Abstract
Background
Psychosocial stress profoundly impacts long-term cardiovascular health through adverse effects on sympathetic nervous system activity, endothelial dysfunction, and atherosclerotic development. Recreational Music Making (RMM) is a unique stress amelioration strategy encompassing group music-based activities that has great therapeutic potential for treating patients with stress-related cardiovascular disease.
Material/Methods
Participants (n=34) with a history of ischemic heart disease were subjected to an acute time-limited stressor, then randomized to RMM or quiet reading for one hour. Peripheral blood gene expression using GeneChip® Human Genome U133A 2.0 arrays was assessed at baseline, following stress, and after the relaxation session.
Results
Full gene set enrichment analysis identified 16 molecular pathways differentially regulated (P<0.005) during stress that function in immune response, cell mobility, and transcription. During relaxation, two pathways showed a significant change in expression in the control group, while 12 pathways governing immune function and gene expression were modulated among RMM participants. Only 13% (2/16) of pathways showed differential expression during stress and relaxation.
Conclusions
Human stress and relaxation responses may be controlled by different molecular pathways. Relaxation through active engagement in Recreational Music Making may be more effective than quiet reading at altering gene expression and thus more clinically useful for stress amelioration.
Keywords: relaxation response, recreational music making, stress, gene expression, cardiovascular disease
Background
Psychosocial stress has been shown to increase risk for chronic diseases such as cardiovascular disease and cancer [1]. A growing body of evidence indicates that psychosocial stressors are associated with hypertension [2], decreased heart rate variability [3], progression of atherosclerosis [4], and cardiovascular events [5], and thus contribute significantly to cardiovascular morbidity and mortality [6,7]. Psychosocial stress elicits a number of physiological, behavioral, and metabolic responses by stimulating production of stress-related hormones and pro-inflammatory factors [8,9], as well as changes in transcription of various genes that function in molecular pathways relevant to the stress response [10,11].
The relaxation response reduces psychological distress through physiological changes, including decreased oxygen consumption and lower heart and respiratory rate, which are distinctly different from responses that occur while resting quietly or sleeping [12]. Thus, the relaxation response has great therapeutic potential for treating patients with stress-related cardiovascular disease [13]. Recreational Music Making (RMM) is a unique stress amelioration strategy encompassing group music-based activities for individuals without prior musical experience. RMM focuses on personal expression and group support, rather than mastery and performance [14]. Research has shown that RMM activities are effective bio-behavioral modulators for reducing stress, limiting occupational burnout and attrition, and enhancing quality of life [15,16]. Although acute psychological stress has been shown to induce changes in signaling cascades, cellular defense mechanisms, and peripheral blood gene expression, little is known about molecular responses that accompany relaxation strategies.
In this study we characterized peripheral blood gene expression in the context of stress induction and amelioration. We used a novel randomized two-phase investigation to explore the impact of a RMM protocol on molecular responses to an acute time-limited stressor and the potential for ameliorating stress at the genomic level in individuals with a history of cardiovascular disease.
Material and Methods
Participants
This research was conducted under protocols approved by the Windber Medical Center Institutional Review Board. Subjects voluntarily agreed to participate and gave written informed consent. Interviews were conducted with participants at the time of recruitment to assess their eligibility for the study and to collect information on demographic and clinical factors including age, ethnicity, gender, and family and personal medical history.
The study population consisted of 34 Caucasian participants (15 women and 19 men) ranging in age from 41–83 (mean age =67.7) with a history of ischemic cardiovascular disease, defined as having at least one of the following: prior history of myocardial infarction, angina pectoris, coronary artery bypass surgery/stent placement, or documented ischemia based upon cardiac catheterization data. Individuals were excluded from the study if they had a recent cardiac event (within 60 days) or met any of the following criteria: (1) current chemotherapy, (2) use of steroids or beta blockers, (3) current smoker, (4) consumption of more than one alcoholic beverage per day, (5) strong aversion to blood drawing, (6) enjoyment of jigsaw puzzles, or (7) prior music-making experience.
