SUMMARY
Proper arrangement of axonal projections into topographic maps is crucial for brain function, especially in sensory systems. An important mechanism for map formation is pre-target axon sorting, in which topographic ordering of axons appears in tracts before axons reach their target, but this process remains poorly understood. Here, we show that selective axon degeneration is used as a correction mechanism to eliminate missorted axons in the optic tract during retinotectal development. We find that retinal axons are not precisely ordered during initial pathfinding but become corrected later, with missorted axons selectively fragmenting and degenerating. We further show that heparan sulfate is required non-cell-autonomously to correct missorted axons, and that restoring its synthesis at late stages in a deficient mutant is sufficient to restore topographic sorting. These findings unravel a novel function for developmental axon degeneration in ordering axonal projections, and identify heparan sulfate as a key regulator of that process.
INTRODUCTION
Organization of neuronal connections into topographic maps is crucial for processing information. One widely accepted mechanism that determines the topographic order of axon terminals relies on specific axon-target interactions, in which axons with a unique profile of receptors interpret guidance cues distributed in a gradient within the target (Feldheim and O’Leary, 2010). Another mechanism far less well understood but also contributing to map formation is pre-target topographic sorting of axons along tracts. In many systems, axons are pre-ordered en route to their target according to their identity and/or positional origin. For instance, olfactory sensory neurons expressing specific odorant receptors and projecting to different locations in the olfactory bulb are pre-sorted in the axon bundle (Bozza et al., 2009; Imai et al., 2009; Satoda et al., 1995). Similarly in the visual system, retinal axons are pre-ordered along the dorsoventral axis in the optic tract before reaching the optic tectum (or superior colliculus in mammals) (Plas et al., 2005; Scholes, 1979). This specific ordering of axons is well conserved among vertebrates and likely involves local regulatory mechanisms independent from brain targets, since sorting of retinal and olfactory axons is preserved in the complete absence of tectum or olfactory bulb respectively (Imai et al., 2009; Reh et al., 1983; St John et al., 2003).
While it appears to have an instructive role in map formation (Imai et al., 2009), how pre-target axon sorting is established and regulated during development is poorly understood. Some signals have been implicated in organizing axons along tracts (Imai et al., 2009; Plas et al., 2008), but the cellular mechanisms by which axons are precisely ordered have never been described. Do axons segregate during initial growth cone guidance, or are their trajectories refined at later stages? If axonal projections are corrected, what are the cellular and molecular mechanisms involved? Exploring these questions requires the ability to directly visualize growing axons in live embryos, an approach that can be challenging in mammalian models. We took advantage of the unique accessibility and transparency of the zebrafish embryo to monitor pre-target sorting of retinal axons in vivo as they elongate along the optic tract. In all vertebrates, axons originating from the dorsal and ventral retina are topographically reorganized after crossing the chiasm so that dorsal and ventral axons segregate respectively into the ventral and dorsal branches of the optic tract (Chan and Guillery, 1994; Plas et al., 2005; Scholes, 1979). Here we report that some dorsal axons misroute along the dorsal branch as they first elongate along the tract, indicating that sorting is not precisely established by initial growth cone guidance. Instead, topographic order is achieved through the selective degeneration of missorted dorsal axon trajectories. In contrast to correctly sorted axons, missorted dorsal axons stop their elongation before reaching the tectum and rapidly fragment all along their length. We further demonstrate that this specific degeneration does not require neuronal activity of retinal ganglion cells (RGCs) or the activation of p53-dependent apoptotic pathways. It depends however on the presence of heparan sulfate (HS) that acts non-cell autonomously for correcting missorted axons and establishing pre-target topographic sorting. Thus, our study not only unravels a novel function for developmental axon degeneration in ordering axonal projections, but also identifies HS as a key regulator required for topographic sorting error correction.
