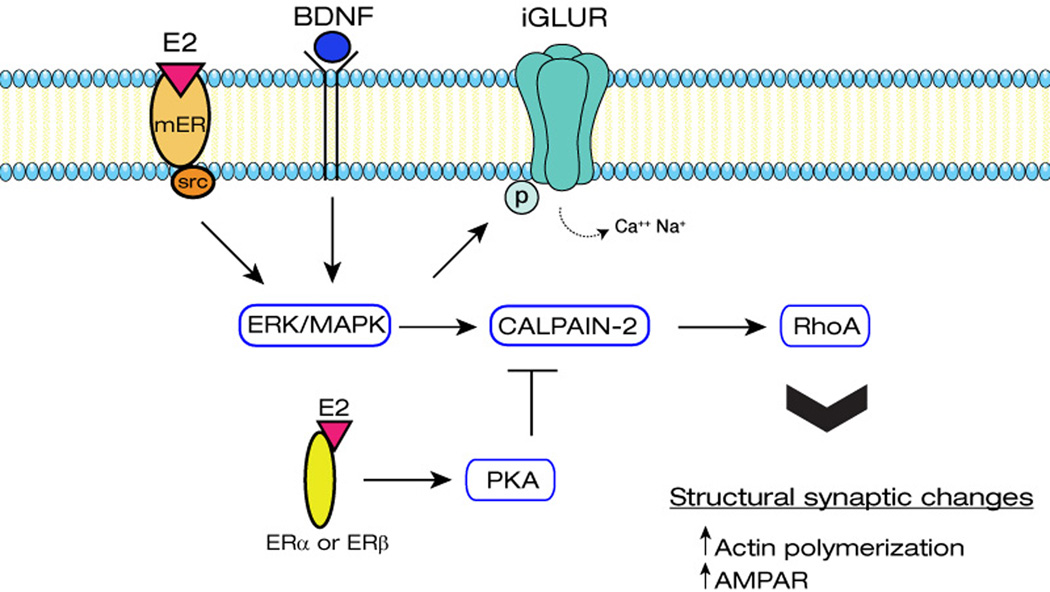
Figure 2. Schematic illustration of the links between E2 and synaptic plasticity.
E2 activation of membrane receptors leads to structural synaptic changes that result in increased synaptic activity and facilitation of LTP induction/consolidation. Part of these effects involve calpain activation via ERK/MAPK-mediated phosphorylation as well as calpain inhibition via PKA-mediated phosphorylation. E2 also regulate actin polymerization via the RhoA-LIMK-cofilin pathway.
The FRET-based calpain assay also provided information regarding the localization of E2- mediated calpain activation within neurons. Confocal analysis of the FRET signal following application of E2 indicated that calpain was activated within dendrites and dendritic spine-like structures. Calpain has previously been localized in dendritic spines (Perlmutter et al., 1988) and there is also evidence for the presence of E2 receptors in dendrites (Milner et al., 2005). These results further support a critical role for E2 and calpain in regulating structural changes at synapses following the induction and maintenance of LTP. They also fit well with the evidence that E2 participates in the regulation of synaptic contacts in hippocampus (Gould et al., 1990).
Both ERα and ERβ receptors are present in dendrites of hippocampal neurons, although their respective roles in mediating various effects of E2 in adult hippocampus are still debated. E2-mediated calpain activation could be elicited by stimulation of either ERα or ERβ receptors in cultured neurons. Activation of either ERα or ERβ receptors has been shown to lead to MAP kinase activation (Nilsen et al., 2002), a result consistent with the idea that calpain-2 is activated by MAP kinase-dependent phosphorylation. In addition to MAP kinase, E2 has been shown to activate CaMKII as well as PKA. In fibroblasts, stimulation of adenylate cyclase or PKA inhibited calpain activation by phosphorylating calpain-2 at serine/threonine 369–370 (Shiraha et al., 2002). Interestingly, when neurons were treated with inhibitors of either adenylate cyclase or PKA, E2-induced calpain activation in cultured neurons was greatly enhanced, suggesting that, like in fibroblasts, PKA-mediated calpain-2 phosphorylation in neurons results in inhibition of calpain activation. Thus, E2, by activating different intracellular signalling pathways, has complex effects on calpain activity. On one hand, by activating MAP kinase, E2 elicits calpain-2 activation, while by activating PKA, it produces calpain inhibition (Fig. 2). The location and balance between these two opposing effects of E2 on calpain activity are likely to be important to determine the location, extent and duration of calpain activation. In any event, these effects could participate in several of the reported cellular effects of E2, including regulation of synaptic plasticity and dendritic spine morphology.
As mentioned earlier, E2 inactivates cofilin by stimulating LIM kinase, thereby leading to actin polymerization and increased formation of filopodia (Kramar et al., 2009; Sanchez et al., 2009). Since calpain inhibition prevented E2-induced increase in actin polymerization, calpain activation could be an important step leading to actin polymerization, possibly through cofilin phosphorylation/inactivation. Such a role for calpain has been clearly demonstrated in fibroblasts and other cell types (Fox, 1999). In addition, E2 treatment of hippocampal slices for 1 h has also been shown to increase the levels of GluR1 subunits of membrane AMPA receptors, suggesting that E2 increased AMPA receptor trafficking (Liu et al., 2008). E2-mediated increase in GluR1 levels was completely blocked with a calpain inhibitor, indicating that calpain activation plays a critical role in the regulation of AMPA receptor membrane insertion (Zadran et al., 2010).
Interestingly, the effect of E2 on basal synaptic transmission in field CA1 of hippocampal slices was also completely blocked by calpain inhibition (Zadran et al., 2010). As mentioned above, it has been proposed that E2-mediated increased excitability in CA1 was due to increase in actin polymerization, possibly leading to increased surface expression of AMPA receptors (Kramar et al., 2009; Liu et al., 2008; Srivastava et al., 2008). In other words, E2 would reproduce some of the events associated with LTP induction, with the major differences that these effects are reversible, whereas LTP induction leads to a long lasting increase in synaptic efficacy. It has recently been demonstrated that LTP consolidation requires the activation of two separate cascades, both of which involve the Rho family of small GTPases, leading to cytoskeletal modifications (Rex et al., 2009), and it was proposed that E2 would activate only one of the two cascades, consisting in RhoA and its downstream effector, LIM Kinase. This selectivity could account for its reversible effects on cytoskeleton and synaptic function (Kramar et al., 2009). This interpretation is completely consistent with the hypothesis that E2-mediated increased actin polymerization, increased membrane levels of GluR1 subunits of AMPA receptors and increased synaptic responses are all blocked by calpain inhibition. Moreover, in a recent study, we found that down-regulation of calpain-2 or LIMK1 by systemic injection of a vector consisting of the Rabies Virus Glycoprotein (RVG) and siRNA against calpain 2 or LIMK1 respectively resulted in LTP impairment in acute hippocampal slices, further supporting a critical role for the calpain-LIMK cascade in LTP regulation (Zadran et al., 2012).
2.4. LTP and the estrus cycle
In another series of animal studies, estrus cycle changes in rats were correlated with changes in synaptic plasticity. Hippocampal slices from cycling female rats in diestrus (low E2 concentration) and proestrus (high E2 concentration) were prepared, and LTP was elicited by high-frequency stimulation. The difference in LTP magnitude between these groups following high-frequency stimulation was highly significant: slices prepared from rats in proestrus exhibited LTP representing about a 50% increase over baseline, whereas slices prepared from rats in diestrus had LTP values representing about a 25% increase over baseline (Bi et al., 2001). These findings are consistent with those of Teyler et al (1980), who reported that baseline synaptic transmission varied with the phase of the estrus cycle in female rats at the time of hippocampal slice preparation (Teyler et al., 1980). Interestingly, E2 increased the magnitude of LTP in slices prepared from diestrus rats, while it decreased LTP in slices prepared from proestrus rats (Foy et al., 2004). Thus, the effects of E2 on hippocampal LTP in female rats depend on the state of their estrous cycle. In cycling female rats, when endogenous circulating levels of E2 are at their highest (i.e., proestrus), LTP magnitude is high, but exogenously applied E2 decreases LTP magnitude. In contrast, when endogenous circulating levels of E2 are at their lowest (i.e., diestrus), the situation is completely reversed and LTP magnitude is low, and exogenously applied E2 increases LTP magnitude. These results suggest that cyclic changes in E2 levels occurring during the estrous cycle in female rats are associated with changes not only in the magnitude of LTP recorded from hippocampal CA1 cells, but also in the very machinery that is used to produce LTP, including calpain, actin polymerization and levels and functional status of glutamate receptors. However, P4 levels were not recorded in these studies and given that P4 levels also fluctuate during the estrous cycle, it may help explain some of these effects on synaptic transmission. Nevertheless, these results fit well with those mentioned earlier indicating facilitation of LTP induction by E2 in OVX female rats (Cordoba-Montoya and Carrer, 1997), increased LTP in the afternoon of proestrus of female rats (Warren et al., 1995), and support the results of a study showing improved memory performance with high E2 levels in female rats (Leuner et al., 2004).
