Abstract
The purpose of this study was to examine differences in heat pain threshold (HPTh) and heat pain tolerance (HPTo) between temporomandibular joint disorder (TMJD) patients and healthy controls. Using suprathreshold heat pain, this study also examined between-group (i.e. TMJD vs. healthy controls) differences in hyperalgesia and temporal summation (TS) of heat pain. Lastly, whether between-group differences in these heat pain outcomes were mediated by self-reported sleep quality was also tested. A total of 119 participants (41% TMJD) completed the current study. HPTh and HPTo responses were assessed at the ventral forearm with an ascending method of limits, while hyperalgesia and TS responses were assessed at the dorsal forearm at temperatures of 46, 48 and 50 °C. Prior to completion of heat pain procedures, participants completed the Pittsburgh Sleep Quality Index. Significant between-group differences in HPTh and HPTo were not observed. TMJD patients demonstrated significantly greater hyperalgesia than healthy controls at 46 °C only, but there were no differences for TS. Furthermore, TMJD patients reported significantly poorer sleep quality compared with healthy controls. Data analysis revealed a significant simple mediation effect whereby the presence of TMJD was strongly associated with poorer self-reported sleep quality, which, in turn, was related to enhanced hyperalgesia at 46 °C. These findings support the hypothesis that the thermal hyperalgesia demonstrated by TMJD patients may be related to poor quality of their self-reported sleep. The ability of interventions that improve sleep quality to also affect pain sensitivity is currently the topic of ongoing investigation.
1. Introduction
Temporomandibular joint disorder (TMJD) represents a group of conditions characterized by pain in the temporomandibular joint and/or masticatory muscles (Atsu and Ayhan-Ardic, 2006). Alterations in central pain modulation are as one possible factor contributing to hyperalgesic changes in TMJD (Svensson and Graven-Nielsen, 2001), particularly temporal summation (TS) of pain. TS is a pathophysiological mechanism common to many chronic pain conditions, which results in the perception of increased pain despite constant or even reduced peripheral afferent input (Staud et al., 2001), and TS is considered a perceptual manifestation of enhanced central excitability. Hyperalgesia refers to increased pain in response to a previously painful stimulus. Maixner et al. (1995, 1998) reported that TMJD patients showed hyperalgesia and greater TS to thermal stimuli relative to healthy controls. Conversely, Raphael et al. (2009) found no differences in TS of heat pain comparing females with TMJD with healthy female controls, while Sarlani et al. (2007) observed mechanical hyperalgesia and enhanced TS of mechanical pain among females but not males with TMJD. These findings suggest the presence of hyperalgesia and TS among individuals with TMJD; however, additional research into this matter is warranted.
One factor that may contribute to hyperalgesia and enhanced TS is poor sleep because a poor night’s sleep may be accompanied by an increase in pain severity on the subsequent day, and pain during the day is often followed by another poor night’s sleep (Smith and Haythornthwaite, 2004). Such association also extends to TMJD patients (Schutz et al., 2009; Smith et al., 2009a). One mechanism whereby sleep may affect the pain of TMJD patients is via effects on central pain modulation (Smith et al., 2009b). A study of healthy female participants found that partial sleep loss achieved via forced awakenings was not only associated with greater spontaneous pain reports but also diminished endogenous pain inhibition (Smith et al., 2007). Another study of TMJD patients reported that poor sleep efficiency, the ratio of time spent asleep (total sleep time) to the amount of time spent in bed, predicted greater impairment of endogenous pain inhibitory capacity the following day (Edwards et al., 2009). These data together support the relations among poor sleep, greater pain sensitivity and impaired endogenous pain inhibitory processes. No studies have yet evaluated whether poor sleep influences pain facilitatory processes such as hyperalgesia and TS.
The purpose of the current study was to examine differences in heat pain threshold (HPTh), heat pain tolerance (HPTo), hyperalgesia, TS and self-reported sleep quality between TMJD and healthy controls. The study also sought to determine whether differences in heat pain outcomes might be indirectly explained by differences in self-reported sleep quality. The following a priori hypotheses were tested: (1) TMJD patients will demonstrate lower HPTh and HPTo compared with healthy controls; (2) TMJD patients will demonstrate greater hyperalgesia and TS compared with healthy controls; (3) self-reported sleep quality will be poorer for TMJD patients relative to healthy controls; and (4) poor sleep quality will mediate differences in hyperalgesia and TS between TMJD patients and healthy controls.
2. Materials and methods
2.1 Participants
Over the course of three years, potential participants were recruited using two different flyers posted throughout the University of Florida medical campus. The first flyer targeted individuals with a history of orofacial pain, while the second flyer targeted age- and gender-matched individuals without orofacial pain. Potential participants completed an initial telephone screening and eligible participants met the following criteria: (1) between the ages of 18 and 45 years; (2) no ongoing chronic pain problems other than TMJD; (3) no systemic medical condition; (4) not pregnant; (5) use of analgesics, antidepressants or other centrally acting agents; and (6) no mental health disorders requiring hospitalization in the past year. Potential participants with self-reported orofacial pain matriculated through the study only if they reported periodic pain in the face and jaw for at least 2 weeks and also reported 5 or more days with headache within the 30 days prior to study screening. A total of 157 potential participants (68 with orofacial pain, 89 without orofacial pain) were screened for inclusion into the study; 38 potential participants (19 with orofacial pain, 89 without orofacial pain) were found to be ineligible. Potential participants with orofacial pain were deemed ineligible because they reported either less than 2 weeks of facial pain or less than 5 days with headache within the past 30 days. Potential participants without orofacial pain were ineligible either because they did not meet the age requirement (2) or because they failed to meet the other eligibility requirements listed above (17). Following this initial screen, 119 participants (55% women) met the criteria to matriculate through the study (49 were orofacial pain, 70 without orofacial pain).
2.2 Study protocol
Upon arriving at the study site, all participants signed the informed consent and completed sleep and psychological self-report questionnaires to assess mood and perceived sleep quality (see more details in the Measures section). Next, all participants underwent a clinical examination using the Research Diagnostic Criteria (RDC) for the determination of TMJD status (Dworkin and LeResche, 1992) for classification as either TMJD or control. Of the 49 potential participants with orofacial pain that met the criteria for study inclusion, all also met RDC criteria for confirmation of TMJD. Conversely, none of the potential participants without orofacial pain (i.e. healthy controls) met the RDC criteria for TMJD.