RESULTS
Sorting of retinal axons is achieved through a correction mechanism
To determine whether dorsal and ventral axons are first sorted during initial growth cone guidance along the tract, we performed precise topographic dye labeling of the dorsonasal (DN) and ventronasal (VN) quadrants of the retina in zebrafish embryos fixed at early stages (Figure 1A). Corresponding DN and VN axonal projections were visualized along the optic tract after removing the contralateral eye (Figure 1B). At 48 hours-post-fertilization (hpf), when the first axons elongate along the tract and reach the tectum (Burrill and Easter, 1995; Stuermer et al., 1988), DN and VN axons were not precisely sorted. Some DN axons elongated along with VN axons in the most dorsal (anterior) part of the tract (Figure 1C, C’, Figure S1A, A’). Moreover, growth cones were intermingled and did not segregate along distinct paths according to their dorsoventral identity (Figure 1C’). At 54 and 60 hpf, sorting was more apparent, but some DN axons were still visible in the dorsal part of the tract, growing along or sometimes dorsally to VN axons (Figure 1D-E’, Figure S1B-C’). In contrast at 72 hpf, when projections are more mature, DN and VN axons were completely segregated (Figure 1F, F’). We analyzed and quantified the distribution and levels of DN and VN signals across the optic tract (Figure 1G-I), and generated a missorting index (MI) measuring the proportion of missorted DN axons (Figure 1J). While sorting of VN axons was accurate at all stages observed, a significant number of DN axons were clearly missorted at 48 hpf (MI 23.3%). Missorting of DN axons progressively decreased at later stages (MI 10.8% at 54 hpf) and was no longer observed at 72 hpf. These results indicate that pre-target sorting of retinal axons is not precisely established during initial growth cone guidance along the optic tract but rather is achieved by correcting missorted DN projections.
Figure 1. Sorting of retinal axons in the optic tract is achieved through a correction mechanism.
(A) DiD (blue) and DiI (red) were topographically injected into the DN and VN quadrants of the retina respectively. Lateral view, confocal maximal projection. (B) Diagram of corresponding DN (blue) and VN (red) retinal projections observed at 72 hpf in a lateral view after removing the contralateral eye. The optic tract region is demarcated with orange dashed line. (C-F’) Dorsal axon trajectories are progressively corrected between 48 and 72 hpf. At 48 hpf, DN (C, blue in C’) and VN (red in C’) axons are not precisely sorted in the optic tract. Some DN axons (arrows) are observed along VN axons in the most anterior/dorsal part of the tract. DN (yellow arrowheads in C’) and VN (white arrowhead) growth cones leading axons are intermingled. At 54 hpf (D, D’) and 60 hpf (E, E’), some DN axons are still missorted along VN axons (arrows). This missorting is no longer observed at 72 hpf (F, F’). Lateral views, confocal maximal projections; Scale bar, 20 μm. See also Figure S1. (G-I) Representative examples showing measurement of axon sorting in the tract at 48, 54 and 72 hpf. Intensity (Int, y axis) of DiD (blue) and DiI (red) signals was measured along a reference line drawn perpendicular to the tract (as indicated in C’). DiD signals are observed anteriorly to DiI signals at 48 and 54 hpf (arrows), but not at 72 hpf. (J) Quantification of missorting: a missorting index (MI) was calculated as the ratio of the signal intensity corresponding to missorted DN axons (DR) to the signal intensity of all DN axons (DL + DR). DN axons were considered missorted when located anterior to the VN axons with the highest signal intensity. Missorting of DN axons is evident at 48 hpf and becomes progressively corrected by 72 hpf. **p < 0.05, ***p < 0.001.
Missorted DN axons undergo selective developmental degeneration
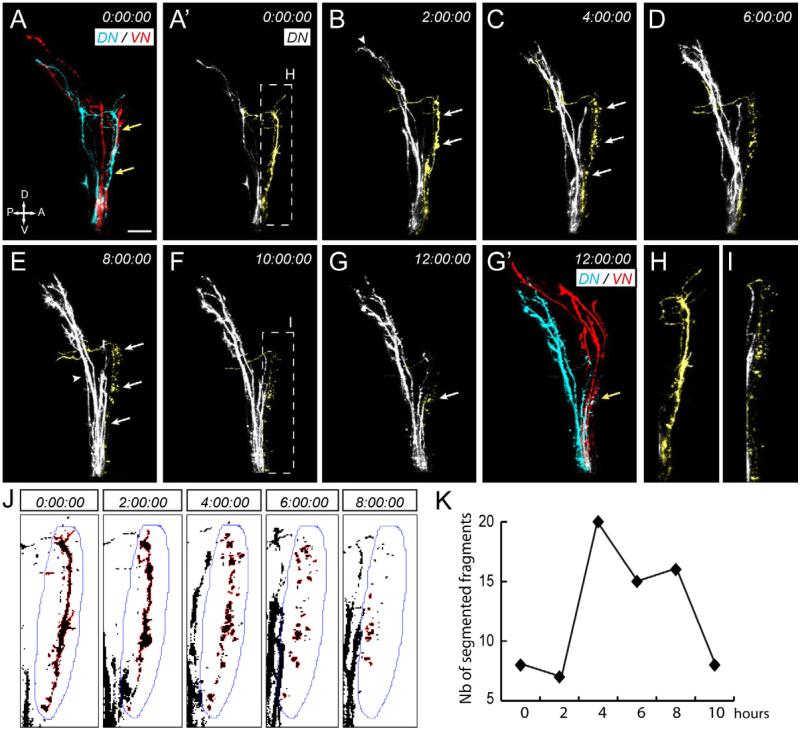
