Abstract
Background
In northeastern Italy, according to Italian legislation, authorized public facilities can accept the donation and preservation of cord blood stem cells (CB-SC). Attitudes and knowledge in pregnant women differs between the local and immigrant (non-European Union [EU]) population. In this study we assessed the choices that pregnant women have with respect to the public and private harvesting system and the main reasons driving their decisions. We examined the ethnic origin of the families and compared tests for syphilis screening and leukocyte (WBC) counts in the CB-SC bags that are required for validation of the collection.
Methods
Out of a population of 3450 pregnant patients at the Institute for Maternal and Child Health of Trieste, northeast Italy, 772 women agreed to cord blood harvesting and the associated lab tests. Of these, 221 women (28.6%) were from immigrant families of non-EU countries. Their ethnic affiliation was recorded, and tests were performed for syphilis screening and for nucleated red blood cell (NRBC) interference with the WBC count in CB-SC bags to assess cellularity and to determine if storage was appropriate.
Results
Of the 772 pregnant women, 648 (84.0%) accessed the public collection system, which is free of charge, and 124 (15.0%) accessed the private fee-based system. One woman from the non-EU group opted for the private fee-based system. Of the 3450 pregnant women screened for syphilis at the Institute for Maternal and Child Health, the Treponema pallidum hemagglutination (TPHA) and Venereal Disease Research Laboratory (VDRL) tests were the main tests performed (66.0% of total cases) because many gynecologists in the public harvesting system apply the Italian regulations of the 1988 Decree, while the private system requires tests on syphilis and leaves the option to the lab physicians to select the best determination method. We found that the chemiluminescence method was more specific (97.0%) than the TPHA (83.0%) and nontreponemal rapid plasma reagin VDRL (75.0%) tests (P < 0.05, χ2 test). The specificity link between the two automatic methods versus microscopes for WBC dosing and NRBC interference was r2 = 0.08 (ADVIA 120) and r2 = 0.94 (XE-2100). The public system does not include human T-cell lymphotropic virus testing; this is reserved for the population from endemic zones.
Conclusion
In northeastern Italy current legislation prevents the establishment of private fee-based banks for storage of CB-SC. The cryopreservation, for future autologous personal or family use, is possible only by sending to foreign private banks, with a further fee of €300. These regulations confirm that Italian legislation tries to increase the anonymous allogenic donations and the number of CB-CS bags stored in the free-cost public system, that are available to anyone with therapeutic needs. Private banking is used almost exclusively by the wealthier local population. In the public system, many physicians continue to use older Italian laws regarding syphilis diagnosis, and NRBC interference on WBC count may have an impact on cord blood harvesting. Our findings suggest that in the EU there is no consensus policy on donor management. The value of storage for potential use within the family is useful only with collaboration between the public and the private systems.
Keywords: cord blood collection, public system, private system, pregnant women’s choice
Introduction
Over the past 20 years, the use of cord blood stem cells (CB-SCs) for transplants (first used by Gluckmann in 1988) has increased.1–6 The use of this alternative hemopoietic source has practical, biologic, and clinical advantages for donors and recipients: prompt availability upon request (a few weeks for placenta blood units versus a number of months for bone marrow donors); provision for ethnic minorities, who account for a low percentage on bone marrow registers; no risk of anesthesia for donors or stimulation with Granulokine®; lower risk of transmissible infective diseases; partially compatible transplants may be performed; and lower immunogenicity and lower risk of severe post-transplant immune response, such as Graft-versus-host disease (GVHD), which is the cause of a high post-transplant death rate.7,8 In contrast to these advantages, the disadvantage of the cord blood is the limited content of hemopoietic stem cells, and consequently it is mainly used for pediatric patients. With a view to overcoming this major barrier, transplants of multiple cord units have recently been performed and have achieved encouraging results.45 The infusion of two cord units is equal to the stem cell dose for adult patients.9,10 Research has been carried out on in vitro cord stem cell expansion techniques to obtain a sufficiently high number of cells for the reconstruction of adult marrow as well as alternative infusion techniques, such as bone infusion.11–13
After the establishment of the first banks in New York State (USA, 1991), Dusseldorf (Germany, 1992), and Milan (Italy, 1993), many public cord blood banks were established all over the world. In Europe, a public collection program was launched (18 banks in Italy, 37 in 21 countries) alongside the private collection (16 cord blood banks in Europe). More than 780,000 cord blood units are stored in over 130 private cord blood banks worldwide, and over 400,000 are stored in more than 100 quality controlled public cord blood banks.14–16
In Italy and in France, current regulations prevent the establishment of private banks. For the harvesting of placenta blood for private use and for sending to foreign private banks, hospital authorization is required for countries allowing banking and cryogenic storage (Decree no 62/CSR/2010).46 The Friuli Venezia Giulia Region (FVGR) has adopted a further provision against collection for private purposes (initial set-up cost €2000), introducing a further fee of €300 and mandatory viral tests for expectant mothers to be performed at least 30 days before delivery (for hospital authorization [Decree no 2321/2010]).47 Voluntary public donation is the basis for the success of unrelated CB-SC transplantation programs. In contrast, in other EU countries, such as England, Spain, Germany, and Switzerland, aggressive marketing techniques by for-profit banks offer collection and personal storage as biologic insurance against future life-threatening conditions. In this private system, validation test updating is faster. CB-SC stored in private banks for a future transplant or therapy (for a related family member) are not searchable by the public system. Indeed the major critical issue of public banks is the nonavailability of the CB-SC.