Participants then underwent a series of heat pain testing procedures to assess pain threshold and pain tolerance. Pain threshold refers to the intensity at which a person first perceives the heat stimulus to be painful. Pain threshold intensity was recorded as the temperature at which the pain threshold was crossed. Pain tolerance refers to the maximum level of pain that a person is able to tolerate and was recorded as the temperature at which the subject discontinued the heat stimulus. Following 5 min, subjects completed a series of tests for suprathreshold heat pain responses, and from these responses, indices of hyperalgesia and TS were derived. That the heat pain for this series of tests was ‘suprathreshold’ indicates that the intensity of the heat was greater than the intensity at which subjects first indicated experiencing pain (i.e. pain threshold). To minimize inconsistency in heat pain assessment due to differential anatomical locations, HPTh and HPTo were tested on the right ventral forearm of all subjects. Hyperalgesia and TS of heat pain were tested on the right dorsal forearm. The decision to test heat pain responses at a peripheral location (forearm), rather than locally (temporomandibular joint), was made because the larger surface area of the forearm allowed for the thermode to be moved between procedures (e.g. ventral for pain threshold and tolerance vs. dorsal for suprathreshold assessment) and between trials. This was important to avoid either sensitization or response suppression of cutaneous heat nociceptors. Assessment of heat pain responses as the temporomandibular joint would have prevented repositioning of the thermode and would have risked sensitization and/or response suppression of cutaneous heat nociceptors.
On the day of examination, participants were instructed to refrain from using alcohol, nicotine products and non-prescription medications on the day of the study. All study procedures were approved by the University of Florida Institutional Review Board, and informed consent was obtained prior to the initiation of study procedures. Participants were compensated for their participation.
3. Measures
3.1 Experimental pain sensitivity
3.1.1 HPTh and HPTo
HPTh and HPTo were assessed on the ventral forearm using a Medoc Pathway Neurosensory Analyzer (Medoc, Ltd, Ramat Yishai, Israel) with a 30 × 30 mm thermode in accordance with an ascending method of limits. From a baseline of 32 °C, probe temperature increased at a rate of 0.5 °C/s until participants responded by pressing a button to indicate when they first felt pain (HPTh) and when they were no longer able to tolerate the pain (HPTo). Three trials of HPTh and HPTo were presented to each participant. The position of the thermode was altered slightly between trials (although it remained on the ventral forearm). For each measure, the average of all three trials was computed for use in subsequent analyses.
3.1.2 Suprathreshold heat pain stimuli
Suprathreshold heat pain stimuli were applied to participants’ right dorsal forearm. Sequences of 10 consecutive heat pulses of <1-s duration at interpulse intervals of 2.5 s were delivered (Fillingim et al., 1998; Edwards and Fillingim, 2001). Participants verbally rated the intensity of each heat pulse on a numerical rating scale from 0 = no pain to 100 = the most intense pain imaginable (Fillingim et al., 1998). The procedure was performed three times using 46, 48 and 50 °C thermal intensities, with 10 heat pulses delivered at each temperature. The testing temperatures were non-randomly allocated following a stepped sequence beginning with 46 °C. Participants were informed that the task would be discontinued prior to completion of the 10 pulses if a rating of 100 was given, or if they wished to discontinue the task and subsequently said, ‘Stop’. To impute missing values created when participants terminated the procedure prematurely, the last observation was carried forward. The temperature for the heat pulses rapidly fluctuated from 38 °C as a baseline temperature for inter-stimulus intervals to a peak of 46, 48 or 50 °C, according to the respective trial. The inter-sequence interval (i.e. time between each testing temperature) was 30 s. The position of the thermode was altered slightly between temperatures (although it remained on the dorsal forearm).
Traditional indices derived from suprathreshold heat pain stimuli assessment reported in the literature include the use of the first pain rating (Staud et al., 2003), the mean pain ratings (Vierck et al., 1997; Edwards et al., 2006), the final pain rating (Farrell and Gibson, 2007), the highest pain rating minus the first pain rating (Edwards et al., 2003b) and the maximal pain rating (Staud et al., 2001). These indices likely reflect different aspects of suprathreshold heat pain perception such as hyperalgesia or TS and, as a result, are not interchangeable. It can be argued that differences in first pain ratings (Staud et al., 2003) and mean pain ratings (Edwards et al., 2006) are perhaps more reflective of heat hyperalgesia, while the highest pain rating minus the first pain rating may be considered as an indicator of TS (Edwards et al., 2003b). However, currently no consensus exists regarding which indices are the most appropriate measures of suprathreshold hyperalgesia and TS, respectively. For this study, the pain sensitivity measures to be analysed as statistical outcomes involved data related to all 10 pulses of suprathreshold heat pain stimuli assessment. Thus, TS was obtained by subtracting the first pain rating from the highest pain rating, as this reflected the slope or the maximum amount of TS obtained (Edwards et al., 2003b). A mean pain rating for the 10 heat pulses was used to represent hyperalgesia (Vierck et al., 1997; Edwards et al., 2006). These TS and hyperalgesia calculations were carried out across the various trials of heat pain intensity (i.e. 46, 48 and 50 °C), similar to that reported in previous studies (Valencia et al., 2011).
3.2 Sleep and psychological questionnaires
3.2.1 Pittsburgh Sleep Quality Index (PSQI)
The PSQI is a self-rated questionnaire that assesses sleep quality and disturbances over a 1-month time interval (Buysse et al., 1989). Nineteen individual items generate seven ‘component’ scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medications and daytime dysfunction. The sum of scores for these seven component scores yields one global score, ranging from 0 to 21, with higher scores indicating poorer sleep quality. The seven component scores of the PSQI have previously been shown to possess good internal consistency (α = 0.83), and the overall global score has demonstrated good test–retest reliability (r = 0.87) (Buysse et al., 1989).
