Abstract
Objectives
To examine the association between successful aging without subsequent cognitive decline (SA-ND) and the Northern Manhattan Study (NOMAS) global vascular risk score (GVRS), which is predictive of stroke, MI, and vascular death.
Design
Prospective cohort study.
Setting
A stroke-free sample of Hispanic, black, and white participants living in the same community enrolled in an MRI substudy of NOMAS, a population-based prospective cohort study.
Participants
A cognitive screen was administered at baseline and at enrollment in the MRI substudy (n=1,290).
Measurements
SA-ND was based on disease, disability, and cognitive function. The GVRS includes age, sex, race-ethnicity, waist circumference, alcohol intake, smoking, physical activity, blood pressure, antihypertensive medication use, fasting blood sugar, lipid levels, and peripheral vascular disease.
Results
Data at baseline and follow-up were available for 1,162 participants (mean age 70 ± 9 years; 61% women; 13% white, 16% black, 69% Hispanic; mean GVRS 8.6 ± 0.9). Logistic regression, adjusted for education, socioeconomic status, and follow-up time, showed that the odds of SA-ND were 38% greater for each additional one-point decrease on the GVRS (OR=1.38, 95% C.I. 1.17-1.61; p<0.0001). We also observed an inverse dose-response for quartiles of GVRS with SA-ND. Greater diastolic blood pressure among those on antihypertensive medication, and a history of claudication or peripheral arterial disease, were inversely associated with SA-ND in our fully adjusted model (p<0.005).
Conclusion
Potentially modifiable vascular risk factors were independently associated with SA-ND in a multi-ethnic community-based sample. Improvements in global vascular risk scores could help promote healthy longevity in the aging population.
Keywords: Successful Aging, Cognitive Aging, Global Vascular Risk, Vascular Risk Factors
INTRODUCTION
Successful aging is a multidimensional process, involving physical, functional, and psychosocial domains [1], and entails living free of diseases that adversely affect quality of life despite some physical limitations. Successful cognitive aging further implies the avoidance of diseases that affect cognition – including vascular cognitive impairment (VCI), defined as cognitive impairment contributed to by vascular disease.[2] While aging is unavoidable, VCI is potentially preventable if means to identify those at risk are available and the risk factors themselves are modifiable.
Heart disease and stroke are the leading causes of death in older adults, and vascular disease is an important contributor to cognitive decline.[3] More than one-quarter of American adults have multiple risk factors for heart disease and stroke, while the percentage of adults with no risk factors, and the proportion that engage in healthy lifestyles, is low.[4] Clustering of cardiovascular risk is associated not only with physical disease and disability, but also with cognitive health. For instance, participants of the Cardiovascular Risk Factors, Aging, and Dementia (CAIDE) study who were obese with high systolic blood pressures and total cholesterol had six times the risk of dementia compared to those with no risk factors.[5] The metabolic syndrome, perhaps the most well known cluster of cardiovascular risk factors, is also a risk factor for accelerated cognitive aging (2).[6-7] A number of cardiovascular risk scores have been developed and include determinants such as age, hypertension, hyperlipidemia, and a history of smoking.[8] More recently, behavioral and anthropometric indices have been used to estimate risk.[9-11] In the Northern Manhattan Study (NOMAS), an urban population that includes whites, blacks, and Hispanics determined to be stroke-free at baseline, continuous measures of obesity, lipids, fasting glucose, and blood pressure, as well as quantification of physical activity and alcohol consumption – the Global Vascular Risk Score (GVRS) – improved upon traditional Framingham measures in predicting incident stroke, MI, or vascular death.[11] In providing improved risk stratification, such models allow for better targeting of preventive therapies.
Central to successful cognitive aging is a protective vascular risk factor profile, yet little is known about the effect of global vascular risk. As the NOMAS GVRS predicts the overall combined risk of adverse vascular outcomes, we hypothesized that it would be inversely associated with having successful aging and no cognitive decline (SA-ND) in this multi-ethnic stroke-free cohort.
