Abstract
Introduction:
Screening for severe sepsis in adult emergency department (ED) patients may involve potential delays while waiting for laboratory testing, leading to postponed identification or over-utilization of resources. The systemic inflammatory response syndrome (SIRS) criteria are inaccurate at predicting clinical outcomes in sepsis. Shock index (SI), defined as heart rate / systolic blood pressure, has previously been shown to identify high risk septic patients. Our objective was to compare the ability of SI, individual vital signs, and the systemic inflammatory response syndrome (SIRS) criteria to predict the primary outcome of hyperlactatemia (serum lactate ≥ 4.0 mmol/L) as a surrogate for disease severity, and the secondary outcome of 28-day mortality.
Methods:
We performed a retrospective analysis of a cohort of adult ED patients at an academic community trauma center with 95,000 annual visits, from February 1st, 2007 to May 28th, 2008. Adult patients presenting to the ED with a suspected infection were screened for severe sepsis using a standardized institutional electronic order set, which included triage vital signs, basic laboratory tests and an initial serum lactate level. Test characteristics were calculated for two outcomes: hyperlactatemia (marker for morbidity) and 28-day mortality. We considered the following covariates in our analysis: heart rate >90 beats/min; mean arterial pressure < 65 mmHg; respiratory rate > 20 breaths/min; ≥ 2 SIRS with vital signs only; ≥2 SIRS including white blood cell count; SI ≥ 0.7; and SI ≥ 1.0. We report sensitivities, specificities, and positive and negative predictive values for the primary and secondary outcomes.
Results:
2524 patients (89.4%) had complete records and were included in the analysis. 290 (11.5%) patients presented with hyperlactatemia and 361 (14%) patients died within 28 days. Subjects with an abnormal SI of 0.7 or greater (15.8%) were three times more likely to present with hyperlactatemia than those with a normal SI (4.9%). The negative predictive value (NPV) of a SI ≥ 0.7 was 95%, identical to the NPV of SIRS.
Conclusion:
In this cohort, SI ≥ 0.7 performed as well as SIRS in NPV and was the most sensitive screening test for hyperlactatemia and 28-day mortality. SI ≥ 1.0 was the most specific predictor of both outcomes. Future research should focus on multi-site validation, with implications for early identification of at-risk patients and resource utilization.
INTRODUCTION
Severe sepsis poses substantial clinical, financial, and logistical challenges. Annual hospitalizations for septicemia or sepsis have more than doubled over the past decade, from 326,000 in 2000 to 727,000 in 2008, without a corresponding increase in overall hospitalizations for that time period.1 Although this represents only 2% of admissions to the hospital, these patients comprise an estimated 17% of inhospital deaths.1 Up to 46% of hospitalized septic patients are admitted through a dwindling number of emergency departments (EDs).1,2 Emergency clinicians now must balance two conflicting forces in the care of patients at risk for sepsis: the recognized need for early intensive therapy and an increasingly resource-strained environment.
When sepsis is identified early in the ED and its severe form is treated aggressively with the protocolized care bundle of early goal directed therapy (EGDT), improvements in mortality are significant.4,5 A number of studies have analyzed the process of implementing EGDT in the ED based on the sepsis definitions outlined in the American College of Chest Physicians / Society for Critical Care Medicine (ACCP/SCCM).4,6–8 The expert consensus panel defined sepsis as the systemic inflammatory response syndrome (SIRS) with evidence of infection (Figure 1). The panel further asserted that SIRS is the physiologic response to inflammation in the body, regardless of cause.8 These parameters were selected for their high sensitivity, to represent the lowest threshold for detection of the early response to inflammation during times of physiologic stress.9
Figure 1.
