Abstract
One of the hallmarks of Alzheimer’s Disease is a significant increase in ventricular volume. To date we and others have shown that a cholesterol-fed rabbit model of Alzheimer’s Disease displays as many as fourteen different pathological markers of Alzheimer’s Disease including amyloid β accumulation, thioflavin-S staining, blood brain barrier breach, microglia activation, cerebrovasculature changes and alterations in learning and memory. Using structural magnetic resonance imaging at 3T we now report that cholesterol-fed rabbits also show a significant increase in ventricular volume following 10 weeks on a diet of 2% cholesterol. The increase in volume is attributable in large part to increases in the size of the third ventricle. These changes are accompanied by significant increases in the number of amyloid β immuno-positive cells in the cortex and hippocampus. Increases in the number of beta amyloid neurons in the cortex also occurred with the addition of 0.24 ppm copper to the drinking water. Together with a list of other pathological markers, the current results add further validity to the value of the cholesterol-fed rabbit as a non-transgenic animal model of Alzheimer’s disease.
Keywords: Amyloid β, animal model, lateral ventricle, MRI, third ventricle, copper
Introduction
Alzheimer’s disease (AD) has significant effects on the brain including changes in volume, structure, extracellular content, intracellular composition and synaptic connectivity [1–10]. Animal models of AD including cholesterol-fed and transgenic mice, cholesterol-fed rats, cholesterol-fed and transgenic rabbits, aged dogs and primates have all approximated one or more aspects of this disease [11–21]. For example, evidence of Alzheimer’s like changes in transgenic mice has been documented using structural MRI to identify amyloid β (Aβ) plaques [22–27] and changes in blood and hippocampal volume [24,28,29]. Amyloid β plaques have also been detected in rabbits post mortem following two years of low-level cholesterol feeding [30]. In rabbits fed 2% cholesterol for as little as eight weeks, there are as many as fourteen different indices of pathology that are similar to those seen in AD including intracellular and extracellular Aβ, breaches of the blood brain barrier, activation of microglia, apoptosis, increased levels of Apolipoprotein E and phosphorylated tau protein [15,31–39].
Among the most consistent gross anatomical changes found in AD is an increase in ventricular volume [9,40–46]. Although a hallmark in humans, surprisingly little has been reported on changes in ventricular volume in animal models of AD. In one imaging study, Andjus and colleagues found that a trimethylin-treated rat model of AD appeared to have enlarged ventricles compared to sham-treated controls when imaged by MRI at 1.5T [47]. In another study, Dror and colleagues observed ventricle enlargement in a thiamine-deficient rat model of neurodegeneration using a 7.0 T magnet [48]. Xie and co-workers found that a transgenic mouse model of AD exhibited ventricular dilation compared to wild-type mice examine at 4.7 T [49]. Interestingly, Chen and co-workers noted ventricular enlargement in normal mice as a function of age using a 7.0 T MRI scanner [50] suggesting enlargement may simply be a function of age in this model [51]. A recent study by Ramesh and colleagues showed that an intracisternal injection of Aβ into the brains of aged rabbits reduced cortical thickness and increased the thickness of the lateral ventricles when measured 45 days after treatment [52]. We have previously used magnetic resonance angiographic imaging at 3.0 T to report changes in the cerebrovasculature of cholesterol-fed rabbits [53]. That report also included initial efforts to quantify total ventricular volume which were based on a comparison of group-averaged images determined by manual tracing of the ventricles. Those efforts suggested a trend but no significant difference in total ventricular volume as a function of a cholesterol diet. In the present study, we used separate, individual, semi-automated measurements of the lateral and third ventricles to provide a total ventricular volume in individual rabbits fed 2% cholesterol for ten weeks and given 0.24 ppm copper in their drinking water to show a significant change in third as well as overall ventricular volume as a result of the high cholesterol diet. The level of copper added to drinking water in our previous experiments was 0.12 ppm. The present experiment increased the copper concentration in distilled water to 0.24 ppm in an effort to increase beta amyloid pathology. Consistent with previous results, cholesterol and copper increased the number of amyloid β immuno-reactive neurons in the cortex and hippocampus [20,35,54–57] and, surprisingly, the higher concentration of copper increased the number of amyloid β immuno-reactive neurons above distilled-water controls.
Materials and Methods
Animals
A total of 26 male New Zealand White rabbits (Oryctolagus cuniculus) 3–4 months of age and weighing approximately 2 kg upon arrival were housed individually, with free access to Purina rabbit chow and water, maintained on a 12-h light/12-h dark cycle and treated following National Institutes of Health guidelines in experiments approved by the West Virginia University Animal Care and Use Committee.
The rabbits received one of four possible treatment conditions in a 2x2 factorial design in which food (cholesterol vs. normal chow) and water (copper vs. distilled water) were manipulated. Rabbits either received Purina 5321 chow plus 2% cholesterol (Dyets, Inc, Bethlehem, PA) and copper in distilled water (n=6), Purina 5321 chow plus 2% cholesterol and distilled water (n=7), Purina 5321 chow (0% cholesterol) and copper in distilled water (n=7), or normal Purina 5321 chow and distilled water (n=6). Rabbits given copper received copper sulfate in their distilled drinking water with a final copper concentration of 0.24 ppm (0.24 mg/liter). Rabbits were kept on their respective diets for 10 weeks.
