Abstract
Type 1 diabetes (T1DM) affects one in every 400 children and adolescents in the US. Due to the limitations of exogenous insulin therapy and whole pancreas transplantation, pancreatic islet transplantation has emerged as a promising therapy for T1DM. However, this therapy is severely limited by donor islet availability and poor islet engraftment and function. We engineered an injectable bio-synthetic, polyethylene glycol-maleimide hydrogel to enhance vascularization and engraftment of transplanted pancreatic islets in a mouse model of T1DM. Controlled presentation of VEGF-A and cell adhesive peptides within this engineered material significantly improved the vascularization and function of islets delivered to the small bowel mesentery, a metabolically relevant site for insulin release. Diabetic mice receiving islets transplanted in proteolytically degradable hydrogels incorporating VEGF-A exhibited complete reversal of diabetic hyperglycemia with a 40% reduction in the number of islets required. Furthermore, hydrogel-delivered islets significantly improved weight gain, regulation of a glucose challenge, and intra-islet vascularization and engraftment compared to the clinical standard of islet infusion through the hepatic portal vein. This study establishes a simple biomaterial strategy for islet transplantation to promote enhanced islet engraftment and function.
1. Introduction
T1DM, a condition that results from the autoimmune destruction of the insulin-producing beta cells in the pancreas, requires careful management of blood glucose through exogenous insulin therapy to control serious complications that result from chronically high blood glucose. In the US, one in every 400 children and adolescents is living with T1DM [1, 2] and the worldwide incidence of T1DM is increasing ~3% per year [3]. Pancreatic islet transplantation has emerged as a promising therapy for T1DM to address the limitations associated with exogenous insulin therapy. Despite initial improvements in metabolic control, less than 20% of transplant recipients remain exogenous insulin-independent after 3–5 years due to islet loss and poor engraftment [4–8]. Many factors contribute to both islet death at transplantation and progressive graft loss [9]. In the current standard of clinical islet transplantation, a large bolus of donor islets is infused into the hepatic portal vein where the transplanted cells lodge downstream in the liver [10]. Islets transplanted in this manner are at major risk of loss from instant blood-mediated inflammatory reaction (IBMIR), an activation of the complement and coagulation cascades, during the injection procedure [11, 12]. Upon lodging in the liver, islets are exposed to acute ischemia and inflammation. Transplanted islets suffer from fibrosis, glucolipotoxicity, exposure to high levels of immunosuppressive drugs, and poor revascularization [13–15]. Engraftment failure of as many as 50–75% of islets transplanted intrahepatically requires a very high number of donor islets from 2–3 cadaver donors and often multiple islet infusions to achieve independent normoglycemia [16]. Despite these disadvantages, hepatic portal transplantation remains the primary target of clinical islet transplantation because the site is metabolically relevant for maintenance of glucose homeostasis, the procedure is considered to be low risk for the patient, and is the only site that has been routinely used successfully in the clinic [17]. If islet transplantation is to become more widely available as a treatment option, strategies are needed that significantly reduce the number of islets required per transplant recipient through improvements to the efficiency of transplanted islet engraftment and alternative implantation sites [16, 18].
Poor islet revascularization after transplantation is one of the major impediments to long-term islet engraftment and function [19, 20]. Native islets in the pancreas are highly vascularized with fenestrated endothelium throughout the islet core and receive 15–20% of pancreatic blood supply while comprising only 1–2% of the total mass [20, 21]. This high degree of vascularization is rarely recapitulated in transplanted islets [22, 23]. Attempts have been made to augment islet vascularization by gene or protein delivery in animal models [24–31], but many of these techniques are difficult to translate due to complex or insufficient protein delivery strategies and raise serious safety concerns associated with exogenous gene expression. Co-delivery of progenitor or endothelial cells has also been shown to augment islet vascularization [32, 33]. In this study, we present a simple and effective biomaterial solution to support grafting and revascularization of transplanted islets combined with a metabolically relevant transplant site that avoids the negative effects of direct injection into the hepatic portal vein.
2. Materials and Methods
2.1 Hydrogel preparation and VEGF release characterization
PEG-MAL (20 kDa MW, Laysan Bio) precursor was pre-functionalized with RGD peptide and/or rhVEGF165 (Invitrogen) for 1 hour at room temperature in PBS + 4 mM triethanolamine and cross-linked with addition of VPM at 1:2 molar ratio of VPM peptide to available MAL groups. The final concentrations of RGD and VEGF in the gel were 2 mM and 10 μg/mL, respectively. PEG-MAL gels were degraded in 100 μg/mL collagenase I at 37 °C. Released VEGF was quantified by ELISA. Gel degradation products were run on SDS-PAGE and Western blotted for VEGF or stained for PEG with 4% BaCl2 and 0.1% iodine. For endothelial cell proliferation, human umbilical vein endothelial cells (HUVEC, Lonza) were grown in endothelial growth media EGM-2 (Lonza) and synchronized in growth factor free basal media (EBM-2, Lonza) with 1% fetal bovine serum overnight followed by addition of VEGF, PEG-MAL-conjugated VEGF, PEG-MAL only, or EGM-2 for 24 hours. Cell proliferation and metabolism were assayed by the Click-iT-Edu kit (Invitrogen) and CellTiter 96 MTS Aqueous Cell Proliferation Assay (Promega).
2.2 PEG-MAL biodistribution and toxicity
PEG-MAL-750 analog degradation product was synthesized by reacting RGD-functionalized PEG-MAL macromers with GCRDVPMS and MRGGDRCG, followed by labeling with VivoTag-S 750 (Perkin Elmer). PEG-MAL-750 (20 mg) in PBS was injected IP into male Lewis rats. After 24 hours organs were scanned on a Xenogen IVIS Lumina II in vivo fluorescence imager. For toxicity, Lewis rats received implants of 100 μL or 1 mL PEG-MAL hydrogel on the small bowel mesentery. Serum and tissue specimens were analyzed by a blinded pathology team at the University of Georgia Veterinary Comparative Pathology Lab.