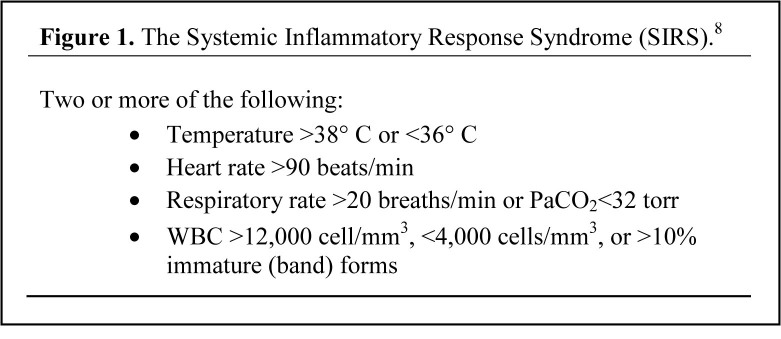
Criteria for systemic inflammatory response syndrome.7
Critics of SIRS assert that the criteria lack specificity for infectious causes of illness and for clinical outcomes.10,11 Shapiro et al12 demonstrated that adult ED patients admitted to the hospital with a suspected infection with 2 or more SIRS criteria were at no greater risk for 28-day mortality than patients with less than 2 SIRS criteria. The non-specific nature of SIRS may in fact hinder the utility of screening practices for the early detection of sepsis; this in turn has implications for enrollment of patients in ED-based sepsis research.
An additional limitation of SIRS to function as an early warning of sepsis is its inclusion of a white blood cell count (WBC). The time required to order, draw, analyze, and report laboratory tests is substantial, particularly when these are used to fulfill criteria for diagnosis or to make clinical decisions. On the one hand, protocol-driven laboratory draws screen low-risk patients, potentially generating many false positives. On the other hand, relying on laboratory information to define treatment strategies causes delays in care. The consequences of delayed recognition of those at risk for sepsis include ongoing volume depletion and microcirculatory inflammation, which lead to end-organ dysfunction.12 Non-laboratory immediate bedside “red flags” for sepsis may alert providers to initial assessment of those at risk for severe sepsis. Congruently, a clinical basis to re-prioritize those with more benign parameters on presentation would direct resources appropriately.
The shock index (SI) is a bedside assessment defined as heart rate divided by systolic blood pressure, with a normal range of 0.5 to 0.7 in healthy adults. Allgöwer and Buri13 first introduced the concept in 1967 as a simple and effective means of gauging the degree of hypovolemia in hemorrhagic and infectious shock states. Experimental and clinical studies have shown that SI is linearly inversely related to physiologic parameters, such as cardiac index, stroke volume, left ventricular stroke work, and mean arterial pressure.14 A SI ≥ 1.0 has been associated with significantly poorer outcomes in patients with acute circulatory failure.14 Furthermore, SI was also shown to indicate persistent failure of left ventricular function during aggressive therapy of shock patients in the ED.15 In 1994, Rady et al16 found that a SI ≥ 0.9 predicted higher illness priority at triage, higher hospital admission rates, as well as intensive therapy on admission than pulse or blood pressure alone. This suggests that SI may be a valuable tool for the early recognition and evaluation of critical illness in the ED,16 as well as a means to track progress of resuscitation.15
As an adjunct to established methods, SI may identify and risk-stratify septic patients early in the ED course. One of these established markers for sepsis severity – hyperlactemia (serum lactate ≥ 4.0 mmol/L) - is an entry criterion for EGDT protocols and is associated with significant short-term mortality risk.5,17 The shock state causes cellular hypoxia, leading to anaerobic metabolism and increased lactate production, as well as decreased clearance, even before vital signs are compromised.17 Persistently high lactate levels are associated with under-resuscitation and have been shown to down-trend with successful resuscitation.15 Our objective was to compare the ability of SI, individual vital signs, and SIRS to predict the outcomes of hyperlactatemia as an objective surrogate for disease severity and 28-day mortality in a cohort of adult ED patients screened for severe sepsis.17–19
METHODS
Study Design
This was an observational cohort of adult ED patients with a suspected infection who were screened for severe sepsis using a standardized clinical protocol. The computerized order set included obtaining a CBC and venous lactate level on these patients’ initial blood draw. The study was conducted from February 1, 2007 to May 28, 2008 at a 450-bed urban teaching hospital with 95,000 ED visits annually. The local institutional review board approved the study with a waiver of informed consent. All data were collected retrospectively by electronic medical record review. The pre-specified primary outcome was hyperlactatemia; the secondary outcome was 28-day mortality.
Selection of Participants
Patients 21 years of age or older were included in the study if screened for severe sepsis by the above protocol. In order not to confound the dataset with repeated measures, the first ED visit was used in patients with mul tiple encounters over the study period. We created and audited a database of patients screened for severe sepsis to confirm inclusion of all potential subjects. A similar cohort from the same data set was analyzed and an examination of markers for sepsis severity has been previously published.20
Methods and Measurements
Initial serum lactate levels (mmol/L) were determined using a serum-based immunoassay (Unicel Synchron, Beckman Coulter Inc, Brea, CA). The study site used venous lactate (over arterial lactate) to improve compliance with its established screening protocol. Only initial laboratory studies performed in the ED were used for analysis.