2.5. P4 effect on LTP
Few studies have examined the acute effects of P4 on synaptic transmission and plasticity, with the results of these early studies being mostly contradictory. In an earlier study, P4 (10 µM) had no effect on LTP in CA1 slices from 4-week-old rats (Ito et al., 1999). On the other hand, P4 (8–10 µM) significantly enhanced basal synaptic transmission in hippocampal slices; however, P4 also decreased epileptiform activity elicited by tetanic stimulation (Edwards et al., 2000). In pyramidal neurons from slices of prelimbic cortex, P4 (100 µM) had no effect on the frequency of excitatory postsynaptic currents (EPSCs), but inhibited dopamine-induced increases in EPSC frequency (Feng et al., 2004). In a more recent study, P4 (1 µM) produced a slowly occurring (within 30 min of application in hippocampal slices) decrease in CA1 fEPSPs, and this effect persisted for at least 45 min following initial P4 application (Foy et al., 2008). Results from intracellular recordings indicated that the effects of P4 on hippocampal synaptic transmission was mediated, at least in part, by activation of GABAA receptors, as P4-mediated decrease in EPSCs was completely blocked by the GABAA-receptor antagonist picrotoxin (100 µM) (Foy et al., 2008). Similar results were obtained in another study linking GABAA receptors with P4 action (Herd et al., 2008). These results are consistent with the idea that the effects of P4 on basal synaptic transmission and LTP are due to a direct action or indirect action through P4 metabolites on GABAA receptors (Fig. 1).
2.6. E2/P4 interactions
Interactions between E2 and P4 on synaptic plasticity have recently been evaluated. Interestingly, chronic treatment with P4 (60 days) was also found to decrease hippocampal synaptic transmission and LTP in hippocampal slices prepared from adult, OVX female rats. In contrast, chronic treatment with P4 and E2 for 60 days in adult, OVX female rats restored both basal synaptic transmission and LTP to levels similar to those found in gonadally intact female rats. Clearly, more studies are needed to understand the complex interactions between E2 and P4 on both basal synaptic transmission and LTP. On one hand, it appears that some of the effects of P4 are mediated through direct or indirect interactions with GABAA receptors. On the other hand, more needs to be known regarding the interactions between P4 and the complex cascades of biochemical pathways activated by E2, including calpain activation and actin polymerization. In particular, P4 was shown to activate µ-calpain in human sperm (Ozaki et al., 2001), while calpain inhibitors prevented P4-mediated down-regulation of P4 receptors (Lange et al., 2000). It will also be interesting to determine whether P4 can antagonize E2-mediated calpain activation, possibly through the activation of PKA and phosphorylation of calpain-2 at serine/threonine 369–370. Similarly, while both E2 and P4 can increase BDNF mRNA and protein levels, P4 can reverse E2-mediated increases in BDNF mRNA and protein levels (Bimonte-Nelson et al., 2004; Aguirre and Baudry, 2009). E2 has also been shown to produce BDNF release (Sato et al., 2007), and it is conceivable that some of the effects of E2 on synaptic plasticity are due to the acute release of BDNF from neurons or astrocytes. Whether P4 also antagonizes the acute effect of E2 on BDNF release remains to be investigated (see below for further discussion of this issue).
3. P4/E2 interactions in neurodegeneration
3.1. E2 effect on excitotoxicity
E2-mediated protection against different neuronal death-inducing events has been well documented both in vivo and in vitro (Bains et al., 2007; Raval et al., 2006; Singer et al., 1999). The detailed mechanisms involved in this action of E2 remain widely unknown, although the use of ERα- and ERβ-specific agonists has indicated that both types of ERs may be involved (Cordey and Pike, 2005; Zhao et al., 2004). In contrast, accumulating evidence suggests that neuroprotection is mediated by the activation of a subpopulation of membrane-localized ERα receptors associated with the rapid activation of several pro-survival signaling cascades, including the induction of anti-apoptotic genes (Alexaki et al., 2006; Honda et al., 2000; Wu et al., 2005) (Fig. 3). Hippocampal CA1 pyramidal neurons have been repeatedly reported to be protected by E2, and experiments with ER knock-out mice have shown that ERα is the critical receptor involved in neuroprotection against ischemia (Dubal et al., 2001; Merchenthaler et al., 2003).
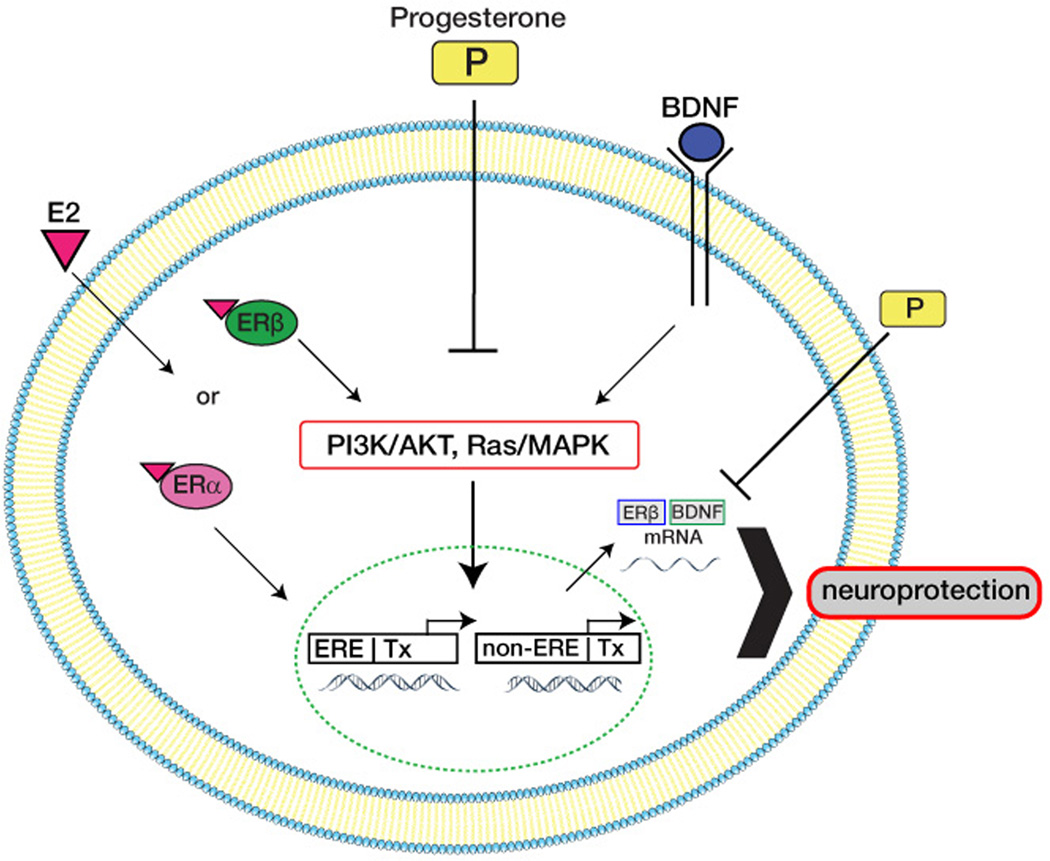
Figure 3. Schematic representation of postulated interactions between E2 and P4.
Both E2 and P4 trigger calpain activation, presumably through ERK-mediated phosphorylation. In addition, both E2 and P4 increase BDNF expression, which further stimulates calpain activation. These responses are involved in the neuroprotective effects of both E2 and P4. However, P4 is proposed to negatively regulate the stimulatory effects of E2 and BDNF on calpain activation as well as through the destabilization of mRNA for ERβ and BDNF.
We were the first to report that E2 treatment of hippocampal slice cultures produced a significant degree of protection against both KA and NMDA neurotoxicity (Bi et al., 2000). This effect was observed whether the steroid was added 24 h or 10 min before the excitotoxins, suggesting that E2’s neuroprotective effect was probably not caused by the activation of a nuclear receptor and subsequent transcription of genes with estrogen regulatory elements. E2 neuroprotective effect was completely blocked by an inhibitor of Src tyrosine kinase or of the MAPK kinase, indicating that the effect is caused by the activation of a pathway that is shared with a variety of neurotrophic factors. These results were in good agreement with another study indicating that E2 neuroprotection against glutamate toxicity in cortical neurons in cultures was mediated by the activation of the same pathways (Singer et al., 1999). Likewise, they matched well with those of Singh et al. (Singh et al., 1999), who showed that E2 rapidly activates src tyrosine kinase and MAPK kinase in cortical organotypic cultures (Fig. 1).