METHODS
Study Design
The Northern Manhattan Study
NOMAS is a population-based cohort study that includes 3,298 stroke-free participants identified from random digit dialing using dual-frame sampling to identify published and non-published telephone numbers. People were eligible if they had never been diagnosed with a stroke, were above 39 years of age, and were residents of Northern Manhattan for at least 3 months in a household with a telephone. Those eligible from the telephone sample were recruited for in-person assessments between 1993 and 2001 with an overall response rate of 68%.[11]
Definition of Successful Aging at Baseline
The current study is limited to NOMAS participants who were administered the MMSE twice, once at baseline and once at the time of enrollment in an MRI substudy. Criteria for inclusion into the substudy were as follows: (1) age older than 55 years; (2) no contraindications to MRI; and (3) provided written informed consent. Participants were enrolled in the substudy an average of 6.1 ± 3.5 years after baseline. Within this sample we classified participants at the time of their baseline enrollment in NOMAS (between 1993 and 2001) based on disease, disability, and cognitive function using definitions of successful aging from other large cohort studies.[1, 12]
Successful aging (SA) at baseline was defined as:
no history of cancer, chronic obstructive pulmonary disease, or cardiac disease (myocardial infarction, coronary artery disease, congestive heart failure, atrial fibrillation, or valvular heart disease) – the sample was stroke-free by NOMAS enrollment criteria and remained stroke free at the time of the second MMSE measurement;
-
a creatinine clearance rate of ≥45 mL/min;
Creatinine clearance rate (CCL) was derived from the Cockcroft-Gault formula [13]:
Excluding those with chronic kidney disease is a novel part of our definition of SA. Chronic kidney disease was found in NOMAS to be a significant risk factor for stroke and combined vascular events, especially in blacks.[14] And, more recently, decreased kidney function was associated with cognitive decline even in those with mild chronic kidney disease in our cohort.[15] We chose a CCL of >45 mL/min based on results from several large cohorts showing that the risks of death, cardiovascular events, and hospitalization rise sharply below a GFR of 45 mL/min/1.73m2, as do the odds of incident cognitive impairment.[16-17]
-
3)
a global score of ≥95 on the Barthel Activities of Daily Living scale; and
-
4)
a baseline Mini-Mental State Examination (MMSE) score above education-specific cutoffs (>17 for those with 8 years or less of education or >23 for at least 9 years).
In addition to advancing age, socioeconomic factors, particularly race/ethnicity and level of education, may influence performance on the MMSE independent of other conditions.[18-19] Therefore, to avoid misclassification, the MMSE cutoff for this study was based on level of education as determined by analyses comparing educational attainment, MMSE score, and in depth cognitive assessments in our multi-ethnic cohort (data not shown).
Participants were then considered to have successful aging without cognitive decline (SA-ND) if they continued to meet the above disease and disability criteria and declined less than 3 points on the MMSE [20-21] between baseline and follow-up cognitive testing at enrollment in the MRI substudy.
Global Vascular Risk Score (GVRS)
The NOMAS GVRS has been described previously and improved upon the Framingham risk score in predicting stroke, MI, and vascular death in the NOMAS multi-ethnic urban sample.[11] In the prior study, a survival model was constructed to predict combined cardiovascular outcomes, which included stroke (ischemic or intracerebral hemorrhage), myocardial infarction (MI), and vascular death. The following variables contributed significantly to model fit and are included in the GVRS: age, sex, black race, Hispanic ethnicity, waist circumference, moderate alcohol use, current and former smoking, moderate to heavy physical activity, the interaction between sex and moderate to heavy physical activity, systolic and diastolic blood pressures, the interaction between diastolic blood pressure and anti-hypertensive medication use, peripheral vascular disease, fasting blood sugar, and total cholesterol to HDL ratio. In the NOMAS cohort, GVRS (calculated at baseline) ranged from 4.4 to 11.6 (mean 8.6 ± 1.0 standard deviation) and each individual's score was calculated from a multivariable regression model.[11] A GVRS of 9.0 implied a 10-year probability of experiencing a vascular event of 0.20, a GVRS of 8.2 implied a 10-year probability of 0.10, and a GVRS of 6.6 implied a 10-year probability of 0.02. Thirty-five percent of our population had a GVRS of at least 9.0.