Data Collection
Trained research associates abstracted the medical records of all patients screened for severe sepsis during the study period. Researchers used standardized data abstraction sheets, were routinely audited, and were blinded to the study hypothesis in keeping with accepted chart-review standards.21 A second blinded reviewer collected all variables on 10% of enrolled subjects to perform inter-rater reliability analysis, which resulted in a Cohen’s kappa coefficient of 0.90 or greater for all variables. Initial lactate level and 28-day mortality information was collected on each subject.
Subjects who were inpatients at 28 days and those discharged prior to 28 days from admission who were not reported as deceased in the Social Security Death Index (SSDI) for that time frame were considered “alive” for this analysis. The SSDI is a database of death records created from the United Stated Social Security Administration’s Death Master File; it has been validated as an acceptable means of collecting mortality data for ED research.22
Data Analysis
For ease of interpretation, we categorized the variables of interest (SI, SIRS criteria, modified SIRS criteria without white blood cell count, and hyperlactatemia) into binary categorical variables. Hyperlactatemia was the primary outcome of interest as a marker for severe sepsis.17–19 We analyzed 4 distinct predictors of hyperlactatemia.
The variables of interest were categorized into binary categorical variables as follows: SI[1.0] (shock index greater than or equal to 1.0); SI[0.7] (shock index greater than or equal to 0.7); SIRS criteria (2 or more SIRS criteria met); modified SIRS criteria without white blood cell count (2 or more SIRS criteria met with no white blood cell information included); and hyperlactatemia (lactate levels greater than or equal to 4.0).
To compare predictors of hyperlactatemia, we stratified frequency tables by variable. We calculated sensitivity, specificity, and positive and negative predictive values for each of the four measures. To compare sensitivities across predictors we used tests of equivalence. All calculations were performed in R: A Language and Environment for Statistical Computing, version 2.14.0 (R Foundation for Statistical Computing, Vienna, Austria, 2011).
We calculated distinct frequency tables for hyperlactatemia and 28-day mortality, stratified by each of the 4 methods of measurement.
RESULTS
There were 2,824 unique adult patients screened for severe sepsis and admitted for a suspected or confirmed infection during the study period. Of these, 2,524 patients (89.4%) had complete records, including triage vital signs and initial CBC and lactate levels measured in the ED. Demographic characteristics were similar in the low lactate (< 4.0 mmol/L) and high lactate (≥ 4.0 mmol/L) groups (Table 1).
Table 1.
Demographics and predictors of the full cohort startified by hyperlacatemia*.
| Lactate < 4.0 mmol/L | Lactate ≥ 4.0 mmol/L | |