The primary aim of this study was to assess the choices of families, the ethnic composition of the sample, and the main reasons for familial decisions as well as to monitor the increasing amount of placenta blood in the public sector for nonprofit purposes (available to anyone with therapeutic needs), and in the private/individual profit-making sector. A secondary aim of this study was to examine some of the tests that are required to validate the collection of placenta blood in the public and private systems in terms of screening methods for syphilis (which has been increasing as a result of immigration from Eastern Europe) and erythroblast interference in leukocyte counts in placenta blood with respect to the minimum cellularity requirement on banking access (leukocyte [WBC] >1.2 × 109/L).48
Syphilis and WBC testing
Syphilis is a reemerging disease, and serological tests are considered to be a milestone in syphilis control. In Italy the incidence of syphilis has recently increased, and the availability of automated systems with improved test specificity has reduced time and labor for syphilis testing. Many gynecologists apply the provisions of the Italian Regulation of 1998 (Decree of October 10, 1998, no 245)49 and require pregnant women to have Treponema pallidum hemagglutination (TPHA) or Venereal Disease Research Laboratory (VDRL) screening. In contrast, the private banks with CB-SC storage require tests for syphilis, leaving the option to the lab physicians to select the best determination method with chemiluminescence method (CLIA) as it is more sensitive and specific.17–21
Collection for cord blood banking is validated with a WBC count that is >1.2 × 109/L, in line with the principles agreed by the Foundation for the Accreditation of Cellular Therapy and the American Society for Blood and Marrow Transplantation.22,23 This is a mandatory requirement for public banks, and if the cell count is below this threshold, cord blood is not stored and the bag is discarded. In the private system, however, it is the family’s decision to decide whether to store cord blood on the basis of the expected future development of expansion techniques. It is important to perform a proper WBC count as placenta blood contains a high percentage of nucleated red blood cells (NRBCs; 0.03–4.8 × 109/L) and their presence may affect the WBC count, resulting in an inaccurate elevation of the count.24,25 Peripheral NRBC counting by automated hematology analyzers has developed in recent years and has been applied in laboratory practice. In contrast to the conventional (manual microscopic) method, the number of counted cells is usually as few as 100 cells, causing problems in terms of accuracy and precision. Analyzers differ in terms of methods and assessment of NRBC “alarms.” The accuracy of the dosing in specific reading channels or cluster analysis determined in the cytochemical reaction of the unstained region of the peroxidase channel versus determination in the baso channel has been improving.26–28 For our study, we used two last-generation hematology instruments, which are the most popular in Italy and in Europe, for the validation of 100 placenta blood samples.
