Abstract
Background
In 2008, the Polish Gynecological Society issued recommendations to screen pregnant women for GBS colonization and offer antibiotic prophylaxis at delivery. The goal of this study was to assess compliance with these recommendations among women delivering very low birth weight infants (VLBW) in Poland.
Material/Methods
The 6 Polish Perinatological Institutions managing infections in the framework of the Polish Perinatological Network were subjected to the analysis. A retrospective case-cohort study for 2009 was conducted using the standard protocols and definitions. The collected data pertained to 812 pregnant women who gave birth to 910 babies with VLBW.
Results
The statistical variation across the 6 studied centers associated with GBS prevention of infections was noted. Bacteriological examinations of samples taken from the vagina were performed only in 273 (34%) of the women, ranging between 2% and 93%, depending on the center. GBS carriage was proven in 19% of these women, ranging between 8% and 27%. The culture method was inadequate because of highly variable results. It was found that the rate of GBS determination is statistically connected with the number of women’s screenings performed in the study centers. The intrapartum antibiotic prophylaxis (IAP) was used only in the half of GBS-positive women (47%). Six cases of early-onset GBS infections (5 blood stream infections and 1 pneumonia) were registered in the studied newborns, of which 4 neonates were born to women who received IAP against GBS. The incidence rate of GBS infection in VLBW neonates was 6.6 per 1000 live births, with a high death rate (up to 33%).
Conclusions
Poor compliance with GBS screening and antibiotic prevention were observed among women delivering very low birth weight infants. GBS infection was noted in a significant proportion of VLBW neonates; we believe a uniform policy should be put in place to manage these high-risk women and babies.
Keywords: Group B Streptococci (GBS), Intrapartum Antibiotic Prophylaxis (IAP), Very Low Birth Weight (VLWB), neonates
Background
A serious and very significant problem of modern medical science is the occurrence of perinatal infections. The main risk factor for infection for women during puerperium is caesarean section (CS) [1], and for neonates the main risk factors are low gestational age and very low birth weights (VLBW) [2,3].
Group B Streptococcus (GBS) is a significant etiological factor of bacterial infections in neonates [4]. Their main reservoirs are the genitourinary tract and the female alimentary tract. According to epidemiological data, the presence of GBS at the vagino-rectal site can be identified in 10% to 40% of healthy women [5]. The GBS carrier rate statement for Poland depends on the region, population, and methodology used, varying from 5% to 19% in central Poland [6,7] and reaching 30% in southern Poland [8]. Several recent reports from Polish hospitals also noted an increase of both colonization and infection rates of neonates with GBS [7,9].
GBS may pose a threat for colonized women, most frequently those in the perinatal period, but above all, they can cause serious infections in fetuses and neonates. Babies are colonized with GBS mostly during deliveries or by ascending spread after rupture of membranes. The risk of transmission of streptococci to a neonate can be as high as 70% and the frequency of disease contraction is 2 to 4 cases per 1000 live births [10]. Infections in neonates are most frequently early-onset diseases (EOD) developing during the first 7 days of life and are characterized by high death rates. The most frequent clinical forms of neonatal GBS-caused infections are sepsis or pneumonia, and, less frequently, cerebrospinal meningitis [4,10].
In the 1990s in the USA, a high number of neonatal infections caused by GBS were noted, with early morbidity and a death rate up to 50%. This phenomenon was the reason why the Centers for Disease Control and Prevention (CDC) set guidelines in 1996 aimed at preventing GBS infections in neonates [4,5]. The American experience indicated that the most effective method to limit the number of infections in neonates is early prophylaxis based on a vaginal-rectal culture screening for GBS carriage of women between the 35th and 37th week of gestation, taking into consideration the existing infection risk factors. In women colonized with GBS, the use of proper intrapartum antibiotic prophylaxis (IAP) was considered to be advisable, along with observation and possible diagnosis of their babies for GBS infection [4,5,11].
