Abstract
Purpose
This study determined how the magnitude of change in positive subjective responses predicts clinical outcome in a treatment setting. Specifically, we attempted to define what constitutes a clinically important difference (CID) in subjective responses.
Methods
A 100-mm visual analog scale (VAS) measured subjective ratings of drug “high,” calculated via an anchor-based method with published data from participants receiving sustained-release naltrexone (NTX) and heroin in a laboratory setting. The data were then compared to clinical outcomes in a treatment trial with sustained-release naltrexone. A distribution-based method subsequently analyzed data from participants who received ALO-01 (extended-release morphine with sequestered NTX) to predict its abuse liability.
Results
Differences in ratings of drug high of approximately 10 mm on a 100-mm line were clinically significant. By extrapolation, CIDs were also found between crushed or intact ALO-01 and immediate-release morphine sulfate (IRMS). No CIDs were found between intact and crushed ALO-01.
Conclusions
From laboratory and treatment trial data involving naltrexone, calculation of CIDs in subjective ratings of high is possible. Consequently, crushing/swallowing or injecting ALO-01 produces clinically significantly less drug high than oral or intravenous morphine alone, suggesting that ALO-01 has lower abuse liability by those routes than morphine formulations.
Keywords: Analgesics, Opioid, Drug formulations, Morphine, Drug high, Abuse liability
Introduction
In light of widespread prescription-drug abuse, US Food and Drug Administration (FDA) has requested that pharmaceutical companies developing new products containing controlled substances quantify the products’ potential for abuse in humans. Abuse liability studies typically compare the drug’s desirability (drug liking) and positive psychotropic effects (euphoria, or drug high, a well-known motivator for abuse) to that of a comparator with known abuse liability (e.g., heroin). Drug liking and drug high are self-reported subjective responses usually measured quantitatively. Differences in responses between the test drug and comparator estimate the drug’s relative abuse liability. Although differences in these subjective responses may be statistically significant, their clinical significance is also important. Consequently, guidelines [1, 2] have been developed to determine the clinically important difference (CID) of specific subject-reported measures. A family of measures has been used to assess drug liking and drug high, although most of them differ in potentially important ways. Common examples include the Drug Effects Questionnaire, the Addiction Research Center Inventory, and a variety of unipolar and bipolar visual analogue scales (VAS), among others. No measure is considered standard. To our knowledge, there have been no previous attempts to define a CID of any drug liking or drug high measure.
CIDs can be calculated with anchor-based [3–5] or distribution-based methods. An anchor, generally an objective measure (e.g., laboratory value, clinical diagnosis), has some relationship with a more subjective measure (e.g., quality of life, VAS score) and evaluates the responsiveness and importance of changes in the subjective measure [3, 6]. Distribution-based methods involve statistical calculations of CID and have been assessed in various therapeutic areas [6], including subjective measures of pain [7]. Because CIDs of abuse liability outcome measures have not yet been evaluated, one goal of this study was to determine CIDs for the subjective ratings of drug high in opioid abuse liability studies.
Abusers often tamper with (chew, crush, dissolve) extended-release (ER) opioid formulations to rapidly deliver high concentrations of opioids. To minimize risk of opioid abuse, manufacturers develop ER formulations that resist tampering. ALO-01, a novel oral capsule formulation of morphine, contains multiple pellets; each pellet contains morphine surrounded by an ER membrane and a core of naltrexone (NTX), an opioid antagonist [8, 9]. Under normal use, morphine is slowly released to control pain for 12–24 h, while NTX remains sequestered in the core. If the formulation is chewed or crushed, NTX is released from the core, reducing morphine’s euphoric effects. Our second goal was to determine whether statistically significant reductions in drug high produced between morphine and ALO-01, and between altered and intact ALO-01, are clinically important, i.e., meet our estimated CID criteria for drug high in opioid liability studies.
Methods
We used anchor- and distribution-based methods to determine CIDs in drug high for opioid products and thereby predict a product’s abuse liability post-marketing.
