Abstract
Bone morphogenetic protein-2 (BMP-2) is a potent osteoinductive factor, yet its clinical use is limited by a short biological half-life, rapid local clearance and propensity for side effects. Heparin (HP), a highly sulfated glycosaminoglycan (GAG) that avidly binds BMP-2, has inherent biological properties that may circumvent these limitations. Here, we compared hyaluronan-based hydrogels formulated to include heparin (Heprasil™) with similar gels without heparin (Glycosil™) for their ability to deliver bioactive BMP-2 in vitro and in vivo. The osteogenic activity of BMP-2 released from the hydrogels was evaluated by monitoring alkaline phosphatase (ALP) activity and SMAD 1/5/8 phosphorylation in mesenchymal precursor cells. The osteoinductive ability of these hydrogels was determined in a rat ectopic bone model by 2D radiography, 3D µ-CT and histological analyses at 8 weeks post-implantation. Both hydrogels sustain the release of BMP-2. Importantly, the inclusion of a small amount of heparin (0.3% w/w) attenuated release of BMP-2 and sustained its osteogenic activity for up to 28 days. In contrast, hydrogels lacking heparin released more BMP-2 initially but were unable to maintain BMP-2 activity at later time points. Ectopic bone-forming assays using transplanted hydrogels emphasized the therapeutic importance of the initial burst of BMP-2 rather than its long-term osteogenic activity. Thus, tuning the burst release phase of BMP-2 from hydrogels may be advantageous for optimal bone formation.
Keywords: Bone morphogenetic protein-2, Growth factor, Burst release, Glycosaminoglycan, Mesenchymal cell, Osteogenesis
1. Introduction
Bone morphogenetic proteins (BMPs) orchestrate the onset of osteogenesis and elicit de novo bone formation at both ectopic [1] and orthotopic sites [2]. These potent anabolic factors, particularly BMP-2 [3] and BMP-7 (OP-1) [4], have been exploited therapeutically in orthopedic devices approved by the FDA for the treatment of bone disorders. Despite BMP’s proven efficacy, its clinical application is complicated by its short biological half-live, systemic side effects and rapid clearance [5]. Delivery systems that minimize BMP diffusion away from its therapeutic target are desirable not only to enhance bone formation, but also to limit unwanted pathologies.
Currently, BMP delivery employs one of four broad strategies: inorganic materials, synthetic polymers, natural polymers or composites of these materials [6,7]. Despite modest success, bone healing using these delivery systems is limited by several methodological shortcomings that include pathogen transmission, the non-physiological nature of the materials, the toxic/inflammatory nature of breakdown products, the difficulty in molding or the brittleness of materials and the complexity of the various manufacturing processes [8]. The natural polymer collagen is the only FDA-approved carrier for the therapeutic delivery of BMP-2, with its low affinity resulting in a rapid burst release such that only 5% of the loaded BMP-2 remains after 14 days [9]. The initial high burst of BMP is in contrast to the gradual increase in levels of endogenous BMPs (BMP-2, BMP-4, BMP-6 and BMP-7) that are produced during normal bone healing that peak at day 21 [10]. Another major drawback with collagen is its bovine origin, which carries the risk of provoking immunological responses. This latter potential risk is another reason for limiting BMP-2 use in patients. These drawbacks and limitations necessitate the development of new carriers with improved release and material characteristics.
In addition to the retention and release of BMP-2, optimization of a carrier should also consider the rate of degradation, potential cell binding capacity and cellular uptake. Hydrogels containing hyaluronic acid (HA) are potential candidates as the structure of hyaluronan is identical in all species and the risk of an immune response is small. HA also lends itself to various mixing strategies to allow the addition of sensitive biomolecules prior to application [11]. Also, endogenous cells have several receptors that bind native hyaluronic acid, such as RHAMM, CD44 and CD54 (ICAM) [12]. BMP-2 induces bone formation primarily by enhancing the differentiation of osteoprogenitors [13]. Some reports also suggest that BMP-2 synergizes with suboptimal levels of receptor activator of NF-kappaB ligand (RANKL) to enhance osteoclast differentiation [14]. Notably, hyaluronic acid has an anabolic advantage, because it inhibits osteoclastogenesis through toll-like receptor-4 [15], thus favoring bone deposition over bone resorbtion. Based on its biocompatibility, HA and crosslinked HA products are used clinically as intra-articular injections to treat joint and cartilage diseases, including osteoarthritis [16]. In addition, HA-based hydrogels have been used for delivery of several growth factors, including basic fibroblast growth factor (FGF-2) [17], vascular endothelial growth factor (VEGF) [18] and BMP-2 [19–21]. However, when FGF-2 is delivered without stabilization under normal physiological conditions, it diffuses rapidly, undergoes proteolysis and consequently loses bioactivity [22]. BMP-2 also diffuses and degrades rapidly [6]. Thus, the pharmacological effects of osteogenic factors such as BMP-2 can be enhanced by sustaining their bioactivity during the delivery phase.
Heparin (HP), a hypersulfated variant of heparan lacking domain structure, has been used for many years to stabilize a wide range of susceptible growth factors, including BMP-2 [23]. HP also enhances the biological activities of both homo and heterodimers of BMP, because its binding allows for the continuous introduction of the peptide factor to its cognate signaling receptors [24,25]. HP also potentiates the in vivo ectopic bone formation induced by BMP-2 [26]. In addition to maintaining and enhancing the bioactivity of BMP-2, the high negative charge of HP [23] regulates BMP-2 release from delivery systems [27,28]. However, current HP-containing delivery systems have to utilize relatively large amounts of this GAG, amounts that may exert undesirable or even dangerous anticoagulant effects.
To address this issue we covalently linked small amounts of HP (0.3% w/w) using thiol-based chemistry to a hyaluronan backbone that was then in turn crosslinked to form a hydrogel containing BMP-2. We hypothesized that small amounts of immobilized HP would be sufficient to bind BMP-2 and potentiate its osteogenic effects. The osteoinductive potential of these hydrogels was then determined both in vitro and in vivo and compared to similar hyaluronan-based hydrogel that lacked HP.
2. Materials and methods
2.1. Materials
All reagents and chemicals were purchased from Sigma (St. Louis, MO) unless otherwise stated. Thiol-modified hyaluronan (Glycosil™), thiol-modified hyaluronan mixed with 0.3% w/w thiol-modified heparin (Heprasil™) and poly (ethylene glycol)-diacrylate were obtained from Glycosan Biosystems (a division of BioTime, Inc., Alameda, CA). Recombinant human bone morphogenetic protein-2 (rhBMP-2) and enzyme-linked immunosorbent assay (ELISA) kits for rhBMP-2 were purchased from R & D Systems (Minneapolis, MN).
2.2. Hydrogel preparation for in vitro experiments
Two separate types of 1% (w/v) GAG Glycosil and Heprasil hydrogels were prepared for testing. Separate solutions of Glycosil and Heprasil were prepared in deionized and degassed water. These were then crosslinked by mixing with a solution of polyethylene glycol diacrylate, in a volume ratio of 4:1 to produce the final 1% (w/v) hydrogels. Polyethylene glycol diacrylate (PEGDA) was the thiol-reactive cross-linker. rhBMP-2 was non-covalently incorporated in these hydrogels by uniformly pre-mixing a solution that also contained the modified GAG, prior to crosslinking. Although gelation proceeded rapidly and transparent hydrogels were formed within 20 min, these solutions were maintained under continuous gentle agitation on an orbital rotator at 4 °C for 16 h (overnight) to ensure fully crosslinked, homogeneous and uniform gels. Both hydrogels (30 µL) appeared transparent.
