Abstract
BACKGROUND
Sensitized heart transplant candidates are evaluated for donor-specific anti-HLA IgG antibody (DSA) by Luminex single-antigen bead (SAB) testing (SAB-IgG) to determine donor suitability and help predict a positive complement-dependent cytotoxicity crossmatch (CDC-XM) by virtual crossmatching (VXM). However, SAB testing used for VXM does not correlate perfectly with CDC-XM results and individual transplant programs have center-specific permissible thresholds to predict crossmatch positivity. A novel Luminex SAB-based assay detecting C1q-binding HLA antibodies (SAB-C1q) contributes functional information to SAB testing, but the relationship between SAB strength and complement-binding ability is unclear.
METHODS
In this retrospective study, we identified 15 pediatric and adult heart allograft candidates with calculated panel-reactive antibody (cPRA) >50% by SAB-IgG and compared conventional SAB-IgG results with SAB-C1q testing.
RESULTS
Pre- and post-transplant DSA by SAB-C1q correlated with DSA by SAB-IgG and also with CDC-XM results and early post-transplant endomyocardial biopsy findings. Individual HLA antibodies by SAB-IgG in undiluted sera correlated poorly with SAB-C1q; however, when sera were diluted 1:16, SAB-IgG results were well correlated with SAB-C1q. In some sera, HLA antibodies with low mean fluorescent intensity (MFI) by SAB-IgG exhibited high SAB-C1q MFIs for the same HLA antigens. Diluting or heat-treating these sera increased SAB-IgG MFI, consistent with SAB-C1q results. In 13 recipients, SAB-C1q–positive DSA was associated with positive CDC-XM and with early clinical post-transplant antibody-mediated rejection (cAMR).
CONCLUSIONS
Risk assessment for positive CDC-XM and early cAMR in sensitized heart allograft recipients are correlated with SAB-C1q reactivity.
Keywords: HLA antibody, complement binding, antibody mediated rejection, heart transplantation, virtual crossmatch
Evaluation of transplant candidates for anti-human leukocyte antigen (HLA) antibodies and their specificities has evolved over time, and currently various forms of solid-phase assays are used routinely to profile pre-formed alloantibodies. Luminex-based methodologies, including single-HLA-antigen-coated beads (SAB), have been adopted to identify HLA antibodies and define unacceptable donor antigens and to mitigate the risk of antibody-mediated rejection (AMR).1–3 However, the sensitive nature of these assays may increase the number of unacceptable antigens and unnecessarily limit the donor pool for sensitized patients. Most routinely used SAB assays do not discriminate between non–complement- and complement-activating antibodies. Therefore, the use of these tests to predict a positive complement-dependent cytotoxicity (CDC) cross-match (XM) is not always accurate.
The Luminex-based HLA SAB assay to detect antibodies binding C1q (SAB-C1q), correlates better with CDC-XM results than conventional SAB-IgG HLA antibody (SAB-IgG) results.4 C1q binding donor-specific anti-HLA IgG antibodies (DSA) have also been shown to be highly correlated with AMR in cardiac allograft recipients, independent of the mean fluorescent intensity (MFI) results of Luminex SAB testing.5 In prior work, we have shown that SAB-C1q can discriminate complement binding and non–complement binding, epitope-specific antibodies toward a single-donor HLA-A2 prior to transplant, during early AMR, and after successful rejection treatment.6 To further elucidate the characteristics of the SAB-C1q assay, we retrospectively analyzed pre- and post-transplant sera from sensitized heart transplant candidates by SAB-IgG and SAB-C1q to determine the predictive value of high-titer HLA antibody for complement binding and the potential inhibitory effect of C1 in patient sera as the cause of low MFI in SAB testing.
Methods
Patient selection
All pediatric and adult cardiac allograft candidates at the University of Pittsburgh Medical Center (UPMC) and Children’s Hospital of Pittsburgh between 2007 and 2011, who had a pre-transplant calculated panel-reactive antibodies (cPRA) >50% for conventional SAB-IgG HLA antibodies were included for study. This investigation was conducted according to protocols approved by the institutional review board of the University of Pittsburgh.
Characterization of HLA antibodies
Pre- and post-transplant sera were analyzed for IgG HLA antibodies (SAB-IgG) using commercially available, Luminex-based SAB kits (LABScreen; One Lambda, Inc., Canoga Park, CA) according to the manufacturer’s protocol and analyzed with HLA FUSION software (One Lambda). Results were expressed as MFI and reactions ≥1,000 MFI were considered positive, unless otherwise indicated. Select sera were tested undiluted and after dilution with phosphate-buffered saline (PBS; 1:8 and 1:16) or after heat treatment (56°C for 30 minutes).