How are missorted DN axons corrected? Missorted axons could respond to a specific cue and retract, as has been shown during pathfinding error correction at the chiasm (Hutson and Chien, 2002). Alternatively, they could “shift” posteriorly and reach the ventral branch of the tract through selective fasciculation. Finally, they could be removed through selective degeneration. To identify the mechanism involved, we observed the behavior of DN and VN axons as they elongate along the tract by confocal time lapse imaging (Figure 2, Movies S1 and S2). We topographically injected dyes intraocularly at 48 hpf, began imaging at ~54 hpf, and collected confocal z-series every 15 min for up to 12 hours. Based on axon morphology and behavior, no evidence of phototoxicity was observed. We also injected different combinations of dyes in various conditions to ensure that the dyes we used did not have any toxic effect (data not shown). In all our time lapse analyses (n=12), axons were very dynamic, elongating rapidly and sometimes pausing during their navigation. During these pauses, growth cones of correctly sorted axons harbored many dynamic filopodia that extended and retracted quickly. Interestingly, missorted DN axons had a different behavior. Although initially as dynamic as axons that were correctly sorted, they then stopped their elongation and became more quiescent. As they stopped, blebbing started to appear uniformly along their length (Figure 2B). This blebbing phase was rapidly followed by an abrupt and uniform fragmentation (Figure 2C-F). Axonal fragments were of different sizes and appeared disconnected. Fragmentation of missorted DN axons was a rapid process, occurring over a period of 30 min (Movie S2, Figure 2J, K). Fragments became smaller over time and eventually disappeared (Figure 2G). This last clearance phase was slow and not always observed in our time lapse conditions, even after 14 hours of imaging. Our observations thus indicate that topographic sorting of retinal axons along the optic tract is achieved through the selective degeneration of missorted DN axons. Interestingly, missorted DN axons always degenerated before reaching the tectum (n=8), suggesting that degeneration is locally regulated.
Figure 2. Missorted dorsal axons selectively degenerate.
(A-G’) DN (blue, DiD) and VN (red, DiO) axons were topographically labeled at 48 hpf and time-lapse imaged at 15 minutes intervals for 12 hours starting at 54 hpf. The organization of DN and VN axons is shown at the beginning (A) and the end (G’) of the time lapse, while time series of confocal image stack projections (A’-G) show DN axons only. Missorted DN axons are pseudo-colored in yellow for the best visualization. Times indicate hours. Some DN axons are clearly missorted at 54 hpf (A’) and have a normal morphology (H). After two hours (B), correctly sorted axons have reached the tectum (arrowhead), while missorted DN axons stop their growth and start blebbing (arrows). They then fragment uniformly along their length over time (C-G, arrows), while correctly sorted axons (arrowhead in E) continue to grow toward the tectum. Fragments appear smaller at later stages (F, zoomed picture in I) and become hard to detect after 12 hours (G). Scale bar, 20 μm. (See also movies S1 and S2). (J) Maximal intensity projections of DiD signals at 0, 2, 4, 6, 8 and 10 (not shown) hours were converted to binary images and a region of interest (ROI, blue line) surrounding missorted DN axons was defined. Axon segments within this region were then selected and analyzed using the “Analyze Particles” option in ImageJ. (K) Quantification of the number of axon fragments over time. Between 0 and 2 hours, the number of fragments is low, indicating that missorted axons are segmented as one main continuous region. The number of fragments then increases abruptly between 2 and 4 hours as axons degenerate and fragment. It then gradually decreases between 4 and 10 hours as axon fragments are progressively cleared. See also Figures S2 and S3.
The degeneration of missorted axons does not require activation of apoptotic pathways or neuronal activity in RGCs
Axon degeneration is thought in other systems to rely on mechanisms distinct from cell body apoptosis (Nikolaev et al., 2009; Whitmore et al., 2003; Yan et al., 2010). To test whether activation of apoptotic pathways was required for correcting missorted DN axons, we analyzed axon sorting in the optic tract of p53 morphants (Figure S2). No missorted DN axons were observed at 72 hpf when p53 was inhibited (MI 0.8 %), indicating that correction occurred normally. Similarly, we did not observed any missorting defects after inhibiting caspase-3 or Bax activity (data not shown). These results suggest that specific signaling pathways distinct from apoptotic cascades or acting in parallel are involved in topographic sorting error correction.
