Abstract
BACKGROUND
This study tested the hypothesis that use of semantic organizational strategy in approaching the Mattis Dementia Rating Scale (MDRS) Complex Verbal Initiation Perseveration (I/P) task, a test of semantic fluency, is the function specifically associated with remission of late-life depression.
METHOD
70 elders with major depression participated in a 12-week escitalopram treatment trial. Neuropsychological performance was assessed at baseline after a 2-week drug washout period. Patients with a Hamilton Depression Rating Scale Score less than or equal to 7 for two consecutive weeks and who no longer met DSM-IV criteria were considered to be remitted. Cox proportional hazards survival analysis was used to examine the relationship between subtests of the I/P, other neuropsychological domains and remission rate. Participants’ performance on the CV I/P was coded for perseverations, and use of semantic strategy.
RESULTS
The relationship of performance on the Complex Verbal I/P and remission rate was significant. No other subtest of the MDRS I/P evidenced this association. There was no significant relationship of speed, confrontation naming, verbal memory or perseveration with remission rate. Remitters’ use of verbal strategy was significantly greater than non-remitters.
CONCLUSIONS
Geriatric depressed patients who showed decrements in performance on a semantic fluency task showed poorer remission rates than those who showed adequate performance on this measure. Executive impairment in verbal strategy explained performance. This finding supports the concept that executive functioning exerts a “top down” effect on other basic cognitive processes, perhaps as a result of frontostriatal network dysfunction implicated in geriatric depression.
Keywords: Executive function, geriatric, depression, remission, semantic strategy, Mattis Dementia Rating Scale
Introduction
Executive dysfunction, a neuropsychological expression of frontal system impairment, is common in geriatric depression (1–5), and is associated with a clinical presentation resembling medial frontal lobe syndrome (1). When mild, it does not appear to progress to dementia, instead it is a stable disturbance that improves only moderately when depressive symptoms are ameliorated (2, 6).
Abnormal performance on some tests of executive function predicts both poor and unstable antidepressant response in late-life depression (7, 8), although some disagreement exists (9). The term “executive functions” encompasses a variety of cognitive abilities such as planning, organizing, self-monitoring, inhibiting prepotent responses, and strategy generation (10, 11). Each of these functions is subserved by shared, but also separate, neural systems. Further, performance on measures of executive function can affect and be affected by performance in non-executive cognitive domains such as processing speed, learning, and memory (12). Therefore, it is likely that some of these functions, and not others, may be relevant to antidepressant response.
The goal of this study is to identify aspects of executive dysfunction relevant to remission following treatment with a selective serotonin reuptake inhibitor. We focus on remission because remission is viewed as the optimal outcome of antidepressant treatment. Depressed patients achieving remission are less likely to experience or suffer relapse of depression compared to patients who are left with residual depressive symptoms (13).
Among tests of executive function, poor performance on the Initiation/Perseveration (I/P) domain of the Mattis Dementia Rating Scale (MDRS) has been consistently associated with little change in depressive symptoms (7, 14), low remission rate, and early relapse (2, 8) of geriatric depression. The appeal of this test is the brevity of its administration, which increases its clinical utility. However, I/P does not test a singular executive function. Instead, it yields a composite score of several executive skills, some of which are likely to be influenced by other cognitive domains. Therefore, the IP offers an opportunity to examine which executive functions predict a low remission rate of geriatric depression and whether this association is specific to executive functions.
This study tests the hypothesis that the use of semantic organizational strategy when approaching the semantic fluency task on the I/P subscale (Complex Verbal I/P task) is the function specifically associated with remission of late-life depression. This hypothesis is based on findings suggesting that verbal strategy requires integrity of frontal systems (15, 16) and on observations that structural and functional abnormalities of frontal and frontal-subcortical systems are associated with poor response to antidepressants (17–19).