3.2.2 Positive and Negative Affect Schedule (PANAS)
Negative affect was measured using the Negative Affect subscale of the PANAS (Watson et al., 1988b). The Negative Affect subscale was used to examine the influence of general negative affect in the proposed models of the current study. The Negative Affect subscale of the PANAS includes 10 negative affects (e.g. distressed, upset), and participants were asked to indicate on a 5-point Likert scale (1 = not at all, 5 = very much so) the strength of the emotion for them. The total score was the sum of the 10 items, with a possible range of 10–50. Previous studies have reported that mood is reliably related to sleep quality and TS (Staud et al., 2004), with negative mood predicting poorer quality sleep and enhanced TS. It can be argued that emotional factors such as depressive symptoms and anxiety are often manifestations of negative affectivity (Watson et al., 1988a). Thus, to statistically control for negative affect partials out the contributions of depressive and anxious features from the study model. The inclusion of negative affect as a control variable, rather than a measure of depression and anxiety, makes for a more parsimonious study model. The PANAS has previously been demonstrated to possess good psychometric properties (Watson et al., 1988b).
3.3 Data reduction and analysis
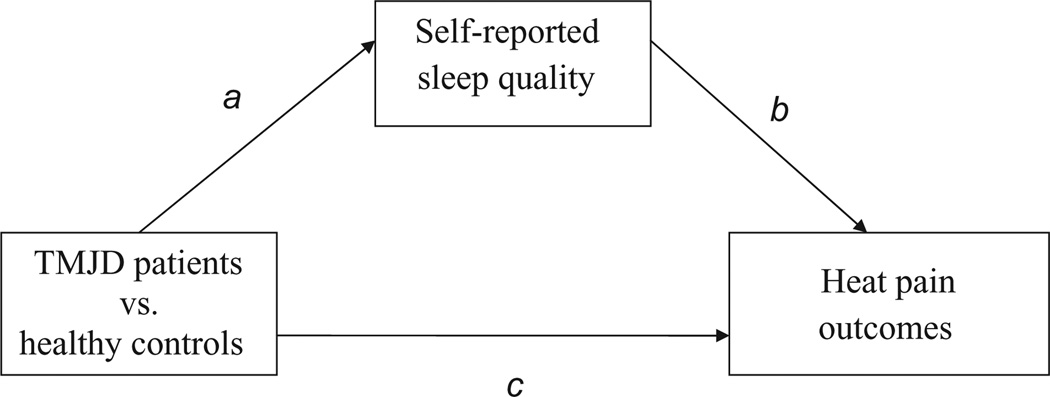
Our first analytical strategy involved comparing TMJD patients and healthy controls for differences in HPTh and HPTo (Hypothesis 1), as well as TS (highest pain rating minus the first pain rating) and hyperalgesia (mean pain ratings) across the three trials of increasing temperature (i.e. 46, 48 and 50°C; Hypothesis 2). For this purpose, the heat pain outcomes were included as the dependent variables in separate analyses of covariance (ANCOVA) controlling for nuisance variables as needed. Next, an additional ANCOVA was carried out to examine between-group differences in self-reported sleep quality assessed via the PSQI (Hypothesis 3). Lastly, simple mediation was tested to determine whether between-group differences in the HPTh, HPTo, hyperalgesia and TS heat pain outcomes were indirectly transmitted through between-group differences in self-reported sleep quality (Hypothesis 4; see Fig. 2 for putative study model).
Figure 2.
Simple mediation model. The indirect relationship explaining study group differences in suprathreshold heat pain responses at 46 °C through self-reported sleep quality.
The present study followed the four criteria put forth by Baron and Kenny (1986) for establishing mediation: (1) significant between-group differences for the suprathreshold heat pain outcomes (hyperalgesia, TS); (2) between-group difference for self-reported sleep quality (mediator); (3) to show that the mediator was significantly related to the heat pain outcome controlling for group; and (4) to test the significance of the indirect effect of between-group differences on heat pain outcomes through self-reported sleep quality. If all four of these criteria are met, then the data are consistent with the hypothesis that sleep quality mediates between-group differences in heat pain outcomes. If any of the first three criteria are not met, then mediation is not supported.
Analysis of the mediation model was completed using the SPSS (SPSS Inc., Chicago, IL, USA) macro for simple mediation (Preacher and Hayes, 2004) to obtain a 95% bootstrapped confidence interval (CI) for an unconditional indirect effect. Conceptually, bootstrapping is a non-parametric approach to effect-size estimation and hypothesis testing that makes no assumptions about the shape of the distribution of the variables within a given model (i.e. normal vs. skewed). Furthermore, bootstrapping has been recommended as a means of circumventing the power problem introduced by asymmetries and other forms of non-normality in the sampling distribution of ab (Lockwood and MacKinnon, 1998; Shrout and Bolger, 2002). In the current study, the term ab represents the indirect effect of between-group differences on heat pain responses through sleep quality and is defined as the product of the between-group difference to sleep quality path (1) and the sleep quality to heat pain response path (2), or ab.
The bootstrapping approach is completed through taking a large number of samples of size n (where n is the original sample size) from the data, sampling with replacement (and therefore an observation that appears only once in the original data set can appear multiple times in a bootstrapped dataset), and computing the indirect effect, ab, in each sample. The point estimate of ab is the mean ab calculated over the bootstrap samples, and the estimated standard error is the standard deviation of the ab estimates. For CI estimation, it is advantageous to take 1000 bootstrap samples (Preacher and Hayes, 2004). To create the 95% CI, the elements of the vector of 1000 estimates of ab are sorted from low to high. The lower limit of the CI is the 25th score and the upper limit is the 976th score in this distribution.
Bootstrapped CIs were used to test the significance of the mediated effect because recent statistical research has suggested that bootstrapping is more appropriate than a normal-theory test (i.e. Sobel’s test) for studies with smaller sample sizes and non-normally distributed variables (MacKinnon et al., 2002; Shrout and Bolger, 2002; Preacher and Hayes, 2004). Specifically, a bias-corrected (BC) bootstrapped CI was used because type I error rates and statistical power for this method have been shown to be better than other methods, such as the series of regression analyses recommended by Baron and Kenny (1986). The incorporation of bootstrapped CIs to test for statistical mediation of pain-related outcomes has previously been described (Goodin et al., 2009; Caes et al., 2011).