Dataset for CID calculation
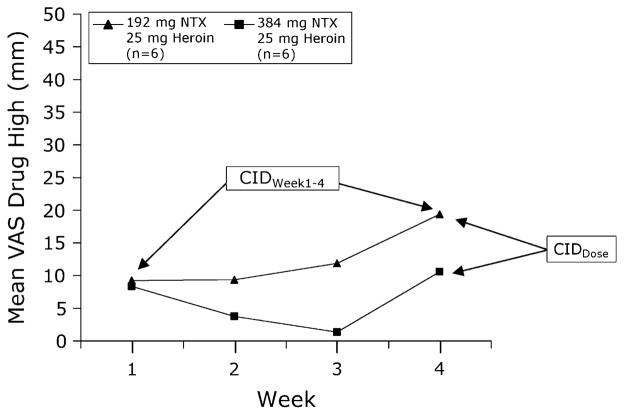
Data from Comer [10] were used to establish CIDs in subjective ratings of drug high (Table 1). Twelve heroin-dependent men received a single dose of 192 mg (n = 6) or 384 mg (n = 6) sustained-release depot NTX (Depotrex®). All participants then received 0, 6.25, 12.5, 18.75, and 25 mg intravenous (IV) heroin. The entire heroin dose range was tested each week (1 dose tested each day). Subjective effects of heroin were evaluated using a VAS for “I feel … high” (0 mm: “Not at all,” to 100 mm: “Extremely”). Ratings of “feeling high” were completely suppressed 1 week after injection of NTX 192 mg and then re-emerged 4 weeks later (Fig. 1).
Table 1.
Datasets and studies used in this investigation
| Study | Subjects | Study design | Measure | Role in this study |
|---|---|---|---|---|
| Datasets analyzed | ||||
| ALO-01-07-106 | 27 opioid-experienced, non-dependent men | Crossover study: MS 30 mg IV; MS 30 mg + NTX 1.2 mg IV; placebo IV | VAS Drug High score 0–100 mm | Drug high dataset analyzed to determine CID Dataset used in distribution-based method |
| ALO-01-07-205 | 32 opioid-experienced, non-dependent adults | Crossover study: IRMS 120 mg PO; crushed ALO-01 PO; intact ALO-01 PO; placebo PO | VAS Drug High score 0–100 mm | Drug high dataset analyzed to determine CID Dataset used in distribution- based method |
| Comer et al. [10] | 12 heroin-dependent men | Single dose of sustained-release depot NTX 192 mg or 384 mg followed by heroin 0, 6.25, 12.5, 18.75, and 25 mg IV once per week for 6 weeks | VAS Drug High score 0–100 mm with 25 mg heroin | Determination of CIDDose and CIDWeek1–4 Dataset used in anchor-based approach |
| Studies used to provide anchors for determining CID | ||||
| Comer et al. [11] | 60 heroin-dependent adults | Sustained-release depot NTX 192 mg or 384 mg at Week 1 and Week 5 | Retention rates | Evaluation of retention rate as anchor for Comer et al. [10] drug high CIDDose |
| Sullivan et al. [12] | 5 heroin-dependent adults | Sustained-release depot NTX 384 mg at Week 1 plus doses of 0, 6.25, 12.5, 18.75, and 25 mg heroin IV for 6 weeks; each week, subjects received 1 of 4 heroin doses or placebo on any given day | Heroin self- administration (mean heroin break point) | Evaluation of heroin self- administration rates at Week 4 versus Week 1 as anchor for Comer et al. [10] drug high CIDWeek1–4 |
CID clinically important difference, NTX naltrexone, VAS visual analog scale, MS morphine sulphate, IV intravenous, PO by mouth, IRMS immediate-release morphine sulphate
Fig. 1.
Subjective ratings. Ratings of drug high with heroin 25 mg as a function of week after administration of 192 mg (triangle) and 384 mg (square) depot NTX. Data points represent mean peak ratings (n = 6 per group). Data from Comer et al. [10]. NTX naltrexone, CID clinically important difference, VAS visual analog scale
Differences in drug high over time (Weeks 1–4) and between NTX doses (192 mg vs. 384 mg) in subjects receiving heroin 25 mg were statistically significant (Fig. 1); we evaluated whether these statistically significant differences over time and in doses were CIDs (CIDWeek1–4 and CIDDose, respectively) using anchor- or distribution-based methods (described below).
Anchor-based methods
Two objective measures (break point and retention rate) served as anchors for subjective measure of drug high (Table 1).