Methods and Materials
Participants
The participants were 70 depressed, older (≥60 years) patients from a university Geriatric Psychiatry clinic who were consecutively recruited for an escitalopram treatment trial. Neuropsychological tests were performed during a 2-week single-blind psychotropic washout/placebo lead-in phase. Participants were screened by both a Ph.D. psychologist and a psychiatrist, and given a full SCID upon initial evaluation. All participants met DSM-IV-TR criteria for unipolar major depression and had a score ≥17 on the 24-item Hamilton Depression Rating Scale (HDRS) (20). Exclusion criteria were: 1) Major depression with psychotic features (according to DSM-IV-TR); 2) history of other psychiatric disorders (except personality disorders) before the onset of depression; 3) severe medical illness (i.e., metastatic cancer, unstable cardiac, hepatic, or renal disease, myocardial infarction, or stroke) within the 3 months preceding the study; 4) neurological disorders (i.e., dementia or delirium according to DSM-IV criteria, history of head trauma, Parkinson’s disease, brain tumors, and multiple sclerosis); 5) conditions often associated with depression (i.e., endocrinopathies other than diabetes, lymphoma, and pancreatic cancer); 6) drugs causing depression (i.e., steroids, α-methyl-dopa, clonidine, reserpine, tamoxifen, and cimetidine); and 7) Mini-Mental State Examination (21) score < 25. 8) Current psychotherapeutic treatment. These criteria resulted in a group of elderly subjects with non-psychotic unipolar major depression without a diagnosable dementing disorder.
The Weill Cornell Medical Institutional Review Board approved all procedures. All subjects signed written informed consent.
Treatment
Subjects were informed that they would receive placebo at some point during their 14-week trial. After a 2-week drug wash-out and single blind placebo lead-in, subjects who still met DSM-IV-TR criteria for major depression and had an HDRS score of 17 or greater received controlled treatment with fixed-dose escitalopram 10 mg daily for 12 weeks. Subjects were instructed to take a single dose of escitalopram in the morning, and were administered medication in one-week supply blisters that permitted dispensation of their daily dosage separately.
The subjects were followed with weekly meetings with a research psychiatrist and a research assistant who administered rating instruments over a period of 14 weeks (2 on placebo followed by 12 on escitalopram). The psychiatrist followed a medication clinic format consisting of a review of symptoms, explanations related to the need for treatment, and encouragement of treatment adherence. The research assistant administered the HDRS, the UKU, obtained vital signs, questioned the subjects about medication adherence, and counted the remaining tablets. No subject received psychotherapy during the study. The subjects were considered in remission if they no longer met DSM-IV-TR criteria for depression and had an HDRS score of 7 or below for two consecutive weeks.
Measures
Depressive symptoms were assessed using the 24-item HDRS at baseline and during each follow up week. Side effects of escitalopram were monitored with the UKU side effect scale (22) following the same schedule as the HDRS assessment. Neuropsychological instruments relevant to hypothesis testing were administered at the end of the 2-week, placebo lead-in phase and consisted of the MDRS (23) as well as tests of language, attention and processing speed, verbal memory, and perseveration.
The MDRS initiation/perseveration subscale contains eleven items where a subject can earn up to 37 points. The IP tasks include: Complex verbal initiation and perseveration (e.g., rapid oral generation of a list of supermarket items), simple verbal initiation and perseveration (e.g., rapid self-generated naming of items of clothing), vowel and consonant perseveration(e.g., repetition of “bee-kee-gee” and “bee-bah-boh”), alternating movements with fingers/hands (e.g. repetition of “palm up, palm down”), and copying alternating shapes and figures (e.g., “XOXO”)(23). Simple Verbal Initiation/Perseveration (23) was used to test confrontation naming. Trails A (24) was administered to assess attention and processing speed. The Hopkins Verbal Learning Test-Revised (HVLT-R) delayed recall was used to assess verbal memory (25). The Wisconsin Card Sorting Test (WCST-64) (26) perseverative errors score was used to assess perseveration as this is the category is the most sensitive to executive dysfunction in the elderly and represents one of the most commonly reported WCST measures (27). The research assistants were trained and supervised by a clinical neuropsychologist.
Statistical Analyses
The MDRS was separated into its subtests: Attention, Initiation/Perseveration, Construction, Conceptualization, and Memory. The relationship of these subtests with time to remission was tested using Cox’s proportional hazards survival analysis. Scores on the Initiation Perseveration (I/P) portion of the MDRS were then separated into subscales. The relationships of the subscales of the I/P with time to remission were also assessed with Cox’s proportional hazards survival analysis. In these models, age and education were entered as covariates because of their possible influence on performance. Because these clinical measures are often multidimensional, we also assessed the relationship of other component cognitive abilities (confrontation naming, processing speed, verbal memory, and perseveration) with time to remission with survival analysis. Finally, scores on all other subtests of the I/P portion of the MDRS were summed and examined for their collective association with time to remission. To look specifically at the way subjects approached the CVI/P subscale, that item was recoded for perseverations (repeat of a previously stated word), and for strategy (clusters of 3 or more words in a category, serially). Each cluster of three or more words was given a score of “1”. If a perseveration occurred in the midst of a cluster, that cluster was not counted.