We next examined whether neuronal activity in RGCs was required for optic tract sorting by analyzing retinal projections in macho (mao) mutants (Figure S3). The mao mutant was originally isolated in a screen for motility (Granato et al., 1996) and is characterized by a lack of voltage-gated Na+ current in RGCs and other neuronal types. As previously reported (Gnuegge et al., 1999; Trowe et al., 1996), we did not observe any sorting defects in mao (MI 0.9%), indicating that neuronal activity is not required for correcting missorted DN axons along the optic tract.
Heparan sulfate is required for topographic sorting error correction
To identify which molecular mechanisms might regulate the degeneration of missorted DN axons, we decided to examine optic tract sorting in dackel (dak) mutants (Trowe et al., 1996). Our previous studies indicated that some DN axons are missorted at 60 hpf and 5 days-post-fertilization (dpf) in dak as a result of impaired heparan sulfate (HS) synthesis (Lee et al., 2004). However, it was not clear whether this was due to failure of correcting missorted DN axons at earlier stages. We found that sorting of retinal axons in dak mutants at 48 hpf was similar to that observed in wild-type (WT) embryos (Figure 3A, A’). Some DN axons elongated along or dorsally to VN axons in the most dorsal part of the tract, and growth cones leading axons were intermingled. As in WT embryos, missorted DN axons were still visible at 54 hpf, elongating along or dorsally to the dorsal branch of the tract (data not shown). However, in contrast to WT embryos, missorted axons were not corrected in dak mutants by 72 hpf (Figure 3B, B’). MIs were comparable at 48, 54 and 72 hpf (Figure 3E), indicating that the mechanism for correcting missorted DN axons is impaired in dak mutants.
Figure 3. HS is required for correcting missorted DN axons in the tract.
(A-B’) DN (A, B, blue in A’-B’) and VN (red in A’-B’) retinal axons were topographically labeled at 48 and 72 hpf in dak mutants lacking HS. As observed in WT (Figure 1), some missorted DN axons are observed along VN axons at 48 hpf (arrows). DN (yellow arrowheads) and VN (white arrowhead) growth cones navigate along mixed trajectories (A’). In contrast to WT however, missorted DN axons remain clearly visible at 72 hpf (arrows). Lateral views, confocal maximal projections; Scale bar, 20 μm. (C, D) Measurement of DiD and DiI signal intensities across the tract shows that missorted DN axons remain from 48 to 72 hpf in dak. (E) Missorting index (MI) is similar at 48, 54 and 72 hpf in dak, showing that missorted DN axons are not corrected. (F-H) Restoring HS synthesis at late stages in dak mutants corrects missorted DN axons. (F, G) Tg(hsp70l:ext2-2A-TagRFP); dak mutant embryos were heat-shocked at 39°C for 1 hour at 48 hpf, fixed at 4 dpf and their DN axons were topographically labeled with DiO. dak mutants carrying the rescue ext2 transgene (Tg+, F) do not show any missorted DN axons, whereas dak sibling mutants lacking the transgene (Tg-, G) do (arrows). Lateral views, confocal maximal projections; Scale bar, 20 μm. (H) The proportion of dak mutants with missorted DN axons was scored at 4 dpf after embryos were heat-shocked at various stages. In absence of heat-shock, around 80% of dak mutants exhibit missorted DN axons. Heat-shocks as late as 64 hpf correct missorting of DN axons in Tg+ but not in Tg-dak mutants.
If the correction mechanism required for sorting of retinal axons is deficient in dak, we predicted that restoring HS synthesis after axons have grown along the tract should rescue the phenotype. To test this hypothesis, we generated a rescue transgenic line heterozygous for the dak mutation and expressing the WT ext2 gene (mutated in dak) under the control of a heat shock (hsp70l) inducible promoter. We crossed this line to dak heterozygotes, performed heat shocks at different developmental times, and quantified the proportion of dak mutant embryos with missorted DN axons (Figure 3H). As expected, heat-shocks performed before or during axon elongation along the tract (between 30 and 48 hpf) fully rescued the phenotype (Figure 3F, G), indicating that restoring Ext2 activity at early times is sufficient to produce HS that rescues sorting at later stages. Interestingly, heat-shocks performed after axons had grown along the tract, from 48 to 69 hpf, also rescued missorting defects in dak. Rescue was no longer observed when heat-shocks were performed after 72 hpf. This later period coincides with the onset of visually evoked responses from tectal neurons (Niell and Smith, 2005), suggesting that missorted axons might be stabilized by connections with synaptic partners. Thus, restoring HS synthesis at late stages in dak is sufficient to restore pre-target topographic sorting, indicating that the correction mechanism editing missorted axons along the tract requires the presence of HS.
