Abstract
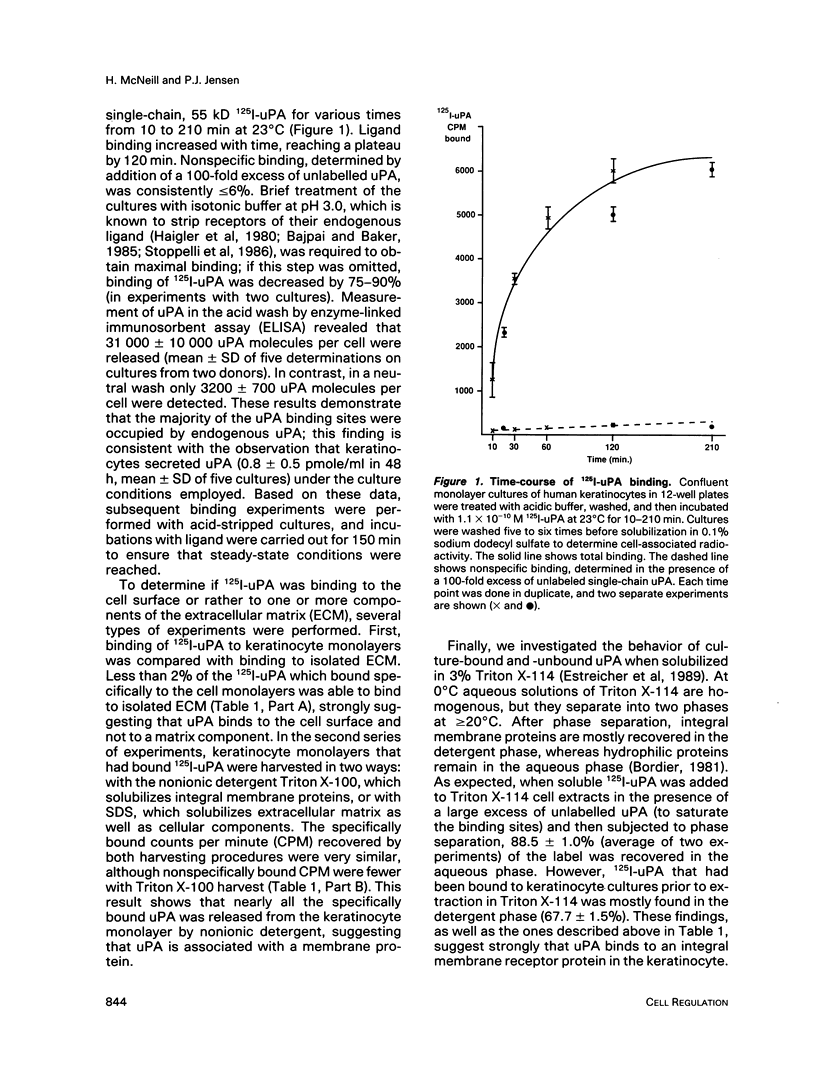
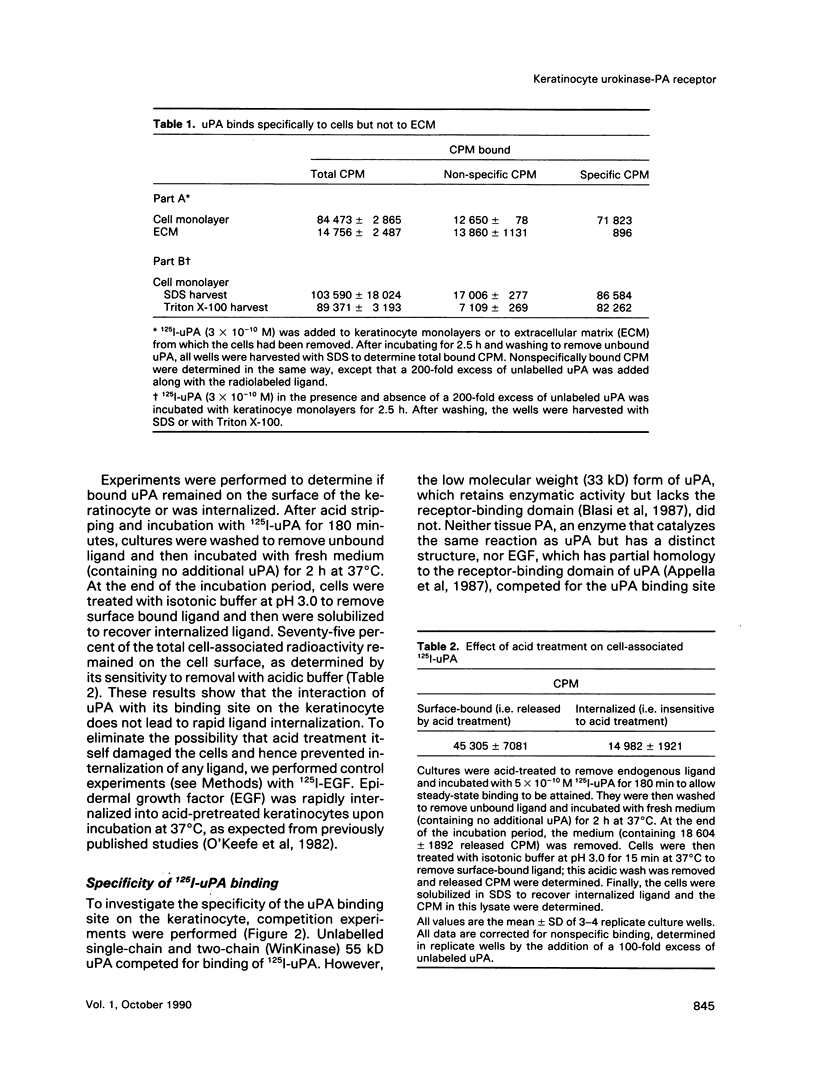
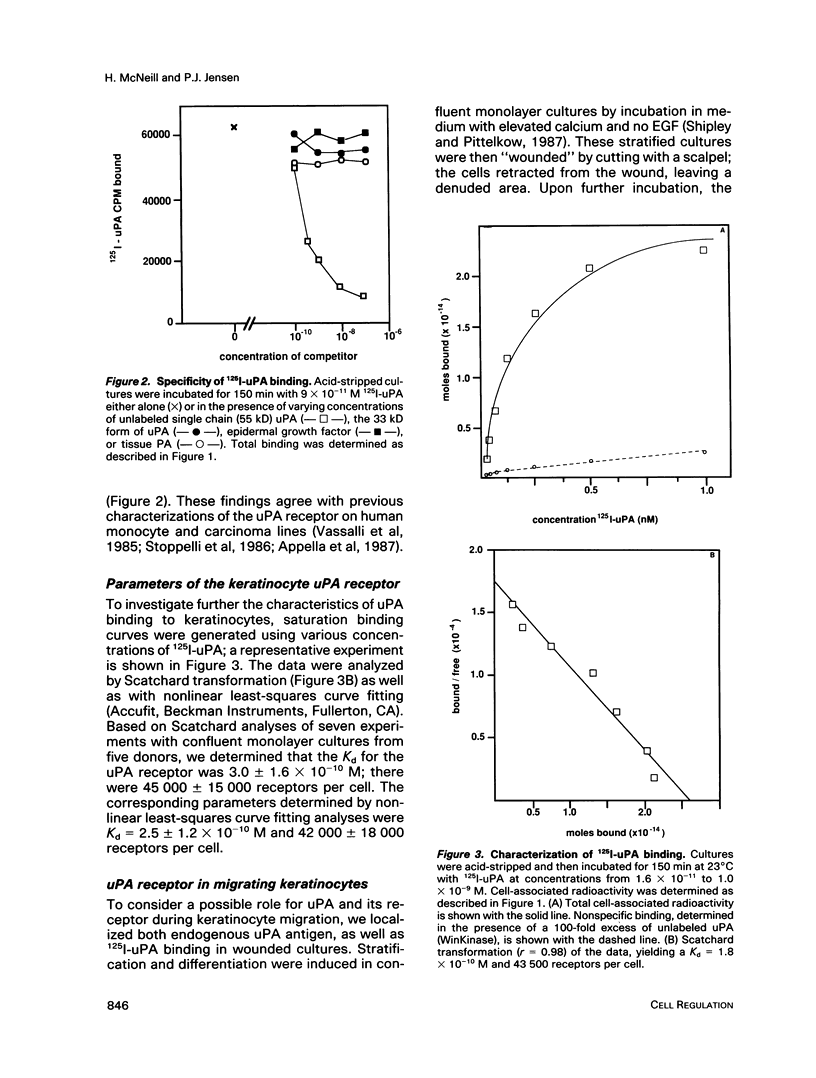
Low passage cultures of normal human keratinocytes produce several components of the plasminogen activator/plasmin proteolytic cascade, including urokinase plasminogen activator (uPA), tissue plasminogen activator (tPA), and two specific inhibitors. Studies here presented demonstrate that these cells also contain a high-affinity (Kd = 3 x 10(-10) M) plasma membrane-binding site for uPA. High molecular weight uPA, either as the single-chain precursor or two-chain activated form, bound to the receptor; however, low molecular weight (33 kD) uPA, tPA, or epidermal growth factor did not compete for binding, demonstrating specificity. Acid treatment, which removed endogenous uPA from the receptor, was required to detect maximal binding (45,000 sites per cell). To investigate the possibility that the uPA receptor on keratinocytes may be involved in epithelial migration during wound repair, cultures were wounded and allowed to migrate into the wounded site. Binding sites for uPA were localized by autoradiographic analysis of 125I-uPA binding as well as by immunocytochemical studies using anti-uPA IgG. With both techniques uPA binding sites were detected selectively on the plasma membrane of cells at the leading edge of the migrating epithelial sheet. This localization pattern suggests that uPA receptor expression on keratinocytes may be coupled to cell migration during cutaneous wounding.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appella E., Robinson E. A., Ullrich S. J., Stoppelli M. P., Corti A., Cassani G., Blasi F. The receptor-binding sequence of urokinase. A biological function for the growth-factor module of proteases. J Biol Chem. 1987 Apr 5;262(10):4437–4440. [PubMed] [Google Scholar]
- Bajpai A., Baker J. B. Cryptic urokinase binding sites on human foreskin fibroblasts. Biochem Biophys Res Commun. 1985 Dec 17;133(2):475–482. doi: 10.1016/0006-291x(85)90931-3. [DOI] [PubMed] [Google Scholar]
- Baker J. B., Low D. A., Simmer R. L., Cunningham D. D. Protease-nexin: a cellular component that links thrombin and plasminogen activator and mediates their binding to cells. Cell. 1980 Aug;21(1):37–45. doi: 10.1016/0092-8674(80)90112-9. [DOI] [PubMed] [Google Scholar]
- Barnhart M. I. Role of blood coagulation in acute inflammation. Biochem Pharmacol. 1968 Mar;(Suppl):205–219. doi: 10.1016/0006-2952(68)90307-9. [DOI] [PubMed] [Google Scholar]