Additional studies showed that E2-mediated neuroprotection and ERK activation were PTX sensitive, and were due to the existence of ERα-mediated G-protein- and β-arrestin-dependent mechanisms in neurons, which regulate some of E2-mediated functions (Dominguez et al., 2009). Many E2-dependent pathways are coupled to G-protein effector systems (Gu and Moss, 1996; Simoncini et al., 2006; Wyckoff et al., 2001). In neurons and other cell types, E2 enhanced expression and activity of Gαi and Gβγ proteins and modulated direct interactions between G-protein subunits and ERα receptors (Thompson and Certain, 2005; Wyckoff et al., 2001). In addition, ERα-like receptors coupled to G-protein subunits are responsible for the activation of the ERK pathway and other signaling cascades (Belcher et al., 2005; D'Souza et al., 2004; Moss and Gu, 1999). In fact, a recent study found that ERα exhibits Gαi- and Gβγ- binding domains (251–260 and 271–585) and that during stimulation, G-protein subunits are released from ERα; this interaction was found to be involved in ERK activation and disrupted by PTX (Kumar et al., 2007). Studies have shown that acute E2 stimulation resulted in interactions between ERα and Gαi; however, the role of such interactions is unclear, although it has been suggested that ERα stimulation of Gβγ leads to activation of the ERK pathway (Lu et al., 2004; Razandi et al., 2003; Wyckoff et al., 2001). Activation of G-protein-induced ERK activation is a major regulatory mechanism of GPCR-mediated effects (Navarro et al., 2003). Free Gβγ subunits have been reported to activate Src kinase and GRK2, resulting in phosphorylation of GPCRs and β-arrestin-1 and formation of a GPCR/β-arrestin-1 receptor signaling complex (Lopez-Ilasaca, 1998; Luttrell et al., 1996; Pitcher et al., 1999). The formation of the receptor complex initiates the activation of ERK pathway components. Formation of the GPCR/β-arrestin-1/ERK complex is required for clathrin-mediated endocytosis, where receptors are sorted to be recycled or sent for degradation (Eichmann et al., 2003; Luttrell et al., 1999). These pathways are involved in GPCR desensitization and down-regulation. Rapid and transient activation of ERK by E2 has been repeatedly shown in different cellular systems. The signal is initiated within minutes (<5 min), reaching a maximum after 30 min of hormone stimulation, and returning toward basal levels after 60–120 min; this action of E2 is known to involve receptor tyrosine kinases (Migliaccio et al., 1996; Miller et al., 2000; Singer et al., 1999; Singh et al., 1999). It could also be accounted for by activation of GPCR-signaling systems (Filardo et al., 2007). The current hypothesis is that ERα and Src kinase form a complex along with adaptor proteins, such as Shc/Grb2, modulator of non-genomic actions of the estrogen receptor (MNAR), and striatin (Honda et al., 2000; Razandi et al., 2003; Singh et al., 1999; Song et al., 2005). Thus, it is now generally admitted that E2-induced ERK activation and possibly neuroprotection requires G-protein- and β-arrestin-mediated mechanisms.
We previously showed that NMDA or KA treatment of hippocampal slice cultures was accompanied by calpain activation and the resulting truncation of the C-terminal domains of several subunits of AMPA and NMDA receptors (Bi et al., 1998b; Bi et al., 1998c). Although the functional significance of the truncation remains unclear, we also found that phosphorylation of the subunits protects them from calpain-mediated truncation (Bi et al., 1998a). Moreover, we demonstrated that Srcmediated phosphorylation of NR2 subunits of NMDA receptors completely protected the subunits from such truncation (Rong et al., 2001). E2 treatment of hippocampal slice cultures resulted in activation of Src tyrosine kinase and tyrosine phosphorylation of NR2A subunits and protected them from calpainmediated truncation of their C-terminal domains that takes place after excitotoxin treatment (Bi et al., 2000). Interestingly, E2 treatment also protected the C-terminal domain of GluR1 receptors from KA- or NMDA-induced truncation, an effect also blocked by inhibitors of Src tyrosine kinase or MAPK kinase (Bi et al., 2000). Because GluR1 is not known to be a substrate of tyrosine kinases, it is possible that tyrosine kinases activate other protein kinases capable of phosphorylating AMPA receptor subunits and thereby of protecting them from calpain-mediated truncation of their C-terminal domains. All these results are consistent with the idea that E2, by activating Src/MAPK pathway is involved in phosphorylating ionotropic glutamate receptors, resulting in enhanced function of the receptors and these effects could account for the increase in basal synaptic transmission and in LTP (Fig. 2).
E2-mediated increase in NMDA receptor function resulting from tyrosine phosphorylation should have been expected to enhance NMDA neurotoxicity; in contrast, E2-mediated activation of the MAPK pathway provided neuroprotection, presumably by activating neuroprotective cascades downstream from NMDA receptor activation. This idea fits well with the notion that E2 and neurotrophins, and in particular BDNF, might share similar signaling pathways (Yu et al., 1997) (see below for further discussion of this point).
3.2. E2/P4 interactions in excitotoxicity
A wealth of data suggests a neuroprotective role for E2 in various animal models of neurodegeneration. Furthermore, emerging data also suggest that P4 has potent neuroprotective effects against glutamate-induced cell death (Kaur et al., 2007; Nilsen and Brinton, 2002; Nilsen and Brinton, 2003) and traumatic brain injury (Robertson et al., 2006; Roof et al., 1997). As with E2, P4 likely stimulates several different mechanisms of neuroprotection, including activation of neurotrophins. In addition, reduced metabolites of progesterone, such as 3-α-hydroxy-5α-pregnan-20-one (THP, allopregnanolone) have been shown to mediate, at least in part, the neuroprotective effects of progesterone (Ciriza et al., 2004). Although there is an abundant literature illustrating the neuroprotective effects of female sex hormones, the possible regulation by P4 of E2-mediated neuroprotection has not been extensively investigated. Indeed, the paucity of studies exploring the effects of P4 on estrogenic actions may account for some of the inconsistencies in the literature. Recent studies have suggested inhibitory effects on E2 action by P4, highlighting the importance of E2-P4 interactions on the neurobiological processes that influence brain systems involved in learning and memory, and regulating neuroprotection.
P4 has been shown to have antagonistic effects on estrogenic actions, as illustrated by studies on dendritic spine density (Murphy and Segal, 2000), and spatial memory (Bimonte-Nelson et al., 2006), reversing estrogenic neuroprotection against kainate toxicity (Rosario et al., 2006), NMDA toxicity (Aguirre and Baudry, 2009), and mitochondrial toxicity (Brinton et al., 2008). Recent studies have uncovered potential biological mechanisms underlying this antagonism, and many have implicated neurotrophins as a key component of this cross-regulation.
3.3. E2, P4, BDNF and neuroprotection
First isolated from pig brain, brain-derived neurotrophic factor (BDNF) was initially characterized as a key effector in mediating long-term survival and promoting neuronal differentiation in developing neurons, as well as neuronal viability in adult brain (Barde et al., 1982; Lu and Chow, 1999). The past twenty years have been witness to a growing compendium of functions assigned to BDNF. In mammalian brain, the neurotrophin family is composed of nerve growth factor (NGF), BDNF, neurotrophin 3 (NT3) and neurotrophin 4 (NT4). Their functions are dependent upon activation of two distinct classes of transmembrane receptors, the p75 neurotrophin receptor and the Trk family of receptor tyrosine kinases (Lu and Martinowich, 2008; Scharfman and MacLusky, 2006). While the p75 neurotrophin receptor is non-selective, each Trk receptor selectively binds to a different neurotrophin, with BDNF selectively binding to the TrkB receptor. Given that downstream effects of neurotrophin activation influence learning, memory and cognition, numerous studies have explored the role of BDNF in hippocampal functions. Age-related declines in BDNF, as well as impaired BDNF regulation in agerelated neurodegenerative diseases such as Parkinson’s disease (Mogi et al., 1999; von Bohlen und Halbach et al., 2005) and Alzheimer’s disease (Hock et al., 2000; Soontornniyomkij et al., 1999), signify the importance of understanding neurotrophin interactions with steroid hormones to resolve the complexities underlying age-related cognitive deficits and neurodegenerative diseases.
BDNF is neuroprotective against certain toxic stimuli and several reports have demonstrated that E2 can modulate BDNF expression (Gibbs, 1999; Sohrabji and Lewis, 2006; Solum and Handa, 2002). However, several studies have shown that P4 can attenuate E2-induced increase in neurotrophin levels (Aguirre and Baudry, 2009; Bimonte-Nelson et al., 2006), even though P4 treatment alone has been shown to increase BDNF mRNA and protein levels (Gonzalez et al., 2005; Kaur et al., 2007). P4 treatment also decreased the number of cells undergoing chromatolysis, a feature of motoneuron degeneration, thus illustrating the clinical relevance of P4, particularly for spinal cord injury.