In 2008 in Poland, following the CDC guidelines and under the auspices of the Polish Gynecological Society, recommendations for prevention of perinatal GBS diseases were developed and published [12]. The Polish guidelines recommended universal culture-based screening of all pregnant women at 35–37 weeks’ gestation to optimize the identification of women who should receive IAP. If the GBS colonization status from the current pregnancy is not known, and if onset of labor or rupture of membranes occurred before 37 weeks’ gestation with a substantial risk for preterm delivery, then GBS screening should be performed and IAP for GBS should be provided pending culture results [12]. The Polish IAP against GBS infection corresponds with the CDC recommendations from 2002 [11]. For IAP for women with GBS carriage, IV administration of Penicillin G or, as an alternative, Ampicillin, was recommended. In case of Penicillin-allergic patients, in whom the use of Cephalosporins was admissible, Cephazolin should be administered. In patients with a high risk of anaphylactic shock after the administration of Penicillins, in the event of isolation of GBS strains with macrolide resistance phenotype, Vancomycin should be administered, and in other cases Erythromycin or Clindamycin is preferred [4,12].
Unfortunately, in Poland there is no data concerning putting the aforementioned recommendations into practice. Moreover, epidemiological surveillance of GBS infections in newborns is not conducted. However, in the framework of the Polish Perinatological Network it was possible to obtain data pertaining to VLBW neonates (≤1500 g), which constitute a high risk group for early-onset GBS infections [13]. Therefore, the purpose of this retrospective study was to analyze the utilization of the perinatal prophylaxis of infections caused by GBS in 6 Polish perinatological centers, which monitor infections in VLBW neonates.
Material and Methods
Six Polish perinatological institutions were subjected to the analysis: 3 centers from central Poland (A, B, E), and 1 center each from northern (C), southern (F), and north-western Poland (D) supervising infections in the framework of the Polish Perinatological Network (SN/080/08). The study was carried out from January to December 2009, applying the standardized protocols and definitions. Data were collected from 812 women (including 137 with multiple pregnancies) who gave birth to 910 babies with very low birth weight (VLBW). Almost 50% of these women came from the E (n=210) and D (n=205) centers, and the rest were from centers B (n=137), A (n=83) and C (n=38).
The inclusion criteria of the study were for a live newborn to be of birth weight <1500 g, and the exclusion criteria were for a newborn to achieve a weight >1800 g, death, or discharge from the Neonatal Intensive Care Unit (NICU).
In the group of 812 studied women, 100% had preterm labor (PTL) (≤30 week of pregnancy, 25% (n=203) had preterm premature rupture of membranes (PPROM), 1% (n=10) had diabetes, 3% (n=26) had preeclampsia, 8% (n=66) had amnionitis, and 5% (n=42) had bacterial vaginosis. Twenty-nine percent (n=237) of the studied women had taken steroid-based medications, 21% (n=170) took immunosuppressive drugs, and 15% (n=122) took antimicrobial drugs.
The surveillance covered 910 newborns, including 166 (18%) very low birth weight infants with a birth weight up to 750 grams, 250 (28%) with the birth weight ranging from 751 to 999 grams, and 494 (54%) with the birth weight ranging from 1000 to 1500 grams; 223 (24%) neonates were born from multiple pregnancies. The average gestational age (GA) for the studied children was 28 weeks, while average birth weight was 1024 grams (Table 1).
Table 1.
Characteristics of the 910 neonates with VLBW.
| Average | 95% CL | |
|---|---|---|
| Gestational age [weeks] | 28.3 | 28.1; 28.6 |
| Birth weight of neonates [g] | 1024 | 1004; 1044 |
| Cases | Percent [%] | |
| Female gender | 427 | 47.0% |
| The newborns delivered by Caesarean Section (CS) | 623 | 68.0% |
| The newborns delivered with preterm premature rupture of membranes (PPROM) | 228 | 25.0% |
| Early-onset bloodstream infections (EO BSI) | 64 | 7.0% |
| Late-onset bloodstream infections (LO BSI) | 206 | 23.0% |
| Early-onset pneumonia (EO PNU) | 78 | 7.0% |
| Late-onset pneumonia (LO PNU) | 164 | 18.% |
| GBS specific infection | 6 | 0.7% |
| Death of the neonates | 175 | 19.0% |
| Death of the neonates connected with GBS infection | 2 | 0.2% |
For bacteriological examinations, in most of cases the standard culture method (not the recommended recto-vaginal screening) was done. The vaginal swabs were taken in the clinic at the time of registration of the patient for childbirth or during hospitalization at the pregnancy pathology ward. To check for vaginal microflora, a vaginal swab was streaked on a plate of Columbia blood agar with 5% sheep blood; plates were then incubated for 24 hours at 37°C in aerobic atmosphere. Standard laboratory methods were used to identify the microorganisms that grew from blood cultures. Mycoplasma spp. and Candida spp. were isolated and identified according to conventional cultures laboratory methods.
