Abstract
Cutaneous myoepithelial tumors demonstrate heterogeneous morphologic and immunophenotypic features. We previously described, in brief, seven cases of cutaneous myoepithelioma showing solid syncytial growth of ovoid, spindled or histiocytoid cells. We now present the clinicopathologic features in a series of 38 cases of this distinctive syncytial variant, which were diagnosed between 1997–2012 (mostly seen in consultation). There were 27 men and 11 women, with a median age of 39 years (range 2 months to 74 years). Primary anatomic sites were upper extremity (11, including 2 on the hand), upper limb girdle (3), lower extremity (14; 3 on the foot), back (6), face (2), chest (1), and buttock (1); the typical presentation was as either a polypoid or papular lesion. Tumors were well-circumscribed and centered in the dermis and ranged in size from 0.3 to 2.7 cm (median 0.8 cm). Microscopically all tumors showed a solid sheet-like growth of uniformly sized ovoid to spindled or histiocytoid cells with palely eosinophilic syncytial cytoplasm. Nuclei were vesicular with fine chromatin and small or inconspicuous nucleoli, and exhibited minimal to no atypia. Mitoses ranged from 0 to 4 per 10 HPF; 28 tumors showed no mitoses. Necrosis and lymphovascular invasion were consistently absent. Adipocytic metaplasia, appearing as superficial fat entrapped within the tumor, was seen in 12 cases. Chondro-osseous differentiation was seen in one tumor. All tumors examined were diffusely positive for EMA, and the majority showed diffuse staining for S-100 protein (5 showing focal staining). Keratin staining was focal in 1 of 33 tumors and seen in rare cells in 3 other cases. There was also positivity for GFAP (14/33), SMA (9/13), and p63 (6/11). Most lesions were treated by local excision. The majority of tumors tested (14/17; 82%) were positive by fluorescence in situ hybridization for EWSR1 gene rearrangement; testing for potential fusion partners (PBX1, ZNF444, POU5F1, DUX4, ATF1, CREB1, NR4A3, DDIT3, and NFATc2) was negative in all EWSR1-rearranged tumors. No FUS gene rearrangement was detected in two tumors lacking EWSR1 rearrangement. Follow-up information is available for 21 patients (mean follow-up 15 months). One patient with a positive deep margin developed a local recurrence 51 months after initial biopsy. All other patients with known follow-up, including 11 who had positive deep margins, are alive with no evidence of disease and no reported metastases. In summary, cutaneous syncytial myoepithelioma is a morphologically distinct variant that more frequently affects men, occurs over a wide age range, and usually presents on the extremities. Tumors are positive for S-100 protein and EMA, and unlike most myoepithelial neoplasms, keratin staining is infrequent. EWSR1 gene rearrangement is present in nearly all tumors tested, and likely involves a novel fusion partner. Prior reports describe some risk of recurrence and metastasis for cutaneous myoepithelial tumors; however, the syncytial variant appears to behave in a benign fashion and only rarely recurs locally.
Keywords: myoepithelioma, syncytial, skin, soft tissue, EWSR1
INTRODUCTION
Cutaneous myoepithelial tumors demonstrate heterogeneous morphologic and immunophenotypic features, similar to primary myoepithelial neoplasms in other sites. Myoepithelial neoplasms in skin and soft tissue have been increasingly characterized over the past ten years (1–9), and are classified as chondroid syringoma/benign mixed tumor (defined by tubulo-ductal differentiation), myoepithelioma, and myoepithelial carcinoma. In common with lesions showing myoepithelial differentiation, a wide variety of cytologic and architectural features may be present both within a given lesion and between different tumors. Myoepithelial tumors typically coexpress epithelial antigens (keratin and/or EMA), S100 protein and/or GFAP; staining for p63, SMA and calponin is variable. To date, studies have shown that approximately 40–50% of myoepithelial tumors (more often carcinomas) in soft tissue sites harbor EWSR1 rearrangement (and rarely alternate FUS rearrangement) (10), and documented fusion partners include POU5F1, PBX1, and ZNF444 (10–12). Reports of the frequency of EWSR1-rearrangement has been variable in cutaneous myoepithelial tumors, ranging from 29% (10) to 44% (13), while mixed tumors more often show PLAG1 gene rearrangement, comparable to their salivary counterparts (14). Up to 20% of myoepitheliomas and myoepithelial carcinomas in skin and soft tissue are known to recur, and the risk appears higher with incomplete resection. Myoepithelial carcinoma, which is distinguished by the presence of at least moderate cytologic atypia, has a significant risk of metastatic spread to distant sites.
The majority of cutaneous myoepitheliomas show a typically lobular or multinodular architecture with epithelioid, spindled, plasmacytoid, or clear cells associated with a prominent myxoid or hyalinized stroma. In 2004, among a series of 14 cutaneous myoepitheliomas, we described a morphologically and immunohistochemically distinct subset (7 cases) showing solid syncytial growth of ovoid, spindled or histiocytoid cells with eosinophilic cytoplasm, with an immunophenotype of EMA and S-100 positivity but infrequent keratin staining (7). Herein we describe the clinicopathologic and molecular features of 38 cases of this distinctive syncytial variant of cutaneous myoepithelioma with the goal of more completely characterizing these lesions.
MATERIALS AND METHODS
Thirty-eight cases of cutaneous syncytial myoepithelioma identified between 1997 and 2012 were retrieved from surgical and consultation files (C.D.M.F. and J.L.H.) at Brigham and Women’s Hospital, Department of Pathology, Boston, MA. Hematoxylin and eosin (H&E) stained sections and immunohistochemical stains performed at the time of diagnosis were re-examined, with attention to cytomorphologic features, growth pattern, cytologic atypia, the presence of necrosis, and mitotic count. Clinical and follow-up data were obtained from referring pathologists using a standardized data sheet (see Acknowledgements). Seven cases have been published previously in a preliminary study (cases 1–7) (7, 15).
Immunohistochemistry had been performed in our laboratory on 4-µm-thick formalin-fixed paraffin-embedded tissue sections using the antibodies and conditions listed in Table 1. The Envision Plus detection system (Dako, Carpinteria, CA) was used for all antibodies. Appropriate positive and negative controls were used throughout.
Table 1.
Immunohistochemistry: Sources, Clones, Dilutions, and Pretreatment Conditions
| Antibody | Source | Clone | Dilution | Pretreatment |
|---|---|---|---|---|
| EMA | Dako (Carpinteria, CA | E29 | 1:200 | None |
| S100 protein | Dako | Polyclonal | 1:3000 | None |
| CK (pankeratin) | Dako | MNF-116 | 1:700 | 10 min protease digestion |
| CK (AE1/AE3) | Dako | AE1 + AE3 | 1:200 | 10 min protease digestion |
| CK (CAM 5.2) | Dako | CAM5.2 | 1:50 | 10 min protease digestion |
| GFAP | Dako | Polyclonal | 1:15,000 | Citrate buffer, pressure cooker |
| p63 | Neomarkers, (Fremont, CA) | 4A4 | 1:600 | Citrate buffer, microwave |
| SMA | Sigma (St. Louis, MO) | 1A4 | 1:20,000 | None |
Abbreviations: EMA, epithelial membrane antigen; CK, cytokeratin; SMA, smooth muscle actin; GFAP, glial fibrillary acidic protein
FISH on interphase nuclei from paraffin embedded 4-micron sections was performed in 17 cases applying custom probes using bacterial artificial chromosomes (BAC), covering and flanking EWSR1 in 22q12, FUS in 16p11, PBX1 in 1q23, ZNF444 in 19q13, POU5F1 in 6p21, DDIT3 in 12q13.3, ATF1 in 12q13.3, CREB1 in 2q33.3, NR4A3 in 9q31.1, DUX4 in 4q35.2, and NFATc2 in 20q13.2 (Table 2). Two of these cases (cases 26 and 35) and two other cases (cases 17 and 27) had initially been tested for EWSR1 rearrangement at Brigham and Women’s Hospital using similar methods. No cases had been submitted for conventional cytogenetic analysis.
Table 2.