Sera were also tested for C1q-binding HLA antibody (SAB-C1q) using commercially available kits (C1qScreen; One Lambda).4 Briefly, sera were heat treated (56°C for 30 minutes) to denature endogenous C1 and then spiked with 150 μg/μl purified human C1q (hC1q). SAB-C1q reactions ≥500 MFI were considered positive.
cPRA was determined for the 14 patients with pre-transplant sera available based on a threshold of ≥2,000 for SAB-IgG and ≥500 MFI for SAB-C1q assays, respectively, using the OPTN cPRA calculator (http://optn.transplant.hrsa.gov/resources/allocationcalculators.asp?index=78).
Diagnosis of antibody-mediated rejection and acute cellular rejection
In all recipients, surveillance endomyocardial biopsies (EMBs) were performed per protocol during the first 6 weeks. Acute cellular rejection (ACR) was diagnosed based on current ISHLT guidelines.7,8 The diagnosis of antibody-mediated rejection (AMR) was made on clinical grounds based on serial, post-transplant DSA profiles, allograft functional assessments by echocardiogram and catheterization, and EMB findings, including histologic and immunopathologic findings.8 For the purposes of this report, we have indicated where patients were diagnosed as having graft dysfunction and/or EMB features of AMR. Patients with clinical concern for AMR were treated with plasmapheresis (PP) and high-dose intravenous immunoglobulin (IVIg), with some patients also receiving rituximab.
Statistical analysis
Whenever applicable, data are presented as the mean ± standard deviation. Output measures included sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), receiver-operator characteristic (ROC) analysis and correlations. Analysis was conducted using statistical (SPSS, version 20; IBM Corporation, Armonk, NY). Continuous data were statistically analyzed by paired t-test analysis and categorical data analyzed in 2 × 2 contingency tables using Fisher’s exact tests. Statistical significance was set at p < 0.05.
Results
Patient demographics
We identified 15 sensitized heart transplant recipients with cPRA >50%, including 8 pediatric (mean age 10.73 ± 6.13) and 7 adult (mean age 46.57 ± 8.89) patients (Table 1). Pediatric patients had a longer post-transplant follow-up (1,222 ± 365 post-operative days [PODs]) than did the adult recipients (321 ± 282 PODs). Most adult patients were female (6 of 7), whereas the pediatric group had an equal gender distribution.
Table 1.
Patient Demographics, Pre-transplant PRA and Number of HLA Mismatches
| Pt ID (Tx year) | Age at Tx (years) | Gender | Pre-Tx conventional IgG cPRA Class I/II (%) | Pre-Tx C1q cPRA Class I/II (%) | Number of HLA mismatchesa | Follow-up PODb |
|---|---|---|---|---|---|---|
| 1p (2008) | 14.86 | F | 81/51 | ND | 7 | 1,134 |
| 2p (2009) | 14.84 | F | 74/37 | 55/0 | 9 | 1,079 |
| 3p (2009) | 6.98 | M | 97/99 | 38/86 | 11 | 891 |
| 4p (2008) | 2.74 | M | 98/98 | 46/19 | 7 | 1,409 |
| 5p (2008) | 1.24 | M | 0/88 | 0/0 | ND | 1,338 |
| 6p (2010) | 16.47 | M | 88/73 | 4/0 | 8 | 617 |
| 7p (2007) | 13.34 | F | 0/57 | 0/0 | ND | 1,724 |
| 8p (2007) | 15.42 | F | 46/0 | 0/0 | 9 | 1,584 |
| 9a (2010) | 34.66 | F | 99/0 | 96/0 | 8 | 649 |
| 10a (2010) | 47.05 | F | 74/92 | 1/42 | 9 | 591 |
| 11a (2011) | 44.09 | F | 86/23 | 0/0 | 9 | 234 |
| 12a (2011) | 56.96 | F | 96/48 | 80/0 | 8 | 70 |
| 13a (2011) | 40.17 | F | 56/0 | 0/0 | 11 | 62 |
| 14a (2011) | 56.5 | F | 75/0 | 63/0 | 8 | 15 |
| 15a (2011) | 60 | M | 75/80 | 74/70 | NA | NA |
a, adult; p, pediatric; NA, not available or not yet transplanted; ND, not determined.
HLA-A, -B, -C, -DR (DRB1 and DRB3, 4, 5), -DQ.
Follow-up through February 29, 2012.