2.3 Islet transplantation into diabetic mice
Islets were isolated from healthy 8–10 week old male C57BL/6J mice (Jackson Laboratories) following standard islet isolation procedures [34]. Male C57BL/6J mice were made diabetic by 5 successive daily intraperitoneal injections of 30 mg/kg streptozotocin. Mice were monitored daily for weight loss and blood glucose but did not receive supplemental insulin dosing during the course of the study. For transplantation surgery, mice were laparotomized with a 1 cm two-layer incision through the skin and abdominal muscle along the linea alba. A small section of small bowel and associated mesentery were gently exteriorized. PEG-MAL macromer (100 μL) pre-functionalized with 2 mM RGD and ± 1 μg VEGF was mixed with islets (isolated the previous day) and 2.6 mM peptide cross-linker. The mixture was immediately pipetted onto the small bowel mesentery and allowed to cross-link for 5 minutes. For hepatic portal vein injection, a minor mesenteric tributary vein was selected for injection of islets in 100 μL of PBS. After hydrogel formation or islet injection, the small bowel was reinserted into the abdomen and the animal closed. Glucose tolerance testing was performed by IP injection of 2 g/kg D-glucose and corresponding changes in blood glucose levels were measured by tail tip blood.
2.4 Immunolabeling and histology
Prior to euthanasia, animals were injected IV with FITC-labeled tomato lectin to label perfused vasculature. Explanted islet grafts were embedded in ImmunoBed (PolySciences). Sections were stained with chicken anti-insulin primary antibody (Abcam). Vascularization was quantified in ImageJ as the ratio of lectin-positive intra-islet vessel area to total insulin-positive islet area.
2.5 Animal care and use
All animal experiments were performed with the approval of the Georgia Tech Animal Care and Use Committee (IACUC) under the supervision a research veterinarian and within the guidelines of the Guide for the Care and Use of Laboratory Animals.
2.6 Statistics
Results were analyzed by analysis of variance (ANOVA) in GraphPad Prism 6. If deemed significant, post-hoc pairwise comparisons were performed, and a confidence level of 95% was considered significant.
3.0 Results
3.1 Bioactive VEGF-releasing PEG-MAL hydrogel
We engineered a bioactive PEG hydrogel to achieve controlled, in situ delivery of islets and angiogenic factors to the small bowel mesentery of diabetic mice. This bioactive hydrogel is based on a maleimide-functionalized 4-arm PEG macromere (PEG-MAL) which is functionalized with bioactive molecules in a plug-and-play manner (Fig. 1A). We previously showed that this material offers significant advantages over other types of Michael-type addition or UV-cross-linked PEG hydrogels for cell delivery [35]. The maleimide-functionalized PEG precursors react with available thiols in target biomolecules to generate rapid, cyto-compatible curing of a hydrogel that self-adheres to tissue surfaces with minimal inflammation (no sutures or staples are required). To promote angiogenesis PEG-MAL macromer was pre-functionalized with the angiogenic growth factor VEGF-A165 (VEGF) and GRGDSPC (RGD) peptide, a ligand for the angiogenic cell-adhesion integrin receptor αvβ3. The peptide-functionalized PEG-MAL macromer was cross-linked into a gel with a cysteine-flanked protease-degradable peptide GCRDVPMS↓MRGGDRCG (VPM), where ↓ indicates the cleavage point [36]. VPM is a synthetic peptide readily cleavable by a wide repertoire of protease enzymes. The protease-degradable cross-links were designed to provide for cell-demanded VEGF release and tissue ingrowth.
Fig. 1.
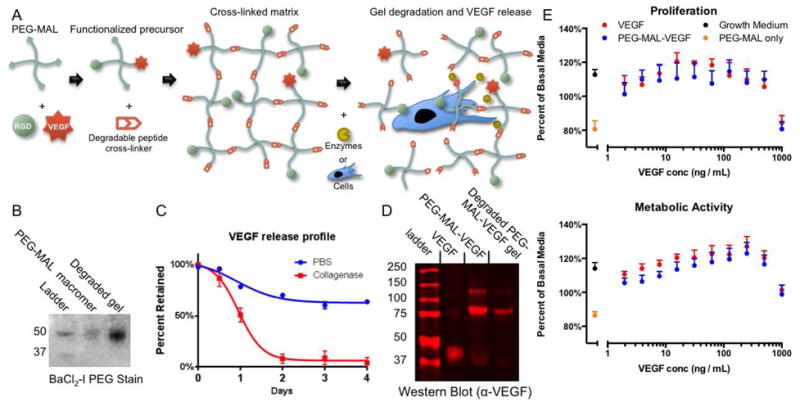
PEG-MAL hydrogels for on-demand release of VEGF. (A) 4-arm PEG-MAL macromer is functionalized by maleimide-thiol Michael-type addition reactions with RGD peptide and VEGF growth factor. The matrix is cross-linked by a cysteine-flanked degradable peptide cross-linker GCRDVPMSMRGGDRCG. Proteases or protease-expressing cells degrade the hydrogel by cleaving cross-linker peptides and release VEGF. (B) BaCl2-I stain for PEG showing MW of PEG-MAL precursor and gel degradation product. (C) VEGF release profile from degrading gels or gels treated in PBS as measured by ELISA (± S.E.M. n=6). (D) Western blot of PEG-MAL-VEGF functionalized precursor or gel degradation product compared to native VEGF showing molecular weight change of VEGF once PEGylated. (E) Endothelial cell proliferation assay for VEGF vs. PEG-MAL-VEGF bioactivity measured by metabolic activity (MTS) and proliferation (EdU incorporation) (± S.E.M. n=6).
PEG-MAL gels could be degraded with collagenase to generate a uniform molecular weight PEG product of equivalent size to the PEG-MAL macromer precursor (Fig. 1B). This result indicates that the hydrogel can completely degrade into a small product likely to be easily cleared through renal filtration. Upon degradation of the gel with collagenase, 100% of incorporated VEGF was released, whereas gels incubated in PBS retained 75% of the incorporated VEGF (Fig. 1C). While the VEGF released from enzyme-degraded gels remained PEG macromer-bound (Fig. 1D), it retained full bioactivity compared to free VEGF as shown by dose-dependent effects on endothelial cell proliferation and metabolic activity (Fig. 1E). These results indicate that PEG-MAL hydrogels retain VEGF bound to the gel and release it in a bioactive form in an on-demand manner when proteases expressed through tissue remodeling processes cleave the degradable peptide cross-links.