For the statistical analysis, the likelihood ratio test (G2) was used. The assumed level of significance was P<0.05.
Results
In the group of 812 women undergoing this analysis, there was a high percentage of parturients delivering their babies by CS, which amounted to 71% (n=576) (Table 2); depending on the center, it ranged between 61% and 95%. In 180 (31%) of them, antibiotic perinatal prophylaxis connected with CS was used. The highest percentage (68%) of such patients was registered in the A center, which was 3 times the number in the E center (22%). In total, 298 (37%) from those included in the study underwent antibiotic perinatal prophylaxis because of various clinical indications. The highest percentage of women subjected to the antibiotic perinatal prophylaxis was 72% (in center A), whereas the lowest percentage of female patients who were administered an antibiotic after the start of labor was 26% (in center E). By analyzing the use of the antibiotic perinatal prophylaxis irrespective of clinical indications, significant differences between individual centers were found (P<0.0001).
Table 2.
Characteristics of the 810 mothers of VLBW neonates with consideration to antibiotic perinatal prophylaxis applied in the study centers.
| Number (%) | Range between the study centers (%) | |
|---|---|---|
| The women, to whom the antibiotic prevention was applied of various clinical indications | 298 (37%) | 26–72% |
| The women delivering by Caesarian Section | 576 (71%) | 67–95% |
| The women, to whom the antibiotic prevention was applied in relation to the Caesarian Section | 180 (31%) | 22–68% |
| The women, to whom bacteriological examining of the genital tract was performed during pregnancy | 273 (34%) | 13–93% |
| The women with GBS carriage | 53 (19%) | 8–22% |
| The number of women with GBS carriage to whom the perinatal antibiotic prevention was applied | 25 (47%) | 0–100% |
| The number of women with GBS carriage to whom the perinatal antibiotic prevention was not applied | 28 (53%) | 0–100% |
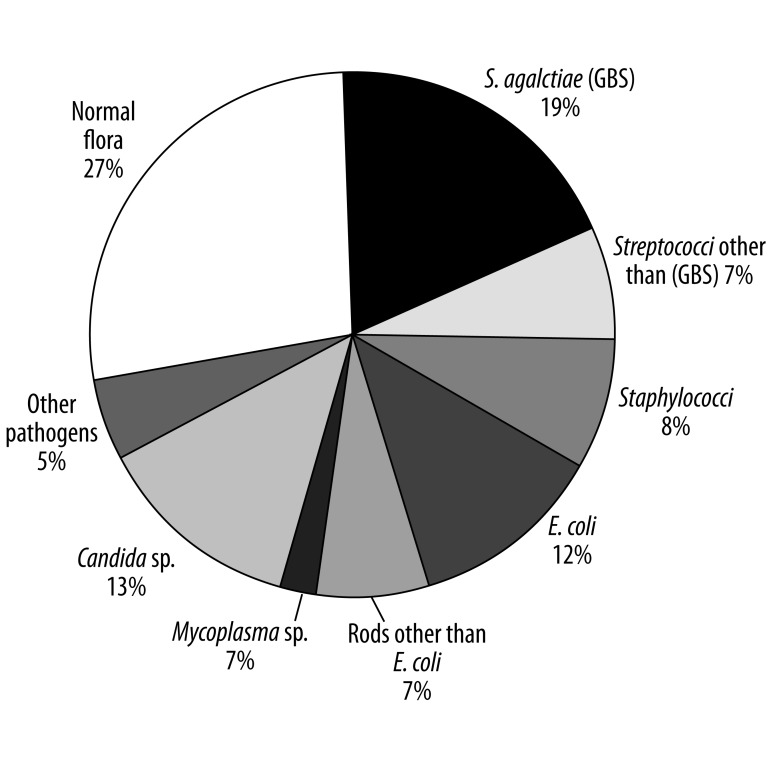
Bacteriological examinations of samples taken from the vagina were performed in 273 (34%) pregnant women, with the percentage of examined women ranging between 2% and 93%, depending on the center. The observed differences between centers were significant (P<0.0001). The potentially pathogenic microbes were cultured from vaginal swabs from 203 (74%) of the studied women: 53 (19%) had GBS, 35 (13%) had Candida spp., 32 (12%) had E. coli, 22 (8%) had staphylococci, 20 (7%) had streptococci other than GBS, 20 (7%) had rods other than E. coli, 6 (2%) had Mycoplasma spp., and 15 (5%) had other pathogens (Figure 1).