Methods
Our study sample consisted of 3450 pregnant women who responded to questionnaires. Of these, 772 women, who had given birth from September 2010 to September 2012, were studied by questionnaires at the Hospital for Maternal and Child Health of Trieste (capital of FVGR) to determine their reasons for their choice of public or private sector, and to establish the family nationality. This was a sample of women (in particular from North Africa and East Europe) based on the availability of personnel at the Institute. The research was approved by the Hospital Bioethics Committee of the Institute of Maternal and Child Health. The interview was confidential and concerned the patients’ country of origin, socio-economic status, modality of information about CB-SC preserving (physician, obstetric, friends, medical journals, internet), motivations, and presence of genetically determined disease in the family. To validate placenta blood, syphilis screening of expectant mothers was performed with the following tests and methods: VDRL (Veneral Disease Reagin nontreponemal Research Laboratory) (RPR FAR, Verona, Italy), TPHA (Treponema Palladium HAEMOAG-GLUTINATION, FAR) and SYP-CLIA (CLIA [chemiluminescence], Abbott, Rome, Italy). Western blot immunoglobulin (Ig)G-IgM (Bio-Rad Laboratories, Padua, Italy) was used for the control. Erythroblast dosing in the placenta blood for the detection of interference on leukocyte count was performed with two automated hematology systems (ADVIA 120; Siemens, Milan, Italy, for routine analysis; XE-2100; Sysmex, Milan, Italy, for analysis at night or over holiday periods) and an NRBC alarm in a count range between 2% and 50%. Results were compared with a manual microscope count that was performed under National Committee for Clinical Laboratory Standards (NCCLS) H20-A1 guidelines by two operators. At delivery, an umbilical cord venous blood sample was taken after cord clamping and was placed immediately into ethylenediaminetetraacetic acid (EDTA) tripotassium (K3) tubes (KIMA, Azergrande, Padua, Italy) to be analyzed with the automated hematology cell-counting machines (Siemens, Milan, Italy). The different specificity and alarms of 50 samples of cord blood were assessed, and the data were examined using the χ2 test.
Results
The FVGR informs pregnant women and their families about CB-SC harvesting by using posters in hospitals and the assistance of voluntary associations and their interpreters. However, educational differences exist between EU and non-EU populations, and “collection” is a concept difficult to explain and understand.
Out of the 772 pregnant women who agreed to placenta blood collection, 124 (16.0%) chose the private system and the remaining 648 (84.0%) chose the public system. The main reasons for choosing the private system were: (a) autologous transplant or transplant for the family and/or for the future treatment of nonhematological diseases (genetic and multiorgan illnesses) that require nonstandardized therapies (52.4%); (b) the highest degree of test safety and cryologic storage (24.1%); (c) the fact that, in the public system, cord blood is not collected if delivery occurs during holiday periods (13.7%); and (d) previous experiences with no collection in the public system as a result of organization issues (8.0%) (Figure 1). Reasons for collections for future treatments included the possibility of treating metabolic pathologies, such as diabetes (50.0%), celiac disease (19.0%), cystic fibrosis (18.0%), or cardiovascular disease (8.0%) in addition to degenerative diseases, such as Alzheimer’s (5.0%) (Figure 2). In contrast, the relevant factors for the public choice were: (a) more confidence in the public sanitary system (27.4%); (b) advice from friends (20.1%) and obstetricians (16.1%); and (c) no specific motivation (36.2%).
Figure 1.
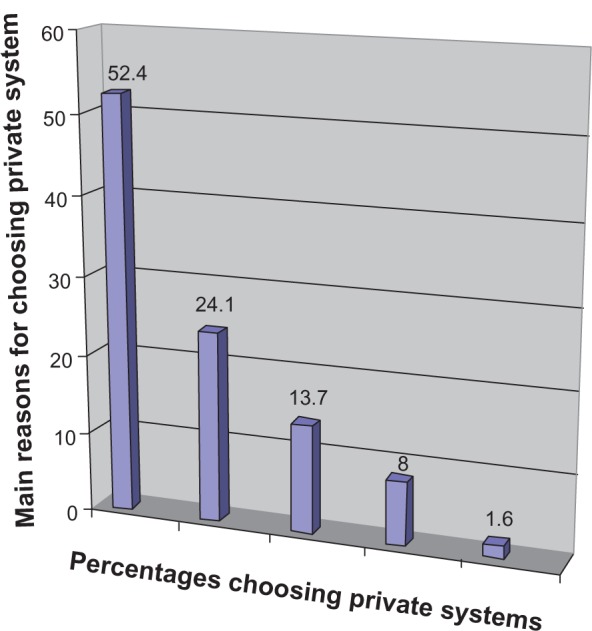
Main reasons for choosing private system on 124 cases: 52.4% non hematological diseases, 24.1% highest degree of safety test, 13.7% non collection in festive or pre-festive days, 8.0% technical problems in previous experience, 1.6% other.
Figure 2.