4. Results
4.1 Sample characteristics and examination of covariates
Among those who met the RDC criteria for TMJD, the average duration since self-reported TMJD onset was 42 ± 31 months. Chi-square tests showed that the group distribution of TMJD patients versus healthy controls significantly differed as a function of gender [χ2 (1) = 3.84; p = 0.05] such that there were a greater number of women than men with TMJD. The number of men in the TMJD and healthy control groups was comparable, as it was for women. The group distribution of TMJD versus healthy control did not significantly differ as a function of ethnicity [χ2 (3) = 7.15; p > 0.05]. Among the total sample, 50% identified as non-Hispanic white, 10% as African American, 17% as Asian/Pacific Islander and 23% as Hispanic. Because exploration of ethnic differences was not a primary aim in the current study, ethnicity was coded as 0 = non-white, 1 = white for analytical purposes and statistically controlled. The mean age of the participants was 24.6 years (standard deviation = 5.5), and all participants were either employed or studying for completion of a degree.
Descriptive statistics for demographics, sleep, and psychological measures, and sensory heat pain testing from TMJD patients and healthy controls are presented in Table S1. Results showed that TMJD patients reported significantly greater negative mood than did controls [F(3,115) = 4.24; p < 0.05]. Furthermore, negative affectivity, ethnicity and gender were all significantly associated with heat pain responses (p’s < 0.05; Table S2); therefore, ethnicity, gender and the PANAS-neg were statistically controlled in all subsequent analyses examining differences in heat pain responses between TMJD patients and healthy controls.
4.2 HPTh and HPTo
The TMJD patients showed slightly lower HPTh and HPTo compared with controls (Table S1); however, between-group differences did not reach statistical significance for either threshold [F(1,114) = 1.03; p > 0.05] or tolerance [F(1,114) = 1.47; p > 0.05]. The lack of significant between-group differences for HPTh and HPTo did not fulfil the first criterion of mediation; no further analyses were completed using HPTh or HPTo.
4.3 TS of heat pain
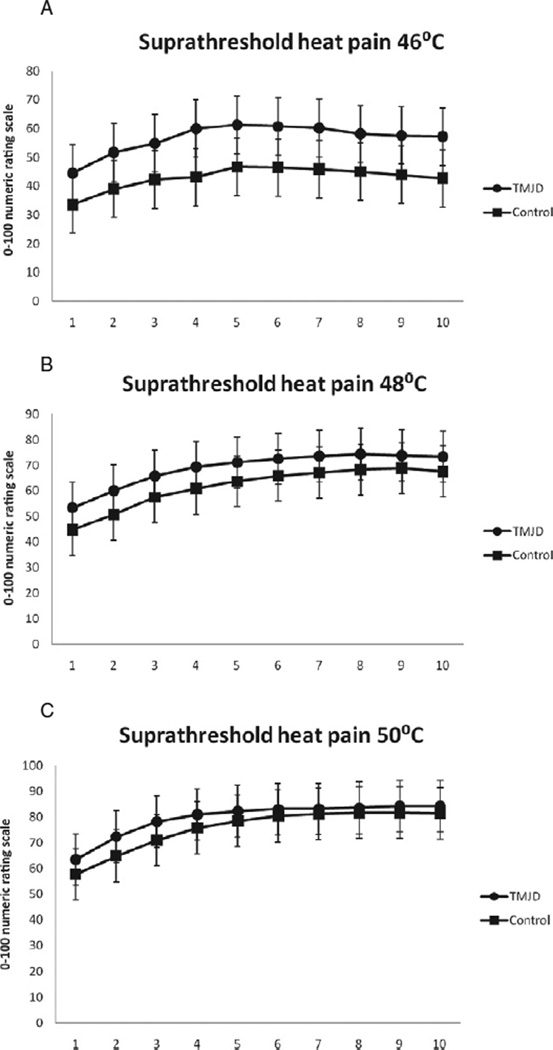
For all temperatures, pain ratings significantly increased across trial for TMJD patients and healthy controls (p’s < 0.05) indicating TS of heat pain (Fig. 1, panels A, B and C). However, the magnitudes of TS did not significantly differ. Specifically, the TS variable created by subtracting the first pain rating from the highest pain rating did not significantly differ between TMJD patients and healthy controls at 46 °C [F(1,114) = 0.07; p > 0.05], 48 °C [F(1,114) = 0.39; p = > 0.05] or 50 °C [F(1,114) = 0.44; p > 0.05]. Levene’s tests were all non-significant (p’s > 0.05), suggesting that the parametric assumption of equality of variance was not violated. The lack of significant between-group differences for TS did not fulfil the first criterion of mediation; a subsequent test of mediation using TS as the outcome was not supported.
Figure 1.
(A) Mean ratings of suprathreshold heat pain responses across the 10 stimuli at 46 °C. (B) Mean ratings of suprathreshold heat pain responses across the 10 stimuli at 48 °C. (C) Mean ratings of suprathreshold heat pain responses across the 10 stimuli at 50 °C.
4.4 Suprathreshold heat pain hyperalgesia
TMJD patients reported significantly greater heat pain ratings (i.e. hyperalgesia) at 46 °C for TMJD patients compared with healthy controls [F(1,114) = 6.56; p < 0.01]. As the temperature of the thermal stimuli increased, these between-group differences became less pronounced, such that there were no significant between-group differences for hyperalgesia at 48 °C F(1,114) = 1.58; p > 0.05] or 50 °C [F(1,114) = 0.94; p > 0.05]. The significant between-group difference for hyperalgesia at 46 °C met the first criterion for continued consideration of simple mediation. Again, all Lavene’s tests were non-significant for this analysis (p’s > 0.05).
4.5 Heat pain responses and sleep quality
Controlling for negative mood, ethnicity and gender, TMJD patients reported significantly poorer sleep quality than did the healthy controls [F(1,114) = 8.16; p < 0.01]; Levene’s test was non-significant (p > 0.05). The between-group difference in sleep quality fulfilled the second criterion of mediation. The correlations presented in Table S2 show that poor sleep quality was significantly associated with greater hyperalgesia at 46 °C [r(119) = 0.27; p < 0.01]. The significant relationship between sleep quality and hyperalgesia at 46 °C provided initial fulfilment of the third criterion for mediation. Sleep quality did not significantly relate to hyperalgesia or TS at any other temperature.
