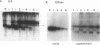Abstract
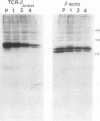
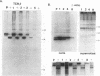
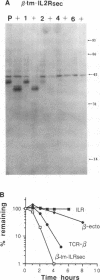
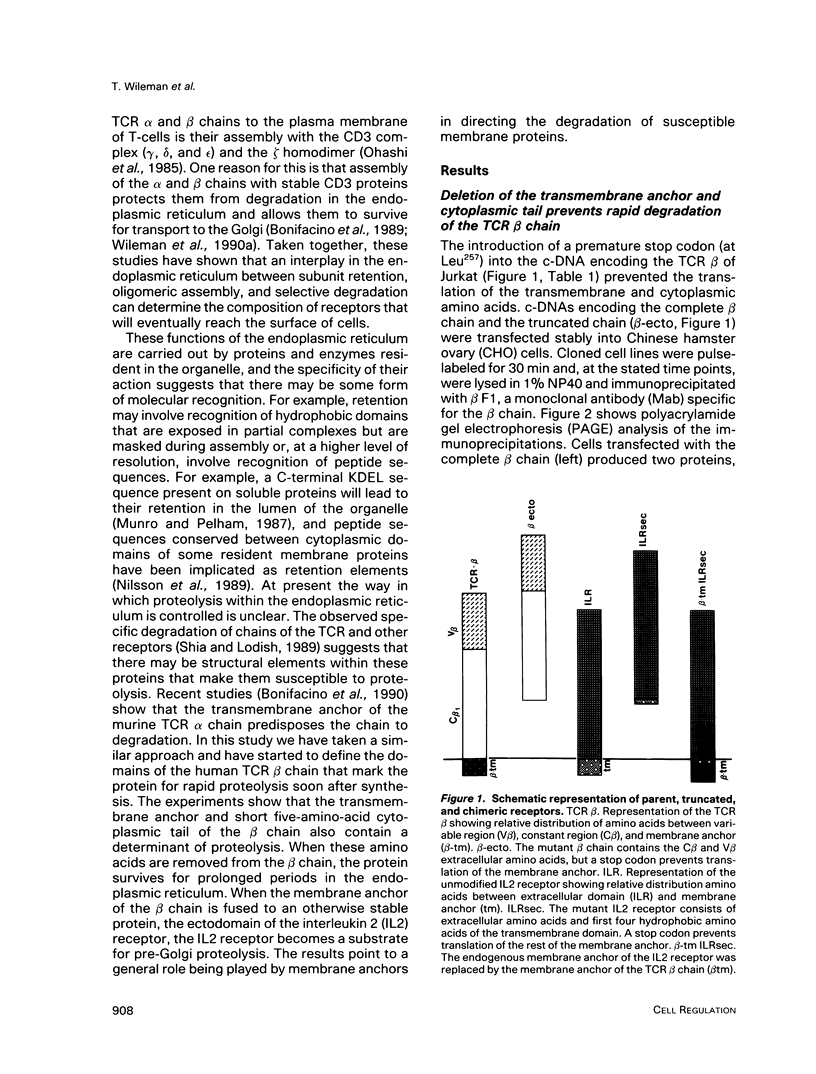
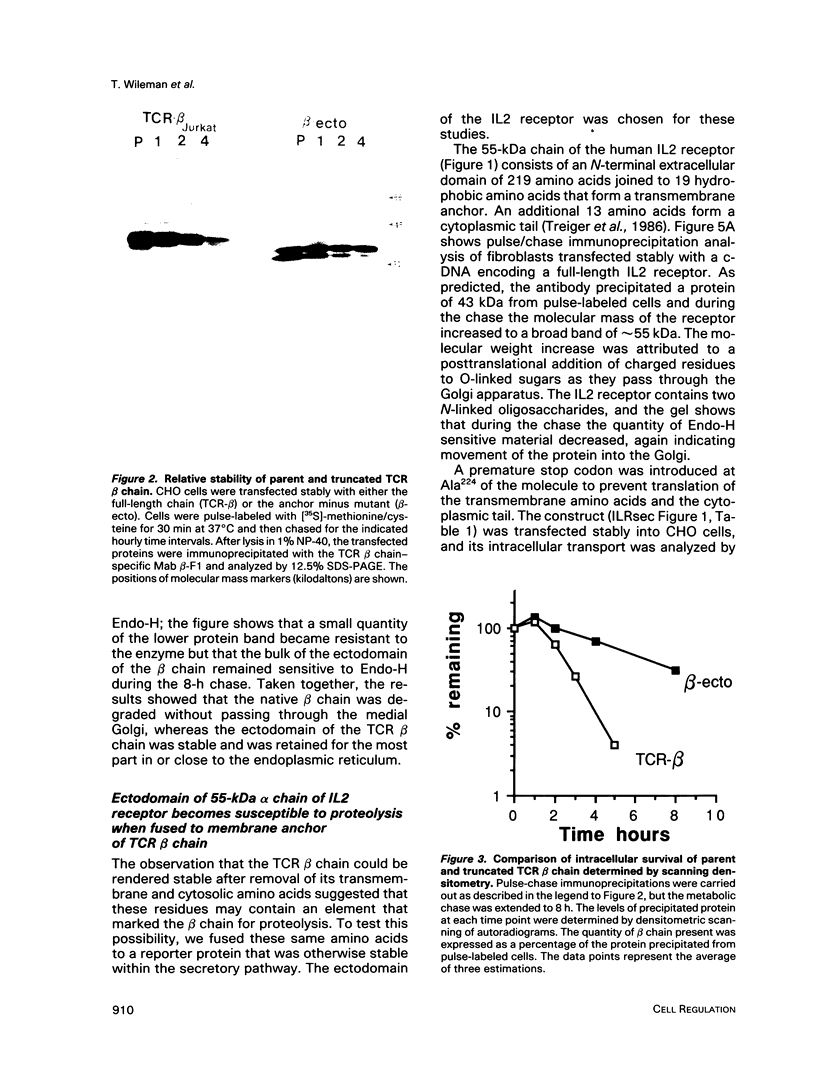
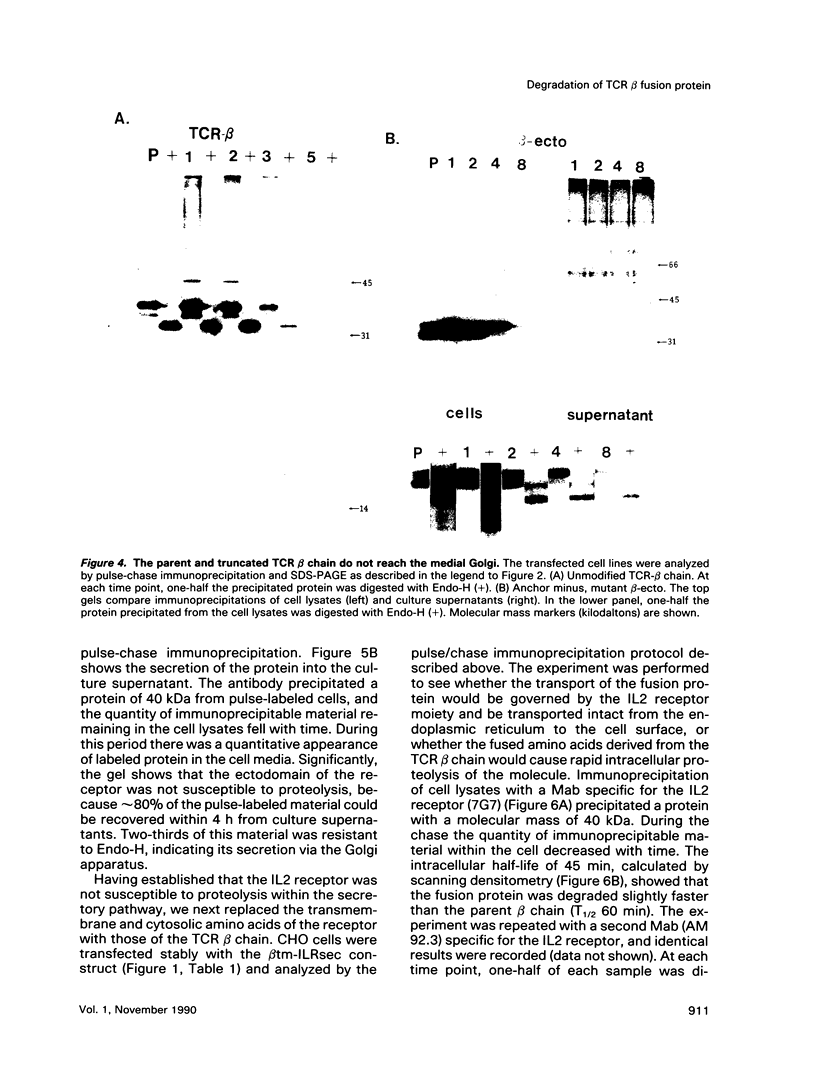
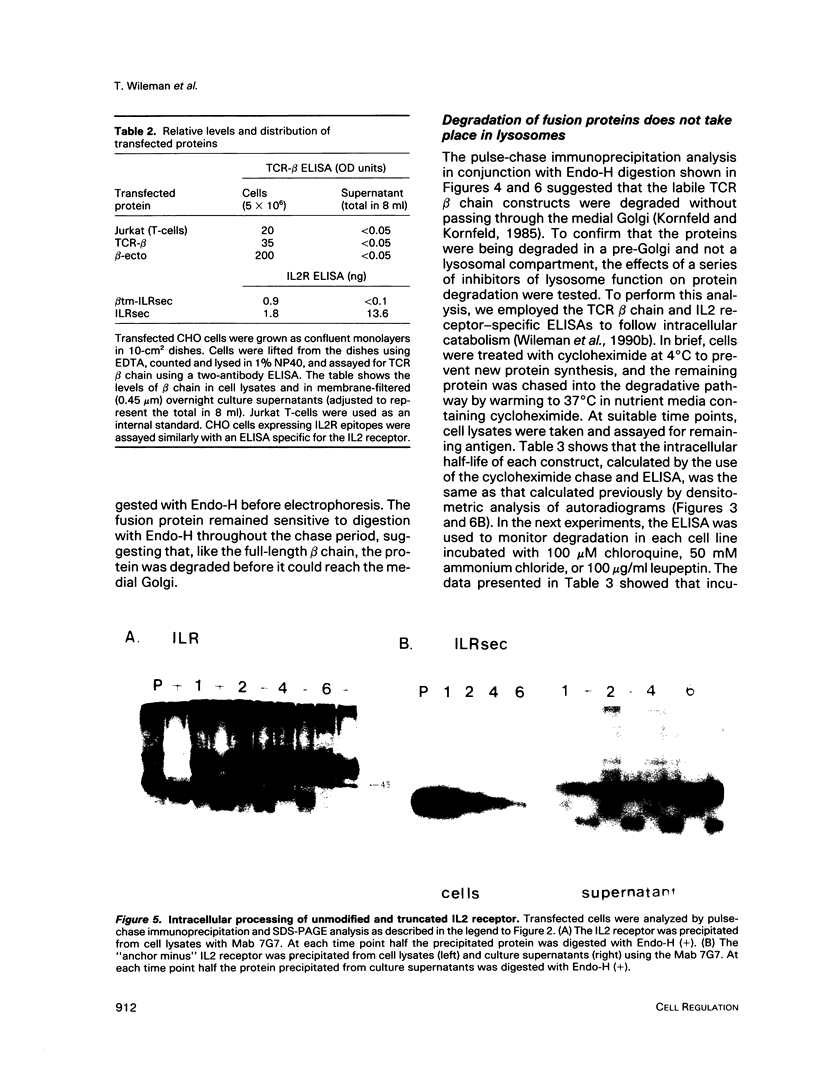
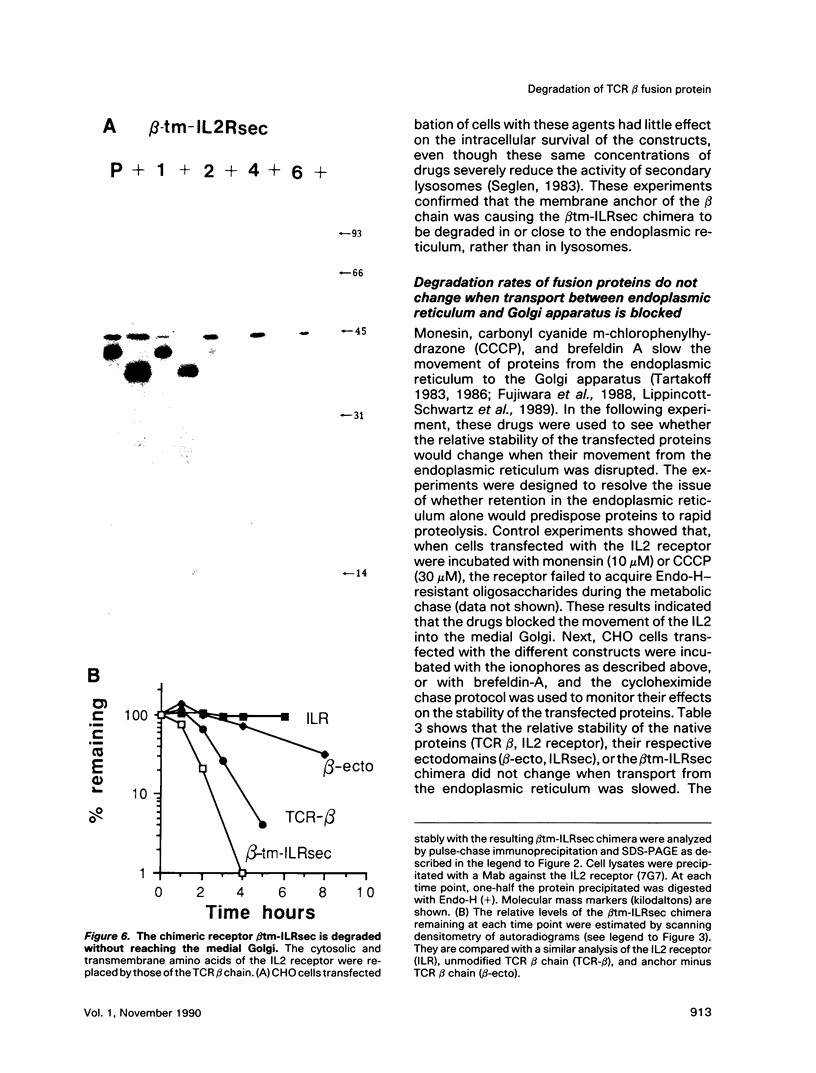
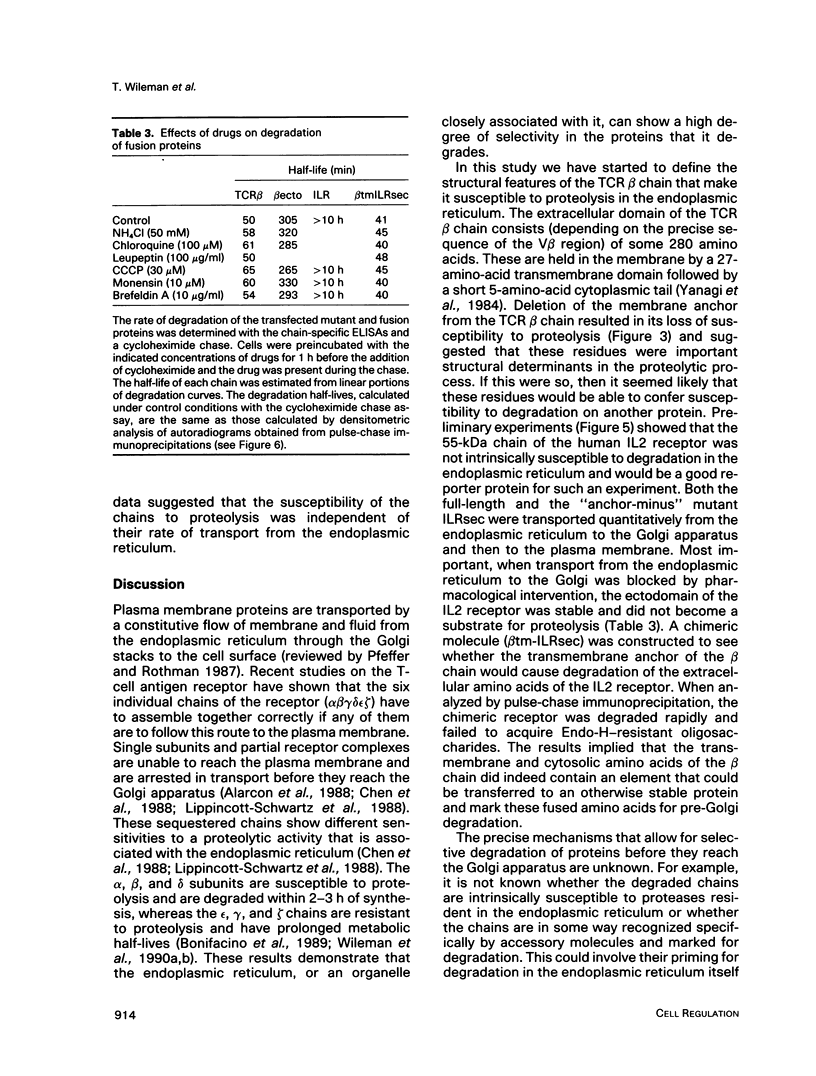
Studies with the T-cell antigen receptor (TCR) have shown that the endoplasmic reticulum, or an organelle closely associated with it, can retain and degrade membrane proteins selectively. The observation that only three (alpha, beta, and delta) of the six (alpha beta gamma delta epsilon zeta) subunits of the TCR are susceptible to proteolysis implies that structural features within the labile proteins mark them for degradation. The TCR beta chain is degraded in the endoplasmic reticulum, and, in this study, we have started to define the domains of the protein that make it susceptible to proteolysis. The experiments show that the transmembrane anchor and short five-amino-acid cytoplasmic tail of the protein contain a dominant determinant of proteolysis. When these residues were removed from the beta chain, the protein became resistant to proteolysis. Even though the resulting ectodomain of the beta chain lacked a transmembrane anchor, it was not secreted by cells and was retained in the endoplasmic reticulum. We conclude that retention in the endoplasmic reticulum alone