MRI Methods
MRI studies were performed immediately before euthanasia and subsequent histological analysis. Animals were anesthetized using 27.7 mg/kg ketamine and 5.7 mg/kg of xylazine injected subcutaneously 15 minutes before imaging. A General Electric Medical Systems 3.0 T MRI long-bore Signa clinical scanner was used with a 12cm quadrature transmit/receive radio-frequency (RF) coil from Nova Medical Systems (Wilmington, MA). The rabbit’s head was supported on a Plexiglas table to position each rabbit in the same prone position. The rabbit’s body was extended onto foam supports with a heated Delta Phase Thermal pad (Braintree Scientific, Braintree, MA) under the torso to maintain a core temperature of 36–38°C. Each rabbit MRI image data set was collected in less than 30 minutes.
The rabbits underwent 4 MRI scans: 1) a 3-plane localizer scan to check animal positioning and prescribe the axial slices, 2) an axial T1-weighted 3D inversion-recovery spoiled gradient echo sequence (3D SPGR) for anatomical scanning with good gray/white matter differentiation and dark cerebrospinal fluid (CSF); 3) an axial T2-weighted fast spin-echo sequence (FSE) for anatomical scanning with good grey/white matter differentiation and some sensitivity to stroke lesions and bright CSF; and 4) the axial time-of-flight (TOF) magnetic resonance angiography sequence to image vessels. All axial image sets covered from below the most caudal end of the cerebellum to a slice beyond the rostral end of the olfactory bulb.
The 3D SPGR images were used for the current ventricle volume analysis and were 1.6mm in slice thickness interpolated in the slice direction and reconstructed every 0.8mm. The 3D SPGR images had an in-plane resolution of 0.3mm x 0.3mm with image acquisition parameters as follows: field-of-view = 80 x 60 mm; matrix = 256 x 192; TR/TE/TI = 10.4ms./2.3ms/300ms, flip angle = 15 degrees, bandwidth = 11.9 kHz, number of acquisitions=4.
Image Analysis
To make ventricular volume measurements, the following image processing steps were performed. The MRI image contrast was enhanced using Imaging software (ImageJ 1.43, NIH) to saturate the pixels and normalize all images of each rabbit in the dataset. The lateral ventricles and the third ventricle were located with reference to a rabbit brain atlas [58,59]. Using the editing function of 3D Slicer – an MRI three-dimensional reconstruction program [60], the ventricles were manually selected using intensity-based threshold painting. The lateral ventricles were fully selected. The third ventricle was selected from the anterior edge of the cerebellum through the cerebral cortex. The statistics module of 3D Slicer was used for quantification of ventricular volume. The module derives the volume from the number of pixels selected with the editing module multiplied by the image spacing and volume per pixel. To validate the intensity-based threshold painting method, a subsection of the third ventricle was manually traced and the mean volume of three separate tracings for each rabbit was analyzed [61].
Histological analysis
The histological procedures and equipment for measurement of accumulation of Aβ peptide have been described previously [57]. Briefly, rabbits were anesthetized deeply with a mixture of ketamine (500 mg/kg) and xylazine (100 mg/kg) and the rabbits were perfused transcardially with 0.5% paraformaldehyde. Brains were extracted and post fixed for 14 days in 4% paraformaldehyde. Fifty-micrometer vibratome sections of hippocampus and parahippocampal cortex of the brain were immunostained with an antibody to Aβ (10D5; provided by Dale Schenk of Elan Pharmaceuticals, San Diego, CA) by using standard peroxidase-antiperoxidase immunohistochemical methods. Immunoreactive neurons were counted as described [35]. Specifically, neurons positively stained for the 10D5 Aβ antibody within a 0.5 x 0.5 mm square grid were counted in at least six randomly selected areas of the cortex and at least three areas of the hippocampus within a randomly selected section using a 20X objective. All neuronal counts were made blind to the treatment conditions.
Statistical Analysis
Lateral and third ventricle volumes as well as the total ventricular volumes for all subjects were subjected to analysis of variance with factors of food (cholesterol vs. normal chow) and water (copper vs. distilled water) with the significance level set at p < .05 SYSTAT, Version 8). The dependent variables were volume of the lateral and third ventricles as well as total ventricular volume expressed in cubic millimeters and the average number of Aβ immuno-positive neurons in the cortex and hippocampus.
Results
Ventricular Volume
The panels of Figure 1 show sample sequential MRI axial scans from left to right through the brain between the superior colliculus and temporal cortex of a rabbit that received chow and distilled water (A), chow and 0.24 ppm copper in distilled water (B), chow plus 2% cholesterol and distilled water (C), or chow with 2% cholesterol and 0.24 ppm copper in distilled water (D). Compared to the controls, the MRI scans show degenerating brain regions and ventricular enlargement that was produced by the cholesterol diet. Between-subject variations in the representative MRI scans are due to slight differences in the position of each rabbit in the scanner.
Figure 1.
Panels show sample sequential MRI axial scans from left to right through the brain from the colliculus to the temporal cortex of a rabbit that received chow and distilled water (A), chow and 0.24 ppm copper in distilled water (B), chow plus 2% cholesterol and distilled water (C), or chow with 2% cholesterol and 0.24 ppm copper in distilled water (D).