Break point
Data from Sullivan [12] were used to anchor ratings of drug high with break point (heroin dose at which the abuser prefers getting heroin over money, in this case, $20). Five heroin-dependent adults received a single 384 mg dose of sustained-release depot NTX (Depotrex®), and the effects of various heroin doses (0, 6.25, 12.5, and 25 mg) were evaluated for 6 weeks. The entire dose range was tested each week (1 dose tested each day). A statistically significant difference in break point occurred between Weeks 1 and 4 (Fig. 2); therefore, break point was used as anchor for the CIDWeek1–4 in Comer [10]. The break point anchor was further justified by a meta-analysis of NTX studies [13].
Fig. 2.
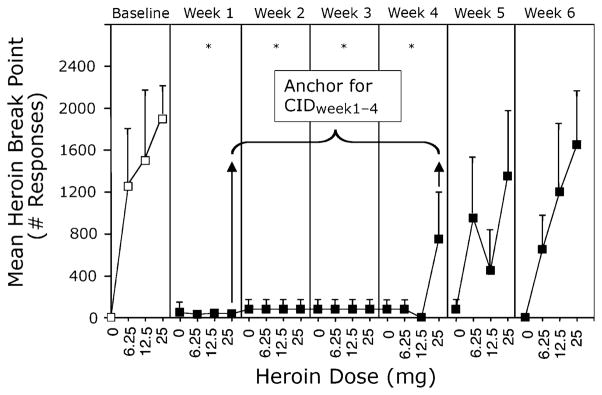
Self-administration of heroin. Self-administration of IV heroin as a function of heroin dose (0–25 mg) and week at baseline (white squares) and following 384 mg depot NTX (black squares). Asterisks indicate a significant difference in mean heroin break point values across heroin dose conditions for a given week versus baseline. The data points for heroin self-administration behavior between Week 4 and Week 1 labeled as “Anchor for CIDWeek1–4” indicates that these observations provide clinically relevant justification (anchor) for the parameters chosen in the Comer et al. [10] study. With kind permission from Springer Science + Business Media: Psychopharmacology, Sullivan et al. [12] © Springer-Verlag 2006
Retention rate
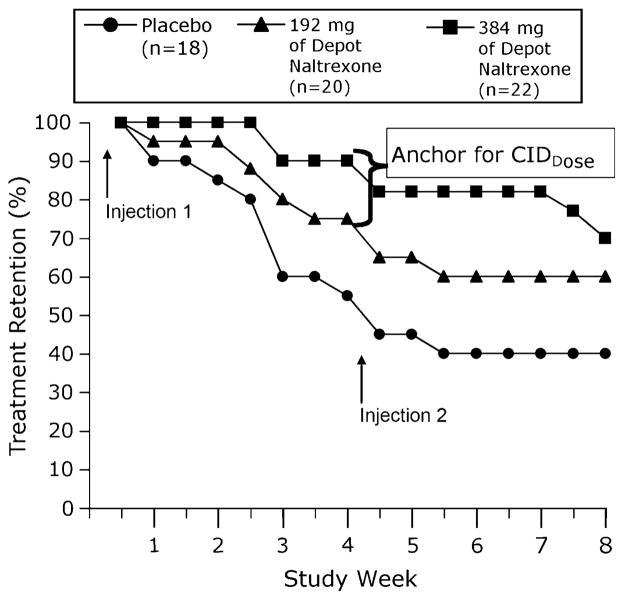
Data from Comer [11] were used to anchor ratings of drug high with the rates of retention in treatment. Sixty heroin-dependent adults were randomized to receive placebo, or 192 mg or 384 mg of sustained-release depot NTX (Depotrex ®) at the beginning of Weeks 1 and 5. The retention rate (percent of randomized subjects still present in the study) was used to evaluate NTX effectiveness. A statistically significant difference in retention rate was observed between 192 and 384 mg doses (Fig. 3); therefore, retention rate was used as anchor for the CIDDose in Comer [10]. The 95% confidence interval (CI) of the CID was calculated for both anchor-based methods.
Fig. 3.
Treatment retention. Percentage of patients retained in treatment across 8 weeks for the 3 treatment conditions (placebo, 192 mg NTX, and 384 mg NTX). Retention rates were measured twice a week. The data points labeled with the bracket indicate a clinically meaningful difference that provides justification (anchor) for the parameters chosen in the Comer et al. [10] study. Reprinted with permission from Comer et al. [11]
Distributional methods
Studies ALO-01-07-205 and ALO-01-07-106 were used in the distribution-based analysis of CID (Table 1).