3.2 Biodistribution and toxicity of PEG-MAL hydrogel
We investigated the biodistribution and toxicity of PEG-MAL hydrogel delivered in vivo because these are important considerations regarding the safety and translation potential of these biomaterials. For biodistribution analyses, PEG-MAL precursors, functionalized with RGD and pre-cleaved VPM peptides, were labeled with the near-IR dye VivoTagS-750 to generate PEG-MAL-750, a fluorescently labeled analog of the hydrogel degradation product (Fig. 2A). We verified conjugation, labeling, and purity of this analog degradation product (Fig. 2B). We delivered a single bolus injection of PEG-MAL-750 to the intraperitoneal cavity of rats and collected urine and feces over 24 hours. Animals were sacrificed and the organs were visualized for tracing of the dye with an IVIS fluorescence imager (Fig. 2C,D). We observed the strongest IR signals in urine. When normalized to compartment volume, 80% of the degradation product was excreted in the urine within the first 24 hours. We observed some accumulation in the gut, liver, kidneys, epididymal fat, lungs, and bladder. Other organs had minimal fluorescence above background. While the main route of excretion was in the urine, low levels of accumulation in other organs over short time frames prompted us to investigate any potential toxicity of the cross-linked PEG-MAL hydrogel.
Fig. 2.
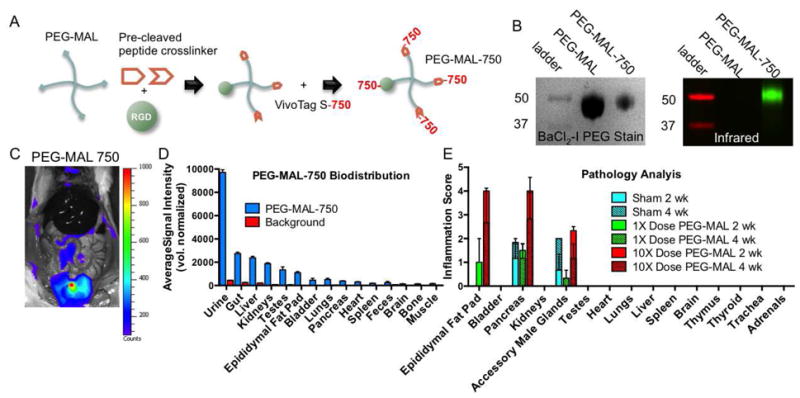
PEG-MAL biodistribution and toxicity. (A) PEG-MAL degradation product analog generated by reaction with pre-cleaved cross-linker and labeled with IR dye. (B) Molecular weight of labeled PEG-MAL-750 and corresponding IR fluorescence. (C) In vivo fluorescence 24 hours after injection of PEG-MAL-750 showing localization to urine in the bladder (D) Quantification of average organ fluorescence intensity. (E) Rats were implanted with 100 μL (1X dose) or 1 mL (10X dose) PEG-MAL hydrogel. Histopathology analysis of major organ systems at 2 and 4 weeks (± S.E.M. D n=2; E n=3).
To investigate toxicity, PEG-MAL hydrogels pre-functionalized with RGD (no VEGF) were cross-linked in situ on the surface of the small bowel mesentery of healthy adult rats at the same delivery dose subsequently used for islet transplantation (100 μL) or ten times the islet delivery dose (1 mL). Control animals received a sham surgery but no hydrogel. Animals were sacrificed at 2 and 4 weeks post-implantation for analysis of blood serum biochemistry and organ histopathology. Inflammation was scored by an independent histopathology laboratory. At 2 weeks post-implantation, moderate inflammation was present in the epididymal fat, para-pancreatic adipose tissue, and accessory male glands for the 10X dose hydrogel. Low levels of inflammation were observed in the epididymal fat and para-pancreatic adipose tissue for the 1X dose hydrogel (Fig. 2E). Sham animals presented similar levels of inflammation in the pancreas as the 1X dose hydrogel and in the accessory male glands as the 10X dose hydrogel. At 4 weeks post-implantation, the inflammation was significantly reduced for the 10X dose hydrogel and inflammation was mostly resolved for the 1X dose hydrogel. All other organs examined, including kidney and liver, were free of inflammation.
Both biodistribution of the degradation product and the organ histopathology indicate that the PEG-MAL hydrogel degradation products are mostly and rapidly excreted via the urine although low levels accumulate transiently in adipose tissue and other organs in the IP space. To examine any systemic toxicity, serum biochemistry panels were performed at 2 and 4 weeks and were within normal limits and did not differ from sham animals (Fig. S1). Together, these analyses demonstrate that PEG-MAL hydrogel products are excreted in the urine without sustained localized inflammation or toxicity.
3.3 PEG-MAL islet transplantation into diabetic mice
To examine islet engraftment and function in diabetic subjects, recipient mice were made diabetic with multiple low dose streptozotocin (STZ) intraperitoneal injections 15 days prior to transplantation. Islets for transplantation were isolated from healthy syngeneic mice 24 hours prior to transplantation. To deliver islets into diabetic animals, we developed a novel islet transplantation technique where pancreatic islets were delivered to the surface of the small bowel mesentery within PEG-MAL hydrogels cross-linked in situ onto the surface of the mesentery (Fig. 3). Islets were mixed with a precursor solution of PEG-MAL pre-functionalized with RGD and ± VEGF. The precursor solution was cross-linked in situ to the surface of the small bowel mesentery by addition of VPM peptide just before application. After 5 minutes, islets were embedded in a fully cross-linked PEG-MAL hydrogel that was well adhered to the surface of the mesentery. For hepatic delivery, islets were injected in sterile PBS into the hepatic portal vein. In larger animals and in humans, islets are routinely injected to the hepatic portal vein laparoscopically; we expect that PEG-MAL hydrogel application to the mesentery will be easily translatable to laparoscopic procedures.
Fig. 3.
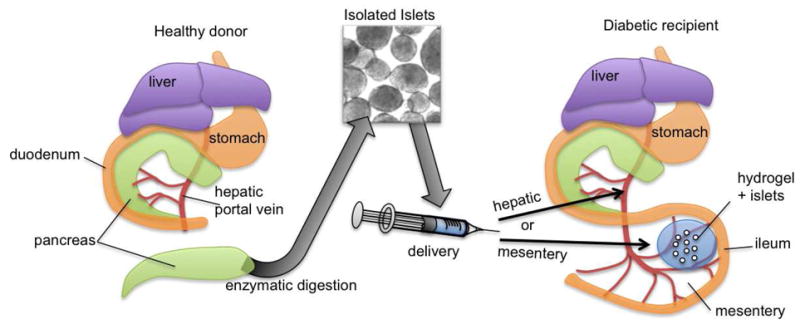
Transplantation of pancreatic islets. Islets are isolated from healthy donors and delivered to diabetic recipients either by injection into the hepatic portal vein or by adhesion to the small bowel mesentery with an engineered delivery hydrogel.