|---|---|---|
| 2234 (86%) | 2234 (86%) | |
| Admitted to the hospital [n(%)] | 1996 (89.4%) | 279 (96.2%) |
| 28-day mortality | 267 (12.0%) | 94 (32.4%) |
| Demographics | ||
| Age (years) | 72.9 ± 17.0 | 74.5 ± 15.4 |
| Male gender [n (%)] | 1013 (45%) | 145 (50%) |
| Race [n (%)] | ||
| Caucasian | 1189 (53%) | 151 (52%) |
| Asian | 440 (20%) | 68 (23%) |
| African-American | 156 (7%) | 17 (6%) |
| Hispanic | 267 (12%) | 33 (11%) |
| Other | 182 (8%) | 21 (7%) |
| Vital signs | ||
| Temperature (°C) | 37.7 ± 1.2 | 37.7 ± 1.3 |
| Heart rate (beats/min) | 97.1 ± 26.5 | 109.4 ± 25.7 |
| Respiratory rate (breaths/min) | 21 ± 6 | 23 ± 6 |
| Mean arterial pressure (mmHg) | 87 ± 19.9 | 77.3 ± 21.7 |
| Shock index** | 0.8 ± 0.3 | 1.1 ± 0.5 |
| Laboratory data | ||
| White blood cell count (cells/mm3) | 12.3 ± 8.9 | 16.1 ± 10.4 |
| Bands (Cells/mm3) | 2.8 ± 6.5 | 10.3 ± 13.0 |
| Platelets (1,000 cells/mm3) | 257 ± 125 | 258 ± 138 |
| SIRS criteria*** | 0.6 ± 0.5 | 0.8 ± 0.4 |
SIRS, systemic inflammatory response syndrome
Continuous data expressed as the mean and standard deviation; categorical data expressed as n (percentage)
Defined as heart rate / systolic blood pressure
Defined as 1 point for each: heart rate >90 beats/min; respiratory rate >20 breaths/min; temperature ≥ 38°C or < 36°C; White blood cell ≥ 12,000, < 4,000 or bands ≥ 10%
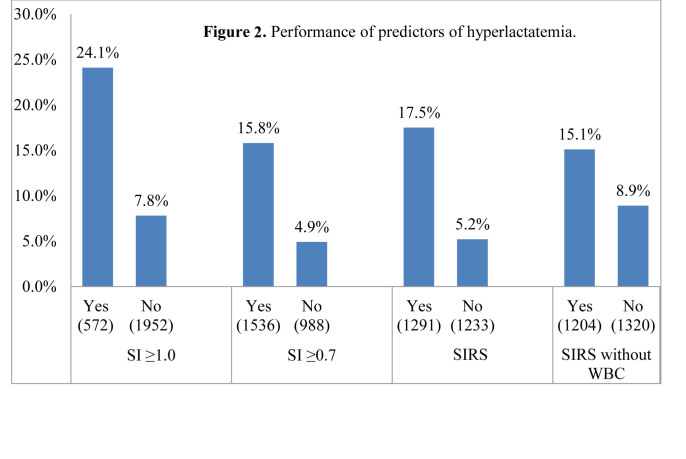
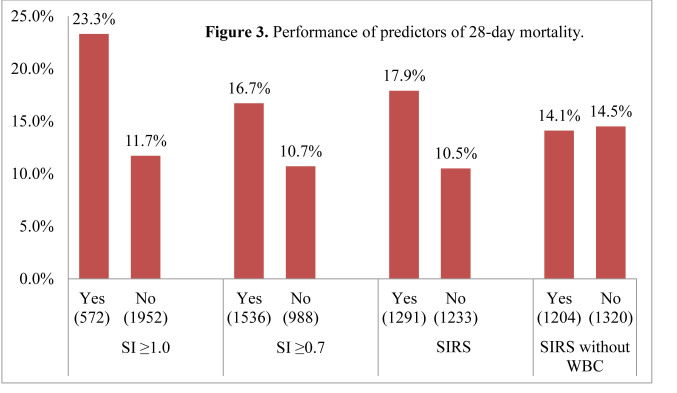
Shock index performed well as a predictor of hyperlactatemia and 28-day mortality (Figures 2 and 3). Subjects with a shock index of 0.7 or greater (15.8%) were 3 times more likely to have hyperlactatemia than those with a shock index of less than 0.7 (4.9%) (Table 2). Similarly, subjects with a shock index of 1.0 or greater (24.1%) were 3 times more likely to present with hyperlactatemia than those with a shock index of less than 1.0 (7.8%). Hyperlactemia and 28-day mortality frequencies were similar across groups, consistent with previous studies.17
Figure 2.
Performance of predictors of hyperlactatemia. SI, shock index; SIRS, systemic inflammatory response syndrome; WBC, white blood cell count
Figure 3.
Performance of predictors of 28-day mortality. SI, shock index; SIRS, systemic inflammatory response syndrome; WBC, white blood cell count
Table 2.
Frequency tables for each measurement method and outcome.
| Hyperlactatemia (≥ 4.0 mmol/L) | 28-Day Mortality | ||
|---|---|---|---|
| Shock index ≥ 1.0 | Yes (n=572) | 138 (24.1%) | 133 (23.3%) |
| No (n=1952) | 152 (7.8%) | 228 (11.7%) | |
| Shock index ≥ 0.7 | Yes (n=1536) | 242 (15.8%) | 256 (16.7%) |
| No (n=988) | 48 (4.9%) | 105 (10.7%) | |
| SIRS | Yes (n=1291) | 226 (17.5%) | 231 (17.9%) |
| No (n=1233) | 64 (5.2%) | 130 (10.5%) | |
| SIRS without white blood cell | Yes (n=1204) | 182 (15.1%) | 170 (14.1%) |
| No (n=1320) | 108 (8.9%) | 170 (14.1%) |
SIRS, systemic inflammatory response syndrome
All data are presented as n(%) of the total sample.
