Abstract
Neither the innate nor adaptive immune system “responds” unless leukocytes cross blood vessels. This process occurs through diapedesis, in which the leukocyte moves in an ameboid fashion through tightly apposed endothelial borders and, in some cases, through the endothelial cell itself. This review focuses on the active role of the endothelial cell in diapedesis. Several mechanisms play a critical role in transendothelial migration, including signals derived from clustering of apically disposed intercellular adhesion molecule 1 and vascular cell adhesion molecule 1, disruption or loosening of adherens junctions, and targeted recycling of platelet/endothelial cell adhesion molecule and other molecules from the recently described lateral border recycling compartment. Surprisingly, many of the same molecules and mechanisms that regulate paracellular migration also control transcellular migration. A hypothesis that integrates the various known mechanisms of transmigration is proposed.
Keywords: inflammation, lateral border recycling compartment (LBRC), endothelial cell, diapedesis, platelet/endothelial cell adhesion molecule (PECAM), cell junctions
INTRODUCTION
The inflammatory response is the body’s stereotyped reaction to tissue damage. It involves the rapid and transient delivery of preformed soluble elements in the blood to the site of injury, followed by a more prolonged delivery of leukocytes. Because leukocytes cannot swim, they are recruited locally at the site of inflammation in a series of adhesive steps that allow them to attach to the vessel wall, locomote along the wall to the endothelial borders, traverse the endothelium and the subendothelial basement membrane, and migrate through the interstitial tissue (1, 2). Transendothelial migration (TEM), or diapedesis, is arguably the point of no return in the inflammatory response. All the preceding steps—leukocyte rolling, activation, adhesion, and locomotion—are reversible, and most of the leukocytes that attach to the postcapillary venule at the site of inflammation reenter the circulation. However, once the leukocyte commits to diapedesis, it does not go back—at least not as the same cell type (3). The regulation of the leukocyte recruitment steps of capture, rolling, activation, and adhesion have been well studied and reviewed. Although less is known about diapedesis, our knowledge of the molecules and mechanisms controlling TEM has increased relatively recently. Most TEM takes place at endothelial borders (paracellular migration). Recently there has been a flurry of interest in TEM through the endothelial cell body (transcellular migration). The mechanisms behind transcellular migration are less well established. In this review, I focus first on some general types of molecules and mechanisms that have been implicated in paracellular TEM and try to show how these observations may be related. Then I review transcellular migration and argue that these two routes of transmigration may have more in common than originally thought.
MOLECULES REGULATING PARACELLULAR TRANSMIGRATION
Numerous endothelial cell molecules have been implicated in transmigration, given that genetic deletion or antibody blockade of these molecules impairs diapedesis. All these molecules are enriched at or restricted to the endothelial cell borders. In addition to adhesive functions, these molecules have signaling functions that contribute to their role in TEM. Intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) are discussed later in this section. Although they are not involved in diapedesis per se, they seem to be involved in events that directly precede diapedesis and are recruited to the endothelial cell border during transmigration. These interactions are summarized in Table 1.
Table 1.
Endothelial cell molecules that participate in leukocyte transmigrationa
| Endothelial molecule | Leukocyte ligand | Endothelial ligand |
|---|---|---|
| ICAM-1 | CD11a/CD18, CD11b/CD18 | N/A |
| VCAM-1 | CD49d/CD29 | N/A |
| JAM-A | CD11a/CD18 | JAM-A |
| JAM-B | Not described on leukocytes | JAM-B, JAM-C |
| JAM-C | CD11b/CD18 | JAM-C, JAM-B |
| ESAM | None known | ESAM |
| PECAM-1 | PECAM-1 | PECAM-1 |
| CD99 | CD99 | CD99 |
| CD99L2 | CD99L2 | CD99L2 |
| VE-cadherin | None known | VE-cadherin |
The main endothelial molecules and their ligands on endothelial cells and leukocytes are shown.
Abbreviations: ESAM, endothelial cell–selective adhesion molecule; ICAM-1, intercellular adhesion molecule 1; JAM, junctional adhesion molecule; N/A, not applicable; PECAM, platelet/endothelial cell adhesion molecule; VCAM-1, vascular cell adhesion molecule 1; VE-cadherin, vascular endothelial cell–specific cadherin.
Luminal Surface Molecules
ICAM-1 is involved in the firm adhesion of leukocytes to the apical surface of endothelial cells through interactions with leukocyte CD11a/CD18 and/or CD11b/CD18. Dimers of ICAM-1 on the endothelial surface (i.e., in cis) are the preferential ligands for CD11/18 (4, 5). Once adherent, ICAM-1 becomes enriched under the leukocyte as it migrates to the endothelial cell border and continues to surround it during transmigration (6). The actin cytoskeleton is involved in this process; specifically, Src-dependent phosphorylation of the actin-binding molecule cortactin is required for ICAM-1 clustering (7, 8).
VCAM-1 is involved in the firm adhesion of monocytes and lymphocytes bearing CD49d/CD29. VCAM-1 clustering has been observed in the steps leading up to diapedesis. Both ICAM-1 and VCAM-1 are enriched over actin-rich docking structures that form prior to TEM (9, 10). Engagement of VCAM-1 activates intracellular calcium release and the small GTPase Rac-1, which in turn activates the endothelial NADPH oxidase Nox2 (11). Reactions downstream of this process affect the adherens junctions (see section entitled Loosening the Junctions, below).
ICAM-2, another CD11a/CD18 ligand, is constitutively expressed on endothelial cells, where it is concentrated at cell borders but retains considerable surface expression. Antibodies against ICAM-2 do not seem to have a major effect on TEM in vitro. Compared with ICAM-1, ICAM-2 seems to play a lesser role (12). However, in some inflammatory models in vivo, blocking antibodies or genetic deletion of ICAM-2 inhibits the transmigration of neutrophils (13, 14).
Junctionally Enriched Molecules
Junctional adhesion molecule (JAM)-A is concentrated at endothelial cell borders. Although it normally engages in homophilic adhesion, during inflammation it can bind to CD11a/CD18 on the leukocyte (15). On the one hand, blocking JAM-A on human endothelial cells in vitro using antibodies generally has no reported effect on TEM (6, 16, 17), with one notable exception (15). On the other hand, in vivo studies show decreased inflammation (18) and TEM (14) when JAM-A is blocked. JAM-C is likewise concentrated at endothelial cell borders. It can engage in homophilic adhesion with JAM-C or heterophilic adhesion with JAM-B or CD11b/CD18. The latter interaction is implicated in TEM both in vitro (19) and in vivo (20). For an extensive review of the roles of JAM family members in the inflammatory response, see Reference 21.
Endothelial cell–selective adhesion molecule (ESAM) is molecularly related to the JAMs but has a long cytoplasmic domain. As its name implies, its distribution is limited mostly to endothelial junctions, but it is also expressed on activated platelets (22). It binds homophilically; no ligand on leukocytes has been described. ESAM-deficient mice have no defect in lymphocyte extravasation, but they do show a transient decrease in neutrophil emigration in response to thioglycollate broth injected intraperitoneally. Compared with wild-type mice, there was markedly reduced neutrophil emigration into the peritoneal cavity at 2 h, but normal levels of emigration were observed by 4 h (23).
Platelet/endothelial cell adhesion molecule 1 (PECAM-1, also known as CD31) is an immunoglobin superfamily member that is concentrated at the borders of endothelial cells and expressed diffusely on platelets and leukocytes. Homophilic interaction between leukocyte PECAM and endothelial PECAM is required for TEM (24, 25). Blockade with monoclonal antibody against the amino-terminal homophilic interaction domain, soluble PECAM-Fc chimeras, and genetic deletion of PECAM inhibit TEM in vitro and in vivo (reviewed in Reference 3). When PECAM is transfected into cells that normally lack it, expression of PECAM allows them to support TEM (26). This gain of function has not been demonstrated with other adhesion molecules. When PECAM-PECAM interactions are blocked, leukocytes are arrested tightly adherent to the apical surface of the cell (27) and actively migrate along the junctions as if searching for a place to transmigrate (17). In vivo, at sites of inflammation leukocytes can get to the postcapillary venules at the site of inflammation but are unable to transmigrate efficiently. They are observed in vastly increased numbers apparently adherent to the endothelial cell luminal surface (28, 29), which is reminiscent of the block to TEM observed in vitro (24, 27).
This phenotype is observed with human cells and in all mouse strains examined except for C57BL/6 (29, 30). Interestingly, this mouse strain seems to be unique. Genetic deletion of PECAM or administration of blocking antibody or mouse PECAM-Fc to these mice has no effect in a variety of inflammatory models (29, 31, 32). Even the closely related C57BL/10 strain responds to anti-PECAM therapy (30). The ability to circumvent the need for PECAM in the thioglycollate peritonitis model of inflammation has been linked to a small locus at the proximal end of chromosome 2 (30). Therefore, earlier studies carried out in C57BL/6 mice that found no role or only a minor role for PECAM in inflammation need to be reevaluated. See Reference 3 for a detailed discussion of the role of PECAM in various in vivo models.
There is a role for leukocyte PECAM in traversing the basal lamina (33). C57BL/6 mice in which PECAM has been knocked out (31) or blocked with antibody (14) are defective in terms of their ability to migrate across this extravascular barrier.