Intervention
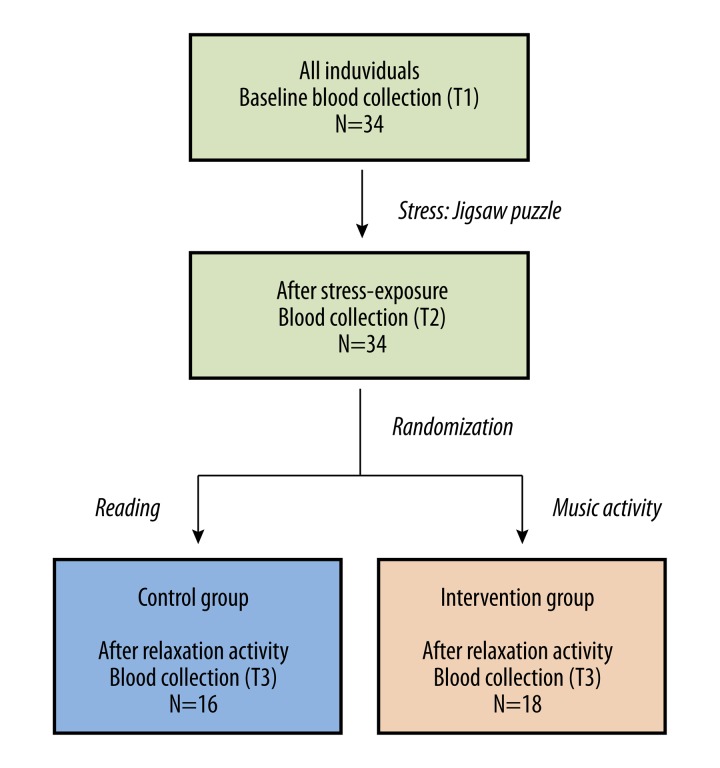
A diagram of the study design is shown in Figure 1. During the stress phase, all subjects were tasked with assembling a 500–1,000-piece jigsaw puzzle classified as “most difficult” by the manufacturer based on several attributes: repetitive images, dual-sided images offset by 90°, extra pieces, and photo-mosaics within each piece. The objective was to assemble as many pieces of the puzzle as possible during the ensuing hour. To intensify stress perception, subjects were reminded at irregular intervals of the time remaining and a monetary reward was offered to the individual who assembled the most pieces within one hour.
Figure 1.
Diagram of the study design.
Participants were subsequently randomized to one of two groups for the one-hour relaxation phase using a random number generator (with integer values between one and two) available at http://www.random.org/integers/. Subjects randomized to the control group (n=16) sat quietly in an isolated room reading magazines or newspapers of their choice. The intervention group (n=18) participated in recreational music-making (the Clavinova Connection) in an isolated room, with each session consisting of no more than seven participants at individual electronic keyboards and the facilitator physician sitting in close proximity.
The RMM protocol followed a multifaceted bio-behavioral group-based stress amelioration algorithm that included the following integral components: (1) Arrival Song played while the facilitator welcomed participants, (2) Mind-Body Wellness Warm-up featuring relaxing music, movement, imagery and awareness, (3) Drum Circle – a keyboard simulation of rhythm-based communication, (4) Improvisation – pentatonic-based melodic expression with calming background environmental accompaniments, (5) Musical Insight using a brief discussion of a relevant musical concept/metaphor, (6) Song of the Day where participants performed a designated song guided by keyboard lights, (7) Mind-Body Wellness Cool-down, (8) Reflection – a group discussion focused on awareness and personal progress, and (9) a Farewell Song.
Blood collection and RNA isolation
Heparin locks were inserted intravenously and secured in the left antecubital fossa using standard phlebotomy techniques. Peripheral blood samples at three time points [baseline (T1), end of stress (T2), and end of relaxation (T3)] were obtained from participants using the PAXgene™ Blood RNA System (Qiagen, Venlo, The Netherlands). PAXgene™ tubes were incubated at room temperature for 16 hours (overnight) following blood collection and then stored at −80°C. Prior to RNA isolation, tubes were removed from −80°C and allowed to thaw at room temperature overnight. RNA was isolated following the manufacturer’s recommended protocol with on-column DNase digestion. Quality of the resulting RNA samples was assessed on an Agilent® 2100 Bioanalyzer Nanochip (Agilent Technologies, Palo Alto, CA), and RNA concentrations were determined using a NanoDrop® ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE).
Globin depletion, RNA amplification, and microarray analysis
Globin mRNA transcripts were depleted from a portion of each total RNA sample (2.5±0.7 μg) using the GLOBINclear™-Human kit (Ambion, Austin, TX) and quality of the resulting samples was determined on the Bioanalyzer. Globin-depleted RNA aliquots (1 μg) were then amplified using the MessageAmp™ II aRNA Amplification System (Ambion) following the manufacturer’s recommended procedure. Eukaryotic Poly-A RNA (Affymetrix, Santa Clara, CA), used for quality control during the amplification process as a sensitive indicator of target preparation and labelling reaction efficiency, showed the expected linear relationships across all samples according to the default Affymetrix Expression Consol® parameters. The resulting double-stranded cDNA was purified, amplified, and labeled with biotin-11-UTP (PerkinElmer, Wellesley, MA) during a 14-hour reaction at 37°C. Quality of the labeled aRNA was assessed using the Bioanalyzer and quantity was measured on the NanoDrop® ND-1000. Labeled aRNA (15 μg) was then fragmented and hybridized to GeneChip® Human Genome U133A 2.0 arrays (Affymetrix) according to the manufacturer’s protocol. Following hybridization, arrays were washed, stained, and scanned on a GeneChip® Scanner 3000. Quality control analysis was performed on the arrays using Expression Consol® to determine aRNA and array performance.