- Blasi F., Stoppelli M. P., Cubellis M. V. The receptor for urokinase-plasminogen activator. J Cell Biochem. 1986;32(3):179–186. doi: 10.1002/jcb.240320303. [DOI] [PubMed] [Google Scholar]
- Blasi F., Vassalli J. D., Danø K. Urokinase-type plasminogen activator: proenzyme, receptor, and inhibitors. J Cell Biol. 1987 Apr;104(4):801–804. doi: 10.1083/jcb.104.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Clark R. A., Lanigan J. M., DellaPelle P., Manseau E., Dvorak H. F., Colvin R. B. Fibronectin and fibrin provide a provisional matrix for epidermal cell migration during wound reepithelialization. J Invest Dermatol. 1982 Nov;79(5):264–269. doi: 10.1111/1523-1747.ep12500075. [DOI] [PubMed] [Google Scholar]
- Cubellis M. V., Nolli M. L., Cassani G., Blasi F. Binding of single-chain prourokinase to the urokinase receptor of human U937 cells. J Biol Chem. 1986 Dec 5;261(34):15819–15822. [PubMed] [Google Scholar]
- Del Rosso M., Dini G., Fibbi G. Receptors for plasminogen activator, urokinase, in normal and Rous sarcoma virus-transformed mouse fibroblasts. Cancer Res. 1985 Feb;45(2):630–636. [PubMed] [Google Scholar]
- Del Rosso M., Fibbi G., Dini G., Grappone C., Pucci M., Caldini R., Magnelli L., Fimiani M., Lotti T., Panconesi E. Role of specific membrane receptors in urokinase-dependent migration of human keratinocytes. J Invest Dermatol. 1990 Mar;94(3):310–316. doi: 10.1111/1523-1747.ep12874442. [DOI] [PubMed] [Google Scholar]
- Eaton D. L., Scott R. W., Baker J. B. Purification of human fibroblast urokinase proenzyme and analysis of its regulation by proteases and protease nexin. J Biol Chem. 1984 May 25;259(10):6241–6247. [PubMed] [Google Scholar]
- Ellis V., Scully M. F., Kakkar V. V. Plasminogen activation initiated by single-chain urokinase-type plasminogen activator. Potentiation by U937 monocytes. J Biol Chem. 1989 Feb 5;264(4):2185–2188. [PubMed] [Google Scholar]
- Estreicher A., Wohlwend A., Belin D., Schleuning W. D., Vassalli J. D. Characterization of the cellular binding site for the urokinase-type plasminogen activator. J Biol Chem. 1989 Jan 15;264(2):1180–1189. [PubMed] [Google Scholar]