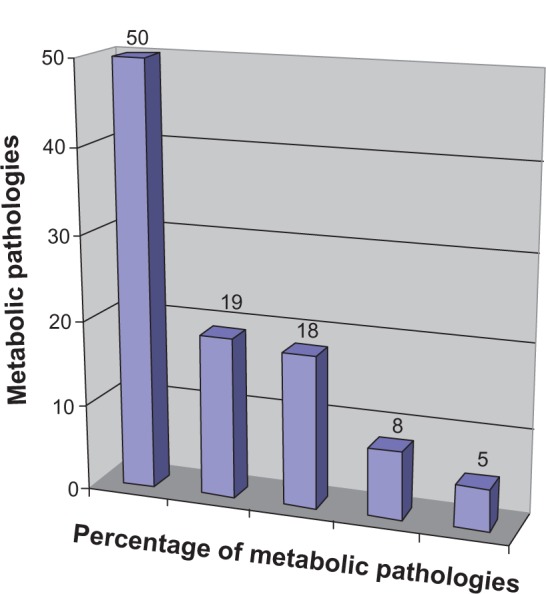
Metabolic pathologies of 65 cases.
Notes: 50% diabetes, 19% celiac disease, 18% cystic fibrosis, 8% cardiovascular disease, 5% degenerative diseases, such as Alzheimer’s disease.
Of the 772 interviewed pregnant women, 221 (28.6%) were from non-EU countries: 150 (68.0%) from Eastern Europe, 33 (15.0%) from Asia, 26 (11.8%) from Africa, and 11 (5.0%) from South America (Table 1). Out of these non-EU immigrant families, only one chose the private fee-based system. Among non-EU populations the major deciding factor was economic so only the public collection was a possibility, because it was free of cost.
Table 1.
Total and percentages of immigrant subjects according to area of origin
| Origin | Total numbers | Percentage |
|---|---|---|
| Africa | 26 | 11.8% |
| Asia | 33 | 15.00% |
| Latin America | 11 | 5.00% |
| East Caucasus | 11 | 5.00% |
| West Caucasus | 139 | 62.9% |
| Total | 221 | 28.6% |
Required tests for the validation of placental blood and collection authorization and viral tests did not show any difference in the two validation systems (negative result for B and C hepatitis and human immunodeficiency virus tests). The private system also requires the T-cell lymphotropic (HTLV I–II) test, which in the public system is only required for individuals who have travelled or lived in endemic areas.
Our lab research showed good specificity (97.0%) for the CLIA method for syphilis screening; this was higher than the TPHA (83.0%) and VDRL (75%) tests, which are mainly used in the public system (66.0% of total cases). Western blot IgG–IgM was used for controls (Table 2). Our data showed a statistically significant difference comparing CLIA method and Western blot IgG-IgM, in agreement with the international literature (P < 0.05).29,30
Table 2.
Method and diagnostic reagent for syphilis screening of 3450 patients
| Method and reagent | Total number (%) | Number in agreement with WB | % agreement with WB |
|---|---|---|---|
| Architect CLIA* | 42 (12) | 41 | 0.97 |
| TPHA | 36 (10) | 30 | 0.83 |
| VDRL | 29 (8) | 22 | 0.75 |
Notes: Western blot IgG-IgM (WB) was used for Controls.
Statistically significant difference P < 0.05.
Abbreviation: IgG, immunoglobulin G; TPHA, treponemal hemoagglutination; VDRL, veneral disease nontreponamal reagin laboratory.
Out of the 3450 pregnant women registered at the hospital over a 2-year period, syphilis (lues venerea) was diagnosed in three women from Eastern Europe who were positive to TPHA dosing (requested by the gynecologist) and had positive confirmation from Western blot IgG–IgM analysis. There were also 20 false-positive results with VDRL and TPHA tests (Table 3).
Table 3.
Total numbers and pathologies of false-positive reactions in serologic tests for syphilis in 3450 cases
| Disease | VDRL false positive | TPHA false positive | CLIA false positive |
|---|---|---|---|
| Autoimmune disease | 3 | 2 | – |
| Drug abuse | 3 | – | – |
| Lyme disease | – | 2 | – |
| Pregnancy | 6 | 4 | – |
Note: Total numbers.
Abbreviations: CLIA, Architect CLIA; TPHA, treponemal hemoagglutination; VDRL, veneral disease nontreponamal reagin laboratory.
The specificity between WBC dosing and NRBC interference from 50 tests using two automatic methods versus a microscope was: r2 = 0.08 (ADVIA 120) and r2 = 0.94 (XE-2100). The NRBC alarm of both instruments was exceeded in 25.0% of the tests (Figure 3).
Figure 3.