Statistical Analysis
We used logistic regression to examine the relationship between GVRS (using data at enrollment in the MRI substudy) and the odds of achieving the SA-ND outcome. Generalized linear models were used for regression, adjusting for duration of follow-up time (time between baseline and follow-up cognitive assessment), with other covariates included if associated with SA-ND and GVRS at a p-value ≤0.20. We used SAS statistical software (version 9.2, SAS Institute, Cary, NC) and significance was assessed according to a pre-set two-sided alpha level of 0.05, with the exception of covariate selection.
RESULTS
Sample Characteristics
Of the 1,290 individuals in the NOMAS MRI substudy cohort, data to determine SA-ND status were available in 1,162 (90%). The prevalence of SA-ND in this sample was 37%. The number of persons meeting each of the disease, disability, and cognition criteria in our definition of SA-ND is shown in Table 1. Characteristics, including components of the GVRS, of the overall sample and by SA-ND, status are presented in Table 2. The average age of participants was 70 ± 9 years, with 51% between ages 70 and 96; 13% were white, 16% black, and 69% Hispanic, and 61% were women. Mean GVRS score was 8.6 ± 0.9.
Table 1.
Criteria for Successful Aging – No Decline
| % (N) | |
|---|---|
| Disease and Disability | 49 (565) |
| No cancer | 93 (1076) |
| No chronic obstructive pulmonary disease | 91 (1053) |
| No cardiac disease | 83 (966) |
| Creatinine clearance rate ≥45 mL/min | 82 (957) |
| Barthel ADL score ≥95 | 95 (1098) |
| Cognition | 87 (1011) |
| Baseline MMSE >17 for ≤8 years of education or >23 for ≥9 years of education | 96 (1112) |
| ≤3-point decline on MMSE at follow-up | 89 (1037) |
Table 2.
Sample Characteristics*
| Global Vascular Risk Score (GVRS) | SA–ND** | |||
|---|---|---|---|---|
| And Its Components^ | Overall (N=1,162) | Yes (N=430) | No (N=732) | p-value |
| GVRS | 8.6 ± 0.9 | 8.5 ± 0.9 | 8.8 ± 0.8 | <0.001 |
| Age (years) | 70 ± 9 | 70 ± 8 | 71 ± 10 | 0.12 |
| Sex | <0.001 | |||
| Male | 39 (451) | 45 (195) | 35 (256) | |
| Race/ethnicity | 0.60 | |||
| Non-Hispanic white | 14 (157) | 13 (54) | 14 (103) | |
| Non-Hispanic black | 16 (186) | 17 (72) | 16 (114) | |
| Hispanic | 70 (796) | 69 (299) | 68 (497) | |
| Moderate alcohol drinker | 41 (476) | 43 (186) | 40 (290) | 0.30 |
| Current smoker | 16 (190) | 18 (78) | 15 (112) | 0.24 |
| Former smoker | 36 (419) | 36 (153) | 36 (266) | 0.69 |
| Never smoker | 48 (553) | 47 (202) | 48 (351) | 0.62 |
| Moderate or heavy physical activity | 10 (112) | 11 (46) | 9 (66) | 0.38 |
| Among men | 4 (52) | 5 (23) | 4 (29) | 0.29 |
| Waist circumference (inches) | 38 ± 5 | 38 ± 5 | 38 ± 5 | 0.17 |
| History of leg pain or arterial disease | 12 (143) | 8 (33) | 15 (110) | <0.001 |
| Systolic blood pressure (mmHg) | 136 ± 17 | 137 ± 17 | 136 ± 17 | 0.31 |
| Diastolic blood pressure (mmHg) | 78 ± 10 | 78 ± 9 | 78 ± 10 | 0.24 |
| Diastolic blood pressure (mmHg) among those on anti-hypertensive medication (N=472) | 80 ± 10 | 81 ± 9 | 79 ± 10 | 0.12 |
| Glucose (mg/dL) | 101 ± 34 | 101 ± 36 | 101 ± 33 | 0.89 |
| Total cholesterol to HDL ratio | 4 ± 2 | 4 ± 2 | 4 ± 1 | 0.06 |
|
Other Covariates | ||||
| Education (years) | 10 ± 5 | 10 ± 5 | 9 ± 5 | 0.14 |
| Medicaid or no insurance | 49 (469) | 45 (194) | 38 (275) | 0.03 |
| Time between baseline and follow-up cognitive testing (years) | 6 ± 4 | 7 ± 2 | 5 ± 4 | <0.001 |
% (N) or Mean ± SD
Successful aging without cognitive decline
All GVRS components from validated vascular risk prediction model are included [9].