- Fibbi G., Ziche M., Morbidelli L., Magnelli L., Del Rosso M. Interaction of urokinase with specific receptors stimulates mobilization of bovine adrenal capillary endothelial cells. Exp Cell Res. 1988 Dec;179(2):385–395. doi: 10.1016/0014-4827(88)90277-7. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Billingham R. E., Burgess L. Distribution of fibronectin during wound healing in vivo. J Invest Dermatol. 1981 Mar;76(3):181–189. doi: 10.1111/1523-1747.ep12525694. [DOI] [PubMed] [Google Scholar]
- Grøndahl-Hansen J., Lund L. R., Ralfkiaer E., Ottevanger V., Danø K. Urokinase- and tissue-type plasminogen activators in keratinocytes during wound reepithelialization in vivo. J Invest Dermatol. 1988 Jun;90(6):790–795. doi: 10.1111/1523-1747.ep12461511. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., Maxfield F. R., Willingham M. C., Pastan I. Dansylcadaverine inhibits internalization of 125I-epidermal growth factor in BALB 3T3 cells. J Biol Chem. 1980 Feb 25;255(4):1239–1241. [PubMed] [Google Scholar]
- Hashimoto K., Wun T. C., Baird J., Lazarus G. S., Jensen P. J. Characterization of keratinocyte plasminogen activator inhibitors and demonstration of the prevention of pemphigus IgG-induced acantholysis by a purified plasminogen activator inhibitor. J Invest Dermatol. 1989 Mar;92(3):310–314. doi: 10.1111/1523-1747.ep12277087. [DOI] [PubMed] [Google Scholar]
- Hébert C. A., Baker J. B. Linkage of extracellular plasminogen activator to the fibroblast cytoskeleton: colocalization of cell surface urokinase with vinculin. J Cell Biol. 1988 Apr;106(4):1241–1247. doi: 10.1083/jcb.106.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isseroff R. R., Rifkin D. B. Plasminogen is present in the basal layer of the epidermis. J Invest Dermatol. 1983 Apr;80(4):297–299. doi: 10.1111/1523-1747.ep12534677. [DOI] [PubMed] [Google Scholar]
- Jensen P. J., John M., Baird J. Urokinase and tissue type plasminogen activators in human keratinocyte culture. Exp Cell Res. 1990 Mar;187(1):162–169. doi: 10.1016/0014-4827(90)90131-s. [DOI] [PubMed] [Google Scholar]
- Kirchheimer J. C., Christ G., Binder B. R. Growth stimulation of human epidermal cells by urokinase is restricted to the intact active enzyme. Eur J Biochem. 1989 Apr 15;181(1):103–107. doi: 10.1111/j.1432-1033.1989.tb14699.x. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Goldfarb R. H., Brundage R., Siegal G. P., Terranova V., Garbisa S. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 1981 Nov;41(11 Pt 1):4629–4636. [PubMed] [Google Scholar]
- Loskutoff D. J. Effects of acidified fetal bovine serum on the fibrinolytic activity and growth of cells in culture. J Cell Physiol. 1978 Sep;96(3):361–369. doi: 10.1002/jcp.1040960312. [DOI] [PubMed] [Google Scholar]