2.3. In vitro release kinetics of rhBMP-2
To study its release kinetics, 5 µg of rhBMP-2 was loaded into 30 µL of separate Glycosil (1%) and Heprasil (1%) hydrogels prior to crosslinking. The hydrogels were then incubated in media containing 0.5 mL phosphate buffered saline (PBS) supplemented with 1% bovine serum albumin (BSA), 10 µg/mL heparin (#H3149) and 1 mm EDTA at 37 °C/150 rpm. Samples of media (50 µL) were collected after 1, 2 and 6 h, and 1, 2, 3, 7, 14, 21 and 28 days. Fresh media (50 µL) was re-introduced to maintain constant volume. Concentrations of rhBMP-2 in the collected media samples were determined by ELISA as per the manufacturer’s instructions (R & D Systems). The cumulative release of rhBMP-2 was then expressed as a percentage of the initial loading amount (5 µg). At the endpoint (28 days), the remaining hydrogel in each tube was digested with 0.5 mL of hyaluronidase (500 U/mL) at 37 °C for 48 h, and the amount of BMP-2 retained by the hydrogel determined as above. The hyaluronidase solution was also supplemented with 1% BSA and 10 µg/mL heparin in order to minimize the degradation of rhBMP-2 [26] during hydrogel digestion. The BMP-2 remaining in the hydrogels after 28 days was also expressed as a percentage of the initial loading amount. We note however that the cumulative release values reported by ELISA typically underestimate the actual amount of BMP-2 released [29], most likely because the BMP-2 antibody fails to recognize degraded BMP-2. To address this, we present the values here as normalized BMP-2 release (%) after multiplying the cumulative release by a normalization factor. The normalization factor for Glycosil and Heprasil hydrogels were 1.97 and 2.32 respectively and were calculated as 100%/[release(%) + retained(%)].
2.4. In vitro bioactivities
2.4.1. Alkaline phosphatase activity assay
As BMP-2 induces differentiation of C2C12 mesenchymal progenitor cells into osteoblasts [30]. We used this in vitro model to study the bioactivity of released BMP-2 over a period of 28 days. Hydrogels (of 30 µL, incorporating 5 µg rhBMP-2) were fabricated on culture inserts (Transwell®, Corning) and cells cultured in their presence. Two different strategies were used to assess the ALP activity induced by released BMP-2. In the first, the inserts containing hydrogels were transferred into new cell cultures every three days in order to determine the ALP response over consecutive periods; this was continued over 28 days. We refer to this ALP experimental set up as “consecutive treatment”. Briefly, the cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 1000 mg/L of glucose, 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. For each consecutive time point, 40,000 cells were seeded into 24-well plates. One day after plating, the medium was replaced with fresh medium containing 5% FBS and cells exposed for a period of 3 days to the BMP-2-loaded hydrogels. Additional cultures with no treatment (negative control) were used to determine the basal ALP activity of C2C12 cells, with cultures treated with BMP-2 at 100 ng/mL used as positive control. For each time point, the trans-wells were transferred to a new cell culture and the exposed cultures then lysed in radio-immunoprecipitation assay (RIPA) buffer (150 mm NaCl, 10 mm Tris, pH 7.4, 2 mm EDTA, 0.5% Igepal/NP40, 0.1% SDS, 1% Triton X-100, protease inhibitor mixture), with the bicinchoninic acid assay (BCA Protein Assay Kit, Pierce) used to determine protein concentration. ALP activity was determined using equal amounts of total protein. Briefly, proteins (20 µg) were incubated with assay buffer (40 µL) including p-nitrophenyl phosphate substrate (Zymed Laboratories Inc.) at 37 °C for 1 h. Calf intestinal alkaline phosphatase (New England Biolabs) was used as positive control. The absorbance at 405 nm was measured using the Victor3 1420 Multilabel Counter (PerkinElmer Life Sciences).
In the second strategy, BMP-2-loaded hydrogels fabricated onto the culture inserts were left in contact with the same cell culture for 3, 7, 14, 21 and 28 days. Separate sets of 24-well plates were prepared for each time point of study. We refer to this ALP set up as continuous treatment. Briefly, 40,000 C2C12 cells were plated into 24-well plates (Corning Incorporated, New York, NY, USA) and cultured in the same media. One day after plating, the medium was replaced with fresh medium containing 5% FBS and BMP-2-loaded hydrogels inserted. The medium was changed after every 3 days. ALP activities were measured as described above. Additional cultures without treatment (negative control) were used to determine the basal ALP activity of C2C12 cells while cultures treated with BMP-2 at 100 ng/mL in each media change were used as positive control.
2.4.2. Phosphorylated SMAD 1/5/8 activity assay
The bioactivity of BMP-2 released from hydrogels was further assessed by determining their ability to induce SMAD 1/5/8 phosphorylation [31]. C2C12 cells (40,000) were seeded in 24-well plates as described earlier. Media containing released BMP-2 (‘release media’) was obtained by incubating hydrogels in PBS supplemented with BSA (1%) for either 7 days or 28 days at 37 °C/150 rpm. Media samples obtained after 7 days or 28 days were diluted 10- or 20-fold, respectively, prior to administration to cells. The SMAD 1/5/8 assay is only sensitive at low BMP-2 concentrations as phosphoSMAD 1/5/8 (pSMAD 1/5/8) Western blot signals saturate at high BMP-2 concentrations (data not shown). Therefore, it was important to dilute the collected release media so as to lower the BMP-2 levels to be within the sensitivity range of the assay. The cell layer was pre-incubated with fresh media (DMEM, 1000 mg/L glucose with 5% FBS) for 24 h. The cells were then exposed to BMP-2-containing media for 15 min and the cell layer lysed in Laemmli buffer, resolved on 4–12% SDS-PAGE gels (Life Technologies), and immunoblotted with antibodies against SMAD 1/5/8 (Santa Cruz Biotechnology) and phosphorylated SMAD 1/5/8 (Cell Signaling Technology).
2.5. Animals and surgery
All animal experiments were performed in strict accordance to guidelines approved by A*STAR’s Institutional Animal Care and Use Committee. Bilateral hind limb intramuscular pockets in six female Sprague Dawley rats (120–150 g) were used as an ectopic bone formation model. Rats were anaesthetized with isoflurane via an induction chamber and maintained throughout the procedure using a facemask. The entire hind limb region was shaved, aseptically prepared for surgery and two transverse skin incisions of 1 cm each were made in both hind limbs of the rat. Bilateral intramuscular pockets (2 on each side) were created by blunt dissections parallel to the longitudinal axis of the muscle fibers and the implants inserted. Thus, each rat received a total of 4 implants, randomly assigned. Sterile polycaprolactonetricalcium phosphate (PCL-TCP) tubes (4.5 mm inner diameter, 3 mm in height, 1 mm wall thickness) were used to deliver the following treatments: Glycosil only, Heprasil only, Glycosil with 5 µg BMP-2 or Heprasil with 5 µg BMP-2. 30 µL of 1% Glycosil hydrogel or 1% Heprasil hydrogel was used for implantation. Each treatment group consisted of six replicates. Incisions were sutured at the muscular layer and sealed with wound clips, and the animals subsequently allowed to recover for 8 weeks post-surgery. After animals were sacrificed by CO2 inhalation, only the implants were harvested, fixed and assessed by 2D x-ray, µ-CT and histology for mineral deposition.
2.6. In vivo data analysis
2.6.1. Radiographic analysis
After surgery and again at week 8 post-implantation, 2D radiographs were taken of all animals and specimens using an Imaging Radiographic System (MUX-100, Shimadzu). Digital micrographs were then taken of the x-rays.