Data pre-processing and quality control
Data from 102 Affymetrix U133A v2.0 CEL files were analyzed using the affyQCReport R Package to ensure a consistent level of quality [17,18]. Percent-present calls and 3′: 5′ ratios for all samples were within the 95% confidence intervals. Probe summarization and normalization were then performed using the MAS5.0 algorithm with median centering across the dataset. Individual intensity measurements <10 were set to a threshold of 10, and those with fewer than 20% detection calls were excluded. Probe set identifications were annotated using the Affymetrix Human Genome U133A 2.0 Array annotation R package version 2.4.5 [17] and redundancy was eliminated by selecting the probe with the highest mean expression across all samples.
Software & statistical methods
GeneChip quality control and data analysis were performed using BRB-ArrayTools [19], R (2.12.0) [20], and Bioconductor (2.9) [17]. Cluster (3.0) [21] and Java Treeview (1.1.6) [22] were used for hierarchical clustering and visualization. All statistical tests were two-sided with a P-value <0.05 considered statistically significant.
Using predetermined gene sets, such as the BioCarta (http://www.biocarta.com) and Kyoto Encyclopedia of Genes and Genomes (KEGG) [23] databases, a functional class scoring analysis was used to identify differential expression between classes [24]. This approach is considered more powerful for identifying differential expression compared to the more common over-representation analysis or annotation of gene lists based on individually analyzed genes [25]. Gene sets containing more differentially expressed genes than would be expected by chance were identified using the Fisher (LS) and Kolmogorov-Smirnov (KS) permutation test statistics (P<0.005) [19].
Results
RNA extraction and globin depletion
Average total RNA yields (±SD) using the PAXgene™ Blood RNA System were 8.7±3.8 μg per blood collection tube. RNA yields in RMM participants (8.5±4.3 μg) did not differ significantly (p=0.61) from yields obtained from controls (8.9±3.2 μg). The OD 260/280 ratio, which measures RNA purity with respect to DNA and protein contamination, ranged from ~2.06 to ~2.26 (average 2.13±0.04) and RNA Integrity Numbers (RINs), which measure RNA degradation, averaged 8.3±0.4 indicating good overall RNA quality.
Following globin reduction, the average recovery of total RNA was 83±1.3%, comparable to the manufacturer’s reported yield. Yields for the globin-depleted RNA samples determined by the NanoDrop® ND-1000 spectrophotometer were 2.5±0.8 μg. The average OD 260/280 ratio of globin-depleted samples was 2.06±0.06 and RIN values determined by the Agilent® 2100 Bioanalyzer Nanochip were 7.46±0.4.
RNA amplification and microarray analysis
Amplification and labeling of globin-depleted RNA (1 μg) using the MessageAmp™ II aRNA Amplification System (Ambion) produced 53±20 μg of aRNA with an average OD 260/280 ratio of 2.22±0.04. Labeled aRNA (15 μg) was then fragmented and hybridized to the U133A 2.0 arrays, yielding an average of 10,407±339 present calls, or 57.8±1.9% of the 18,000 probes on the array. Call rates did not differ significantly (p=0.39) between participants (57.7±2.1%) and controls (58.0±1.6%).
Hierarchical clustering
During the relaxation phase (T2 to T3), 140 genes were found to exhibit significantly different patterns of expression between RMM participants and controls. These genes had a nominal P-value <0.05 in a univariate two-sample T-test comparing cases and controls and a fold-change ≥1.5. Using this set of genes, we conducted hierarchical clustering using Cluster 3.0. The resulting heat map, shown in Figure 2, clearly partitioned RMM participants and controls into separate and distinct clusters.
Figure 2.