Figure 1.
Pathogenic agents isolated from the genital tracts of the examined mothers (n=273) of VLBW neonates.
Having analyzed the group of GBS-positive pregnant women (n=53; 19%) studied in 6 centers, the highest percentage of GBS carriage was noted in center D (27%) and the lowest percentages were in centers A and C (8% each). The observed differences between centers were significant (P<0.0001). The rate of GBS determination was statistically connected with the number of women’s screenings performed in the study centers (P<0.0001); the higher the number of examined women in a given center, the higher the probability to obtain a result positive for GBS. For example, in center B screening of 128 (93%) gravidae revealed GBS colonizations in 28 (22%).
The antibiotic perinatal prophylaxis against GBS-caused infections was used only in the half of the female patients showing colonizations by GBS (n=25; 47%) (Table 2). Statistically significant differences between centers were stated in terms of using antibiotic prophylaxis against GBS infections (P<0.0001).
Six cases of EOD caused by GBS were registered in the group of 910 VLBW neonates (Table 1). Four cases of GBS infections were noted in center B, where bacteriological examinations of the genital tracts of women were performed significantly more often than other centers, and where there was a significantly higher GBS detectability. Mothers of 4 babies from center B, in whom GBS-caused infections were identified, were carriers of GBS, and they were subjected to antibiotic perinatal prophylaxis. The remaining 2 cases of neonates with GBS-caused infections were registered in centers C and E, but at the time of birth their mothers’ GBS-carrier statuses were unknown.
The incidence rate of EOD caused by GBS in the group of VLBW neonates was 6.6 per 1000 live births. The prevailing clinical forms of these infections were blood stream infection (BSI) (n=5; 83%) and pneumonia (n=1; 17%). In total, early onset GBS infections made up 4.2% of all early infections, including both BSI and pneumonia (6/142). In the group of babies with GBS infections, 2 deaths were registered; thus the death rate reached no less than 33%.
Discussion
Perinatal infections remain a significant problem in infection control. The recommendation of universal antenatal screening for group B streptococcus is an important policy shift that poses challenges in implementation [4,5,14]. As an example of an effective GBS prophylaxis in neonates in the United States prior to 1996 (the year in which recommendations pertaining to the antibiotic perinatal prophylaxis of GBS were introduced), the incidence of early infections in neonates was 1.8 per 1000 live births, whereas in 2003 it fell to 0.3 [4] and in term infants in 2011 to 0.26 [13]. In the USA, the broad implementation of universal screening, after the CDC guidelines were issued, shows that public health policy can be translated into action. Recommendations were rapidly adopted and coincided with a decline in the incidence of early-onset disease [13,15].
Our results are the first to be reported by the Polish Neonatology Network and are the first such report from Central Europe regarding a national program for infection control among neonatal units. Altogether, surveillance covered 19.1% of the VLBW infants born in 2009 in Poland and their mothers, which is over half of all VLBW infants born in regions where NICUs were located.
In the group of women subjected to this analysis, the bacteriological examination of samples taken from the vagina was performed in only 34% of patients. Because of preterm labor, the studied women were not GBS screened (the average gestational age was 28.3 weeks). The 2010 CDC revised guideline for perinatal GBS prevention contains specific recommendations about how to manage women who present in preterm labor, and separate recommendations for preterm rupture of membranes [5].Therefore, a similar uniform policy should be put in place in Poland to management these high-risk women and babies.
In this study, GBS carriage was proven in 19% of pregnant women, and the percentage was significantly different depending on the center. Unfortunately, we had only limited information about methods used and were unable to assess clinical and laboratory procedures. There may also be differences in policies and practices among studied centers and their laboratories. Therefore, the interpretation of the obtained results is difficult. Due to the highly variable results obtained, we conclude that the culture method was inadequate. According to Welch & Aldridge (2005), GBS detection largely depends on laboratory experience, and this may explain the differences among various centres and countries [16]. Similar results were obtained in our previous study, which demonstrated that the GBS colonization rate depends on the detection procedure used (cultured method vs. recto-vaginal screening) [8,9].