Negative predictive values (NPVs) of shock index for hyperlactatemia performed well (Table 3). The NPV for a shock index of ≥ 0.7 was 95%, which was the same for the full SIRS criteria (including WBC). Positive predictive values (PPVs) were consonantly low across predictors (SI[1.0], SI[0.7], SIRS, and modified SIRS).
Table 3.
Performance of predictors for hyperlactatemia.
| SI ≥ 1.0 | SIRS | SI ≥ 0.7 | SIRS without WBC | |
|---|---|---|---|---|
| Positive predictive value | 0.24 | 0.18 | 0.16 | 0.15 |
| Negative predictive value | 0.92 | 0.95 | 0.95 | 0.92 |
| Sensitivity | 0.48 | 0.78 | 0.83 | 0.63 |
| Specificity | 0.81 | 0.52 | 0.42 | 0.54 |
SI, shock index; WBC, white blood cell; SIRS, systemic inflammatory response syndrome
Negative predictive values of shock index for 28-day mortality performed similarly well (Table 4). The NPV for a shock index of ≥ 0.7 was 89%, also the same NPV for the full SIRS criteria (including WBC).
Table 4.
Performance of predictors for 28-day mortality.
| SI ≥ 1.0 | SIRS | SI ≥ 0.7 | SIRS without WBC | |
|---|---|---|---|---|
| Positive predictive value | 0.23 | 0.18 | 0.17 | 0.14 |
| Negative predictive value | 0.88 | 0.89 | 0.89 | 0.86 |
| Sensitivity | 0.37 | 0.64 | 0.71 | 0.47 |
| Specificity | 0.8 | 0.51 | 0.41 | 0.52 |
SI, shock index; WBC, white blood cell; SIRS, systemic inflammatory response syndrome
We compared sensitivity probabilities between the 2 highest-performing measurements: SIRS criteria and SI[0.7]. A test of equality showed no evidence that the 2 probabilities were significantly different (P = 0.11). The 95% confidence interval for the difference in performance between the SIRS criteria and SI[0.7] included the null value of 1.0 (−0.4% to 10.4%). That is, there was no statistically significant difference between the sensitivity of SIRS and the sensitivity of SI[0.7] for the primary outcome.
DISCUSSION
To our knowledge this is the first study to explore the relationship between shock index, severe sepsis, and clinical outcome. With the advent of early identification and treatment strategies for sepsis in the context of ever-shrinking resources, fast, reliable screening tools are needed. Haas23 described the ideal triage tool as simple to use, accurate, rapid, reproducible, and discriminative. In the high-acuity and high-uncertainty setting of the ED, the goal is to avoid potentially dangerous under-triage while appropriately assigning higher priority to sicker patients.24
SI emphasizes current physiologic dynamics, rather than static criteria. To illustrate the use of traditional methods of triage and interpretation of vital signs, consider a common presentation: a small-framed, otherwise well elderly woman with a cough and fever arrives to the ED with a heart rate of 88 beats/min and a systolic blood pressure of 110 mmHg. She may not fulfill SIRS criteria on presentation, let alone be “flagged” for sepsis. However, from a clinician’s inherently Bayesian perspective, her chief complaint of cough and fever informs him of a high pre-test likelihood of infectious disease; her increased risk for deterioration is instantaneously and objectively recognized. What would otherwise be deemed a fairly benign heart rate and systolic blood pressure now can be viewed through the lens of the shock index – in her case, we refocus our clinical gaze to see a grossly abnormal SI of 0.8 (normal: 0.5 to 0.7). She is identified as being at-risk for severe sepsis on initial presentation, before any laboratory testing is performed.
Since many factors affect abnormal vital signs, sensitivities and positive predictive values will vary. The true reliability of our findings lies in the negative predictive values of the shock index. As screening for sepsis popularizes in our hospitals, many institutions have begun to draw and send laboratory specimens from triage in an attempt to “save time” in disposition and through-put.25 Screening many for a disease with varying prevalence may introduce many false positives; these “abnormal” tests are followed by a costly and lengthy remediation process. This study shows that patients with a normal SI (less than 0.7) are 95% likely not to present with an established marker for severe sepsis – a high lactate level.