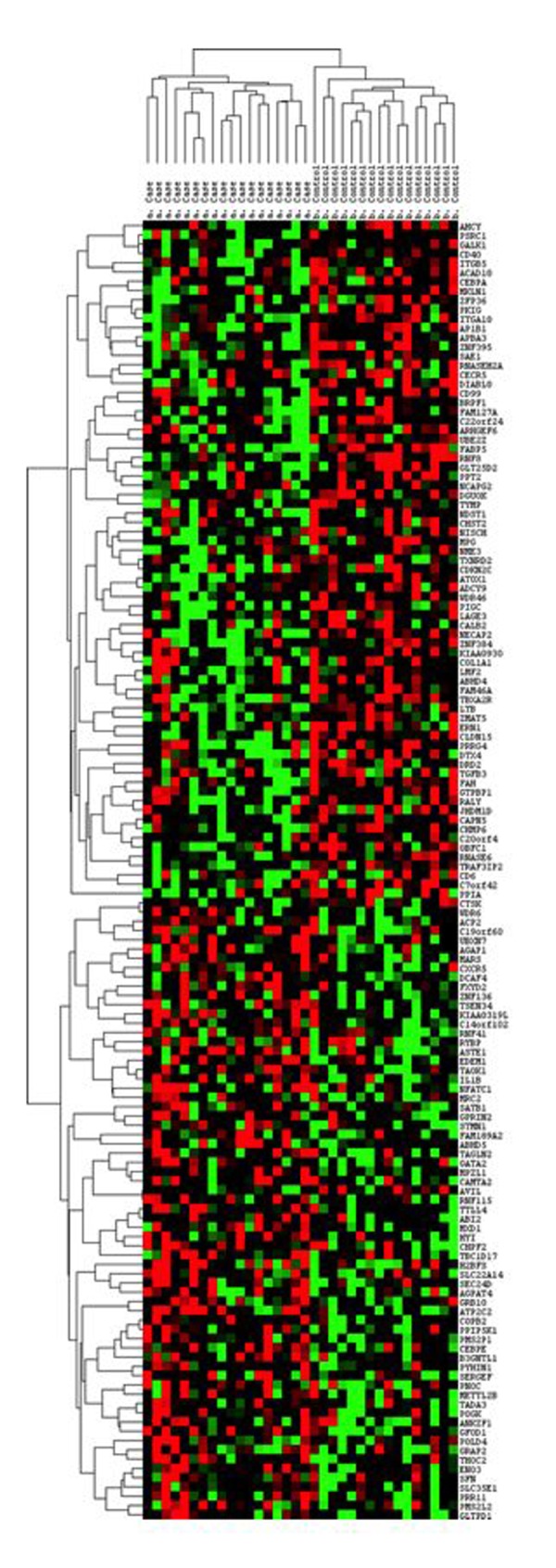
Heat map showing hierarchical clustering of RMM participants and controls based on changes in expression of 140 genes during the relaxation phase.
Gene set enrichment analysis
Gene set enrichment analysis examining expression differences between T1 and T2 identified 10 BioCarta pathways and six KEGG pathways that were differentially regulated (P<0.005) during the stress phase (Table 1). These pathways function primarily in molecular processes such as immune response, cell motility/migration and adhesion, and transcription.
Table 1.
BioCarta and KEGG molecular pathways differentially expressed during stress.
| ID | Name | No. Genes | Function |
|---|---|---|---|
| BioCarta | |||
| h_sm | Spliceosomal assembly | 14 | Assembly of spliceosome; mRNA processing |
| h_integrin | Integrin signaling pathway | 27 | Cell signaling; cellular shape, mobility; cell cycle |
| h_pten | PTEN dependent cell cycle arrest and apoptosis | 17 | Tumor suppression; apoptosis; inhibition of migration |
| h_ucalpain* | uCalpain and friends in cell spread | 11 | Formation of lamellipodia, motility; cell adhesion |
| h_fMLP | fMLP induced chemokine gene expression in HMC-1 cells | 28 | Immune response; chemokine activation |
| h_eponfkb* | Erythropoietin mediated neuroprotection through NF-κB | 9 | Neuroprotection, brain development |
| h_akt* | AKT signaling pathway | 20 | Cell survival/signaling; response to stress |
| h_rarrxr | Nuclear receptors…transcription in carcinoma** | 6 | Chromatin remodeling; initiation of transcription |
| h_nkcells | Ras-independent pathway in NK cell-mediated cytotoxicity | 17 | Immune response; cytotoxicity |
| h_mef2d* | Role of MEF2D in T-cell apoptosis | 15 | T-cell calcium induced apoptosis; immune response |
| KEGG | |||
| hsa00190* | Oxidative phosphorylation | 97 | Metabolism; energy metabolism |
| hsa03010 | Ribosome | 85 | Genetic information processing; translation |
| hsa03060 | Protein export | 11 | Protein transport across cell membrane |
| hsa00193 | ATP synthesis | 32 | Cellular energy/function |
| hsa04810* | Regulation of actin cytoskeleton | 137 | Regulation of cell shape, motility, adhesion |
| hsa04670 | Leukocyte transendothelial migration | 77 | Immune surveillance; inflammation; migration |
Available at: http://www.biocarta.com/genes/index.asp and http://www.genome.jp/kegg/pathway.html. The LS or KS permutation test statistic for all pathways was <0.005. There were 8,857 genes used in the pathway analysis; 261 BioCarta and 155 KEGG pathways (416 total) were tested. Pathway IDs highlighted in bold were differentially expressed during stress and relaxation.