Clinical Features of 38 Cases of Cutaneous Syncytial Myoepithelioma
| Case | Age (years) |
Se x |
Site, Laterality |
Size (cm) |
Durati on (mo) |
Biopsy/Treatment (Margin Status) |
Follow-up Status (mo) |
|---|---|---|---|---|---|---|---|
| 1 | 16 | M | Thigh, L | 0.7 | 4.5 | Excision (wide) | NED (56) |
| 2 | 41 | M | Palm, L | 0.8 | 240 | Excisional bx (wide) | NA |
| 3 | 52 | M | "Digit", R | 1.3 | 3 | Excision (wide) | NED (28) |
| 4 | 15 | M | Back | 0.5 | NA | Bx (positive) | NA |
| 5 | 20 | M | Leg, L | 0.5 | 6 | Excision (wide) | NED (18) |
| 6 | 45 | F | Leg, L | 0.8 | 12 | Excision (wide) | NED (6) |
| 7 | 29 | M | Arm, R | 0.6 | NA | Bx (wide) | Lost to f/u |
| 8 | 24 | M | Shoulder, L | 0.3 | NA | Bx (wide) | NA |
| 9 | 64 | M | Back | 0.3 | NA | Bx (positive) | NA |
| 10 | 29 | M | Thigh, R | 1.9 | NA | Excisional bx (positive) | NA |
| 11 | 45 | M | Arm, L | 0.6 | NA | Bx and excision (positive) | Lost to f/u |
| 12 | 59 | M | Thigh, L | 0.7 | 48 | Bx and excision (wide) | NED (1) |
| 13 | 50 | M | Axilla, R | 0.9 | NA | Bx (positive) | NA |
| 14 | 68 | F | Toe, R | 1.0 | NA | Excisional bx (positive) | NED (7) |
| 15 | 26 | M | Cheek, L | 0.4 | NA | Bx (positive) | NA |
| 16 | 35 | M | Heel, L | 0.9 | 24 | Excision (wide) | NED (7) |
| 17 | 26 | M | Back | 2.7 | 24 | Excision (wide) | NED (36) |
| 18 | 43 | F | Buttock, R | 1.0 | 12 | Excision (positive) | NED (22) |
| 19 | 74 | F | Thigh, u/k | 1.2 | NA | Excisional bx (wide) | NED (4) |
| 20 | 18 | F | Thigh, L | 0.5 | NA | Excisional bx (wide) | NA |
| 21 | 25 | M | Thigh, R | 1.4 | NA | Excision (wide) | NED |
| 22 | 2 mo. | M | Arm, R | 2.5 | 2 | Excision (marginal) | NED (30) |
| 23 | 28 | F | Arm, R | 0.8 | 1 | Bx and Excision (wide) | NED (9) |
| 24 | 60 | M | Arm, L | 1.3 | NA | Bx (positive) | NA |
| 25 | 34 | M | Shoulder, L | 1.6 | NA | Excisional bx (positive) | NED (15) |
| 26 | 13 | M | Arm, R | 0.6 | NA | Shave bx (positive) | Lost to f/u |
| 27 | 36 | F | Arm, L | 1.5 | 2 | Bx and Excision (wide) | NED (14) |
| 28 | 51 | M | Nose | 0.5 | 3 | Bx and Excision (wide) | NED (14) |
| 29 | 50 | F | Thigh, R | 0.7 | 12 | Excisional bx(wide) | NED (10) |
| 30 | 39 | M | Chest | 0.3 | NA | Bx (wide) | NA |
| 31 | 29 | M | Thigh, L | 1.3 | 24 | Excision (wide) | NA |
| 32 | 47 | F | Toe, R | 0.7 | NA | Bx (positive) | NA |
| 33 | 8 | M | Arm, R | 0.7 | NA | Shave bx (positive) | NED (NA) |
| 34 | 49 | F | Arm, L | 1.0 | NA | Shave bx (positive) | NED (NA) |
| 35 | 47 | F | Back | 1.1 | NA | Bx (positive) | NA |
| 36 | 13 | M | Back | 0.6 | NA | Shave bx (positive) | NED (1) |
| 37 | 39 | M | Leg, L | 0.6 | NA | Shave bx (positive) | Recurrence (51) |
| 38 | 62 | M | Back | 0.9 | 24 | Bx (positive) | NA |
Abbreviations: Mo., months; L, left; R, right; NA, not available; F/U, follow up; Bx, biopsy; NED, no evidence of disease
BAC clones were chosen according to USCS genome browser (http://genome.uscs.edu). The BAC clones were obtained from BACPAC sources of Children's Hospital of Oakland Research Institute (CHORI) (Oakland, CA) (http://bacpac.chori.org). DNA from individual BACs was isolated according to the manufacturer’s instructions, labeled with different fluorochromes in a nick translation reaction, denatured, and hybridized to pretreated slides. Slides were then incubated, washed, and mounted with DAPI in an antifade solution. The genomic location of each BAC set was verified by hybridizing them to normal metaphase chromosomes. Two hundred successive nuclei were examined using a Zeiss fluorescence microscope (Zeiss Axioplan, Oberkochen, Germany), controlled by Isis 5 software (Metasystems). A positive score was interpreted when at least 20% of the nuclei showed a break-apart signal. Nuclei with incomplete set of signals were omitted from the score. All cases were first tested with an EWSR1 probe. The EWSR1-rearranged tumors were then evaluated for break-apart signals using probes for PBX1, ZNF444, POU5F1, etc. The EWSR1 negative tumors were then tested for FUS break-apart, since FUS may substitute for the EWSR1 gene in certain translocation-associated sarcomas (10, 16, 17). All 17 cases were tested for NR4A3 break-apart.
This study was performed with the approval of the Institutional Review Board at the Brigham and Women’s Hospital.
RESULTS
Clinical Features
Clinical data are summarized in Table 2. The patient group comprised 27 men and 11 women (2.5:1 ratio), with a median age of 39 years (range 2 months to 74 years). Over half (60%) of the patients were in the third through fifth decades in age. The anatomic distribution was as follows: upper extremity (11, including two on the hand), upper limb girdle (3), lower extremity (14; 3 on the foot), back (6), face (2), chest (1), and buttock (1). These skin lesions were described as either polypoid or papular. Reported preoperative duration ranged from two months to four years. Clinical diagnoses offered included: acrochordon, cutaneous cyst, wart, seborrheic keratosis, actinic keratosis, Kaposi sarcoma, hemangioma, neurofibroma, keratoacanthoma, dermatofibroma, basal cell carcinoma, squamous cell carcinoma, dermal nevus (with or without inflammation), amelanotic melanocytic lesion, Spitz nevus, adnexal tumor, sebaceous hyperplasia, schwannoma, leiomyoma, spiradenoma, and angiolipoma. Proposed diagnoses by submitting pathologists, when provided, included epithelioid sarcoma (3 cases), neurofibroma (2), perineurioma/sclerosing perineurioma (5), cellular neurothekeoma (3), granular cell tumor, myofibroma/myopericytoma (4), smooth muscle neoplasm (2), angiomyolipoma, meningioma, dermatofibrosarcoma protuberans, proliferative fasciitis, cutaneous epithelioid angiomatous nodule, fibrous histiocytoma, epithelioid fibrous histiocytoma (2), fibroma, juvenile fibrous histiocytoma, ossifying (non-ossifying) fibromyxoid tumor, and nevus. In only five cases, a myoepithelial neoplasm was suggested.
Most patients were treated by local excision (or re-excision after initial biopsy sampling). Follow-up information was available for 21 patients (mean follow-up 15 months). Many patients were not followed longer because of the benign diagnosis. One patient with a positive deep margin developed a local recurrence 51 months after initial shave biopsy without formal excision (case 37). All other patients with known follow-up, including 6 others who had positive margins and 1 with marginal excision, were reported to be alive with no evidence of disease. No patients received radiation or chemotherapy, and none developed metastases.