Pilot studies of the islet transplant dose in this model indicated that diabetes could be reversed in mice with 400 islets transplanted by PEG-MAL hydrogel but only by 700 islets delivered intrahepatically (Fig. S2). For further study, we selected a dose of 400 islets, corresponding to the islet mass that we were able to isolate from a single donor mouse under optimal conditions. Single-donor islet transplants in humans are a major goal of islet transplantation research because of extremely limited donor availability and increased risk associated with multiple foreign HLA alleles [16]. Animals were monitored daily for blood glucose and weight for 28 days post-transplantation. Control healthy animals maintained blood glucose levels on average of 155 mg/dL while sham transplant animals remained diabetic at 470 mg/dL. Remarkably, hyperglycemia was reversed in mice receiving 400 islets in PEG-MAL + VEGF with an average blood glucose 187 mg/dL, whereas 400 islets transplanted intrahepatically only reduced blood sugar to 327 mg/dL and did not reverse hyperglycemia (Fig. 4A). This marked difference in blood sugar was also reflected in weight gain, which is intrinsically linked to insulin expression. Healthy mice gained weight at a rate of 58 mg/day whereas sham animals gained weight at a rate of 12 mg/day. Mice receiving islets in PEG-MAL + VEGF gained weight at 70 mg/day while mice receiving hepatically transplanted islets only gained weight at 36 mg/day (Fig. 4B). Because we hypothesized that vascularization is critical for islet function and grafting, we also tested the effect of exclusion of the angiogenic signal, VEGF, from the PEG-MAL delivery hydrogel. Animals receiving islets in PEG-MAL without VEGF had average blood glucose of 259 mg/dL, significantly higher than islets transplanted with VEGF but lower than islets transplanted intrahepatically. Islets transplanted in PEG-MAL without VEGF also reduced weight gain by half to 35 mg/day compared to PEG-MAL + VEGF. These results indicate a significant improvement in islet function when transplanted in PEG-MAL + VEGF hydrogel.
Fig. 4.
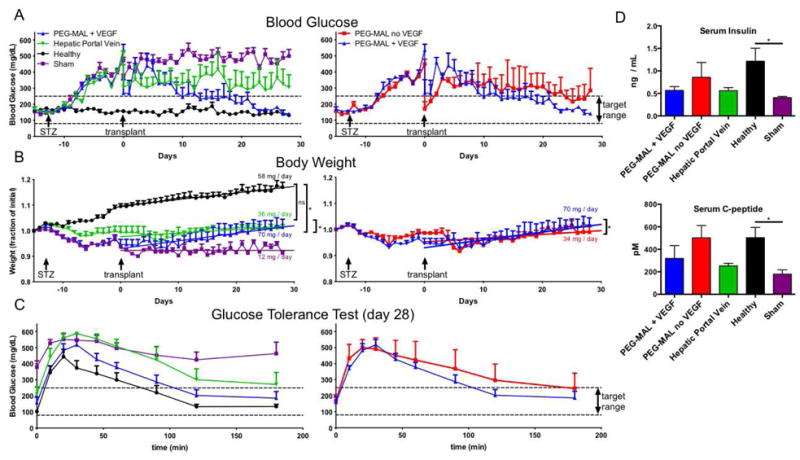
STZ-induced diabetic mice were monitored for weight and blood sugar after transplantation of 400 islets. Data from treatment groups were separated into two panels to aid visualization. (A) Random daily blood sugar measurements (B) Body weight plotted as a fraction of initial weight. Linear curve fit to body weight highlights differences in rate of weight gain between treatment groups. (C) Glucose tolerance test performed on day 28 to measure islet glucose responsiveness. (D) Blood serum ELISA measurements for insulin and C-peptide at 28 days (± S.E.M. A-C n=4–6; D n=3).
At 4 weeks post-transplant, a glucose tolerance test was performed to examine glucose responsiveness of the islet graft. Animals were fasted for 6 hours prior to intraperitoneal injection of a glucose bolus. Healthy animals responded with a normal peak in blood glucose followed by rapid return to normoglycemia. Diabetic animals with no islet transplantation exhibited a glucose spike 100 points higher than healthy animals and did not return to normoglycemia. Mice with islets transplanted in PEG-MAL + VEGF returned to normoglycemia at the same rate as healthy animals while mice with islets transplanted intrahepatically responded much more slowly and did not return to a blood glucose level below 250 mg/dL. Islets transplanted in PEG-MAL without VEGF were slower to respond to the glucose challenge than islets in PEG-MAL + VEGF and had higher overall blood sugar at all time points after the initial spike (Fig. 4C). These results indicate greater glucose responsiveness in islets transplanted with PEG-MAL + VEGF hydrogel than without angiogenic growth factors or intrahepatic delivery.
We measured serum insulin and C-peptide levels at 4 weeks post-transplantation as a measure of islet secretory activity. Diabetic sham mice secreted very little insulin and C-peptide indicating nearly complete knockdown of beta cell activity. Higher levels of insulin and C-peptide were measured in animals transplanted with islets in PEG-MAL + VEGF and in PEG-MAL without VEGF than in islets transplanted intrahepatically (Fig. 4D). These results indicate better systemic availability of insulin produced by islets transplanted in PEG-MAL either with or without VEGF than by islets transplanted intrahepatically.