Evidently, both E2 and P4 may exert their neurotrophic effects by stimulating BDNF. Understanding the interactions between E2 and P4 may provide further insight as to the mechanisms underlying the hormonal-neurotrophin relationship. Cyclic changes in hormone levels are a key element in hormonal effects throughout the organism, and have been linked to numerous physiological processes; likewise, levels of BDNF mRNA have been shown to be dependent on the estrous cycle (Gibbs, 1998), with BDNF protein levels high at proestrous and estrous stages. Moreover, the increased neuronal excitability observed in CA1 and CA3 regions of hippocampus at these stages of the estrous cycle coincided with changes in BDNF expression, suggesting that synaptic responses could be regulated by E2-mediated BDNF increase (Scharfman et al., 2003). However, these results disagree with previous data showing that BDNF protein levels declined during estrous and proestrous, even though mRNA levels increased (Gibbs, 1998). The discrepancy between these studies may be due to methodological differences, including detection procedures and antibody selectivity.
Recent data examining the roles of ERs in regulating BDNF expression indicated that, in the mouse auditory system, activating ERβ with the agonist DPN increased BDNF mRNA and protein levels, presenting the first evidence that directly correlated ERβ expression with neuroprotection of auditory function (Meltser et al., 2008).
Despite the wealth of data indicating a direct interaction between E2, P4 and BDNF, the methodological differences and treatment paradigms between the various studies underscore the needs to control for numerous variables, such as age, sex, length of ovariectomy, and anatomical structures. In addition, the interactions observed at the level of second messenger systems could reflect the existence of indirect mechanisms for hormonal regulation of neurotrophins. Thus, hormones and BDNF could independently activate different signal transduction pathways. For example E2, P4 and BDNF have all been shown to mediate their actions through the activation of second messengers systems and signal transduction pathways. As discussed, one of the major pathways involved in synaptic plasticity, learning and memory is the MAPK pathway (Fig. 3). Along with its many other functions, BDNF is also implicated in the ERK/MAPK signaling cascade (Zheng and Quirion, 2004). Furthermore, cyclic changes in E2 have been shown to exert powerful effects on the ERK/MAPK signaling cascade. Ovariectomy results in decreased levels of activated ERK2 and E2 replacement reverses this loss. Moreover, these cyclic changes are closely associated with changes in the phosphorylated state of the NR2 subunit of NMDA receptors (Bi et al., 2001). Several other groups have also examined estrogenic effects on this pathway (Bi et al., 2001; Kim et al., 2002; Nilsen et al., 2002). However, cyclic changes in hormonal state also involve P4, and therefore it is not surprising that this hormone may also able to affect the MAPK pathway (Nilsen et al., 2002; Singh, 2001). Accordingly, a possible mechanism to account for steroid hormone action could involve interactions between E2 and P4 to modulate NMDAR function by regulating the ERK/MAPK signaling cascade. Another important pathway critical for various cell processes is the PI3K/AKT pathway, which is also activated by E2 (Zhang et al., 2001; Znamensky et al., 2003), P4 (Kaur et al., 2007), and BDNF (Zheng and Quirion, 2004). Similarly, the Ca2+/CREB second messenger system is involved in estrogenic functions (Panickar et al., 1997; Segal and Murphy, 1998; Zhou et al., 2005), as well as with BDNF (Alonso et al., 2002; Blanquet et al., 2003; Ying et al., 2002), and P4 (Nilsen and Brinton, 2002). As mentioned above, E2 can activate calpain-2 through ERK-mediated phosphorylation; P4 has also be shown to activate ERK (Orr et al., 2012) and could therefore also activate calpain-2, as was shown in human sperm (Ozaki et al., 2001). The relationships between P4 and BDNF appear to be more complex, as P4 can stimulate BDNF expression in motoneurons, while BDNF has been shown to inhibit P4 synthesis in non-neuronal tissues (Gonzalez et al., 2005; Jensen and Johnson, 2001). The inconsistencies in these interactions remain to be elucidated. Whether E2 and BDNF act synergistically or in parallel to exert neuroprotective effects in the CNS has not been clarified.
Perhaps a look at studies performed in non-neuronal tissues might elucidate certain mechanisms of action underlying the antagonistic effect of P4 on estrogenic induction of growth factors and subsequent neuroprotection. P4, along with other progestins, has been shown to inhibit E2-induced increase in vascular endothelial growth factor (VEGF) in the endometrium (Okada et al., 2011). A recent study suggests that the increased breast cancer risk following hormone therapy in post-menopausal women may be due to treatment with E2 plus P4, rather than E2 treatment alone. This combination enhanced tissue proliferation more than E2 treatment alone, an effect mediated through the increased production of amphiregulin, an epidermal growth factor receptor (EGFR) ligand (Kariagina et al., 2010). These studies illustrate the point that interactions between E2, P4 and growth factors in the pathology of diseases are not restricted to brain. However, as regulation of growth factors is downstream from E2-P4 interactions, it is important to first understand the nature of hormonal interaction at the molecular level.
As previously mentioned, evidence has emphasized the role of ERβ in cognition and behavior (Bodo and Rissman, 2006; Liu et al., 2008; Walf et al., 2008; Walf et al., 2009). Recently, estrogenic effects on neuroprotection and BDNF upregulation were shown to be mediated by activation of ERβ receptors, as well as with ERα receptors. E2 treatment was neuroprotective against NMDA toxicity in wild-type (WT) but not in ERβ KO mice. Furthermore, the ERβ selective agonist DPN, but not the ERα agonist PPT, mimicked E2 effects on both neuroprotection against NMDA toxicity and BDNF protein expression (Aguirre et al., 2010). This suggests that activation of ERβ is necessary for E2-mediated upregulation of BDNF and neuroprotection against NMDA toxicity. P4 has also been shown to down-regulate ER expression within hours, suggesting a possible mechanism of action that involves transcriptional activity (Jayaraman and Pike, 2009). Although further experiments have to be done to confirm receptor stability and/or degradation, such a rapid effect on mRNA levels suggests that P4 interferes with mRNA stability or degradation rather than transcription (Fig. 3).
The length of the poly(A) tail of mRNAs has important roles in mRNA stability, degradation and translational efficiency (Meijer et al., 2007). P4 has been shown to shorten the poly(A) tail of ovine luteinizing hormone subunits, resulting in mRNA down-regulation. In addition, significant shortening was observed following treatment with 7–10 nM P4 and after 3–10 h of treatment (Wu and Miller, 1991), the same range of time and concentration for which P4 was able to inhibit E2-mediated neuroprotection, BDNF induction and ERβ activation (Aguirre et al., 2010). These observations point to a possible mechanism to account for these effects and support the hypothesis that P4 stimulates mRNA degradation or destabilization, suggesting a post-transcriptional control of P4 in regulating neuroprotection (Fig. 3). Future studies exploring the length of poly(A) tails of various mRNAs, such as BDNF and ERβ, following P4 treatment would further increase our understanding of hormonal relationships with respect to neuroprotection, and possibly enable new drug developments of hormone therapy without detrimental effects. Clearly, it is important to elucidate the hormonal-neurotrophin interactions to better understand how these interactions may be contributing to cognition and neurodegenerative diseases.
4. Conclusions
Both E2 and P4 activate intracellular pathways that have been linked to both synaptic plasticity and neuroprotection. Thus, E2 and P4 activate the MAPK/ERK pathway, which could account for the beneficial effects of these steroid hormones on learning and memory. As ERK activation has been shown to activate calpain-2, this pathway could also account for the effects of both hormones on dendritic spine structure and function. As discussed, because calpain activation is also regulated by protein kinases and phosphatases, which could in turn be differentially modified by both hormones, the relative timing of changes in these two hormones during the estrus cycle could result in significant differences in the overall activation of this pathway under physiological conditions. Likewise, both steroid hormones are linked to BDNF regulation providing a further node of interaction for their effects both on synaptic plasticity and neuroprotection. Again, subtle differences in the spatio-temporal regulation of BDNF expression produced by changes in these hormones could result in profound changes in local levels of BDNF and therefore in their neuroprotective effects. As indicated, E2/P4 interactions can occur at various sites in these complex pathways, including the expression of various receptors, the activity of various enzymes, and the levels of neurotrophins. Clearly, more studies are required to unravel these complex interactions, with the potential to provide new insights into the molecular mechanisms underlying both synaptic plasticity and neuroprotection.
Highlights.
E2 and P4 exhibit complex effects on cellular mechanisms involved in synaptic plasticity and learning and memory.
E2 and P4 stimulate ERK, leading to calpain activation and regulation of dendritic spine structure and function.
E2 and P4 increase BDNF expression, possibly accounting for their neuroprotective effects.