Gross and Microscopic Features
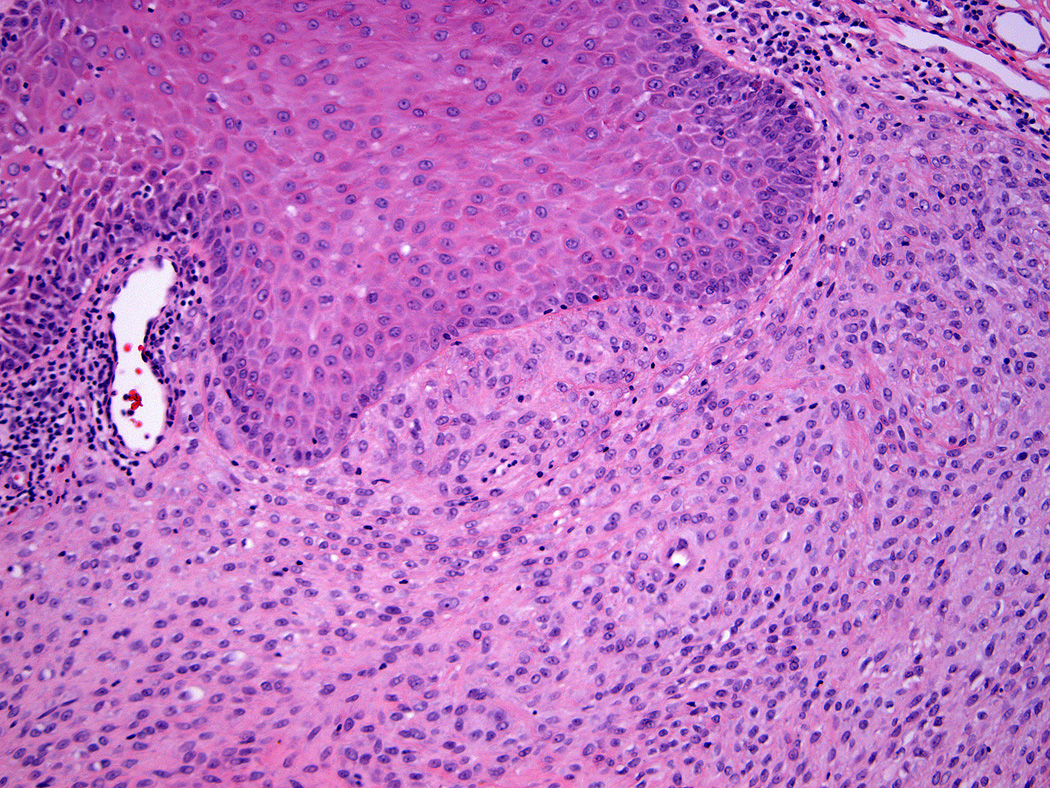
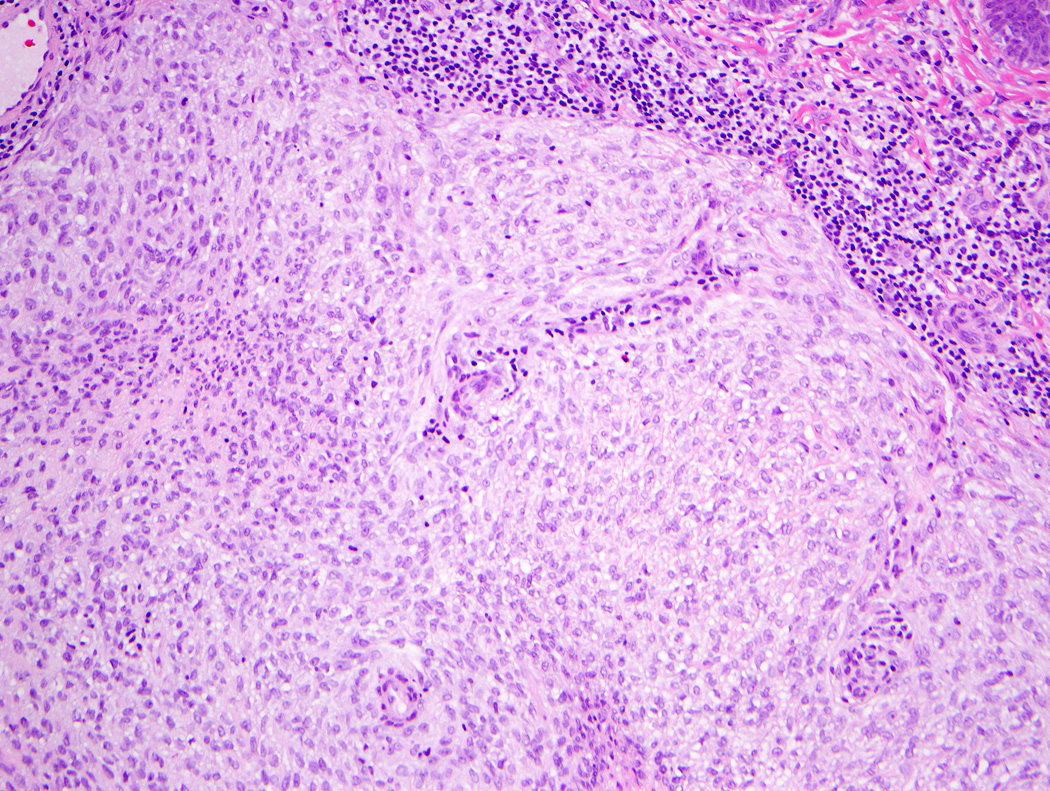
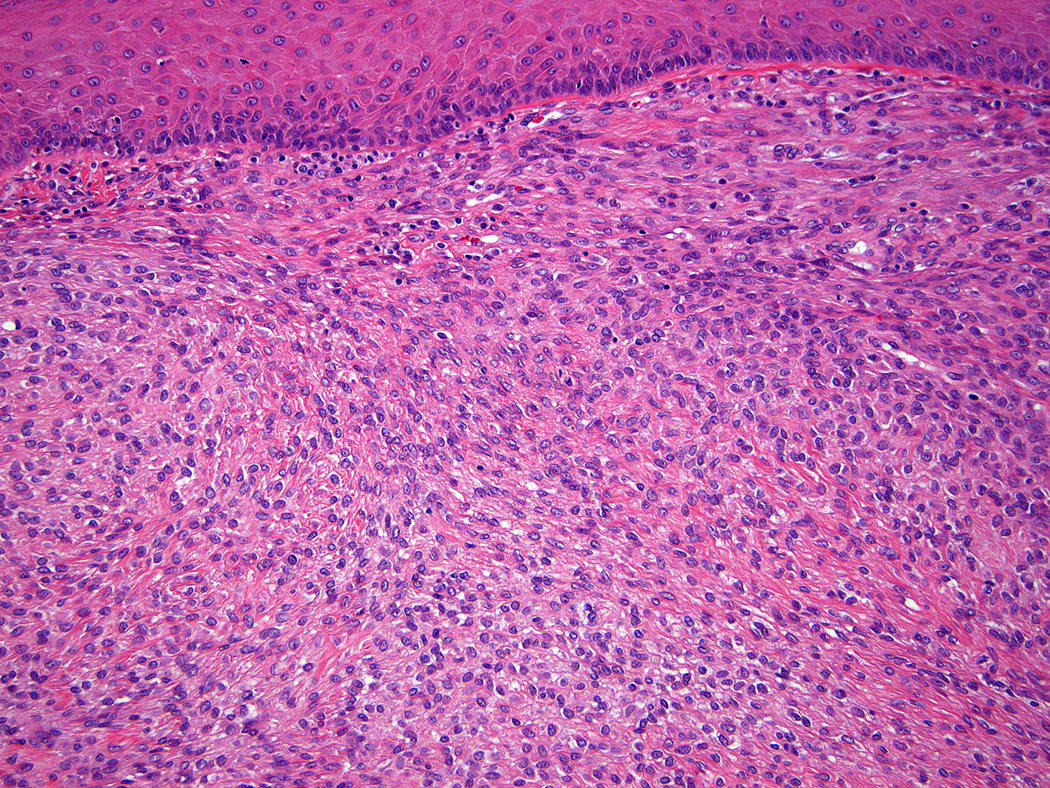
Lesions ranged in size from 0.3 to 2.7 cm (median 0.8 cm). Most tumors were well-circumscribed but unencapsulated (Figure 1). All tumors were centered in the dermis and often abutted the overlying epidermis (Figure 2). Microscopically all tumors showed a solid sheet-like growth of uniformly sized ovoid to spindled or histiocytoid cells with palely eosinophilic syncytial cytoplasm (Figures 3a and b). Only occasional focal fascicular growth of spindle cells was seen (Figure 3c). Nuclei were vesicular with fine chromatin and smooth nuclear contours had small or inconspicuous nucleoli, and showed minimal to no atypia. Mitoses ranged from 0 to 4 per 10 high power fields (HPF); 28 tumors showed no mitoses. All tumors lacked evident necrosis and lymphovascular invasion. In 17 cases, minimal-to-moderate lymphoplasmacytic infiltrates were observed at the periphery of or within tumor, frequently surrounding vessels (Figure 4). Adipocytic metaplasia, appearing as superficial fat seemingly entrapped within the tumor, was seen in 12 cases (Figures 1b and 5). Chondro-osseous differentiation was seen in 1 tumor (Figure 6). All tumors lacked ductal differentiation, although entrapment of adnexal structures was common. For the recurrent tumor observed in case 37, no mitoses were found in the primary or recurrent tumor, which appeared morphologically identical to one another.
Figure 1.