The four panels of Figure 2 depict a sample MRI image from a rabbit in each of the four groups to illustrate how the ventricular volume measurements were made. The enlarged inset in each panel shows the area of the third ventricle (in red) selected using intensity-based threshold painting. The insets illustrate the larger third ventricle volume in rabbits fed cholesterol (Panels C and D) compared to those fed normal chow (Panels A and B). The difference in size of the ventricles is illustrated in Figure 2 by the white arrows in each panel and inset indicating the lateral ventricle that is barely visible in these images. This loss of clear differentiation of tissue types, called volume averaging, between the ventricle and the surrounding brain tissue made the measurement of the extremely narrow ventricular structure difficult in this data set.
Figure 2.
Panels show analysis of ventricular volume in a sample MRI axial scan for a rabbit that received chow and distilled water (A), chow and 0.24 ppm copper in distilled water (B), chow plus 2% cholesterol and distilled water (C), or chow with 2% cholesterol and 0.24 ppm copper in distilled water (D). The inset of each panel shows the third ventricle selected using the intensity-based threshold painting method. The white arrows indicate the barely detectable lateral ventricle.
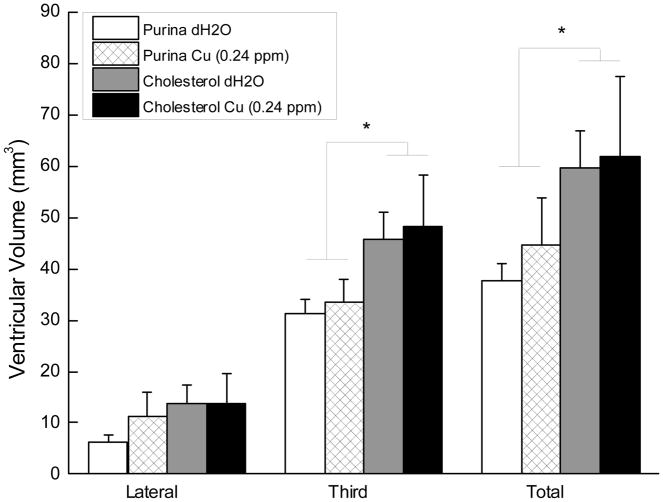
Figure 3 shows the average volume of the third and lateral ventricles as well as the total ventricular volume for rabbits in each of the four treatment groups. The figure shows clear differences in third and total ventricular volume but not in the lateral ventricle for rabbits given cholesterol in their food without there being any significant effect of adding copper to their drinking water. Analysis of variance confirmed a significant main effect of cholesterol for both third ventricle volume and total volume (F’s (1, 22) = 6.93 and 4.96, respectively, p’s < .05) without any other significant effects (F’s < 1). An analysis of the volumes of the manually traced section of the third ventricle [61] also yielded a significant difference between rabbits fed cholesterol and those fed normal chow (F(1, 22) = 5.11, p < .05) without any other significant effects (F’s < 1) providing confirmation of the results of the intensity-based threshold painting method.
Figure 3.
Mean (±SEM) volume in cubic millimeters of the lateral and third ventricle and total ventricular volume for rabbits that received chow and distilled water (Purina dH2O, n=6), chow and 0.24 ppm copper in distilled water (Purina Cu, n=7), chow plus 2% cholesterol and distilled water (Cholesterol dH2O, n=7), or chow with 2% cholesterol and 0.24 ppm copper in distilled water (Cholesterol Cu, n=6).
Amyloid β-positive neurons
The panels of Figure 4 show Aβ immuno-positive neurons in the cortex of a rabbit that received chow and distilled water (A), chow and 0.24 ppm copper in distilled water (B), chow plus 2% cholesterol and distilled water (C), or chow with 2% cholesterol and 0.24 ppm copper in distilled water (D). The figure shows that relative to the chow/distilled water control where there were relatively few stained neurons and what staining there was a relatively light, there were significant numbers of darkly stained Aβ immuno-positive neurons in the cortex of the other three groups with most staining in the cholesterol-fed rabbits.
Figure 4.
Panels show amyloid β immuno-positive neurons (5D10 antibody) in the cortex a rabbit that received chow and distilled water (A), chow and 0.24 ppm copper in distilled water (B), chow plus 2% cholesterol and distilled water (C), or chow with 2% cholesterol and 0.24 ppm copper in distilled water (D).
These impressions were confirmed in Figure 5 which shows the mean number of Aβ immuno-positive neurons in the cortex and hippocampus for the four groups. The number of Aβ-labeled cells in both the cortex and hippocampus was significantly higher in rabbits fed cholesterol than in those fed normal chow (F’s (1, 21) = 36.96, p < .001 and 9.55, p < .01, respectively). In addition, rabbits with copper added to their drinking water had more immuno-positive Aβ cells in the cortex than rabbits given distilled water (F(1,21) = 7.93, p < .05). There were no other significant effects.
Figure 5.
Mean (±SEM) number of amyloid β immuno-positive neurons in the cortex and hippocampus for rabbits that received chow and distilled water (Purina dH2O, n=6), chow and copper in distilled water (Purina Cu, n=7), chow plus 2% cholesterol and distilled water (Cholesterol dH2O, n=7), or chow with 2% cholesterol and copper in distilled water (Cholesterol Cu, n=6).
Discussion
The principal finding of the current experiment was that rabbits fed a 2% cholesterol diet for ten weeks showed an overall increase in ventricular volume due, in large part to a significant increase in the volume of the third ventricle. Consistent with previous experiments, the cholesterol diet also significantly increased the number of Aβ immuno-positive neurons in the cortex and hippocampus [20,21,35,54–57]. In addition, a concentration of copper in the drinking water twice that previously examined, significantly increased the number of Aβ immuno-positive neurons in the cortex [20] without significantly affecting neurons in the hippocampus or affecting ventricular volume.