Of the 14 patients with both SAB-IgG and SAB-C1q antibodies, 8 had Class I and II SAB-IgG antibodies with mean cPRAs of 86 ± 10% and 69 ± 29%, respectively. By SAB-C1q, the cPRA was significantly lower (37 ± 32% and 27 ± 35% for Class I and II, respectively; p < 0.001). Four candidates had only Class I antibodies by SAB-IgG (mean cPRA 69 ± 29) and they also had significantly lower SAB-C1q cPRA (40 ± 48; p = 0.05). Two patients had only Class II antibodies by SAB-IgG (mean cPRA 72 ± 22), neither of whom were SAB-C1q positive.
Correlation of conventional IgG SAB MFI with C1q reactivity
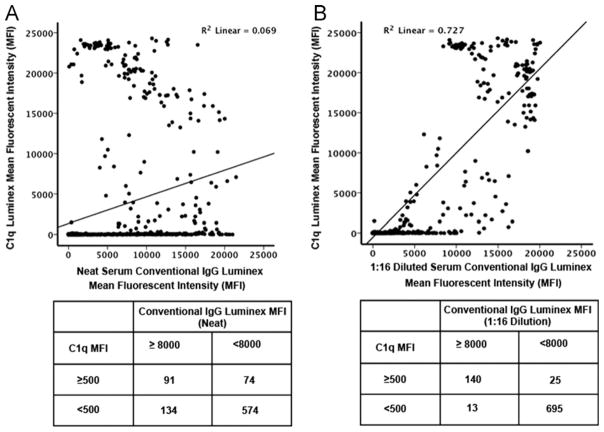
Correlations between the MFI values for individual HLA Class I antigens measured by SAB-IgG using undiluted or 1:16 diluted sera and SAB-C1q were performed on 9 serum samples from 5 heart transplant recipients (5 pre- and 4 post-transplant specimens) (Figure 1). On undiluted sera the pattern of HLA antigens with SAB-IgG positivity correlated poorly with the same HLA antigens detected by SAB-C1q (Pearson’s correlation = 0.262; Figure 1A). Using a value of ≥8,000 MFI in the SAB-IgG assay to predict a positive SAB-C1q, the sensitivity and specificity were only 40% and 88%, respectively, with a PPV of 55% and a NPV of 81%. In contrast, when SAB-IgG was performed using sera diluted 1:16, the correlation between the SAB-IgG and undiluted SAB-C1q results was enhanced (Pearson’s correlation = 0.853; Figure 1B). With diluted sera, the sensitivity and specificity improved to 85% and 95.5%, respectively, with a PPV of 85% and NPV of 98%. By ROC curve analysis the SAB-IgG using 1:16 diluted sera was a significantly better predictor of a positive SAB-C1q value than undiluted sera (area under the curve = 0.988 vs 0.821, respectively; Figure 2).
Figure 1.
Correlation of the HLA Class I antibody single-antigen bead (SAB) mean fluorescent intensity (MFI) results of 873 beads from 9 individual sera assessed by conventional IgG and C1q testing. (A) Comparison of SAB results using undiluted sera vs C1q results. The HLA alleles were segregated using cutoff values of 8,000 MFI in the conventional IgG SAB assay using undiluted sera and 500 MFI for C1q testing. This was used to generate positive and negative predictive values. (B) Comparison of SAB results using sera diluted 1:16 vs C1q results. The HLA alleles were segregated using cutoff values of 8,000 MFI in the conventional IgG SAB assay using 1:16 diluted sera and 500 MFI for the C1q testing for use in generating positive and negative predictive values.
Figure 2.
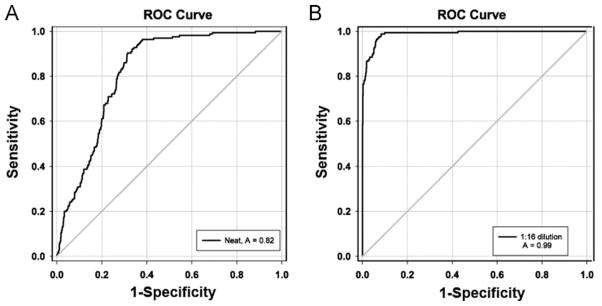
Receiver–operator characteristic curve analysis examining the ability of a mean fluorescent intensity (MFI) value from conventional single-antigen bead testing using (A) undiluted sera or (B) sera diluted 1:16 to predict a positive C1q test value (MFI >500). A better area under the curve (AUC) was found using the 1:16 diluted sera (AUC = 0.988) compared with the undiluted sera (AUC = 0.821).