2.6.2. Micro-computed tomography (µ-CT) analysis
Harvested specimens were scanned with a µ-CT scanner (Skyscan 1076; Skyscan, Belgium) at a resolution of 35 µm, a scanning width of 68 mm, a voltage of 104 kV and a current of 98 µA. Cone-Beam CT-reconstruction® A Sasov software (Skyscan) was used to convert the isotropic slice data generated into 2D images. For this, the lower and upper threshold values for bone were set as −315 and 543 Hounsfield units. The data was subsequently analyzed and remodeled in 3D using Mimics 13.1 software (Materialise, Belgium). For all specimens, both the cylindrical region of interest (ROI) and number of slices were kept constant. Quantification of the total volume of newly-formed bone in each specimen was achieved by assigning a predetermined threshold for total bone content. The data are reported as total bone volume (mm3).
2.6.3. Histological analysis
For all histological preparations, harvested specimens were fixed for 1 week in 10% neutral buffered formalin under vacuum. For paraffin sections, specimens were decalcified in 30% formic acid for 2 weeks at room temperature. After processing in a vacuum infiltration processor (Sakura Finetek, Japan) with a 14 h program, followed by dehydration, specimens were embedded in paraffin. Sections (5 µm) were cut and subsequently stained with Hematoxylin & Eosin (H & E) and Ralis Tetrachrome. For undecalcified resin sections, specimens were dehydrated through an ascending alcohol series and subjected to resin processing/embedding in methylmethacrylate. Transverse sections (5 µm) were cut and stained with MacNeal/von Kossa to identify new bone formation. All histological sections were examined under bright field microscopy using an Olympus Stereo (SZX12) and upright fluorescence microscope (BX51).
2.7. Statistical analysis
All data are given as mean ± standard deviation (SD) for n = 4 (in vitro experiments) and n = 6 (in vivo experiments). Two-Way ANOVA with Tukey HSD’s post hoc tests were used for pairwise comparison between hydrogels and their mean BMP-2 release at each time point and also to determine any between-subjects effects of time and hydrogel on release behaviors. Time-dependent ALP activity induced by released BMP-2 from the hydrogels was also compared in a pairwise manner using Two-Way ANOVA with Tukey HSD’s post hoc testing. These tests were also performed to determine between-subject effects of time and hydrogel on ALP activity. The Student t-test (GraphPad software) was used to assess differences in BMP-2-induced SMAD 1/5/8 phosphorylation at 7-day and 28-day. Student t-test was also used to determine differences between the amount of subcutaneous bone induced by the BMP-2-containing hydrogels. This analysis was performed with SPSS software (version 13.0, SPSS Inc., Chicago, IL). In all cases, differences were considered significant at p < 0.05.
3. Results
3.1. Hydrogel fabrication
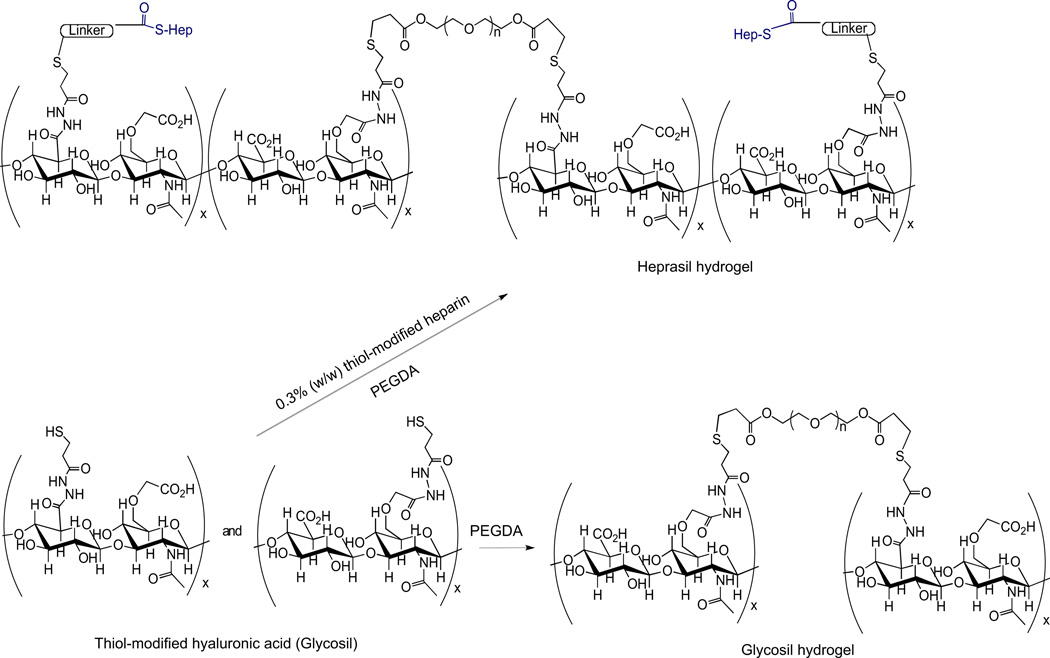
The Glycosil hydrogels for this study were prepared with thiolated derivatives of HA, and the Heprasil hydrogels prepared with thiolated derivatives of HA together with a small amount of a thiol derivatized heparin. HA is a chemically versatile starting material for preparing clinical-grade hydrogels with a variety of biological and mechanical properties for cell and molecule delivery [32]. These HA hydrogels are synthetic mimics of the extracellular matrix, and have been designed for efficient translation from the laboratory to the clinic [33]. Addition of these thiolated derivatives and PEGDA at neutral, or slightly basic pH (Fig. 1) is advantageous for crosslinking [34]. Notably, gelation occurs within 20–30 min, yielding disulfide-crosslinked gels that are stable and highly cytocompatible, both in vitro and in vivo [34,35]. Also the gels have robust biomechanical properties, and can be dehydrated down into a flexible, dry, durable film, which swells without degradation when reversibly rehydrated in aqueous solutions [36]. Such HA-based systems also can be injected during the gelation period without the need for surgical implantation [36]. Importantly, heparin when covalently co-crosslinked into these gels retains its binding affinity for growth factors [17,37].
Fig. 1.
Chemical structures of crosslinked HA hydrogels (Glycosil) and crosslinked HA-HP hydrogels (Heprasil).
3.2. In vitro BMP-2 release and bioactivity
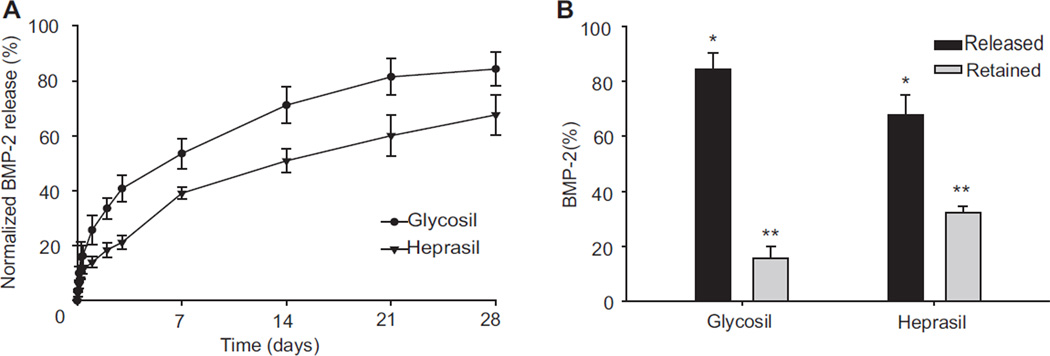
To assess the ability of Glycosil and Heprasil hydrogels to sustain the delivery of mitogenic factors, BMP-2-loaded hydrogels were allowed to release peptide in vitro at 37 °C as described in Materials and Methods. Several additives were required in the release buffer to maintain BMP-2 activity and prevent its surface adsorption. Heparin (10 µg/mL) was added to minimize the degradation of released BMP-2 [26] and to help maintain its activity [24] after release, 1% BSA was added to prevent adsorption onto the tube surface, and 1 mm EDTA was added as a chelator to prevent trace metal-induced disulfide exchange between the hydrogels and the BMP-2 [38]. Both the hydrogel formulations were able to sustain the release of BMP-2 over a period of at least 28 days (Fig. 2A). Both displayed an initial burst release that was followed by near-linear sustained release profiles. The Glycosil hydrogel showed an initial burst release of 25.74 (±5.28) % of the contained BMP-2 during the first 24 h, which subsequently plateaued over 3 days to assume a sustained release (R2 = 0.93). In contrast, the BMP-2 release profile of Heprasil hydrogels yielded an initial burst release of only 14.0 (±1.94) % during the first 24 h, which also leveled off over 3 days to assume an almost linear sustained release (R2 = 0.93). The Glycosil hydrogels released a total of 84.32 (±6.07) % of the loaded BMP-2 over 28 days, whereas the Heprasil hydrogels released only 67.63 (±7.31) % of the loaded BMP-2 over the same period. We also analyzed the amount of BMP-2 retained in the hydrogels after 28 days by digesting them with hyaluronidase, and found that 15.67 (±4.24) % and 32.35 (±2.27) % was still present in the Glycosil and Heprasil hydrogels respectively (Fig. 2B and Table 1). There was also a significant (p = 0.003) between-subjects effect of time on BMP-2 release, suggesting that both time and carrier effects interact to govern BMP-2 release.
