Abstract
Most events promoting early melanoma development are yet to be identified but deregulation of the B-Raf and Akt3 signaling cascades are important regulators of this process. Approximately 90% of normal moles and ~60% of early invasive cutaneous melanomas contain a T1799A B-Raf mutation (V600EB-Raf), leading to 10X higher enzyme activity and constitutive activation of the MAP kinase pathway. Furthermore, ~70% of melanomas have elevated Akt3 signaling due to increased gene copy number and PTEN loss. Therefore, targeting V600EB-Raf and Akt3 signaling is necessary to prevent or treat cutaneous melanocytic lesions. Agents specifically targeting these proteins are needed, having fewer side effects than those inhibiting both normal and mutant B-Raf protein or targeting all three Akt isoforms. In this study, a unique nanoliposomal-ultrasound mediated approach has been developed for delivering siRNA specifically targeting V600EB-Raf and Akt3 into melanocytic tumors present in skin to retard melanoma development. Novel cationic nanoliposomes stably encapsulate siRNA targeting V600EB-Raf or Akt3, providing protection from degradation and facilitating entry into melanoma cells to decrease expression of these proteins. Low-frequency ultrasound using a lightweight 4-cymbal transducer array enables penetration of nanoliposomal-siRNA complex throughout epidermal and dermal layers of laboratory-generated or animal skin. Nanoliposomal-mediated siRNA targeting of V600EB-Raf and Akt3 led to a cooperatively acting ~65% decrease in early or invasive cutaneous melanoma compared to inhibition of each singly with negligible associated systemic toxicity. Thus, cationic nanoliposomes loaded with siRNA targeting V600EB-Raf and Akt3 provide an effective approach for targeted inhibition of early or invasive cutaneous melanomas.
Keywords: melanoma, ultrasound, B-Raf, nanoliposomes, siRNA
INTRODUCTION
Melanoma is a cancer of pigmented skin cells called melanocytes residing at the epidermal-dermal junction (1). Most genetic alterations promoting development of early melanomas are yet to be identified. One frequent change occurring in ~90% of normal moles is a T1799A mutation of B-Raf in the MAP kinase pathway (2–5). Approximately 60% of advanced stage metastatic melanomas also contain the activating V600E mutation leading to 10.7 times higher activity than occurs in normal cells, thereby promoting cellular proliferation (2–5).
A second frequent alteration promoting melanoma development is increased Akt3 activity in ~70% of melanomas (6). Akt3 is activated through loss of the PTEN phosphatase and increased gene copy number, leading to deregulation of apoptosis thereby promoting chemoresistance (6, 7). Targeting V600EB-Raf or Akt3 signaling decreases melanoma tumor development and simultaneous inhibition can lead to cooperatively acting tumor inhibition (6, 8, 9). Thus, agents specifically targeting V600EB-Raf and/or Akt3 could have significant potential to treat moles, early melanocytic lesions or skin metastases in which these proteins are deregulated.
Inhibition of B-Raf or Akt3 using siRNA specifically designed to inhibit V600EB-Raf but not wild-type B-Raf or Akt3 but not Akt1 or Akt2 protein expression can effectively decrease melanoma tumor development in mice (6, 8, 10). Since these agents target specific genes, there would be fewer potential side effects than drugs inhibiting both mutant and wild-type B-Raf or all three Akt isoforms (6, 8, 10). Introduction of siRNA in cultured cells is easily accomplished using standard transfection methods (11–13). However, use as a targeted chemopreventive or therapeutic agent is limited due to degradation in animal systems (14, 15).
Encapsulating siRNA into nanoliposomes can be used for protection and systemic delivery, leading to tumor inhibition (16–18). In contrast, topical administration on skin leads to uptake confined to the upper epidermal layers without complete skin penetration (19). This is due in part to skin architecture with epidermis and dermis protected by a surface keratinized layer (19, 20), which prevents most topically applied agents from reaching early melanocytic lesions developing at the epidermal-dermal junction or from reaching locally invasive cutaneous melanoma metastases in the skin dermis (21). One promising strategy for delivery through the keratinized layer utilizes low-frequency ultrasound to permeabilize skin enabling passage of macromolecules including antisense oligonucleotides without significant skin damage (21–26). Combining the protective qualities of nanoliposomes with the permeabilizing activities of low-frequency ultrasound is a novel unexplored strategy for delivering siRNA into melanocytic lesions present in skin to prevent or treat cutaneous melanoma.
This study, details development of a unique nanoliposomal-ultrasound mediated approach for delivering siRNA-targeting V600EB-Raf and Akt3 protein expression into melanocytic lesions present in skin to inhibit early lesion development or prevent cutaneous melanoma metastasis.
MATERIALS AND METHODS
Cell lines and culture conditions
Human melanoma cell lines UACC 903, UACC 903-GFP, 1205 Lu, C8161.Cl9, and human fibroblasts, FF2441, were maintained in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% FBS (Hyclone, Logan, UT). WM35 and WM35-GFP cells were grown as described previously (27). Human keratinocytes, HFK, were grown in Epilife Medium with Human Keratinocyte Growth Supplement (Cascade Biologics, Portland, OR). Mouse melanocytes, melan-a, were grown in RPMI 1640 (Mediatech, Herndon, VA) supplemented with 10% FBS, 2 mM L-glutamine, and 200 nM TPA (Sigma-Aldrich, St. Louis, MO).
Liposome composition, characterization and siRNA loading
Nanoliposomes were created by combining DOTAP (1,2-Dioleoyl-3-Trimethylammonium-Propane), DOPE (1,2-Dioleoyl-sn-Glycero-3-Phosphoethanolamine) and DSPE-PEG(2000) (1,2-Distearoyl-sn-Glycero-3-Phosphoethanolamine-N-[Amino(Polyethylene Glycol)[2000]) (Avanti Polar Lipids, Alabaster, AL) in chloroform at a 4.75:4.75:0.5 molar ratio (28, 29). siRNA and nanoliposomes complexing occurred at specific weight ratios of 1:5, 1:10, and 1:15 for 0.5–24 h. A quasielastic light scattering system (Malvern Nanosizer, Malvern Instruments, UK) was used to measure particle diameter of nanoliposomes ±siRNA 1-d after preparation. Nanoliposomal loading of siRNA was measured by mixing fluorescent, Alexa Fluor 546 tagged-siRNA (Qiagen, Valencia, CA) with nanoliposomes at specific weight ratios (1:5, 1:10, 1:15) and complexing at room temperature for 0.5, 3 or 6 h. Loading was considered complete when no free siRNA was evident with complex remaining in well of a 2% agarose gel. To measure liposome-mediated protection of siRNA, nanoliposomes complexed with siRNA overnight were treated with 50% FBS for 10, 30, 60, 180, or 360 m. Following FBS treatment, half of the complex at each time point was treated with 0.5% SDS for 10 m at 37°C to disrupt the complexes and release free siRNA. siRNA alone was also digested with 50% FBS at each time point to serve as a control. All samples were run on a 2% agarose gel at 100 V for 20 m and visualized using UV.
Nanoliposomal toxicity
To assess cytotoxicity of nanoliposomes complexed with siRNA in normal or cancer cells; 5×103 fibroblasts (FF2441), keratinocytes (HFK), melanocytes (melan-a), and melanoma (1205 Lu) cells were plated into 96-well plates and 48 h later treated with ghost nanoliposomes for 24 h at nanoliposomal concentrations of 12.5, 25, and 50 μM or exposed to 125, 250, 500, 1000, or 2000 nM of siRNA complexed with nanoliposomes and compared to untreated control cells. Cytotoxicity was analyzed using the Cell Titer 96 aqueous nonradioactive cell proliferation assay (Promega, Madison, WI).
Uptake of nanoliposomal siRNA into cells
Uptake and internalization of nanoliposomal siRNA into cells was determined using Alexa Fluor 546, and/or fluorescein tagged siRNA (Invitrogen, Carlsbad, CA). 1×105 1205 Lu cells were plated and following overnight growth, nanoliposomal-siRNA complex (100 nM) or ghost nanoliposomes added. 3 h later, cells were trypsinzed and plated onto coverslips followed by overnight incubation. Uptake of siRNA-nanoliposomal complex by fibroblasts (FF2441), keratinocytes (HFK), or melanocytes (melan-a) was measured by plating 5×103 cells onto coverslips and 2-d later, adding nanoliposomal siRNA (50, 100, or 200 nM) for 3 h. Following all conditions, cells were rinsed with PBS, fixed in 4% parafomaldehyde (Fisher Scientific, Waltham, MA) for 30 m, washed in PBS, mounted in DAPI containing mountant (Vector Laboratories, Burlingame, CA) and fluorescence microscopy used to measure uptake.