mRNA – messenger RNA; PTEN – phosphatase and tensin homolog; fMLP – N-formyl-methionyl-leucyl-phenylalanine; HMC-1 – human mastocytoma cell line; NF-κB – nuclear factor-kappaB; AKT – protein kinase B; NK – natural killer; MEF2D – myocyte enhancer factor 2D.
Showed differential expression in a genomic study of acute stress and recovery [37];
Nuclear receptors coordinate the activities of chromatin remodeling complexes and coactivators to facilitate initiation of transcription in carcinoma cells.
During the relaxation phase from T2–T3, only one BioCarta pathway and one KEGG pathway were differentially regulated in the control group (Table 2). These pathways function in immune response and translation. In contrast, eight BioCarta pathways and four KEGG pathways demonstrated a significant change in expression among RMM participants (Table 2). The majority of these pathways govern immune function and genetic information processing.
Table 2.
BioCarta and KEGG molecular pathways differentially expressed during relaxation.
| ID | Name | No. Genes | Function |
|---|---|---|---|
| Control BioCarta | |||
| h_cb1r | Metabolism of anandamide, an endogenous cannabinoid | 5 | Immune function; vasodilation; pain relief |
| Control KEGG | |||
| hsa03010 | Ribosome | 85 | Genetic information processing, translation |
| RMM BioCarta | |||
| h_erk* | Erk1/Erk2 Mapk signaling pathway | 22 | Cell signaling, growth, differentiation; transcription |
| h_ahsp | Hemoglobin’s chaperone | 11 | Hemoglobin synthesis/homeostasis; O2 transport |
| h_egfr_smrte | Map kinase inactivation of SMRT corepressor | 10 | Cell proliferation; apoptosis; transcription |
| h_fMLP | fMLP induced chemokine gene expression in HMC-1 cells | 30 | Immune response; chemokine activation |
| h_sppa* | Aspirin blocks signaling pathway…platelet activation** | 14 | Inhibition of platelet activation/vasoconstriction |
| h_fcer1* | Fc epsilon receptor I signaling in mast cells | 34 | Immune/inflammatory response; cytokine expression |
| h_lymphocyte | Adhesion molecules on lymphocyte | 8 | Immune/inflammatory response; cell adhesion |
| h_vip | Neuropeptides VIP and PACAP inhibit the apoptosis…*** | 20 | Immune function; inhibition of T-cell apoptosis |
| RMM KEGG | |||
| hsa03010 | Ribosome | 85 | Genetic information processing; translation |
| hsa04650* | Natural killer cell mediated cytotoxicity | 90 | Immune response; cytotoxicity, defense |
| hsa04612 | Antigen processing and presentation | 56 | Immune system; cytotoxicity |
| hsa00450* | Selenoamino acid metabolism | 25 | Amino acid metabolism |
Available at: http://www.biocarta.com/genes/index.asp and http://www.genome.jp/kegg/pathway.html. The LS or KS permutation test statistic for all pathways was <0.005. There were 8,857 genes used in the pathway analysis; 261 BioCarta and 155 KEGG pathways (416 total) were tested. Pathway IDs highlighted in bold were differentially expressed during stress and relaxation.
Erk – extracellular signal-regulated kinase; Mapk – mitogen-activated protein kinase; SMRT – silencing mediator for retinoid or thyroid-hormone receptor; fMLP – N-formyl-methionyl-leucyl-phenylalanine; HMC-1 – human mastocytoma cell line; Fc – fragment, crystallizable; VIP – vasoactive intestinal peptide; PACAP – pituitary adenylate cyclase-activating polypeptide.
Showed differential expression in a genomic study of acute stress and recovery [37];
Aspirin blocks signaling pathway involved in platelet activation;
Neuropeptides VIP and PACAP inhibit the apoptosis of activated T-cells.
Discussion
In this study we characterized changes in peripheral blood gene expression during a two-phase experiment, stress and subsequent relaxation, to investigate molecular pathways relevant to stress-relaxation modulation. During the acute stress phase, we observed differential expression in 16 molecular pathways, which control diverse physiological processes including innate immunity, cell mobility, and gene transcription. During the relaxation phase, 140 genes were informative for distinguishing RMM participants from controls. Controls who sat reading quietly showed differential regulation of only two pathways, while participants randomized to the RMM protocol demonstrated changes in expression for 12 pathways governing immune function and genetic information processing. Our observation that RMM can induce greater molecular change than quiet reading suggests that RMM may serve as a more effective tool for stress amelioration with important implications for management of cardiovascular patients.