Complex interactions between E2 and P4 could occur at a variety of sites in these molecular cascades.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre C, Jayaraman A, Pike C, Baudry M. Progesterone inhibits estrogen-mediated neuroprotection against excitotoxicity by down-regulating estrogen receptor-beta. J Neurochem. 2010;115:1277–1287. doi: 10.1111/j.1471-4159.2010.07038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre CC, Baudry M. Progesterone reverses 17beta-estradiol-mediated neuroprotection and BDNF induction in cultured hippocampal slices. Eur J Neurosci. 2009;29:447–454. doi: 10.1111/j.1460-9568.2008.06591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexaki VI, Charalampopoulos I, Kampa M, Nifli AP, Hatzoglou A, Gravanis A, Castanas E. Activation of membrane estrogen receptors induce pro-survival kinases. J Steroid Biochem Mol Biol. 2006;98:97–110. doi: 10.1016/j.jsbmb.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Ali S, Coombes RC. Estrogen receptor alpha in human breast cancer: occurrence and significance. J Mammary Gland Biol Neoplasia. 2000;5:271–281. doi: 10.1023/a:1009594727358. [DOI] [PubMed] [Google Scholar]
- Alonso M, Vianna MR, Izquierdo I, Medina JH. Signaling mechanisms mediating BDNF modulation of memory formation in vivo in the hippocampus. Cell Mol Neurobiol. 2002;22:663–674. doi: 10.1023/A:1021848706159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves SE, McEwen BS, Hayashi S, Korach KS, Pfaff DW, Ogawa S. Estrogen-regulated progestin receptors are found in the midbrain raphe but not hippocampus of estrogen receptor alpha (ER alpha) gene-disrupted mice. J Comp Neurol. 2000;427:185–195. doi: 10.1002/1096-9861(20001113)427:2<185::aid-cne2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Bains M, Cousins JC, Roberts JL. Neuroprotection by estrogen against MPP+-induced dopamine neuron death is mediated by ERalpha in primary cultures of mouse mesencephalon. Exp Neurol. 2007;204:767–776. doi: 10.1016/j.expneurol.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry M, Lynch G. Remembrance of arguments past: how well is the glutamate receptor hypothesis of LTP holding up after 20 years? Neurobiol Learn Mem. 2001;76:284–297. doi: 10.1006/nlme.2001.4023. [DOI] [PubMed] [Google Scholar]
- Baudry M, Bi X, Gall C, Lynch G. The biochemistry of memory: The 26year journey of a 'new and specific hypothesis'. Neurobiol Learn Mem. 2011;95:125–133. doi: 10.1016/j.nlm.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Belcher SM, Le HH, Spurling L, Wong JK. Rapid estrogenic regulation of extracellular signal-regulated kinase 1/2 signaling in cerebellar granule cells involves a G protein- and protein kinase A-dependent mechanism and intracellular activation of protein phosphatase 2A. Endocrinology. 2005;146:5397–5406. doi: 10.1210/en.2005-0564. [DOI] [PubMed] [Google Scholar]
- Berlucchi G, Buchtel HA. Neuronal plasticity: historical roots and evolution of meaning. Exp Brain Res. 2009;192:307–319. doi: 10.1007/s00221-008-1611-6. [DOI] [PubMed] [Google Scholar]
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Bi R, Bi X, Baudry M. Phosphorylation regulates calpain-mediated truncation of glutamate ionotropic receptors. Brain Res. 1998a;797:154–158. doi: 10.1016/s0006-8993(98)00433-8. [DOI] [PubMed] [Google Scholar]
- Bi R, Broutman G, Foy MR, Thompson RF, Baudry M. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc Natl Acad Sci U S A. 2000;97:3602–3607. doi: 10.1073/pnas.060034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci U S A. 2001;98:13391–13395. doi: 10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, Chen J, Baudry M. Calpain-mediated proteolysis of GluR1 subunits in organotypic hippocampal cultures following kainic acid treatment. Brain Res. 1998b;781:355–357. doi: 10.1016/s0006-8993(97)01365-6. [DOI] [PubMed] [Google Scholar]
- Bi X, Rong Y, Chen J, Dang S, Wang Z, Baudry M. Calpain-mediated regulation of NMDA receptor structure and function. Brain Res. 1998c;790:245–253. doi: 10.1016/s0006-8993(98)00067-5. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci. 2006;24:229–242. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- Blanquet PR, Mariani J, Derer P. A calcium/calmodulin kinase pathway connects brain-derived neurotrophic factor to the cyclic AMP-responsive transcription factor in the rat hippocampus. Neuroscience. 2003;118:477–490. doi: 10.1016/s0306-4522(02)00963-6. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. New roles for estrogen receptor beta in behavior and neuroendocrinology. Front Neuroendocrinol. 2006;27:217–232. doi: 10.1016/j.yfrne.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: form and function in brain. Front Neuroendocrinol. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Pike CJ. Selective estrogen receptor modulators differentially regulate Alzheimer-like changes in female 3xTg-AD mice. Endocrinology. 2008;149:2607–2611. doi: 10.1210/en.2007-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WC, Pak TR, Weiser MJ, Hinds LR, Andersen ME, Handa RJ. Progestin receptor expression in the developing rat brain depends upon activation of estrogen receptor alpha and not estrogen receptor beta. Brain Res. 2006;1082:50–60. doi: 10.1016/j.brainres.2006.01.109. [DOI] [PubMed] [Google Scholar]
- Ciriza I, Azcoitia I, Garcia-Segura LM. Reduced progesterone metabolites protect rat hippocampal neurones from kainic acid excitotoxicity in vivo. J Neuroendocrinol. 2004;16:58–63. doi: 10.1111/j.1365-2826.2004.01121.x. [DOI] [PubMed] [Google Scholar]
- Cordey M, Pike CJ. Neuroprotective properties of selective estrogen receptor agonists in cultured neurons. Brain Res. 2005;1045:217–223. doi: 10.1016/j.brainres.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Cordoba-Montoya DA, Carrer HF. Estrogen facilitates induction of long term potentiation in the hippocampus of awake rats. Brain Res. 1997;778:430–438. doi: 10.1016/s0006-8993(97)01206-7. [DOI] [PubMed] [Google Scholar]
- Croall DE, DeMartino GN. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol Rev. 1991;71:813–847. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- D'Souza DN, Zhang Y, Damjanoska KJ, Carrasco GA, Sullivan NR, Garcia F, Battaglia G, Doncarlos LL, Muma NA, Van de Kar LD. Estrogen reduces serotonin-1A receptor-mediated oxytocin release and Galpha(i/o/z) proteins in the hypothalamus of ovariectomized rats. Neuroendocrinology. 2004;80:31–41. doi: 10.1159/000080795. [DOI] [PubMed] [Google Scholar]
- Dominguez R, Hu E, Zhou M, Baudry M. 17beta-estradiol-mediated neuroprotection and ERK activation require a pertussis toxin-sensitive mechanism involving GRK2 and beta-arrestin-1. J Neurosci. 2009;29:4228–4238. doi: 10.1523/JNEUROSCI.0550-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards HE, Epps T, Carlen PL, N JM. Progestin receptors mediate progesterone suppression of epileptiform activity in tetanized hippocampal slices in vitro. Neuroscience. 2000;101:895–906. doi: 10.1016/s0306-4522(00)00439-5. [DOI] [PubMed] [Google Scholar]
- Eichmann T, Lorenz K, Hoffmann M, Brockmann J, Krasel C, Lohse MJ, Quitterer U. The amino-terminal domain of G-protein-coupled receptor kinase 2 is a regulatory Gbeta gamma binding site. J Biol Chem. 2003;278:8052–8057. doi: 10.1074/jbc.M204795200. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Feng XQ, Dong Y, Fu YM, Zhu YH, Sun JL, Wang Z, Sun FY, Zheng P. Progesterone inhibition of dopamine-induced increase in frequency of spontaneous excitatory postsynaptic currents in rat prelimbic cortical neurons. Neuropharmacology. 2004;46:211–222. doi: 10.1016/j.neuropharm.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148:3236–3245. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- Forstrom L. The influence of sex hormones on acne. Acta Derm Venereol Suppl (Stockh). Suppl. 1980;89:27–31. [PubMed] [Google Scholar]
- Fox JE. On the role of calpain and Rho proteins in regulating integrin-induced signaling. Thromb Haemost. 1999;82:385–391. [PubMed] [Google Scholar]
- Foy M, Baudry M, Thompson R. Estrogen and hippocampal synaptic plasticity. Neuron Glia Biol. 2004;1:327–338. doi: 10.1017/S1740925X05000165. [DOI] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Foy MR, Akopian G, Thompson RF. Progesterone regulation of synaptic transmission and plasticity in rodent hippocampus. Learn Mem. 2008;15:820–822. doi: 10.1101/lm.1124708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3alpha,5alpha-THP. Pharmacol Biochem Behav. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Res. 1998;787:259–268. doi: 10.1016/s0006-8993(97)01511-4. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Treatment with estrogen and progesterone affects relative levels of brain-derived neurotrophic factor mRNA and protein in different regions of the adult rat brain. Brain Res. 1999;844:20–27. doi: 10.1016/s0006-8993(99)01880-6. [DOI] [PubMed] [Google Scholar]