Finally, we evaluated islet vascularization and engraftment at four weeks post-transplantation. Blood vessels in the islet graft were labeled by an intravenous injection of FITC-lectin prior to euthanasia to label perfused tissues. Macroscopic observation of the mesentery graft site revealed significant remodeling of the PEG-MAL matrix with a dense vascular influx into both PEG-MAL + VEGF and PEG-MAL without VEGF. Whole islets could be readily observed in the graft zone (Fig. 5). Histological sections of mesentery and liver were immuno-stained for insulin, imaged on a laser confocal microscope (Fig. 6A), and scored for fraction of islet area staining positive for vascularization (Fig. 6B). Islets delivered within PEG-MAL hydrogel maintained their normal shape with little to no fragmentation. Lectin-positive vessels heavily infiltrated islets within PEG-MAL + VEGF hydrogels at levels similar to native islets in the pancreas. Islets in PEG-MAL without VEGF exhibited less vascularization. Hepatically transplanted islets were fragmented and exhibited poor intra-islet vascularization, despite the close proximity of perfused vessels in the surrounding liver tissue. Taken together these results indicate a marked improvement in the function, engraftment, and vascularization for islets transplanted in the angiogenic PEG-MAL hydrogel compared to islets transplanted intrahepatically.
Fig. 5.
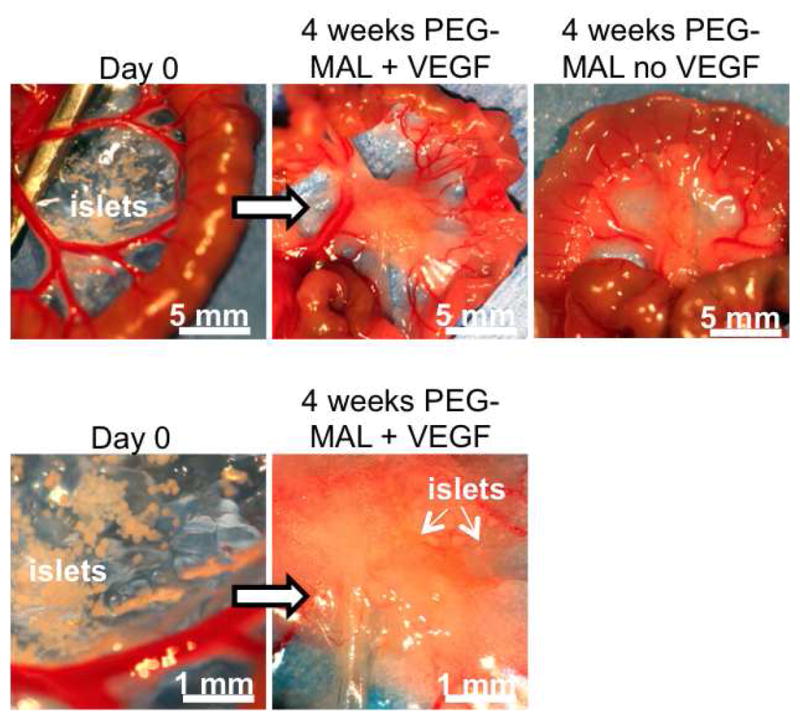
Transplant site in small bowel mesentery at day 0 and at 4 weeks demonstrating significant remodeling of the PEG-MAL matrix.
Fig. 6.
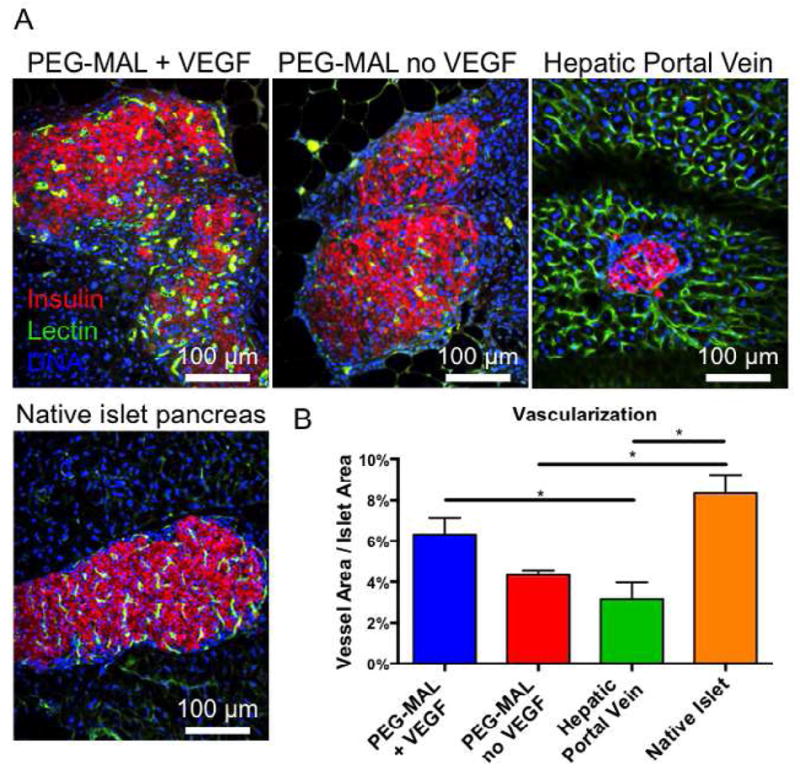
Islet Transplants, 4 weeks (A) Islet graft explants with patent vascular structures stained with IV-perfused FITC-lectin (green), DAPI (blue), and immunostained for insulin (red). (B) Quantification of vascular area normalized to islet area (± S.E.M. n=6).
4. Discussion
Across a number of physiological indicators including daily blood glucose, weight gain, and glucose responsiveness, islets transplanted into diabetic mice performed better when implanted to the small bowel mesentery in PEG-MAL + VEGF hydrogel than when transplanted intrahepatically. We attribute these functional improvements to several factors. The vascular-inductive properties of the engineered matrix encourage controlled revascularization of the intra-islet endothelial bed. We posit that the improved intra-islet vascularization that we observed histologically in PEG-MAL + VEGF hydrogel is critical for maintenance of healthy physiological glucose homeostasis by enhancing islet viability, glucose sensing by beta cells, and insulin secretion into the blood stream. Faster glucose responsiveness by beta cells allows for tighter control of overall glucose homeostasis. Previous studies have linked intra-islet vascularization to better glucose homeostasis as well as long-term islet health [37–39]. Our results indicate that increasing the level of vascularization in transplanted islets reduces the total number of donor islets required to achieve normoglycemia, supporting the notion that the islets have a higher rate of engraftment and function better when transplanted using the PEG-MAL + VEGF biomaterial. Serum insulin in VEGF containing gels was slightly lower than without VEGF, which may be an indication of increased insulin sensitivity associated with more highly vascularized islets. Additionally, the PEG-MAL based transplantation procedure is extremely gentle on the delicate islet cells and leaves the whole islets intact without subjecting them to the rigors of hepatic portal injection. Islets delivered intrahepatically are often fragmented [37]. Delivering islets to a tissue surface instead of injecting into the blood stream eliminates islet loss due to IBMIR.