A normal shock index may serve as an adjunct to inform the clinician of which patients to prioritize for care. Since an elevated SI (SI ≥ 0.7) performed identically to the full SIRS criteria (including WBC), we can report that this no-cost bedside triage tool (SI) predicted absence of a reliable marker of severe sepsis (lactate) at least as well as an established rubric that uses laboratory information as part of its criteria (SIRS). This would suggest that low-risk patients with a normal SI may forgo (or not urgently need) routine triage laboratory screening for sepsis, especially from triage and before full evaluation. Additionally, using SI for triage decisions regarding severe sepsis screening can be made without waiting for results of the WBC. This has corresponding implications for efficiency and cost-effectiveness in ED protocols.
LIMITATIONS
This exploratory study has several important limitations. Providers in the ED identified patients during their initial evaluation based on a suspected infection, and all data were collected via retrospective computerized chart review. To mitigate potential misclassification bias, previously established recommendations were followed for chart abstraction. The main factors studied (vital signs, laboratory data, hospital admission and mortality), however, are less likely to be subject to misclassification.17 It is conceivable that the ED lactate results affected some downstream diagnostic and therapeutic care decisions (such as admission); however, the predictors studied and primary outcome were all based on initial presentation.
Due to the study’s preliminary nature, concurrent medication information was not collected or available to the data extractors, and any potential influence on vital signs is not controlled. Since many of these medications would tend to “normalize” vital signs (e.g. relative bradycardia from beta- or calcium-channel blockade in a nevertheless sick patient), this omission would tend to yield conservative estimates. Furthermore, as this information is frequently not known upon arrival of acutely ill patients, this particular limitation may reflect more closely real-world conditions that affect decision-making and initiating EGDT.
The cohort was elderly, with a mean age of 73 years, reflecting the study setting with many nursing home affiliations. It should be noted, however, that the majority of patients hospitalized for sepsis (approximately two-thirds) in the U.S. are aged 65 and older;1 in this light, our single site roughly reflects the at-risk population for sepsis. A larger, multi-site, prospective study is necessary to control for multiple confounders and to expand the cohort to younger patients.
CONCLUSION
In summary, the shock index is an effective, no-cost modality in the initial assessment of patients at risk for sepsis. Patients presenting with a presumed infection and a normal SI were found to be at very low risk (high NPV) for occult severe sepsis on presentation (as defined by a surrogate marker for morbidity, hyperlactatemia). SI may be used as an additional bedside assessment tool – a “red flag” for severe disease; this is particularly useful when traditional vital signs are seemingly relatively benign. Multisite prospective work is needed to clarify its role in resource utilization, risk stratification of patients with sepsis, and in tracking resuscitation progress.
Footnotes
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
- 1.Hall MJ, Williams SN, DeFrances CJ, et al. Inpatient Care for Septicemia or Sepsis: A Challenge for Patients and Hospitals. U.S. Department of Health and Human Services - National Center for Health Statistics. 2011;62:1–7. [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble W, Lidicker J, et al. Epidemiology of Severe Sepsis in the United States: Analysis of Incidence, Outcome, and Associated Costs of Care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Strehlow M, Emond S, Shapiro N, et al. National Study of Emergency Department Visits for Sepsis, 1992 to 2001. Ann Emerg Med. 2006;48:326–331. doi: 10.1016/j.annemergmed.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Rivers EP, Nguyen B, Havstad S, et al. Early Goal Directed Therapy in the Treatment of Severe Sepsis and Septic Shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 5.Osborn T, Nguyen B, Rivers EP. Emergency Medicine and the Surviving Sepsis Campaign: An International Approach to Managing Severe Sepsis and Septic Shock. Ann Emerg Med. 2005;46:228–231. doi: 10.1016/j.annemergmed.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro N, Howell M, Talmor D, et al. Implementation and Outcomes of the Multiple Urgent Sepsis Therapies (MUST) Protocol. Crit Care Med. 2006;34:1025–1032. doi: 10.1097/01.CCM.0000206104.18647.A8. [DOI] [PubMed] [Google Scholar]