- Glading A, Bodnar RJ, Reynolds IJ, Shiraha H, Satish L, Potter DA, Blair HC, Wells A. Epidermal growth factor activates m-calpain (calpain II), at least in part, by extracellular signal-regulated kinase-mediated phosphorylation. Mol Cell Biol. 2004;24:2499–2512. doi: 10.1128/MCB.24.6.2499-2512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Gonzalez SL, Labombarda F, Deniselle MC, Mougel A, Guennoun R, Schumacher M, De Nicola AF. Progesterone neuroprotection in spinal cord trauma involves up-regulation of brain-derived neurotrophic factor in motoneurons. J Steroid Biochem Mol Biol. 2005;94:143–149. doi: 10.1016/j.jsbmb.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammer M, Kuchay S, Chishti A, Baudry M. Lack of phenotype for LTP and fear conditioning learning in calpain 1 knock-out mice. Neurobiol Learn Mem. 2005;84:222–227. doi: 10.1016/j.nlm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Grant SG, O'Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- Gu Q, Moss RL. 17 beta-Estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci. 1996;16:3620–3629. doi: 10.1523/JNEUROSCI.16-11-03620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Moss RL. Novel mechanism for non-genomic action of 17 beta-oestradiol on kainateinduced currents in isolated rat CA1 hippocampal neurones. J Physiol. 1998;506(Pt 3):745–754. doi: 10.1111/j.1469-7793.1998.745bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guennoun R, Meffre D, Labombarda F, Gonzalez SL, Deniselle MC, Stein DG, De Nicola AF, Schumacher M. The membrane-associated progesterone-binding protein 25-Dx: expression, cellular localization and up-regulation after brain and spinal cord injuries. Brain Res Rev. 2008;57:493–505. doi: 10.1016/j.brainresrev.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Guerriero G. Vertebrate sex steroid receptors: evolution, ligands, and neurodistribution. Ann N Y Acad Sci. 2009;1163:154–168. doi: 10.1111/j.1749-6632.2009.04460.x. [DOI] [PubMed] [Google Scholar]
- Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28:726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- Henderson VW. The epidemiology of estrogen replacement therapy and Alzheimer's disease. Neurology. 1997;48:S27–S35. doi: 10.1212/wnl.48.5_suppl_7.27s. [DOI] [PubMed] [Google Scholar]
- Henderson VW, Brinton RD. Menopause and mitochondria: windows into estrogen effects on Alzheimer's disease risk and therapy. Prog Brain Res. 2010;182:77–96. doi: 10.1016/S0079-6123(10)82003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd MB, Haythornthwaite AR, Rosahl TW, Wafford KA, Homanics GE, Lambert JJ, Belelli D. The expression of GABAA beta subunit isoforms in synaptic and extrasynaptic receptor populations of mouse dentate gyrus granule cells. J Physiol. 2008;586:989–1004. doi: 10.1113/jphysiol.2007.146746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata S, Osada T, Hirai M, Hagihara K, Kato J. Expression of estrogen receptor in the rat brain: detection of its mRNA using reverse transcription-polymerase chain reaction. J Steroid Biochem Mol Biol. 1992;41:583–587. doi: 10.1016/0960-0760(92)90388-y. [DOI] [PubMed] [Google Scholar]
- Hock C, Heese K, Hulette C, Rosenberg C, Otten U. Region-specific neurotrophin imbalances in Alzheimer disease: decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch Neurol. 2000;57:846–851. doi: 10.1001/archneur.57.6.846. [DOI] [PubMed] [Google Scholar]
- Honda K, Sawada H, Kihara T, Urushitani M, Nakamizo T, Akaike A, Shimohama S. Phosphatidylinositol 3-kinase mediates neuroprotection by estrogen in cultured cortical neurons. J Neurosci Res. 2000;60:321–327. doi: 10.1002/(SICI)1097-4547(20000501)60:3<321::AID-JNR6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Ito K, Skinkle KL, Hicks TP. Age-dependent, steroid-specific effects of oestrogen on long-term potentiation in rat hippocampal slices. J Physiol. 1999;515(Pt 1):209–220. doi: 10.1111/j.1469-7793.1999.209ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman A, Pike CJ. Progesterone attenuates oestrogen neuroprotection via downregulation of oestrogen receptor expression in cultured neurones. J Neuroendocrinol. 2009;21:77–81. doi: 10.1111/j.1365-2826.2008.01801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen T, Johnson AL. Expression and function of brain-derived neurotrophin factor and its receptor, TrkB, in ovarian follicles from the domestic hen (Gallus gallus domesticus) J Exp Biol. 2001;204:2087–2095. doi: 10.1242/jeb.204.12.2087. [DOI] [PubMed] [Google Scholar]
- Kariagina A, Xie J, Leipprandt JR, Haslam SZ. Amphiregulin mediates estrogen, progesterone, and EGFR signaling in the normal rat mammary gland and in hormone-dependent rat mammary cancers. Horm Cancer. 2010;1:229–244. doi: 10.1007/s12672-010-0048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P, Jodhka PK, Underwood WA, Bowles CA, de Fiebre NC, de Fiebre CM, Singh M. Progesterone increases brain-derived neuroptrophic factor expression and protects against glutamate toxicity in a mitogen-activated protein kinase- and phosphoinositide-3 kinase-dependent manner in cerebral cortical explants. J Neurosci Res. 2007;85:2441–2449. doi: 10.1002/jnr.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Kim HY, Kim JH, Shin HK, Lee SH, Lee YS, Son H. Enhancement of rat hippocampal long-term potentiation by 17 beta-estradiol involves mitogen-activated protein kinase-dependent and -independent components. Neurosci Lett. 2002;332:65–69. doi: 10.1016/s0304-3940(02)00902-3. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G. Cytoskeletal changes underlie estrogen's acute effects on synaptic transmission and plasticity. J Neurosci. 2009;29:12982–12993. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Wu Q, Chambliss KL, Yuhanna IS, Mumby SM, Mineo C, Tall GG, Shaul PW. Direct interactions with G alpha i and G betagamma mediate nongenomic signaling by estrogen receptor alpha. Mol Endocrinol. 2007;21:1370–1380. doi: 10.1210/me.2006-0360. [DOI] [PubMed] [Google Scholar]
- Lancaster B, Rogers MV. A peptide activator of endogenous tyrosine kinase enhances synaptic currents mediated by NMDA receptors. Eur J Neurosci. 1998;10:2302–2308. doi: 10.1046/j.1460-9568.1998.00241.x. [DOI] [PubMed] [Google Scholar]
- Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci U S A. 2000;97:1032–1037. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecain E, Yang TH, Tran Ba Huy P. Steroidogenic enzyme expression in the rat cochlea. Acta Otolaryngol. 2003;123:187–191. doi: 10.1080/0036554021000028106. [DOI] [PubMed] [Google Scholar]
- Lee YS, Silva AJ. The molecular and cellular biology of enhanced cognition. Nat Rev Neurosci. 2009;10:126–140. doi: 10.1038/nrn2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Shors TJ. High levels of estrogen enhance associative memory formation in ovariectomized females. Psychoneuroendocrinology. 2004;29:883–890. doi: 10.1016/j.psyneuen.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. Long-term potentiation: outstanding questions and attempted synthesis. Philos Trans R Soc Lond B Biol Sci. 2003;358:829–842. doi: 10.1098/rstb.2002.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Fallon JR. What maintains memories? Science. 1999;283:339–340. doi: 10.1126/science.283.5400.339. [DOI] [PubMed] [Google Scholar]
- Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Lopez-Ilasaca M. Signaling from G-protein-coupled receptors to mitogen-activated protein (MAP)-kinase cascades. Biochem Pharmacol. 1998;56:269–277. doi: 10.1016/s0006-2952(98)00059-8. [DOI] [PubMed] [Google Scholar]
- Lu B, Chow A. Neurotrophins and hippocampal synaptic transmission and plasticity. J Neurosci Res. 1999;58:76–87. [PubMed] [Google Scholar]