CD99 is unrelated to any other molecule in the human genome except the closely related paralog CD99-like 2 (CD99L2), which may have arisen from a common ancestral gene (34). The gene encoding CD99 is in the pseudoautosomal region of the human X chromosome (35). In mice, the region of the genome syntenic to the pseudoautosomal region of the human X chromosome is on chromosome 4 (36), where mouse CD99 is encoded. Similar to PECAM interactions, homophilic interaction between CD99 at the endothelial cell border and CD99 on monocytes (37) and neutrophils (38) is required for transmigration. However, CD99 regulates a later step in transmigration than PECAM does. Leukocytes in which PECAM has been blocked can still be prevented from transmigrating if anti-CD99 is added after the anti-PECAM block has been removed. Conversely, when CD99 interaction is first blocked, leukocytes cannot be inhibited from transmigrating by anti-PECAM antibody after the anti-CD99 block is removed (37). In support of this mechanism, confocal images of leukocytes blocked in the act of transmigration by anti-CD99 show their leading edge under the endothelial cytoplasm, their cell body lodged at the border between endothelial cells, and their trailing uropod on the apical surface (37, 38). As long as the block continues, the leukocytes migrate along the junctions over the surface of the endothelium in this manner, unable to finish transmigration (38). There is indirect evidence that CD99 cannot function unless PECAM acts first (26). Blocking antibodies against mouse CD99 inhibit inflammation in several animal models. Migration of T lymphocytes into skin (39) and migration of neutrophils and monocytes into the peritoneal cavity (40) are blocked by interference with CD99 function.
The CD99L2 molecule is ancestrally related to CD99. It is encoded by a gene on the X chromosome, as is CD99, but unlike CD99, the gene encoding CD99L2 is not in the pseudoautosomal region (36). CD99L2 expression in mice seems similar to that of CD99; that is, it is expressed on the vascular endothelium of all tissues examined (41, 42) and at the borders of endothelial cells. It is expressed to varying degrees on all circulating blood cells. Only polyclonal antibodies against murine CD99L2 have been tested in vivo. They block neutrophil and monocyte influx in the thioglycollate peritonitis model (41, 42). The incomplete blockade of inflammation observed when interfering with either CD99 or CD99L2 may be due to partial redundancy of the function of these molecules.
Vascular endothelial cell–specific cadherin (VE-cadherin) is the major adhesion molecule of the endothelial adherens junction. It negatively regulates transmigration. Antibodies against VE-cadherin enhance early migration into a site of inflammation in vivo (43). In vitro studies show that VE-cadherin is transiently removed from the site of transmigration at the cell junction (44, 45). Mutation of the cytoplasmic tail of VE-cadherin so that it cannot interact with p120 or β-catenin, or overexpression of p120 to outcompete the kinases that would phosphorylate it (see the section entitled Loosening the Junctions, below), prevents clearance of VE-cadherin from the cell border and blocks transmigration (46, 47).
Why So Many Molecules?
Antibodies against the following molecules block TEM, implicating their role in transmigration: poliovirus receptor (48), MUC18 (49), activated leukocyte cell adhesion molecule (50), integrin-associated protein (51), and nepmucin (52). There have been numerous recent discoveries of molecules on endothelial cells or leukocytes that are implicated in diapedesis. When added to the well-characterized molecules discussed in the previous section, these discoveries raise the question of why so many molecules are required for TEM. Are they simply an artifact of clogging up the junction with antibody or turning the cell junctions into immune complexes? This conjecture is unlikely, as most of these studies used control antibody, Fab or F(ab′)2 fragments, soluble recombinant adhesion molecules, small interfering RNA knockdown, or genetic deletion to buttress their claims.
The process of diapedesis itself can be further broken down into a series of molecularly defined steps controlled by specific molecules acting in sequence. Sequential blocking experiments demonstrated that PECAM regulates a step in diapedesis that is “upstream” of the step regulated by CD99 (37). As mentioned above, images of the blocked cells show that blocking PECAM arrests leukocytes before the start of transmigration; blocking CD99 arrests leukocytes during the process of transmigration. Sequential blockade analysis has not been performed with other pairs of molecules, but images of leukocytes blocked by antibodies in vivo in C57BL/6 mice show that ICAM-2 arrests neutrophils on the apical surface of the endothelium, anti-JAM-A arrests them at the cell junctions, and anti-PECAM arrests them between the endothelial cell and basal lamina (14). These findings beg the questions of whether each molecule controls its own defined step in the sequence, whether multiple molecules control each step, and how many steps there are. Until sequential blockade studies can be performed with each of these molecules, these questions will remain unanswered. The answers are likely to be different for different leukocyte types, vascular beds, and inflammatory stimuli, as well as the amount of time elapsed after the initiation of the stimulus. However, it seems unlikely that there is a separate unique step in diapedesis controlled by each molecule reported to be important for transmigration.
What if most of the endothelial molecules that are reported to control transmigration were part of a large, multimolecular transmigration complex, or a series of multimolecular transmigration complexes (one for each successive step in diapedesis) that combine to make a platform to support transmigration, analogous to the way that multiple transcription factors and coactivators combine to make DNA accessible to transcription? In that case, loss of or interference with any one of the molecules could make the complex less efficient at supporting diapedesis and could account for the published results.
MECHANISMS REGULATING PARACELLULAR TRANSMIGRATION
Clustering Surface ICAM-1 and VCAM-1
The adhesion step immediately upstream of diapedesis is an obvious prerequisite for diapedesis, and some of the events that occur during this step may signal the events that regulate transmigration. Clustering of ICAM-1 and VCAM-1 on the endothelial cell has been observed as the leukocyte approaches the endothelial cell border (9, 10). The initial leukocyte-facilitated clustering of ICAM-1 requires Src-dependent phosphorylation of the actin-binding protein cortactin, which is also associated with the actin filament remodeling that takes place during transmigration (7). However, ICAM-1 engagement or clustering induces Src-dependent phosphorylation of cortactin (53). These observations, rather than being at odds, may belie a self-amplification cycle: The initial recruitment of ICAM-1 and VCAM-1 may be due to adhesion to their leukocyte ligands. This clustering induces phosphorylation of cortactin, which leads to actin polymerization and recruitment of more ICAM-1 to the site of leukocyte adhesion, which in turn induces more cortactin phosphorylation. Clustering of ICAM-1 and VCAM-1 stimulates signaling in the endothelial cells that promote diapedesis in ways that are discussed below.
Clustering of ICAM-1 and VCAM-1 may occur in three dimensions. So-called docking structures or transmigratory cups are finger-like projections of endothelial apical surface membrane reported to surround the lower portion of adherent leukocytes. The membrane is enriched in ICAM-1 and VCAM-1 and overlies cytoplasm enriched in F-actin and actin-binding proteins. Barreiro et al. (9) first used the term docking structures to describe these projections, which engage polyclonally activated lymphocytes and lymphoblasts adherent to cytokine-activated human umbilical vein endothelial cells (HUVEC). Most of these authors’ observations were made with a cell line (4M7) that expressed very late antigen 4 (VLA-4) but not lymphocyte function–associated antigen 1 (LFA-1). These cells adhered strongly but did not transmigrate, and thus there was plenty of time to strengthen VLA-4/VCAM-1 interactions and recruit more VCAM-1, actin, and ERM (ezrin, radixin, moesin) proteins to the site of adhesion. However, similar extensions bearing ICAM-1 were observed around adherent LFA-1-expressing lymphoblasts.
Subsequently, Carman et al. (54) reported similar projections of ICAM-1 that seemed to rise up off the endothelial surface and surround at least the lower part and sides of leukocytes engaging either cytokine-activated endothelial cells or ICAM-1 transfected Chinese hamster ovary cells. Disruption of the cytoskeleton abolished these structures but had no effect on leukocyte adhesion. The authors commented that this observation might belie a role in transmigration. Interestingly, however, Barreiro et al. (9) found that the docking structures rapidly vanished as lymphocytes began to migrate through the monolayers. Carman & Springer (10) showed that these projections are associated with transmigrating neutrophils, monocytes, and lymphocytes, at least under experimental conditions that involved apical application of a chemokine or leukocyte activator and interaction with activated endothelium. They referred to these structures, which are associated with both paracellular and transcellular migration, as transmigratory cups. Subsequently, most transmigratory cups were reported in association with transcellular migration.
The experimental conditions used for these studies involved monolayers of tumor necrosis factor α (TNF-α)-activated human umbilical vein endothelial cells grown on glass coverslips. Transmigration experiments were performed in the presence or absence of fluid shear, but this did not seem to make a difference (10, 55). In all cases, the investigators observed a ring of enriched ICAM-1 (and VCAM-1) fluorescence on the apical surface of the endothelial cell at the point of contact with the adherent or transmigrating leukocyte.
However, not everyone who reported rings of ICAM-1 enrichment around transmigrating leukocytes observed docking structures or transmigratory cups. For example, Ridley’s group (56), using a similar system (but without application of apical SDF-1), found distinct ICAM-1 enrichment around transmigrating lymphoblasts, but no docking structures. Luscinskas’s group (6) also demonstrated local enrichment of ICAM-1 around transmigrating neutrophils undergoing transmigration, but found no actin-rich microvilli.
At sites of documented ongoing transcellular migration in vivo (57), cup-like structures were not observed during migration. Instead, the neutrophil appeared to be migrating through a fenestra in the flat endothelial cell. A similar electron microscopy image was taken for a neutrophil migrating through an ICAM-1-enriched zone of endothelial cell cytoplasm in vitro (8). A true apical cup was reported (58) through the use of scanning electron microscopy. The apical cup surrounded a differentiated HL60 cell adherent to a TNF-α-activated HUVEC for 30 min. The cell adhered but did not transmigrate.
What do these docking structures represent, and why are they not universally seen? One possibility is that they represent a response of the endothelial cell to leukocytes that are either highly activated or tightly adherent. The structures were observed under conditions in which (a) the leukocytes adhered but could not transmigrate, which allowed time for the recruitment of additional ICAM-1 and/or VCAM-1 molecules (9, 58), or (b) the leukocytes were additionally activated by the exogenous application of platelet-activating factor or chemokines on the apical surface of the endothelial cells (10, 54). Under these conditions, enhanced leukocyte integrin activation could result in greater recruitment of counterreceptors from the endothelial surface. The scanning electron micrograph (58) is reminiscent of ligand-mediated phagocytosis in macrophages, and endothelial cells are known to be phagocytic under certain conditions. In contrast, under conditions in which transmigrating neutrophils (6) or lymphoblasts (56) were activated by interactions with the cytokine-activated endothelium without additional apical chemokine, ICAM-1 enrichment was not accompanied by the formation of transmigratory cups.