Nucleofection of siRNA into cells
Stealth siRNA (1.5–200 pmoles) (Invitrogen, Carlsbad, CA) was introduced via an Amaxa Nucleofector (Koeln, Germany) into 1×106 UACC 903, UACC 903-GFP or C8161.Cl9 cells using Solution R/Program K-17 or WM35-GFP cells using NHEM-NEO Solution/Program U-20. Resulting transfection efficiency was ~99% (8). Duration of siRNA-mediated protein knockdown at 0, 2, 4, 6, and 8 d following nucleofection was measured for B-Raf or C-Raf by Western blot analysis. Cells used in reconstructed skin were nucleofected with buffer, siMutB-Raf (100, 50, or 12.5 pmoles), siC-Raf (100 pmoles), or scrambled (100 pmoles) siRNA, plated into culture dishes and 2 d later mixed with keratinocytes, which was added onto the dermis as detailed below. Protein lysates of remaining cells were analyzed by Western blotting.
Western Blot Analysis
Western blot analysis, was conducted as described previously (30). Blots were probed with primary antibodies to B-Raf, Erk2, and C-Raf and secondary antibodies conjugated with horseradish peroxidase from Santa Cruz Biotechnology (Santa Cruz, CA). Immunoblots were developed using the enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, Piscataway, NJ). Blots were normalized to Erk2 and quantified using ImageJ software (31).
Nanoliposomal-siRNA mediated protein knockdown
5×105 1205 Lu cells were plated in 6-well plates for 2 d and nanoliposomal-siMutB-Raf or -siScrambled complex (1μM) added. Protein lysates were collected 18 and 32 h later for Western blot analysis measuring B-Raf and Erk2 expression. Protein levels were quantified from three independent blots using ImageJ software, normalized to Erk2 and average B-Raf levels calculated (31).
Generation of skin containing melanocytic lesions
To create skin in a culture dish, human fibroblasts, were trypsinized and resuspended in 10% reconstitution buffer, 10% 10× DMEM (Mediatech, Herndon, VA), 2.4 μl/ml of 10 M NaOH, and 80% collagen I (BD, Bedford, MA) at a concentration of 2.5×105 cells/ml on ice (32). Mixture was then aliquoted into 6 or 12 well plates and incubated at 37°C for 3 h. E-media was added to each well to equilibrate the dermal matrix (32). After 2 d growth, keratinocytes and melanoma cells (WM35-GFP or UACC 903-GFP) were trypsinized and resuspended at a 1:10 ratio of melanoma cells (nucleofected or untreated) to keratinocytes in E-media. 1 ml of cell suspension added to each well on top of the dermal layer. Following 2 d growth, reconstructed skin was transferred onto wire grids and fed via diffusion from E-media below the platforms.
Ultrasound transducer design and calibration
The cymbal transducer is a novel thin flextensional transducer capable of producing very low frequencies (33, 34). It has a compact, lightweight structure with an adjustable resonance frequency. Caps on the lead zirconate-titanate ceramic contain a shallow cavity beneath the inner surface. The fundamental mode of vibration is flexing of the end caps caused by radial motion of the ceramic. Therefore, the overall displacement of the device is a combination of the axial motion of disk plus radial motion amplified by the end caps (23, 24). A radio frequency signal was generated by a frequency pulse/function generator (Agilent 32250A, Palo Alto, CA) and amplified using an amplifier (Model 40A12, Amplifier Research, Souderton, PA). Electrical impedance of the array was tuned to the output impedance of the amplifier by an external inductor-capacitor tuning network. Pulse period, duty cycle and exposure time of radio frequency signal from the frequency generator was monitored using an oscilloscope (Tektronix 2213A, Beaverton, OR). For experiments, signal generator operated at 20 kHz with pulse duration of 200 ms and pulse repetition period of 1 second (i.e. 20% duty cycle); the amplifier gain was set to 50 dB. Pulsed ultrasound was used to avoid heat generation, which could damage array or skin.
Ultrasound treatment and analysis of damage, nanoliposomal siRNA uptake, and tumor inhibition in reconstructed skin
Skin reconstructs were treated with ultrasound at a frequency of 20 kHz and duty cycle of 20% for 20 m by removing media and submersion in PBS (26). 1 μM nanoliposomal-siRNA complex was then applied topically and allowed to soak into the skin. Skin damage was assessed following ultrasound treatment, addition of ghost liposome and fixation in 4% paraformaldehyde at 4°C overnight. H&E stained paraffin embedded cross sections were examined for skin damage using microscopy. Skin reconstruct uptake studies involved treating with nanoliposomes containing 1 μM Alexa Fluor 546 siRNA or ghost nanoliposomes and uptake analyzed using stereo fluorescence microscopy 1-h later. Skin reconstructs were treated with ultrasound followed by treatment with nanoliposomes containing B-Raf siRNA or ghost nanoliposomes on alternate days from days 10–21. Skin reconstructs from all experiments were fixed in 4% paraformaldehyde at 4°C overnight. After fixation skin was stored in 0.5 M EDTA pH 8.0 (Fisher Scientific, Waltham, MA). Total average area occupied by GFP-tagged tumor nodules present in 4–6 skin images were used to quantify differences between treatment groups using IP Lab software.
Cell doubling time
In vitro doubling time of UACC 903 cells nucleofected with siRNA was estimated by plating 3×103 UACC 903 cell in 96-well plates and at 24-h intervals, using the SRB assay (Sigma, St. Louis, MO) to measure growth rate and calculate doubling times (8).
In vivo cell proliferation
For mechanistic studies, 5×106 UACC 903 cells nucleofected with siRNA were injected into mice and tumors harvested 4 d later to quantify cell proliferation in formalin-fixed tumor sections using the RPN 20 cell proliferation kit (Amersham Pharmacia Biotech) (8).
Anchorage independent growth
1×106 UACC 903 cells were nucleofected with siAkt3 (200 pmoles), siMutB-Raf (3, 6, or 12 pmoles), siScrambled or buffer, allowed to recover for 2 d and then plated in serum free DMEM (1×104/well) into polyHEMA-coated 96 well plates. Cell viability was assessed 3-d later using the MTS assay (9).
Ultrasound and siRNA-nanoliposomal complex treatment of tumors
1×106 UACC 903-GFP cells were injected subcutaneously into the right flank of nude mice. After 24 h, mice were ultrasound treated for 15 minutes followed by topical application of 25 μg nanoliposomal-siRNA (siMutB-Raf, siAKt3 or siScrambled) complex on alternate days. For combination nanoliposomal V600EB-Raf and Akt3 siRNA studies, mice were treated with the same regime as the single agents. Single agents were compensated with extra liposomes so equivalent amounts were used. Prior to ultrasound treatment, mice were anesthetized using 2:1 ketamine/xylazine, and area sterilized using alcohol pads. Aquaflex Gel Pads (Parker Laboratories, Inc., Fairfield, NJ) served as an ultrasound standoff. Ultrasound was administered for 15 m at a frequency of 20 kHz and duty cycle of 20% (26). Nanoliposomal siRNA was applied to ultrasound treated area overlying tumor for penetration into skin.
Toxicity analysis in mice
To establish toxicity associated with ultrasound plus siRNA-nanoliposomal complex, nude mice were ultrasound treated for 15 m followed by topical addition of nanoliposomal-siRNA (siMutB-Raf, siAkt3, siScrambled or siMutB-Raf + siAkt3) complex on alternate days. Mice were weighed on alternate days to ascertain toxicity. Following euthanization on day 23, blood was collected from siMutB-Raf and siScrambled mice and serum separated to measure serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), glucose, and alkaline phosphatase. Livers were fixed in formalin, sectioned and stained with H&E to assess liver morphology changes.
Statistical analysis
One-Way or Two-Way ANOVA followed by appropriate post hoc test (Tukey’s or Bonferroni) or t-test was used to ascertain whether significant differences occurred between groups. Differences were considered significant at p<0.05. Combination Index (CI) was calculated using the Chou-Talalay method with CalcuSyn software to establish mechanistic basis for cooperative inhibition mediated by siRNA targeting V600EB-Raf and Akt3 (35).