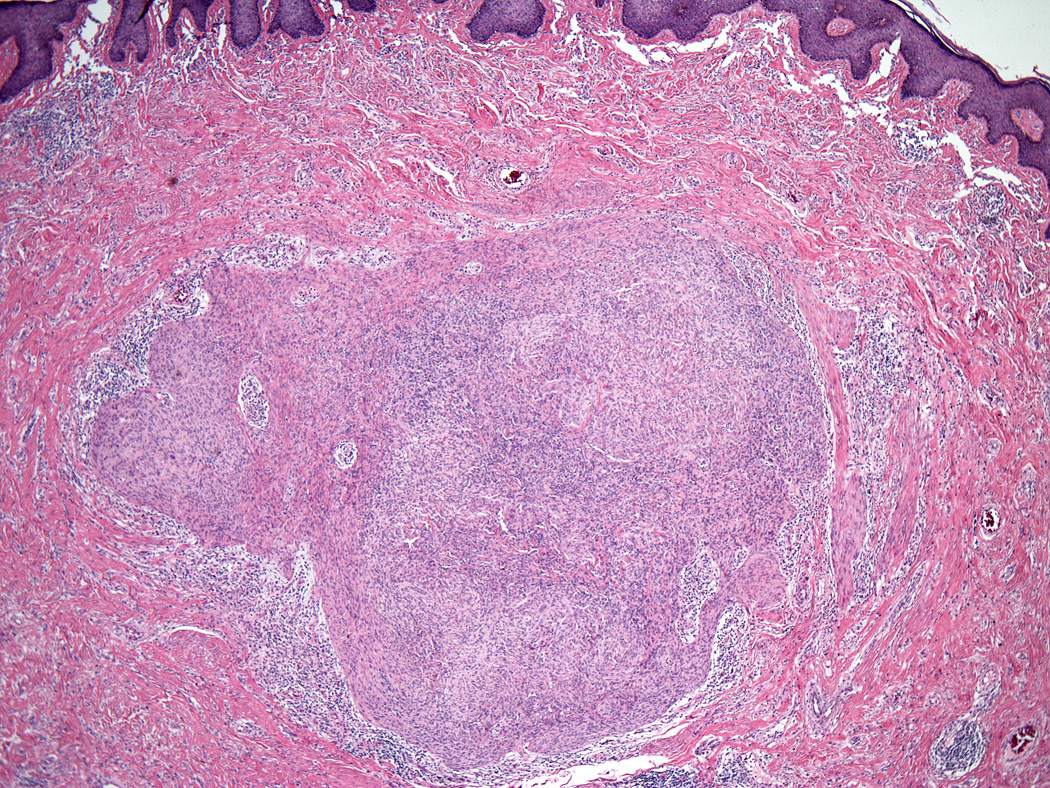
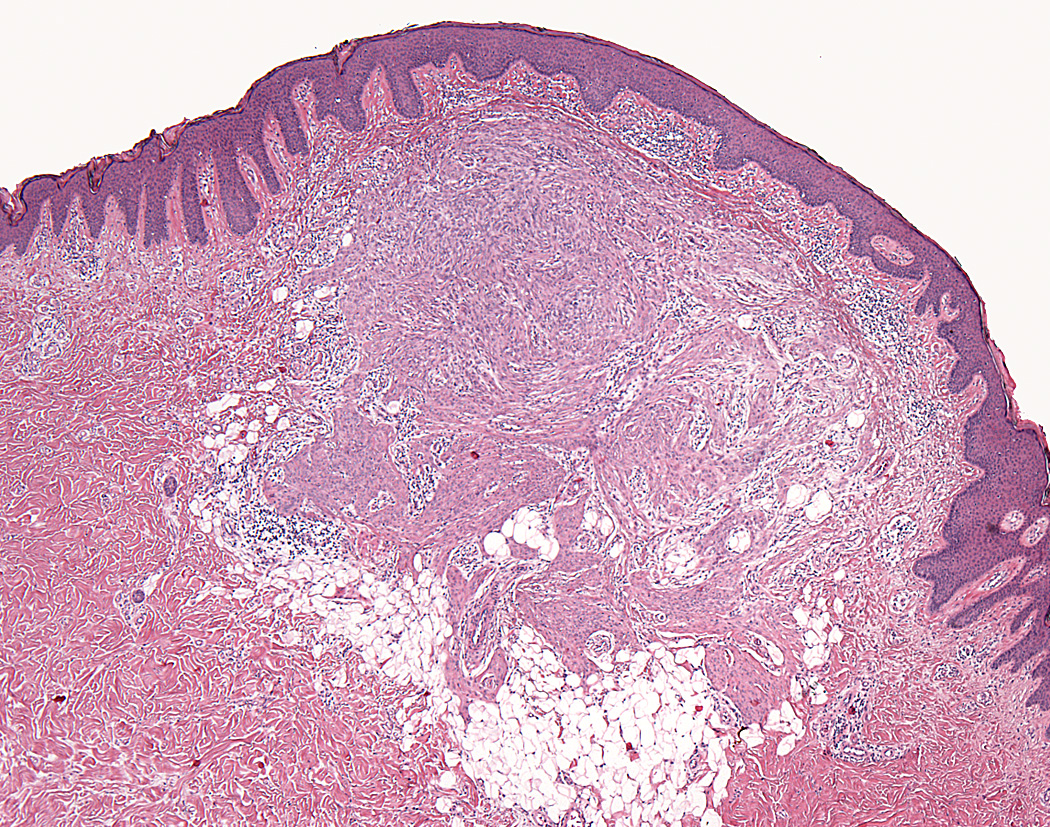
Most cutaneous syncytial myoepitheliomas were well-circumscribed and unencapsulated (a); some tumors showed adipocytic metaplasia (b).
Figure 2.
Tumors were centered in the dermis and often abutted overlying epidermis (a & b).
Figure 3.
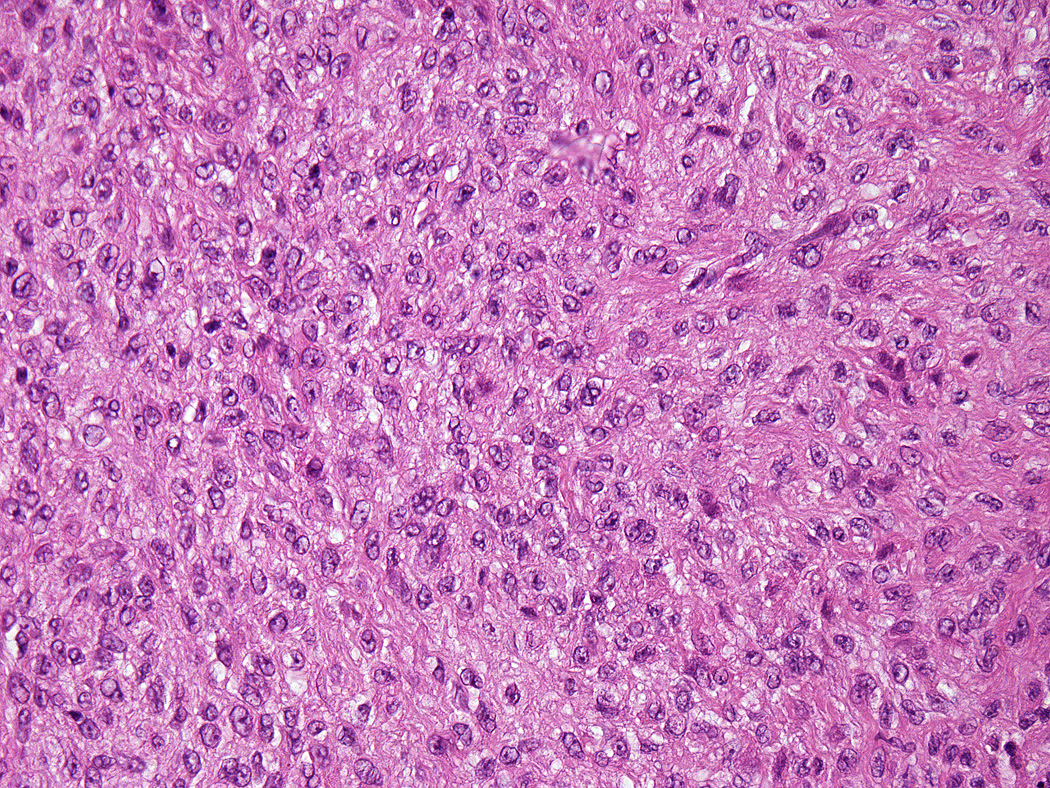
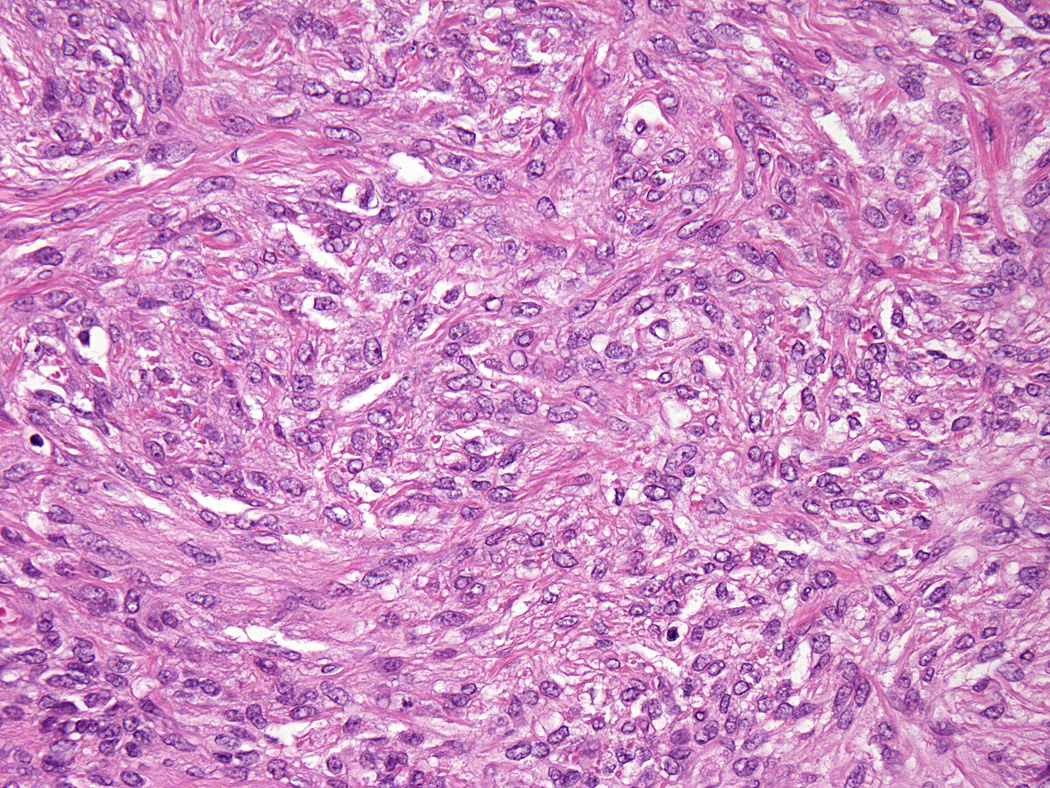
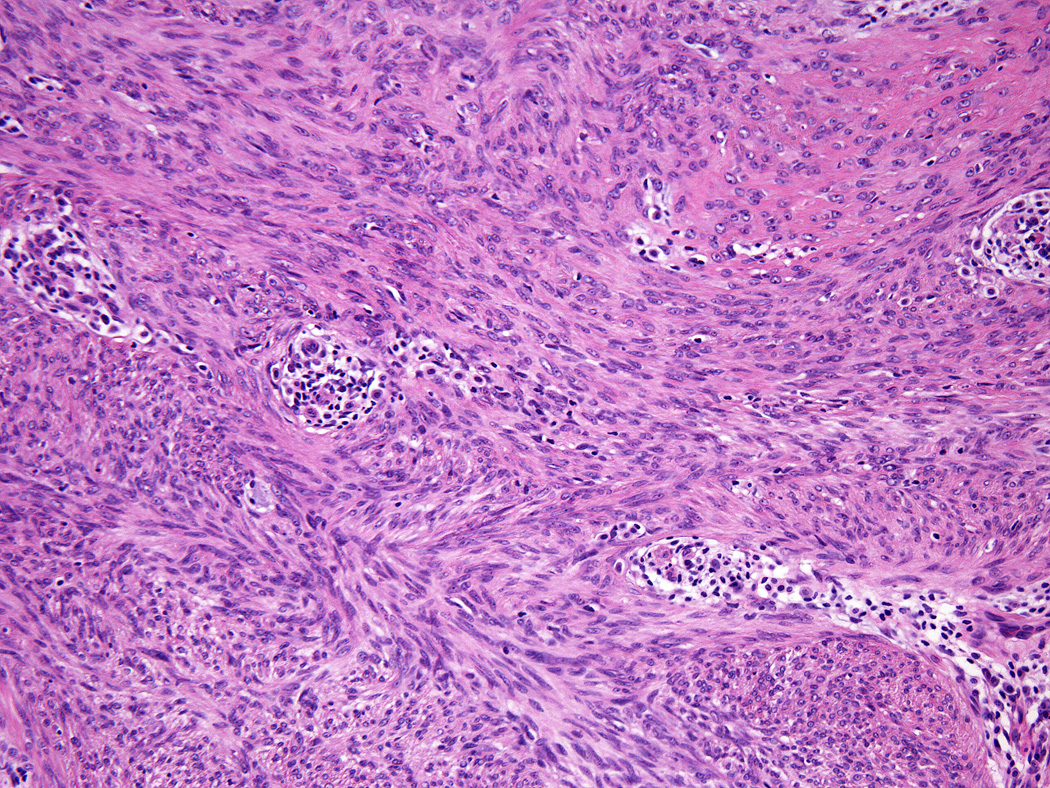
All tumors showed a solid sheet-like growth of uniformly sized ovoid to spindled or histiocytoid cells with palely eosinophilic syncytial cytoplasm (3a); most tumors showed