Loosening the Junctions
Several lines of evidence show that loosening the endothelial cell junctions is important for efficient transmigration. Clustering of ICAM-1 and VCAM-1 on endothelial cells transmits a number of signals into the endothelial cell (reviewed in Reference 59), some of which appear to be relevant to diapedesis. Cross-linking VCAM-1 (60) and ICAM-1 (59) on the endothelial cell stimulates an increase in cytosolic free calcium ions, which has long been known to be a requirement for diapedesis (61). The increase in cytosolic free calcium ion activates myosin light-chain kinase (MLCK), which leads to actin–myosin fiber contraction. This contraction is believed to help endothelial cells separate (62).
Stimulation of ICAM-1 leads to phosphorylation of VE-cadherin, which is a prerequisite for adherens junction disassembly (63). In HUVEC, the kinases Src and Pyk2 phosphorylate VE-cadherin on the p120- and β-catenin-binding sites, tyrosine residues 658 and 731, respectively (46). This process inhibits the binding of p120 and β-catenin to VE-cadherin. Because the interaction of these proteins with VE-cadherin is critical for retaining VE-cadherin at the adherens junction, phosphorylation of VE-cadherin at the p120- and β-catenin-binding sites destabilizes the junctions. Alternatively, if p120 is overexpressed, VE-cadherin levels at the cell border remain high, and transmigration is inhibited (47). The authors of this study (47) hypothesized that overexpression of p120 interferes with or outcompetes the kinases that normally phosphorylate VE-cadherin on Y658 and inhibit p120 binding.
Cross-linking VCAM-1 also activates Rac1 (64) and stimulates an increase in reactive oxygen species in endothelial cells (11) that leads to loosening of adherens junctions. In other systems, Rac1 activation leads to phosphorylation of VE-cadherin on serine 665, which signals its clathrin-dependent internalization (65). The net result is loosening of junctional structures.
Under resting conditions, the vascular endothelial protein tyrosine phosphatase (VE-PTP) associates with VE-cadherin via plakoglobin (γ-catenin) and maintains VE-cadherin in a hypophosphorylated state at the junction. Interaction between leukocytes and cytokine-activated endothelial cells triggers rapid dissociation of VE-PTP from VE-cadherin, which allows it to be phosphorylated on tyrosine, thereby increasing junctional permeability and facilitating TEM (66). A role for another VE-cadherin accessory molecule, p120-catenin, was recently demonstrated (47). Overexpression of p120 prevented VE-cadherin phosphorylation and the formation of gaps in VE-cadherin staining along the endothelial junction during engagement of leukocytes. (These gaps were not spaces between cells but rather disruption of the staining pattern of VE-cadherin.) Overexpression of p120 was associated with a significant decrease in transmigration. Interestingly, these authors (47) did not find evidence for VE-cadherin internalization during gap formation.
Similarly, clustering of ICAM-1 activates RhoA, which activates Rho kinase (reviewed in Reference 67). This process in turn phosphorylates and inactivates PP1c, the major phosphatase inactivating MLCK. The end result is potentiation of actin-myosin contraction. Importantly, although intercellular gaps that are visible in the light microscope can be produced on endothelial cells cultured on glass coverslips, in vivo the gaps produced between endothelial cells by even the strongest inducers of vascular permeability (e.g., histamine and serotonin) are on the order of hundreds of angstroms (68) and are resealed by the time most leukocytes are recruited. These gaps are not unimportant, but leukocytes must still crawl through closely adherent endothelial cells; they do not fall into holes between endothelial cells.
The Lateral Border Recycling Compartment
Even under steady-state conditions, considerable membrane movement takes place at the endothelial cell borders. Membrane is internalized into and recycled from an interconnected reticulum of tubulovesicular structures that resides just beneath the plasma membrane of the endothelial cell borders (25). Electron microscopy shows that most of the components appear to be 50-nm vesicles. The compartment, termed the lateral border recycling compartment (LBRC) (69), is distinct from caveolae, typical recycling endosomes, and vesiculo-vacuolar organelles (VVOs) (25). The LBRC remains closely localized to the endothelial cell border and does not colocalize for staining with either transferrin receptor (a marker of recycling endosomes) or caveolin-1 (a marker of caveolae and VVOs) (25). Furthermore, virtually all PECAM in HUVEC was extracted by cold Triton and sank in sucrose gradients in which caveolin-1 floated, demonstrating that PECAM does not reside in lipid rafts in resting endothelial cells (25). Whereas vesicles of the LBRC are mostly uniform in size and recycle locally and constitutively at the cell border (25), VVOs vary greatly in size and are larger than caveolae and LBRC vesicles; provide a channel across the cell cytoplasm (70); and, rather than being constantly in communication with the exterior of the cell, open in response to stimulation by vascular endothelial cell growth factor (VEGF), histamine, or serotonin (71). Furthermore, VVOs do not form in vitro under the conditions in which one can easily detect the LBRC (72).
Approximately 30% of the cell’s PECAM resides in this compartment and recycles with a half time of roughly 10 min (25). This compartment also contains CD99 and JAM-A but not VE-cadherin (73). In high–endothelial venule endothelium, the immunoglobin superfamily molecule nepmucin, which promotes lymphocyte TEM, is in the LBRC (52).
The purpose of this constitutive recycling is not known. However, when a leukocyte transmigrates, membrane from the LBRC is redirected (Figure 1). It is targeted to and exteriorized at the cell border at the site of leukocyte transmigration (25, 69). Blocking homophilic PECAM-PECAM interactions between leukocyte and endothelial cell blocks targeted recycling from the LBRC and blocks transmigration. Moreover, there is accumulating evidence that targeted recycling from the LBRC is an essential step in TEM: The LBRC membrane is trafficked to the site of transmigration by kinesin molecular motors along microtubules (69). Disrupting or bundling microtubules, or inhibiting the motor domain of kinesin, blocks targeted recycling and blocks TEM. Lymphocytes and activated lymphoblasts transmigrate in a manner that cannot be blocked by anti-PECAM antibodies (74, 75). Nevertheless, transmigration of lymphoblasts can be efficiently blocked by disrupting the targeted recycling of the LBRC (69). A tyrosine-to-phenylalanine mutation on the cytoplasmic tail of PECAM blocks the ability of PECAM to support TEM. This mutation interferes with the ability of PECAM to enter and leave the LBRC and markedly diminishes its ability to participate in targeted recycling (26). In confluent endothelial cell monolayers, a minority of PECAM is phosphorylated; however, essentially all of the phosphorylated PECAM resides in the LBRC (76).
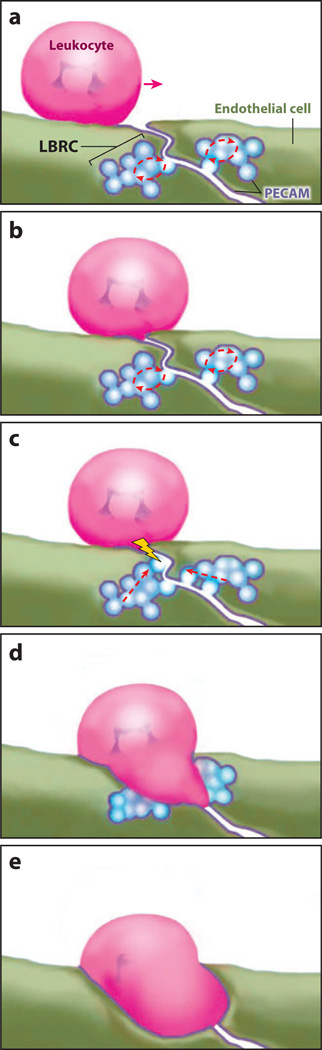
Figure 1.
Schematic view of the movement of the lateral border recycling compartment (LBRC) during paracellular transmigration. (a) Constitutive recycling of the LBRC is ongoing as the leukocyte locomotes toward the intercellular border. (b) Upon reaching the apical side of the endothelial border, leukocyte platelet/endothelial cell adhesion molecule (PECAM) engages endothelial cell PECAM, which is enriched in plasma membrane at the cell borders (and in the LBRC). (c) In cases of PECAM-dependent transmigration, leukocyte PECAM–endothelial cell PECAM interaction triggers a signal (lightning bolt) that redirects recycling of the LBRC to the site of leukocyte engagement. In cases of PECAM-independent transmigration, some other interaction triggers this signal. (d) Membrane from the interconnected vesicles of the LBRC moves to surround the leukocyte. (e) Recruitment of the LBRC continues as the leukocyte crosses the endothelial cell border. Endothelial cell thickness is exaggerated to allow depiction of the LBRC and various signaling molecules. In reality, the endothelial cell is ≤0.5 µm thick, and the leukocyte is ~7–10 µm in diameter. Modified from Reference 77 with permission.
Targeted recycling of LBRC membrane during TEM potentially solves many of the problems inherent in the process. Rather than having to “unzip” high-density homophilic adhesions of VE-cadherin, PECAM, JAM-A, CD99, and so on, these molecules (and other structural components of the junction) may be pushed aside by membrane from the LBRC. The LBRC membrane then presents unligated molecules (e.g., PECAM, JAM-A, CD99, nepmucin) that the leukocyte must interact with on its path across the endothelial cell while removing structural barriers to transmigration (e.g., the adherens junction complex of VE-cadherin and associated catenins). The absence of VE-cadherin from the LBRC could explain the observation that when a leukocyte crosses the endothelial border, VE-cadherin at the border appears to move out of the way (44, 45). As the transmigrating leukocyte is surrounded by membrane coming from the LBRC, VE-cadherin at the cell border may be either diluted or pushed out of the way. Hypothetically, once the leukocyte has moved across the junction, the LBRC may be pulled back into the cell, which allows the other components to diffuse back into place, thereby reestablishing the endothelial junction without having to reform all of the complex three-dimensional interactions. VE-cadherin undergoes endocytosis via a clathrin-dependent pathway (78); apparently it is not internalized into the LBRC.
A Unifying Model of Paracellular Transmigration?