Results
A total of 116 patients met eligibility criteria and entered the 2-week single-blind, placebo lead in period. Of these, 70 completed baseline neuropsychological measures, met the depression severity criterion (HDRS ≥ 17) after the 2-week placebo phase, and entered the 12-week escitalopram treatment phase. Of these 70, 56 completed the 12 week treatment trial and 14 exited prior to completion. Of the 14 subjects, 2 had 11 weeks of treatment (both exited because they found the treatment ineffective), 1 had 10 weeks of treatment (exited because she found the treatment ineffective) 2 had 9 weeks of treatment (one was lost to follow-up and one exited because of worsening depression), 2 had 8 weeks of treatment (both exited because they found the treatment ineffective), 2 had 7 weeks of treatment (one exited because he found the treatment ineffective, and one withdrew because she developed hyponatremia), 1 had 6 weeks of treatment (no longer wanted to participate in research), and 4 had 4 weeks of treatment (three exited due to worsening depression, and one was lost to follow-up).
At exit from the escitalopram trial, 35 subjects achieved remission and 35 remained symptomatic. At baseline, there was no difference in severity of depression (HDRS) (t(69) =0.694, p=.5), number of previous episodes(t(53)=−0.9;p=0.4), length of current episode(t(37.6)=−0.3;p=0.78), level of disability (WHODAS-II)(t(66)=1.5;p=0.13), or age of onset(t(57)=−1.3;p=0.19) between remitters and non-remitters (Table 1).
Table 1.
Baseline Demographic and Clinical Data of 65 Elderly Patients with Major Depression Treated with Escitalopram 10 mg Daily.
| Remitter | Non-R | Wilcoxon | Statistic | |
|---|---|---|---|---|
| Variable | Mean(SD) | Mean(SD) | Z | p |
| Age (years) | 70.1(5.8) | 70.4(7.1) | −.22 | 0.8 |
| Education (years) | 15.7(3.5) | 16.1(3.6) | −.73 | 0.5 |
| Baseline HDRS | 21.8(4.1) | 22.4(3.7) | −1.1 | 0.3 |
| Age of Onset (years) | 58.1(16.4) | 52.5(21.0) | −.92 | 0.4 |
| Length of current episode (months) | 35.9(92.0) | 31.3(32.6) | −1.35 | 0.2 |
| Number of Previous Episodes | 3.3(3.4) | 2.7(2.2) | −.92 | 0.4 |
| WHODAS-II Total | 35.4(10.4) | 39.5(12.3) | −1.71 | 0.8 |
| Mini Mental State Exam | 28.5(1.5) | 27.9(1.5) | −1.69 | 0.1 |
| Psychomotor Speed (Trails A) | 44.2(15.3) | 48.6(44.4) | −.38 | 0.7 |
Survival analysis revealed that a higher MDRS-IP score was associated with the occurrence of remission when age and education were taken into consideration (Hazard ratio (95% CI) = 1.26 (1.04–1.54), χ2 =8.97, df=3, p=0.03) (Table 2). Other subtests of the MDRS when covaried for age and education, were not significantly associated with remission: Attention (χ2=2.2, df=3, p=0.53), Construction (χ2=1.8, df=3, p=0.6), Conceptualization(χ2=3.4, df=3, p=0.3)and Memory (χ2=3.8, df=3, p=0.3).
Table 2.
Complex Initiation Perseveration: Relationship to Remission in 70 Elderly Patients with Major Depression Treated with Escitalopram.