Global Vascular Risk Score and Successful Aging
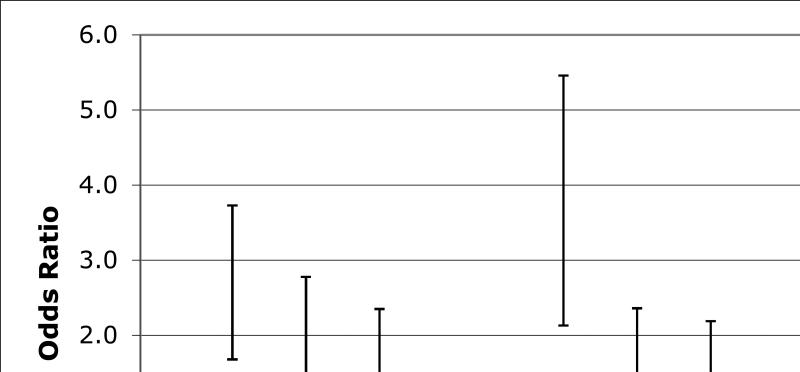
Examining the GVRS as a continuous measure, we found an inverse association such that that there was a greater odds of SA-ND with lower (better) GVRS. For each additional one-point decrease on the GVRS, the odds of SA-ND were 38% greater (OR=1.38, 95% C.I. 1.17-1.61; p<0.0001). The association was strengthened after adjustment for level of education and socioeconomic status (OR=1.56, 95% C.I. 1.32-1.85; p<0.0001). Results of logistic regression analyses comparing the odds of SA-ND across quartiles of GVRS, adjusted for length of follow-up time, are shown in Figure 1. Compared to the fourth quartile of GVRS (>9.3), the odds of SA-ND were 1.5 times greater for the third quartile of GVRS, 1.6 times greater for the second quartile, and more than three-fold greater for the lowest quartile of GVRS.
Figure 1.
Odds of Successful Aging – No Decline by Quartile of GVRS
We observed a dose response for increasing quartiles of GVRS. Compared to the highest quartile of GVRS (9.3+), the odds of successful aging without cognitive decline (SA-ND) were 1.50 (95% C.I. 1.03-2.19) times greater for the third quartile of GVRS, 1.63 (1.12-2.36) times greater for the second quartile, and 3.25 (2.13-4.94) times greater for the lowest quartile. Odds ratios in Model 1 are adjusted for length of time between baseline and follow-up cognitive testing only, and in Model 2 for years of education, health insurance status, and follow-up time.
GVRS Components and Successful Aging
We also examined individual components of the GVRS to determine key factors associated with SA-ND, adjusting for education, health insurance status, and length of follow-up time. Older age, greater diastolic blood pressure among those on antihypertensive medication, and a history of claudication or peripheral arterial disease were each independently associated with a lower likelihood of SA-ND (Table 3).