As with previous studies, the present study confirmed one of the most consistent findings in the cholesterol-fed rabbit – an increase in cortical Aβ [12,30,31,33,35,39,54,62]. A recent study by Ramesh and colleagues used an acute intracisternal injection of Aβ in aged rabbits to determine changes in the brain [52]. Of particular relevance to the present study was a finding of increased ventricular width that occurred 45 days after the injection of Aβ. The current data appear to confirm this overall finding although the differences observed here were in the third ventricle and not the lateral ventricle and were based on ventricular volume and not ventricular width. Given the variety of species and effects of Aβ [63–68], the present differences may be the result of slowly accumulating, endogenous, intracellular Aβ compared to acutely injected, exogenous, extracellular Aβ [52].
Nevertheless, the larger volume of the third ventricle compared to the lateral ventricle in the present study is different from anatomical atlases [58,59] and casting methods [69] which show the lateral ventricle to be considerably larger than the third ventricle. This issue turns upon how little of the lateral ventricle is visible in the current MRI scans indicated by the white arrows shown in Figure 2. For example, although Levinger (1971) documents the rabbit lateral ventricle to be between 20 and 25 mm in length when measured with a polyester resin cast, Fellows-Mayle et al. (2005) were only able to see the lateral ventricle in one or two axial MRI scans that were spaced a millimeter apart. In the present study we were able to see parts of the lateral ventricle in up to three axial scans spaced 1.3 millimeters apart. In contrast, the third ventricle was completely and clearly visible in at least four to five axial scans. The second aspect of this issue concerns the thickness of the ventricle that can be resolved in the MRI image. In contrast to the third ventricle which is relatively wide with clearly defined edges, the lateral ventricles are thinner with ill-defined edges (Figure 2). Thus, each individual volume element (voxel) in the MRI image may contain white matter, grey matter or ventricular cerebrospinal fluid in varying proportions depending on the angle of the slice, the lateral ventricle width and its curvature. If the MRI voxel measures 0.2 mm on each side, for example, a 0.1 mm-wide part of the ventricle might not be visible.
The present results also show that the addition of .24 ppm copper to the drinking water significantly elevated the number of Aβ-positive neurons in rabbits fed a normal chow diet. Although we have shown previously that the addition of .12 ppm copper to drinking water can significantly increase the level of Aβ accumulation in cholesterol-fed rabbits [20,35], this is the first evidence that copper by itself is capable of elevating the number of Aβ-positive neurons. These data suggest that elevated copper levels prevent clearance of Aβ from the brain of rabbits fed normal chow [20] – an effect we did not see when rabbits were given only .12 ppm copper in their drinking water [20,35]. One potential mechanism that might explain this effect is copper-induced interference with Aβ clearance across the blood brain barrier via low density lipoprotein receptor-related proteins [70,71]. There has been considerable debate about the role of trace metals including copper in AD [72–78] with some proposing that high levels are problematic whereas others suggesting that low levels are of concern. Whatever role copper plays, it is clear that there is a need for tight control of trace metals in the CNS and that imbalances, as in the present case, can be detrimental [72,78–80].
In sum, the current data add increased ventricular volume – a hallmark of Alzheimer’s Disease – to the fourteen other indices of pathology in rabbits fed 2% cholesterol that are similar to those seen in AD including intracellular and extracellular Aβ, breaches of the blood brain barrier, activation of microglia, apoptosis, increased levels of Apolipoprotein E, phosphorylated tau protein and neurovascular changes [15,31–39,53].
Acknowledgments
Preparation of the manuscript and the described experiment was supported by NIH grant AG023211. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIA.
Reference List
- 1.Yankner BA, Lu T. Amyloid β-protein toxicity and the pathogenesis of Alzheimer’s disease. J Biol Chem. 2008;284:4755–4759. doi: 10.1074/jbc.R800018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar-Singh S. Cerebral amyloid angiopathy: pathogenetic mechanisms and link to dense amyloid plaques. Genes, Brain and Behavior. 2007;7 (Suppl 1):67–82. doi: 10.1111/j.1601-183X.2007.00380.x. [DOI] [PubMed] [Google Scholar]