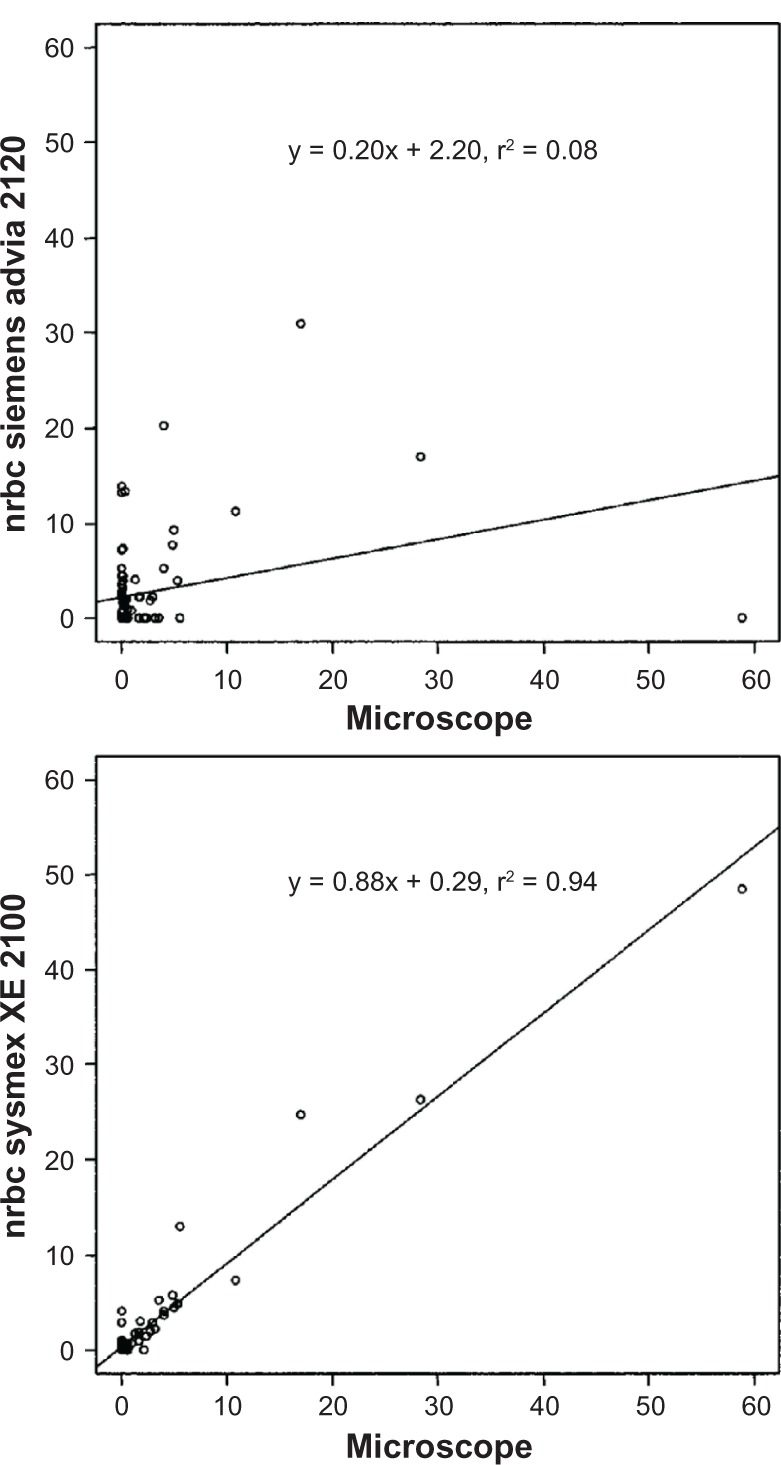
White blood cell dosing and nucleated red blood cell interference: specificity between two automatic analyzers and microscope count.
Note: Y and X axes: units of measurement: 103 μL.
Abbreviation: NRBC, nucleated red blood cell.
Discussion
This research showed that the public system prevails in northeastern Italy and that private banking is only used by the local population not immigrants from non-EU countries. The non-EU population, because of their low economic conditions and the difficulty understanding the Italian language, can only afford the public system that is free of cost. In fact, the private system has an additional fee imposed by the Regional Authority and other costs for storage in foreign banks. Immigrants are occupied by different, often economic as well as current problems and do not want to consider hypothetical future issues such as the use of CB-SC. Professionals and public institutions should make an effort to provide unbiased information and education about both CB-SC donation and preservation, focusing on the utilization of stem cells; in particular, obstetricians should encourage CB-SC storage, providing detailed information especially to pregnant women and to future parents.