We demonstrate effective conjugation of VEGF to PEG-MAL macromer with 75% retention in a cross-linked hydrogel after washing in PBS over multiple days. When the gels are degraded with proteases, the VEGF is released. In vivo, the hydrogel is degraded through tissue remodeling and cell-invasion processes which are mediated by the RGD cell-adhesive ligands. As the hydrogel is degraded by invading cells, more VEGF is released creating positive pro-angiogenic feedback. When islets were delivered in PEG-MAL gels without VEGF, the islets survived but were less vascularized and performed less effectively to control glucose homeostasis. Islets themselves secrete high levels of angiogenic growth factors [22], which likely help to stabilize the vasculature once the supply of incorporated VEGF has been depleted and may also explain the vascularization that occurs, albeit to a lesser extent, in gels without VEGF. Others have made efforts to augment transplanted islet vascularization in the liver by artificially inducing expression of angiogenic genes or in the omentum with basic material scaffolds [18, 24–26, 28, 40]. However gene delivery raises serious safety concerns for translation to humans and materials such as PLGA are slow-degrading and do not actively induce angiogenesis. Here, we achieved reversal of chemically-induced diabetes with a modular material that actively encourages vessel ingrowth from surrounding tissue. We and others have previously shown that VEGF bound to proteolytically degradable PEG matrices supports a robust angiogenic response in vivo [41–43]. The PEG-maleimide hydrogels used in this study offer several advantages over other previously studied types of PEG-based engineered matrices such as PEG-diacrylate and PEG-vinyl-sulfone: The PEG-maleimide cross-linking reaction occurs at neutral pH and is specific for thiols in the cross-linker peptides; neither UV light nor free-radicals are required resulting in greater viability of encapsulated cells; PEG-maleimide hydrogels incorporate higher percentages of functional ligands and cross-link more rapidly; and PEG-maleimide hydrogels integrate well with tissue when cross-linked in situ allowing for facile delivery in surgical applications. The release kinetics controlled by cell-mediated proteolytic degradation offers an alternative strategy of “on-demand” therapeutics which contrasts with release triggered by hydrolysis or other chemical means.
As an implantable material, it is important to investigate potentially toxic effects and the excretion route of the degradation products of PEG-MAL. We tracked excretion of IR dye-labeled PEG-MAL degradation product to the urine. Urinary clearance is the predominate mode of excretion for linear PEG molecules up to 190,000 molecular weight [44, 45]. Importantly, histopathology and serum biochemistry did not indicate any inflammation or toxicity in kidneys or liver. Inflammation in the epididymal fat pad and para-panceatic adipose tissue seen in histopathology analysis suggests some transient irritation to fat tissue. However, this early inflammation is quickly resolved.
The small bowel mesentery is an intrinsically difficult site to transplant cells by traditional means such as injection because it is an extremely thin and delicate tissue. To our knowledge, the mesentery has never been used as a site of islet transplantation. The rapid tissue-bonding capabilities of the PEG-MAL hydrogel enabled us to make use of this otherwise well-situated site for islet transplantation for the first time. This approach is fundamentally different from techniques that use biomaterials to achieve immune-isolation through encapsulation or islet coatings [46–49]. Encapsulation prohibits engraftment and vascularization but avoids the need for immunosuppression. However encapsulation leads to poorer long-term islet survival and poorer glucose responsiveness, especially in large animals. In this study we utilized syngeneic islets to focus on the role of vascularization in transplanted islet function. The plug-and-play modular design of the PEG-MAL matrix platform is readily amenable to further studies with allogeneic islets combined with immune-suppression or other tolerance-inducing techniques such as blocking lymphangiogenesis [50] or attracting regulatory T-cells. Additionally, PEG-MAL could be used to deliver engineered insulin-producing autologous cells such as induced pluripotent stem cells.
5. Conclusions
We demonstrate a robust biomaterial strategy for islet transplantation that achieves improved islet engraftment and function with fewer total islets compared to the current clinically standard method by augmenting islet vascularization and avoiding many of the stresses of intrahepatic islet transplantation. The degradation products of this resorbable material are excreted mainly in the urine and the material itself is non-toxic. Islets transplanted using this engineered pro-vascularization hydrogel have superior glycemic function in diabetic animals when compared to islets delivered intrahepatically. Our results demonstrate the significant potential of engineered hydrogels for enhancing islet engraftment and function. Further studies with allogenic transplantation models and in large animals are warranted to investigate this promising strategy.
Supplementary Material
Fig. S1. Blood Serum biochemistry panel for renal and hepatic toxicity markers at 2 and 4 weeks post implantation of 100 μL (1X dose) or 1 mL (10X dose) PEG-MAL hydrogel (± S.E.M. n=3).
Fig. S2. STZ-induced diabetic mice were monitored for weight and blood sugar after transplantation of 400 or 700 islets. Random daily blood sugar, body weight plotted as a fraction of initial weight, and glucose tolerance test performed on day 28 are displayed as comparison of 700 to 400 islets for hepatic portal injected islets (left panel) and PEG-MAL + VEGF transplanted islets (right panel) (± S.E.M. n=4–6).
Acknowledgments
E.A.P. designed and performed the experiments, analyzed the data, and wrote the paper with input from the other authors. D.M.H. designed and performed experiments. W.R.T. provided conceptual and technical input and designed experiments. P.M.T. provided conceptual and technical input, designed experiments and surgical protocols, and analyzed the data. A.J.G. conceived the project, designed experiments, analyzed the data, and edited the paper.