- Low D. A., Baker J. B., Koonce W. C., Cunningham D. D. Released protease-nexin regulates cellular binding, internalization, and degradation of serine proteases. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2340–2344. doi: 10.1073/pnas.78.4.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks R., Nishikawa T. Active epidermal movement in human skin in vitro. Br J Dermatol. 1973 Mar;88(3):245–248. doi: 10.1111/j.1365-2133.1973.tb07542.x. [DOI] [PubMed] [Google Scholar]
- Morioka S., Jensen P. J., Lazarus G. S. Human epidermal plasminogen activator. Characterization, localization, and modulation. Exp Cell Res. 1985 Dec;161(2):364–372. doi: 10.1016/0014-4827(85)90093-x. [DOI] [PubMed] [Google Scholar]
- Morioka S., Lazarus G. S., Baird J. L., Jensen P. J. Migrating keratinocytes express urokinase-type plasminogen activator. J Invest Dermatol. 1987 Apr;88(4):418–423. doi: 10.1111/1523-1747.ep12469754. [DOI] [PubMed] [Google Scholar]
- Nielsen L. S., Kellerman G. M., Behrendt N., Picone R., Danø K., Blasi F. A 55,000-60,000 Mr receptor protein for urokinase-type plasminogen activator. Identification in human tumor cell lines and partial purification. J Biol Chem. 1988 Feb 15;263(5):2358–2363. [PubMed] [Google Scholar]
- O'Keefe E., Battin T., Payne R., Jr Epidermal growth factor receptor in human epidermal cells: direct demonstration in cultured cells. J Invest Dermatol. 1982 Jun;78(6):482–487. doi: 10.1111/1523-1747.ep12510246. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Allen T. D. The fine structure and cell kinetics of mouse epidermis after wounding. J Cell Sci. 1975 Mar;17(3):413–447. doi: 10.1242/jcs.17.3.413. [DOI] [PubMed] [Google Scholar]
- Pöllänen J., Saksela O., Salonen E. M., Andreasen P., Nielsen L., Danø K., Vaheri A. Distinct localizations of urokinase-type plasminogen activator and its type 1 inhibitor under cultured human fibroblasts and sarcoma cells. J Cell Biol. 1987 Apr;104(4):1085–1096. doi: 10.1083/jcb.104.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley J. P., Gold L. I., Schwimmer R., Sullivan L. M. Limited cleavage of cellular fibronectin by plasminogen activator purified from transformed cells. Proc Natl Acad Sci U S A. 1987 May;84(9):2776–2780. doi: 10.1073/pnas.84.9.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O. Plasminogen activation and regulation of pericellular proteolysis. Biochim Biophys Acta. 1985 Nov 12;823(1):35–65. doi: 10.1016/0304-419x(85)90014-9. [DOI] [PubMed] [Google Scholar]