Table 3.
| Component | OR (95% C.I.) | p-value |
|---|---|---|
| Age (per year) | 0.95 (0.94-0.97) | <0.0001 |
| Men | 1.06 (0.77-1.46) | 0.74 |
| Race-ethnicity | ||
| Hispanic | 1.59 (0.94-2.70) | 0.08 |
| Black | 1.26 (0.75-2.13) | 0.38 |
| White | reference | -- |
| Moderate alcohol consumption | 1.13 (0.84-1.52) | 0.44 |
| Current smoker | 0.99 (0.72-1.37) | 0.97 |
| Former smoker | 1.10 (0.73-1.65) | 0.65 |
| Never smoker | reference | -- |
| Moderate or heavy physical activity | 0.90 (0.47-1.75) | 0.77 |
| Among men | 1.28 (0.49-3.38) | 0.61 |
| Waist circumference (per inch) | 1.02 (0.99-1.05) | 0.15 |
| History of leg pain or arterial disease | 0.39 (0.25-0.61) | <0.0001 |
| Systolic blood pressure (per 10 mmHg) | 1.05 (0.96-1.16) | 0.29 |
| Interaction between diastolic blood pressure (per 10 mmHg) and | 1.06 (0.89-1.27) | 0.52 |
| anti-hypertensive medication use | 0.94 (0.91-0.98) | 0.003 |
| Glucose (per 10 mg/dL) | 0.99 (0.95-1.03) | 0.67 |
| Total cholesterol to HDL ratio | 1.00 (0.91-1.12) | 0.86 |
All GVRS components from validated vascular risk prediction model are included [9].
Multivariable model further adjusted for education, health insurance status, and time between baseline and follow-up cognitive testing.
Comparison with Framingham Risk Score
We also explored the relationship between the Framingham Risk Score for coronary heart disease (FRS) and SA-ND to compare the GVRS with an established risk prediction tool [22]. Data were available to calculate the FRS in 1,138 (98%) of the 1,162 participants with SA-ND data. The mean FRS score was 8.2 ± 3.6 (range 1.3 to 12.8), and was significantly lower (better) in those who experienced SA-ND (7.8 ± 3.6 vs. 8.4 ± 3.5; p=0.003). Examining the FRS as a continuous variable, we found an inverse association such that each point decrease on the FRS was associated with 6% greater odds of SA-ND (OR=1.06, 95% C.I. 1.02-1.10; p=0.0009). The odds were marginally decreased after adjusting for level of education and socioeconomic status (OR=1.05, 95% C.I. 1.01-1.09; p=0.007).
DISCUSSION
A low vascular risk factor profile does not guarantee successful aging, but the elevated mortality and morbidity associated with vascular outcomes suggests it may be a key determinant. We found that for NOMAS participants the odds of SA-ND was 38% greater for each additional one-point decrease on the GVRS, and observed an inverse dose-response with greater odds of SA-ND for the lowest quartiles of GVRS. The odds of SA-ND was also greater for lower FRS, but the GVRS was more strongly associated with SA-ND in our cohort, further suggesting that it may be more applicable to a race/ethnically diverse urban sample.
While the literature supports an association between the presence of vascular risk factors and a lower likelihood of SA, this study is one of the first, to our knowledge, to examine the effect of global vascular risk. Given the importance of avoiding vascular outcomes such as stroke, myocardial infarction, peripheral vascular disease, and vascular death, global risk scores such as the GVRS add value if they identify those at risk early on. A greater burden of cardiovascular risk factors increases with age, limiting healthy longevity, and a poor cardiovascular risk profile is strongly associated with cardiovascular events and cardiovascular mortality.[3, 23] Individuals with more cardiovascular risk factors also have lower health-related quality of life, independent of sociodemographic characteristics and other comorbidities, and are at least 40% less likely to be employed, whereas a beneficial cardiovascular risk profile is related to greater productivity and lower Medicare costs in later years.[24-26]
The value of the GVRS and other risk scores is that it quantifies risk and translates comorbidities into a useful number that provides a target for intervention. Here, we also explored the individual components that were most associated with SA-ND in our cohort and found that age, a history of claudication or arterial disease, and diastolic blood pressure among hypertensives, were each independently associated with SA-ND. Peripheral arterial disease, along with other markers of generalized atherosclerosis such as carotid artery intima and media thickness, angina pectoris, and ankle-arm index, has implications for overall cardiovascular health, including an increased risk of vascular and non-vascular events, and has been related to disability, cognitive impairment, and unsuccessful aging.[27-28] Up to 90% of PAD patients have angiographically-determined coronary artery atherosclerosis, and up to half have evidence of cerebrovascular disease. Comorbid cardiovascular risk factors including smoking and diabetes are more common in these individuals as well [29]. Yet, patients with PAD tend to be undertreated in regards to risk factor modification, and screening for PAD is non-invasive and widely available [30].