Fig. 2.
In vitro release of BMP-2 from Glycosil and Heprasil hydrogels over a 28 days period (A) and BMP-2 (%) remaining or released from the hydrogels over 28 days (B).
Table 1.
Glycosil and Heprasil hydrogels and their release characteristics after 28 days.
| Hydrogel | Released BMP-2 (%) | Retained BMP-2 (%) | |
|---|---|---|---|
| Burst | Sustained | ||
| Glycosil | 40.81 ± 8.85 | 43.52 ± 5.70 | 15.67± 4.24 |
| Heprasil | 21.21 ± 2.69 | 46.43 ± 9.21 | 32.36± 2.28 |
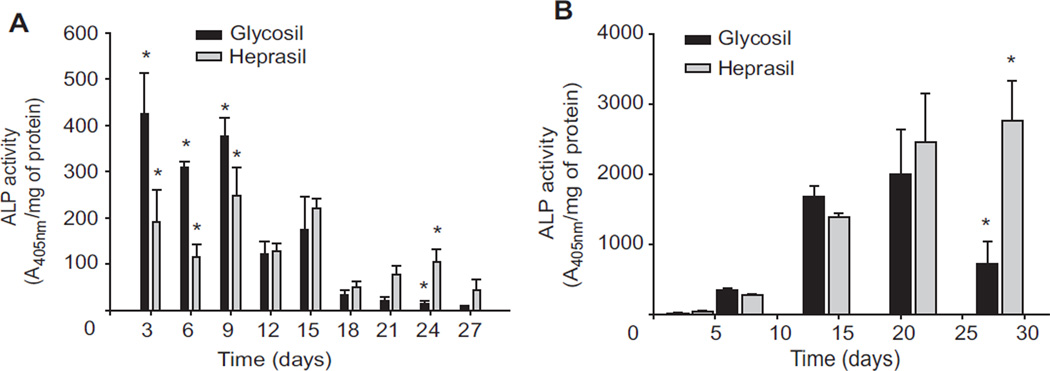
Next, the biological activity of BMP-2 released from Glycosil and Heprasil hydrogels was determined by measuring ALP activity in consecutive 3-day cultures of C2C12 cells over 28 days (Fig. 3A). C2C12 cells are a classical cell culture model for differentiation of mesenchymal progenitor cells [30]. C2C12 cells adopt an osteogenic cell fate upon BMP-2 administration and ALP is a well-established marker for the initial stages of osteoblast differentiation. Overall, BMP-2 released from both hydrogels significantly (p < 0.05) increased ALP activity at all time points. However, the increased ALP activity for Glycosil hydrogels was significantly (p < 0.05) higher over the first 9 days compared to that of Heprasil hydrogels. Yet by days 12 and 15 no differences we observed. At all time points after day 18, significantly higher ALP activity was observed for the Heprasil gels loaded with BMP-2.
Fig. 3.
Individual hydrogels releasing BMP-2 were exposed to fresh cultures every 3 days (consecutive treatment) for up to 27 days and ALP activities were determined (A). In a separate experiment, cells were cultured for up to 28 days (continuous treatment) in the presence of BMP-2 releasing hydrogels and ALP activities were determined (B).
As BMP-2 releases continuously from both hydrogels over 28 days, we assessed the ability of hydrogel-released BMP-2 to enhance ALP activity in continuous culture (Fig. 3B). The BMP-2 released from both hydrogels was able to significantly enhance ALP activity at all time points. The increases in ALP activity for early cultures at 3, 7 and 14 days with Glycosil hydrogels were greater than those of cultures with Heprasil hydrogels. Interestingly, in contrast to consecutive exposure, levels of ALP activity after continuous exposure to BMP-2 for up to 28 days were significantly higher (p < 0.05) with Heprasil hydrogels compared to cultures with Glycosil hydrogels. Also, as observed for the “BMP-2 release”, there was a significant (p < 0.008) interaction between the time-related and carrier-related effects on ALP activity (both consecutive and continuous treatments). As expected, the BMP-2 released from the hydrogels in the continuous treatment system resulted in much higher ALP activity level as compared to that in the consecutive treatment system due to overall exposure to the BMP-2 (Fig. 3B).
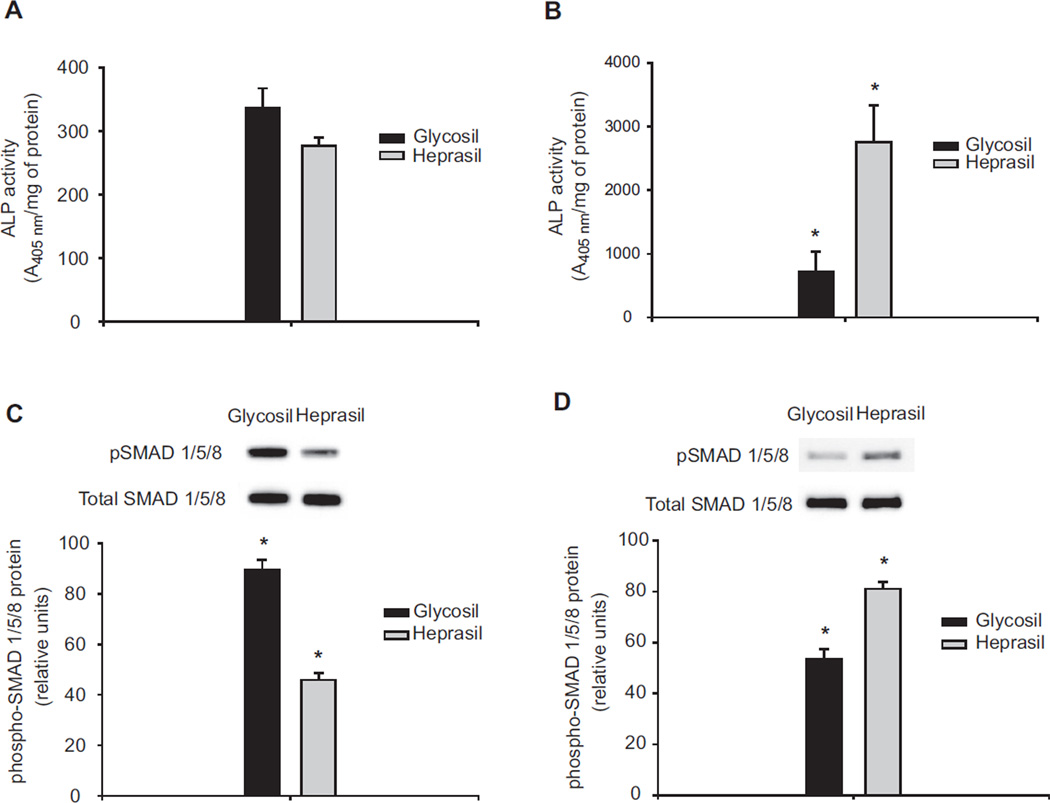
Phosphorylation of SMAD 1/5/8, a molecular event that monitors the intracellular response to BMP-2 signaling, was also analyzed to confirm the biological activity of the BMP-2 released from the hydrogels. Most studies rely on in vitro ALP assays to assess BMP-2 activity. As ALP is only an indirect measure of BMP-2 activity, the need exists to more fully benchmark measures of responsiveness. This motivated us to assay SMAD 1/5/8 phosphorylation as a more precise molecular marker for the bioactivity of BMP-2. We chose days 7 and 28 to study the time-dependency of the bioactivity of released BMP-2. Phosphorylation of SMAD 1/5/8 directly measures the serine/threonine kinase activities of the BMP receptors (BMPR-I and R-II) due to their productive interactions with biologically active BMPs at the cell surface [31]. Overall, the BMP-2 released from both hydrogels at early (day 7) and late (day 28) time points induces phosphorylation of SMAD 1/5/8 in C2C12 cells. Phosphorylated SMAD 1/5/8 levels for BMP-2 released from Glycosil hydrogels by day 7 (Fig. 4C) were also significantly (p < 0.05) greater than observed for BMP-2 released from Heprasil hydrogels. In contrast, phosphorylated SMAD 1/5/8 levels by day 28 (Fig. 4D) and ALP levels were significantly (p < 0.05) higher upon incubation of C2C12 cells with media containing BMP-2 released from Heprasil compared to Glycosil.