Inhibition of conventional IgG SAB MFI in the presence of high C1q reactivity
To further elucidate the correlations between SAB-IgG with SAB-C1q reactivity, we identified 5 patients having both high and low MFI SAB-C1q antibodies and performed the SAB-IgG assay using sera diluted at 1:8 or heat-treated sera (Table 2). In these patients, SAB-C1q–positive HLA antigens typically had a SAB-IgG strength <5,000 MFI; however, after dilution (Patients 4p and 6p) or heat treatment (Patients 12a, 13a and 14a), the SAB-IgG MFIs increased to >10,000, suggesting an inhibiting factor within the sera whose potency was reduced by dilution or was heat-labile. All HLA antigens displaying this increase had strong SAB-C1q reactivity (>17,000 MFI). In contrast, HLA antigens with low or negative C1q reactivity had unchanged or decreased SAB-IgG MFI values after heat treatment or dilution. For example, in Patient 13a, the SAB-IgG MFIs of the Class I alleles with low/no C1q reactivity using sera with and without heat treatment were 13,561 ± 898 and 10,831 ± 511, respectively. In contrast, in Patient 4p, alleles having low or negative C1q reactivity using sera diluted 1:8 had a significant drop in SAB-IgG MFI (18,702 ± 1,783 and 9,677 ± 371, respectively).
Table 2.
Inhibition of Conventional IgG Reactivity in Class I Alleles Exhibiting High C1q Reactivity Is Reversed by Serum Dilution or Heat Treatment
| Patient group and ID (number of Class I HLA alleles) | Mean fluorescent intensity (MFI, mean ± SD)
|
p-valuea | ||
|---|---|---|---|---|
| 1:8 dilution | Luminex IgG | Luminex IgG 1:8 dilutiona | Luminex C1q | |
| 4p (12) | 4,871 ± 2,733 | 19,790 ± 1,141 | 20,780 ± 1,307 | <0.001 |
| 6p (11) | 8,800 ± 2,680 | 18,135 ± 625 | 21,601 ± 203 | <0.001 |
|
| ||||
| Heat treatment | Luminex IgG | Luminex IgG heat treateda | Luminex C1q | p-valuea |
| 12a (7) | 3,000 ± 970 | 18,218 ± 1,022 | 15,920 ± 2,580 | <0.001 |
| 13a (6) | 2,500 ± 1,176 | 20,438 ± 728 | 19,876 ± 2,294 | <0.001 |
| 14a (10) | 10,361 ± 3,233 | 19,745 ± 1,222 | 21,482 ± 1,659 | <0.001 |
Paired t-test: undiluted conventional IgG MFI vs 1:8 serum dilution or heat-treated sera conventional IgG MFI.
Correlation of DSA C1q reactivity with early AMR
Table 3 shows the associations of pre- and post-transplant SAB-IgG and SAB-C1q with CDC-XM, EMB results and graft function in the 13 heart allograft recipients (Table 3). Changes in SAB-IgG and SAB-C1q reactivity after treatment of clinical AMR (cAMR) are also shown for each patient.
Table 3.
Pre- and Post-transplant IgG DSA Strength and C1q Reactivity in Pediatric (p) and Adult (a) Heart Transplant Recipients: Correlation With AMR, ACR and Response to AMR Treatment
| Pre-transplant
|
Post-transplant
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pts | CXM | DSA | C1q |
1 week
|
2 weeks
|
3 weeks
|
4 to 5 weeks
|
6 weeks
|
|||||||||||||||
| cAMR | ACR | DSA | C1q | cAMR | ACR | DSA | C1q | cAMR | ACR | DSA | C1q | cAMR | ACR | DSA | C1q | cAMR | ACR | DSA | C1q | ||||
| 1p | + | A2 (M) | ND | − | 0R | A2 (M) | − | + | 0R | A2 (M) | − | ||||||||||||
| A23 (M) | ND | A23(M) | − | A23 (M) | + | ||||||||||||||||||
| DR15(M) | − | DR15(M) | − | ||||||||||||||||||||
| DR51(M) | − | DR51(M) | − | ||||||||||||||||||||
| DQ4 (W) | + | ||||||||||||||||||||||
| 2p | + | A2 (M) | + | + | 0R | A2 (M) | + | −p | 0R | A2 (S) | − | ||||||||||||
| B60 (M) | − | ||||||||||||||||||||||