- 3.Lockhart A. Imaging Alzheimer’s disease pathology: one target, many ligands. Drug Discovery Today. 2006;11:1093–1099. doi: 10.1016/j.drudis.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Fassbender K, Master C, Beyreuther K. Alzheimer’s disease: molecular concepts and therapeutic targets. Naturwissenschaften. 2001;88:261–267. doi: 10.1007/s001140100237. [DOI] [PubMed] [Google Scholar]
- 5.Raskind MA, Peskind ER. Alzheimer’s disease and related disorders. Med Clin North Am. 2001;85:803–817. doi: 10.1016/s0025-7125(05)70341-2. [DOI] [PubMed] [Google Scholar]
- 6.Mattson MP, Chan SL. Dysregulation of cellular calcium homeostasis in Alzheimer’s disease. J Mol Neurosci. 2001;17:205–224. doi: 10.1385/JMN:17:2:205. [DOI] [PubMed] [Google Scholar]
- 7.Albert MS. Cognitive and neurobiologic markers of early Alzheimer disease. Proceedings of the National Academy of Sciences, USA. 1996;93:13547–13551. doi: 10.1073/pnas.93.24.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braak H, Braak E. Staging of Alzheimer’s Disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–284. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 9.Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YL, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi RH, Almeida CG, Kearney PF, Yu F, Lin MT, Milner TA, Gouras GK. Oligomerization of Alzheimer’s β-amyloid within processes and synapses of cultured neurons and brain. The Journal of Neuroscience. 2004;24:3592–3599. doi: 10.1523/JNEUROSCI.5167-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Philipson O, Lord A, Gumucio A, O’Callaghan P, Lannfelt L, Nilsson LNG. Animal models of amyloid-β-related pathologies in Alzheimer’s disease. FEBS Journal. 2010;277:1389–1409. doi: 10.1111/j.1742-4658.2010.07564.x. [DOI] [PubMed] [Google Scholar]
- 12.Beach TG. Physiologic origins of age-related β-amyloid deposition. Neurodegenerative Diseases. 2008;5:143–145. doi: 10.1159/000113685. [DOI] [PubMed] [Google Scholar]
- 13.Cheng H, Zhou Y, Holtzman D, Han X. Apolipoprotein E mediates sulfatide depletion in animal models of Alzheimer’s disease. Neurobiol Aging. 2008;31:1188–1196. doi: 10.1016/j.neurobiolaging.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gotz J, Ittner LM. Animal models of Alzheimer’s disease and frontotemporal dementia. Nature Reviews Neuroscience. 2008;9:532–544. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- 15.Woodruff-Pak DS. Animal models of Alzheimer’s disease: therapeutic implications. Journal of Alzheimer’s Disease. 2008;17:507–521. doi: 10.3233/jad-2008-15401. [DOI] [PubMed] [Google Scholar]
- 16.McDonald MP, Overmier JB. Present imperfect: a critical review of animal models of the mnemonic impairments in Alzheimer’s disease. Neurosci Biobehav Rev. 1998;22:99–120. doi: 10.1016/s0149-7634(97)00024-9. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annu Rev Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- 18.Woodruff-Pak DS, Trojanowski JQ. The older rabbit as an animal model: implications for Alzheimer’s disease. Neurobiol Aging. 1996;17:283–290. doi: 10.1016/0197-4580(95)02064-0. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Basha MR, Brock B, Cox DP, Cardozo-Palaez F, McPherson CA, Harry J, Rice DC, Maloney B, Chen D, Lahiri DK, Zawia NH. Alzheimer’s disease (AD)-like pathology in aged monkeys after infantile exposure to environmental lead (Pb): evidence for the developmental origin and environmental link for AD. J Neurosci. 2008;28:3–9. doi: 10.1523/JNEUROSCI.4405-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sparks DL, Friedland R, Petanceska S, Schreurs BG, Shi J, Perry G, Smith MA, Sharma A, DeRosa S, Ziolkowski C, Stankovic G. Trace copper levels in the drinking water, but not zinc or aluminum influence CNS Alzheimer-like pathology. Journal of Nutrition, Health and Aging. 2006;10:247–254. [PMC free article] [PubMed] [Google Scholar]
- 21.Refolo LM, Pappolla MA, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 22.Hooijmans CR, Van Der Zee CEEM, Deeg DJH, Brouwer KM, Reijmer YD, van Groen T, Broersen LM, Lutjohann D, Heerschap A, Kiliaan AJ. DHA and cholesterol containing diets influence Alzheimer-like pathology, cognition and cerebral vasculature in APPSWE/PS1dE9 mice. Neurobiol Dis. 2009;33:482–498. doi: 10.1016/j.nbd.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 23.El Tayara NT, Volk A, Dhenain M, Delatour B. Transverse relaxation time reflects brain amyloidosis in young APP/PS1 transgenic mice. Magn Reson Imaging. 2007;58:179–184. doi: 10.1002/mrm.21266. [DOI] [PubMed] [Google Scholar]
- 24.Hooijmans CR, Rutters F, Dederen PJ, Gambarota G, Veltien A, van Groen T, Broersen LM, Lutjohann D, Heerschap A, Tanila H, Kiliaan AJ. Changes in cerebral blood volume and amyloid pathology in aged Alzheimer APP/PS1 mice on a docohexaeonic acid (DHA) diet or cholesterol enriched typical Western diet (TWD) Neurobiol Dis. 2007;28:16–29. doi: 10.1016/j.nbd.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Wadghiri YZ, Sigurdsson EM, Wisniewski T, Turnbull DH. Magnetic resonance imaging of amyloid plaques in transgenic mice. Methods Mol Biol. 2005;299:365–379. doi: 10.1385/1-59259-874-9:365. [DOI] [PubMed] [Google Scholar]