RESULTS
Development of stable cationic nanoliposomes that load siRNA
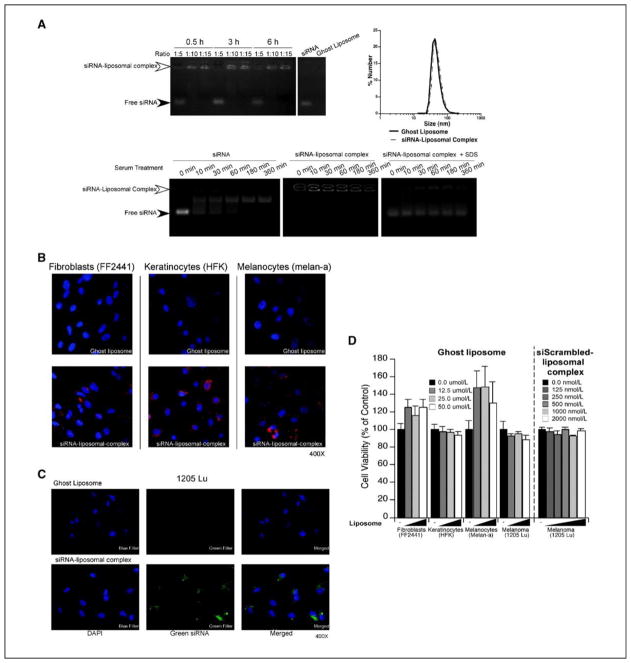
Since nanoliposomes have been reported as potential siRNA delivery vehicles, a number of possible formulations were evaluated and a novel DOTAP, DOPE and DSPE-PEG(2000) ratio of 4.75:4.75:0.5 was found to efficiently load siRNA. Loading was measured by adding Alexa Fluor 546 tagged siRNA to nanoliposomes at ratios of 1:5, 1:10, or 1:15 (siRNA: liposome, by weight) and complexed for 0.5, 3, or 6 h. Measuring free fluorescently tagged siRNA present in the gel or as a siRNA-liposome complex retained in the gel well was used to directly assess siRNA loading. A 1:5 ratio following 0.5 h incubation showed presence of both loaded and unloaded siRNA, which was in contrast to the 1:10 and 1:15 ratios where siRNA remained in gel wells indicating complete complexing with nanoliposomes (Fig. 1A; top left panel). Since maximal loading occurred following 30 m incubation at a 1:10 ratio, this siRNA-nanoliposomal complex formulation was used for subsequent experiments. Diameter of siRNA-liposome complexes one day after preparation was measured using dynamic light scattering, showing an average diameter of ~50 nm with a range of 34–67 nm (Fig. 1A; top right panel). Ghost nanoliposomes had a similar diameter averaging ~48 nm with a range of 32–64 nm. Thus, stable nanoliposomes were generated that loaded siRNA.
Figure 1. Characterization of cationic siRNA-liposome complexes.
A. Loading of fluorescently tagged siRNA into nanoliposomes. Fluorescently tagged siRNA was complexed with cationic nanoliposomes at ratios of 1:5, 1:10, or 1:15, for 0.5, 3, or 6 h and run on a 2% agarose gel to determine loading efficiency. Maximal loading was reached at a 1:10 ratio following a 0.5-h incubation (upper left panel). siRNA at a 1:10 ratio with nanoliposomes were sized using dynamic light scattering and similar size ranges observed for ghost or nanoliposomes loaded with siRNA (upper right panel). siRNA protection by cationic nanoliposomes was measured by complexing fluorescent siRNA with nanoliposomes overnight followed by exposure to serum for 10, 30, 60, 180, or 360 m. Free fluorescent siRNA alone was used as a control (lower left & middle panels). Release of siRNA in nanoliposomes was accomplished by collapsing serum treated siRNA-nanoliposomal complexes with SDS to release free siRNA, which was then run on an agarose gel (lower right panel). B. siRNA-nanoliposomal complex is taken up into normal cells. Uptake of siRNA-nanoliposomal complex into normal cells was measured by adding fluorescently tagged siRNA-nanoliposomal complex (200 nM) to fibroblasts, keratinocytes, and melanocytes for 3 h followed by fixation and imaging using fluorescence microscopy (magnification; 400X). Ghost nanoliposomes lacking fluorescent siRNA were used as a control. C. siRNA-nanoliposomal complex is taken up into the cytoplasm of cells and is not merely surface bound. siRNA-nanoliposomal complex localization in cells following treatment with siRNA-nanoliposomal complexes (100 nM) was ascertained by exposing 1205 Lu melanoma cells for 3 h after which cells were trypsinized to remove surface bound complex and replated overnight onto coverslips. Cells were fixed and imaged using fluorescent microscopy (magnification; 400X). D. Ghost liposome or siRNA-nanoliposomal complex exerted negligible toxicity on normal or cancer cells. Cellular toxicity of ghost liposome and siRNA-nanoliposomal complex was measured by adding nanoliposomes (12.5, 25, and 50 μM) to fibroblasts, keratinocytes, melanocytes, or melanoma cells for 24 h followed by MTS assay analysis. Untreated cells (−) served as controls for comparison. Results are mean±SE
siRNA in nanoliposomes is protected from serum degradation
To evaluate the protective effects conferred by nanoliposomes on siRNA, fluorescently tagged free siRNA or siRNA-liposome complexes were exposed to 50% FBS, which is rich in RNA degrading factors, for 10, 30, 60, 180, or 360 m. Partial degradation of free siRNA was observed after 10 m and complete degradation at 60 m (Fig. 1A; lower left panel). In contrast, siRNA-nanoliposomal complexes remained in gel wells at all time points, indicating protection from degradation (Fig. 1A; lower middle panel). Half the original sample was treated with 0.5% SDS for 10 m, disrupting the complex and releasing siRNA, which was then run on an agarose gel. Similar levels of free siRNA were observed at all time points indicating protection by the complex (Fig. 1A; lower right panel). Thus, siRNA in nanoliposomes was protected from degradation by external factors.
Nanoliposomal-siRNA complex is non-toxic and taken-up into the cytoplasm of normal as well as cancer cells
To determine whether nanoliposomes mediated cellular uptake of siRNA, fibroblasts, keratinocytes, and melanocytes were treated with ghost nanoliposomes or nanoliposomes containing Alexa 546-tagged siRNA (50, 100, or 200 nM). Fluorescence microscopy showed red Alexa fluorescence in ~100% of all cells indicating uptake of nanoliposomal siRNA (Fig. 1B). To verify siRNA was taken into the cytoplasm of cells, 1205 Lu cells were treated with 100 nM fluorescein-tagged siRNA-nanoliposomal complex or ghost nanoliposomes. Cells were then trypsinzed to remove cell surface bound nanoliposomes and replated overnight followed by fixation (36). Fluorescence microscopy showed presence of green fluorescein-tagged siRNA in cytoplasm of cells surrounding a nuclear shadow indicating nanoliposomal siRNA was internalized (Fig. 1C). In contrast, no green fluorescence was observed in cells treated with ghost nanoliposomes. Next, toxicity mediated by ghost nanoliposomes was examined in fibroblast, keratinocyte, melanocyte, or 1205 Lu melanoma cells. Cells were treated with increasing concentrations of ghost nanoliposomes (12.5, 25, and 50 μM) for 24 h and analyzed by MTS assay (37). Compared to untreated cells, no significant decrease in cellular viability was observed; indicating ghost nanoliposomes were not toxic at concentrations examined (Fig. 1D). Toxicity of siRNA-nanoliposomal complex was examined next in 1205 Lu cells comparing untreated cells to those treated with increasing concentrations of complex loaded with scrambled siRNA (Fig. 1D). No change in cell viability was observed indicating negligible toxicity. Thus, the siRNA-liposome complex was non-toxic and taken up into the cytoplasm of normal and cancer cells.