A growing body of research suggests that psychosocial stress may contribute significantly to the development and pathogenesis of cardiovascular disease [26]. Humans typically react to stress through the autonomic nervous system, which controls a wide range of cardiovascular and endocrine functions [27]. However, little is known about mechanisms that link psychosocial stress to cellular dysfunction. Fortunately, peripheral blood is an easily accessible tissue that may be useful for examining complex biological processes associated with health and disease [28]. Since peripheral blood mononuclear cells (PBMC) are essential mediators of stress that play a multi-faceted role in vascular disease, inflammation, and immune responses, monitoring changes in PBMC gene expression may prove useful for quantifying and/or monitoring effects of stress and relaxation [29].
Episodes of psychological stress, even if relatively short in duration, have been shown to trigger acute gene expression responses in peripheral blood. Stress responsive genes include cytokines, adhesion molecules, heat shock proteins, and growth- or apoptosis-related genes with a wide range of biological functions including cell signaling, inflammation, immuno-modulation, and neuroprotection [10,11]. During the first phase of this study, dysregulation of gene expression was noted in multiple molecular pathways that govern a variety of biological processes including immune responses, cell adhesion and migration, and transcription.
While volunteers in this research study were not subjected to the high levels of psychosocial stress encountered by many cardiovascular patients, the number of molecular pathways showing dysregulation during the stress phase suggests that assembling a complex puzzle under a time constraint was sufficiently stressful to influence transcriptional activity over a broad range of molecular pathways. Behavioral interventions targeting stress reduction demonstrate health benefits that extend beyond usual clinical care in patients with cardiovascular disease. For example, stress management has been associated with a significant reduction in clinical cardiovascular events relative to usual care in patients with myocardial ischemia [30]. Similarly, reducing emotional stress has been associated with improved prognosis in coronary heart disease patients [31] and significant reductions in carotid atherosclerosis in hypertensive African Americans [32].
The relaxation response can be effective in offsetting the negative psychobiological effects of stress through mind-body approaches such as meditation, guided imagery, and controlled breathing [33,34]. Research has shown that relaxation training elicits changes in peripheral blood gene expression [35], while others have shown significant differences in gene expression between Qigong practitioners and normal controls [36]. Molecular pathways previously found to be most frequently altered during relaxation include immunity, cellular metabolism, oxidative phosphorylation, and apoptosis. During the relaxation phase of this study, we observed gene expression modulation in similar molecular pathways governing immune function, genetic information processing, cell signaling, and cytotoxicity. In fact, of the 13 unique pathways showing dysregulation during the relaxation phase, five showed differential expression in another genomic study of acute stress and recovery [37].
Recreational Music Making as a mode of stress reduction following stress induction was more effective at altering the expression of molecular pathways than quiet reading. In participants randomized to RMM, 12 pathways showed significant dysregulation, compared to only two pathways in participants randomized to quiet reading.
Psychosocial stress may function as an important risk factor for cardiovascular disease and a variety of chronic diseases through negative effects on the immune system [38,39] and transcriptional control pathways [40]. Our observation that RMM can elicit molecular changes in pathways governing immune function and transcriptional activity suggests that RMM may be an effective tool for stress amelioration with potentially greater benefit to cardiovascular patients than traditional modes of relaxation. While determining the impact of metabolic pathway activation on the actual development of pathology is beyond the scope of this study, it is interesting to note that pathways highly relevant to cardiovascular disease including Hemoglobin’s chaperone and Aspirin blocks signaling pathway involved in platelet activation were differentially expressed exclusively in RMM participants.
Acute stress and relaxation share some commonalities in molecular response, but for the most part, different and distinct molecular pathways appear to govern stress and relaxation responses. In a study of psychosocial stress involving public speaking and mental arithmetic, Nater et al. observed that only 8 of 49 (16%) pathways differentially expressed during acute stress also showed modulation during subsequent recovery [37]. Likewise, of 16 unique pathways differentially expressed during the stress phase of this study, only 2 (13%) were significantly altered during relaxation. These results support the hypothesis that multiple biological processes involving different sets of genes play important roles in both the human stress and relaxation response. While certain pathways may be classified in similar functional categories, the actual gene sets demonstrating dysregulation may be different between stress and relaxation.