- 26.Clifford RJ, Jr, Wengenack TM, Reyes DA, Garwood M, Curran GL, Borowski BJ, Lin J, Preboske GM, Holasek SS, Adriany G, Podulso JF. In vivo magnetic resonance microimaging of individual amyloid plaques in Alzheimer’s transgenic mice. J Neurosci. 2005;25:10041–10048. doi: 10.1523/JNEUROSCI.2588-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van BB, Vanhoutte G, Pirici D, Van DD, Wils H, Cuijt I, Vennekens K, Zabielski M, Michalik A, Theuns J, De Deyn PP, van der LA, Van BC, Kumar-Singh S. Intraneuronal amyloid beta and reduced brain volume in a novel APP T714I mouse model for Alzheimer’s disease. Neurobiol Aging. 2008;29:241–252. doi: 10.1016/j.neurobiolaging.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Redwine JM, Kosofsky B, Jacobs RE, Games D, Reilly JF, Morrison JH, Young WG, Bloom FE. Dentate gyrus volume is reduced before onset of plaque formation in PDAPP mice: a magnetic resonance microscopy and stereologic analysis. Proceedings of the National Academy of Sciences, USA. 2003;100:1381–1386. doi: 10.1073/pnas.242746599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss C, Venkatsubramanian PN, Aguado AS, Power JM, Tom BC, Li L, Chen KS, Disterhoft JF, Wyrwicz AM. Impaired eyeblink conditioning and decreased hippocampal volume in PDAPP V717F mice. Neurobiol Dis. 2002;11:425–433. doi: 10.1006/nbdi.2002.0555. [DOI] [PubMed] [Google Scholar]
- 30.Ronald JA, Chen Y, Bernas L, Kizler HH, Rogers KA, Hegele RA, Rutt BK. Clinical field-strength MRI of amyloid plaques induced by low-level cholesterol feeding in rabbits. Brain. 2009;132:1346–1354. doi: 10.1093/brain/awp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prasanthi JR, Dasari B, Marwarha G, Larson T, Chen X, Geiger JD, Ghribi O. Caffeine protects against oxidative stress and Alzheimer’s disease-like pathology in rabbit hippocampus induced by cholesterol-enriched diet. Free Radical Biology & Medicine. 2010;49:1212–1220. doi: 10.1016/j.freeradbiomed.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prasanthi JRP, Schommer E, Thomasson S, Thompson A, Feist G, Ghribi O. Regulation of β-amyloid levels in the brain of cholesterol-fed rabbit, a model system for sporadic Alzheimer’s disease. Mech Ageing Dev. 2008;29:649–655. doi: 10.1016/j.mad.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woodruff-Pak DS, Agelan A, Del Valle L. A rabbit model of Alzheimer’s disease: valid at neuropathological, cognitive, and therapeutic levels. Journal of Alzheimer’s Disease. 2007;11:371–383. doi: 10.3233/jad-2007-11313. [DOI] [PubMed] [Google Scholar]
- 34.Ghribi O, Golovko MY, Larsen B, Schrag M, Murphy EJ. Deposition of iron and β-amyloid plaques is associated with cortical cellular damage in rabbits fed with long-term cholesterol-enriched diets. J Neurochem. 2006;99:438–449. doi: 10.1111/j.1471-4159.2006.04079.x. [DOI] [PubMed] [Google Scholar]
- 35.Sparks DL, Schreurs BG. Trace amounts of copper in water induce β-amyloid plaques and learning deficits in a rabbit model of Alzheimer’s disease. Proceedings of the National Academy of Sciences, USA. 2003;100:11065–11069. doi: 10.1073/pnas.1832769100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zatta P, Zambenedetti P, Stella MP, Licastro F. Astrocytosis, microgliosis, metallothionein-I-II and amyloid expression in high cholesterol-fed rabbits. Journal of Alzheimer’s Disease. 2002;4:1–9. doi: 10.3233/jad-2002-4101. [DOI] [PubMed] [Google Scholar]
- 37.Sparks DL, Kuo Y-M, Roher AE, Martin TA, Lukas RJ. Alterations of Alzheimer’s disease in the cholesterol-fed rabbit, including vascular inflammation. Preliminary observations. Annals New York Academy of Sciences. 2000;903:335–344. doi: 10.1111/j.1749-6632.2000.tb06384.x. [DOI] [PubMed] [Google Scholar]
- 38.Sparks DL. Dietary cholesterol induces Alzheimer-like β-amyloid immunoreactivity in rabbit brain. Nutrition, Metabolism and Cardiovascular Diseases. 1997;7:255–266. [Google Scholar]
- 39.Sparks DL, Scheff SW, Hunsaker JC, III, Liu H, Landers T, Gross DR. Induction of Alzheimer-like β-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp Neurol. 1994;126:88–94. doi: 10.1006/exnr.1994.1044. [DOI] [PubMed] [Google Scholar]
- 40.Holland D, Brewer JB, Hagler DJ, Fenema-Notestine C, Dale AM. Subregional neuroanatomical change as a biomarker for Alzheimer’s disease. Proceedings of the National Academy of Sciences. 2009;106:20954–20959. doi: 10.1073/pnas.0906053106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fox NC, Crum WR, Scahill RI, Stevens JM, Janssen JC, Rossor MN. Imaging of onset and progression of Alzheimer’s disease with voxel-compression mapping of serial magnetic resonance images. Lancet. 2001;358:201–205. doi: 10.1016/S0140-6736(01)05408-3. [DOI] [PubMed] [Google Scholar]