siRNA targeting V600EB-Raf specifically decreases mutant but not wild-type protein expression in cells
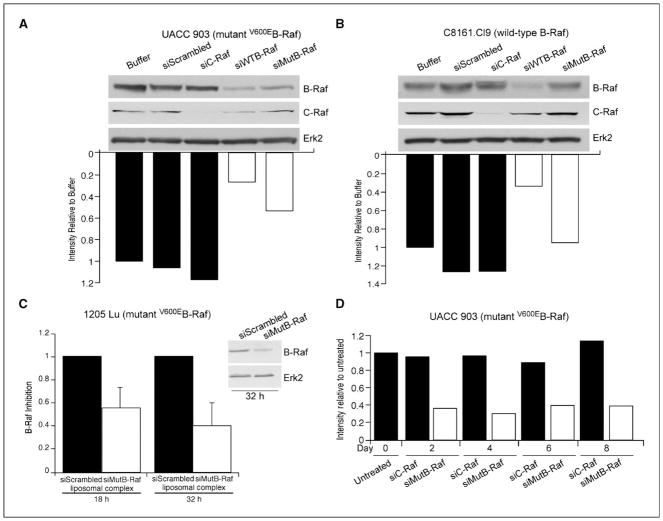
siRNA can be designed overlapping the T1799A mutation site of V600EB-Raf, which has potential to selectively decrease expression of mutant but not wild-type protein (8, 10, 30). To verify specificity of siRNA targeting V600EB-Raf, 100 pmoles (1 μM) was nucleofected into melanoma cells containing mutant (UACC 903) or wild-type protein (C8161.Cl9). Western blot and densitometric analysis of cellular protein lysates showed that siRNA designed specifically against V600EB-Raf reduced protein levels ~50% in mutant UACC 903 (Fig. 2A) but not in wild-type B-Raf expressing C8161.Cl9 cells (Fig. 2B). In contrast, siRNA designed to sequences present in both mutant and wild-type B-Raf decreased protein expression by ~75% in both cell lines (Figs. 2A & 2B). Similarly, siRNA has been reported that specifically targets Akt3 but not Akt1 or Akt2 that can effectively decrease melanoma tumor development (6). Thus, siRNA designed to specifically inhibit mutant V600EB-Raf or Akt3 reduces expression of mutant but not wild-type B-Raf protein and Akt3 but not Akt1 or Akt2, respectively (6).
Figure 2. siMutB-Raf decreases protein expression of V600EB-Raf.
A., B. siRNA can be designed to decrease expression of V600EB-Raf but not normal protein expression. To verify specificity of siMutB-Raf for decreasing expression of mutant but not wild-type protein, melanoma cells containing mutant (UACC 903) (A.) or normal (C8161.Cl9) (B.) B-Raf protein were nucleofected with buffer, siScrambled, siC-Raf, siWTB-Raf, or siMutB-Raf siRNA, protein lysates harvested 48 hours later, and analyzed by Western blot analysis for B-Raf and C-Raf knockdown. Erk2 served as a loading control. C. siMutB-Raf-nanoliposomal complex decreased expression of V600EB-Raf protein in cells. 1205 Lu cells were exposed to siMutB-Raf or siScrambled-nanoliposomal complex, protein lysates harvested at 18 and 32 hours and analyzed by Western blot analysis. Erk2 served as a control for protein loading. Densitometry results are mean±SE. D. Duration of V600EB-Raf protein knockdown following exposure to siRNA targeting mutant protein is beyond 8-d. Cells were nucleofected with C-Raf or V600EB-Raf siRNA, replated in culture dishes and protein harvested 2, 4, 6 and 8 d later to measure duration of protein knockdown. Untreated cells or cells nucleofected with siRNA targeting C-Raf served as controls.
siRNA targeting V600EB-Raf can be loaded into nanoliposomes, delivered into cells and decrease expression of mutant protein
Having validated the specificity of siRNA targeting V600EB-Raf and Akt3, 1μM siRNA was loaded into nanoliposomes and protein knockdown measured in 1205 Lu cells containing V600EB-Raf in three independent experiments by densitometric analysis and using Western blotting (Fig. 2C). Examples of protein knockdown at 18 and 32 h following a single treatment showed a 50–60% decrease of V600EB-Raf protein compared to cells treated with scrambled siRNA-nanoliposomal complex (Fig. 2C). Typically, protein knockdown ranged from 25–60% indicating some variability but knockdown was reproducibly observed. Duration of knockdown following a single siRNA treatment was measured by introducing siRNA into cells and using Western blotting to measure protein knockdown 2, 4, 6, and 8 days later. Western blots were densitometrically scanned and knockdown of B-Raf protein compared to control cells nucleofected with siRNA targeting C-Raf. A consistent ~60% decrease in protein expression was observed through day 8 (Fig. 2D). Thus, siRNA specific to V600EB-Raf can be loaded into cationic nanoliposomes, delivered into melanoma cells and decrease protein expression by 25–60% following a single treatment.
Ultrasound treatment of laboratory-generated skin containing melanocytic lesions enables delivery of siRNA-nanoliposomal complex into tumors
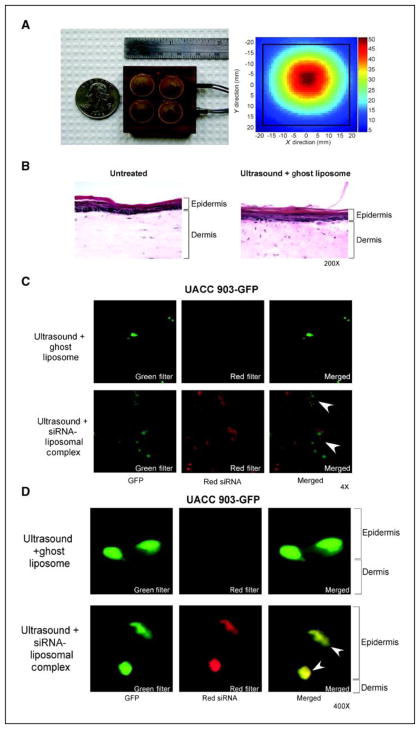
Skin forms a natural barrier preventing uptake of agents, including lipids and siRNA (19, 21). Therefore, skin was permeabilized prior to addition of siRNA-liposomal complex using a lightweight four-cymbal array ultrasound developed originally to deliver insulin through skin and into the blood stream (Fig. 3A; left panel) (23–26). A representative exposimetry analysis showed a gaussian shaped peak field intensity with an overall spatial peak-temporal intensity of 50.6 ± 1.5 mW/cm2 (Fig. 3A; right panel). Possible skin damaged by ultrasound was evaluated on reconstructed skin treated with ultrasound for 20 m followed by addition of topically added ghost liposome for 1 h followed by fixation. H&E stained skin sections comparing untreated control to ultrasound and ghost liposome treated skin showed no detectable damage (Fig. 3B). Next, skin penetration by siRNA-nanoliposomal complex following ultrasound treatment was measured. Skin was ultrasound treated for 20 m followed by topical addition of liposome loaded with 1 μM Alexa Fluor 546-tagged siRNA and 1-h later fixed in 4% paraformaldehyde followed by analysis using fluorescence stereomicroscopy. Top down views of the skin showed uptake of red Alexa Fluor 546 tagged siRNA in both keratinocytes and UACC 903-GFP melanoma cells (Fig. 3C). Cross sections of skin showed presence of red Alexa Fluor 546 tagged siRNA in melanoma cells located in both epidermis and epidermal-dermal junction (Fig. 3D). Similar results were seen in skin reconstructs containing WM35-GFP (data not shown). Thus, permeabalizing skin using ultrasound prior to topical application of siRNA-nanoliposomal complex enabled delivery of siRNA to melanocytic lesions located within skin.
Figure 3. Ultrasound treatment permeabilizes skin enabling melanocytic lesions to take up siRNA-nanoliposomal complexes.
A. Ultrasound assembly. A lightweight, low-profile ultrasound array was constructed using four cymbal transducers, which were connected in parallel and encased in polymer. The temporal peak intensity was determined in a spatial plane 1 mm from the face of the transducer for exposure conditions. B. Ultrasound treatment does not damage skin. Laboratory generated skin was ultrasound treated followed by addition of ghost liposome, skin sectioned and H&E stained. No changes cellular structure or in skin morphology was observed compared to untreated control skin (magnification; 200X). C., D. Following ultrasound treatment of skin, siRNA-nanoliposomal complex is taken up by melanocytic lesions in the epidermis and at the epidermal-dermal junction. Following 20 m ultrasound treatment, fluorescent siRNA-nanoliposomal complex or ghost nanoliposomes were applied topically onto reconstructed skin. 1-h later, skin was fixed and analyzed using fluorescent microscopy looking down at the skin (Magnification; 4X) (C.), or by cross sections (magnification; 400X) (D.). Fluorescence (red) indicating presence of siRNA-nanoliposomal complex was evident in melanocytic lesions in both epidermis and at epidermal-dermal junction (arrows).