does not lead to degradation. The results suggest that structural features within the membrane anchor of the protein predispose the beta chain to proteolysis. This was confirmed by replacing the membrane anchor of the interleukin 2 (IL2) receptor, a protein that was stable within the secretory pathway, with that of the TCR beta chain. The unmodified IL2 receptor was transported efficiently to the surface of cells, and an "anchor minus" construct was secreted quantitatively into the culture media. When the membrane anchor of the IL2 receptor was replaced with that of the TCR beta chain, the chimera was unable to reach the Golgi apparatus and was degraded rapidly.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcon B., Berkhout B., Breitmeyer J., Terhorst C. Assembly of the human T cell receptor-CD3 complex takes place in the endoplasmic reticulum and involves intermediary complexes between the CD3-gamma.delta.epsilon core and single T cell receptor alpha or beta chains. J Biol Chem. 1988 Feb 25;263(6):2953–2961. [PubMed] [Google Scholar]
- Amara J. F., Lederkremer G., Lodish H. F. Intracellular degradation of unassembled asialoglycoprotein receptor subunits: a pre-Golgi, nonlysosomal endoproteolytic cleavage. J Cell Biol. 1989 Dec;109(6 Pt 2):3315–3324. doi: 10.1083/jcb.109.6.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger M. J., Lane M. D. Assembly of very low density lipoprotein in the hepatocyte. Differential transport of apoproteins through the secretory pathway. J Biol Chem. 1988 Aug 25;263(24):11868–11878. [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J. S., Suzuki C. K., Klausner R. D. A peptide sequence confers retention and rapid degradation in the endoplasmic reticulum. Science. 1990 Jan 5;247(4938):79–82. doi: 10.1126/science.2294595. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Suzuki C. K., Lippincott-Schwartz J., Weissman A. M., Klausner R. D. Pre-Golgi degradation of newly synthesized T-cell antigen receptor chains: intrinsic sensitivity and the role of subunit assembly. J Cell Biol. 1989 Jul;109(1):73–83. doi: 10.1083/jcb.109.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boström K., Borén J., Wettesten M., Sjöberg A., Bondjers G., Wiklund O., Carlsson P., Olofsson S. O. Studies on the assembly of apo B-100-containing lipoproteins in HepG2 cells. J Biol Chem. 1988 Mar 25;263(9):4434–4442. [PubMed] [Google Scholar]
- Chen C., Bonifacino J. S., Yuan L. C., Klausner R. D. Selective degradation of T cell antigen receptor chains retained in a pre-Golgi compartment. J Cell Biol. 1988 Dec;107(6 Pt 1):2149–2161. doi: 10.1083/jcb.107.6.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland C. S., Doms R. W., Bolzau E. M., Webster R. G., Helenius A. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J Cell Biol. 1986 Oct;103(4):1179–1191. doi: 10.1083/jcb.103.