ALO-01-07-205 (www.clinicalTrial.gov; No. NCT0075-1478). Thirty-two healthy, opioid-experienced, non-dependent adults were enrolled in a randomized, double-blind, crossover study with oral administration of immediate-release morphine sulfate (IRMS), crushed ALO-01, intact ALO-01, or placebo. Measurements of drug high (100-mm VAS) were used for CID evaluations.
ALO-01-07-106. Twenty-nine opioid-experienced, nondependent men were enrolled in a randomized, double-blind, crossover study with IV administration of morphine sulphate (MS) alone, MS + NTX, or placebo. The Drug Effects Questionnaire (DEQ) question, “How high are you now?” (on a 100-mm VAS) was the primary pharmacodynamic endpoint for CID evaluations.
Distributional approaches to clinical significance involve quantifying changes in the distribution of measure (e.g., drug high) upon intervention (e.g., administration of NTX). Usually, changes ≥ 0.5 standard deviation (SD) [14] or 1 standard error of the mean (SEM) [15] between 2 interventions are considered clinically meaningful. We used both pooled SD [14] and pooled SEM approaches [15].
The pooled SD (Sp) for various conditions in ALO-01 studies used the formula: , where n is sample size and s is variance. One-half of the pooled SD (CID½ Sp) was then compared with the means for the pairwise comparisons of interest.
The pooled SEM method was adapted from Wyrwich [15] using the formula: , extended to compare several conditions. The process described above was used to calculate differences between group means using the pooled SEM to obtain CIDSEM.
Analysis of ALO-01 clinical data
To determine whether the decreases in drug high scores observed with the NTX interventions in the 2 ALO-01 studies were clinically important, we compared the differences in VAS Drug High scores between the groups to the anchor-based calculated CIDs (CIDWeek1–4 and CIDDose) and the distribution-based calculated CIDs (CIDSEM and CID½ Sp) at Emax (maximum effect) for each condition. We also compared the differences between groups to the upper 95% CI for anchor-based CIDs and to the lower 95% CI for distributional CIDs.
Results
CID calculations
Anchor-based calculations from Comer [10] yielded similar CIDs (overlapping CIs): CIDWeek1–4 was 10.17 mm (95% CI 3.00, 17.33; N = 5), and CIDDose was 8.83 mm (95% CI 1.14, 16.53; N = 60) (Table 2).
Table 2.
CID values in VAS Drug High score [95% CI] calculated using anchor- and distribution-based methods
| Anchor-based methods
|
Distribution-based methods
|
||||
|---|---|---|---|---|---|
| Week 1 versus Week 4 (CIDWeek1–4) | 384 versus 192 mg NTX (CIDDose) | PO CID½ Sp | IV CID½ Sp | PO CIDSEM | IV CIDSEM |
| 10.17 mm (3.00, 17.33) | 8.83 mm (1.14, 16.53) | 13.46 mm | 8.54 mm | 9.52 mm | 5.73 mm |
| N = 5 | N = 60 | ||||
PO CID: CID calculated with data using oral administration (ALO-01-07-205)
IV CID: CID calculated with data using IV administration (ALO-01-07-106)
CI confidence interval, CID clinically important difference, NTX naltrexone, ½Sp one-half of the pooled standard deviation, SEM standard error of the mean, VAS visual analog scale, PO by mouth, IV intravenous
Distributional methods using oral data also yielded similar CIDs (9.52 mm and 13.46 mm for SEM and Sp methods, respectively) (Table 2). Distributional CIDs with IV data were somewhat smaller (SEM, 5.73 mm; Sp, 8.54 mm).
Clinical importance of changes in drug high in ALO-01 studies
ALO-01-07-106 (IV)
The difference in mean VAS Drug High scores between placebo and MS (85.20 mm), between placebo and MS + NTX (29.80 mm), and between MS and MS + NTX (55.40 mm) (Table 3) were all higher than the mean anchor-based CIDs (10.17 mm [CIDWeek1–4] and 8.83 mm [CIDDose]; Table 2), indicating these differences are probably clinically important. These differences were also greater than the upper 95% confidence limits of both mean anchor-based CIDs, the most conservative limit.
Table 3.