We also found that, among individuals on antihypertensive medication, each 10-mm Hg of diastolic blood pressure was associated with a 6% lower odds of SA-ND. While both systolic and diastolic blood pressures have been related to cardiovascular morbidity and mortality, in older adults cardiovascular risk appears to be directly proportional to systolic blood pressure, and eventual health outcome inversely proportional to diastolic blood pressure [31]. However, we have recently reported that diastolic and not systolic pressure was associated with greater white matter lesion load. Diastolic blood pressure has also been independently associated with cognitive decline, and subclinical cerebral infarction and ischemic white matter damage may mediate this relationship [32]. Diastolic blood pressure may reflect peripheral vascular resistance, and thus be more specific to small vessel damage than systolic pressure that is most affected by large vessel stiffness. However, a history of hypertension treatment confers cardiovascular risk even after adjustment for blood pressure and other traditional risk factors, though it is not known whether this is due to an incomplete reversal of hypertension-induced end-organ damage, or a possible deleterious effect of medications.[33] The findings from our study indicate that greater diastolic blood pressure may decrease the likelihood of SA in those on antihypertensive drugs (i.e., with established hypertension). However, because hypotension, too, affects brain perfusion and has been shown to worsen vascular and cognitive outcomes, more research is needed to determine the optimal blood pressure levels that should be targeted to achieve SA.[34]
We acknowledge several study limitations. The NOMAS GVRS has not been validated in other cohorts, although it is a strength that the score is applicable to this urban multi-ethnic U.S. population. We only included people who were enrolled in the MRI substudy, and therefore the substudy sample was somewhat healthier than the overall cohort due to a survivor effect typical of MRI substudies (i.e., participants had to be well enough to visit our center). We acknowledge that our methods would tend to underestimate the effect of vascular risk factors because of this healthy cohort effect. However, our findings do not depend on loss to follow-up related to the outcome.
Successful cognitive aging is a multidimensional construct for which there is no currently accepted standard definition. For this study, we created a definition of successful aging that incorporates both physical well-being and cognitive abilities. We considered only those diseases that are major causes of mortality among older adults – cancer, chronic lung disease, heart disease, and stroke. Additionally, our definition of SA is unique in that we included chronic kidney disease as a novel component - not including this in our definition would have increased the number of people in the SA category. However, we believe this would bias our findings toward the null by including less successful agers in the SA category. Otherwise, we chose similar criteria to other large studies and this aids in comparing across them. As the manner in which successful cognitive aging is defined and measured affects its prevalence and observed associations, further research is needed to refine the concept of successful cognitive aging. Consideration of biopsychosocial factors including socioeconomic status, social support, quality of life, and depressive symptoms alongside novel indicators of disease and disability such as global vascular risk, may lead to a more robust definition of successful cognitive aging replete with opportunities to modify the aging process. Current estimates suggest that 44 million adults in the United States are obese and 50 million are hypertensive, and the prevalence of cardiovascular disease is expected to rise as other risk factors, such as obesity, diabetes, and dyslipidemia become ever more prevalent.[35-37] Our data suggest that the GVRS provides meaningful information about the risk of SA-ND in a multi-ethnic urban U.S. sample, and that identification of hypertension and PAD may be of particular importance in estimating risk of unsuccessful cognitive aging. Application of existing treatments for cardiovascular risk reduction, coupled with primary prevention efforts, could potentially modify the course of cognitive aging and further studies are needed to clarify this. Our results stress the importance of considering the range of global vascular risk instead of isolated cardiovascular risk factors, given that even moderate increases in risk are met with substantial decreases in the odds of SA. With early detection of at-risk persons crucial to the success of medical management and behavioral interventions, comprehensive global risk assessment tools such as the GVRS, if found to predict SA, may be an effective means of guiding comprehensive public health efforts.