- 42.Killiany RJ, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, Tanzi R, Jones K, Hyman BT, Albert MS. Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Ann Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- 43.Schuff N, Woerner N, Boreta L, Kornfield T, Shaw LM, Trojanowski JQ, Thompson PM, Jack CR, Jr, Weiner MW the Alzheimer’s Disease Neuroimaging Initiative . MRI of hippocampal volume loss in early Alzheimer’s disease in relation to ApoE genotype and biomarkers. Brain. 2009;132:1067–1077. doi: 10.1093/brain/awp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.den Heijer T, Geerlings MI, Hoebeek FE, Hoffman A, Koudstaal PJ, Breteler MMB. Use of hippocampal and amygdalar volumes on magnetic resonance imaging to predict dementia in cognitively intact elderly people. Arch Gen Psychiatry. 2006;63:57–62. doi: 10.1001/archpsyc.63.1.57. [DOI] [PubMed] [Google Scholar]
- 45.Murphy DG, DeCarli CD, Daly E, Gillette J, McIntosh AR, Haxby JV, Teichburg D, Schapiro M, Rapoport SI, Horwitz B. Volumetric magnetic resonance imaging in men with dementia of the Alzheimer type: correlations with disease severity. Biol Psychiatry. 2003;34:612–621. doi: 10.1016/0006-3223(93)90153-5. [DOI] [PubMed] [Google Scholar]
- 46.Nestor SM, Rupsingh R, Borrie M, Smith M, Accomazzi V, Wells JL, Fogarty J, Bartha R. Ventricular enlargement as a possible measure of Alzheimer’s disease progression validated using the Alzheimer’s disease neuroimaging initiative database. Brain. 2008;131:1443–2454. doi: 10.1093/brain/awn146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andjus PR, Bataveljic D, Vanhoutte G, Mitrecic D, Pizzolante F, Djogo N, Nicaise C, Gankam KF, Gangitano C, Michetti F, van der LA, Pochet R, Bacic G. In vivo morphological changes in animal models of amyotrophic lateral sclerosis and Alzheimer’s-like disease: MRI approach. Anat Rec. 2009;292:1882–1889. doi: 10.1002/ar.20995. [DOI] [PubMed] [Google Scholar]
- 48.Dror V, Eliash S, Rehavi M, Assaf Y, Biton IE, Fattal-Valevski A. Neurodegeneration in thiamine deficient rats - a longitudinal MRI study. Brain Res. 2010;1308:176–184. doi: 10.1016/j.brainres.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 49.Xie Z, Yang D, Stephenson D, Morton D, Hicks C, Brown T, Bocan T. Characterizing the regional structural difference of the brain between tau transgenic (rTg4510) and wild-type mice using MRI. Medical Image Computing and Computer Assisted Intervention. 2010;13:308–315. doi: 10.1007/978-3-642-15705-9_38. [DOI] [PubMed] [Google Scholar]
- 50.Chen C-CV, Tung Y-Y, Chang C. A lifespan MRI evaluation of ventricular enlargement in normal aging mice. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.01.013. In press. [DOI] [PubMed] [Google Scholar]
- 51.Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM. One-year brain atrophy evident in healthy aging. J Neurosci. 2009;29:15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramesh BN, Raichurkar KP, Shamsaundar NM, Rao TSS, Rao KSJ. Aβ(42) induced MRI changes in aged rabbit brain resembles AD brain. Neurochem Int. 2011 doi: 10.1016/j.neuint.2011.06.003. In press. [DOI] [PubMed] [Google Scholar]
- 53.Lemieux SK, Smith-Bell CA, Wells JR, Ezerioha NM, Carpenter JS, Sparks DL, Schreurs BG. Neurovascular changes measured by TOF-MRA in cholesterol-fed rabbits with cortical amyloid beta-peptide accumulation. J Magn Reson Imaging. 2010;32:306–314. doi: 10.1002/jmri.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schreurs BG, Smith-Bell CA, Darwish DS, Stankovic G, Sparks DL. High dietary cholesterol facilitates classical conditioning of the rabbit’s nictitating membrane response. Nutritional Neuroscience. 2007;10:31–43. doi: 10.1080/10284150701232034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schreurs BG, Smith-Bell CA, Darwish DS, Wang D, Burhans L, Gonzales-Joekes J, Deci S, Stankovic G, Sparks DL. Cholesterol enhances classical conditioning of the rabbit heart rate response. Behav Brain Res. 2007;181:52–63. doi: 10.1016/j.bbr.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schreurs BG, Smith-Bell CA, Darwish DS, Stankovic G, Sparks DL. Classical conditioning of the rabbit’s nictitating membrane response is a function of the duration of dietary cholesterol. Nutritional Neuroscience. 2007;10:159–168. doi: 10.1080/10284150701565540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schreurs BG, Smith-Bell CA, Lochhead J, Sparks DL. Cholesterol modifies classical conditioning of the rabbit (Oryctolagus cuniculus) nictitating membrane response. Behav Neurosci. 2003;117:1220–1232. doi: 10.1037/0735-7044.117.6.1220. [DOI] [PubMed] [Google Scholar]
- 58.Shek JW, Wen GY, Wisniewski HM. Atlas of the Rabbit Brain and Spinal Chord. Krager; 1986. pp. 1–135. [Google Scholar]
- 59.Girgis M, Shih-Chang W. In: Stereotaxic Atlas of the Rabbit Brain. Warren H, editor. Green, Inc; 1981. pp. 1–70. [Google Scholar]