Summary of difference in VAS Drug High score in studies ALO-01-07-205 and ALO-01-07-106
| Group comparisons | Mean difference in VAS Drug High score, mm* | Lower 95% CI | Upper 95% CI |
|---|---|---|---|
| Study ALO-01-07-205 (oral dosing) | |||
| Placebo versus IRMS | 75.2 | 63.62 | 86.78 |
| Placebo versus intact ALO-01 | 45.7 | 29.24 | 62.16 |
| Placebo versus crushed ALO-01 | 39.8 | 21.98 | 57.62 |
| Crushed versus intact ALO-01 | 5.6 | −3.54 | 24.74 |
| IRMS versus intact ALO-01 | 29.8 | 16.27 | 43.33 |
| IRMS versus crushed ALO-01 | 35.4 | 20.24 | 50.56 |
| Study ALO-01-07-106 (IV dosing) | |||
| Placebo versus MS | 85.20 | 79.33 | 91.07 |
| Placebo versus MS + NTX | 29.80 | 17.73 | 41.87 |
| MS versus MS + NTX | 55.40 | 41.98 | 68.82 |
VAS Drug High at Emax were the following: 29.8 mm for MS + NTX; 85.2 mm for MS alone; and 0.0 for placebo
ALO-01 extended-release morphine with sequestered naltrexone, CI confidence interval, IRMS immediate-release MS, MS morphine sulfate, NTX naltrexone, VAS visual analog scale
The differences in mean VAS Drug High scores between groups (Table 3) were also greater than the CIDs calculated by distributional methods (8.54 and 5.73 mm; Table 2). The lower 95% CI limits of these mean differences (range = 17.73–79.33 mm) were all greater than the IV CIDs calculated by distributional methods (5.73 and 8.54 mm).
ALO-01-07-205 (PO)
Except for crushed versus intact ALO-01, all the mean differences in VAS Drug High scores (Table 3) were greater than both anchor- and distribution-based CIDs (Table 2). All pairwise differences (except intact vs. crushed ALO-01) were greater than the upper 95% confidence limit of the anchor-based CIDs. Further, all lower 95% confidence limits for the mean between-group comparisons (Table 3) were greater than the distribution-based CIDs (Table 2).
Discussion
This study aimed to determine whether statistically significant reductions in drug high produced by tampered ALO-01 versus comparators were clinically important. We first determined CIDs in drug high—the difference in VAS Drug High score that predicts the product’s abuse liability post-marketing. Such novel CID analyses for drug high in abuse liability studies help provide a criterion for determining a product’s clinically significant abuse liability.
We measured CID using anchor- and distribution-based approaches. Anchor-based CIDs ranged from 8.83 to 10.17 mm; distribution-based estimates from oral dosing were 9.52 and 13.46 mm (100-mm VAS). The results’ similarity suggests that the distribution-based approach supports the findings from the anchor-based approach. Distributional CIDs with IV data were smaller (5.73 and 8.54 mm) because data from IV studies are less variable, leading to a smaller difference that can be considered clinically meaningful. Although anchor-based methods are generally preferred [6, 16], distributional CID estimates are recommended when anchor-based estimates are unavailable. We considered, but did not use, 2 other studies [9, 17] evaluating the abuse liability of NTX + morphine. One study did not measure drug high [17]; the other used multidimensional measures of drug high.
CIDs between IRMS and crushed or intact ALO-01 (Table 4) demonstrated that euphoria produced by crushed ALO-01 is clinically significantly less than euphoria produced by IRMS. Furthermore, because no CID existed between intact and crushed ALO-01 (Table 4), abusers may not be interested in tampering with ALO-01. The results also suggest that ALO-01 has an IV abuse liability closer to placebo than to morphine.
Table 4.
Summary of CID calculations
| Comparisons | Meets CID criteria?*
|
|
|---|---|---|
| Anchor CID | Distribution CID | |
| Study ALO-01-07-205 (oral dosing) | ||
| Placebo versus IRMS | Yes | Yes |
| Placebo versus intact ALO-01 | Yes | Yes |
| Placebo versus crushed ALO-01 | Yes | Yes |
| Crushed versus intact ALO-01 | No | No |
| IRMS versus intact ALO-01 | Yes | Yes |
| IRMS versus crushed ALO-01 | Yes | Yes |
| Study ALO-01-07-106 (IV dosing) | ||
| Placebo versus MS | Yes | Yes |
| Placebo versus MS + NTX | Yes | Yes |
| MS versus MS + NTX | Yes | Yes |
See Table 3 for data
ALO-01 extended-release morphine with sequestered naltrexone, CID clinically important difference, IRMS immediate-release MS, MS morphine sulfate, NTX naltrexone
The time to drug-induced euphoria (Table 5) dictates a drug’s potential for abuse: Drugs with shorter time to euphoria have higher abuse potential [16]. Crushed ALO- 01 had a longer time to euphoria (3.03 h) than IRMS (1.69 h), suggesting that crushed/chewed ALO-01 has a lower abuse potential than IRMS. Moreover, subjective effects produced by crushed ALO-01 (Emax = 3.03 h) are delayed when compared with the peak of morphine (Tmax = 1.1 h), suggesting that NTX released from crushed ALO-01 attenuates morphine’s euphoric effects.