Heparan sulfate acts non-cell-autonomously to correct missorted axons
HS is carried by diverse core proteins present at the cell surface or in the extracellular matrix. It could therefore act non-cell-autonomously in the environment and/or cell-autonomously at the surface of missorted DN axons. To determine where HS is required to correct missorted DN axons, we performed topographic transplantations of DN RGCs between WT and dak embryos carrying the isl2b:EGFP or isl2b:TagRFP transgenes, which are expressed in RGCs (Pittman et al., 2008) (Figure 4A). DN donor RGCs were transplanted between 30 and 34 hpf into the DN quadrant of the host retina, and their axonal projections were analyzed at 4 dpf. As in WT>WT transplants, dak RGCs transplanted into a WT host projected axons that were correctly sorted into the ventral branch of the tract (Figure 4C, C’, E, E’). In contrast, some axons of WT RGCs transplanted into a dak host were clearly missorted and elongated along the dorsal branch of the tract, as observed in dak>dak transplants (Figure 4B, B’, D, D’). Thus, HS is required non-cell-autonomously for correcting missorted DN axons and establishing pre-target topographic sorting.
Figure 4. HS is required non-cell-autonomously for correct sorting of DN axons.
(A) DN RGCs from an isl2b:TagRFP donor embryo were topographically transplanted between 30 and 34 hpf into the DN retina of an isl2b:EGFP host embryo. EGFP is shown in blue in the figure for the best visualization. (B-F’) Diagrams (B) and pictures (C-F’) of corresponding DN donor axons observed at 4 dpf. Lateral views, confocal maximal projections. In WT>WT (C, C’) and dak >WT (E, E’) transplants, DN donor axons (TagRFP+) pass through the ventral branch of the optic tract. In contrast in dak >dak (D, D’) and WT>dak (F, F’) transplants, some donor axons are missorted along the dorsal branch of the tract (arrows) before reaching the tectum. Insets show transplanted DN TagRFP+ RGCs localized in the DN part of the host retina. (G) Quantification of missorting in transplants. The proportion of hosts with missorted donor DN axons was scored at 4 dpf. Missorting is observed in dak mutants but not WT hosts, indicating that HS is required non-cell-autonomously for correct sorting of retinal axons.
DISCUSSION
Pre-target axon sorting is an important process contributing to the formation of topographic maps, yet the cellular and molecular mechanisms ordering axonal projections remain largely unknown. In this report, we took advantage of the unique accessibility of the zebrafish embryo to determine how retinal axons are sorted along the dorsoventral axis in the optic tract before reaching the tectum. We showed that topographic sorting of retinal axons is not precisely established during initial growth cone pathfinding along the tract but is rather achieved through the selective degeneration of missorted axons. We further demonstrated that this specific developmental degeneration is regulated non-cell-autonomously by HS.
The selective elimination of axons or dendrites is an essential process refining neuronal connections during development and establishing precise wiring of the nervous system. While programmed cell death has long been recognized as an important strategy for removing exuberant projections, it has become apparent that other mechanisms also remodel connections by locally eliminating inappropriate or misguided axons. Selective retraction of axon collaterals has been described in many systems as a pruning strategy to generate accurate patterns of connectivity. More recently, selective degeneration has emerged as another pruning mechanism for eliminating specific axons during development. It has mostly been described in Drosophila, where axons of the γ neurons in the mushroom bodies undergo fragmentation and locally degenerate during metamorphosis (Watts et al., 2003). In vertebrates, there are a limited number of studies suggesting that selective degeneration is used for eliminating inappropriate axon branches during the development of layer 5 subcortical projections and the retinotopic map after brain target has been reached (Luo and O’Leary, 2005). The contribution of selective axon degeneration to other developmental processes remains largely unknown, mostly because of the limited experimental approaches available in vivo in mammals. Using the zebrafish embryo as a model, we discovered a novel function for developmental degeneration in establishing precise pre-target topographic ordering of axons along a tract. Missorted retinal projections are selectively eliminated, leading to accurate and precise organization of axon fibers along the tract before they reach their brain target.