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland C. S., Zimmer K. P., Wagner K. R., Healey G. A., Mellman I., Helenius A. Folding, trimerization, and transport are sequential events in the biogenesis of influenza virus hemagglutinin. Cell. 1988 Apr 22;53(2):197–209. doi: 10.1016/0092-8674(88)90381-9. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Oda K., Yokota S., Takatsuki A., Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem. 1988 Dec 5;263(34):18545–18552. [PubMed] [Google Scholar]
- Gascoigne N. R. Transport and secretion of truncated T cell receptor beta-chain occurs in the absence of association with CD3. J Biol Chem. 1990 Jun 5;265(16):9296–9301. [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Gold D. P., Puck J. M., Pettey C. L., Cho M., Coligan J., Woody J. N., Terhorst C. Isolation of cDNA clones encoding the 20K non-glycosylated polypeptide chain of the human T-cell receptor/T3 complex. Nature. 1986 May 22;321(6068):431–434. doi: 10.1038/321431a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kreis T. E., Lodish H. F. Oligomerization is essential for transport of vesicular stomatitis viral glycoprotein to the cell surface. Cell. 1986 Sep 12;46(6):929–937. doi: 10.1016/0092-8674(86)90075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krissansen G. W., Owen M. J., Fink P. J., Crumpton M. J. Molecular cloning of the cDNA encoding the T3 gamma subunit of the mouse T3/T cell antigen receptor complex. J Immunol. 1987 May 15;138(10):3513–3518. [PubMed] [Google Scholar]