In the studied group of VLBW neonates, the value of early GBS-caused morbidity rate was 6.6 per 1000 live births. These infections were accompanied by a high death rate (up to 33%), which was most probably connected with the group of neonates studied, in whom there existed an array of other risk factors, including pre-term birth and low birth weight. Similar results were described in a group of VLBW babies in which the morbidity rate was 10.1 per 1000 live births and the death rate was 37% [17]. In contrast, in the USA, preterm infants had an incidence of early-onset GBS disease of only 0.73 per 1000 live births, and term infants had a rate of 0.26 per 1000 [13].
In our study most of the GBS infections were registered in center B, the same center in which there existed an effective procedure for GBS prophylaxis. This situation might be connected not so much with high incidence in center B, but rather with correct and reliable microbiological diagnostics directed towards not only finding GBS in samples coming from women, but also in clinical samples coming from babies from risk groups. Based on this, one might surmise that the described value of intensity of incidence of early GBS-caused infections was rather understated in other centers participating in this study.
In the presented study, the IAP against GBS-caused infections was used only in the half of the female patients showing colonizations by GBS, and significant differences between centers were found. Unfortunately, data collected in the framework of the 2009 Polish Neonatological Network contain only information about the use of IAP, while there is no detailed information on the kind of antibiotics and the patterns of their administration. Similar results were demonstrated in Germany (2011), where IAP was administered only to 39% of GBS-positive women. In that study, despite national guidelines for universal GBS screening, a lack of adherence to this recommendation was demonstrated [18]. A different situation was observed in France, where mandatory measures quickly resulted in a significant increase of the screening rate at the correct term [19].
In the group of VLBW and pre-term neonates in this study, administration of the IAP to mothers was ineffective in 4 cases (67%), 2 of which resulted in a newborn’s death. Comparable results were described by Goins et al. (2010), where 25% of neonates with EOD were born to women who received screening at 35 weeks of gestation or later and, when indicated, optimal chemoprophylaxis [14]. Our study is limited by the lack of information on which antibiotics were given to the women, and for how long before delivery mothers received antibiotic treatment. Without this information, as well as other details such as duration of rupture of membranes, it is difficult to interpret the fact that 4/6 infected babies were born to mothers who received intrapartum antibiotics.
Conclusions
In the Polish standards of care for pregnant women, even though the Polish Gynecological Society recommendations were introduced in 2008, there is still no evaluation of GBS carriage of gravidae, and no recommended antibiotic perinatal prophylaxis is used. In the light of American data, it seems that the introduction of routine exams for GBS carrier status and using proper antibiotic prophylaxis are the best protection against GBS-caused infections [4,5]. The data presented show that even the introduction of proper IAP does not exempt one from surveillance of EOD caused by GBS in VLBW neonates, and it suggests that alternative strategies such as vaccination are needed. Developing a new GBS conjugate vaccine for the prevention of neonatal disease has been listed as a top priority for vaccine development by the CDC [20]. The key importance of prophylaxis of GBS infections in Poland is that costs of these examinations should be covered for every pregnant woman by the National Health Fund. Appropriate cooperation between the obstetrician, the microbiological lab, and the neonatologist is crucial. Screening test performance needs to be regularly evaluated and monitored for improved compliance.
Acknowledgements
The authors would like to thank all of the collaborators of the Polish Perinatological Network for the execution of the network tasks.