Fig. 4.
Bioactivity of BMP-2 released from the hydrogels. Alkaline phosphatase activities of the cells which is continuously exposed to BMP-2 releasing hydrogels for either 7 days (A) or 28 days (B). In a parallel experiment, BMP-2 was released from the hydrogels for either 7 days (C) or 28 days (D) and the conditioned media was dosed onto fresh cultures and levels of stimulated pSMAD 1/5/8 assessed.
3.3. In vivo implantation
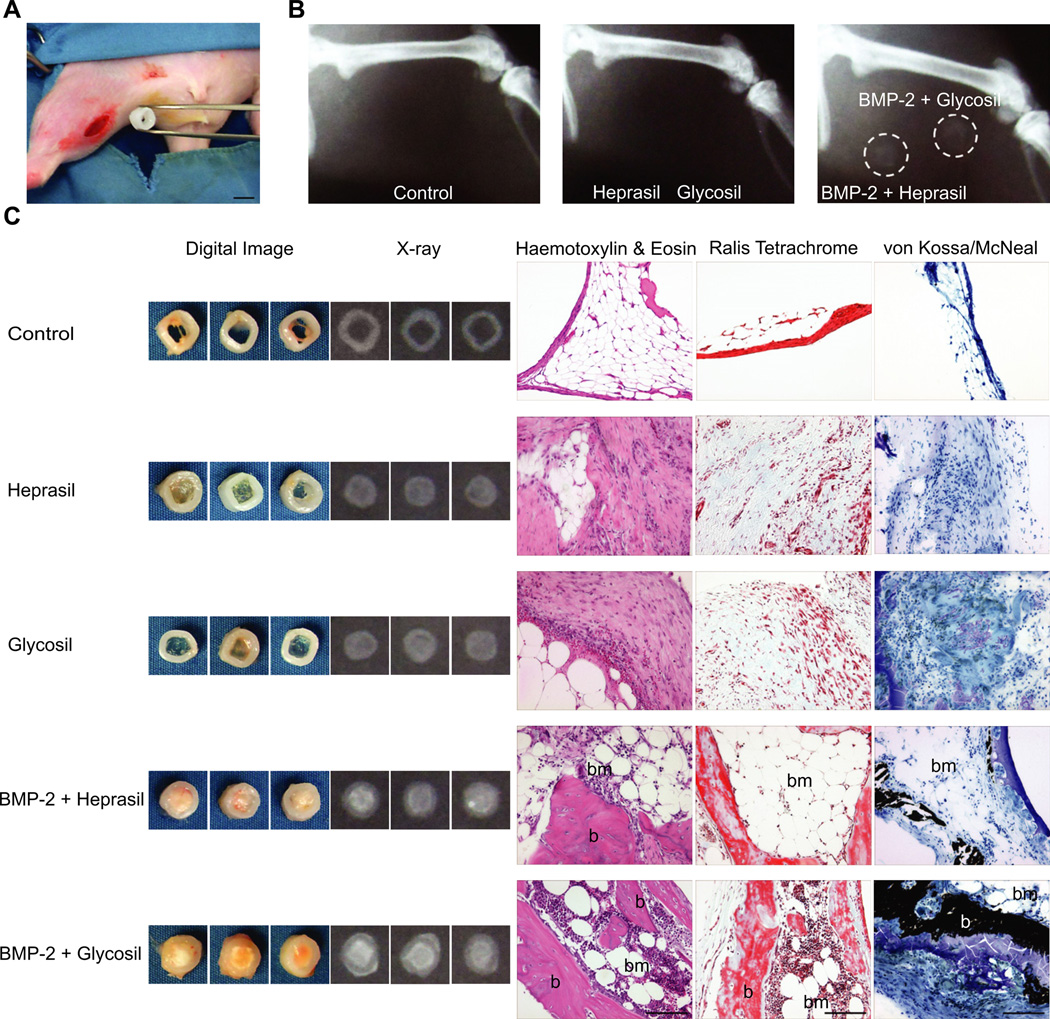
We next explored the ability of the BMP-2-loaded hydrogels to form bone in a rat ectopic (hind limb muscle) model (Fig. 5A). At 8 weeks, transplanted samples were harvested and assessed. It was readily apparent that hydrogels without BMP-2 did not form any hard tissue, as opposed to the hydrogels incorporating BMP-2 (Fig. 5B–C). X-ray analysis confirmed this result and areas of mineralization were noted for both hydrogel/BMP-2 groups. Also, Glycosil/BMP-2 hydrogels yielded more mineral deposition than Heprasil/BMP-2 hydrogels, a finding supported by hematoxylin and eosin stained paraffin sections (Fig. 5C). Meanwhile, hydrogels lacking BMP-2 produced abundant fibroblast/fatty tissue. The Heprasil and Glycosil hydrogels loaded with BMP-2 stimulated robust bone deposition that morphologically resembled woven bone containing osteoblasts and osteocytes as well as infiltrating bone marrow elements characterized by cellular masses of developing blood cells lying between large, round fat cells. We also observed more marrow-like tissue with Glycosil-based treatment as compared to Heprasil.
Fig. 5.
In vivo implantation of hydrogels into muscle of the rat hind limb. (A) Digital micrograph showing surgical implantation of a PCL-TCP tube containing the BMP-2 releasing hydrogel. Scale bar indicates 5 mm (B) X-ray micrographs 8 weeks post-implantation showing new bone formation only in the presence of BMP-2 releasing hydrogels (dotted circles). (C) Representative digital and x-ray images of hydrogels post-harvest (8 weeks post-implantation) along with corresponding histological sections stained with H & E, Ralis tetrachrome (red: bone, blue: osteoid) and von Kossa/McNeal (black: mineral deposits). b: bone, bm: bone marrow. Scale bar indicates 100 µm.