no mitotic activity. Nuclei were vesicular with fine chromatin and smooth nuclear contours and had small or inconspicuous nucleoli, with minimal to no atypia (3b). Focally fascicular growth was observed in only one tumor (3c).
Figure 4.
A subset of tumors showed a minimal-to-moderate lymphoplasmacytic infiltrate, mostly at the periphery.
Figure 5.
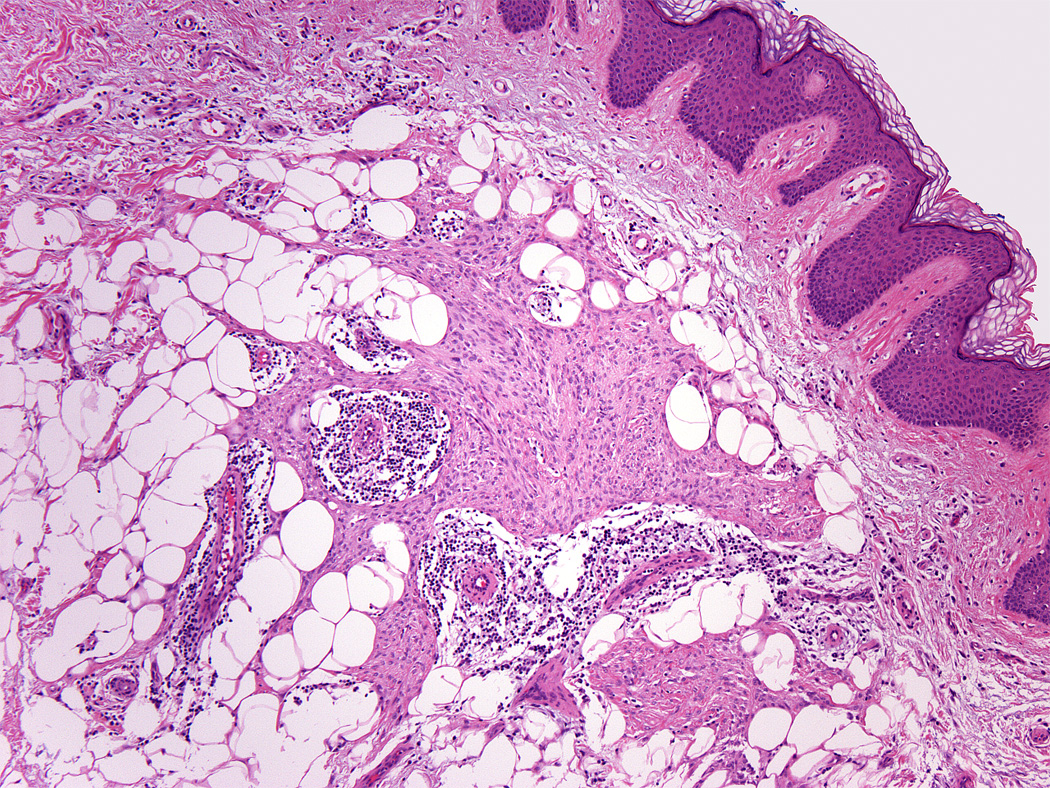
Adipocytic metaplasia, appearing as superficial fat seemingly entrapped within the tumor, was observed in twelve cases.
Figure 6.
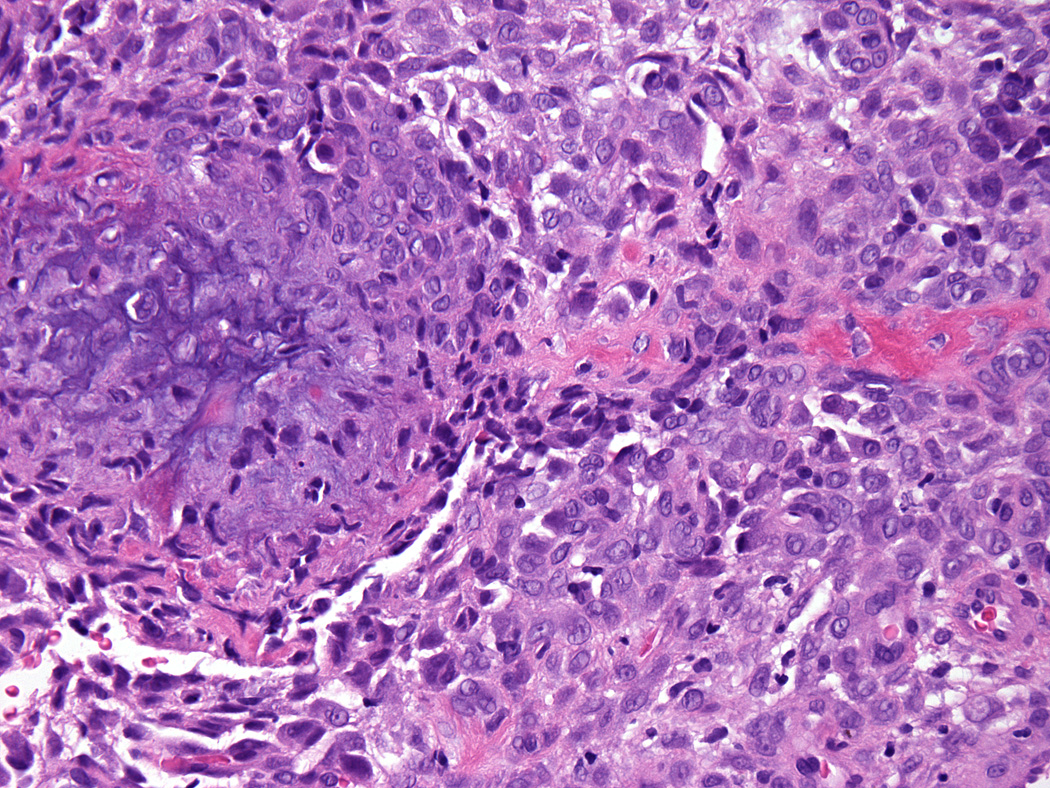
Chondro-osseous differentiation was seen in one tumor.