ICAM-1 and VCAM-1 signaling, cytosolic free calcium flux, RhoA and Rac1 activation, VE-cadherin removal from the junction, MLCK activation, and targeted recycling of the LBRC are necessary for efficient transmigration. How are these diverse phenomena related? Are they sequential links in a chain or events occurring in parallel, where all are required for TEM to occur? Considering that many second messenger signaling systems interact with each other and that feedback loops exist, these questions may be purely semantic. However, the following undoubtedly oversimplified scheme seems to be consistent with all of the published data and at least provides a testable hypothesis (Figure 2).
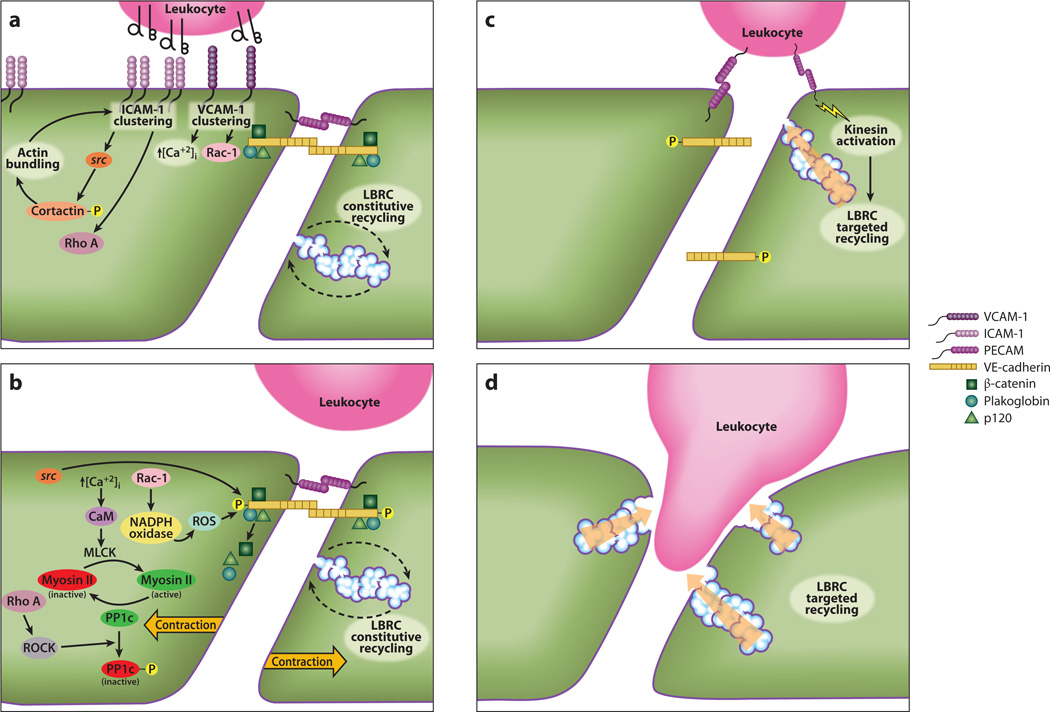
Figure 2.
A unified schematic view of paracellular transendothelial migration. (a) Clustering of intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) through engagement of their leukocyte integrin counterreceptors (αβ in diagram) initiates activation of src, Rho A, and Rac-1, as well as increased cytosolic free calcium ion. Phosphorylation of cortactin by src stimulates F-actin rearrangements in the cortical cytoplasm, which facilitates more ICAM-1 clustering. (b) These signals lead to activation of myosin light-chain kinase (MLCK), inactivation of PP1c, and phosphorylation of vascular endothelial cell–specific cadherin (VE-cadherin), inducing release of the associated catenins. (c) Leukocyte platelet/endothelial cell adhesion molecule (PECAM) engagement of endothelial cell PECAM, and/or other leukocyte/endothelial cell interactions at the apical surface of the endothelial border, activates kinesin molecular motors in the endothelial cell and stimulates targeted trafficking of LBRC membrane to the vicinity of the leukocyte. (d) Targeted trafficking of LBRC membrane continues as the leukocyte moves into the border between endothelial cells, which is now enlarged by the contribution of membrane from the LBRC. This process continues until transmigration is complete. Abbreviations: CaM, calmodulin; ROCK, Rho kinase; ROS, reactive oxygen species; circled P, phosphorylated state. Reprinted from Reference 79 with permission.
LFA-1 preferentially binds to ICAM-1 dimers (4, 5), which initiates clustering of ICAM-1. This process stimulates phosphorylation of cortactin, enhancing further actin-induced clustering of ICAM-1. This self-enhancing cycle leads to the enrichment of ICAM-1 around tightly adherent leukocytes. ICAM-1 multimerization leads to increases in cytosolic free calcium and activation of RhoA (Figure 2a).
In the meantime, if the leukocytes express VLA-4 and the endothelial cells express VCAM-1, clustering of VCAM-1 also stimulates an increase in cytosolic free calcium, activation of Rac-1, and production of reactive oxygen species in endothelial cells (80, 81). The latter activates protein kinase C α (82). The net result is loosening of endothelial cell junctions (Figure 2a,b).
The simultaneous signaling of ICAM-1 and VCAM-1 causes weakening of the endothelial junctions due to effects on the phosphorylation of VE-cadherin. This signaling dissociates VE-cadherin from its links to the actin cytoskeleton, and it potentially (but not necessarily) becomes subject to endocytosis in a clathrin-dependent manner (Figure 2b).
The increase in cytosolic free calcium activates MLCK to induce tension in the endothelial cells. The activation of MLCK is augmented by the inactivation of PP1 phosphatase, which is mediated by the RhoA activation stimulated by signals originated through ICAM-1 clustering. The net result of the contraction of the endothelial cell body against weakened junctions is to allow for the easier passage of leukocytes (Figure 2b).
With leukocytes poised over weakened adherens junctions, the other homophilic junctional adhesion molecules continue to hold the endothelial borders apposed. PECAM-PECAM interactions between leukocytes and endothelial cells (25), or other signals (69), stimulate targeted trafficking of LBRC membrane to surround the leukocytes (Figure 2c). Targeted recycling of the LBRC may displace components of the adherens junction laterally, providing increased surface area and unligated molecules with which the leukocytes want to interact (Figure 2d). It is possible, and even likely, that some of the many additional junctional molecules discussed above are also part of the LBRC or that they function to recruit it. That is, the LBRC may be one of the hypothetical multimolecular complexes that control transmigration, whereas other multimolecular complexes may function to recruit the LBRC to the site of TEM and reinternalize it after TEM.
The signals that trigger targeted recycling are not known, nor is it clear how the membrane is directed to the site of transmigration. However, weakening of the endothelial cell adherens junctions by brief calcium chelation leads to diffuse exteriorization of the LBRC along the endothelial cell border (S.K. Muller & W.A. Muller, unpublished data). Local weakening of the adherens junctions at the site of leukocyte engagement may allow for localized exteriorization of the LBRC.
TRANSCELLULAR MIGRATION
Although it is well accepted that leukocytes cross the endothelium at the cell borders (paracellular route), there is increasing evidence that leukocytes can also pass directly through endothelial cells (transcellular route). Much of the original evidence was indirect and was based on single transmission electron micrographs that appeared to show leukocytes deeply indenting endothelial cells and/or passing across endothelial cells through a membrane-lined channel next to an intact junction (83–85). However, endothelial junctions are serpentine, and the leukocytes may have been passing through a less structured junction (75). For a good historical review of transcellular migration, the reader is referred to Reference 86.
Transcellular migration was initially viewed with skepticism despite (or perhaps because of) the large amount of circumstantial evidence that supported it. The skeptics were to a great extent appeased by a paper published by the Dvorak lab in 1998 (57), which arguably provided the first indisputable evidence of neutrophil transcellular migration in vivo. In these studies, neutrophil emigration was stimulated by direct injection of fMLP, a neutrophil chemoattractant and β2 integrin activator, into the skin of guinea pigs (57). The authors presented a collection of electron microscope serial sections in which neutrophils were observed to pass entirely across an endothelial cell without ever contacting a recognizable junction (57). Although this study did not address the absolute frequency of transcellular migration, it demonstrated that transcellular migration was possible in vivo.
Molecules Implicated in Transcellular Migration
Recently, several in vitro models produced reliable transcellular migration (8, 10, 56, 73). These studies provided data on the molecules and mechanisms involved in this process. Perhaps surprisingly, many of the same molecules that are important for paracellular migration also play a role in transcellular migration.
During transcellular TEM, ICAM-1 that is uniformly expressed on the luminal surface of HUVEC redistributes and is concentrated at the site of diapedesis. Furthermore, it is enriched in the membrane channel that surrounds the crossing leukocyte as it goes through the endothelial cell body (8, 10, 55, 56, 73). However, for transcellular migration, as for paracellular migration, some investigators (6, 8, 69, 73) did not observe the docking structures or transmigratory cups formed by projections of ICAM-1 above the plane of the endothelial cell membrane that were reported by others (9, 10).
In addition to ICAM-1, other molecules normally considered restricted to the cell borders, including PECAM, JAM-A, and CD99, are observed around leukocytes migrating transcellularly (8, 10, 55, 56, 73). In all cases, VE-cadherin is absent. These molecules are not only present but appear to be functional. Somewhat surprisingly, transcellular migration is dependent on PECAM and CD99. Blocking antibodies arrest transcellular migration (73). The reason for this finding may be explained by the mechanism discussed below.
MECHANISM(S) OF TRANSCELLULAR MIGRATION
Despite the progress made in uncovering some of the molecules involved in the transcellular route of diapedesis, it remains unclear why leukocytes that appear to use the same mechanisms for rolling and adhesion transmigrate through the endothelial cell rather than at the junctions.
What Determines the Site of Transmigration?