- Schleef R. R., Podor T. J., Dunne E., Mimuro J., Loskutoff D. J. The majority of type 1 plasminogen activator inhibitor associated with cultured human endothelial cells is located under the cells and is accessible to solution-phase tissue-type plasminogen activator. J Cell Biol. 1990 Jan;110(1):155–163. doi: 10.1083/jcb.110.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppä H. E., Yamada K. M., Seppä S. T., Silver M. H., Kleinman H. K., Schiffmann E. The cell binding fragment of fibronectin is chemotactic for fibroblasts. Cell Biol Int Rep. 1981 Aug;5(8):813–819. doi: 10.1016/0309-1651(81)90253-8. [DOI] [PubMed] [Google Scholar]
- Stephens R. W., Pöllänen J., Tapiovaara H., Leung K. C., Sim P. S., Salonen E. M., Rønne E., Behrendt N., Danø K., Vaheri A. Activation of pro-urokinase and plasminogen on human sarcoma cells: a proteolytic system with surface-bound reactants. J Cell Biol. 1989 May;108(5):1987–1995. doi: 10.1083/jcb.108.5.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppelli M. P., Corti A., Soffientini A., Cassani G., Blasi F., Assoian R. K. Differentiation-enhanced binding of the amino-terminal fragment of human urokinase plasminogen activator to a specific receptor on U937 monocytes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4939–4943. doi: 10.1073/pnas.82.15.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppelli M. P., Tacchetti C., Cubellis M. V., Corti A., Hearing V. J., Cassani G., Appella E., Blasi F. Autocrine saturation of pro-urokinase receptors on human A431 cells. Cell. 1986 Jun 6;45(5):675–684. doi: 10.1016/0092-8674(86)90782-8. [DOI] [PubMed] [Google Scholar]
- Thiery J. P. Mechanisms of cell migration in the vertebrate embryo. Cell Differ. 1984 Nov;15(1):1–15. doi: 10.1016/0045-6039(84)90024-1. [DOI] [PubMed] [Google Scholar]
- Vassalli J. D., Baccino D., Belin D. A cellular binding site for the Mr 55,000 form of the human plasminogen activator, urokinase. J Cell Biol. 1985 Jan;100(1):86–92. doi: 10.1083/jcb.100.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Mainardi C. L., Vater C. A., Harris E. D., Jr Endogenous activiation of latent collagenase by rheumatoid synovial cells. Evidence for a role of plasminogen activator. N Engl J Med. 1977 May 5;296(18):1017–1023. doi: 10.1056/NEJM197705052961801. [DOI] [PubMed] [Google Scholar]