Psychological stress induces a series of coordinated processes regulated by the Hypothalamic Pituitary Adrenal Axis (HPA) axis and direct sympathetic nervous system innervation that controls a wide range of metabolic, circulatory, and inflammatory/immunological functions. Repeated stressful events (chronic stress) over a long period of time with failure to habituate may contribute to the development of cardiovascular disease. Therefore, physiological and molecular changes resulting from stress reduction may be important to long-term cardiovascular health. Our findings of statistically significant changes in the expression of gene sets associated with known biological pathways represent an initial step toward further understanding molecular changes that correlate with creative musical expression. While the majority of clinical interventions typically focus on nutrition and exercise [41], a limited scope of relaxation strategies is typically offered to cardiovascular patients. We believe that active engagement or participation in creative musical expression may ultimately play an important role in stress reduction within the realm of chronic disease management.
Prior research identified individualized patterns of stress-induced genomic expression with subsequent amelioration associated with RMM in normal subjects [42]. This study advances our knowledge by demonstrating that RMM induced more gene expression changes than quiet reading across of a cohort of cardiovascular patients. Our work represents a practical preliminary step for investigating the role of active music participation as an integrative stress amelioration strategy in the clinical realm. Additional research is needed with larger sample sizes and ongoing experimental refinement to further decipher and characterize molecular responses, as well as the potential benefits, associated with active music participation.
Conclusions
An activity as simple as assembling a complex puzzle under a time constraint is sufficiently stressful to induce genomic expression changes in a variety of molecular pathways.
The human stress and relaxation responses are controlled, for the most part, by different molecular pathways.
Stress reversal through RMM is more effective than quiet reading at altering the expression of immune response and transcriptional control pathways, suggesting that RMM may be more clinically useful for stress amelioration and may confer greater benefit to cardiovascular patients than traditional modes of relaxation.
Additional studies are required to identify and validate bio-markers that can quantify molecular changes associated with stress-amelioration and define the long-term benefits of integrating creative musical expression strategies in comprehensive treatment protocols for individuals facing the challenges of cardiovascular disease.
Acknowledgements
We acknowledge the late Karl T. Bruhn for his pioneering contributions to Recreational Music Making. Support for this research was provided by Yamaha Corporation of America.
Footnotes
Source of support: Yamaha Corporation of America
References
- 1.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5:466–75. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 2.Player MS, Peterson LE. Anxiety disorders, hypertension, and cardiovascular risk: a review. Int J Psychiatry Med. 2011;41:365–77. doi: 10.2190/PM.41.4.f. [DOI] [PubMed] [Google Scholar]
- 3.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141:122–31. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 4.Paterniti S, Zureik M, Ducimetière P, et al. Sustained anxiety and 4-year progression of carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:136–41. doi: 10.1161/01.atv.21.1.136. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Hawken S, Ôunpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 6.Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- 7.Olafiranye O, Jean-Louis G, Zizi F, et al. Anxiety and cardiovascular risk: review of epidemiological and clinical evidence. Mind Brain. 2011;2:32–37. [PMC free article] [PubMed] [Google Scholar]
- 8.Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240–48. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strike PC, Magid K, Whitehead DL, et al. Pathophysiological processes underlying emotional triggering of acute cardiac events. Proc Natl Acad Sci USA. 2006;103:4322–27. doi: 10.1073/pnas.0507097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morita K, Saito T, Ohta M, et al. Expression analysis of psychological stress-associated genes in peripheral blood leukocytes. Neurosci Lett. 2005;381:57–62. doi: 10.1016/j.neulet.2005.01.081. [DOI] [PubMed] [Google Scholar]
- 11.Kawai T, Morita K, Masuda K, et al. Gene expression signature in peripheral blood cells from medical students exposed to chronic psychological stress. Biol Psychol. 2007;76:147–55. doi: 10.1016/j.biopsycho.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Benson H. The relaxation response: its subjective and objective historical precedents and physiology. Trends Neurosci. 1983;6:281–84. [Google Scholar]
- 13.Esch T, Fricchione GL, Stefano GB. The therapeutic use of the relaxation response in stress-related diseases. Med Sci Monit. 2003;9(2):RA23–34. [PubMed] [Google Scholar]