To further confirm the deposition of bone-like tissue in the ectopic site, histological sections were stained with Ralis Tetrachrome and von Kossa/McNeal respectively (Figs. 5C and 6). Control, Heprasil- and Glycosil-alone groups resulted in negligible bone deposition as evident from the lack of bright red staining (for Ralis Tetrachrome) or black deposits (for von Kossa/McNeal). In contrast, hydrogels loaded with BMP-2, contained multiple areas stained bright red by Ralis Tetrachrome and von Kossa-stained black deposits, indicative of bone mineral. Also, bone marrow elements were found throughout the ectopic bone tissue for both groups, with the marrow in the Glycosil/BMP-2 group appearing more cellular. Similar observations were found with the hematoxylin-stained paraffin sections.
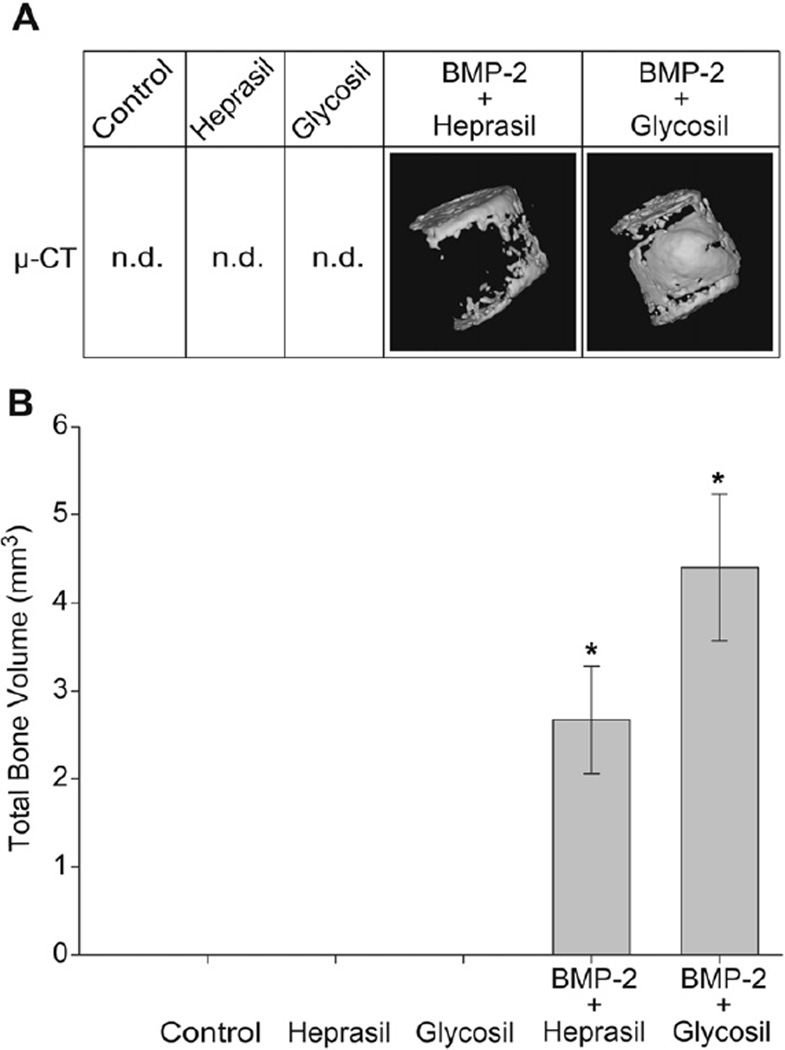
Fig. 6.
Reconstructed 3D images from harvested tissue showing robust bone formation in the presence of BMP-2 releasing hydrogels (A). Bone volume measurements (mm3) from µ-CT scan of harvested tissues. N = 6/group, *p < 0.05, n.d.: not detected.
The bone-forming capacity of Glycosil/BMP-2 or Heprasil/BMP-2 hydrogels were quantitatively assessed by µ-CT bone volume (mm3) measurements. The data strongly support the histological evidence with negligible bone formation for the control, or hydrogels containing only Heprasil or Glycosil However, the Glycosil/BMP-2 hydrogel yielded approximately 1.5-fold more bone than the Heprasil/BMP-2 hydrogel (4.4 ± 0.8 mm3 versus 2.7 ± 0.6 mm3 respectively) (p < 0.05). Reconstructed 3D images of the µ-CT slices showed bone mainly on the top and bottom surfaces of the Heprasil/BMP-2 hydrogel implant, whereas the Glycosil/BMP-2 hydrogels exhibited significantly more bone formation throughout the gel matrix.
4. Discussion
Although the therapeutic effects of BMP-2 are well recognized, a clinically effective means of delivering BMP-2 has not yet been realized. While supra-physiological doses of BMP-2 have adverse effects on tissues [39], insufficient amounts are of no use either. From a pharmacokinetic perspective, a successful delivery device must maintain a sustained drug release within the therapeutic window [40]. Hence we are interested in developing heparin-functionalized hyaluronan hydrogels that can retain BMP-2, sustain its release and preserve or even enhance its biological activities. To achieve this objective, we prepared an HA-based hydrogel with 0.3% (w/w) of covalently linked HP. This concentration was selected based on experience with VEGF delivery using similar hydrogels [37]. Rates of release from 0.3% hydrogels were essentially the same as those composed of 3% HP. Although the incorporation of covalently linked HP in the hydrogels regulates susceptible growth factor release, high concentrations can be problematic. The ionic character of the gel increases, altering buffer exchange and protein diffusion non-specifically. Increased HP levels can also slow the rate of crosslinking and decrease the stability of the resulting hydrogel. Most significantly, high levels of HP markedly increase a gel’s avidity for a particular growth factor, ultimately slowing its release to the point that a major fraction may not be released. Therefore, it is important to minimize the HP content in these hydrogels.
Our in vitro release studies revealed a biphasic profile in which an initial burst of BMP-2 is followed by a sustained release for both Glycosil and Heprasil hydrogels. HA itself binds BMP-2 electrostatically [41], albeit weakly and non-specifically. Thus, the initial burst can be attributed to the dissociation of weakly bound BMP-2 near or on the surface of the hydrogels, with subsequent depolymerization of the hydrogel network permitting a steady release thereafter. Both Glycosil and Heprasil hydrogels exhibit similar profiles except for the initial burst. The Glycosil hydrogel showed an initial burst release of 25.73 (±5.28) % of the contained BMP-2 during the first 24 h, while Heprasil yielded an initial burst of only 14.0 (±1.94) %. This reduction is most likely due to covalently linked HP. HP associates with a variety of growth factors with comparatively high affinity and via electrostatic interactions between the clusters of positively charged basic amino acids on the growth factor and specific, spatially grouped, negatively charged sulfate groups on the sugar [42]. Because BMP-2 has a defined heparin-binding domain [23], the release kinetics and biological functions of HBGFs can be regulated via the strategic incorporation of HP into synthetic scaffolds or delivery systems [37,43]. We conclude that the incorporation of a small amount of HP in Heprasil reduces the magnitude of the initial burst release of BMP-2 by approximately 45% (Table 1).
Despite the widespread use of BMP-2 as a pro-osteogenic factor, few studies have been conducted that correlate the effect of sustained release on subsequent biological activity [44]. In this study, we have combined both release and bioactivity assays in one experimental model to determine their dose- and time-related effects. Clearly, while evaluating the utility of a growth factor delivery system, it is important to consider the combination of dose delivered per unit time and subsequent potency. Therefore, ALP activity was determined here under two different cell culture conditions. Under our consecutive treatment regimen, cells cultured in the presence of either hydrogel showed enhanced ALP activity, albeit it was greater for Glycosil hydrogels at earlier time points. In contrast, at later time points, when less BMP-2 is being released, Glycosil hydrogels trigger less ALP activity. Conversely, ALP activity in the Heprasil group was less at early time points, but greater as time progressed.