Table 5.
VAS Drug High, Cmax, and Tmax in study ALO-01-07-205 (oral dosing)
| Placebo | Intact ALO-01 120 mg | Crushed ALO-01 120 mg | IRMS 120 mg | |
|---|---|---|---|---|
| VAS Drug High Emax, mm | ||||
| Mean (SD) | 15.2 (25.36) | 60.6 (30.43) | 55.0 (34.59) | 90.4 (11.60) |
| Median | 1.0 | 68.5 | 64.0 | 97.0 |
| Range | 0–74 | 0–100 | 0–100 | 61–100 |
| Time to VAS Drug High Emax, h | 1.48 | 6.41 | 3.03 | 1.69 |
| Cmax, mean (SD) (pg/ml) | – | 19,256 (7,683) | 80,588 (38,805) | 92,516 (38,051) |
| Tmax, median (h) | – | 8.1 | 1.1 | 1.2 |
The calculated differences between groups are provided in Table 3
ALO-01 extended-release morphine with sequestered naltrexone, Cmax plasma concentration to maximum subjective rating, Emax maximum subjective rating, IRMS immediate-release morphine sulfate, SD standard deviation, Tmax time to maximum subjective rating, and VAS visual analog scale definitive evidence of the abuse deterrence of ALO-01 in the community has not yet been determined.
Several considerations arise from this study. First, anchor-based CIDs were calculated from 2 studies using small sample sizes. Thus, CIDs from opioid studies with larger samples, other classes of abused drugs, or other subjective measures are recommended, to validate the present study. Second, the different populations for the anchor studies (heroin-dependent) and ALO-01 studies (non-dependent) may bias CID calculation. Third, injecting morphine plus NTX (as in the ALO-01 IV study) may not exactly mimic the crushing and IV administration of ALO-01.
The 8–10 mm differences in VAS Drug High are clinically important. Using this CID criterion, we found that intact or crushed ALO-01 has significantly lower oral and IV abuse liability than IR morphine formulations. However, definitive evidence of the abuse deterrence of ALO-01 in the community has not yet been determined.
Acknowledgments
The authors thank Kevin Flynn from Analgesic Solutions and Florence Paillard, PhD from Focus BioCom for editorial support. Funding for this project was provided by Alpharma Pharmaceuticals, LLC, a wholly owned subsidiary of King Pharmaceuticals, Inc. No author received compensation for writing this manuscript.
Abbreviations
- CI
Confidence interval
- CID
Clinically important difference
- DEQ
Drug effects questionnaire
- ER
Extended-release
- H
hours
- FDA
Food and Drug Administration
- IRMS
Immediate-release morphine sulfate
- IV
Intravenous
- MS
Morphine sulfate
- NTX
Naltrexone
- Sp
Pooled standard deviation
- SD
Standard deviation
- SEM
Standard error of the mean
- VAS
Visual analog scale
Contributor Information
Thomas A. Eaton, Analgesic Solutions, Inc, 232 Pond St, Natick, MA 01760, USA. Department of Psychology, Adjunct Faculty, University of Connecticut, Storrs, CT, USA
Sandra D. Comer, Department of Psychiatry, Columbia University, New York State Psychiatric Institute, New York, NY, USA
Dennis A. Revicki, Center for Health Outcomes Research, United BioSource Corporation, Bethesda, MD, USA
Jeremiah J. Trudeau, Email: jtrudeau@analgesicsolutions.com, Analgesic Solutions, Inc, 232 Pond St, Natick, MA 01760, USA. Hampshire College, School of Cognitive Science, Amherst, MA, USA
Richard G. van Inwegen, Analgesic Solutions, Inc, 232 Pond St, Natick, MA 01760, USA
Joseph W. Stauffer, Alpharma Pharmaceuticals, Piscataway, NJ, USA
Nathaniel P. Katz, Analgesic Solutions, Inc, 232 Pond St, Natick, MA 01760, USA. Program on Opioid Risk Management, Department of Anesthesiology, Tufts University School of Medicine, Boston, MA, USA
References
- 1.Kweder SL. Congressional testimony before the Subcommittee on Criminal Justice, Drug Policy and Human Resources. Food and Drug Administration; 2006. [Google Scholar]