- 14.Bittman B, Bruhn KT, Stevens C, et al. Recreational music-making: a cost-effective group interdisciplinary strategy for reducing burnout and improving mood states in long-term care workers. Adv Mind Body Med. 2003;19:4–15. [PubMed] [Google Scholar]
- 15.Bittman BB, Snyder C, Bruhn KT, et al. Recreational music-making: an integrative group intervention for reducing burnout and improving mood states in first year associate degree nursing students: insights and economic impact. Int J Nurs Educ Scholarsh. 2004;1 doi: 10.2202/1548-923x.1044. Article 12. [DOI] [PubMed] [Google Scholar]
- 16.Bittman B, Dickson L, Coddington K. Creative musical expression as a catalyst for quality-of-life improvement in inner-city adolescents placed in a court-referred residential treatment program. Adv Mind Body Med. 2009;24:8–19. [PubMed] [Google Scholar]
- 17.Gentleman RC, Carey VJ, Bates DM, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson CL, Miller CJ. Simpleaffy: a BioConductor package for Affymetrix quality control and data analysis. Bioinformatics. 2005;21:3683–85. doi: 10.1093/bioinformatics/bti605. [DOI] [PubMed] [Google Scholar]
- 19.Simon R, Lam A, Li M-C, et al. Analysis of gene expression data using BRB-ArrayTools. Cancer Inform. 2007;3:11–17. [PMC free article] [PubMed] [Google Scholar]
- 20.R Development Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2010. http://softlibre.unizar.es/manuales/aplicaciones/r/fullrefman.pdf. [Google Scholar]
- 21.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–68. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saldanha AJ. Java Treeview – extensible visualization of microarray data. Bioinformatics. 2004;20:3246–48. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
- 23.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavlidis P, Qin J, Arango V, et al. Using the gene ontology for microarray data mining: a comparison of methods and application to age effects in human prefrontal cortex. Neurochem Res. 2004;29:1213–22. doi: 10.1023/b:nere.0000023608.29741.45. [DOI] [PubMed] [Google Scholar]
- 25.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 27.Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–84. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- 28.Whitney AR, Diehn M, Popper SJ, et al. Individuality and variation in gene expression patterns in human blood. Proc Natl Acad Sci USA. 2003;100:1896–901. doi: 10.1073/pnas.252784499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bierhaus A, Wolf J, Andrassy M, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci USA. 2003;100:1920–25. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blumenthal JA, Babyak M, Wei J, et al. Usefulness of psychosocial treatment of mental stress-induced myocardial ischemia in men. Am J Cardiol. 2002;89:164–68. doi: 10.1016/s0002-9149(01)02194-4. [DOI] [PubMed] [Google Scholar]
- 31.Denollet J, Brutsaert DL. Reducing emotional distress improves prognosis in coronary heart disease: 9-year mortality in a clinical trial of rehabilitation. Circulation. 2001;104:2018–23. doi: 10.1161/hc4201.097940. [DOI] [PubMed] [Google Scholar]
- 32.Castillo-Richmond A, Schneider RH, Alexander CN, et al. Effects of stress reduction on carotid atherosclerosis in hypertensive African Americans. Stroke. 2000;31:568–73. doi: 10.1161/01.str.31.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang B-H, Dusek JA, Benson H. Psychobiological changes from relaxation response elicitation: long-term practitioners vs. novices. Psychosomatics. 2011;52:550–59. doi: 10.1016/j.psym.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Iglesias SL, Azzara S, Argibay JC, et al. Psychological and physiological response of students to different types of stress management programs. Am J Health Promot. 2012;26:e149–58. doi: 10.4278/ajhp.110516-QUAL-199. [DOI] [PubMed] [Google Scholar]
- 35.Dusek JA, Otu HH, Wohlhueter AL, et al. Genomic counter-stress changes induced by the relaxation response. PLoS One. 2008;3:e2576. doi: 10.1371/journal.pone.0002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li QZ, Li P, Garcia GE, et al. Genomic profiling of neutrophil transcripts in Asian Qigong practitioners: a pilot study in gene regulation by mind-body interaction. J Altern Complement Med. 2005;11:29–39. doi: 10.1089/acm.2005.11.29. [DOI] [PubMed] [Google Scholar]
- 37.Nater UM, Whistler T, Lonergan W, et al. Impact of acute psychosocial stress on peripheral blood gene expression pathways in healthy men. Biol Psychol. 2009;82:125–32. doi: 10.1016/j.biopsycho.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathews HL, Konley T, Kosik KL, et al. Epigenetic patterns associated with the immune dysregulation that accompanies psychosocial distress. Brain Behav Immun. 2011;25:830–39. doi: 10.1016/j.bbi.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith AK, Conneely KN, Kilaru V, et al. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:700–8. doi: 10.1002/ajmg.b.31212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller GE, Chen E, Sze J, et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kB signaling. Biol Psychiatry. 2008;64:266–72. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 42.Bittman B, Berk L, Shannon M, et al. Recreational music-making modulates the human stress response: a preliminary individualized gene expression strategy. Med Sci Monit. 2005;11(2):BR31–40. [PubMed] [Google Scholar]