| 3p | + | DR15 (S) | − | + | 0R | DR15 (S) | − | + | 0R | DR15(S) | + | + | 0R | DR15(M) | − | − | 0R | DR15 (S) | − | ||||
| DR7 (M) | − | ||||||||||||||||||||||
| DR51(M) | − | DR51(W) | − | ||||||||||||||||||||
| DQ9 (S) | + | DQ9(S) | + | DQ9(S) | + | DQ9(M) | + | DQ9 (S) | − | ||||||||||||||
| 4p | + | A24 (S) | + | + | 0R | A24 (W) | + | + | 0R | A24 (W) | + | −p | 1R | A24 (M) | − | − | 0R | A24(W) | − | ||||
| B35 (S) | + | B35 (S) | + | B35 (S) | − | B35 (M) | − | ||||||||||||||||
| B64 (S) | − | B64 (S) | − | B64 (S) | − | ||||||||||||||||||
| A33 (M) | − | A33 (S) | − | A33 (M) | − | ||||||||||||||||||
| DR1 (S) | + | DR1 (M) | + | DR1 (M) | + | DR1 (M) | − | ||||||||||||||||
| DQ5 (S) | − | DQ5 (S) | − | DQ5 (S) | + | ||||||||||||||||||
| DQ6 (M) | − | DQ6 (S) | − | DQ6 (S) | + | ||||||||||||||||||
| 5p | − | DQ7 (M) | − | + | 2R | DQ7(S) | + | + | 0R | DQ7(S) | + | ||||||||||||
| A1 (M) | − | A1 (M) | − | ||||||||||||||||||||
| B60(M) | − | B60(M) | − | ||||||||||||||||||||
| DR7(M) | − | DR7(M) | + | ||||||||||||||||||||
| DQ9(M) | − | DQ9(M) | − | ||||||||||||||||||||
| 6p | − | B35 (M) | − | + | B35 (S) | + | + | 0R | B35 (M) | + | −p | 0R | B35 (S) | + | |||||||||
| DR15(M) | − | DR15(S) | − | DR15(W) | − | ||||||||||||||||||
| DR53(M) | − | DR53(S) | + | DR53(S) | + | DR53(S) | − | ||||||||||||||||
| DR4 (W) | − | DR4 (S) | − | DR4 (W) | − | ||||||||||||||||||
| A11 (S) | − | A11 (M) | − | ||||||||||||||||||||
| Cw12(W) | − | ||||||||||||||||||||||
| 7p | − | DQ7 (S) | − | − | 3R | DQ7 (S) | − | − | 3R | DQ7 (S) | − | ||||||||||||
| DR4 (W) | − | DR4 (W) | − | ||||||||||||||||||||
| 8p | − | A24 (W) | − | − | 3R | A24 (W) | − | − | 3R | A24 (W) | − | ||||||||||||
| DR13 (M) | − | ||||||||||||||||||||||
| 9aa | + | A2 (S) | + | − | 0R | A2 (M) | − | − | 0R | A2 (M) | − | ||||||||||||
| A11 (S) | − | A11 (M) | − | A11 (M) | − | ||||||||||||||||||
| Cw12 (S) | − | Cw12 (S) | − | Cw12 (S) | − | ||||||||||||||||||
| 10aa | − | B42 (W) | − | − | 1R | B42 (W) | − | − | 1R | B42 (W) | − | − | 1R | B42 (W) | − | ||||||||
| Cw17(M) | − | ||||||||||||||||||||||
| DR12(W) | − | DR12(W) | − | ||||||||||||||||||||
| DQ5 (W) | − | DQ5 (M) | − | DQ5 (M) | − | DQ5 (M) | − | ||||||||||||||||
| 11a | − | B7 (W) | − | None | B7 (S) | + | + | 0R | B7 (M) | + | + | 1R | B7 (S) | + | −p | 0R | B7 (S) | − | |||||
| DR10(W) | − | DR10(W) | − | DR10(W) | − | DR10(M) | − | ||||||||||||||||
| DR9 (S) | − | DR9 (S) | + | DR9 (M) | − | ||||||||||||||||||
| DQ2 (M) | − | DQ2 (M) | − | DQ2 (M) | − | ||||||||||||||||||
| 12aa | − | DR1 (W) | − | − | 0R | − | 0R | ||||||||||||||||
| 13a | − | DR4 (W) | − | None | DR4 (S) | + | −f | 1R | |||||||||||||||
| DR53(W) | − | DR53(S) | − | ||||||||||||||||||||
| A2 (W) | − | ||||||||||||||||||||||
| A29 (W) | − | ||||||||||||||||||||||
| B62 (M) | − | ||||||||||||||||||||||
ACR, acute cellular rejection; CXM, cytotoxic crossmatch; ND, not done; cAMR, clinical antibody-mediated rejection determined by circulating DSA, pathology and clinical findings (Patients 1p and 3p) and graft dysfunction (Patients 2p, 4p, 5p, 6p and 11a), all treated. S, strong DSA, >8000 MFI; M, moderate DSA, 2,000 to 8,000 MFI; W, weak DSA, 1,000 to 2,000 MFI. None, no biopsy performed at the time of sera analysis (3 to 5 days post-transplant); −p, no cAMR, only pathologic pAMR defined as having diffuse C4d endothelial cell staining ; −f, no cAMR, only focal C4d endothelial staining.