Following ultrasound treatment, nanoliposomal complex containing siRNA targeting V600EB-Raf, inhibits melanocytic lesion development in skin reconstructs
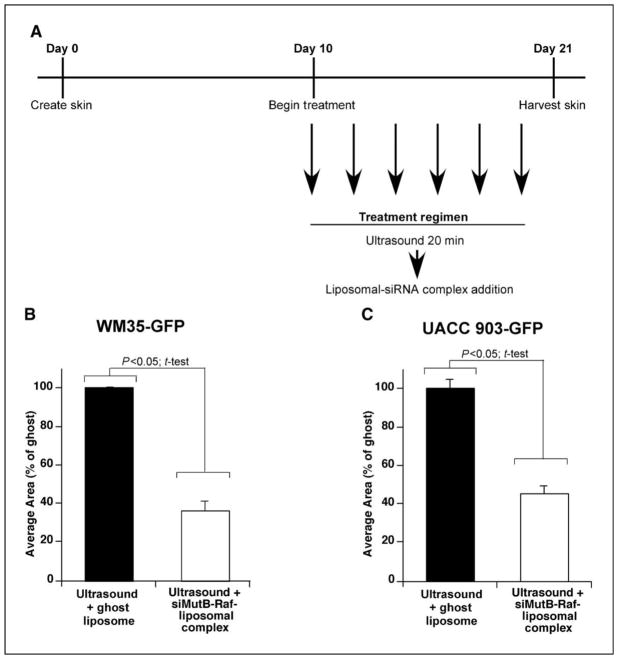
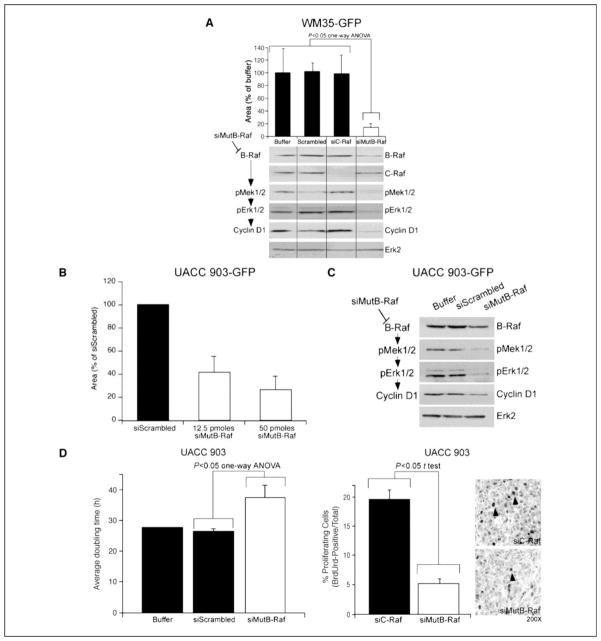
Effectiveness of siRNA-nanoliposomal complex for inhibiting early melanocytic skin lesions or cutaneous metastasis development, was examined by treating skin containing GFP tumors on alternate days with ultrasound for 20 m followed by addition of nanoliposomes containing siRNA to V600EB-Raf or ghost liposome (Fig. 4A). On day 21, stereo fluorescence microscopy was used to quantify average area occupied by GFP fluorescent skin lesions and compared to ultrasound + ghost liposome treated skin. A statistically significant 50–65% reduction in area occupied was observed for WM35-GFP (Fig. 4B) and UACC 903-GFP (Fig. 4C) tumor nodules (p<0.05; One-Way ANOVA). The WM35 cell line represents early non-invasive melanocytic lesions while UACC 903 cells represent invasive melanocytic lesions in the reconstructed skin model. Thus, siRNA mediated inhibition of V600EB-Raf decreased proliferative capacity of tumor cells reducing skin nodule development.
Figure 4. Ultrasound treatment followed by topical application of siMutB-Raf-nanoliposomal complex inhibits melanocytic lesion development in reconstructed skin.
A. Schematic showing treatment regime. Beginning on d 10 and on alternate days thereafter up to d 20, reconstructed skin was treated with ultrasound for 20 m followed by topical administration of siMutB-Raf-nanoliposomal complex (100 pmoles) or ghost nanoliposomes. B., C. Ultrasound followed by addition of siMutB-Raf-nanoliposomal complex decreases melanocytic lesion development in skin. Reconstructed skin containing UACC 903-GFP or WM35-GFP cells were treated with ultrasound for 20 m followed by topical administration of siMutB-Raf-nanoliposomal complex on alternated days from d 10–20. Skin was harvested on d 21, and average area occupied by GFP-tagged tumors calculated for each group. Ultrasound treatments followed by exposure to ghost nanoliposomes served as a control. Results are mean±SE.
Mechanistically, siRNA targeting V600EB-Raf decreases the proliferative potential of melanoma cell by inhibiting MAP kinase signaling in laboratory generated skin
To establish the mechanistic basis by which siRNA-targeting V600EB-Raf inhibited melanoma development, siRNA was introduced into GFP-tagged WM35 or UACC 903 cells, which were seeded into the epidermis of reconstructed skin. 10 d later, skin was fixed and fluorescence microscopy used to quantify average area occupied by GFP expressing tumor nodules. Compared to cells nucleofected with buffer, scrambled or C-Raf siRNA, a 3–7-fold reduction in nodule development was observed by cells exposed to siRNA targeting V600EB-Raf (Figs. 5A & 5B, One-Way ANOVA p<0.05). Knockdown of V600EB-Raf protein expression was confirmed by Western blotting of protein lysates collected following creation of reconstructed skin (Figs. 5A & 5C).
Figure 5. siMutB-Raf inhibits melanocytic lesion growth in reconstructed skin.
A. Targeting melanocytic lesions using siRNA against V600EB-Raf decreases melanocytic lesion development in laboratory-generated skin. Effectiveness of siRNA targeting V600EB-Raf for decreasing cutaneous tumor development was established by nucleofecting GFP-tagged WM35 cells with buffer, scrambled siRNA or siRNA targeting C-Raf, or V600EB-Raf (100 pmoles). Cells were then seeded into laboratory-generated skin at time of creation and 10 d later, average area occupied by green melanocytic lesions quantified. A statistically significant reduction in green fluorescent lesions was observed following siMutB-Raf treatment (p<0.05; One-Way ANOVA) (upper panel). Results are mean±SE. Protein lysates harvested from cells were analyzed by Western blot for B-Raf, C-Raf, p-Mek1/2, pErk1/2, and Cyclin D1 protein expression. Erk2 served as a control for protein loading (lower panel). B. siRNA-mediated inhibition of V600EB-Raf protein expression in GFP-tagged UACC 903 cells decreases lesion formation in skin reconstrtucts. UACC 903-GFP cells were nucleofected with siScrambled or siB-Raf (12.5 or 50 pmoles) and cells seeded into laboratory generated skin at time of creation. Reconstructed skin was analyzed using fluorescence microscopy 10 d later and area occupied by developing GFP lesions quantified. Results are mean±SE. C. Inhibition of V600EB-Raf decreased MAP kinase signaling in UACC 903-GFP cells. UACC 903-GFP cells were nucleofected with buffer, siScrambled or siB-Raf (50 pmoles) and harvested at 48 h for Western blot analysis. Westerns were probed with B-Raf, p-Mek1/2, p-Erk1/2, and cyclin D1 to show decreased MAP kinase pathway signaling. Erk2 served as a loading control. D. Mechanistically, siRNA-mediated targeting of V600EB-Raf protein decreased the proliferative capacity of cells. Cultured UACC 903 melanoma cells treated with siMutB-Raf had an increased doubling time indicating cells were proliferating at a slower rate (left panel). Quantifying proliferating cells showed a 2–3 fold decrease following siMutB-Raf treatment of tumor cells in size and time matched tumor controls treated with siRNA to C-Raf (middle and right panels). Results are mean±SE.
Mechanism leading to decreased cutaneous tumor development was established by calculating in vitro doubling times as well as comparing differences in proliferation and measuring effect on MAP kinase signaling. siRNA-mediated inhibition of V600EB-Raf decreased mutant protein expression causing corresponding decreases in pMek and pErk as well as decreased cyclin D1 expression, indicating reduction in the proliferative signaling pathways of these cells (Figs. 5A & 5C). The doubling time of cultured cells containing siRNA targeting V600EB-Raf was found to be ~1.3 fold higher than control cells treated with buffer or scrambled siRNA (Fig. 5D, left panel). Furthermore, a 4–5-fold decrease in proliferating cells was observed in tumor cells treated with siRNA targeting V600EB-Raf compared to control cells treated with C-Raf siRNA (Fig. 5D, right panels). This growth inhibitory mechanism is consistent with prior reports (8, 30). Thus, siRNA in nanoliposomes can decrease expression of V600EB-Raf protein, reducing MAP kinase signaling and the proliferative potential of melanoma cells.