Whether the leukocyte migrates paracellularly or transcellularly may depend on the relative tightness of the endothelial junctions and the ability of the leukocyte to breach them. Carman & Springer (87) speculated that the leukocytes take the path of least resistance across the endothelium. If junctions are very tight, as in, for example, the blood-brain barrier, migration across the cell at a thin point may be easier, as observed in cerebral inflammation (88, 89). However, that cannot be the only explanation because postcapillary venules, which are the sites of most inflammatory diapedesis [including transcellular migration (57)], have very leaky junctions. Postcapillary venules are specialized for permeability, given that they are the site of fluid reuptake from the interstitium (90). Endothelial cells in culture, where transcellular migration has been best demonstrated, form a monolayer of low electrical resistance even under the most optimal culture conditions (91).
Transcellular migration may also occur if the leukocyte has difficulty reaching the junction, such as in cells in which CD11b is nonfunctional (17) or deficient (92). Phillipson et al. (92) reported that neutrophils deficient in CD11b/CD18 adhere to the endothelium at a site of inflammation but transmigrate poorly due to their inability to locomote along the endothelial surface. However, the neutrophils that transmigrated tend to go transcellularly, perhaps because they achieve sufficient activation to transmigrate before they reached the cell border. Similarly, T cells deficient in the Rac activator Tiam1 are deficient in polarization and locomotion on endothelium and tend to migrate transcellularly (93).
The Role of Leukocyte Activation
We hypothesize that, at least for low-resistance endothelia, transcellular migration may occur when leukocytes are highly and/or directly activated. The first indisputable evidence of neutrophil transcellular migration in vivo came from studies in which emigration was stimulated by direct injection of fMLP, a neutrophil chemoattractant and β2 integrin activator, into the skin of guinea pigs (57). The published in vitro studies of transcellular migration either used MCP-1, platelet activating factor, or SDF-1 to stimulate migration of monocytes, neutrophils, and T cells, respectively (10), or employed mitogen-activated T lymphoblasts (56, 87). Furthermore, these agents were added to the apical side of the endothelium, where they would activate leukocytes but would not provide a chemotactic gradient. In the standard TEM assay system in our lab (94), paracellular migration predominates, and transcellular migration is almost never seen across cytokine-activated HUVEC. However, when we applied chemokine or chemoattractant to the apical side of endothelial monolayers, 10–30% of the leukocytes migrated transcellularly (73).
Leukocyte activation would promote polymerization of actin in the leukocytes’ lamellipodia, which is associated with transcellular migration in vivo (57) and is necessary for transcellular migration in vitro (55). Leukocytes probe the apical surface of endothelial cells with so-called invasive podosomes that contain polymerized actin. These podosomes deeply invaginate the surface in the areas of eventual transcellular migration (55).
The Role of the Lateral Border Recycling Compartment
Membrane vesicles accumulate in the region of transcellular migration (55). Millan et al. (56) reported that caveolin-1 accumulates in the regions of transcellular migration, implying that these regions might be caveolae. However, other groups (10, 55, 73) did not observe any enrichment in caveolin-1 at the site of diapedesis.
Mamdouh et al. (73) recently reported that the LBRC is critical for transcellular migration of leukocytes, similar to its involvement in paracellular migration. The LBRC membrane components PECAM, CD99, and JAM-A move in concert to surround neutrophils and monocytes migrating transcellularly. The source of the membrane is the LBRC and, as in paracellular migration (69), depends on functioning microtubules (73). Microtubule-depolymerizing agents block targeted recycling and block transcellular as well as paracellular migration (73).
Using transfected cell lines, Yang et al. (8) showed that overexpression of ICAM-1 promotes transcellular migration, implicating a role for ICAM-1 in this process. However, when LBRC recycling is inhibited, there is no effect on ICAM-1 enrichment around adherent leukocytes, even though both paracellular and transcellular migration are blocked (73). Therefore, although enrichment of ICAM-1 may help promote transcellular migration and may even be a necessary prerequisite, it is not sufficient to promote transmigration in the absence of a functional LBRC.
The role of the LBRC in transcellular migration explains a number of observations made by several groups of investigators. The appearance in electron micrographs of membrane vesicles clustered around the leukocyte as it transmigrates (55) is consistent with recruitment of the LBRC. Many studies that provide evidence for transcellular migration in vivo often show electron micrographs of leukocytes passing through the cell within 1 or 2 µm of an intact endothelial junction (88, 89). This is exactly where the LBRC is situated and would place it in a prime location for its role in promoting transcellular as well as paracellular migration. Transcellular migration also depends on PECAM and CD99 (73). Because expression of PECAM and CD99 on the cell surface is essentially restricted to the cell borders, this finding was unexpected. However, once the LBRC is recruited and becomes part of the membrane forming the transmigration pore, interaction between leukocyte and endothelial cell PECAM and CD99 is required for transcellular passage.
A UNIFYING MODEL OF (PARACELLULAR AND) TRANSCELLULAR TRANSMIGRATION?
Paracellular migration involves an elaborate series of rolling, adhesion, and locomotion events designed to bring the leukocyte close to the endothelial border. Transcellular migration appears to use the same initial steps. However, for some reason the leukocytes migrate through the cell rather than at the border. A unifying mechanism may involve clustering of ICAM-1 and recruitment of the LBRC.
A signal from the leukocyte may trigger recruitment of the LBRC to the sites of both paracellular and transcellular migration. It could be the same signal in both cases. If the leukocyte were so activated that it transmits the signal prematurely before reaching the lateral border, the signaling cascade that sets the recruitment of the LBRC in motion might be directed to the apical surface of the cell, where the leukocyte makes contact (Figure 3). That transcellular migration is more common among highly and directly activated leukocytes is consistent with this hypothesis. The mechanism by which the LBRC is redirected from the lateral border to the apical surface is not known. However, the clustering of ICAM-1 that precedes LBRC recruitment suggests that it might be involved in transmitting this signal through interactions with the leukocyte. Transcellular migration may also occur if the leukocytes have difficulty reaching the junction, such as (a) in cells in which CD11b is nonfunctional (17) or deficient (92); (b) in cells that are unable to polarize (93); or (c) in vasculature in which the junction is particularly tight, such as the blood-brain barrier (88, 89). A mechanism in which homophilic adhesion molecules such as PECAM, CD99, and JAM-A tightly engage leukocytes as they pass through the endothelial cell could allow leukocyte passage without plasma leakage. The LBRC could provide such a route.
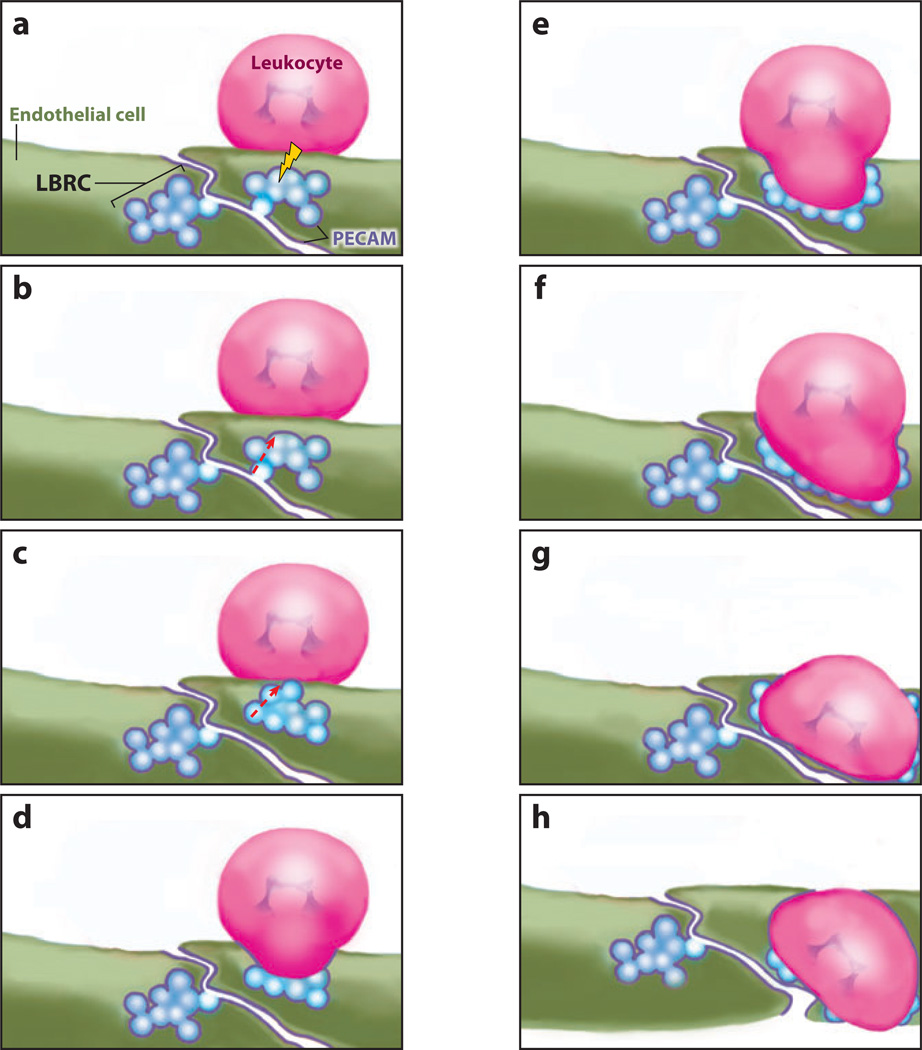
Figure 3.
Schematic view of the movement of the lateral border recycling compartment (LBRC) during paracellular transmigration. (a) The signal that recruits targeted recycling to the vicinity of the leukocyte (lightning bolt) is transmitted while the leukocyte is on the apical surface of the endothelial cell. (b) This process causes targeting of the LBRC membrane along cortical microtubules toward the leukocyte. (c) A single fusion event may be sufficient to allow the entire interconnected LBRC to come into contact with the leukocyte. (d–f) As the leukocyte passes through the endothelial cell, additional LBRC membrane is recruited to surround the leukocyte. The transmigration pore is thus essentially a parajunctional junction lined by molecules the leukocyte needs to interact with [e.g., platelet/endothelial cell adhesion molecule (PECAM), CD99, junctional adhesion molecule (JAM)-A] and lacking those it needs to bypass [e.g., vascular endothelial (VE)-cadherin and associated proteins]. (g) At the basal surface, a second fusion event may be necessary to allow the leukocyte to complete its migration across the endothelial cell. (h) The leukocyte is now in communication with the subendothelial basement membrane and completes diapedesis as if it had migrated paracellularly.