Patients 9a, 10a and 12a were desensitized pre-transplant; Patients 9a and 10a were also treated first week post-transplant with PP/IVIg per protocol when crossing single or multiple S/M DSAs.
A total of 35 DSA were detected by SAB-IgG testing pre-transplant (1 to 7 DSA/patient). HLA IgG DSA specificity and strength were classified by MFI (strong: >8,000 MFI; moderate: 2,000 to 8,000 MFI; weak: 1,000 to 2,000 MFI). Only 5 of 35 DSA (in 4 patients) were positive by SAB-C1q testing. CDC-XM was positive in 5 of 13 patients, including all 4 recipients with pre-transplant SAB-C1q–positive DSA (p = 0.002, Fisher’s exact test). Within the first month post-Tx, cAMR was diagnosed in 7 patients (Patients 1p to 6p and 11a); all had DSA by SAB-IgG and SAB-C1q. Of the 4 AMR-free patients, all had DSA by SAB-IgG but none had DSA by SAB-C1q, and only the relationship between AMR and C1q DSA was significant (p = 0.005 by Fisher’s exact test). Overall, the presence of circulating C1q-positive DSA post-transplant significantly predicted the development of cAMR, but not ACR, with a positive predictive value (PPV) of 87.5% and a negative predictive value (NPV) of 100%. All 7 patients with the diagnosis of clinical AMR had been treated with PP and high-dose IVIg with and without rituximab. Persistent C1q DSA (Patients 3p, 4p, 5p and 6p) after treatment was associated with resistant clinical AMR, including persistent C4d in biopsy. C1q-negative DSA was associated with resolution of clinical graft dysfunction symptoms (Patients 2p and 4p) but persistent C4d on biopsy (pathologic AMR [pAMR]).
Three adult recipients underwent desensitization (Patients 9a, 10a and 12a) and Patients 9a and 10a, who exhibited a strong single DSA or multiple DSA pre-transplant were treated with PP/IVIg during the first post-transplant week per protocol. Their SAB-IgG remained positive during this period, ranging from strong to moderate, whereas SAB-C1q DSA post-Tx was negative and they had no AMR (cAMR and pAMR). In contrast, Patient 11a developed early cAMR with increased DSA strength becoming C1q positive within 5 days and persisting for >1 month despite treatment. Class II DSA in Patient 13a also increased in strength post-transplant and became C1q positive. PP/IVIg was initiated prior to the first biopsy. This patient had only focal C4d without clinical dysfunction at biopsy. Patient 12a was highly sensitized (96% cPRA), but only had weak IgG DSA that was C1q negative pre-transplant, did not require treatment, and was rejection-free in the first month post-transplant.
Discussion
HLA antibody testing has grown increasingly more sensitive with the transition from cell-based antibody detection to solid-phase methodologies. However, more sensitive assays have caused concern about the significance of the results generated. The Luminex C1q assay combines the sensitivity of SAB testing to identify HLA antibodies with a measure of antibody function by detecting C1 binding ability, the first complement component. Initial reports found the Luminex C1q test was poorly associated with the strength (MFI) of antibodies detected by conventional IgG testing.4 In this study, we also observed a poorer correlation between the MFI of HLA antibodies by IgG testing using undiluted sera (Figure 1). However, an IgG MFI in sera diluted 1:16 was more significantly correlated and a better predictor of C1q reactivity. In our experience, as well in another study,4 C1q reactivity was unbiased with respect to HLA Class I and II alleles. Although we did not determine composition of IgG subclass, C1q binding likely indicates the presence of complement-fixing IgG1 and/or IgG3 subclasses. In contrast, non–C1q binding HLA antibodies may reflect more IgG2 with reduced complement-binding ability.