- Lu B, Martinowich K. Cell biology of BDNF and its relevance to schizophrenia. Novartis Found Symp. 2008;289:119–129. doi: 10.1002/9780470751251.ch10. discussion 129-35, 193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Pallas DC, Surks HK, Baur WE, Mendelsohn ME, Karas RH. Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor alpha. Proc Natl Acad Sci U S A. 2004;101:17126–17131. doi: 10.1073/pnas.0407492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu WY, Xiong ZG, Lei S, Orser BA, Dudek E, Browning MD, MacDonald JF. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat Neurosci. 1999;2:331–338. doi: 10.1038/7243. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Hawes BE, van Biesen T, Luttrell DK, Lansing TJ, Lefkowitz RJ. Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gbetagamma subunit-mediated activation of mitogen-activated protein kinases. J Biol Chem. 1996;271:19443–19450. doi: 10.1074/jbc.271.32.19443. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- Lynch G, Baudry M. The biochemistry of memory: a new and specific hypothesis. Science. 1984;224:1057–1063. doi: 10.1126/science.6144182. [DOI] [PubMed] [Google Scholar]
- Lynch G, Rex CS, Chen LY, Gall CM. The substrates of memory: defects, treatments, and enhancement. Eur J Pharmacol. 2008;585:2–13. doi: 10.1016/j.ejphar.2007.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- Meijer HA, Radford HE, Wilson LS, Lissenden S, de Moor CH. Translational control of maskin mRNA by its 3' untranslated region. Biol Cell. 2007;99:239–250. doi: 10.1042/BC20060112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltser I, Tahera Y, Simpson E, Hultcrantz M, Charitidi K, Gustafsson JA, Canlon B. Estrogen receptor beta protects against acoustic trauma in mice. J Clin Invest. 2008;118:1563–1570. doi: 10.1172/JCI32796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I, Dellovade TL, Shughrue PJ. Neuroprotection by estrogen in animal models of global and focal ischemia. Ann N Y Acad Sci. 2003;1007:89–100. doi: 10.1196/annals.1286.009. [DOI] [PubMed] [Google Scholar]
- Micevych P, Sinchak K. Estradiol regulation of progesterone synthesis in the brain. Mol Cell Endocrinol. 2008;290:44–50. doi: 10.1016/j.mce.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, Auricchio F. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- Miller WE, Maudsley S, Ahn S, Khan KD, Luttrell LM, Lefkowitz RJ. beta-arrestin1 interacts with the catalytic domain of the tyrosine kinase c-SRC. Role of beta-arrestin1-dependent targeting of c-SRC in receptor endocytosis. J Biol Chem. 2000;275:11312–11319. doi: 10.1074/jbc.275.15.11312. [DOI] [PubMed] [Google Scholar]
- Mills SJ, Ashworth JJ, Gilliver SC, Hardman MJ, Ashcroft GS. The sex steroid precursor DHEA accelerates cutaneous wound healing via the estrogen receptors. J Invest Dermatol. 2005;125:1053–1062. doi: 10.1111/j.0022-202X.2005.23926.x. [DOI] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Mogi M, Togari A, Kondo T, Mizuno Y, Komure O, Kuno S, Ichinose H, Nagatsu T. Brainderived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson's disease. Neurosci Lett. 1999;270:45–48. doi: 10.1016/s0304-3940(99)00463-2. [DOI] [PubMed] [Google Scholar]
- Moss RL, Gu Q. Estrogen: mechanisms for a rapid action in CA1 hippocampal neurons. Steroids. 1999;64:14–21. doi: 10.1016/s0039-128x(98)00092-0. [DOI] [PubMed] [Google Scholar]
- Murakami G, Tanabe N, Ishii HT, Ogiue-Ikeda M, Tsurugizawa T, Mukai H, Hojo Y, Takata N, Furukawa A, Kimoto T, Kawato S. Role of cytochrome p450 in synaptocrinology: endogenous estrogen synthesis in the brain hippocampus. Drug Metab Rev. 2006;38:353–369. doi: 10.1080/03602530600724068. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Segal M. Progesterone prevents estradiol-induced dendritic spine formation in cultured hippocampal neurons. Neuroendocrinology. 2000;72:133–143. doi: 10.1159/000054580. [DOI] [PubMed] [Google Scholar]
- Navarro CE, Saeed SA, Murdock C, Martinez-Fuentes AJ, Arora KK, Krsmanovic LZ, Catt KJ. Regulation of cyclic adenosine 3',5'- monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotrophin-releasing hormone neurons. Mol Endocrinol. 2003;17:1792–1804. doi: 10.1210/me.2003-0040. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;143:205–212. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Chen S, Brinton RD. Dual action of estrogen on glutamate-induced calcium signaling: mechanisms requiring interaction between estrogen receptors and src/mitogen activated protein kinase pathway. Brain Res. 2002;930:216–234. doi: 10.1016/s0006-8993(02)02254-0. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci U S A. 2003;100:10506–10511. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Okamoto R, Tsuzuki T, Tsuji S, Yasuda K, Kanzaki H. Progestins inhibit estradiol-induced vascular endothelial growth factor and stromal cell-derived factor 1 in human endometrial stromal cells. Fertil Steril. 2011;96:786–791. doi: 10.1016/j.fertnstert.2011.06.048. [DOI] [PubMed] [Google Scholar]
- Orban PC, Chapman PF, Brambilla R. Is the Ras-MAPK signalling pathway necessary for longterm memory formation? Trends Neurosci. 1999;22:38–44. doi: 10.1016/s0166-2236(98)01306-x. [DOI] [PubMed] [Google Scholar]
- Orr PT, Rubin AJ, Fan L, Kent BA, Frick KM. The progesterone-induced enhancement of object recognition memory consolidation involves activation of the extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin (mTOR) pathways in the dorsal hippocampus. Horm Behav. 2012;61:487–495. doi: 10.1016/j.yhbeh.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki Y, Blomgren K, Ogasawara MS, Aoki K, Furuno T, Nakanishi M, Sasaki M, Suzumori K. Role of calpain in human sperm activated by progesterone for fertilization. Biol Chem. 2001;382:831–838. doi: 10.1515/BC.2001.101. [DOI] [PubMed] [Google Scholar]
- Panickar KS, Guan G, King MA, Rajakumar G, Simpkins JW. 17beta-estradiol attenuates CREB decline in the rat hippocampus following seizure. J Neurobiol. 1997;33:961–967. [PubMed] [Google Scholar]
- Perlmutter LS, Siman R, Gall C, Seubert P, Baudry M, Lynch G. The ultrastructural localization of calcium-activated protease "calpain" in rat brain. Synapse. 1988;2:79–88. doi: 10.1002/syn.890020111. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Tesmer JJ, Freeman JL, Capel WD, Stone WC, Lefkowitz RJ. Feedback inhibition of G protein-coupled receptor kinase 2 (GRK2) activity by extracellular signal-regulated kinases. J Biol Chem. 1999;274:34531–34534. doi: 10.1074/jbc.274.49.34531. [DOI] [PubMed] [Google Scholar]
- Raval AP, Bramlett H, Perez-Pinzon MA. Estrogen preconditioning protects the hippocampal CA1 against ischemia. Neuroscience. 2006;141:1721–1730. doi: 10.1016/j.neuroscience.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Park ST, Levin ER. Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem. 2003;278:2701–2712. doi: 10.1074/jbc.M205692200. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Oyama RK, Schlinger BA. Elevated aromatase activity in forebrain synaptic terminals during song. J Neuroendocrinol. 2009;21:191–199. doi: 10.1111/j.1365-2826.2009.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Chen LY, Sharma A, Liu J, Babayan AH, Gall CM, Lynch G. Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. J Cell Biol. 2009;186:85–97. doi: 10.1083/jcb.200901084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CL, Puskar A, Hoffman GE, Murphy AZ, Saraswati M, Fiskum G. Physiologic progesterone reduces mitochondrial dysfunction and hippocampal cell loss after traumatic brain injury in female rats. Exp Neurol. 2006;197:235–243. doi: 10.1016/j.expneurol.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Rong Y, Lu X, Bernard A, Khrestchatisky M, Baudry M. Tyrosine phosphorylation of ionotropic glutamate receptors by Fyn or Src differentially modulates their susceptibility to calpain and enhances their binding to spectrin and PSD-95. J Neurochem. 2001;79:382–390. doi: 10.1046/j.1471-4159.2001.00565.x. [DOI] [PubMed] [Google Scholar]
- Roof RL, Hoffman SW, Stein DG. Progesterone protects against lipid peroxidation following traumatic brain injury in rats. Mol Chem Neuropathol. 1997;31:1–11. doi: 10.1007/BF02815156. [DOI] [PubMed] [Google Scholar]
- Rosario ER, Ramsden M, Pike CJ. Progestins inhibit the neuroprotective effects of estrogen in rat hippocampus. Brain Res. 2006;1099:206–210. doi: 10.1016/j.brainres.2006.03.127. [DOI] [PubMed] [Google Scholar]