- Krissansen G. W., Owen M. J., Verbi W., Crumpton M. J. Primary structure of the T3 gamma subunit of the T3/T cell antigen receptor complex deduced from cDNA sequences: evolution of the T3 gamma and delta subunits. EMBO J. 1986 Aug;5(8):1799–1808. doi: 10.1002/j.1460-2075.1986.tb04429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le A., Graham K. S., Sifers R. N. Intracellular degradation of the transport-impaired human PiZ alpha 1-antitrypsin variant. Biochemical mapping of the degradative event among compartments of the secretory pathway. J Biol Chem. 1990 Aug 15;265(23):14001–14007. [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Crabtree G. R., Rudikoff S., Pumphrey J., Robb R. J., Krönke M., Svetlik P. B., Peffer N. J., Waldmann T. A. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):626–631. doi: 10.1038/311626a0. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Bonifacino J. S., Yuan L. C., Klausner R. D. Degradation from the endoplasmic reticulum: disposing of newly synthesized proteins. Cell. 1988 Jul 15;54(2):209–220. doi: 10.1016/0092-8674(88)90553-3. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L. C., Bonifacino J. S., Klausner R. D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989 Mar 10;56(5):801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolios N., Bonifacino J. S., Klausner R. D. Transmembrane helical interactions and the assembly of the T cell receptor complex. Science. 1990 Jul 20;249(4966):274–277. doi: 10.1126/science.2142801. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Nilsson T., Jackson M., Peterson P. A. Short cytoplasmic sequences serve as retention signals for transmembrane proteins in the endoplasmic reticulum. Cell. 1989 Aug 25;58(4):707–718. doi: 10.1016/0092-8674(89)90105-0. [DOI] [PubMed] [Google Scholar]