Immunohistochemical Findings
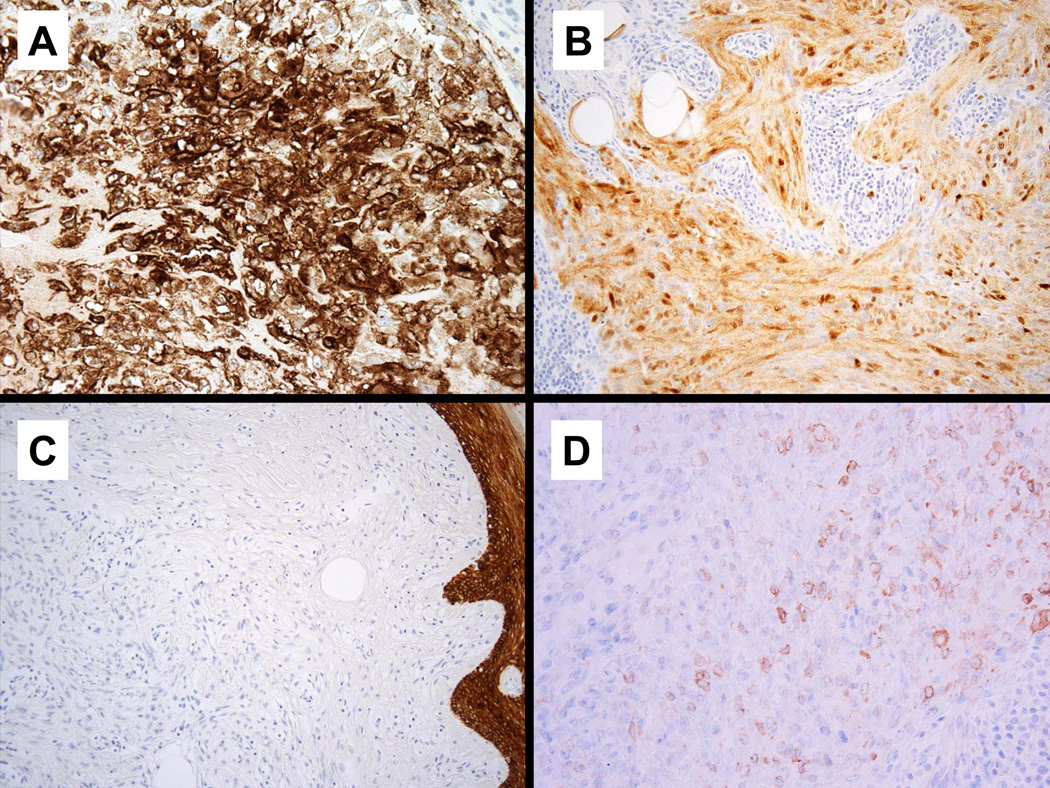
All tumors examined were diffusely positive for EMA (38/38) (Figure 7a) and the majority showed diffuse staining for S-100 protein (33/38); five showing more focal staining (Figure 7b). Keratin was negative in 31/36 cases tested (Figure 7c) ; there was focal positivity in one tumor rare cells were positive in four other cases. Of 33 tumors tested for GFAP, 14 were positive and most commonly showed weak multifocal staining (Figure 7d), although as few as rare positive cells were observed in some cases. SMA was positive in 9 of 13 cases. p63 was positive in 6 of 11 tumors and typically showed a focal to multifocal nuclear staining pattern.
Figure 7.
Immunohistochemical findings. All tumors showed diffuse positivity for EMA (7a) and S100 (7b). The majority of tumors were negative for keratin expression (7c). GFAP staining, when positive, was typically weak and multifocal (7d).
Molecular genetic findings
Fourteen of seventeen tumors tested showed EWSR1 rearrangement by FISH (Figure 8).
Figure 8.
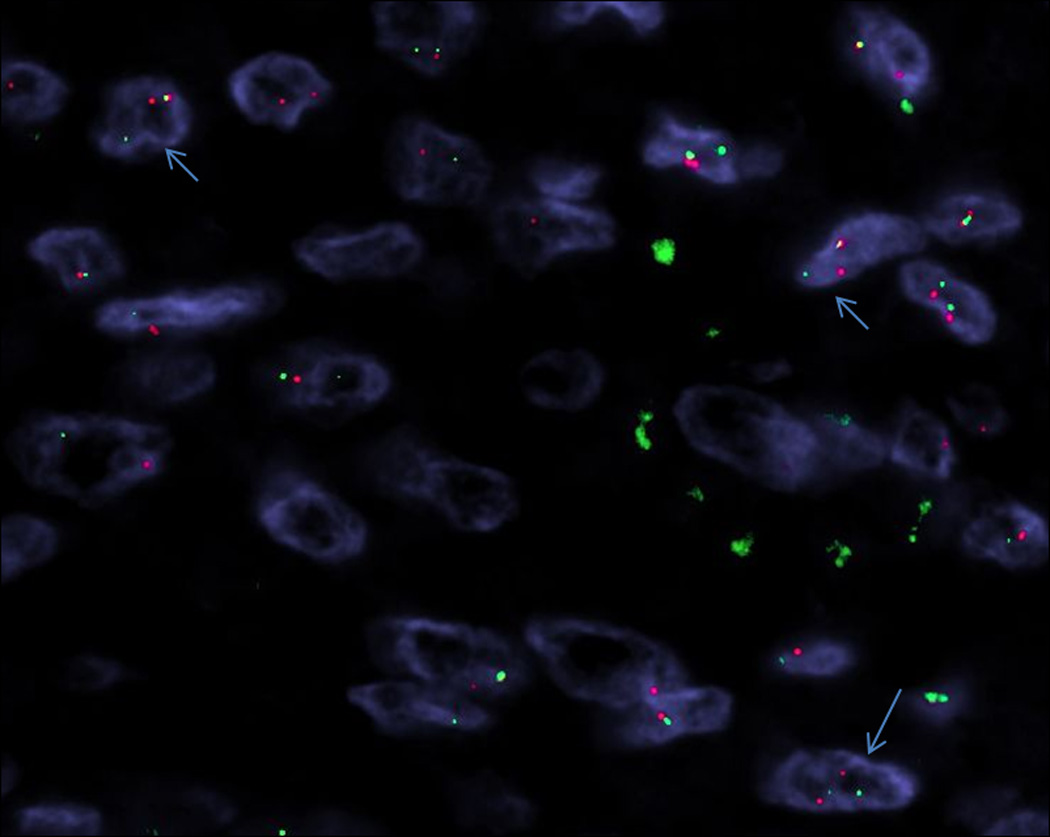
Fluorescence in situ hybridization (FISH) for the EWSR1 gene demonstrated a break-apart signal in 14 cases (arrows) (Case 10).
Two cases did not show break-apart signals for EWSR1, and further testing for FUS break-apart was negative in both tumors. For the last case, no hybridization signal could be obtained, likely due to DNA degradation. Subsequent testing of all EWSR1-rearranged tumors, in hopes of identifying a fusion partner, was negative for all break-apart signals for PBX1, ZNF444, POU5F1, ATF1, CREB1, NR4A3, DDIT3 and NFATc2, as well as for EWSR1-DUX4 fusion
DISCUSSION
Cutaneous syncytial myoepithelioma is a morphologically distinct subtype of myoepithelial neoplasm that is characterized by solid growth of spindled, ovoid, or histiocytoid cells with palely eosinophilic cytoplasm and a distinct immunophenotype of EMA and S-100 positivity and infrequent cytokeratin staining. The tumor affects patients over a wide age range (2 months to 74 years) with a male predominance (2.5:1, M:F). While the anatomic distribution is broad, these tumors are most common on the extremities (74%). The majority of cutaneous syncytial myoepitheliomas tested show EWSR1 gene rearrangement.
While myoepithelial neoplasms show a wide range of morphologic and immunophenotypic heterogeneity, most tumors at least focally demonstrate characteristic features of epithelioid, plasmacytoid, or spindled cells showing either nested or reticular growth within a collagenous or chondromyxoid stroma. The syncytial variant consistently showed syncytial solid growth with no significant stroma. Most tumors were cytologically bland, with benign-appearing nuclei. The majority of tumors (28/38; 73%) showed no mitotic activity; and the highest mitotic count was 4 per 10 HPF. No necrosis or lymphovascular invasion was observed. Adipocytic metaplasia was seen in approximately one-third of tumors. Interestingly, one prior case report (18) of what was regarded as a cutaneous myoepithelial carcinoma showing “a plexiform growth pattern” showed similar pathologic features, including predominance of solid growth of spindled cells, positivity for EMA and S100, and negativity for keratin; the tumor exhibited adipocytic metaplasia which accounts for the plexiform appearance. The histologic features of spindled and histiocytoid cells and lack of significant stroma, atypia, and necrosis are useful in recognizing the syncytial variant.