This work was funded by the Georgia Tech/Emory Center for the Engineering of Living Tissues and the Atlanta Clinical and Translational Science Institute under PHS Grant UL RR025008 from the Clinical and Translational Science Award Program; the Center for Pediatric Healthcare Technology Innovation at Georgia Tech and Children’s Hospital of Atlanta; a VA Merit Review; NIH NIDDK R01 DK076801–01; JDRF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National diabetes fact sheet. Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 2.Dabelea D, Bell RA, D’Agostino RB, Jr, Imperatore G, Johansen JM, Linder B, et al. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–24. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 3.Cizza G, Brown RJ, Rother KI. Rising incidence and challenges of childhood diabetes. A mini review. J Endocrinol Invest. 2012;35:541–6. doi: 10.3275/8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rickels MR, Schutta MH, Mueller R, Markmann JF, Barker CF, Naji A, et al. Islet cell hormonal responses to hypoglycemia after human islet transplantation for type 1 diabetes. Diabetes. 2005;54:3205–11. doi: 10.2337/diabetes.54.11.3205. [DOI] [PubMed] [Google Scholar]
- 5.Robertson RP. Islet transplantation as a treatment for diabetes - a work in progress. N Engl J Med. 2004;350:694–705. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
- 6.Ryan EA, Lakey JR, Paty BW, Imes S, Korbutt GS, Kneteman NM, et al. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51:2148–57. doi: 10.2337/diabetes.51.7.2148. [DOI] [PubMed] [Google Scholar]
- 7.Ryan EA, Lakey JR, Rajotte RV, Korbutt GS, Kin T, Imes S, et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50:710–9. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- 8.Barton FB, Rickels MR, Alejandro R, Hering BJ, Wease S, Naziruddin B, et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care. 2012;35:1436–45. doi: 10.2337/dc12-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibly RF, Graham JG, Luo X, Lowe WL, Jr, Hering BJ, Shea LD. Advancing islet transplantation: from engraftment to the immune response. Diabetologia. 2011;54:2494–505. doi: 10.1007/s00125-011-2243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro AM. State of the art of clinical islet transplantation and novel protocols of immunosuppression. Curr Diab Rep. 2011;11:345–54. doi: 10.1007/s11892-011-0217-8. [DOI] [PubMed] [Google Scholar]
- 11.Azzi J, Geara AS, El-Sayegh S, Abdi R. Immunological aspects of pancreatic islet cell transplantation. Expert Rev Clin Immunol. 2010;6:111–24. doi: 10.1586/eci.09.67. [DOI] [PubMed] [Google Scholar]
- 12.Giraldo JA, Weaver JD, Stabler CL. Tissue engineering approaches to enhancing clinical islet transplantation through tissue engineering strategies. J Diabetes Sci Technol. 2010;4:1238–47. doi: 10.1177/193229681000400525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson JD, Ao Z, Ao P, Li H, Dai LJ, He Z, et al. Different effects of FK506, rapamycin, and mycophenolate mofetil on glucose-stimulated insulin release and apoptosis in human islets. Cell Transplant. 2009;18:833–45. doi: 10.3727/096368909X471198. [DOI] [PubMed] [Google Scholar]
- 14.Lakey JR, Mirbolooki M, Shapiro AM. Current status of clinical islet cell transplantation. Methods Mol Biol. 2006;333:47–104. doi: 10.1385/1-59745-049-9:47. [DOI] [PubMed] [Google Scholar]
- 15.Vaithilingam V, Sundaram G, Tuch BE. Islet cell transplantation. Curr Opin Organ Transplant. 2008;13:633–8. doi: 10.1097/MOT.0b013e328317a48b. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro AM. Strategies toward single-donor islets of Langerhans transplantation. Curr Opin Organ Transplant. 2011;16:627–31. doi: 10.1097/MOT.0b013e32834cfb84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantarelli E, Piemonti L. Alternative transplantation sites for pancreatic islet grafts. Curr Diab Rep. 2011;11:364–74. doi: 10.1007/s11892-011-0216-9. [DOI] [PubMed] [Google Scholar]
- 18.Gibly RF, Zhang X, Graham ML, Hering BJ, Kaufman DB, Lowe WL, Jr, et al. Extrahepatic islet transplantation with microporous polymer scaffolds in syngeneic mouse and allogeneic porcine models. Biomaterials. 2011;32:9677–84. doi: 10.1016/j.biomaterials.2011.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jansson L, Carlsson PO. Graft vascular function after transplantation of pancreatic islets. Diabetologia. 2002;45:749–63. doi: 10.1007/s00125-002-0827-4. [DOI] [PubMed] [Google Scholar]
- 20.Ballian N, Brunicardi FC. Islet vasculature as a regulator of endocrine pancreas function. World J Surg. 2007;31:705–14. doi: 10.1007/s00268-006-0719-8. [DOI] [PubMed] [Google Scholar]
- 21.Brissova M, Powers AC. Revascularization of transplanted islets: can it be improved? Diabetes. 2008;57:2269–71. doi: 10.2337/db08-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, et al. Pancreatic islet production of vascular endothelial growth factor--a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55:2974–85. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- 23.Olsson R, Carlsson PO. The pancreatic islet endothelial cell: emerging roles in islet function and disease. Int J Biochem. 2006;38:492–7. doi: 10.1016/j.biocel.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Cheng K, Fraga D, Zhang C, Kotb M, Gaber AO, Guntaka RV, et al. Adenovirus-based vascular endothelial growth factor gene delivery to human pancreatic islets. Gene Ther. 2004;11:1105–16. doi: 10.1038/sj.gt.3302267. [DOI] [PubMed] [Google Scholar]
- 25.Narang AS, Cheng K, Henry J, Zhang C, Sabek O, Fraga D, et al. Vascular endothelial growth factor gene delivery for revascularization in transplanted human islets. Pharm Res. 2004;21:15–25. doi: 10.1023/b:pham.0000012147.52900.b8. [DOI] [PubMed] [Google Scholar]
- 26.Sigrist S, Mechine-Neuville A, Mandes K, Calenda V, Braun S, Legeay G, et al. Influence of VEGF on the viability of encapsulated pancreatic rat islets after transplantation in diabetic mice. Cell Transplant. 2003;12:627–35. doi: 10.3727/000000003108747109. [DOI] [PubMed] [Google Scholar]
- 27.Zhang N, Richter A, Suriawinata J, Harbaran S, Altomonte J, Cong L, et al. Elevated vascular endothelial growth factor production in islets improves islet graft vascularization. Diabetes. 2004;53:963–70. doi: 10.2337/diabetes.53.4.963. [DOI] [PubMed] [Google Scholar]