ACKNOWLEDGMENT
This work was supported by the Evelyn F. McKnight Brain Institute, NIH/NINDS R37 NS 29993, K02 NS 059729, and AHA SDG 0735387N.
Jessica R. L. Warsch: Employee of the Department of Neurology, University of Miami
T. Rundek – Employee of Columbia University
M.C. Paik - Funded by NINDS/NIH grant R37 NS 29993
Sponsor's Role: None.
Footnotes
Conflict of Interest
REFERENCES
- 1.Rowe JW, Kahn RL. Successful Aging. Dell Publishing; New York: 1998. [Google Scholar]
- 2.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beeri MS, Ravona-Springer R, Silverman JM, et al. The effects of cardiovascular risk factors on cognitive compromise. Dialogues Clin Neurosci. 2009;11:201–212. doi: 10.31887/DCNS.2009.11.2/msbeeri. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenlund KJ, Zheng ZJ, Keenan NL, et al. Trends in self-reported multiple cardiovascular disease risk factors among adults in the United States, 1991–1999. Arch Intern Med. 2004;164:181–188. doi: 10.1001/archinte.164.2.181. [DOI] [PubMed] [Google Scholar]
- 5.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 6.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 7.Vieira JR, Elkind MS, Moon YP, et al. The metabolic syndrome and cognitive performance: The northern Manhattan study. Neuroepidemiology. 2011;37:153–159. doi: 10.1159/000332208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 9.Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Buring JE, Rifai N, et al. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: The Reynolds Risk Score. JAMA. 2007;297:611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 11.Sacco RL, Khatri M, Rundek T, et al. Improving global vascular risk prediction with behavioral and anthropometric factors: The multi-ethnic Northern Manhattan Cohort Study. J Am Coll Cardiol. 2009;54:2303–2311. doi: 10.1016/j.jacc.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Depp CA, Jeste DV. Definitions and predictors of successful aging: A comprehensive review of larger quantitative studies. Am J Geriatr Psychiatry. 2006;14:6–20. doi: 10.1097/01.JGP.0000192501.03069.bc. [DOI] [PubMed] [Google Scholar]
- 13.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 14.Nickolas TL, Khatri M, Boden-Albala B, et al. The association between kidney disease and cardiovascular risk in a multi-ethnic cohort: Findings from the Northern Manhattan Study (NOMAS). Stroke. 2008;39:2876–2879. doi: 10.1161/STROKEAHA.107.513713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khatri M, Nickolas T, Moon YP, et al. CKD associates with cognitive decline. J Am Soc Nephrol. 2009;20:2427–2432. doi: 10.1681/ASN.2008101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etgen T, Sander D, Chonchol M, et al. Chronic kidney disease is associated with incident cognitive impairment in the elderly: The INVADE study. Nephrol Dial Transplant. 2009;24:3144–3150. doi: 10.1093/ndt/gfp230. [DOI] [PubMed] [Google Scholar]
- 17.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 18.Tombaugh TN, McDowell I, Kristhansson B, et al. Mini-Mental State Examination (MMSE) and the Modified MMSE (3MS): A psychometric comparison and normative data. Psychol Assess. 1996;8:48–59. [Google Scholar]
- 19.Crum RM, Anthony JC, Bassett SS, et al. Mini-Mental State Examination by age and educational level. JAMA. 1993;269:2386–2391. [PubMed] [Google Scholar]
- 20.Das D, Orengo CA, Kunik ME, et al. Cognitive decline in patients on an acute geropsychiatric unit. J Neuropsychiatry Clin Neurosci. 1998;10:205–209. doi: 10.1176/jnp.10.2.205. [DOI] [PubMed] [Google Scholar]