Given significant differences between TMJD patients and healthy controls for hyperalgesia at 46 °C (mediation criterion 1) and self-reported sleep quality (mediation criterion 2), and because sleep quality was significantly related to hyperalgesia at 46 °C (mediation criterion 3), a mediational analysis was completed to determine whether between-group differences in hyperalgesia may have been indirectly transmitted through sleep quality. The model shown in Fig. 2 and Table 1 examined whether self-reported sleep quality partially or completely mediated the between-group difference in mean ratings of hyperalgesia at 46 °C controlling for negative mood, ethnicity and gender. In this model, the indirect effect (i.e. ab path) was found to be significant using a BC bootstrapped CI (95% BC CI: 0.23–6.52 with 5000 resamples), particularly because this CI excluded zero. This finding supports simple mediation by indicating that the between-group difference in hyperalgesia at 46 °C (TMJD patients demonstrated greater hyperalgesia than controls) may have been indirectly affected by poor sleep quality.
Table 1.
Simple mediation model representing case–control differences in mean ratings of hyperalgesia at 46 °C through self-reported sleep quality.
| Effect | Coefficient | SE | t | Sig. | 95% CI bias corrected |
|---|---|---|---|---|---|
| c | 11.98 | 4.68 | 2.56 | <0.05 | |
| a | 1.32 | 0.46 | 2.86 | <0.01 | |
| b | 1.82 | 0.93 | 1.96 | <0.05 | |
| c′ | 9.57 | 4.78 | 2.00 | <0.05 | |
| a × b | 2.34 | 1.51 | d | (LL = 0.23, UL = 6.52) |
This shows unstandardized coefficients for the unconditional indirect effect of group differences in suprathreshold heat pain ratings through self-reported sleep quality.
a, IV to mediator; b, direct effect of mediator on DV; c, total effect of IV on DV through proposed mediator; c′, direct effect of IV on DV; a × b, indirect effect of IV on DV through proposed mediator; IV, independent variable (i.e. TMJD-control status); DV, dependent variable (i.e. hyperalgesia at 46 °C; LL, lower limit; UL, upper limit.
A p-value for the indirect effect is not provided because such a p-value is contingent upon a normal distribution of the indirect effect. Given that the product of the a and b path coefficients is always positively skewed, interpretation of this p-value can be misleading.
Examination of the path coefficients for the current study’s mediational model (see Table 1) shows that the path representing the between-group difference in self-reported sleep quality (path A) is statistically significant (t = 2.86; p < 0.01). Additionally, the path representing the relation between sleep quality and hyperalgesia at 46 °C (path B) is statistically significant (t = 1.96; p < 0.05) and positive. Partial mediation was demonstrated because the path representing the total effect (i.e. indirect and direct) of TMJD versus healthy control affiliation on hyperalgesia at 46 °C (path C; t = 2.56; p < 0.01) remained significant after controlling for the indirect effect of sleep quality (path C’; t = 2.00; p < 0.05). It is important to note that the association between sleep quality and hyperalgesia at 46 °C did not appreciably differ as a function of TMJD or healthy status. Rather, it was the case that, compared with healthy controls, TMJD patients demonstrated greater hyperalgesia at 46 °C and self-reported poorer sleep quality; poor sleep quality subsequently related to greater hyperalgesia at 46 °C.
5. Discussion
This investigation of heat pain responses in TMJD patients and healthy controls showed that it was possible to elicit thermal TS via multiple temperatures of noxious heat; however, this was the case for both TMJD and controls, and TS did not significantly differ between the two cohorts. Likewise, HPTh and HPTo did not differ between groups. These findings are in contrast with the previous studies, which have demonstrated significant differences in pain threshold, tolerance and TS between TMJD and healthy controls using multiple modalities of repeated noxious stimuli (i.e. thermal and mechanical) tested at multiple anatomical locations (i.e. trigeminal and extratrigeminal; Maixner et al., 1998; Ayesh et al., 2007; Sarlani et al., 2007). Most importantly, this study showed that suprathreshold hyperalgesia (calculated as the mean of the 10 heat pulses) was significantly greater for TMJD patients, compared with controls, when tested at 46 °C, but not at 48 or 50 °C. Furthermore, TMJD patients self-reported significantly poorer sleep quality relative to controls and, in turn, poorer sleep quality was positively associated with hyperalgesia at 46 °C. All of these between-group differences and associations remained significant even after statistically controlling the variance accounted for by negative affect and participants’ gender and ethnicity.
One possible explanation for why TS was not found to significantly differ between TMJD patients and controls was due to the presence of ‘ceiling effects’, particularly at the two higher temperatures. Indeed, chi-square tests revealed that, compared with controls, greater proportions of TMJD patients rated the first heat pulse as the maximum (i.e. 100) at 48 °C [χ2 (1) = 6.09; p < 0.05] and 50 °C [χ2 (1) = 7.33; p < 0.01], but not 46 °C [χ2 (1) = 1.96; p > 0.05]. Further analyses of TS omitting those participants demonstrating a ‘ceiling effect’ were not indicated given that removal of these data resulted in parametric violations of the assumption of homogeneity of variance (i.e. Levene’s tests were significant; p’s < 0.05). Methodological differences may also explain the discrepancy between this study and prior studies that found differences in TS between TMJD patients and controls (Maixner et al., 1998; Ayesh et al., 2007; Sarlani et al., 2007). Non-thermal stimuli were used in two of the previous studies (Ayesh et al., 2007; Sarlani et al., 2007) and the thermal stimulus used by Maixner et al. (1998) differed in both size and in temporal characteristics from the one used in the present experiment. Also, Ayesh et al. (2007) tested intra-articular summation thresholds in TMJD patients with arthralgia, contrasted with our assessment of TS on the forearm in patients with TMJD and healthy controls. Furthermore, Sarlani et al. (2007) only observed TS in women with TMJD and not in men. It is worth nothing that recent investigation using methods similar to that of the current study reported equal magnitudes of TS in TMJD patients and controls (Raphael et al., 2009). Another potential reason for the lack of differences in the magnitude of TS between TMJD patients and controls in the current study is that TMJD patients generally self-reported only mild to moderate and infrequent clinical pain intensity on measures assessing pain experiences within the past 6 months (data not shown). Thus, although TMJD patients met the RDC (Dworkin and LeResche, 1992) for their clinical diagnosis, the mild level of chronic pain severity experienced suggests that our TMJD patients may be characterized by less robust alterations in nociceptive processing relative to samples from previous studies. The mild to moderate level of chronic pain severity present in this study’s TMJD cohort may have been the result of the inclusion criteria, which omitted subjects using prescriptions opioids, who may have been doing so to treat more severe chronic pain. It may be that differences in the hyperexcitability of central nociceptive processing between TMJD patients and controls are particularly detectable once the symptoms of clinical pain that often accompany TMJD have become more chronic and moderate to severe in quality.