Following ultrasound treatment, siRNA-nanoliposomal complex targeting V600EB-Raf and Akt3 cooperatively decreased melanocytic lesion development in animal skin
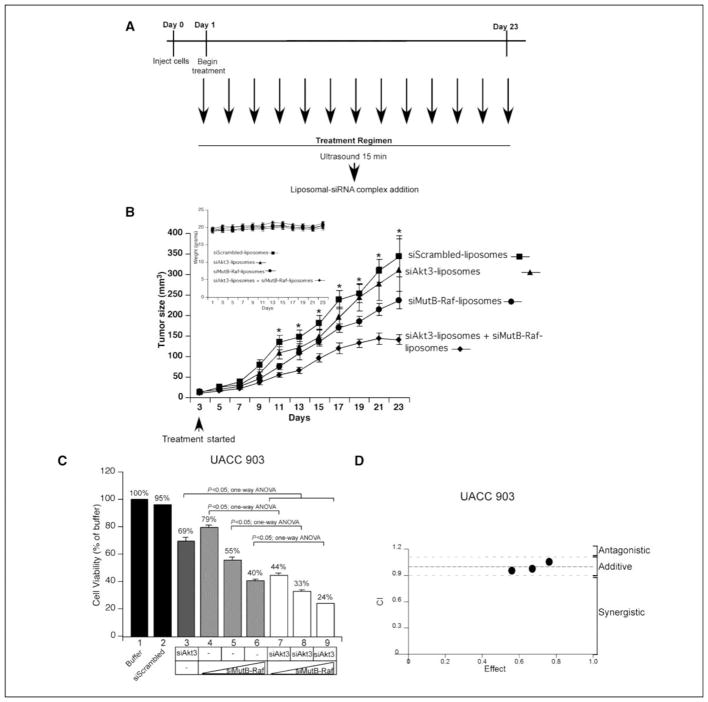
Effect of ultrasound followed by topical application of nanoliposomal-siRNA complex on melanoma development in animal skin was examined next. 1×106 UACC 903-GFP cells were subcutaneously injected into the right flank of nude mice and, under anesthesia, treated on alternate days at the tumor site with ultrasound for 15 m followed by topical application of siRNA-nanoliposomal complex (Fig. 6A). Along with measurement of tumor size (Fig. 6B), mice were weighed (Fig. 6B, inset) on alternate days. Effect of liposomes containing siRNA targeting V600EB-Raf or Akt3 alone or V600EB-Raf and Akt3 together on tumor development were compared. Ghost liposomes were added to single treatments to compensate for differences in the amount of liposomal vehicle. A statistically significant ~30% reduction in tumor size was observed from d 15 in mice treated with complex containing siRNA targeting V600EB-Raf compared to animals exposed to complex containing scrambled siRNA (Fig. 6B, p<0.05; Two-Way ANOVA). In contrast, no statistically significant difference was observed on cutaneous melanoma development following treatment with liposomes containing siRNA targeting Akt3 even though treated tumors were 10–15% smaller than controls treated with liposomes containing scrambled siRNA (Fig. 6B, p>0.05; Two-Way ANOVA). Significantly, the combination treatment showed cooperatively acting tumor inhibition from d 11 when compared to siScrambled-liposomal complex (Fig. 6B, p<0.05; Two-Way ANOVA).
Figure 6. Ultrasound treatment followed by topical application of siMutB-Raf-nanoliposomal complex alone or in combination with siAkt3-nanoliposomal complex inhibits melanocytic lesion development in animal skin.
A. Schematic showing treatment regime. Ultrasound treatment followed by topical application of siMutB-Raf-nanoliposomal complexes started the day after injection of melanoma cells and continued on alternate days to day 23. During the procedure, anesthetized mice were treated with ultrasound at the injection site for 15 m followed by topical application of siMutB-Raf-nanoliposomal complex. B. Ultrasound treatment followed by topical application of siAkt3-liposomal complex + siMutB-Raf-liposomal complex decreased melanoma development in animal skin. UACC 903-GFP cells (1×106), were injected subcutaneously into nude mice and after 24 h, tumors forming at injection sites were treated on alternate days with ultrasound for 15 m followed by topical administration of siMutB-Raf-liposomal complex, siAkt3-liposomal complex, or siAkt3-liposomal complex + siMutB-Raf liposomal complex. Tumors were measured on alternate days beginning on d-3. Control mice were ultrasound treated followed by addition of siScrambled-liposomal complex. Ghost liposomes were added to single treatments so that mice were treated with equivalent amounts of liposomal vehicle. Statistically significant differences between control and siMutB-Raf-liposomal complex + siAkt3-liposomal complex treated tumors were observed beginning on day 11 (Two-Way ANOVA; p<0.05). Results are mean±SE. Ultrasound treatment followed by topical application of siAkt3-liposomal complex + siMutB-Raf-liposomal complex does not cause significant change in animal body weight. Animal weights were measured on alternate days beginning on d 1 to determine whether any weight-related toxicity occurred. No significant weight loss was observed between control and experimental groups (Two-Way ANOVA; p>0.05) (B. inset). Results are mean±SE. C. siMutB-Raf and siAkt3 cooperate to reduce anchorage independent growth in cell culture. UACC 903 cells were nucleofected with siAkt3 (200 pmoles) and siMutB-Raf (1.5, 3, 6, or 12 pmoles) in combination and compared to single siRNAs to siAkt3 (200 pmoles), siMutB-Raf (1.5, 3, 6, or 12 pmoles), siScrambled, or Buffer only for the ability to inhibit anchorage independent growth. D. siAkt3 and siMutB-Raf act additively to inhibit cell viability. Calculation of the CI index for the combination of siAkt3 and siMutB-Raf showed additive inhibition of cell viability with CI values between 0.94 and 1.10.
Weights were monitored on alternate days to ascertain weight-related toxicity (Fig. 6B, inset). No differences were seen in weights indicating a lack of toxicity (Two-Way ANOVA, p>0.05). Lack of systemic toxicity was confirmed by analyzing blood for SGPT, SGOT, alkaline phosphatase, and glucose levels (data not shown), which showed no significant differences between animal groups (p>0.05; t-test). Additionally, no changes in liver morphology were detected in H&E stained sections (data not shown).
Targeting V600EB-Raf and Akt3 leads to additively cooperative inhibition
Since liposomal-mediated targeting of Akt3 and V600EB-Raf suggested cooperatively acting tumor inhibition, mechanistic basis for inhibition was determined. siRNA targeting Akt3 and/or V600EB-Raf was nucleofected into UACC 903 cells and effect on in vitro anchorage independent growth examined. For these studies and subsequent Chou-Talalay analysis for measuring the mechanistic basis underlying inhibition, Akt3 siRNA was maintained at 200 pmoles B-Raf siRNA was titrated at doses of 3, 6, and 12 pmoles. Simultaneous siRNA-mediated inhibition of Akt3 and B-Raf significantly decreased growth of cells compared to either Akt3 or B-Raf targeting alone (Fig 6C, Lanes 3, 4 versus 7, Lanes 3, 5 versus 8 and Lanes 3, 6 versus 9 ; One-Way ANOVA, p<0.05) (38).
To establish whether growth inhibition would be additive or synergistic following simultaneous inhibition of Akt3 and V600EB-Raf, the Chou-Talalay method was used for determining the combination index (CI) using Calcusyn software (35). Using this approach, CI values < 0.9 are synergistic, > 1.1 is antagonistic, and values 0.9–1.1 are additive (Fig. 6D). The estimated CI values were 0.94, 0.97, and 1.10, for 3, 6, and 12 pmoles of B-Raf siRNA plus 200 pmoles of Akt3 siRNA, suggesting additive cooperation (Fig. 6D). Thus, targeting V600EB-Raf and Akt3 in culture or in animals should lead to cooperatively additive inhibition. However, tumor inhibition following liposome-mediated delivery of siRNA targeting V600EB-Raf and Akt3 in Fig. 6B seems to be more than additive suggesting it may tend towards being synergistic.
DISCUSSION
Novel therapeutic regimens need development for treating early or cutaneous metastatic melanoma since aside from surgical excision, few treatment options are available (39). Current systemic therapeutics cause toxicities and have off-target effects (19). Topical or localized treatments of agents could permit use of high local concentrations with minimal toxicity and be useful for treating cutaneous lesions not amenable to surgical removal (19, 40). A case in point is use of interlesional injections of IL-2, and electroporation for improved topical delivery of bleomycin for melanoma treatment (40, 41). Effective topical agents could provide additional, currently unavailable, treatments options for patients with early or invasive cutaneous melanoma.