Observing the behavior of DN and VN axons directly in vivo as they elongate along the tract allowed us to show for the first time that pre-target topographic sorting of retinal axons is not precisely established during initial pathfinding. Growth cones are not precisely segregrated according to their dorsoventral identity, and some DN axons elongate along with VN axons in the most dorsal part of the tract. Interestingly, only DN axons appear missorted, perhaps because VN axons act as pioneers and elongate along the tract first (Burrill and Easter, 1995). Missorted axons are initially very dynamic but eventually stop their elongation before reaching the tectum and rapidly fragment and degenerate. Axons always seem to pause before degenerating, suggesting that they might encounter a “stop” signal possibly leading to their degeneration. Whether stopping and degeneration are triggered coincidently by the same signal or independently by different cues remains to be determined. Blebbing and fragmentation are uniform along axonal length, indicating that degeneration is not initiated at the growth cone by a signal derived from the target but is rather locally regulated along the tract by spatially restricted cues. Interestingly, rotating the presumptive optic tract induces a corresponding turning of retinal axons (Harris, 1989), suggesting that axons react to local positional information on the neuroepithelium that might trigger their degeneration. That this information is independent from the brain target is further supported by the absence of sorting defects along the tract following ablation of the tectal primordia in frog (Reh et al., 1983).
As has been described in other developmental processes involving axon degeneration (Nikolaev et al., 2009; Whitmore et al., 2003; Yan et al., 2010), inhibiting p53, Bax, or caspase-3 activity did not prevent the selective degeneration of missorted DN axons, indicating that mechanisms distinct from cell body apoptosis or acting in parallel of apoptotic cascades are involved in topographic sorting error correction. Another parameter known to regulate developmental axon degeneration in some systems is neuronal activity. For instance, intrinsic neuronal activity is required for the selective elimination of callosal or subcortical axons (Luo and O’Leary, 2005). Similarly, retinal waves of activity are necessary for axon elimination leading to topographic map refinement in the superior colliculus in mammals (McLaughlin et al., 2003). In contrast, pre-target topographic sorting of retinal axons is normal in mao mutants that lack neuronal activity in RGCs. A possible explanation for this result is that correction of missorted axons occurs at early stages of development, before sensory stimulation or activity competition between axons might play a role.
We identified HS as a key regulator for correcting pre-target topographic sorting errors along the optic tract. While the importance of HS in axon guidance has been well described (Bulow et al., 2008; Lee and Chien, 2004), this is the first description, to our knowledge, of its role in modulating selective developmental degeneration. Interestingly, missorted DN axons pause and degenerate in WT but continue growing to the tectum in dak, suggesting that HS might also regulate a “stop” signal acting in parallel or prior to axonal degeneration.
HS is present at the cell surface or in the extracellular matrix as the glycosaminoglycan part of heparan sulfate proteoglycans (HSPGs). Interestingly, it is required non-cell-autonomously for correcting DN axons, suggesting different models for its mode of action. First, HS might function in the neuroepithelium as the specific cue triggering the degeneration of DN axons. HS could then be carried by a core protein specifically expressed along the dorsal pathway, or have specific structural motifs provided by HS-modifying enzymes themselves expressed preferentially at that location. Whether specific enzymes or core proteins are expressed in such distinctive patterns remains actually unknown. Alternatively, HS could act indirectly in the neuroepithelium by regulating the secretion or diffusion of a signaling molecule such as a guidance cue or a morphogen. Such cue would then be present in higher concentration along the dorsal branch of the tract. Finally, HS might be present at the surface of ventral axons and signals to the missorted dorsal axons elongating alongside. Such “communication” between ventral and dorsal axons would involve the presence of specific receptors at the surface of dorsal axons.
Whether HSPGs act directly on missorted axons or indirectly by modulating a signaling pathway remains to be determined. Interestingly, factors regulating map topography along the dorsoventral axis in the tectum such as Ephrin-Bs or Semaphorin-D (Hindges et al., 2002; Liu et al., 2004; Mann et al., 2002) do not seem to be involved in ordering axonal projections along the optic tract (Liu et al., 2004; Plas et al., 2008). These observations further suggest that the selective degeneration of missorted axons is locally regulated by an independent, specific pathway involving HSPGs. Syndecans and Glypicans are highly expressed in the nervous system and are known to modulate the signaling of guidance cues like slits (Johnson et al., 2004; Rhiner et al., 2005; Steigemann et al., 2004) or of morphogens such as Wnt (Han et al., 2005; Munoz et al., 2006). While Slit/Robo2 signaling does not seem to regulate sorting along the optic tract (data not shown), the Wnt pathway appears as an interesting candidate, as it has been shown to modulate developmental axon pruning in C. elegans and maintain axon stability in the olfactory system in the adult fly (Chiang et al., 2009; Hayashi et al., 2009). Determining whether specific Syndecans or Glypicans regulate similar pathways will be essential for a better understanding of axon tract formation and the etiology of related neurological disorders.
EXPERIMENTAL PROCEDURES
Detailed experimental procedures are available in supplemental information.
Zebrafish
A detailed description of the strains used and manipulations of embryos are available in supplemental information.