Our findings of enhanced hyperalgesia at 46 °C heat stimulation in TMJD patients are consistent with others that have previously demonstrated similar findings in patients with chronic musculoskeletal pain conditions (Staud et al., 2003, 2008). However, as the temperature of the heat stimuli increased, these between-group differences became less pronounced, such that there were no significant between-group differences in hyperalgesia at 48 or 50 °C. Chi-square tests revealed that, relative to controls, a greater proportion of TMJD patients prematurely discontinued the series of 46 °C heat pulses prior to completion of the 10th pulse [χ2 (1) = 6.30; p < 0.05]. However, the proportions of TMJD patients and controls that discontinued prior to completion of all 10 pulses were comparable for 48 °C [χ2 (1) = 3.33; p > 0.05] and 50 °C [χ2 (1) = 2.56; p > 0.05]. These results suggest that a larger number of TMJD patients were less willing than controls to endure the full series of 10 heat pulses at 46 °C, perhaps because TMJD patients experienced greater hyperalgesia at this lower temperature. However, as the temperature increased (and subsequently became more painful), a relatively equal number of TMJD patients and controls seemed unwilling to endure the full series of 10 heat pulses at 48 and 50 °C, perhaps due to comparable hyperalgesic experiences. It may be that the poorer sleep reported by TMJD patients resulted in differential hyperalgesia at the least noxious temperature, such that poor sleeping TMJD patients perceived greater pain severity at 46 °C stimulation. However, as the temperature increased (48 and 50 °C), the resulting hyperalgesia became more comparable between TMJD patients and healthy controls, while the influence of poor sleep quality became less pronounced.
The current study adds to the emerging evidence base emphasizing the deleterious effects of sleep disturbance and poor sleep quality on pain sensitivity and the overall experience of pain (Okura et al., 2008; O’Donoghue et al., 2009; Smith et al., 2009b). Poor self-reported sleep quality was significantly related to greater hyperalgesia at 46 °C but not hyperalgesia at 48 or 50 °C. These findings suggest that perceived sleep quality may be contribute to variability in hyperalgesia at lower heat temperatures, and this association is minimal or non-existent at higher heat temperatures. To our knowledge, these data are some of the first to suggest that self-reported sleep quality may indirectly explain differences in suprathreshold heat pain responses between TMJD patients and healthy controls. Support for simple mediation was found in an analysis using a BC bootstrapped CI, such that the presence of TMJD was strongly associated with poorer self-reported sleep quality, which, in turn, was related to enhanced hyperalgesia at a 46 °C stimulus. On balance, these associations provide support for the ability of poor sleep quality to mediate the hyperalgesia and increased pain sensitivity often demonstrated by TMJD patients (Sarlani and Greenspan, 2003). However, this interpretation of the data is tentative and should be considered with caution until future research replicates the current study’s findings.
As in prior studies involving a variety of chronic musculoskeletal pain disorders, TMJD patients in our sample reported poorer sleep quality than did healthy controls. Poorer overall sleep quality in TMJD patients compared with healthy controls may predispose individuals with TMJD to more negative experimental and clinical pain outcomes (Edwards et al., 2009; Smith et al., 2009a). At this time, the mechanisms underlying how sleep quality might affect suprathreshold heat pain responses is not clear; however, ongoing research continues to address this matter. Ukponmwan et al. (1984) reported that the analgesic action of endogenous and exogenous opioids is dependent on undisturbed sleep architecture and undisrupted sleep continuity, as selective rapid eye movement (REM) sleep deprivation prevents opioid analgesia. This suggests that REM sleep deprivation and disrupted sleep continuity can exert an influence on the activity of the opioid system. Sleep deprivation might inhibit opioid protein synthesis (Shapiro and Girdwood, 1981) while concomitantly reducing the affinity of µ- and δ-opioid receptors (Fadda et al., 1991). Furthermore, REM sleep deprivation has been shown to produce changes in other neurotransmitter systems including the serotonergic raphe system (see Longordo et al., 2009 for review), which is also involved in pain control. Indeed, animal models have shown that injection of a 5-HT1A receptor antagonist attenuated mechanical sensitivity in sleep-deprived animals (Wei et al., 2008). Such findings lend support to the involvement of the raphespinal serotonergic system in the maintenance of sleep deprivation-induced hypersensitivity. In humans, it has been suggested that hyperalgesic states resulting from poor and disrupted sleep may be attributable, at least in part, to alterations of endogenous pain inhibitory processes (Smith et al., 2009a). Edwards et al. (2009) found that TMJD patients with poor sleep efficiency (i.e. greater percentage of wake time during the sleep period) and reduced total sleep time recorded by polysomnography (PSG) demonstrated an impaired capacity for endogenous pain inhibition during the day following PSG assessment. That poor sleep quality was related to impaired endogenous pain inhibitory capacity is particularly salient given previous studies demonstrating that endogenous pain inhibition relies heavily upon opioid- and serotonergic-mediated supraspinal mechanisms (Chitour et al., 1982; Le Bars et al., 1992; Wilder-Smith et al., 2004).