- Ohashi P. S., Mak T. W., Van den Elsen P., Yanagi Y., Yoshikai Y., Calman A. F., Terhorst C., Stobo J. D., Weiss A. Reconstitution of an active surface T3/T-cell antigen receptor by DNA transfer. Nature. 1985 Aug 15;316(6029):606–609. doi: 10.1038/316606a0. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Doms R. W. Regulation of protein export from the endoplasmic reticulum. Annu Rev Cell Biol. 1988;4:257–288. doi: 10.1146/annurev.cb.04.110188.001353. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989 Nov 17;59(4):591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Rubin L. A., Kurman C. C., Biddison W. E., Goldman N. D., Nelson D. L. A monoclonal antibody 7G7/B6, binds to an epitope on the human interleukin-2 (IL-2) receptor that is distinct from that recognized by IL-2 or anti-Tac. Hybridoma. 1985 Summer;4(2):91–102. doi: 10.1089/hyb.1985.4.91. [DOI] [PubMed] [Google Scholar]
- Sato R., Imanaka T., Takatsuki A., Takano T. Degradation of newly synthesized apolipoprotein B-100 in a pre-Golgi compartment. J Biol Chem. 1990 Jul 15;265(20):11880–11884. [PubMed] [Google Scholar]
- Seglen P. O. Inhibitors of lysosomal function. Methods Enzymol. 1983;96:737–764. doi: 10.1016/s0076-6879(83)96063-9. [DOI] [PubMed] [Google Scholar]