| Variables | Hazard Ratio (95% CI) | Likelihood Ratio χ2 | p |
|---|---|---|---|
| Model A1 | |||
| DRS-IP | 1.26 (1.04 – 1.54) | 5.39 | 0.02 |
| Age | 0.98 (0.93 – 1.03) | 0.71 | 0.40 |
| Education | 0.91 (0.82 – 1.02) | 2.42 | 0.12 |
| Model B2 | |||
| Complex IP | 1.57 (1.06 – 2.33) | 5.1 | 0.02 |
| Age | 0.98 (0.93 – 1.03) | 0.72 | 0.40 |
| Education | 0.92 (0.83 – 1.03) | 2.06 | 0.15 |
Proportional Hazards Likelihood Ratio χ2=8.97, df=3, p<0.03
Proportional Hazards Likelihood Ratio χ2=12.53, df=3, p<0.0006
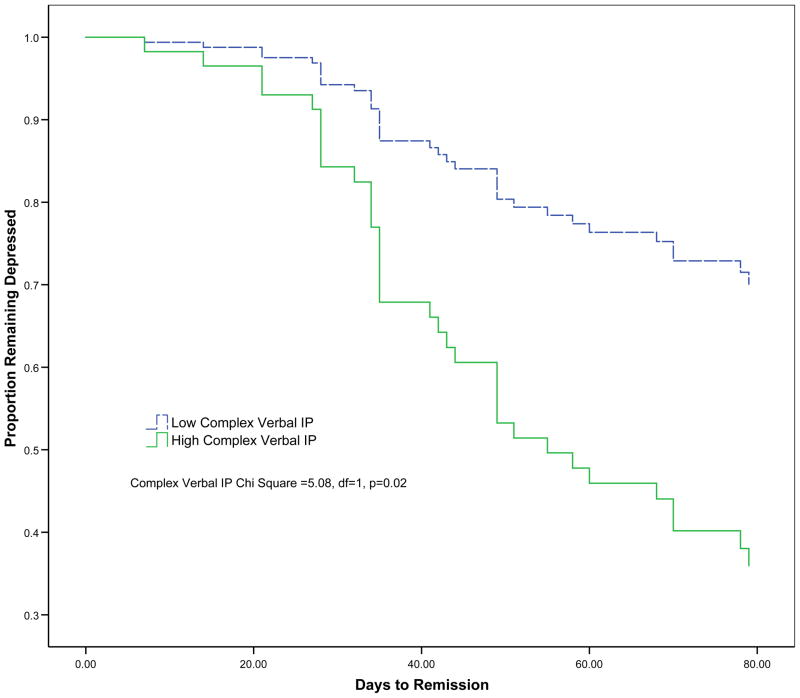
Subsequent analyses focused on the components of the IP scale. Only the Complex Verbal subscale (CV I/P) was associated with the occurrence of remission when taking into account age and education (Hazard ratio (95% CI) = 1.57 (1.06–2.3), χ2 =12.5, df=3, p=0.006) (Table 2, Figure 1). Remission was not significantly associated with other subscales of the I/P (Simple Verbal I/P, Consonant Perseveration, Double Alternating Movements, Alternate Tapping, Graphomotor Design 1–4), or Vowel Perseveration (VP).
Figure 1.
Remission rates in 70 elderly patients with major depression with high and low scores (median split) on the Complex portion of the Mattis Dementia Rating Scale Initiation Perseveration (DRS IP). The Complex IP Chi-Square was estimated after adjustment for age and education (Hazard ratio (95% CI) = 1.57 (1.06–2.3)).
To ensure the relationship between CV I/P and remission rate was not merely due to the larger number of items of the CV I/P, a sensitivity analysis was completed whereby the CV I/P subscale score was subtracted from the MDRS-IP total score in order to assess cumulative effects of the remaining subscales on remission rate. Survival analysis determined that when summed together, the remaining subscales of the MDRS-IP were not associated with time to remission (Hazard ratio (95% CI) = 1.02 (0.76–1.39), χ2 =1.37, df=3, p=0.71).
To identify the cognitive components with the strongest association to remission, we examined performance on neuropsychological measures for their unique relationship with remission. Remission could not be attributed to the effects of psychomotor speed (Trails A) (Hazard ratio (95% CI) = 0.33 (0.98–1.00), χ2 =2.9, df=3, p=0.42), confrontation language retrieval (Simple Verbal I/P) (Hazard ratio (95% CI) = 1.5 (0.76–2.96), χ2 =2.9, df=3, p=0.40), verbal memory (HVLT- Delayed recall) (Hazard ratio (95% CI) = 1.03 (0.92–1.16), χ2 =1.18, df=3, p=0.76) or perseveration (WCST- Perseverative Errors) (Hazard ratio (95% CI) = 1.05 (0.99–1.11), χ2 =4.4, df=3, p=0.22).
Independent samples t-tests revealed that when the CV I/P subscale was recoded for perseverations and for strategy (semantic clusters), subjects who remitted utilized more clusters of related words, when completing the task than non-remitters (t=2.319; df=66; p=.028). Remitters and non-remitters did not differ in the number of perseverations (t=.257; df=66; p=.79) (Table 1).