Future research should address some important limitations within the current study. First, sleep quality was subjectively reported using the PSQI. Although the PSQI is a measure commonly used to capture individuals’ subjective reports of sleep quality, future studies would benefit from a multi-method approach to sleep assessment that includes objectively derived s leep parameters (e.g. via PSG and actigraphy). Such assessment would allow for greater understanding of how specific domains of sleep quality (e.g. waking after sleep onset, latency and sleep duration) may affect hyperalgesic changes and also centrally mediated pain inhibitory and facilitatory processes. Second, although our data are consistent with prospective findings in which poor sleep quality at baseline predicted later modulation of pain sensitivity and centrally mediated pain processes (Schey et al., 2007), the current study does not rule out the possibility that the associations among sleep quality and hyperalgesia may be bidirectional or co-occurring. That is, greater hyperalgesia to noxious stimuli, in the context of a clinical pain condition, may act to drive subsequent sleep disruption and heightened perception of poor sleep quality. Despite the body of evidence suggesting that poor sleep exerts a deleterious impact on pain and, in turn, greater pain experiences can lead to further impoverished sleep, the current data seem to be more suggestive of a unidirectional association. The PSQI asked participants to report on their sleep quality over the month leading up to the assessment of TS; thus, the temporal precedence of the assessment of sleep quality and TS in the current study suggests that poor sleep quality likely exerted a negative influence on individuals’ experiences of the experimental pain procedures and not vice versa. Third, when assessing TS and hyperalgesia, future studies would perhaps benefit from using thermal temperatures tailored to each person’s tolerance level as previously described (Edwards and Fillingim, 2001; Edwards et al., 2003a). Tailoring the temperatures of the heat stimuli to each person’s tolerance level should help minimize ‘ceiling effects’ and premature discontinuations from the series of 10 heat pulses. Finally, our study sample size was relatively small and may have lacked sufficient power for detecting small (effect size) differences in heat pain outcomes between TMJD patients and healthy controls. We also conducted a large number of statistical tests, which increases the possibility of chance findings. However, a strength of this study is the use of BC 95% bootstrapped CIs for examination of a simple mediation effect. It has been shown that bootstrapping is more appropriate for studies with smaller sample sizes and non-normally distributed variables, resulting in improved type I error rates and statistical power (MacKinnon et al., 2002; Shrout and Bolger, 2002; Preacher and Hayes, 2004). Still, the findings of the present study require replication before being considered definitive.
The current study extends prior research suggesting the potentially important role of sleep in shaping the perception of pain and how it is processed in the central nervous system. Additional studies examining the correlates of sleep and centrally mediated pain modulatory systems such as TS and endogenous pain inhibition within clinical and healthy samples are needed. Whether treatments to improve sleep quality also influence pain outcomes via effects on biological mechanisms such as alteration of ascending or descending pain modulatory pathways is not yet known. However, future studies addressing this topic may further support the primary role of improved sleep quality in the modulation of central pain processing and pain outcomes. Additionally, given the current study’s support for self-reported sleep quality as a partial mediator of hyperalgesia, future studies should examine potential mechanisms how impaired sleep might come to affect pain sensitivity. The opioidergic and serotonergic systems, and their interaction, may be mechanisms worthy of further consideration.
Supplementary Material
Footnotes
Conflicts of interest
None declared.
Author contributions
M.C. Ribeiro-Dasilva: Data collection, manuscript writing, concept and design.
B.R. Goodin: Data analysis and manuscript writing.
R.B. Fillingim: Concept and design.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Descriptive data.
Table S2. Zero-order correlations.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Atsu SS, Ayhan-Ardic F. Temporomandibular disorders seen in rheumatology practices: a review. Rheumatol Int. 2006;26:781–787. doi: 10.1007/s00296-006-0110-y. [DOI] [PubMed] [Google Scholar]
- Ayesh EE, Jensen TS, Svensson P. Hypersensitivity to mechanical and intra-articular electrical stimuli in persons with painful temporomandibular joints. J Dent Res. 2007;86:1187–1192. doi: 10.1177/154405910708601209. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Caes L, Vervoot T, Eccleston C, Vandenhende M, Goubert L. Parental catastrophizing about child’s pain and its relationship with activity restriction: the mediating role of parental distress. Pain. 2011;152:212–222. doi: 10.1016/j.pain.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Chitour D, Dickenson AH, Le Bars D. Pharmacological evidence for the involvement of serotonergic mechanisms in diffuse noxious inhibitory controls (DNIC) Brain Res. 1982;236:329–337. doi: 10.1016/0006-8993(82)90718-1. [DOI] [PubMed] [Google Scholar]
- Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications critique. J Craniomandib Disord. 1992;6:301–355. [PubMed] [Google Scholar]
- Edwards RR, Fillingim RB. Effects of age on temporal summation and habituation of thermal pain: clinical relevance in healthy older and younger adults. J Pain. 2001;2:307–317. doi: 10.1054/jpai.2001.25525. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain. 2003a;101:155–165. doi: 10.1016/s0304-3959(02)00324-x. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Grace EG, Petersen S, Klick B, Haythornthwaite JA, Smith MT. Sleep continuity and architecture: associations with pain-inhibitory processes in patients with temporomandibular joint disorder. Eur J Pain. 2009;13:1043–1047. doi: 10.1016/j.ejpain.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RR, Ness TJ, Weigent DA, Fillingim RB. Individual differences in diffuse noxious inhibitory controls (DNIC): association with clinical variables. Pain. 2003b;106:427–437. doi: 10.1016/j.pain.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Smith MT, Stonerock G, Haythornthwaite JA. Pain-related catastrophizing in healthy women is associated with greater temporal summation of and reduced habituation to thermal pain. Clin J Pain. 2006;22:730–737. doi: 10.1097/01.ajp.0000210914.72794.bc. [DOI] [PubMed] [Google Scholar]