The era of personalized, targeted cancer treatment is near, but specific proteins playing key roles in particular cancer types need identification and demonstration that simultaneous targeting inhibits tumor development in a synergistically acting manner. Thus study demonstrates that V600EB-Raf and Akt3 are two such targets in melanoma whose inhibition led to cooperative tumor inhibition. Furthermore, a novel approach has been developed for delivering therapeutic siRNA into early or invasive cutaneous melanocytic lesions targeting these important proteins. Ultrasound treatment of skin followed by topical application of nanoliposomes containing siRNA-targeting V600EB-Raf and Akt3 reduced expression of these proteins significantly decreasing melanoma development in a cooperatively additive manner.
Simultaneous inhibition of the MAP and PI3 kinase pathways has previously been reported to enhance the inhibitory effect on both signaling cascades leading to increased tumor cell apoptosis, which supports the feasibility of this approach (47–49). Furthermore, siRNA-mediated inhibition of V600EB-Raf and Akt3 in aggressive melanoma cells has been shown to cooperatively inhibit tumor development through enhanced effects on the signaling cascades; specifically leading to decreased cyclin D1 expression as well as increased p27 and cleaved caspase-3 levels (9). These results are not surprising considering discoveries linking the two pathways in early melanoma development (9). Approximately 90% of nevi express V600EB-Raf but very few ever develop into melanoma due to the inhibitory effects associated with high MAP kinase pathway activity caused by the mutant protein. Akt3 has been shown to overcome this block by phosphorylating V600EB-Raf these melanocytes in order to lower activity of mutant protein and downstream MAP kinase pathway signaling to levels promoting rather than inhibiting melanoma development (9). Phosphorylation of V600EB-Raf enabled melanocytes to overcome the growth inhibitory effects associated with high pathway activity and to develop melanoma cell like characteristics (9). Thus, this discovery demonstrates that siRNA can be used as a therapeutic, specifically targeting multiple aberrantly expressed or mutant cancer causing genes without inhibiting activity of normal proteins to more effectively treat melanoma.
Systemic administration of free unmodified siRNA by intravenous injection is not clinically feasible since serum factors rapidly degrade it preventing clinically useful concentrations from being reached (42). Development of novel vehicles for loading, protecting and delivering siRNA to distant sites around the body is one strategy (16–18, 43, 44). This study demonstrates that cationic nanoliposomes can load, protect and deliver therapeutic siRNA into melanoma cells. Nanoliposomes are taken up by all cell types but only effects those containing V600EB-Raf or overexpression of Akt3. Specificity of siRNA for its target reduces toxicity and potential side effects. Furthermore, use of pegylated lipids and small average nanoliposomal diameter of ~50 nm decreases immunological or inflammatory responses enabling the particles to bypass the reticulo-endothelial system (45, 46).
Ultrasound was used to permeabilize skin enabling delivery of siRNA-liposomal complex throughout epidermal and dermal layers since it had been reported to be effective for delivering various macromolecules into and throughout skin (19, 21–26). A light-weight transducer with demonstrated capabilities of delivering insulin or antisense oligonucleotides into or through skin was used, showing that low-frequency ultrasound can permeabilize skin enabling delivery of nanoliposomal siRNA into melanoma cells residing at the dermal-epidermal junction to inhibit protein expression and melanoma development.
Mechanistically, a therapeutic nanoliposomal-siRNA complex targeting V600EB-Raf and Akt3 can effectively decrease expression of both proteins and downstream signaling cascades to inhibit development or spread of cutaneous melanoma (8, 10, 30). Thus, use of a nanoliposomal-siRNA complex targeting these proteins could be used as a non-surgical approach to decrease number of benign moles or inhibit early cutaneous melanocytic lesions development (2–5, 9). The latter would have the consequence of decreasing the spread at the earliest stages thereby prolonging survival by preventing metastases. Ultrasound in conjunction with a therapeutic topical nanoliposomal-siRNA complex targeting V600EB-Raf and Akt3 could also provide a treatment option for patients whose disease is not amenable to surgical removal or limb perfusion when widespread cutaneous lesions are present on the trunk. No therapeutic agents for treating these patients are available. Furthermore, advanced stage melanoma patients could benefit by administering these agents at the time of sentinel lymph node biopsy or surgery to target cells that may not have been removed during the procedure (50). Finally, this approach could be combined with more traditional therapeutic agents to improve clinical efficacy through synergistically acting mechanisms.
In conclusion, topical delivery of cationic nanoliposomes loaded with siRNA targeting V600EB-Raf and Akt3 in combination with low-frequency ultrasound has potential to decrease early melanocytic lesion development in skin or prevent spread of cutaneous melanoma metastases.
Acknowledgments
Grant support: NIH/NCI 1-RO3-CA128033-01, The American Cancer Society (RSG-04-053-01-GMC), The Foreman Foundation for Melanoma Research, State of Pennsylvania Non-formulary Tobacco Settlement Funds, Department of Defense Technologies for Metabolic Monitoring (W81XWH-05-1-0617).
We would like to thank James Kaiser, Julie Anderson, Nishit Trivedi, Mitchel Cheung and SubbaRao V. Madhunpantula for technical assistance. We would also like to thank Shantu Amin for his reading this of manuscript.
References
- 1.Satyamoorthy K, Herlyn M. Cellular and molecular biology of human melanoma. Cancer Biol Ther. 2002;1:14–7. doi: 10.4161/cbt.1.1.32. [DOI] [PubMed] [Google Scholar]
- 2.Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000. [PubMed] [Google Scholar]
- 3.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 4.Yazdi AS, Palmedo G, Flaig MJ, et al. Mutations of the BRAF gene in benign and malignant melanocytic lesions. J Invest Dermatol. 2003;121:1160–2. doi: 10.1046/j.1523-1747.2003.12559.x. [DOI] [PubMed] [Google Scholar]
- 5.Miller CJ, Cheung M, Sharma A, et al. Method of mutation analysis may contribute to discrepancies in reports of (V599E)BRAF mutation frequencies in melanocytic neoplasms. J Invest Dermatol. 2004;123:990–2. doi: 10.1111/j.0022-202X.2004.23468.x. [DOI] [PubMed] [Google Scholar]
- 6.Stahl JM, Sharma A, Cheung M, et al. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64:7002–10. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- 7.Stahl JM, Cheung M, Sharma A, Trivedi NR, Shanmugam S, Robertson GP. Loss of PTEN promotes tumor development in malignant melanoma. Cancer Res. 2003;63:2881–90. [PubMed] [Google Scholar]
- 8.Sharma A, Trivedi NR, Zimmerman MA, Tuveson DA, Smith CD, Robertson GP. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res. 2005;65:2412–21. doi: 10.1158/0008-5472.CAN-04-2423. [DOI] [PubMed] [Google Scholar]
- 9.Cheung M, Sharma A, Madhunapantula SV, Robertson GP. Akt3 and mutant (V600E) B-Raf cooperate to promote early melanoma development. Cancer Res. 2008;68:3429–39. doi: 10.1158/0008-5472.CAN-07-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hingorani SR, Jacobetz MA, Robertson GP, Herlyn M, Tuveson DA. Suppression of BRAF(V599E) in human melanoma abrogates transformation. Cancer Res. 2003;63:5198–202. [PubMed] [Google Scholar]
- 11.Brazas RM, Hagstrom JE. Delivery of small interfering RNA to mammalian cells in culture by using cationic lipid/polymer-based transfection reagents. Methods Enzymol. 2005;392:112–24. doi: 10.1016/S0076-6879(04)92007-1. [DOI] [PubMed] [Google Scholar]