- 60.Gering DT, Nabavi A, Kikinis R, Hata N, O’Donnell LJ, Grimson WE, Jolesz FA, Black PM, Well WM., III An integrated visualization system for surgical planning and guidance using image fusion and an open MR. J Magn Reson Imaging. 2001;13:967–975. doi: 10.1002/jmri.1139. [DOI] [PubMed] [Google Scholar]
- 61.Fellows-Mayle W, Hitchens TK, Simplaceanu E, Horner J, Barbano T, Nakaya K, Losee JE, Losken HW, Siegel MI, Mooney MP. Age-related changes in lateral ventricle morphology in craniosynostotic rabbits using magnetic resonance imaging. Childs Nerv Syst. 2005;21:385–391. doi: 10.1007/s00381-004-1107-z. [DOI] [PubMed] [Google Scholar]
- 62.Sparks DL, Martin T, Stankovic G, Waggoner T, Van Adel R. Influence of water quality on cholesterol induced systemic pathology. The Journal of Nutrition, Health & Aging. 2007;11:189–193. [PubMed] [Google Scholar]
- 63.Robakis NK. Are Aβ and Its derivatives causative agents or innocent bystanders in AD? Neurodegenerative Diseases. 2010;7:32–37. doi: 10.1159/000266476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sakono M, Zako T. Amyloid oligomers: formation and toxicity on Aβ oligomers. FEBS Journal. 2010;277:1348–1358. doi: 10.1111/j.1742-4658.2010.07568.x. [DOI] [PubMed] [Google Scholar]
- 65.Garcia-Osta A, Alberini CM. Amyloid beta mediates memory formation. Learning & Memory. 2009;16:267–272. doi: 10.1101/lm.1310209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chiang H-C, Iijima K, Hakker I, Zhong Y. Distinctive roles of different β-amyloid 42 aggregates in modulation of synaptic functions. FASEB J. 2009;23:1969–1977. doi: 10.1096/fj.08-121152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giuffrida ML, Caraci F, Pignataro G, Cataldo S, De Bona P, Bruno V, Molinaro G, Pappalardo G, Messina A, Palmigiano A, Garozzo D, Nicoletti F, Rizzarelli E, Copani A. β-amyloid monomers are neuroprotective. J Neurosci. 2009;29:10582–10587. doi: 10.1523/JNEUROSCI.1736-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rauk A. Why is the amyloid beta peptide of Alzheimer’s disease neurotoxic? Dalton Transactions. 2008;2008:1273–1282. doi: 10.1039/b718601k. [DOI] [PubMed] [Google Scholar]
- 69.Levinger IM. Special features of the rabbit cerebroventricular system, studied by the casting method. J Anat. 1971;109:527–533. [PMC free article] [PubMed] [Google Scholar]
- 70.Deane R, Bell RD, Sagare A, Zlokovic BV. Clearance of amyloid-β across the blood-brain barrier: implications for therapies in Alzheimer’s disease. CNS & Neurological Disorder - Drug Targets. 2009;8:16–30. doi: 10.2174/187152709787601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, Marky A, Lenting PJ, Wu Z, Zarcone T, Goate A, Mayo K, Perlmutter D, Coma M, Zhong Z, Zlokovic BV. Clearance of amyloid-β by circulating lipoprotein receptors. Nat Med. 2007;13:1029–1031. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin C-J, Huang H-C, Jiang Z-F. Cu(II) interaction with amyloid-β peptide: a review of neuroactive mechanisms in AD brains. Brain Res Bull. 2010;82:235–242. doi: 10.1016/j.brainresbull.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 73.Hung YH, Bush AI, Cherny RA. Copper in the brain and Alzheimer’s disease. Journal of Biological Inorganic Chemistry. 2010;15:61–76. doi: 10.1007/s00775-009-0600-y. [DOI] [PubMed] [Google Scholar]
- 74.Zatta P, Drago D, Bolognin S, Sensi SL. Alzheimer’s disease, metal ions and metal homeostasis therapy. Trends Pharmacol Sci. 2009;xx:xxx–xxx. doi: 10.1016/j.tips.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 75.Kessler H, Pajonk F-G, Bach D, Schneider-Axmann T, Falkai P, Herrmann W, Multhaup G, Wiltfang J, Schafer S, Wirths O, Bayer TA. Effect of copper intale on CSF parameters in patients with mild Alzheimer’s disease: a pilot phase 2 clincial trial. J Neural Transm. 2008;115:1651–1659. doi: 10.1007/s00702-008-0136-2. [DOI] [PubMed] [Google Scholar]
- 76.Sparks DL. Cholesterol metabolism and brain amyloidosis: evidence for a role of copper in the clearance of Aβ through the liver. Current Alzheimer Research. 2007;4:165–169. doi: 10.2174/156720507780362119. [DOI] [PubMed] [Google Scholar]
- 77.Squitti R, Barbati G, Rossi L, Ventriglia M, Dal Forno G, Cesaretti S, Moffa F, Caridi I, Cassetta E, Pasqualetti P, Calabrese L, Lupoi D, Rossini PM. Excess of nonceruloplasmin serum copper in AD correlates with MMSE, CSF β-amyloid, and h-tau. Neurology. 2006;67:76–82. doi: 10.1212/01.wnl.0000223343.82809.cf. [DOI] [PubMed] [Google Scholar]
- 78.Bayer TA, Schafer S, Breyhan H, Wirths O, Treiber C, Multhaup G. A vicious circle: role of oxidative stress, intraneuronal Aβ and Cu in Alzheimer’s disease. Clin Neuropathol. 2006;25:163–171. [PubMed] [Google Scholar]
- 79.Duce JA, Bush AI. Biological metals and Alzheimer’s disease: implications for therapeutics and diagnostics. Prog Neurobiol. 2010;92:1–18. doi: 10.1016/j.pneurobio.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 80.Brewer GJ. Iron and copper toxicity in diseases of aging, particularly atherosclerosis and Alzheimer’s disease. Exp Biol Med. 2007;232:323–335. [PubMed] [Google Scholar]