- 28.Stendahl JC, Wang LJ, Chow LW, Kaufman DB, Stupp SI. Growth factor delivery from self-assembling nanofibers to facilitate islet transplantation. Transplantation. 2008;86:478–81. doi: 10.1097/TP.0b013e3181806d9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olerud J, Johansson M, Lawler J, Welsh N, Carlsson PO. Improved vascular engraftment and graft function after inhibition of the angiostatic factor thrombospondin-1 in mouse pancreatic islets. Diabetes. 2008;57:1870–7. doi: 10.2337/db07-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su D, Zhang N, He J, Qu S, Slusher S, Bottino R, et al. Angiopoietin-1 production in islets improves islet engraftment and protects islets from cytokine-induced apoptosis. Diabetes. 2007;56:2274–83. doi: 10.2337/db07-0371. [DOI] [PubMed] [Google Scholar]
- 31.Chow LW, Wang LJ, Kaufman DB, Stupp SI. Self-assembling nanostructures to deliver angiogenic factors to pancreatic islets. Biomaterials. 2010;31:6154–61. doi: 10.1016/j.biomaterials.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta R, Sefton MV. Application of an endothelialized modular construct for islet transplantation in syngeneic and allogeneic immunosuppressed rat models. Tissue Eng Part A. 2011;17:2005–15. doi: 10.1089/ten.tea.2010.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Figliuzzi M, Cornolti R, Perico N, Rota C, Morigi M, Remuzzi G, et al. Bone marrow-derived mesenchymal stem cells improve islet graft function in diabetic rats. Transplant Proc. 2009;41:1797–800. doi: 10.1016/j.transproceed.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. A practical guide to rodent islet isolation and assessment. Biol Proced Online. 2009;11:3–31. doi: 10.1007/s12575-009-9021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phelps EA, Enemchukwu NO, Fiore VF, Sy JC, Murthy N, Sulchek TA, et al. Maleimide cross-linked bioactive PEG hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv Mater. 2012;24:64–70. 2. doi: 10.1002/adma.201103574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patterson J, Hubbell JA. SPARC-derived protease substrates to enhance the plasmin sensitivity of molecularly engineered PEG hydrogels. Biomaterials. 2011;32:1301–10. doi: 10.1016/j.biomaterials.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Henriksnas J, Lau J, Zang G, Berggren PO, Kohler M, Carlsson PO. Markedly decreased blood perfusion of pancreatic islets transplanted intraportally into the liver: disruption of islet integrity necessary for islet revascularization. Diabetes. 2012;61:665–73. doi: 10.2337/db10-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaufman-Francis K, Koffler J, Weinberg N, Dor Y, Levenberg S. Engineered vascular beds provide key signals to pancreatic hormone-producing cells. PloS One. 2012;7:e40741. doi: 10.1371/journal.pone.0040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lau J, Svensson J, Grapensparr L, Johansson A, Carlsson PO. Superior beta cell proliferation, function and gene expression in a subpopulation of rat islets identified by high blood perfusion. Diabetologia. 2012;55:1390–9. doi: 10.1007/s00125-012-2476-6. [DOI] [PubMed] [Google Scholar]
- 40.Cheng Y, Liu YF, Zhang JL, Li TM, Zhao N. Elevation of vascular endothelial growth factor production and its effect on revascularization and function of graft islets in diabetic rats. World J Gastroenterol. 2007;13:2862–6. doi: 10.3748/wjg.v13.i20.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phelps EA, Landazuri N, Thule PM, Taylor WR, Garcia AJ. Bioartificial matrices for therapeutic vascularization. Proc Natl Acad Sci U S A. 2010;107:3323–8. doi: 10.1073/pnas.0905447107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, et al. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17:2260–2. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 43.Leslie-Barbick JE, Saik JE, Gould DJ, Dickinson ME, West JL. The promotion of microvasculature formation in poly(ethylene glycol) diacrylate hydrogels by an immobilized VEGF-mimetic peptide. Biomaterials. 2011;32:5782–9. doi: 10.1016/j.biomaterials.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 44.Yamaoka T, Tabata Y, Ikada Y. Distribution and tissue uptake of poly(ethylene glycol) with different molecular weights after intravenous administration to mice. J Pharm Sci. 1994;83:601–6. doi: 10.1002/jps.2600830432. [DOI] [PubMed] [Google Scholar]
- 45.Webster R, Didier E, Harris P, Siegel N, Stadler J, Tilbury L, et al. PEGylated proteins: evaluation of their safety in the absence of definitive metabolism studies. Drug Metab Dispos. 2007;35:9–16. doi: 10.1124/dmd.106.012419. [DOI] [PubMed] [Google Scholar]
- 46.Yun LD, Hee NJ, Byun Y. Functional and histological evaluation of transplanted pancreatic islets immunoprotected by PEGylation and cyclosporine for 1 year. Biomaterials. 2007;28:1957–66. doi: 10.1016/j.biomaterials.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 47.Weber LM, Anseth KS. Hydrogel encapsulation environments functionalized with extracellular matrix interactions increase islet insulin secretion. Matrix Biol. 2008;27:667–73. doi: 10.1016/j.matbio.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weber LM, Cheung CY, Anseth KS. Multifunctional pancreatic islet encapsulation barriers achieved via multilayer PEG hydrogels. Cell Transplant. 2008;16:1049–57. [PubMed] [Google Scholar]
- 49.Wilson JT, Cui W, Chaikof EL. Layer-by-layer assembly of a conformal nanothin PEG coating for intraportal islet transplantation. Nano Lett. 2008;8:1940–8. doi: 10.1021/nl080694q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin N, Zhang N, Xu J, Shi Q, Ding Y, Bromberg JS. Targeting lymphangiogenesis after islet transplantation prolongs islet allograft survival. Transplantation. 2011;92:25–30. doi: 10.1097/TP.0b013e31821d2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Blood Serum biochemistry panel for renal and hepatic toxicity markers at 2 and 4 weeks post implantation of 100 μL (1X dose) or 1 mL (10X dose) PEG-MAL hydrogel (± S.E.M. n=3).
Fig. S2. STZ-induced diabetic mice were monitored for weight and blood sugar after transplantation of 400 or 700 islets. Random daily blood sugar, body weight plotted as a fraction of initial weight, and glucose tolerance test performed on day 28 are displayed as comparison of 700 to 400 islets for hepatic portal injected islets (left panel) and PEG-MAL + VEGF transplanted islets (right panel) (± S.E.M. n=4–6).