The greater ALP activity in the presence of Heprasil at later time points is likely due to the two factors. First, the Heprasil hydrogels release more BMP-2 at later time points; secondly, the BMP-2 released from Heprasil is likely to be more active due the protection afforded by heparin [25,26] inside the gel matrix. As a corollary, Heprasil hydrogels also release more heparin at later time points when the gel starts to degrade (data not shown). Takada et al. [24] has also reported that heparin at 5 µg/mL enhances ALP activity induced by BMP-2. In a similar study, Zhao et al. [26] showed that heparin strongly stimulates induction of ALP activity by BMP-2 (or BMP-4 or BMP-6) at concentrations ranging from 50 to 400 ng/mL. It is not clear why heparin is unable to enhance the ALP activity of BMP-2 released by Heprasil hydrogels at earlier time points, but this unexpected finding is most likely due to intricate and time-dependent differences in the release kinetics of HA, HP and BMP-2 as hydrogels decompose.
Phosphorylation of SMAD 1/5/8, which represents a highly effective marker that directly reflects the actual bioactivity of BMP-2 in the external cellular micro-environment, was significantly greater with Glycosil hydrogels than with Heprasil hydrogels at 7 days. This result is presumably due to the initially greater burst in BMP-2 activity for Glycosil. In contrast, SMAD 1/5/8 phosphorylation was significantly greater with Heprasil hydrogels than Glycosil hydrogels at day 28, most likely due to the presence of heparin [26]. Zhao et al. [26] have also demonstrated that heparin enhances the levels of phosphorylated SMAD 1/5/8 induced by BMP-2 in C2C12 cells, but only after 12 h. In our study, we exposed C2C12 cells for only 15 min to BMP-2 released from either hydrogel. In contrast, the release media collected from Heprasil at day 28 also contains free heparin from the process of gel depolymerization, which may also contribute to enhanced phosphorylation of SMAD 1/5/8. The SMAD 1/5/8 phosphorylation results are thus in agreement with the ALP activity data.
One prominent finding of our study is that Glycosil hydrogels form more and better quality bone in vivo than Heprasil hydrogels, as is expected from the fact that Glycosil releases more BMP-2 initially than Heprasil. Our in vivo results indicate that Heprasil’s ability to sustain the release of BMP-2 over longer periods is not sufficient for faster and better bone formation. Rather, this study demonstrates that the initial release of BMP-2 over the first few days is critical for improved bone formation. This interpretation is supported by a recent study by Brown et al. [45] where a burst followed by the sustained release of rhBMP-2 from a polyurethane scaffolds generated 50% more new bone formation compared to an instant release, yet a sustained release without the burst did not form significant bone. It would appear that the burst release of BMP-2 from Glycosil triggers a more efficient recruitment of MSCs and pre-osteoblasts from the surrounding tissues compared to Heprasil.
5. Conclusions
This study demonstrates that BMP-2 can be incorporated into Glycosil and Heprasil hydrogels to permit sustained release over prolonged periods of time. The presence of small amounts of heparin in Heprasil gels retains BMP-2 more effectively and releases BMP-2 with a more measured initial burst compared to Glycosil hydrogels. Entrapment of BMP-2 into these hydrogels results in the release of biologically active BMP-2 for at least 28 days in vitro. BMP-2 delivered both via Glycosil or Heprasil induces osteogenesis upon implantation in an in vivo rat ectopic bone formation model. Thus, although both these hyaluronan-based carriers appear useful for delivery of bioactive BMP-2 in bone tissue-engineering applications, Glycosil resulted in a more efficacious outcome in vivo. This study demonstrates the importance of the initial burst release in addition to release maintenance in clinically relevant strategies involving biomaterials that ultimately strive for optimal osteoinductive effects of BMP-2 in human patients. The availability of cGMP manufactured HyStem-Rx based on the Glycosil and Heprasil materials will accelerate translation of these results to a clinical setting [32,33].
Acknowledgments
The authors would like to acknowledge the funding support of Singapore’s Agency for Science, Technology and Research (A*STAR) and Institute of Medical Biology (IMB). We thank all members of our laboratory especially Andrew Krishna Ekaputra, Diah Saraswati Bramono, Murali Sadasivam and Ling Ling for stimulating discussions and technical advice. The authors are grateful to Prof. Jöns Hilborn from the Department of Chemistry, Ångström, Uppsala University, Sweden for his critique of this manuscript.
References
- 1.Chen B, Lin H, Wang JH, Zhao YN, Wang B, Zhao WX, et al. Homogeneous osteogenesis and bone regeneration by demineralized bone matrix loading with collagen-targeting bone morphogenetic protein-2. Biomaterials. 2007;28(6):1027–1035. doi: 10.1016/j.biomaterials.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Sandhu HS, Khan SN. Animal models for preclinical assessment of bone morphogenetic proteins in the spine. Spine. 2002;27(Suppl. 1)(16):S32–S38. doi: 10.1097/00007632-200208151-00008. [DOI] [PubMed] [Google Scholar]
- 3.Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Jt Surg Am. 2002;84-A(12):2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Cook SD. Preclinical and clinical evaluation of osteogenic protein-1 (BMP-7) in bony sites. Orthopedics. 1999;22(7):669–671. [PubMed] [Google Scholar]
- 5.Ruhe PQ, Boerman OC, Russel FG, Mikos AG, Spauwen PH, Jansen JA. In vivo release of rhBMP-2 loaded porous calcium phosphate cement pretreated with albumin. J Mater Sci Mater Med. 2006;17(10):919–927. doi: 10.1007/s10856-006-0181-z. [DOI] [PubMed] [Google Scholar]
- 6.Seeherman H, Wozney J, Li R. Bone morphogenetic protein delivery systems. Spine. 2002;27(Suppl. 1)(16):S16–S23. doi: 10.1097/00007632-200208151-00005. [DOI] [PubMed] [Google Scholar]
- 7.Crouzier T, Sailhan F, Becquart P, Guillot R, Logeart-Avramoglou D, Picart C. The performance of BMP-2 loaded TCP/HAP porous ceramics with a polyelectrolyte multilayer film coating. Biomaterials. 2011;32(30):7543–7554. doi: 10.1016/j.biomaterials.2011.06.062. [DOI] [PubMed] [Google Scholar]
- 8.Li RH, Wozney JM. Delivering on the promise of bone morphogenetic proteins. Trends Biotechnol. 2001;19(7):255–265. doi: 10.1016/s0167-7799(01)01665-1. [DOI] [PubMed] [Google Scholar]
- 9.Uludag H, D’Augusta D, Palmer R, Timony G, Wozney J. Characterization of rhBMP-2 pharmacokinetics implanted with biomaterial carriers in the rat ectopic model. J Biomed Mater Res. 1999;46(2):193–202. doi: 10.1002/(sici)1097-4636(199908)46:2<193::aid-jbm8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 10.Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17(3):513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 11.Piskounova S, Rojas R, Bergman K, Hilborn J. The effect of mixing on the mechanical properties of hyaluronan-based injectable hydrogels. Macromol Mater Eng. 2011;296(10):944–951. [Google Scholar]