Visualization of optic tract sorting in fixed embryos
RGCs in embryos fixed at 4 dpf were anterogradely labeled with the lipophilic dyes DiI or DiO (Molecular probes, Invitrogen) using a vibrating needle injector (Baier et al., 1996). RGCs in embryos fixed at earlier stages were labeled with DiD and DiI using a dye-coated glass microneedle (Poulain et al., 2010). The contralateral eye was removed for imaging lateral views.
Quantification of optic tract sorting
Confocal images of the optic tract were acquired with constant PMT voltage and gain throughout the z-series. Stack images were imported in ImageJ and sum projected. Intensities of DiD (DN axons) and DiI (VN axons) signals were plotted along a reference line drawn perpendicular to the tract, 50 μm from the point where axons turn caudally to the tectum. A missorting index (MI) was calculated as a ratio of signal intensities: (missorted DN axons)/(total DN axons). Statistical comparisons of MI used two-tailed Student’s t tests.
Time-lapse imaging of live embryos
Embryos were anesthetized at 24 and 32 hpf to remove about half of the yolk and their left eye, and at 48 hpf to perform topographic injection of DiD and DiO into the retina. Embryos were then mounted laterally at 54 hpf for time-lapse imaging. Z-series were acquired at 15 min intervals for up to 14 hours. Maximal intensity projections for each time point were compiled, pseudo-colored and aligned using ImageJ software and StackReg plugin.
Quantification of missorted DN axon fragmentation
For each time lapse, maximal intensity projections of DiD signals at 0, 2, 4, 6, 8 and 10 hours were converted to binary images. A region of interest (ROI) was defined at t=0 along the dorsal branch of the optic tract as an ellipse surrounding missorted dorsal axons. The number of axonal segments within the ROI were quantified over time using the “Analyze Particles” option in ImageJ. A threshold of 4pixel^2 was used to eliminate background signal.
Heat-shock
Embryos were left at room temperature for 30 min and then transferred at 39°C for 1 hour at different developmental times. They were fixed at 4 dpf, and dorsal retinal projections were labeled by injection of DiO. The proportion of dak−/− mutants with missorted DN axons was scored in three independent experiments for each time point embryos were heat-shocked.
Topographic transplantations
Topographic transplantations were performed as recently described (Poulain et al., 2010). Projections of donor axons were imaged at 4 dpf by live confocal microscopy.
Supplementary Material
HIGHLIGHTS.
-
>
Pre-target sorting of retinal axons is not completed by initial growth cone guidance.
-
>
Selective axon degeneration corrects missorted axons prior to reaching the target.
-
>
Degeneration of missorted axons does not depend on apoptosis or neuronal activity.
-
>
Heparan sulfate is required for topographic sorting error correction.
ACKNOWLEDGMENTS
We thank A.B. Ribera for providing the mao mutant. We thank C. Stacher Hörndli and J.A. Gaynes for technical assistance. We are grateful to M.L. Vetter, R.I. Dorsky, and K.M. Kwan for critical reading of the manuscript. This study was supported by grants from the Fyssen Foundation (to F.E.P.), the Mizutani Foundation for Glycoscience (to C.B.C.) and the NEI (R01-EY012873 to C.B.C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Baier H, Klostermann S, Trowe T, Karlstrom RO, Nüsslein-Volhard C, Bonhoeffer F. Genetic dissection of the retinotectal projection. Development. 1996;123:415–425. doi: 10.1242/dev.123.1.415. [DOI] [PubMed] [Google Scholar]
- Bozza T, Vassalli A, Fuss S, Zhang JJ, Weiland B, Pacifico R, Feinstein P, Mombaerts P. Mapping of class I and class II odorant receptors to glomerular domains by two distinct types of olfactory sensory neurons in the mouse. Neuron. 2009;61:220–233. doi: 10.1016/j.neuron.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulow HE, Tjoe N, Townley RA, Didiano D, van Kuppevelt TH, Hobert O. Extracellular sugar modifications provide instructive and cell-specific information for axon-guidance choices. Curr Biol. 2008;18:1978–85. doi: 10.1016/j.cub.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrill JD, Easter SS., Jr. The first retinal axons and their microenvironment in zebrafish: cryptic pioneers and the pretract. J. Neurosci. 1995;15:2935–2947. doi: 10.1523/JNEUROSCI.15-04-02935.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SO, Guillery RW. Changes in fiber order in the optic nerve and tract of rat embryos. J Comp Neurol. 1994;344:20–32. doi: 10.1002/cne.903440103. [DOI] [PubMed] [Google Scholar]