Carman et al. (55) observed leukocytes appearing to probe the endothelial surface with lamellipodia (podosomes), often deeply invaginating the surface of the endothelial cell. They were highly enriched for actin filaments (55, 57), which are required for their function (55). Actin polymerization and lamellipodia formation are events triggered in leukocytes by integrin activation, so prominent podosomes in cells migrating transcellularly could be manifestations of high levels of leukocyte activation. The podosomes were hypothesized to initiate the formation of the transcellular channel. In previous reports, such podosomes were observed invaginating the endothelial surface away from the junctions, even under conditions in which leukocytes crossed at the cell borders (95, 96) and in which the leukocytes were blocked in their attempts to cross at the junctions (figure 4a of Reference 27). These lamellipodia may be a general mechanism for leukocytes to crawl across the endothelial surface. However, if they play a role in promoting transmigration, they may signal the recruitment of LBRC membrane to the leukocytes when they are at the cell borders as well as when they are not. In this case, the mechanisms of paracellular and transcellular migration would have even more in common.
At least one potential mechanistic difference between paracellular and transcellular migration remains. Because the LBRC is connected to the lateral endothelial cell surface at the cell borders, fusion of the LBRC membrane with the plasma membrane is not necessarily required to bring the LBRC in contact with the leukocyte for paracellular TEM. However, to bring this membrane compartment in contact with the leukocyte on the apical surface for transcellular migration would require membrane fusion. Carman et al. (55) provide evidence that membrane fusion is required for transcellular migration. However, because the LBRC vesicles are connected to each other (25), transcellular migration would not require multiple fusion events involving dozens or hundreds of vesicles, but rather the entire surface area of the LBRC could be made to surround the leukocyte with perhaps only two fusion events necessary: one at the apical surface and one at the basal surface of the endothelial cell.
SUMMARY POINTS.
Transmigration can be broken down into sequential steps controlled by different molecules on the surface of the endothelial cell and within the cytoplasm. How the sequential steps are temporally and spatially controlled is a topic for future research.
TEM involves an orchestrated series of events within the endothelial cell to facilitate passage of the leukocyte while maintaining tight apposition to prevent plasma leakage. Clustering of endothelial surface ICAM-1 and VCAM-1, loosening of adherens junctions, and recruitment of membrane from the LBRC are critical, and interfering with any of them is sufficient to block transmigration.
Endothelial surface molecules such as ICAM-1 and VCAM-1 appear to be involved in steps that facilitate transendothelial passage itself, such as (a) stimulating an increase in endothelial cell cytosolic free calcium ion, which activates MLCK, and (b) activating RhoA, which inhibits deactivation of myosin II. Molecules enriched at the cell border, and in particular those in the LBRC such as PECAM and CD99, are involved in the diapedesis step.
The LBRC is a recently described reticulum of interconnected tubulovesicular structures that lies along the cell border at the periphery of the endothelial cell. This compartment communicates with the junction at 37°C. The LBRC is enriched for molecules involved in diapedesis, such as PECAM, CD99, and JAM-A. The PECAM and CD99 in this compartment, rather than the molecules already on the surface of the cell junctions, are critical for transmigration. During diapedesis, membrane from the LBRC is targeted to the site of leukocyte diapedesis.
Targeted recycling of the LBRC is critical for transmigration, even under conditions in which transmigration does not depend on PECAM. Disrupting the microtubules on which it is moved, or blocking the molecular motors that guide the LBRC, blocks transmigration.
The majority of transmigration appears to occur paracellularly. However, there are situations in vivo and certainly in vitro where transcellular migration occurs.
Transcellular migration requires active protrusion of leukocyte pseudopods into the endothelial cell body. Transcellular migration tends to occur when the endothelial cell junctions are particularly tight (as in the blood-brain barrier), when the leukocytes have difficulty reaching the cell junctions, or when the leukocytes are strongly activated.
Transcellular migration involves clustering of ICAM-1 and can be blocked by blocking PECAM and CD99. It depends on recruitment of the LBRC along microtubules to the site of leukocyte migration. Thus, transcellular and paracellular migration may have more mechanistic similarities than differences.
FUTURE ISSUES.
Why are so many endothelial cell molecules implicated in the process of transendothelial migration? Do they function individually or as part of multicomponent complexes?
Where is actin-myosin contraction tension exerted in vivo, given that endothelial cells in postcapillary venules do not have stress fibers?
What directs LBRC targeted recycling?
How are all of the molecules and mechanisms that have been identified to participate in TEM coordinated to ensure efficient leukocyte emigration with minimal leakage of soluble vascular contents?
ACKNOWLEDGMENTS
I am supported by grant numbers R01HL046849 and R37HL064774 from the National Institutes of Health. I thank Gillian Muller for drawing Figures 1 and 3 and Sari Kadison-Shapiro for drawing Figure 2.
Glossary
- Transendothelial migration (TEM)
migration of leukocytes across the endothelial cell barrier
- Diapedesis
the squeezing of the leukocyte through a tiny aperture between or through endothelial cells as it leaves the bloodstream to enter the tissues
- Paracellular migration
migration of leukocytes between endothelial cells
- Transcellular migration
migration of leukocytes through the cytoplasm of endothelial cells
- ICAM-1
intercellular adhesion molecule 1
- VCAM-1
vascular cell adhesion molecule 1
- Docking structures/transmigratory cups
projections of endothelial apical membrane enriched for ICAM-1 and VCAM-1; reported by some investigators to partially surround adherent leukocytes
- JAM-A
junctional adhesion molecule A
- PECAM
platelet/endothelial cell adhesion molecule
- VE-cadherin
vascular endothelial cell–specific cadherin
- HUVEC
human umbilical vein endothelial cell(s)
- MLCK
myosin light-chain kinase
- Lateral border recycling compartment (LBRC)
an interconnected reticulum of 50-nm vesicle-like structures; continuous with the lateral border of endothelial cells at 37°C
- Targeted recycling (trafficking) of the LBRC
microtubule-dependent rapid movement of LBRC membrane to the site of leukocyte transmigration
- Podosomes
actin-rich protrusions of leukocyte membrane and cytoplasm that indent the endothelial surface as the leukocyte crawls over it
Footnotes
DISCLOSURE STATEMENT
The author is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:326–333. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 2.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 3.Muller WA. PECAM: regulating the start of diapedesis. In: Ley K, editor. Adhesion Molecules: Function and Inhibition. Basel: Birkhauser; 2007. pp. 201–220. [Google Scholar]
- 4.Reilly PL, Woska JR, Jr, Jeanfavre DD, McNally E, Rothlein R, Bormann BJ. The native structure of intercellular adhesion molecule-1 (ICAM-1) is a dimer. Correlation with binding to LFA-1. J. Immunol. 1995;155:529–532. Erratum. 1996. J. Immunol. 156:3088. [PubMed] [Google Scholar]
- 5.Miller J, Knorr R, Ferrone M, Houdei R, Carron CP, Dustin ML. Intercellular adhesion molecule 1 dimerization and its consequences for adhesion mediated by lymphocyte function associated 1. J. Exp. Med. 1995;182:1231–1241. doi: 10.1084/jem.182.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw SK, Ma S, Kim MB, Rao RM, Hartman CU, et al. Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompany neutrophil transmigration. J. Exp. Med. 2004;200:1571–1580. doi: 10.1084/jem.20040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Kowalski JR, Zhan X, Thomas SM, Luscinskas FW. Endothelial cell cortactin phosphorylation by Src contributes to polymorphonuclear leukocyte transmigration in vitro. Circ. Res. 2006;98:394–402. doi: 10.1161/01.RES.0000201958.59020.1a. [DOI] [PubMed] [Google Scholar]
- 8.Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-α-activated vascular endothelium under flow. Blood. 2005;106:584–592. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, et al. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J. Cell Biol. 2002;157:1233–1245. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J. Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook-Mills JM, Johnson JD, Deem TL, Ochi A, Wang L, Zheng Y. Calcium mobilization and Rac1 activation are required for VCAM-1 (vascular cell adhesion molecule-1) stimulation of NADPH oxidase activity. Biochem. J. 2004;378:539–547. doi: 10.1042/BJ20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiss Y, Hoch G, Deutsch U, Engelhardt B. T cell interaction with ICAM-1-deficient endothelium in vitro: essential role for ICAM-1 and ICAM-2 in transendothelial migration of T cells. Eur. J. Immunol. 1998;28:3086–3099. doi: 10.1002/(SICI)1521-4141(199810)28:10<3086::AID-IMMU3086>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 13.Huang MT, Larbi KY, Scheiermann C, Woodfin A, Gerwin N, et al. ICAM-2 mediates neutrophil transmigration in vivo: evidence for stimulus specificity and a role in PECAM-1-independent transmigration. Blood. 2006;107:4721–4727. doi: 10.1182/blood-2005-11-4683. [DOI] [PubMed] [Google Scholar]