Discussion
The principal finding of this study is that abnormal scores on the complex verbal subscale of the MDRS I/P are predictive of poorer remission rates in geriatric major depression, and that use of a verbal strategy accounts for this difference in performance. This finding was specific to verbal strategy utilization on semantic fluency measures, as no other measure, nor other cognitive function influencing performance (e.g. speed, perseveration etc.) predicted time to remission. Although previous studies have shown that abnormal I/P scores are associated with poor remission rates, to our knowledge, this is the first study that accounts for the variance contributed by specific cognitive tasks within the I/P subtest.
These findings are consistent with studies suggesting that abnormalities in neural systems related to executive functions are associated with poor remission rate of late-life depression. Structural and functional neuroimaging have documented both frontostriatal impairment and the relationship between frontostriatal impairment and executive dysfunction in geriatric depression (28, 29). A recent study found significant associations between fractional anisotropy and Stroop Color Word Interference performance in multiple frontostriatal limbic regions, providing evidence for the association of these areas with the executive dysfunction often accompanying geriatric depression (28). Another recent functional MRI (fMRI) study demonstrated both hypoactivation of the dorsolateral prefrontal cortex (DLFPC) and reduced functional connectivity between the DLFPC and dorsal anterior cingulate pre treatment, and persistent reduced functional connectivity following treatment on a cognitive control task (29).
Our findings are consistent with the view that impaired executive functioning influences cognitive scores as an effect of frontostriatal compromise in geriatric major depression (30). Previous studies have shown that executive functioning in elderly depressed patients mediated performance on cognitive tasks of verbal learning, and visual spatial memory (30, 31). Our findings further support this premise showing no significant relationship between, psychomotor speed, verbal memory, or confrontation naming and remission rate. Our results indicate a “top down” processing effect. Impairment in executive functioning, specifically in the utilization of a verbal strategy, a rather direct clinical expression of DLPFC dysfunction, appears to diminish performance in semantic fluency. It is possible that lack of effective strategy “slows” the subjects’ selection and production of words. Impairment in patients’ executive use of strategy, which relies directly on oversight by the DLPFC accounts for both observed deficits on this test of semantic fluency, and for poorer remission rates.
This theory of “top down” negative effects of impairments in executive process on semantic fluency performance is supported by both neuropsychological, and functional imaging studies (32). Recent fMRI studies suggest that subjects performing lexical semantic fluency tasks show activity in the anterior left inferior prefrontal cortex (15). Specifically, when participants completing strategic semantic tasks select words from multiple activated responses, and suppress semantically or phonemically related but inapplicable words, fMRI studies show activation in the left inferior prefrontal cortex, middle temporal gyrus (MTG) bilateral anterior cingulate cortex (ACC) (16) and the posterior cingulate (33), areas implicated in the pathophysiology of executive dysfunction in geriatric depression by fMRI and genetics studies.
A potential interpretation of the relationship of poor performance on the CV I/P to Remission rate is that abnormal activity and reduced functional connectivity in the DLPFC, MTG, and posterior ACC contributes to both behaviorally expressed difficulty in strategy generation and selection on the fluency task and with patients’ ability to benefit from antidepressant treatment. The current findings provide some preliminary evidence that pre-treatment semantic fluency performance may be a short, yet useful indicator of treatment response potential. If the current study’s findings are replicated, this subscale could be an adjunct to a clinical assessment of depressive symptoms to improve clinician’s ability to identify patients at risk for poor antidepressant treatment response.
There are several limitations to this study. One limitation is the lack of a placebo control group. In addition, given that remission status was based on both a fixed dose of escitalopram and 12-week course of treatment, it is possible that some subjects may have remitted if either treated with higher dosages of escitalopram or longer treatment were offered. However, 9 of 14 subjects who exited the trial had 7–11 weeks of treatment. Another potential limitation of this study is the use of a limited neurocognitive battery. We were not able to eliminate all possible contributing cognitive factors with the present neuropsychological battery. For example, we did not measure initiation or task persistence as factors in rate of remission, both of which have been previously indicated in this relationship (34). Replication of this study with more comprehensive assessment of executive functions is necessary to further discriminate which are predictive of poor outcomes in geriatric depression.