- Shia M. A., Lodish H. F. The two subunits of the human asialoglycoprotein receptor have different fates when expressed alone in fibroblasts. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1158–1162. doi: 10.1073/pnas.86.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess M., Lodish H. F. Sequence of a second human asialoglycoprotein receptor: conservation of two receptor genes during evolution. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6465–6469. doi: 10.1073/pnas.82.19.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller T. J., Shields D. The propeptide of preprosomatostatin mediates intracellular transport and secretion of alpha-globin from mammalian cells. J Cell Biol. 1989 May;108(5):1647–1655. doi: 10.1083/jcb.108.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983 Apr;32(4):1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M. Temperature and energy dependence of secretory protein transport in the exocrine pancreas. EMBO J. 1986 Jul;5(7):1477–1482. doi: 10.1002/j.1460-2075.1986.tb04385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiger B. F., Leonard W. J., Svetlik P., Rubin L. A., Nelson D. L., Greene W. C. A secreted form of the human interleukin 2 receptor encoded by an "anchor minus" cDNA. J Immunol. 1986 Jun 1;136(11):4099–4105. [PubMed] [Google Scholar]
- Yanagi Y., Yoshikai Y., Leggett K., Clark S. P., Aleksander I., Mak T. W. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature. 1984 Mar 8;308(5955):145–149. doi: 10.1038/308145a0. [DOI] [PubMed] [Google Scholar]