- 14.Woodfin A, Voisin MB, Imhof BA, Dejana E, Engelhardt B, Nourshargh S. Endothelial cell activation leads to neutrophil transmigration as supported by the sequential roles of ICAM-2, JAM-A and PECAM-1. Blood. 2009;113:6246–6257. doi: 10.1182/blood-2008-11-188375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostermann G, Weber KSC, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the β2 integrin LFA-1 involved in transendothelial migration of leukocytes. Nat. Immunol. 2002;3:151–158. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Nusrat A, Schnell FJ, Reaves TA, Walsh S, et al. Human junction adhesion molecule regulates tight junction resealing in epithelia. J. Cell Sci. 2000;113:2363–2374. doi: 10.1242/jcs.113.13.2363. [DOI] [PubMed] [Google Scholar]
- 17.Schenkel AR, Mamdouh Z, Muller WA. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat. Immunol. 2004;5:393–400. doi: 10.1038/ni1051. [DOI] [PubMed] [Google Scholar]
- 18.Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J. Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson-Leger C, Aurrand-Lions M, Beltraminelli N, Fasel N, Imhof BA. Junctional adhesion molecule-2 (JAM-2) promotes lymphocyte transendothelial migration. Blood. 2002;100:2479–2486. doi: 10.1182/blood-2001-11-0098. [DOI] [PubMed] [Google Scholar]
- 20.Chavakis T, Keiper T, Matz-Westphal R, Hersemeyer K, Sachs UJ, et al. The junctional adhesion molecule C promotes neutrophil transendothelial migration in vitro and in vivo. J. Biol. Chem. 2004;279:55602–55608. doi: 10.1074/jbc.M404676200. [DOI] [PubMed] [Google Scholar]
- 21.Weber C, Fraemohs L, Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat. Rev. Immunol. 2007;7:467–477. doi: 10.1038/nri2096. [DOI] [PubMed] [Google Scholar]
- 22.Nasdala I, Wolburg-Buchholz K, Wolburg H, Kuhn A, Ebnet K, et al. A transmembrane tight junction protein selectively expressed on endothelial cells and platelets. J. Biol. Chem. 2002;277:16294–16303. doi: 10.1074/jbc.M111999200. [DOI] [PubMed] [Google Scholar]
- 23.Wegmann F, Petri B, Khandoga AG, Moser C, Khandoga A, et al. ESAM supports neutrophil extravasation, activation of Rho, and VEGF-induced vascular permeability. J. Exp. Med. 2006;203:1671–1677. doi: 10.1084/jem.20060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J. Exp. Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mamdouh Z, Chen X, Pierini LM, Maxfield FR, Muller WA. Targeted recycling of PECAM from endothelial cell surface-connected compartments during diapedesis. Nature. 2003;421:748–753. doi: 10.1038/nature01300. [DOI] [PubMed] [Google Scholar]
- 26.Dasgupta B, Dufour E, Mamdouh Z, Muller W. A novel and critical role for tyrosine 663 in PECAM trafficking and transendothelial migration. J. Immunol. 2009;182:5041–5051. doi: 10.4049/jimmunol.0803192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao F, Huynh HK, Eiroa A, Greene T, Polizzi E, Muller WA. Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1. J. Exp. Med. 1995;182:1337–1343. doi: 10.1084/jem.182.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogen S, Pak J, Garifallou M, Deng X, Muller WA. Monoclonal antibody to murine PECAM-1 [CD31] blocks acute inflammation in vivo. J. Exp. Med. 1994;179:1059–1064. doi: 10.1084/jem.179.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schenkel AR, Chew TW, Muller WA. Platelet endothelial cell adhesion molecule deficiency or blockade significantly reduces leukocyte emigration in a majority of mouse strains. J. Immunol. 2004;173:6403–6408. doi: 10.4049/jimmunol.173.10.6403. [DOI] [PubMed] [Google Scholar]
- 30.Seidman MA, Chew TW, Schenkel AR, Muller WA. PECAM-independent thioglycollate peritonitis is associated with a locus on murine chromosome 2. PLoS ONE. 2009;4:e4316. doi: 10.1371/journal.pone.0004316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, et al. Genetic evidence for functional redundancy of platelet/endothelial cell adhesion molecule 1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J. Immunol. 1999;162:3022–3030. [PubMed] [Google Scholar]
- 32.Schenkel AR, Chew TW, Chlipala E, Harbord MW, Muller WA. Different susceptibilities of PECAM-deficient mouse strains to spontaneous idiopathic pneumonitis. Exp. Mol. Pathol. 2006;81:23–30. doi: 10.1016/j.yexmp.2005.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakelin MW, Sanz M-J, Dewar A, Albelda SM, Larkin SW, et al. An antiplatelet/endothelial cell adhesion molecule 1 antibody inhibits leukocyte extravasation from mesenteric microvessels in vivo by blocking the passage through basement membrane. J. Exp. Med. 1996;184:229–239. doi: 10.1084/jem.184.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suh YH, Shin YK, Kook MC, Oh KI, Park WS, et al. Cloning, genomic organization, alternative transcripts and expression analysis of CD99L2, a novel paralog of human CD99, and identification of evolutionary conserved motifs. Gene. 2003;307:63–76. doi: 10.1016/s0378-1119(03)00401-3. [DOI] [PubMed] [Google Scholar]
- 35.Smith MJ, Goodfellow PJ, Goodfellow PN. The genomic organisation of the human pseudoautosomal gene MIC2 and the detection of a related locus. Hum. Mol. Genet. 1993;2:417–422. doi: 10.1093/hmg/2.4.417. [DOI] [PubMed] [Google Scholar]
- 36.Park SH, Shin YK, Suh YH, Park WS, Ban YL, et al. Rapid divergency of rodent CD99 orthologs: implications for the evolution of the pseudoautosomal region. Gene. 2005;353:177–188. doi: 10.1016/j.gene.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 37.Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat. Immunol. 2002;3:143–150. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- 38.Lou O, Alcaide P, Luscinskas FW, Muller WA. CD99 is a key mediator of the transendothelial migration of neutrophils. J. Immunol. 2007;178:1136–1143. doi: 10.4049/jimmunol.178.2.1136. [DOI] [PubMed] [Google Scholar]
- 39.Bixel G, Kloep S, Butz S, Petri B, Engelhardt B, Vestweber D. Mouse CD99 participates in T cell recruitment into inflamed skin. Blood. 2004;104:3205–3213. doi: 10.1182/blood-2004-03-1184. [DOI] [PubMed] [Google Scholar]
- 40.Dufour EM, Deroche A, Bae Y, Muller WA. CD99 is essential for leukocyte diapedesis in vivo. Cell Commun. Adhes. 2008;15:351–363. doi: 10.1080/15419060802442191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schenkel AR, Dufour EM, Chew TW, Sorg E, Muller WA. The murine CD99-related molecule CD99-like 2 (CD99L2) is an adhesion molecule involved in the inflammatory response. Cell Commun. Adhes. 2007;14:227–237. doi: 10.1080/15419060701755966. [DOI] [PubMed] [Google Scholar]
- 42.Bixel MG, Petri B, Khandoga AG, Khandoga A, Wolburg-Buchholz K, et al. A CD99-related antigen on endothelial cells mediates neutrophil, but not lymphocyte extravasation in vivo. Blood. 2007;109:5327–5336. doi: 10.1182/blood-2006-08-043109. [DOI] [PubMed] [Google Scholar]
- 43.Gotsch U, Borges E, Bosse R, Boggemeyer E, Simon M, et al. VE-cadherin antibody accelerates neutrophil recruitment in vivo. J. Cell Sci. 1997;110:583–588. doi: 10.1242/jcs.110.5.583. [DOI] [PubMed] [Google Scholar]
- 44.Allport JR, Muller WA, Luscinskas FW. Monocytes induce reversible focal changes in vascular endothelial cadherin complex during transendothelial migration under flow. J. Cell Biol. 2000;148:203–216. doi: 10.1083/jcb.148.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaw SK, Bamba PS, Perkins BN, Luscinskas FW. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J. Immunol. 2001;167:2323–2330. doi: 10.4049/jimmunol.167.4.2323. [DOI] [PubMed] [Google Scholar]
- 46.Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J. Immunol. 2007;179:4053–4064. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
- 47.Alcaide P, Newton G, Auerbach S, Sehrawat S, Mayadas TN, et al. p120-Catenin regulates leukocyte transmigration through an effect on VE-cadherin phosphorylation. Blood. 2008;112:2770–2779. doi: 10.1182/blood-2008-03-147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reymond N, Imbert AM, Devilard E, Fabre S, Chabannon C, et al. DNAM-1 and PVR regulate monocyte migration through endothelial junctions. J. Exp. Med. 2004;199:1331–1341. doi: 10.1084/jem.20032206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bardin N, Blot-Chabaud M, Despoix N, Kebir A, Harhouri K, et al. CD146 and its soluble form regulate monocyte transendothelial migration. Arterioscler. Thromb. Vasc. Biol. 2009;29:746–753. doi: 10.1161/ATVBAHA.108.183251. [DOI] [PubMed] [Google Scholar]
- 50.Masedunskas A, King JA, Tan F, Cochran R, Stevens T, et al. Activated leukocyte cell adhesion molecule is a component of the endothelial junction involved in transendothelial monocyte migration. FEBS Lett. 2006;580:2637–2645. doi: 10.1016/j.febslet.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 51.Stefanidakis M, Newton G, Lee WY, Parkos CA, Luscinskas FW. Endothelial CD47 interaction with SIRPγ is required for human T-cell transendothelial migration under shear flow conditions in vitro. Blood. 2008;112:1280–1289. doi: 10.1182/blood-2008-01-134429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin S, Umemoto E, Tanaka T, Shimomura Y, Tohya K, et al. Nepmucin/CLM-9, an Ig domain–containing sialomucin in vascular endothelial cells, promotes lymphocyte transendothelial migration in vitro. FEBS Lett. 2008;582:3018–3024. doi: 10.1016/j.febslet.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 53.Durieu-Trautmann O, Chaverot N, Cazaubon S, Strosberg AD, Couraud PO. Intercellular adhesion molecule 1 activation induces tyrosine phosphorylation of the cytoskeleton-associated protein cortactin in brain microvessel endothelial cells. J. Biol. Chem. 1994;269:12536–12540. [PubMed] [Google Scholar]