We have defined an association of HLA antibody MFI values measured by IgG testing with CDC-XM results: an MFI ≥8,000 correlated with a positive CDC-XM.9 For thoracic allograft candidates, we consider any potential DSA ≥8,000 MFI to be VXM positive and contraindicated for transplantation. However, in unpublished findings, we found that some patients with DSA <8,000 MFI had a positive CDC-XM and, conversely, others with DSA ≥8,000 MFI were CDC-XM negative (A.Z. and J.L., unpublished observations). These discrepancies have prompted heated debates within the HLA community and led to different definitions of what the appropriate level of DSA by IgG SAB testing is for a thoracic candidate to avoid hyperacute and early AMR. Our data suggest that DSA maintaining a strong MFI after serum dilution are more frequently complement binding and result in a positive CDC-XM. In contrast, DSA with a significant drop in MFI upon dilution are typically non–complement binding and associated with a negative CDC-XM. We recently reported on a pediatric heart transplant recipient (Patient 2p) with high-titer HLA-A2 epitope–specific HLA antibodies pre- and post-transplant that were C1q-binding.6 The titer of IgG DSA, after treatment for AMR, dropped and subsequently the HLA-A2 antibodies became C1q negative.
Although Luminex-based SAB assays detect HLA antibodies with excellent sensitivity, in some instances false-negative reactions occur. This phenomenon is typically known as the “prozone effect,” due to the presence of high-titer HLA antibodies10 or HLA-specific IgM antibodies.11 Herein we have identified patients with low MFI HLA antibodies by IgG SAB testing in whom, upon C1q testing, exhibited high C1q reactivity in the same HLA antigens. Diluting sera or heat treatment restored the MFI values in conventional IgG testing (Table 2). Our findings are consistent with those of Schnaidt et al,10 who demonstrated that high levels of endogenous C1 inhibit the detection of IgG HLA antibodies by SAB testing. Treating sera with ethylene-diamine tetraacetic acid (EDTA), heat, or the addition of a C1 inhibitor abolished the prozone effect.9 No significant increase in conventional IgG MFI was observed in sera with low C1q reactivity after dilution or heat treatment. Therefore, the inhibition observed in our cohort was likely a result of high endogenous C1q reactivity interfering with IgG HLA antibody detection. The inhibition was reproducible; similar reactivity patterns were seen in desensitized adult heart transplant recipients (data not shown). Importantly, any inhibition can influence VXM when conventional IgG testing using undiluted serum defines unacceptable antibodies. We observed that DSA <4,000 MFI might be considered “acceptable,” but may still be complement binding and result in strong positive CDC-XM (Table 2). Other non–complement-binding antibodies >8,000 MFI might be acceptable, especially for highly sensitized thoracic patients urgently in need of an allograft.
The Organ Procurement and Transplant Network cPRA provides a uniform assessment of transplant candidate sensitization based on unacceptable antigens determined by solid-phase methods based on HLA frequencies derived from the U.S. donor population. Implementation of cPRA has greatly impacted the management of highly sensitized candidates, facilitated VXM, and increased sensitized patient transplantation.12 In a recent review, Chang and Kobashigawa discussed the impact of cPRA and VXM in highly sensitized heart transplant recipients.13 Transplantation of highly sensitized thoracic patients may be facilitated by restricting the unacceptable HLA antibodies to those that are high-titer IgG and/or C1q-positive IgG (Table 1).
Previously, Chin and colleagues described a strong correlation between pre-formed C1q-positive DSA, positive CDC-XM and early AMR.5 In our 13 sensitized heart transplant recipients, a similar correlation between C1q-positive DSA and CDC-XM was seen and resulted in C1q DSA-harboring recipients having a greater cAMR incidence within the first month post-transplant compared to patients without C1q-positive DSA (Table 3). Pre-transplant, most patients exhibited multiple DSA toward both HLA Class I and II antigens detected by IgG SAB testing, but the C1q-positive DSA was far more restricted. After transplant, patients with persistent C1q-positive DSA or with DSA that converted from C1q negative to C1q positive developed early cAMR with EMB findings of diffuse C4d (Table 3). The PPV of C1q DSA for cAMR was 87.5% with an NPV of 100%. All clinically based AMR cases were treated with plasmapheresis and IVIg with or without rituximab. Successful resolution of cAMR correlated with the loss of C1q DSA; persistent C1q DSA was associated with prolonged graft dysfunction and diffuse C4d biopsy staining (pAMR) (Table 3). However, pAMR (diffuse C4d staining) persisted in some patients despite clinical improvement and loss of C1q DSA.