Footnotes
Source of support: The study was partially supported by The Polish Perinatological Network (no. SN/080/08) and grant from the Polish Ministry of Research and Higher Education no. N N401 042337
References
- 1.Declercq E, Barger M, Cabral HJ, et al. Maternal outcomes associated with planned primary cesarean births compared with planned vaginal births. Obstet Gynecol. 2007;109:669–77. doi: 10.1097/01.AOG.0000255668.20639.40. [DOI] [PubMed] [Google Scholar]
- 2.Gaynes RP, Martone WJ, Culver DH, et al. Comparison of rates of nosocomial infections in neonatal intensive care units in the United States. National Nosocomial Infections Surveillance System. Am J Med. 1991;91:192–96. doi: 10.1016/0002-9343(91)90368-8. [DOI] [PubMed] [Google Scholar]
- 3.Iams JD, Romero R, Culhane JF, Goldenberg RL. Primary, secondary and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet. 2008;371:164–75. doi: 10.1016/S0140-6736(08)60108-7. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR. 2002;51 / RR-11:1–22. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Prevention of Perinatal Group B Streptococcal Disease Revised Guidelines from CDC. MMWR. 2010;59 / RR-10:1–32. [PubMed] [Google Scholar]
- 6.Serafin M, Prosniewska M, Kalinka J. Group B streptococci (GBS) prevalence in pregnant women in Lodz region: an obstetrical approach and neonatal complications. Arch Perinat Med. 2010;16:194–97. [Google Scholar]
- 7.Kowalska B, Niemiec KT, Drejewicz H, et al. Prevalence of group B streptococcal colonization in pregnant women and their newborns based on the results of examination of patients in the Obstetric and Gynecology Department of the National Research Institute of Mother and Child – a pilot study. Ginekol Pol. 2003;74:1223–27. [PubMed] [Google Scholar]
- 8.Brzychczy-Wloch M, Gosiewski T, Bodaszewska-Lubas M, et al. Molecular characterization of capsular polysaccharides and surface protein genes in relation to genetic similarity of group B streptococci isolated from Polish pregnant women. Epidemiol Infect. 2012;140(2):329–36. doi: 10.1017/S0950268811000616. [DOI] [PubMed] [Google Scholar]
- 9.Strus M, Pawlik D, Brzychczy-Wloch M, et al. Group B streptococcus colonization of pregnant women and their children observed on obstetric and neonatal wards of the University Hospital in Krakow, Poland. J Med Microbiol. 2009;58:228–33. doi: 10.1099/jmm.0.002865-0. [DOI] [PubMed] [Google Scholar]
- 10.Wessels MR, Kasper DL. Group B Streptococcus. In: Gorbach SL, Bartlett JG, Blacklow NR, editors. Infectious Diseases. 2nd ed. Philadelphia: WB Saunders Co; 1998. pp. 1731–35. [Google Scholar]
- 11.Schrag SJ, Zell ER, Lynfield R, et al. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N Engl J Med. 2002;25:233–39. doi: 10.1056/NEJMoa020205. [DOI] [PubMed] [Google Scholar]
- 12.Kotarski J, Heczko PB, Lauterbach R, et al. The Polish Gynecological Society recommendations for the detection of group B streptococcus (GBS) carriage in pregnant women and for prevention of neonatal infections caused by this pathogen. Ginekol Pol. 2008;79:221–23. [PubMed] [Google Scholar]
- 13.Van Dyke MK, Phares CR, Lynfield R, et al. Evaluation of universal antenatal screening for group B streptococcus. N Engl J Med. 2009;18:2626–36. doi: 10.1056/NEJMoa0806820. [DOI] [PubMed] [Google Scholar]
- 14.Veleminsky M, Hrubesova M, Hanzl M. The importance of antibiotic prophylaxis in GBS-positive parturient women. Med Sci Monit. 2009;15(7):CR372–74. [PubMed] [Google Scholar]
- 15.Goins WP, Talbot TR, Schaffner W, et al. Adherence to perinatal group B streptococcal prevention guidelines. Obstet Gynecol. 2010;115:1217–24. doi: 10.1097/AOG.0b013e3181dd916f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welch DF, Aldridge KE. Optimizing the rapid and accurate detection of group B streptococci from antepartum cultures. Infect Med. 2005;22:133–37. [Google Scholar]
- 17.Puopolo KM, Madoff LC, Eichenwald EC. Early-onset group B streptococcal disease in the era of maternal screening. Pediatr. 2005;115:1240–46. doi: 10.1542/peds.2004-2275. [DOI] [PubMed] [Google Scholar]
- 18.Kunze M, Ziegler A, Fluegge K, et al. Colonization, serotypes and transmission rates of group B streptococci in pregnant women and their infants born at a single University Center in Germany. J Perinat Med. 2011;39:417–22. doi: 10.1515/jpm.2011.037. [DOI] [PubMed] [Google Scholar]
- 19.Albouy-Llaty M, Nadeau C, Descombes E, et al. Improving perinatal Group B streptococcus screening with process indicators. J Eval Clin Pract. 2012;18(4):727–33. doi: 10.1111/j.1365-2753.2011.01658.x. [DOI] [PubMed] [Google Scholar]
- 20.Johri AK, Paoletti LC, Glaser P, et al. Group B Streptococcus: global incidence and vaccine development. Nature Reviews Microbiology. 2006;4:932–42. doi: 10.1038/nrmicro1552. [DOI] [PMC free article] [PubMed] [Google Scholar]