Myoepithelial tumors typically express a combination of epithelial antigens (keratin and/or EMA), S-100 protein and/or GFAP, and variably SMA, calponin, and p63. However, the syncytial variant consistently shows positivity for EMA and S-100 protein and most tumors lacked keratin staining; only five cases were positive for keratin and staining was very focal or present in rare cells. Staining for GFAP appeared to be more limited in extent when compared to S-100, with typically multifocal weak positivity. The absence of significant keratin staining may lead to diagnostic pitfalls, and underscores the importance of applying a complete immunohistochemical panel when a myoepithelial neoplasm is suspected.
The differential diagnosis includes, principally, epithelioid benign fibrous histiocytoma (EFH), juvenile xanthogranuloma (JXG), melanocytic lesions, and, less likely, epithelioid sarcoma. EFH frequently presents on the lower extremities of young to middle-aged adults as a well-circumscribed dermal nodule of epithelioid cells with palely eosinophilic cytoplasm, and often shows EMA positivity (19). The predominance of epithelioid cells embedded in collagenous or variably vascular stroma and absence of spindle cells and syncytial architecture favors EFH. Furthermore, binucleate cells are frequently present in EFH but were not observed in any cases of cutaneous syncytial myoepithelioma. While EFH is frequently positive for EMA and negative for keratin, EFH is consistently negative for S-100, GFAP, and p63 (19), and therefore immunohistochemistry can reliably distinguish EFH from cutaneous syncytial myoepithelioma.
Juvenile xanthogranuloma (JXG) in the so-called "early stage" can mimic cutaneous syncytial myoepithelioma, exhibiting an exophytic proliferation of eosinophilic histiocytic cells that lack characteristic lipidization and Touton giant cells (20). While children are most frequently affected by JXG, seven patients with cutaneous syncytial myoepithelioma in this series were aged 18 years or younger, including a 2-month-old male infant. JXG can be distinguished by histiocytic markers CD163 and CD68, and absent expression of myoepithelial markers.
Melanocytic neoplasms may also enter the differential diagnosis, particularly for lesions showing little cytologic atypia and pleomorphism and those in which a junctional component is not present or visualized. Spitz nevi, which are well-circumscribed and occasionally completely intradermal, can raise diagnostic challenges. Careful examination for the presence of a junctional component, nested growth pattern, Kamino body formation, and downward "maturation" into deeper dermis will identify Spitz nevi. While S-100 positivity is shared by both Spitz nevi (and other melanocytic neoplasms) and cutaneous syncytial myoepithelioma, the application of melanocytic markers HMB-45, MelanA, and MiTF and other myoepithelial markers will usually differentiate the two entities.
Cutaneous syncytial myoepithelioma may also show some morphologic overlap with epithelioid sarcoma, which commonly affects young adults on the distal extremities. Epithelioid sarcoma typically shows an admixture of epithelioid and spindle cells with moderate atypia showing infiltrative growth and often discontiguous nodules; however in some cases solid growth and relatively less atypia can be observed. Both lesions are positive for EMA; positivity for pan-keratin and CD34 and negativity for S-100 and GFAP favors epithelioid sarcoma. While 90% of epithelioid sarcomas show loss of nuclear expression of INI1 (21), the product of a tumor suppressor gene mapped to 22q11.2, INI1 loss has been observed in pediatric myoepithelial carcinomas of soft tissue (8). In fact, since initial recognition of INI1 deficiency in malignant rhabdoid tumors (22), INI1 loss has been observed in several other tumor types (23), including a subset of epithelioid malignant peripheral nerve sheath tumors (21), renal medullary carcinoma (24) and another tumor type typically showing EWSR1 rearrangement, extraskeletal myxoid chondrosarcoma (25). The mechanism of loss of INI1 protein expression varies between tumor types and is not necessarily correlated with cytogenetic status of the region on 22q11.2 (23), but INI1 staining is not likely to be helpful in accurate diagnosis of syncytial myoepithelioma.
Based on available follow-up information, a benign clinical course is expected for syncytial myoepithelioma, with only one patient showing local recurrence 4 years after initial shave biopsy with positive margins. All other patients with known follow-up showed no evidence of recurrence or metastasis, including six patients with positive margins and one patient with marginal excision. While criteria for malignancy in cutaneous myoepithelial neoplasms have not been well established, myoepithelial tumors in soft tissue with moderate-to-severe cytologic/nuclear atypia show increased risk of recurrence and metastasis and are thus classified as myoepithelial carcinoma (5). Based on a small number of reports, cutaneous lesions showing cytologic atypia, high mitotic rate, and necrosis show more aggressive behavior (4, 7–9); however prior work also suggested a potential risk of recurrence and metastasis even for cytologically bland cutaneous myoepithelial tumors (7). In our prior study of cutaneous myoepitheliomas, the three cytologically benign tumors that recurred (one of which also metastasized) showed increased mitotic counts (up to 6 per 10 HPF); however none of these tumors were the syncytial variant (7). All tumors in our present series lacked significant cytologic atypia, necrosis, and lymphovascular invasion; most tumors showed negligible mitotic activity, and the highest count was 4 per 10 HPF. For the case in which a recurrence developed, the primary tumor did not exhibit atypia or mitotic activity in the sections examined and the recurrent tumor was morphologically identical and lacked any features of malignancy.
Cytogenetic aberrations were first reported in a series of pediatric myoepithelial carcinoma of soft tissue in 2007, in which one case showed loss of chromosome 22 and EWSR1 rearrangement was detected in another case (8). In the following two years, Brandal et al reported a balanced translocation t(1;22)(q23;q12) and resultant EWSR1-PBX1 gene fusion in one case of soft tissue myoepithelioma (11) and one myoepithelial carcinoma with translocation t(19;22)(q13;q12) resulting in a chimeric gene EWSR1-ZNF444 (12). In a series of 66 myoepithelial neoplasms in skin, soft tissue, bone, and visceral sites, Antonescu et al (10) identified EWSR1 gene rearrangement in 45% (30/66) of tumors, 47% (14/30) of which were myoepitheliomas and 53% (16/30) of which were malignant. One case showed alternative rearrangement of FUS. PBX1 was identified as a partner gene in five cases and ZNF444 in a single case; POU5F1 (mapped on chromosome 6p21) was identified as a partner gene in five additional cases showing distinctive clear cell morphology. Myoepithelial neoplasms showing EWSR1-POU5F1 fusion affected children and young adults and were located in deep soft tissue of the extremities. Of the seven cutaneous tumors examined, only two tumors were positive for EWSR1 gene rearrangement, neither of which had syncytial features. In a 2011 series of 18 cutaneous myoepithelial tumors, Flucke et al (13) reported that seven (7/16; 44%) cases harbored EWSR1 rearrangement, including four mixed tumors, two myoepitheliomas (none of which were reported to be syncytial), and one myoepithelial carcinoma. In Flucke et al’s series, one case description was consistent with the syncytial variant (their case 11), but was negative for EWSR1 rearrangement. A fourth partner gene of EWSR1 was recently reported in a soft tissue myoepithelioma showing EWSR1-ATF1 gene fusion (26). In contrast to Flucke et al's finding of EWSR1 rearrangement in mixed tumors (13), Bahrami et al (14) reported that mixed tumors in skin and soft tissue sites showed PLAG1 aberrations, similar to their salivary gland counterpart.
In summary, cutaneous syncytial myoepithelioma is a distinct variant showing solid growth of spindled, ovoid, or histiocytoid cells with palely eosinophilic cytoplasm that more frequently affects men, occurs over a wide age range, and usually presents on the extremities. Tumors are positive for S-100 protein and EMA, and in contrast to most myoepithelial neoplasms, keratin staining is infrequent. Prior reports describe some risk of recurrence and metastasis for cutaneous myoepithelial tumors; however, based on preliminary follow-up data, the syncytial variant appears to behave in a benign fashion and only rarely recurs locally. The presence of EWSR1 gene rearrangement in most cases supports a close relationship to other subsets of myoepithelial tumors, but we were unable to demonstrate any of the known fusion gene partners, suggesting that this morphologically distinctive tumor type may prove to be associated with a novel fusion gene.