- 7.Trzeciak S, Dellinger P, Abate N, et al. Translating Research to Clinical Practice: A 1-Year Experience With Implementing Early Goal-Directed Therapy for Septic Shock in the Emergency Department. Chest. 2006;129:225–232. doi: 10.1378/chest.129.2.225. [DOI] [PubMed] [Google Scholar]
- 8.Members of the American College of Chest Physicians / Society of Critical Care Medicine Consensus Conference Committee Definitions for Sepsis and Organ Failure and Guidelines for the Use of Innovative Therapies in Sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 9.Dellinger P, Bone R. To SIRS with Love. Crit Care Med. 1998;26:178–179. doi: 10.1097/00003246-199801000-00036. [DOI] [PubMed] [Google Scholar]
- 10.Sibbald W, Doig G, Inman K. Sepsis, SIRS and Infection. Intensive Care Med. 1995;21:299–301. doi: 10.1007/BF01705407. [DOI] [PubMed] [Google Scholar]
- 11.Vincent J. Dear SIRS, I’m Sorry to Say That I Don’t Like You. Crit Care Med. 1997;25:372–374. doi: 10.1097/00003246-199702000-00029. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro NI, Howell MD, Bates D, et al. The Association of Sepsis Syndrome and Organ Dysfunction with Mortality in Emergency Department Patients with Suspected Infection. Ann Emerg Med. 2006;48:583–590. doi: 10.1016/j.annemergmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Allgöwer M, Buri C. Schockindex. Deutsche Medizinische Wodenschrift. 1967;46:1–10. [Google Scholar]
- 14.Rady M, Nightingale P, Little R, et al. Shock Index: A Re-evaluation in Acute Circulatory Failure. Resuscitation. 1992;23:227–234. doi: 10.1016/0300-9572(92)90006-x. [DOI] [PubMed] [Google Scholar]
- 15.Rady M, Rivers EP, Martin G, et al. Continuous Central Venous Oximetry and Shock Index in the Emergency Department: Use in the Evaluation of Clinical Shock. Am J Emerg Med. 1992;10:538–541. doi: 10.1016/0735-6757(92)90178-z. [DOI] [PubMed] [Google Scholar]
- 16.Rady M, Smithline H, Blake H, et al. A Comparison of the Shock Index and Conventional Vital Signs to Identify Acute, Critical Illness in the Emergency Department. Ann Emerg Med. 1994;24:685–690. doi: 10.1016/s0196-0644(94)70279-9. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro NI, Howell MD, Talmor D, et al. Serum Lactate as a Predictor of Mortality in Emergency Department Patients with Infection. Ann Emerg Med. 2005;45:524–528. doi: 10.1016/j.annemergmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Mikkelson M, Miltiades A, Gaieski D, et al. Serum Lactate is Associated with Mortality in Severe Sepsis Independent of Organ Failure and Shock. Crit Care Med. 2009;37:1670–1677. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- 19.Howell MD, Donnino M, Clardy P, et al. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007;33:1892–1899. doi: 10.1007/s00134-007-0680-5. [DOI] [PubMed] [Google Scholar]
- 20.Green JP, Berger T, Garg N, et al. Serum Lactate is a Better Predictor of Short-Term Mortality When Stratified by C-reactive Protein in Adult Emergency Department Patients Hospitalized for a Suspected Infection. Ann Emerg Med. 2011;57:291–295. doi: 10.1016/j.annemergmed.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert EH, Lowenstein SR, Koziol-McLain J, et al. Chart Reviews in Emergency Medicine Research: Where are the Methods? Ann Emerg Med. 1996;27:305–308. doi: 10.1016/s0196-0644(96)70264-0. [DOI] [PubMed] [Google Scholar]
- 22.Quinn J, Kramer N, McDermott D. Validation of the Social Security Death Index (SSDI): An Important Readily-Available Outcomes Database for Researchers. West J Emerg Med. 2008 Jan;9:6–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Haas H. Outils de triage aux urgencies pédiatriques. Archives de Pédiatrie. 2005;12:703–705. doi: 10.1016/j.arcped.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 24.Horeczko T, Enriquez B, McGrath NE, et al. The Pediatric Assessment Triangle: Accuracy of its Application by Nurses in Triage. J Emerg Nurs. 2012 doi: 10.1016/j.jen.2011.12.020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson JL, Smith BL, Jared JD, et al. Prospective Trial of Real-Time Electronic Surveillance to Expedite Early Care of Severe Sepsis. Ann Emerg Med. 2011;57:500–504. doi: 10.1016/j.annemergmed.2010.12.008. [DOI] [PubMed] [Google Scholar]