- Fadda P, Tortella A, Fratta W. Sleep deprivation decreases mu and delta opioid receptor binding in the rat limbic system. Neurosci Lett. 1991;129:315–317. doi: 10.1016/0304-3940(91)90489-g. [DOI] [PubMed] [Google Scholar]
- Farrell M, Gibson S. Age interacts with stimulus frequency in the temporal summation of pain. Pain Med. 2007;8:514–520. doi: 10.1111/j.1526-4637.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Maixner W, Kincaid S, Silva S. Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. Pain. 1998;75:121–127. doi: 10.1016/S0304-3959(97)00214-5. [DOI] [PubMed] [Google Scholar]
- Goodin BR, McGuire L, Allshouse M, Stapleton L, Haythornthwaite JA, Burns N, et al. Associations between catastrophizing and endogenous pain-inhibitory processes: sex differences. J Pain. 2009;10:180–190. doi: 10.1016/j.jpain.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Willer JC, De Broucker T. Morphine blocks descending pain inhibitory controls in humans. Pain. 1992;48:13–20. doi: 10.1016/0304-3959(92)90126-V. [DOI] [PubMed] [Google Scholar]
- Lockwood CM, MacKinnon DP. Bootstrapping the standard error of the mediated effect. Nashville, TN: Presented at the 23rd Annual Meeting of SAS Users Group International; 1998. Mar, [Google Scholar]
- Longordo F, Kopp C, Luthi A. Consequences of sleep deprivation on neurotransmitter receptor expression and function. Eur J Neurosci. 2009;29:1810–1819. doi: 10.1111/j.1460-9568.2009.06719.x. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maixner W, Fillingim RB, Booker D, Sigurdsson A. Sensitivity of patients with painful temporomandibular joint disorders to experimentally evoked pain. Pain. 1995;63:341–351. doi: 10.1016/0304-3959(95)00068-2. [DOI] [PubMed] [Google Scholar]
- Maixner W, Fillingim RB, Sigurdsson A, Kincaid S, Silva S. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain. 1998;76:71–81. doi: 10.1016/s0304-3959(98)00028-1. [DOI] [PubMed] [Google Scholar]
- O’Donoghue GM, Fox N, Heneghan C, Hurley DA. Objective and subjective assessment of sleep in chronic low back pain patients compared with healthy age and gender matched controls: a pilot study. BMC Musculoskelet Disord. 2009;10:122–131. doi: 10.1186/1471-2474-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okura K, Lavigne GJ, Huynh N, Manzini C, Fillipini D, Montplaisir JY. Comparison of sleep variables between chronic widespread musculoskeletal pain, insomnia, periodic leg movements syndrome and control subjects in a clinical sleep medicine practice. Sleep Med. 2008;9:352–361. doi: 10.1016/j.sleep.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Raphael KG, Janal MN, Ananthan S, Cook DB, Staud R. Temporal summation of heat pain in temporomandibular disorder patients. J Orofac Pain. 2009;23:54–64. [PMC free article] [PubMed] [Google Scholar]
- Sarlani E, Garrett PH, Grace EG, Greenspan JD. Temporal summation of pain characterizes women but not men with temporomandibular disorders. J Orofac Pain. 2007;21:309–317. [PMC free article] [PubMed] [Google Scholar]
- Sarlani E, Greenspan JD. Evidence for generalized hyperalgesia in temporomandibular disorders patients. Pain. 2003;102:221–226. doi: 10.1016/S0304-3959(03)00095-2. [DOI] [PubMed] [Google Scholar]
- Schey R, Dickman R, Parthasarathy S, Quan SF, Wendel C, Merchant J, et al. Sleep deprivation is hyperalgesic in patients with gastroesophogeal reflux disease. Gastroenterology. 2007;133:1787–1795. doi: 10.1053/j.gastro.2007.09.039. [DOI] [PubMed] [Google Scholar]
- Schutz TCB, Andersen ML, Tufik S. The influence of orofacial pain on sleep pattern: a review of theory, animal models and future directions. Sleep Med. 2009;10:822–828. doi: 10.1016/j.sleep.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Shapiro C, Girdwood P. Protein synthesis in rat brain during sleep. Neuropharmacology. 1981;20:457–460. doi: 10.1016/0028-3908(81)90177-5. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Smith MT, Quartana PJ, Okonkwo RM, Nasir A. Mechanisms by which sleep disturbance contributes to osteoarthritis pain: a conceptual model. Curr Pain Headache Rep. 2009b;13:447–454. doi: 10.1007/s11916-009-0073-2. [DOI] [PubMed] [Google Scholar]
- Smith MT, Wickwire EM, Grace EG, Edwards RR, Buenaver LF, Petersen S, et al. Sleep disorders and their association with laboratory pain sensitivity in temporomandibular joint disorder. Sleep. 2009a;32:779–790. doi: 10.1093/sleep/32.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ. Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003;102:87–95. doi: 10.1016/s0304-3959(02)00344-5. [DOI] [PubMed] [Google Scholar]
- Staud R, Craggs JG, Perlstein WM, Robinson ME, Price DD. Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls. Eur J Pain. 2008;12:1078–1089. doi: 10.1016/j.ejpain.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staud R, Price DD, Robinson ME, Vierck CJ. Body pain area and pain-related negative affect predict clinical pain intensity in patients with fibromyalgia. J Pain. 2004;5:338–343. doi: 10.1016/j.jpain.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Staud R, Vierck CJ, Cannon RC, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- Svensson P, Graven-Nielsen T. Craniofacial muscle pain: review of mechanisms and clinical manifestations. J Orofac Pain. 2001;15:117–145. [PubMed] [Google Scholar]
- Ukponmwan OE, Rupreht J, Dzoljic MR. REM sleep deprivation decreased the antinociceptive property of enkephalinase inhibition, morphine and cold-water swim. Gen Pharmacol. 1984;15:255–258. doi: 10.1016/0306-3623(84)90170-8. [DOI] [PubMed] [Google Scholar]
- Valencia C, Fillingim RB, George SZ. Suprathreshold heat pain response is associated with clinical pain intensity for patients with shoulder pain. J Pain. 2011;12:133–140. doi: 10.1016/j.jpain.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierck CJ, Cannon RL, Fry G, Maixner W, Whitsel BL. Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J Neurophysiol. 1997;78:992–1002. doi: 10.1152/jn.1997.78.2.992. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Carey G. Positive and negative affectivity and their relation to anxiety and depressive disorders. J Abnorm Psychol. 1988a;97:346–353. doi: 10.1037//0021-843x.97.3.346. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988b;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wei H, Ma A, Wang Y-X, Pertovaara A. Role of spinal 5-HT receptors in cutaneous hypersensitivity induced by REM sleep deprivation. Pharmacol Res. 2008;57:469–475. doi: 10.1016/j.phrs.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and actions of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595–1601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.