The performance of an additional and more accurate TPHA test for screening syphilis is not taken into account by physicians applying the Italian Regulation of 1998 on the protection of responsible pregnancy and the use of TPHA or VDRL tests.29,30 Consequently, out-of-date regulations, use of older technology, and the slow changes to Italian regulations means that screening for syphilis is not based on the most advanced methods.31
In the public system, NRBC interference on the WBC count may have an impact on cord blood collection, particularly if the lab does not require any external quality audit, and collections that could be banked may instead be discarded; in these cases, a microscope control count is recommended.32 In the private system, the final decision on storage in the event of insufficient cellularity is left to the family.
Conclusion
In France and Italy CB-SC cryopreservation is only permitted in the public system. The bag is at the disposal of any patient requiring it, without discrimination; or for adhoc use (family member with genetic disease that can be treated with hemopoietic stem cell transplantation, according to international protocols).33–35
Aggressive advertising by private fee-based banks about the use of CB-SCs for private use and the opportunity to access further regenerative treatments that are still in the clinical trial phase along with the organizational difficulties of the public system (collection is not performed during holiday periods) have resulted in an increase in private collections in the EU. Private cord banks often publish lists of diseases that can be treated by CB-SCs but do not always provide clear information about the difference between autologous and allogenic transplants.
Tests for validation and storage of bags are based only on internationally recognized reliability criteria, although the private system is faster in adapting tests and procedures, while public systems, which are regulated by national laws, are stricter and adapt more slowly. The Association of Blood Donors (Italy and Switzerland) and nonprofit sports associations (ie, Circolo Marina Mercantile, Trieste, Italy) are very active in raising awareness on public free of cost based collection with events and conferences.36,37 The debate on public collection (mainly for the lower income population or for groups who are more sensitive to ethical and solidarity issues) and private collection (mainly for the wealthier population) should not be reduced to the support of State monopolies, rather the debate should address sufficient and effective means to treat future patients. In general, scientific opinions and the lack of comparative studies point out the importance of public cord banks for allogenic transplantation and underline that, at present, the autologous use of CB-SC is limited.38,39 Public–private partnerships can have the added value of storage for potential use within the family as well as for autologous and related allogenic uses.40–44
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gluckman E, Broxmeyer HE, Arlen D, et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical cord blood from an HLA identical sibling. N Engl J Med. 1989;321:1174–1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 2.Gluckman E, Rocha V. Cord blood transplantation: state of the art. Haematologica. 2009;94:451–454. doi: 10.3324/haematol.2009.005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Directorate for the Quality of Medicines and Health Care of the Council of Europe (EDOM) Guide to the preparation, use and quality assurance of blood components. Recommendation No R(95) 15 Available at http://www.eubis-europe.eu/documents.phpAccessed March 9, 2012
- 4.Ballen KK. Challenges in umbilical cord blood stem cell banking for stem cell reviews and reports. Stem Cell Rev. 2010;6:8–14. doi: 10.1007/s12015-009-9105-x. [DOI] [PubMed] [Google Scholar]
- 5.Lubin BH, Shearer WT. Cord bank banking for potential future transplantation. Pediatrics. 2007;119(1):165–170. doi: 10.1542/peds.2006-2901. [DOI] [PubMed] [Google Scholar]
- 6.Querol S, Rubinstein P, Marsh S, et al. Cord blood banking: “providing cord blood banking for a nation”. Br J Haematol. 2009;147:227–235. doi: 10.1111/j.1365-2141.2009.07818.x. [DOI] [PubMed] [Google Scholar]
- 7.Roha V, Wagner JE, Sobocinski KA, et al. Graft-versus-host disease in children who have received a cord blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N Engl J Med. 2000;342(25):1846–1854. doi: 10.1056/NEJM200006223422501. [DOI] [PubMed] [Google Scholar]