ACKNOWLEDGEMENTS
The authors thank the following pathologists who kindly submitted the cases and provided clinical follow-up information when available: Drs. H. Peter Soyer and Steven Kaddu, Graz, Austria; Dr. Heinz Kutzner, Friedrichshafen, Germany; Dr. Edward B. Heilman, Port Chester, NY; Dr. Jennifer McNiff, New Haven, CT; Dr. Jessie Lee, Teterboro, NJ; Dr. Elsa F. Velazquez, New York, NY; Dr. Naghmeh Yousefzadeh, Herndon, VA; Dr. John T. Bickel, Chattanooga, TN; Dr. M. Barry Randall, Memphis, TN; Dr. Melissa K. Li, Portland, OR; Dr. Uma Krishnamurti, Pittsburgh, PA; Dr. Susan Williams, Swansea, United Kingdom; Dr. Gilbert L. Brodsky, Boston, MA; Dr. Gary A. Letts, Stratford, CT; Dr. Michele Moroni, La Spezia, Italy; Dr. Rob Landers, Waterford, Ireland; Dr. William B. Tyler, Danville, PA; Dr. J.M.H.H. van Gorp, Utrecht, The Netherlands; Dr. Uta Flucke, Nijmegen, The Netherlands; Dr. Vilma C. Fabré, Louisville, KY; Dr. Ruby Delgado, Rye Brook, NY; Dr. George W. Elgart, Miami, FL; Dr. Igor Shendrik, Tulsa, OK; Dr. Loren E. Clarke, Hershey, PA; Dr. Torsten Ehrig, Meriden, CT; Dr. Philip Shapiro, Meriden, CT; Dr. Danielle Bouffard, Montréal, Canada; Dr. Gregory Wolgamot, Bellingham, WA; Dr. Aqeel Haidar, Horsham, PA; Dr. Joan M. Mones, New York, NY; Dr. Stephen Somach, Beachwood, OH; Dr. Toru Shoji, Stratford, CT; Dr. Scott Panzer, Newark, DE; Dr. Ning Li, Shelton, CT (2 cases); Dr. Catherine M. Breen, Putnam, CT; Drs. Kevin Long and Tao Yang, Boston, MA; Dr. Pedram Gerami, Chicago, IL; Dr. Jeoffry B. Brennick, Lebanon, NH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest or funding to disclose.
These results were presented in part at the 101st Annual Meeting of the US-Canadian Academy of Pathology, Vancouver, March 2012.
REFERENCES
- 1.Kilpatrick SE, Hitchcock MG, Kraus MD, et al. Mixed tumors and myoepitheliomas of soft tissue: a clinicopathologic study of 19 cases with a unifying concept. Am J Surg Pathol. 1997;21:13–22. doi: 10.1097/00000478-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Michal M, Miettinen M. Myoepitheliomas of the skin and soft tissues. Report of 12 cases. Virchows Archiv. 1999;434:393–400. doi: 10.1007/s004280050358. [DOI] [PubMed] [Google Scholar]
- 3.Kutzner H, Mentzel T, Kaddu S, et al. Cutaneous myoepithelioma: an under-recognized cutaneous neoplasm composed of myoepithelial cells. Am J Surg Pathol. 2001;25:348–355. doi: 10.1097/00000478-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Mentzel T, Requena L, Kaddu S, et al. Cutaneous myoepithelial neoplasms: clinicopathologic and immunohistochemical study of 20 cases suggesting a continuous spectrum ranging from benign mixed tumor of the skin to cutaneous myoepithelioma and myoepithelial carcinoma. J Cutan Pathol. 2003;30:294–302. doi: 10.1034/j.1600-0560.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 5.Hornick JL, Fletcher CD. Myoepithelial tumors of soft tissue: a clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol. 2003;27:1183–1196. doi: 10.1097/00000478-200309000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Neto AG, Pineda-Daboin K, Luna MA. Myoepithelioma of the soft tissue of the head and neck: a case report and review of the literature. Head & Neck. 2004;26:470–473. doi: 10.1002/hed.20044. [DOI] [PubMed] [Google Scholar]
- 7.Hornick JL, Fletcher CD. Cutaneous myoepithelioma: a clinicopathologic and immunohistochemical study of 14 cases. Hum Pathol. 2004;35:14–24. doi: 10.1016/j.humpath.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Gleason BC, Fletcher CD. Myoepithelial carcinoma of soft tissue in children: an aggressive neoplasm analyzed in a series of 29 cases. Am J Surg Pathol. 2007;31:1813–1824. doi: 10.1097/PAS.0b013e31805f6775. [DOI] [PubMed] [Google Scholar]
- 9.Tanahashi J, Kashima K, Daa T, et al. A case of cutaneous myoepithelial carcinoma. J Cutan Pathol. 2007;34:648–653. doi: 10.1111/j.1600-0560.2006.00676.x. [DOI] [PubMed] [Google Scholar]
- 10.Antonescu CR, Zhang L, Chang NE, et al. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. A molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromos Cancer. 2010;49:1114–1124. doi: 10.1002/gcc.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandal P, Panagopoulos I, Bjerkehagen B, et al. Detection of a t(1;22)(q23;q12) translocation leading to an EWSR1-PBX1 fusion gene in a myoepithelioma. Genes Chromos Cancer. 2008;47:558–564. doi: 10.1002/gcc.20559. [DOI] [PubMed] [Google Scholar]
- 12.Brandal P, Panagopoulos I, Bjerkehagen B, et al. t(19;22)(q13;q12) Translocation leading to the novel fusion gene EWSR1-ZNF444 in soft tissue myoepithelial carcinoma. Genes Chromos Cancer. 2009;48:1051–1056. doi: 10.1002/gcc.20706. [DOI] [PubMed] [Google Scholar]
- 13.Flucke U, Palmedo G, Blankenhorn N, et al. EWSR1 gene rearrangement occurs in a subset of cutaneous myoepithelial tumors: a study of 18 cases. Mod Pathol. 2011;24:1444–1450. doi: 10.1038/modpathol.2011.108. [DOI] [PubMed] [Google Scholar]
- 14.Bahrami A, Dalton JD, Krane JF, et al. A subset of cutaneous and soft tissue mixed tumors are genetically linked to their salivary gland counterpart. Genes Chromos Cancer. 2012;51:140–148. doi: 10.1002/gcc.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fletcher CD, Kutzner H, Fernandez-Figueras MT, et al. Cutaneous myoepithelioma? Am J Dermatopathol. 2000;22:360–361. doi: 10.1097/00000372-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Antonescu CR, Dal Cin P, Nafa K, et al. EWSR1-CREB1 is the predominant gene fusion in angiomatoid fibrous histiocytoma. Genes Chromos Cancer. 2007;46:1051–1060. doi: 10.1002/gcc.20491. [DOI] [PubMed] [Google Scholar]
- 17.Shing DC, McMullan DJ, Roberts P, et al. FUS/ERG gene fusions in Ewing's tumors. Cancer Res. 2003;63:4568–4576. [PubMed] [Google Scholar]
- 18.Franklin G, Chen S, Sznyter LA, et al. Cutaneous myoepithelioma with a plexiform pattern of growth: a case report. J Cutan Pathol. 2009;36(Suppl 1):42–45. doi: 10.1111/j.1600-0560.2009.01210.x. [DOI] [PubMed] [Google Scholar]
- 19.Doyle LA, Fletcher CD. EMA positivity in epithelioid fibrous histiocytoma: a potential diagnostic pitfall. J Cutan Pathol. 2011;38:697–703. doi: 10.1111/j.1600-0560.2011.01747.x. [DOI] [PubMed] [Google Scholar]
- 20.Janssen D, Harms D. Juvenile xanthogranuloma in childhood and adolescence: a clinicopathologic study of 129 patients from the kiel pediatric tumor registry. Am J Surg Pathol. 2005;29:21–28. doi: 10.1097/01.pas.0000147395.01229.06. [DOI] [PubMed] [Google Scholar]
- 21.Hornick JL, Dal Cin P, Fletcher CD. Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol. 2009;33:542–550. doi: 10.1097/PAS.0b013e3181882c54. [DOI] [PubMed] [Google Scholar]
- 22.Hoot AC, Russo P, Judkins AR, et al. Immunohistochemical analysis of hSNF5/INI1 distinguishes renal and extra-renal malignant rhabdoid tumors from other pediatric soft tissue tumors. Am J Surg Pathol. 2004;28:1485–1491. doi: 10.1097/01.pas.0000141390.14548.34. [DOI] [PubMed] [Google Scholar]
- 23.Hollmann TJ, Hornick JL. INI1-deficient tumors: diagnostic features and molecular genetics. Am J Surg Pathol. 2011;35:e47–e63. doi: 10.1097/PAS.0b013e31822b325b. [DOI] [PubMed] [Google Scholar]
- 24.Cheng JX, Tretiakova M, Gong C, et al. Renal medullary carcinoma: rhabdoid features and the absence of INI1 expression as markers of aggressive behavior. Mod Pathol. 2008;21:647–652. doi: 10.1038/modpathol.2008.44. [DOI] [PubMed] [Google Scholar]
- 25.Kohashi K, Oda Y, Yamamoto H, et al. SMARCB1/INI1 protein expression in round cell soft tissue sarcomas associated with chromosomal translocations involving EWS: a special reference to SMARCB1/INI1 negative variant extraskeletal myxoid chondrosarcoma. Am J Surg Pathol. 2008;32:1168–1174. doi: 10.1097/PAS.0b013e318161781a. [DOI] [PubMed] [Google Scholar]
- 26.Flucke U, Mentzel T, Verdijk MA, et al. EWSR1-ATF1 chimeric transcript in a myoepithelial tumor of soft tissue: a case report. Hum Pathol. 2012;43:764–768. doi: 10.1016/j.humpath.2011.08.004. [DOI] [PubMed] [Google Scholar]