- 2.Department of Health and Human Services. [Accessed 25 Aug 2011];Guidance for industry: Patient-reported outcome measures: Use in medical product development to support labeling claims: draft guidance. 2009 doi: 10.1186/1477-7525-4-79. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf. [DOI] [PMC free article] [PubMed]
- 3.Norman GR, Sridhar FG, Guyatt GH, Walter SD. Relation of distribution- and anchor-based approaches in interpretation of changes in health-related quality of life. Medical Care. 2001;39:1039–1047. doi: 10.1097/00005650-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR. Methods to explain the clinical significance of health status measures. Mayo Clinic Proceedings. 2002;77:371–383. doi: 10.4065/77.4.371. [DOI] [PubMed] [Google Scholar]
- 5.Wyrwich KW, Bullinger M, Aaronson N, et al. Estimating clinically significant differences in quality of life outcomes. Quality of Life Research. 2005;14:285–295. doi: 10.1007/s11136-004-0705-2. [DOI] [PubMed] [Google Scholar]
- 6.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. Journal of Clinical Epidemiology. 2008;61:102–109. doi: 10.1016/j.jclinepi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL. Defining the clinically important difference in pain outcome measures. Pain. 2000;88:287–294. doi: 10.1016/S0304-3959(00)00339-0. [DOI] [PubMed] [Google Scholar]
- 8.Jones JB, Sokolowska M, Setnik B, Romach M, Johnson F, Stauffer J, et al. ALO-01, an investigational extended-release opioid formulation containing morphine sulfate and sequestered naltrexone: pharmacodynamic (drug liking) effects. Presented at 24th Annual meeting of the American Academy of Pain Medicine; Orlando, FL. 2008. [Google Scholar]
- 9.Webster L, Jones JB, Johnson F, Sekora D, Stauffer J. Relative drug-liking/euphoria effects of intravenous morphine alone and in combination with naltrexone in recreational opioid users. Presented at 12th World Congress on Pain; Glasgow, Scotland. 2008. [Google Scholar]
- 10.Comer SD, Collins ED, Kleber HD, et al. Depot naltrexone: Long-lasting antagonism of the effects of heroin in humans. Psychopharmacology. 2002;159:351–360. doi: 10.1007/s002130100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comer SD, Sullivan MA, Yu E, et al. Injectable, sustained-release naltrexone for the treatment of opioid dependence: A randomized, placebo-controlled trial. Archives of General Psychiatry. 2006;63:210–218. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan MA, Vosburg SK, Comer SD. Depot naltrexone: Antagonism of the reinforcing, subjective, and physiological effects of heroin. Psychopharmacology. 2006;189:37–46. doi: 10.1007/s00213-006-0509-x. [DOI] [PubMed] [Google Scholar]
- 13.Johansson BA, Berglund M, Lindgren A. Efficacy of maintenance treatment with methadone for opioid dependence: A meta-analytical study. Nordic Journal of Psychiatry. 2007;61:288–295. doi: 10.1080/08039480701415251. [DOI] [PubMed] [Google Scholar]
- 14.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Medical Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 15.Wyrwich KW, Tierney WM, Wolinsky FD. Using the standard error of measurement to identify important changes on the asthma quality of life questionnaire. Quality of Life Research. 2002;11:1–7. doi: 10.1023/a:1014485627744. [DOI] [PubMed] [Google Scholar]
- 16.McColl S, Sellers EM. Research design strategies to evaluate the impact of formulations on abuse liability. Drug and Alcohol Dependence. 2006;83(Suppl 1):S52–S62. doi: 10.1016/j.drugalcdep.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Stauffer J, Setnik B, Sokolowska M, Romach M, Johnson F, Sellers E. Subjective effects and safety of whole and tampered morphine sulfate and naltrexone hydrochloride (ALO-01) extended-release capsules versus morphine solution and placebo in experienced non-dependent opioid users: A randomized, double-blind, placebo-controlled, crossover study. Clinical Drug Investigation. 2009;29:777–790. doi: 10.2165/11530800-000000000-00000. [DOI] [PubMed] [Google Scholar]