- Sanchez AM, Flamini MI, Fu XD, Mannella P, Giretti MS, Goglia L, Genazzani AR, Simoncini T. Rapid signaling of estrogen to WAVE1 and moesin controls neuronal spine formation via the actin cytoskeleton. Mol Endocrinol. 2009;23:1193–1202. doi: 10.1210/me.2008-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: complexity of steroid hormone-growth factor interactions in the adult CNS. Front Neuroendocrinol. 2006;27:415–435. doi: 10.1016/j.yfrne.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KL, Pradhan DS, Shah AH, Charlier TD, Chin EH, Soma KK. Neurosteroids, immunosteroids, and the Balkanization of endocrinology. Gen Comp Endocrinol. 2008;157:266–274. doi: 10.1016/j.ygcen.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Segal M, Murphy DD. CREB activation mediates plasticity in cultured hippocampal neurons. Neural Plast. 1998;6:1–7. doi: 10.1155/NP.1998.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraha H, Glading A, Chou J, Jia Z, Wells A. Activation of m-calpain (calpain II) by epidermal growth factor is limited by protein kinase A phosphorylation of m-calpain. Mol Cell Biol. 2002;22:2716–2727. doi: 10.1128/MCB.22.8.2716-2727.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Matzel LD. Long-term potentiation: what's learning got to do with it? Behav Brain Sci. 1997;20:597–614. doi: 10.1017/s0140525x97001593. discussion 614-55. [DOI] [PubMed] [Google Scholar]
- Shughrue P, Scrimo P, Lane M, Askew R, Merchenthaler I. The distribution of estrogen receptorbeta mRNA in forebrain regions of the estrogen receptor-alpha knockout mouse. Endocrinology. 1997;138:5649–5652. doi: 10.1210/endo.138.12.5712. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Scorticati C, Mannella P, Fadiel A, Giretti MS, Fu XD, Baldacci C, Garibaldi S, Caruso A, Fornari L, Naftolin F, Genazzani AR. Estrogen receptor alpha interacts with Galpha13 to drive actin remodeling and endothelial cell migration via the RhoA/Rho kinase/moesin pathway. Mol Endocrinol. 2006;20:1756–1771. doi: 10.1210/me.2005-0259. [DOI] [PubMed] [Google Scholar]
- Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J Neurosci. 1999;19:2455–2463. doi: 10.1523/JNEUROSCI.19-07-02455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Setalo G, Jr, Guan X, Warren M, Toran-Allerand CD. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. Ovarian hormones elicit phosphorylation of Akt and extracellular-signal regulated kinase in explants of the cerebral cortex. Endocrine. 2001;14:407–415. doi: 10.1385/ENDO:14:3:407. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Lewis DK. Estrogen-BDNF interactions: implications for neurodegenerative diseases. Front Neuroendocrinol. 2006;27:404–414. doi: 10.1016/j.yfrne.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J Neurosci. 2002;22:2650–2659. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song RX, Zhang Z, Santen RJ. Estrogen rapid action via protein complex formation involving ERalpha and Src. Trends Endocrinol Metab. 2005;16:347–353. doi: 10.1016/j.tem.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Soontornniyomkij V, Wang G, Pittman CA, Hamilton RL, Wiley CA, Achim CL. Absence of brain-derived neurotrophic factor and trkB receptor immunoreactivity in glia of Alzheimer's disease. Acta Neuropathol. 1999;98:345–348. doi: 10.1007/s004010051092. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Jones KA, Shum CY, Lash LL, Swanson GT, Penzes P. Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc Natl Acad Sci U S A. 2008;105:14650–14655. doi: 10.1073/pnas.0801581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton PK. LTD, LTP, and the sliding threshold for long-term synaptic plasticity. Hippocampus. 1996;6:35–42. doi: 10.1002/(SICI)1098-1063(1996)6:1<35::AID-HIPO7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Su JD, Qiu J, Zhong YP, Li XY, Wang JW, Chen YZ. Expression of estrogen receptor (ER)-alpha and -beta immunoreactivity in hippocampal cell cultures with special attention to GABAergic neurons. J Neurosci Res. 2001;65:396–402. doi: 10.1002/jnr.1166. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Timiras PS. Electrical activity during the estrous cycle of the rat: cyclic changes in limbic structures. Endocrinology. 1968;83:207–216. doi: 10.1210/endo-83-2-207. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, Vardaris RM, Lewis D, Rawitch AB. Gonadal steroids: effects on excitability of hippocampal pyramidal cells. Science. 1980;209:1017–1018. doi: 10.1126/science.7190730. [DOI] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Thompson TL, Certain ME. Estrogen mediated inhibition of dopamine transport in the striatum: regulation by G alpha i/o. Eur J Pharmacol. 2005;511:121–126. doi: 10.1016/j.ejphar.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Minichiello L, Unsicker K. Haploinsufficiency for trkB and trkC receptors induces cell loss and accumulation of alpha-synuclein in the substantia nigra. FASEB J. 2005;19:1740–1742. doi: 10.1096/fj.05-3845fje. [DOI] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile decrease anxiety-like behavior of wildtype, but not estrogen receptor beta knockout, mice. Behav Neurosci. 2008;122:974–981. doi: 10.1037/a0012749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce C, Manley K, Frye CA. Proestrous compared to diestrous wildtype, but not estrogen receptor beta knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav Brain Res. 2009;196:254–260. doi: 10.1016/j.bbr.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SG, Humphreys AG, Juraska JM, Greenough WT. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res. 1995;703:26–30. doi: 10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]
- Wu JC, Miller WL. Progesterone shortens poly(A) tails of the mRNAs for alpha and beta subunits of ovine luteinizing hormone. Biol Reprod. 1991;45:215–220. doi: 10.1095/biolreprod45.2.215. [DOI] [PubMed] [Google Scholar]
- Wu TW, Wang JM, Chen S, Brinton RD. 17Beta-estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: a potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience. 2005;135:59–72. doi: 10.1016/j.neuroscience.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Wyckoff MH, Chambliss KL, Mineo C, Yuhanna IS, Mendelsohn ME, Mumby SM, Shaul PW. Plasma membrane estrogen receptors are coupled to endothelial nitric-oxide synthase through Galpha(i) J Biol Chem. 2001;276:27071–27076. doi: 10.1074/jbc.M100312200. [DOI] [PubMed] [Google Scholar]
- Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XM, Askalan R, Keil GJ, 2nd, Salter MW. NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science. 1997;275:674–678. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]
- Zadran S, Qin Q, Bi X, Zadran H, Kim Y, Foy MR, Thompson R, Baudry M. 17-Beta-estradiol increases neuronal excitability through MAP kinase-induced calpain activation. Proc Natl Acad Sci U S A. 2009;106:21936–21941. doi: 10.1073/pnas.0912558106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadran S, Jourdi H, Rostamiani K, Qin Q, Bi X, Baudry M. Brain-derived neurotrophic factor and epidermal growth factor activate neuronal m-calpain via mitogen-activated protein kinase-dependent phosphorylation. J Neurosci. 2010;30:1086–1095. doi: 10.1523/JNEUROSCI.5120-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Rubinow DR, Xaing G, Li BS, Chang YH, Maric D, Barker JL, Ma W. Estrogen protects against beta-amyloid-induced neurotoxicity in rat hippocampal neurons by activation of Akt. Neuroreport. 2001;12:1919–1923. doi: 10.1097/00001756-200107030-00030. [DOI] [PubMed] [Google Scholar]
- Zhang QG, Han D, Wang RM, Dong Y, Yang F, Vadlamudi RK, Brann DW. C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-alpha and the critical period hypothesis of estrogen neuroprotection. Proc Natl Acad Sci U S A. 2011;108:E617–E624. doi: 10.1073/pnas.1104391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Wu TW, Brinton RD. Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res. 2004;1010:22–34. doi: 10.1016/j.brainres.2004.02.066. [DOI] [PubMed] [Google Scholar]
- Zheng F, Gingrich MB, Traynelis SF, Conn PJ. Tyrosine kinase potentiates NMDA receptor currents by reducing tonic zinc inhibition. Nat Neurosci. 1998;1:185–191. doi: 10.1038/634. [DOI] [PubMed] [Google Scholar]
- Zheng WH, Quirion R. Comparative signaling pathways of insulin-like growth factor-1 and brain-derived neurotrophic factor in hippocampal neurons and the role of the PI3 kinase pathway in cell survival. J Neurochem. 2004;89:844–852. doi: 10.1111/j.1471-4159.2004.02350.x. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhang H, Cohen RS, Pandey SC. Effects of estrogen treatment on expression of brain-derived neurotrophic factor and cAMP response element-binding protein expression and phosphorylation in rat amygdaloid and hippocampal structures. Neuroendocrinology. 2005;81:294–310. doi: 10.1159/000088448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Znamensky V, Akama KT, McEwen BS, Milner TA. Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal CA1 dendrites. J Neurosci. 2003;23:2340–2347. doi: 10.1523/JNEUROSCI.23-06-02340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]