In conclusion, geriatric depressed patients who showed decrements in performance on a semantic fluency task showed poorer escitalopram treatment response rates than those who showed adequate performance on this measure. Executive impairment in verbal strategy explained the difference in performance. This finding supports the concept that executive functioning exerts a “top down” effect on other basic cognitive processes, perhaps as a result of frontostriatal network dysfunction implicated in geriatric depression.
Acknowledgments
This work was supported by National Institute of Mental Health grants P30 MH68638 (GSA), R01 MH079414 (GSA), T32 MH019132 (GSA) K23 MH74818 (FMG), K23 MH067702 (CFM) the Sanchez Foundation, the TRU Foundation, and Forest Pharmaceuticals.
The authors thank Dr. Sibel A. Klimstra M.D. for her work as study psychiatrist, and James D. Weinberg, B.A. for his contribution to this manuscript.
Footnotes
Financial Disclosures
This work was supported by National Institute of Mental Health Grants P030 MH68638, R01 MH65653, T32 MH019132 (GSA), and K23 MH074818 (FMG), K23 MH067702 (CFM) and by the TRU and Sanchez Foundations. Escitalopram and placebo were provided free of cost by Forest Pharmaceuticals, Inc. Dr. Alexopoulos has received research grants by Forest Pharmaceuticals, Inc. and Cephalon and participated in scientific advisory board meetings of Forest Pharmaceuticals and Sanofi Aventis. He has given lectures supported by Forest, Bristol Meyers Squibb, Janssen, and Lilly and owns equity of Johnson and Johnson. Drs. Morimoto, Gunning, Murphy, and Kelly, and Ms. Kanellopoulos report no competing interests.
References
- 1.Alexopoulos GS, Kiosses DN, Klimstra S, et al. Clinical presentation of the “depression-executive dysfunction syndrome” of late life. Am J Geriatr Psychiatry. 2002;10:98–106. [PubMed] [Google Scholar]
- 2.Alexopoulos GS, Kiosses DN, Murphy C, et al. Executive dysfunction, heart disease burden, and remission of geriatric depression. Neuropsychopharmacology. 2004;29:2278–2284. doi: 10.1038/sj.npp.1300557. [DOI] [PubMed] [Google Scholar]
- 3.Potter GG, Kittinger JD, Wagner HR, et al. Prefrontal neuropsychological predictors of treatment remission in late-life depression. Neuropsychopharmacology. 2004;29:2266–2271. doi: 10.1038/sj.npp.1300551. [DOI] [PubMed] [Google Scholar]
- 4.Sneed JR, Roose SP, Keilp JG, et al. Response inhibition predicts poor antidepressant treatment response in very old depressed patients. Am J Geriatr Psychiatry. 2007;15:553–563. doi: 10.1097/JGP.0b013e3180302513. [DOI] [PubMed] [Google Scholar]
- 5.Story TJ, Potter GG, Attix DK, et al. Neurocognitive correlates of response to treatment in late-life depression. Am J Geriatr Psychiatry. 2008;16:752–759. doi: 10.1097/JGP.0b013e31817e739a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakano Y, Baba H, Maeshima H, et al. Executive dysfunction in medicated, remitted state of major depression. J Affect Disord. 2008;111:46–51. doi: 10.1016/j.jad.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Kalayam B, Alexopoulos GS. Prefrontal dysfunction and treatment response in geriatric depression. Arch Gen Psychiatry. 1999;56:713–718. doi: 10.1001/archpsyc.56.8.713. [DOI] [PubMed] [Google Scholar]
- 8.Alexopoulos GS, Meyers BS, Young RC, et al. Executive dysfunction and long-term outcomes of geriatric depression. Arch Gen Psychiatry. 2000;57:285–290. doi: 10.1001/archpsyc.57.3.285. [DOI] [PubMed] [Google Scholar]
- 9.Butters MA, Bhalla RK, Mulsant BH, et al. Executive functioning, illness course, and relapse/recurrence in continuation and maintenance treatment of late-life depression: is there a relationship? Am J Geriatr Psychiatry. 2004;12:387–394. doi: 10.1176/appi.ajgp.12.4.387. [DOI] [PubMed] [Google Scholar]
- 10.Lezak MD. Neuropsychological assessment. New York: Oxford University Press; 1976. [Google Scholar]
- 11.Benton AL. Contributions to neuropsychological assessment: a clinical manual. 2. New York: Oxford University Press; 1994. [Google Scholar]
- 12.Elderkin-Thompson V, Hellemann G, Pham D, et al. Prefrontal brain morphology and executive function in healthy and depressed elderly. Int J Geriatr Psychiatry. 2008 doi: 10.1002/gps.2137. [DOI] [PubMed] [Google Scholar]