- 8.Bone Marrow Donors Worldwide. [homepage on the Internet] Available from: http://www.bmdw.orgAccessed June 26, 2011
- 9.Laughin MJ, Barker J, Bambach B, Koc ON, Rizzieri DA, et al. Hematopoietic engraftment and survival in adult recipients of umbilical cord blood from unrelated donors. N Engl J Med. 2001;344:1815–1822. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 10.Barker JN, Weisdorf DJ, DeFor YE, Blazar BR. Transplantation of two partially HLA-matched umbilical cord blood units to enhance engraftment in adult with haematological malignancy. Blood. 2005:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 11.Yang Li, Teng Ma, Douglas A, et al. Human cord cell hematopoiesis in three-dimensional nonwoven fbrous matrices: in vitro stimulation of the marrow microenvironment. J Hematother and Stem Cells Research. 2004;10:355–368. doi: 10.1089/152581601750288966. [DOI] [PubMed] [Google Scholar]
- 12.De Angeli S, Di Liddo R, Buoro S, et al. New immortalized human stromal cell lines enhacing in vitro expansion of cord blood hematopoietic stem cells. Int J Mol Med. 2004;13:363–371. [PubMed] [Google Scholar]
- 13.Delaney C, Heimfeld S, Brashem-Stein C, et al. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nature Medicine. 2010;16:232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navarrete C, Contreras M. Cord blood banking: a historical perspective. Br J Haematol. 2009;147:236–245. doi: 10.1111/j.1365-2141.2009.07827.x. [DOI] [PubMed] [Google Scholar]
- 15.Wagner JE, Gluckman E. Umbilical cord blood transplantation: the first 20 years. Semin. Hematol. 2010;47:3–12. doi: 10.1053/j.seminhematol.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Butler MG, Menitove JE. Umbilical cord blood banking: an update. J Assist Reprod Genet. 2011;28:669–676. doi: 10.1007/s10815-011-9577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullick S, Watson-Jones D, Beksinska M, Mabey D. Sexually transmitted infections in pregnancy: prevalence, impact on pregnancy outcomes, and approach to treatment in developing countries. Sex Transm infec. 2005;81:294–302. doi: 10.1136/sti.2002.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bush L. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700–708. doi: 10.1086/655832. [DOI] [PubMed] [Google Scholar]
- 19.Binnicker MJ, Jespersen DJ, Rollins LO. Treponema specific tests for serodiagnosis of Syphilis: comparative evaluation of several assays. J Clin Microbiol. 2011;49:1313–1317. doi: 10.1128/JCM.02555-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joung H, Pryde J, Duncan L, Dave J. The Architect Syphilis assay for antibodies to Treponema pallidum: an automated screening assay with high sensitivity in primary syphilis. Sex Transm Infect. 2009;85:19–23. doi: 10.1136/sti.2008.031872. [DOI] [PubMed] [Google Scholar]
- 21.Giuliani M, Suligoi B, STD Surveillance Working Group Sentinel surveillance of sexually transmitted diseases in Italy. EURO Surveillance. 1998;6:55–58. doi: 10.2807/esm.03.06.00097-en. [DOI] [PubMed] [Google Scholar]
- 22.Kara Walker., editor. NETCORD–FACT International Standards for Cord Blood Collection. Processing, Testing, Banking, Selection and Release. 5th ed. Sep, 2012. [Google Scholar]
- 23.Wall DA. Regulatory issues in cord blood banking and transplantation. Best Pract Res Clin Haematol. 2010:171–177. doi: 10.1016/j.beha.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Rolfo A, Maconi M, Cardaropoli S, et al. Nucleated red blood cells in term fetuses: references values using an automated analyzer. Neonatology. 2007;92:205–208. doi: 10.1159/000102096. [DOI] [PubMed] [Google Scholar]
- 25.Shaefer M, Rowan RM. The clinical relevance of nucleated red blood cell counts. Sysmex J International. 2000;10:59–63. [Google Scholar]
- 26.Janyling Li. The preliminary study of nucleated red blood cell counting by automated analyzer. Sysmex J International. 2004:13–17. [Google Scholar]
- 27.Kratz A, Maloum K, O’Malley C, et al. Enumeration of nucleated red blood cells with the ADVIA 2120 hematology system: an international multicenter trial. Lab Hematol. 2006;12:63–70. doi: 10.1532/LH96.06010. [DOI] [PubMed] [Google Scholar]
- 28.Cappelletti P. Analytical performances and clinical aims in laboratory haematology. I J La M. 2008:90–94. [Google Scholar]