Survival in our highly sensitized cohort was 100% with a follow-up period that ranged from 1 to 56 months post-transplant. Two patients without C1q DSA pre-transplant converted to C1q positive post-transplant and developed early cAMR (Patients 6p and 11a). Thus, in patients with SAB-IgG DSA, continued post-transplant C1q monitoring is advisable. In our limited experience, intervention during the first week post-transplant per protocol in 2 patients (Patients 9a and 10a) who exhibited strong or moderate IgG DSA pre-transplant was associated with a lack of C1q DSA post-transplant and the development of any AMR (Table 3). Furthermore, PP/IVIg for Patient 13a during the first week was initiated in response to increased IgG DSA strength that converted to C1q-positive DSA; the early intervention was associated with focal C4d in the subsequent biopsy without clinical dysfunction.
Despite the small cohort of sensitized thoracic recipients and the retrospective nature of the analysis, the data presented herein provide new insights regarding the characteristics of clinically relevant DSA. Furthermore, pre-transplant risk stratification of sensitized patients is best accomplished by testing for both complement (C1q) and non-complement DSA. Close monitoring of DSA strength (IgG MFI) and function (C1q) post-transplant is imperative for identifying recipients at risk for developing early clinical AMR. Future prospective studies in larger cohorts with longer follow-up are needed to further validate these observations.
Acknowledgments
This study was supported by a Thomas E. Starzl Transplant Institute Young Investigator Grant (to J.L.) and a CSL Berhing unrestricted research grant (to A.Z.).
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose.
References
- 1.Zachary AA, Sholander JT, Houp JA, et al. Using real data for a virtual crossmatch. Hum Immunol. 2009;70:574–9. doi: 10.1016/j.humimm.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Kobashigawa J, Mehra M, West L, et al. Report from a consensus conference on the sensitized patient awaiting heart transplantation. J Heart Lung Transplant. 2009;28:213–25. doi: 10.1016/j.healun.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reinsmoen NL, Lai CH, Vo A, et al. Acceptable donor-specific antibody levels allowing for successful deceased and living donor kidney transplantation after desensitization therapy. Transplantation. 2008;86:820–5. doi: 10.1097/TP.0b013e3181856f98. [DOI] [PubMed] [Google Scholar]
- 4.Chen G, Sequeira F, Tyan DB. Novel C1q assay reveals a clinically relevant subset of human leukocyte antigen antibodies independent of immunoglobulin G strength on single antigen beads. Hum Immunol. 2011;72:849–58. doi: 10.1016/j.humimm.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Chin C, Chen G, Sequeria F, et al. Clinical usefulness of a novel C1q assay to detect immunoglobulin G antibodies capable of fixing complement in sensitized pediatric heart transplant patients. J Heart Lung Transplant. 2011;30:158–63. doi: 10.1016/j.healun.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Zeevi A, Marrari M, Feingold B, et al. Human leukocyte antigen epitope analysis to assess complement- and non-complement-binding donor-specific antibody repertoire in a pediatric heart transplant recipient. Hum Immunol. 2012;73:48–51. doi: 10.1016/j.humimm.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–20. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Berry GJ, Angelini A, Burke MM, et al. The ISHLT working formulation for pathologic diagnosis of antibody-mediated rejection in heart transplantation: evolution and current status (2005–2011) J Heart Lung Transplant. 2011;30:601–11. doi: 10.1016/j.healun.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Batal I, Zeevi A, Lunz JG, III, et al. Antihuman leukocyte antigen-specific antibody strength determined by complement-dependent or solid-phase assays can predict positive donor-specific crossmatches. Arch Pathol Lab Med. 2010;134:1534–40. doi: 10.5858/2009-0581-OA.1. [DOI] [PubMed] [Google Scholar]
- 10.Schnaidt M, Weinstock C, Jurisic M, et al. HLA antibody specification using single-antigen beads—a technical solution for the prozone effect. Transplantation. 2011;92:510–5. doi: 10.1097/TP.0b013e31822872dd. [DOI] [PubMed] [Google Scholar]
- 11.Zachary AA, Lucas DP, Detrick B, Leffell MS. Naturally occurring interference in Luminex assays for HLA-specific antibodies: characteristics and resolution. Hum Immunol. 2009;70:496–501. doi: 10.1016/j.humimm.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Cecka JM, Kucheryavaya AY, Reinsmoen NL, et al. Calculated PRA: initial results show benefits for sensitized patients and a reduction in positive crossmatches. Am J Transplant. 2011;11:719–24. doi: 10.1111/j.1600-6143.2010.03340.x. [DOI] [PubMed] [Google Scholar]
- 13.Chang D, Kobashigawa J. The use of the calculated panel-reactive antibody and virtual crossmatch in heart transplantation. Curr Opin Organ Transplant. 2012;17:423–6. doi: 10.1097/MOT.0b013e328355f195. [DOI] [PubMed] [Google Scholar]