- 13.Lecrubier Y. How do you define remission? Acta Psychiatr Scand Suppl. 2002:7–11. doi: 10.1034/j.1600-0447.106.s415.2.x. [DOI] [PubMed] [Google Scholar]
- 14.Alexopoulos GS, Kiosses DN, Heo M, et al. Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58:204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Gold BT, Balota DA, Kirchhoff BA, et al. Common and dissociable activation patterns associated with controlled semantic and phonological processing: evidence from FMRI adaptation. Cereb Cortex. 2005;15:1438–1450. doi: 10.1093/cercor/bhi024. [DOI] [PubMed] [Google Scholar]
- 16.Gold BT, Balota DA, Jones SJ, et al. Dissociation of automatic and strategic lexical-semantics: functional magnetic resonance imaging evidence for differing roles of multiple frontotemporal regions. J Neurosci. 2006;26:6523–6532. doi: 10.1523/JNEUROSCI.0808-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson S, Baldwin RC, Jackson A, et al. Is subcortical disease associated with a poor response to antidepressants? Neurological, neuropsychological and neuroradiological findings in late-life depression. Psychol Med. 1998;28:1015–1026. doi: 10.1017/s003329179800693x. [DOI] [PubMed] [Google Scholar]
- 18.Alexopoulos GS, Kiosses DN, Choi SJ, et al. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry. 2002;159:1929–1932. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- 19.Alexopoulos GS, Murphy CF, Gunning-Dixon FM, et al. Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry. 2008;165:238–244. doi: 10.1176/appi.ajp.2007.07050744. [DOI] [PubMed] [Google Scholar]
- 20.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Lingjaerde O, Ahlfors U, Bech P, et al. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- 23.Mattis S. Dementia Rating Scale Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc; 1988. [Google Scholar]
- 24.Lezak MD. Neuropsychological assessment. 3. New York: Oxford University Press; 1995. [Google Scholar]
- 25.Brandt J, Benedict R. Hopkins Verbal Learning Test-Revised. Professional. Manual Lutz, FL: Psychological Assessment Resources, Inc; 2001. [Google Scholar]
- 26.Heaton R. The Wisconsin Card Sorting Test Manual. Odessa, FL: Psychological Assessment Resources, Inc; 1981. [Google Scholar]
- 27.Rhodes MG. Age-related differences in performance on the Wisconsin card sorting test: a meta-analytic review. Psychol Aging. 2004;19:482–494. doi: 10.1037/0882-7974.19.3.482. [DOI] [PubMed] [Google Scholar]
- 28.Murphy CF, Gunning-Dixon FM, Hoptman MJ, et al. White-matter integrity predicts stroop performance in patients with geriatric depression. Biol Psychiatry. 2007;61:1007–1010. doi: 10.1016/j.biopsych.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aizenstein HJ, Butters MA, Wu M, et al. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. Am J Geriatr Psychiatry. 2009;17:30–42. doi: 10.1097/JGP.0b013e31817b60af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elderkin-Thompson V, Mintz J, Haroon E, et al. Executive dysfunction and memory in older patients with major and minor depression. Arch Clin Neuropsychol. 2007;22:261–270. doi: 10.1016/j.acn.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 31.Elderkin-Thompson V, Kumar A, Mintz J, et al. Executive dysfunction and visuospatial ability among depressed elders in a community setting. Arch Clin Neuropsychol. 2004;19:597–611. doi: 10.1016/j.acn.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Pyykkonen B, Hook JN, Han DY, et al. An Analysis of the Unique Contribution of Executive Functioning, Language and Verbal Memory to Verbal Fluency Tasks Using Hierarchical Regression. Atlanta, GA: 2009. [Google Scholar]
- 33.Binder JR, Desai RH, Graves WW, et al. Where Is the Semantic System? A Critical Review and Meta-Analysis of 120 Functional Neuroimaging Studies. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potter GG, Blackwell AD, McQuoid DR, et al. Prefrontal white matter lesions and prefrontal task impersistence in depressed and nondepressed elders. Neuropsychopharmacology. 2007;32:2135–2142. doi: 10.1038/sj.npp.1301339. [DOI] [PubMed] [Google Scholar]