- 12.Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242(1):27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 13.Pham L, Beyer K, Jensen ED, Rodriguez JS, Davydova J, Yamamoto M, et al. Bone morphogenetic protein 2 signaling in osteoclasts is negatively regulated by the BMP antagonist, twisted gastrulation. J Cell Biochem. 2011;112(3):793–803. doi: 10.1002/jcb.23003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen ED, Pham L, Billington CJ, Jr, Espe K, Carlson AE, Westendorf JJ, et al. Bone morphogenic protein-2 directly enhances differentiation of murine osteoclast precursors. J Cell Biochem. 2010;109(4):672–682. doi: 10.1002/jcb.22462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang EJ, Kim HJ, Ha J, Ryu J, Park KH, Kim UH, et al. Hyaluronan inhibits osteoclast differentiation via Toll-like receptor 4. J Cell Sci. 2007;120(Pt 1):166–176. doi: 10.1242/jcs.03310. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg VM, Buckwalter JA. Hyaluronans in the treatment of osteoarthritis of the knee: evidence for disease-modifying activity. Osteoarthritis Cartilage. 2005;13(3):216–224. doi: 10.1016/j.joca.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Cai S, Liu Y, Zheng Shu X, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26(30):6054–6067. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Elia R, Fuegy PW, VanDelden A, Firpo MA, Prestwich GD, Peattie RA. Stimulation of in vivo angiogenesis by in situ crosslinked, dual growth factor-loaded, glycosaminoglycan hydrogels. Biomaterials. 2010;31(17):4630–4638. doi: 10.1016/j.biomaterials.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hulsart-Billstrom G, Hu Q, Bergman K, Jonsson KB, Aberg J, Tang R, et al. Calcium phosphates compounds in conjunction with hydrogel as carrier for BMP-2: a study on ectopic bone formation in rats. Acta Biomater. 2011;7(8):3042–3049. doi: 10.1016/j.actbio.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Sanz E, Ossipov DA, Hilborn J, Larsson S, Jonsson KB, Varghese OP. Bone reservoir: injectable hyaluronic acid hydrogel for minimal invasive bone augmentation. J Control Release. 2011;152(2):232–240. doi: 10.1016/j.jconrel.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Crouzier T, Ren K, Nicolas C, Roy C, Picart C. Layer-by-layer films as a biomimetic reservoir for rhBMP-2 delivery: controlled differentiation of myoblasts to osteoblasts. Small. 2009;5(5):598–608. doi: 10.1002/smll.200800804. [DOI] [PubMed] [Google Scholar]
- 22.Edelman ER, Mathiowitz E, Langer R, Klagsbrun M. Controlled and modulated release of basic fibroblast growth factor. Biomaterials. 1991;12(7):619–626. doi: 10.1016/0142-9612(91)90107-l. [DOI] [PubMed] [Google Scholar]
- 23.Ruppert R, Hoffmann E, Sebald W. Human bone morphogenetic protein-2 contains a heparin-binding site which modifies its biological activity. Eur J Biochem. 1996;237(1):295–302. doi: 10.1111/j.1432-1033.1996.0295n.x. [DOI] [PubMed] [Google Scholar]
- 24.Takada T, Katagiri T, Ifuku M, Morimura N, Kobayashi M, Hasegawa K, et al. Sulfated polysaccharides enhance the biological activities of bone morphogenetic proteins. J Biol Chem. 2003;278(44):43229–43235. doi: 10.1074/jbc.M300937200. [DOI] [PubMed] [Google Scholar]
- 25.Jiao X, Billings PC, O’Connell MP, Kaplan FS, Shore EM, Glaser DL. Heparan sulfate proteoglycans (HSPGs) modulate BMP-2 osteogenic bioactivity in C2C12 cells. J Biol Chem. 2007;282(2):1080–1086. doi: 10.1074/jbc.M513414200. [DOI] [PubMed] [Google Scholar]
- 26.Zhao B, Katagiri T, Toyoda H, Takada T, Yanai T, Fukuda T, et al. Heparin potentiates the in vivo ectopic bone formation induced by bone morphogenetic protein-2. J Biol Chem. 2006;281(32):23246–23253. doi: 10.1074/jbc.M511039200. [DOI] [PubMed] [Google Scholar]
- 27.Lin H, Zhao Y, Sun W, Chen B, Zhang J, Zhao W, et al. The effect of crosslinking heparin to demineralized bone matrix on mechanical strength and specific binding to human bone morphogenetic protein-2. Biomaterials. 2008;29(9):1189–1197. doi: 10.1016/j.biomaterials.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 28.Xu X, Jha AK, Duncan RL, Jia X. Heparin-decorated, hyaluronic acid-based hydrogel particles for the controlled release of bone morphogenetic protein 2. Acta Biomater. 2011;7(8):3050–3059. doi: 10.1016/j.actbio.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolambkar YM, Dupont KM, Boerckel JD, Huebsch N, Mooney DJ, Hutmacher DW, et al. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials. 2011;32(1):65–74. doi: 10.1016/j.biomaterials.2010.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127(6 Pt 1):1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson GJ, Darshan D. Small-molecule dissection of BMP signaling. Nat Chem Biol. 2008;4(1):15–16. doi: 10.1038/nchembio0108-15. [DOI] [PubMed] [Google Scholar]
- 32.Prestwich GD. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J Control Release. 2011;155(2):193–199. doi: 10.1016/j.jconrel.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prestwich GD. Engineering a clinically-useful matrix for cell therapy. Organogenesis. 2008;4(1):42–47. doi: 10.4161/org.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shu XZ, Liu Y, Luo Y, Roberts MC, Prestwich GD. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules. 2002;3(6):1304–1311. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Zheng Shu X, Prestwich GD. Biocompatibility and stability of disulfide-crosslinked hyaluronan films. Biomaterials. 2005;26(23):4737–4746. doi: 10.1016/j.biomaterials.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Zheng Shu X, Liu Y, Palumbo FS, Luo Y, Prestwich GD. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials. 2004;25(7–8):1339–1348. doi: 10.1016/j.biomaterials.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 37.Pike DB, Cai S, Pomraning KR, Firpo MA, Fisher RJ, Shu XZ, et al. Heparin-regulated release of growth factors in vitro and angiogenic response in vivo to implanted hyaluronan hydrogels containing VEGF and bFGF. Biomaterials. 2006;27(30):5242–5251. doi: 10.1016/j.biomaterials.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 38.Zhu G, Mallery SR, Schwendeman SP. Stabilization of proteins encapsulated in injectable poly (lactide- co-glycolide) Nat Biotechnol. 2000;18(1):52–57. doi: 10.1038/71916. [DOI] [PubMed] [Google Scholar]
- 39.Shields LB, Raque GH, Glassman SD, Campbell M, Vitaz T, Harpring J, et al. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine. 2006;31(5):542–547. doi: 10.1097/01.brs.0000201424.27509.72. [DOI] [PubMed] [Google Scholar]
- 40.Hollinger JO, Uludag H, Winn SR. Sustained release emphasizing recombinant human bone morphogenetic protein-2. Adv Drug Deliv Rev. 1998;31(3):303–318. doi: 10.1016/s0169-409x(97)00126-9. [DOI] [PubMed] [Google Scholar]
- 41.Kim HD, Valentini RF. Retention and activity of BMP-2 in hyaluronic acid-based scaffolds in vitro. J Biomed Mater Res. 2002;59(3):573–584. doi: 10.1002/jbm.10011. [DOI] [PubMed] [Google Scholar]
- 42.Capila I, Linhardt RJ. Heparin-protein interactions. Angew Chem Int Ed Engl. 2002;41(3):391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 43.Chung YI, Tae G, Hong Yuk S. A facile method to prepare heparin-functionalized nanoparticles for controlled release of growth factors. Biomaterials. 2006;27(12):2621–2626. doi: 10.1016/j.biomaterials.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 44.Kempen DH, Lu L, Hefferan TE, Creemers LB, Maran A, Classic KL, et al. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials. 2008;29(22):3245–3252. doi: 10.1016/j.biomaterials.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown KV, Li B, Guda T, Perrien DS, Guelcher SA, Wenke JC. Improving bone formation in a rat femur segmental defect by controlling bone morphogenetic protein-2 release. Tissue Eng Part A. 2011;17(13–14):1735–1746. doi: 10.1089/ten.TEA.2010.0446. [DOI] [PubMed] [Google Scholar]