- 21.Marzona I, O'Donnell M, Teo K, et al. Increased risk of cognitive and functional decline in patients with atrial fibrillation: Results of the ONTARGET and TRANSCEND studies. CMAJ. 2012;184:E329–E336. doi: 10.1503/cmaj.111173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 23.Chang M, Hahn RA, Teutsch SM, et al. Multiple risk factors and population attributable risk for ischemic heart disease mortality in the United States, 1971-1992. J Clin Epidemiol. 2001;54:634–644. doi: 10.1016/s0895-4356(00)00343-7. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan PW, Ghushchyan V, Wyatt HR, et al. Impact of cardiometabolic risk factor clusters on health-related quality of life in the U.S. Obesity. 2007;15:511–521. doi: 10.1038/oby.2007.580. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan PW, Ghushchyan V. Cardiovascular risk factor clusters and employment in the United States. Value Health. 2007;10(S1):S52–S58. doi: 10.1111/j.1524-4733.2007.00199.x. [DOI] [PubMed] [Google Scholar]
- 26.Daviglus ML, Liu K, Greenland P, et al. Benefit of a favorable cardiovascular risk-factor profile in middle age with respect to Medicare costs. N Engl J Med. 1998;339:1122–1129. doi: 10.1056/NEJM199810153391606. [DOI] [PubMed] [Google Scholar]
- 27.Kuller LH, Velentgas P, Barzilay J, et al. Diabetes mellitus: subclinical cardiovascular disease and risk of incident cardiovascular disease and all-cause mortality. Arterioscler Thromb Vasc Biol. 2000;20:823–829. doi: 10.1161/01.atv.20.3.823. [DOI] [PubMed] [Google Scholar]
- 28.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 29.Rafnsson SB, Deary IJ, Fowkes FGR. Peripheral arterial disease and cognitive function. Vasc Med. 2009;14:51–61. doi: 10.1177/1358863X08095027. [DOI] [PubMed] [Google Scholar]
- 30.Rehring TF, Sandhoff BG, Stolcpart RS, et al. Atherosclerotic risk factor control in patients with peripheral arterial disease. J Vasc Surg. 2005;41:816–822. doi: 10.1016/j.jvs.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 31.Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;6:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 32.Tsivgoulis G, Alexandrov AV, Wadley VG, et al. Association of higher diastolic blood pressure levels with cognitive impairment. Neurology. 2009;73:589–595. doi: 10.1212/WNL.0b013e3181b38969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blacher J, Evans A, Arveiler D, et al. Residual cardiovascular risk in treated hypertension and hyperlipidaemia: the PRIME Study. J Hum Hypertens. 2010;24(1):19–26. doi: 10.1038/jhh.2009.34. [DOI] [PubMed] [Google Scholar]
- 34.Novak V, Hajjar I. The relationship between blood pressure and cognitive function. Nat Rev Cardiol. 2010;7:686–698. doi: 10.1038/nrcardio.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonow RO, Smaha LA, Smith SC, Jr, et al. World Heart Day 2002: The international burden of cardiovascular disease: Responding to the emerging global epidemic. Circulation. 2002;106:1602–1605. doi: 10.1161/01.cir.0000035036.22612.2b. [DOI] [PubMed] [Google Scholar]
- 36.National Center for Health Statistics . Health, United States, 2001 With Urban and Rural Health Chartbook. Dept of Health and Human Services, Centers for Disease Control and Prevention; Hyattsville, MD: 2001. DHHS publication 01-1232. [Google Scholar]
- 37.American Heart Association . 2002 Heart and Stroke Statistical Update. American Heart Association; Dallas, Tex: 2001. [Google Scholar]