- 29.Marangoni A, Moroni S, Accardo S, Cevenini R. Diagnosis of syphilis with automated immunoassay. J Clin Lab Anal. 2009;23:1–6. doi: 10.1002/jcla.20268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipinski D, Licita S, Vered K, Bracha S. Screening for Syphilis: validation of the reverse sequence screening. J Clin Microbiol. 2012 doi: 10.1128/JCM.06286-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giuliani M. Legislation about sexually transmitted diseases in Europe. Ann Ist Super Sanità. 2000;36:409–415. [PubMed] [Google Scholar]
- 32.Korst LM, Phelan J P, Ock Ahn M, et al. Nucleated red blood cells: an update on the marker for fetal asphyxia. Am J Obstet Gynecol. 1996;175:843–846. doi: 10.1016/s0002-9378(96)80010-x. [DOI] [PubMed] [Google Scholar]
- 33.Burgio GR, Gluckman E, Locatelli F, et al. Ethical reappraisal of 15 years of cord blood transplantation. Lancet. 2003;361:250–252. doi: 10.1016/S0140-6736(03)12276-3. [DOI] [PubMed] [Google Scholar]
- 34.European Group on Ethics in Science and New Technologies Opinion 19. Ethical aspects of umbilical cord blood banking. March 16, 2004. Accompanying document Available from: http://ec.europa.eu/bepa/european-group-ethics/docs/publ_op19_en.pdfAccessed on January 11, 2011
- 35.France: Comité Consultantif National d’Ethique pour les sciences de la vie et de la santé (National Ethics Advisory Committee for the Life-Sciences and Health). Opinion 74 – Umbilical cord blood banks for autologous use or research December122002Available from: http://www.ccne-ethique.fr/en/avis074.pdfAccessed on January 11, 2011
- 36.Riva S, Dandri G, Sollecito E, et al. Sport and blood donation: solidarity and health. Homepage on the Internet. EcodelMare Available from: http://www.circolomarinamercantile.it/Accessed September 30, 2011
- 37.Cavo A, Parco S.Sport and blood donors. Physician-sporting information. Homepage on the Internet. AVIS NEWS Available from: http://www.avisfriuliveneziagiulia.it/Accessed on July 25, 2011
- 38.Sun J, Allison J, McLaughlin C, et al. Differences in quality between privately and publicly banked umbilical cord blood units: a pilot study of autologous cord blood infusion in children with acquired neurologic disorders. Transfusion. 2010;50:1980–1987. doi: 10.1111/j.1537-2995.2010.02720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cord blood banking for potential future transplantation American academy of pediatrics policy statement. Pediatrics. 2007;19:165–170. doi: 10.1542/peds.2006-2901. [DOI] [PubMed] [Google Scholar]
- 40.Danzer E, Holzgreve W, Troeger C, et al. Attitudes of Swiss mothers toward unrelated umbilical cord blood banking 6 months after donation. Transfusion. 2003;43:604–608. doi: 10.1046/j.1537-2995.2003.00375.x. [DOI] [PubMed] [Google Scholar]
- 41.Manegold G, Meyer-Monard S, Tichelli A, et al. Controversies in hybrid banking: attitudes of Swiss public umbilical cord blood toward private and public banking. Arch Gynecol Obstet. 2011;284(1):99–104. doi: 10.1007/s00404-010-1607-x. [DOI] [PubMed] [Google Scholar]
- 42.Gluckman E, Ruggeri A, Rocha V, et al. Family-directed umbilical cord banking. Haematologica. 2011;96(11):1700–1708. doi: 10.3324/haematol.2011.047050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrini C. Umbilical cord blood collection, storage and use: ethical issue. Blood Transfus. 2010;8(3):139–148. doi: 10.2450/2010.0152-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fish NM, Atun R. Public-private partnership in cord blood banking. Brit Med J. 2008;336(7645):642–644. doi: 10.1136/bmj.39489.454699.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brunstein CG, Wagner JE. Umbilical cord blood transplantation and banking. Annu Rev Med. 2006;57:403–407. doi: 10.1146/annurev.med.57.051804.123642. [DOI] [PubMed] [Google Scholar]
- 46.Petrini C. A comparative analysis of the opinions from European national and international ethics committees regarding the collection, storage and use of umbilical cord blood. Blood Transfusion. 2012;3:279–289. doi: 10.2450/2012.0172-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parco S, Vascotto F. Autologous cord blood harvesting in North Eastern Italy: ethical questions and emerging hopes for curing diabetes and celiac disease. International Journal of General Medicine. 2012;5:511–516. doi: 10.2147/IJGM.S31977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suligoi B, Giuliani M. Sexually transmitted diseases among foreigners in Italy. Migration Medicine Study Group. Epidemiol Infect. 1997;18(3):235–241. doi: 10.1017/s0950268897007449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matteelli A, Dal Punta V, Angeli A, et al. Congenital syphilis in Italy. Sex Transm Infect. 2007;83(7):590–591. doi: 10.1136/sti.2007.025338. [DOI] [PMC free article] [PubMed] [Google Scholar]