- 54.Carman CV, Jun CD, Salas A, Springer TA. Endothelial cells proactively form microvilli-like membrane projections upon intercellular adhesion molecule 1 engagement of leukocyte LFA-1. J. Immunol. 2003;171:6135–6144. doi: 10.4049/jimmunol.171.11.6135. [DOI] [PubMed] [Google Scholar]
- 55.Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, et al. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 2007;26:784–797. doi: 10.1016/j.immuni.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Millan J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat. Cell Biol. 2006;8:113–123. doi: 10.1038/ncb1356. [DOI] [PubMed] [Google Scholar]
- 57.Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM. Neutrophils emigrate from venules by a transendothelial cell pathway in response to fMLP. J. Exp. Med. 1998;187:903–915. doi: 10.1084/jem.187.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Buul JD, Allingham MJ, Samson T, Meller J, Boulter E, et al. RhoG regulates endothelial apical cup assembly downstream from ICAM1 engagement and is involved in leukocyte trans-endothelial migration. J. Cell Biol. 2007;178:1279–1293. doi: 10.1083/jcb.200612053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Buul JD, Kanters E, Hordijk PL. Endothelial signaling by Ig-like cell adhesion molecules. Arterioscler. Thromb. Vasc. Biol. 2007;27:1870–1876. doi: 10.1161/ATVBAHA.107.145821. [DOI] [PubMed] [Google Scholar]
- 60.Lorenzon P, Vecile E, Nardon E, Ferrero E, Harlan JM, et al. Endothelial cell E- and P-selectin and vascular cell adhesion molecule 1 function as signaling receptors. J. Cell Biol. 1998;142:1381–1391. doi: 10.1083/jcb.142.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang AJ, Manning JE, Bandak TM, Ratau MC, Hanser KR, Silverstein SC. Endothelial cell cytosolic free calcium regulates neutrophil migration across monolayers of endothelial cells. J. Cell Biol. 1993;120:1371–1380. doi: 10.1083/jcb.120.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hixenbaugh EA, Goeckeler ZM, Papaiya NN, Wysolmerski RB, Silverstein SC, Huang AJ. Chemoattractant-stimulated neutrophils induce regulatory myosin light chain phosphorylation and isometric tension development in endothelial cells. Am. J. Physiol. 1997;273:H981–H988. doi: 10.1152/ajpheart.1997.273.2.H981. [DOI] [PubMed] [Google Scholar]
- 63.Turowski P, Martinelli R, Crawford R, Wateridge D, Papageorgiou AP, et al. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J. Cell Sci. 2008;121:29–37. doi: 10.1242/jcs.022681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Wetering S, van Buul JD, Quik S, Mul FP, Anthony EC, et al. Reactive oxygen species mediate Rac-induced loss of cell-cell adhesion in primary human endothelial cells. J. Cell Sci. 2002;115:1837–1846. doi: 10.1242/jcs.115.9.1837. [DOI] [PubMed] [Google Scholar]
- 65.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the β-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 66.Nottebaum AF, Cagna G, Winderlich M, Gamp AC, Linnepe R, et al. VE-PTP maintains the endothelial barrier via plakoglobin and becomes dissociated from VE-cadherin by leukocytes and by VEGF. J. Exp. Med. 2008;205:2929–2945. doi: 10.1084/jem.20080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cernuda-Morollon E, Ridley AJ. Rho GTPases and leukocyte adhesion receptor expression and function in endothelial cells. Circ. Res. 2006;98:757–767. doi: 10.1161/01.RES.0000210579.35304.d3. [DOI] [PubMed] [Google Scholar]
- 68.Majno G, Palade GE. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J. Biophys. Biochem. Cytol. 1961;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mamdouh Z, Kreitzer GE, Muller WA. Leukocyte transmigration requires kinesin-mediated microtubule-dependent membrane trafficking from the lateral border recycling compartment. J. Exp. Med. 2008;205:951–966. doi: 10.1084/jem.20072328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dvorak AM, Kohn S, Morgan ES, Fox P, Nagy JA, Dvorak HF. The vesiculo-vacuolar organelle (VVO): a distinct endothelial cell structure that provides a transcellular pathway for macromolecular extravasation. J. Leukoc. Biol. 1996;59:100–115. [PubMed] [Google Scholar]
- 71.Feng D, Nagy JA, Hipp J, Dvorak HF, Dvorak AM. Vesiculo-vacuolar organelles and the regulation of venule permeability to macromolecules by vascular permeability factor, histamine, and serotonin. J. Exp. Med. 1996;183:1981–1986. doi: 10.1084/jem.183.5.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vasile E, Qu H, Dvorak HF, Dvorak AM. Caveolae and vesiculo-vacuolar organelles in bovine capillary endothelial cells cultured with VPF/VEGF on floating Matrigel-collagen gels. J. Histochem. Cytochem. 1999;47:159–167. doi: 10.1177/002215549904700205. [DOI] [PubMed] [Google Scholar]
- 73.Mamdouh Z, Mikhailov A, Muller WA. Transcellular migration of leukocytes is mediated by the endothelial lateral border recycling compartment. J. Exp. Med. 2009;206:2795–2808. doi: 10.1084/jem.20082745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bird IN, Spragg JH, Ager AH, Matthews N. Studies of lymphocyte transendothelial migration: analysis of migrated cell phenotypes with regard to CD31 (PECAM-1), CD45RA and CD45RO. Immunology. 1993;80:553–560. [PMC free article] [PubMed] [Google Scholar]
- 75.Muller WA. Migration of leukocytes across endothelial junctions: some concepts and controversies. Microcirculation. 2001;8:181–193. doi: 10.1038/sj/mn/7800078. [DOI] [PubMed] [Google Scholar]
- 76.Dasgupta B, Muller WA. Endothelial Src kinase regulates membrane recycling from the lateral border recycling compartment during leukocyte transendothelial migration. Eur. J. Immunol. 2008;38:3499–3507. doi: 10.1002/eji.200838605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muller WA, Schenkel AR. Figure 13.2. In: Wedlich D, editor. Cell Migration in Development and Disease. Weinheim, Ger.: Wiley; 2005. p. 246. [Google Scholar]
- 78.Xiao K, Garner J, Buckley KM, Vincent PA, Chiasson CM, et al. p120-Catenin regulates clathrin-dependent endocytosis of VE-cadherin. Mol. Biol. Cell. 2005;16:5141–5151. doi: 10.1091/mbc.E05-05-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Muller WA. How endothelial cells regulate transendothelial migration leukocytes: molecules and mechanisms. Curr. Top. Membr. 2009;64:335–355. [Google Scholar]
- 80.Cook-Mills JM. VCAM-1 signals during lymphocyte migration: role of reactive oxygen species. Mol. Immunol. 2002;39:499–508. doi: 10.1016/s0161-5890(02)00206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Wetering S, van den Berk N, van Buul JD, Mul FP, Lommerse I, et al. VCAM-1-mediated Rac signaling controls endothelial cell-cell contacts and leukocyte transmigration. Am. J. Physiol. Cell Physiol. 2003;285:C343–C352. doi: 10.1152/ajpcell.00048.2003. [DOI] [PubMed] [Google Scholar]
- 82.Abdala-Valencia H, Cook-Mills JM. VCAM-1 signals activate endothelial cell protein kinase Cα via oxidation. J. Immunol. 2006;177:6379–6387. doi: 10.4049/jimmunol.177.9.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Williamson JR, Grisham JW. Electron microscopy of leukocytic margination and emigration in acute inflammation in dog pancreas. Am. J. Pathol. 1961;39:239–256. [PMC free article] [PubMed] [Google Scholar]
- 84.Marchesi VT, Gowans JL. The migration of lymphocytes through the endothelium of venules in lymph nodes: an electron microscope study. Proc. R. Soc. Lond. B. 1964;159:283–290. doi: 10.1098/rspb.1964.0002. [DOI] [PubMed] [Google Scholar]
- 85.Bamforth S, Lightman S, Greenwood J. Ultrastructural analysis of interleukin-1β-induced leukocyte recruitment to the rat retina. Investig. Opthalmol. Vis. Sci. 1997;38:25–35. [PubMed] [Google Scholar]
- 86.Sage PT, Carman CV. Settings and mechanisms for trans-cellular diapedesis. Front. Biosci. 2009;14:5066–5083. doi: 10.2741/3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carman CV, Springer TA. Trans-cellular migration: Cell-cell contacts get intimate. Curr. Opin. Cell Biol. 2008;20:533–540. doi: 10.1016/j.ceb.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lossinsky AS, Shivers RR. Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Histol. Histopathol. 2004;19:535–564. doi: 10.14670/HH-19.535. [DOI] [PubMed] [Google Scholar]
- 89.Wolburg H, Wolburg-Buchholz K, Engelhardt B. Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta Neuropathol. 2005;109:181–190. doi: 10.1007/s00401-004-0928-x. [DOI] [PubMed] [Google Scholar]
- 90.Simionescu N, Simionescu M. The cardiovascular system. In: Weiss L, editor. Histology: Cell and Tissue Biology. New York: Elsevier; 1983. pp. 371–433. [Google Scholar]
- 91.Furie MB, Cramer EB, Napstek BL, Silverstein SC. Cultured endothelial cell monolayers that restrict the transendothelial passage of macromolecules and electrical current. J. Cell Biol. 1984;98:1033–1041. doi: 10.1083/jcb.98.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J. Exp. Med. 2006;203:2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gerard A, van der Kammen RA, Janssen H, Ellenbroek SI, Collard JG. The Rac activator Tiam1 controls efficient T-cell trafficking and route of trans-endothelial migration. Blood. 2009;113:6138–6147. doi: 10.1182/blood-2008-07-167668. [DOI] [PubMed] [Google Scholar]
- 94.Muller WA, Weigl S. Monocyte-selective transendothelial migration: dissection of the binding and transmigration phases by an in vitro assay. J. Exp. Med. 1992;176:819–828. doi: 10.1084/jem.176.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Furie MB, Naprstek BL, Silverstein SC. Migration of neutrophils across monolayers of cultured microvascular endothelial cells: an in vitro model of leukocyte extravasation. J. Cell Sci. 1987;88:161–175. doi: 10.1242/jcs.88.2.161. [DOI] [PubMed] [Google Scholar]
- 96.Migliorisi G, Folkes E, Pawlowski N, Cramer EB. In vitro studies of human monocyte migration across endothelium in response to leukotriene B4 and f-Met-Leu-Phe. Am. J. Pathol. 1987;127:157–167. [PMC free article] [PubMed] [Google Scholar]