- 12.Gresch O, Engel FB, Nesic D, et al. New non-viral method for gene transfer into primary cells. Methods. 2004;33:151–63. doi: 10.1016/j.ymeth.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Muratovska A, Eccles MR. Conjugate for efficient delivery of short interfering RNA (siRNA) into mammalian cells. FEBS Lett. 2004;558:63–8. doi: 10.1016/S0014-5793(03)01505-9. [DOI] [PubMed] [Google Scholar]
- 14.Kim DH, Rossi JJ. Strategies for silencing human disease using RNA interference. Nat Rev Genet. 2007;8:173–84. doi: 10.1038/nrg2006. [DOI] [PubMed] [Google Scholar]
- 15.Leung RK, Whittaker PA. RNA interference: from gene silencing to gene-specific therapeutics. Pharmacol Ther. 2005;107:222–39. doi: 10.1016/j.pharmthera.2005.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C, Tang N, Liu X, Liang W, Xu W, Torchilin VP. siRNA-containing liposomes modified with polyarginine effectively silence the targeted gene. J Control Release. 2006;112:229–39. doi: 10.1016/j.jconrel.2006.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halder J, Kamat AA, Landen CN, Jr, et al. Focal adhesion kinase targeting using in vivo short interfering RNA delivery in neutral liposomes for ovarian carcinoma therapy. Clin Cancer Res. 2006;12:4916–24. doi: 10.1158/1078-0432.CCR-06-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pal A, Ahmad A, Khan S, et al. Systemic delivery of RafsiRNA using cationic cardiolipin liposomes silences Raf-1 expression and inhibits tumor growth in xenograft model of human prostate cancer. Int J Oncol. 2005;26:1087–91. [PubMed] [Google Scholar]
- 19.El Maghraby GM, Williams AC, Barry BW. Can drug-bearing liposomes penetrate intact skin? J Pharm Pharmacol. 2006;58:415–29. doi: 10.1211/jpp.58.4.0001. [DOI] [PubMed] [Google Scholar]
- 20.Lee SH, Jeong SK, Ahn SK. An update of the defensive barrier function of skin. Yonsei Med J. 2006;47:293–306. doi: 10.3349/ymj.2006.47.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ting WW, Vest CD, Sontheimer RD. Review of traditional and novel modalities that enhance the permeability of local therapeutics across the stratum corneum. Int J Dermatol. 2004;43:538–47. doi: 10.1111/j.1365-4632.2004.02147.x. [DOI] [PubMed] [Google Scholar]
- 22.Lavon I, Kost J. Ultrasound and transdermal drug delivery. Drug Discov Today. 2004;9:670–6. doi: 10.1016/S1359-6446(04)03170-8. [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Newnham RE, Smith NB. Short ultrasound exposure times for noninvasive insulin delivery in rats using the lightweight cymbal array. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:176–80. [PubMed] [Google Scholar]
- 24.Park EJ, Werner J, Smith NB. Ultrasound mediated transdermal insulin delivery in pigs using a lightweight transducer. Pharm Res. 2007;24:1396–401. doi: 10.1007/s11095-007-9306-4. [DOI] [PubMed] [Google Scholar]
- 25.Smith NB, Lee S, Maione E, Roy RB, McElligott S, Shung KK. Ultrasound-mediated transdermal transport of insulin in vitro through human skin using novel transducer designs. Ultrasound Med Biol. 2003;29:311–7. doi: 10.1016/s0301-5629(02)00706-8. [DOI] [PubMed] [Google Scholar]
- 26.Smith NB, Lee S, Shung KK. Ultrasound-mediated transdermal in vivo transport of insulin with low-profile cymbal arrays. Ultrasound Med Biol. 2003;29:1205–10. doi: 10.1016/s0301-5629(03)00908-6. [DOI] [PubMed] [Google Scholar]
- 27.Satyamoorthy K, DeJesus E, Linnenbach AJ, et al. Melanoma cell lines from different stages of progression and their biological and molecular analyses. Melanoma Res. 1997;7 (Suppl 2):S35–42. [PubMed] [Google Scholar]
- 28.Stover T, Kester M. Liposomal delivery enhances short-chain ceramide-induced apoptosis of breast cancer cells. J Pharmacol Exp Ther. 2003;307:468–75. doi: 10.1124/jpet.103.054056. [DOI] [PubMed] [Google Scholar]
- 29.Stover TC, Sharma A, Robertson GP, Kester M. Systemic delivery of liposomal short-chain ceramide limits solid tumor growth in murine models of breast adenocarcinoma. Clin Cancer Res. 2005;11:3465–74. doi: 10.1158/1078-0432.CCR-04-1770. [DOI] [PubMed] [Google Scholar]
- 30.Sharma A, Tran MA, Liang S, et al. Targeting Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase Kinase in the Mutant (V600E) B-Raf Signaling Cascade Effectively Inhibits Melanoma Lung Metastases. Cancer Res. 2006;66:8200–9. doi: 10.1158/0008-5472.CAN-06-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasband WS. Health USNIo, editor. ImageJ. Bethesda, Maryland, USA: 1997–2007. [Google Scholar]
- 32.Meyers C. Organotypic (raft) epithelial tissue culture system for the differntiation-dependent replication of papillomavirus. Methods in Cell Science. 1996;18:201–10. [Google Scholar]
- 33.Maione E, Shung KK, Meyer RJ, Jr, Hughes JW, Newnham RE, Smith NB. Transducer design for a portable ultrasound enhanced transdermal drug-delivery system. IEEE Transactions on Ultrasonics, Ferroelectrics and Frequency Control. 2002;49:1430–6. doi: 10.1109/tuffc.2002.1041084. [DOI] [PubMed] [Google Scholar]
- 34.Newnham RE, Dogan A, inventors. 5,729,077. Metal-electroactive ceramic composite transducer patent. 1998
- 35.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 36.RNA Interference - Technical Referecne & Application Guide. Lafayette, CO: Dharmacon, Inc; 2004. [Google Scholar]
- 37.Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3:207–12. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- 38.Christensen C, Guldberg P. Growth factors rescue cutaneous melanoma cells from apoptosis induced by knockdown of mutated (V600E) B-RAF. Oncogene. 2005 doi: 10.1038/sj.onc.1208758. [DOI] [PubMed] [Google Scholar]
- 39.Garbe C, Eigentler TK. Diagnosis and treatment of cutaneous melanoma: state of the art 2006. Melanoma Res. 2007;17:117–27. doi: 10.1097/CMR.0b013e328042bb36. [DOI] [PubMed] [Google Scholar]
- 40.Radny P, Caroli UM, Bauer J, et al. Phase II trial of intralesional therapy with interleukin-2 in soft-tissue melanoma metastases. Br J Cancer. 2003;89:1620–6. doi: 10.1038/sj.bjc.6601320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mir LM, Glass LF, Sersa G, et al. Effective treatment of cutaneous and subcutaneous malignant tumours by electrochemotherapy. Br J Cancer. 1998;77:2336–42. doi: 10.1038/bjc.1998.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Behlke MA. Progress towards in vivo use of siRNAs. Mol Ther. 2006;13:644–70. doi: 10.1016/j.ymthe.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorensen DR, Leirdal M, Sioud M. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J Mol Biol. 2003;327:761–6. doi: 10.1016/s0022-2836(03)00181-5. [DOI] [PubMed] [Google Scholar]
- 44.Derfus AM, Chen AA, Min DH, Ruoslahti E, Bhatia SN. Targeted Quantum Dot Conjugates for siRNA Delivery. Bioconjug Chem. 2007 doi: 10.1021/bc060367e. [DOI] [PubMed] [Google Scholar]
- 45.Gabizon A, Catane R, Uziely B, et al. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994;54:987–92. [PubMed] [Google Scholar]
- 46.Treat J, Damjanov N, Huang C, Zrada S, Rahman A. Liposomal-encapsulated chemotherapy: preliminary results of a phase I study of a novel liposomal paclitaxel. Oncology (Williston Park) 2001;15:44–8. [PubMed] [Google Scholar]
- 47.Bedogni B, O’Neill MS, Welford SM, et al. Topical treatment with inhibitors of the phosphatidylinositol 3′-kinase/Akt and Raf/mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathways reduces melanoma development in severe combined immunodeficient mice. Cancer Res. 2004;64:2552–60. doi: 10.1158/0008-5472.can-03-3327. [DOI] [PubMed] [Google Scholar]
- 48.Bedogni B, Welford SM, Kwan AC, Ranger-Moore J, Saboda K, Powell MB. Inhibition of phosphatidylinositol-3-kinase and mitogen-activated protein kinase kinase 1/2 prevents melanoma development and promotes melanoma regression in the transgenic TPRas mouse model. Mol Cancer Ther. 2006;5:3071–7. doi: 10.1158/1535-7163.MCT-06-0269. [DOI] [PubMed] [Google Scholar]
- 49.Smalley KS, Haass NK, Brafford PA, Lioni M, Flaherty KT, Herlyn M. Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases. Mol Cancer Ther. 2006;5:1136–44. doi: 10.1158/1535-7163.MCT-06-0084. [DOI] [PubMed] [Google Scholar]
- 50.Balch CM. Cutaneous melanoma. 3. St. Louis, Mo: Quality Medical Pub; 1998. [Google Scholar]