- Chiang A, Priya R, Ramaswami M, Vijayraghavan K, Rodrigues V. Neuronal activity and Wnt signaling act through Gsk3-beta to regulate axonal integrity in mature Drosophila olfactory sensory neurons. Development. 2009;136:1273–1282. doi: 10.1242/dev.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldheim DA, O’Leary DD. Visual map development: bidirectional signaling, bifunctional guidance molecules, and competition. Cold Spring Harb Perspect Biol. 2010;2:a001768. doi: 10.1101/cshperspect.a001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnuegge L, Schmid S, Neuhauss SC. Analysis of the activity-deprived zebrafish mutant macho reveals an essential requirement of neuronal activity for the development of a fine-grained visuotopic map. J. Neurosci. 1999;21:3542–3548. doi: 10.1523/JNEUROSCI.21-10-03542.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato M, van Eeden FJ, Schach U, Trowe T, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Nüsslein-Volhard C. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development. 1996;123:399–413. doi: 10.1242/dev.123.1.399. [DOI] [PubMed] [Google Scholar]
- Han C, Yan D, Belenkaya TY, Lin X. Drosophila glypicans Dally and Dally-like shape the extracellular Wingless morphogen gradient in the wing disc. Development. 2005;132:667–679. doi: 10.1242/dev.01636. [DOI] [PubMed] [Google Scholar]
- Harris WA. Local positional cues in the neuroepithelium guide retinal axons in embryonic Xenopus brain. Nature. 1989;339:218–221. doi: 10.1038/339218a0. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Hirotsu T, Iwata R, Kage-Nakadai E, Kunitomo H, Ishihara T, Iino Y, Kubo T. A trophic role for Wnt-Ror kinase signaling during developmental pruning in Caenorhabditis elegans. Nat Neurosci. 2009;12:981–987. doi: 10.1038/nn.2347. [DOI] [PubMed] [Google Scholar]
- Hindges R, McLaughlin T, Genoud N, Henkemeyer M, O’Leary DD. EphB forward signaling controls directional branch extension and arborization required for dorsal-ventral retinotopic mapping. Neuron. 2002;35:475–487. doi: 10.1016/s0896-6273(02)00799-7. [DOI] [PubMed] [Google Scholar]
- Hutson LD, Chien CB. Pathfinding and error correction by retinal axons: the role of astray/robo2. Neuron. 2002;33:205–217. doi: 10.1016/s0896-6273(01)00579-7. [DOI] [PubMed] [Google Scholar]
- Imai T, Yamazaki T, Kobayakawa R, Kobayakawa K, Abe T, Suzuki M, Sakano H. Pre-target axon sorting establishes the neural map topography. Science. 2009;325:585–590. doi: 10.1126/science.1173596. [DOI] [PubMed] [Google Scholar]
- Johnson KG, Ghose A, Epstein E, Lincecum J, O’Connor MB, Van Vactor D. Axonal heparan sulfate proteoglycans regulate the distribution and efficiency of the repellent slit during midline axon guidance. Curr Biol. 2004;14:499–504. doi: 10.1016/j.cub.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Lee JS, Chien CB. When sugars guide axons: insights from heparan sulphate proteoglycan mutants. Nat Rev Genet. 2004;5:923–935. doi: 10.1038/nrg1490. [DOI] [PubMed] [Google Scholar]
- Lee JS, von der Hardt S, Rusch MA, Stringer SE, Stickney HL, Talbot WS, Geisler R, Nüsslein-Volhard C, Selleck SB, Chien CB, Roehl H. Axon sorting in the optic tract requires HSPG synthesis by ext2 (dackel) and extl3 (boxer) Neuron. 2004;44:947–960. doi: 10.1016/j.neuron.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Liu Y, Berndt J, Su F, Tawarayama H, Shoji W, Kuwada JY, Halloran MC. Semaphorin3D guides retinal axons along the dorsoventral axis of the tectum. J Neurosci. 2004;24:310–318. doi: 10.1523/JNEUROSCI.4287-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, O’Leary DD. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- Mann F, Ray S, Harris W, Holt C. Topographic mapping in dorsoventral axis of the Xenopus retinotectal system depends on signaling through ephrin-B ligands. Neuron. 2002;35:461–473. doi: 10.1016/s0896-6273(02)00786-9. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Torborg CL, Feller MB, O’Leary DD. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40:1147–1160. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- Muñoz R, Moreno M, Oliva C, Orbenes C, Larraín J. Syndecan-4 regulates non-canonical Wnt signalling and is essential for convergent and extension movements in Xenopus embryos. Nat Cell Biol. 2006;8:492–500. doi: 10.1038/ncb1399. [DOI] [PubMed] [Google Scholar]
- Niell CM, Smith SJ. Functional imaging reveals rapid development of visual response properties in the zebrafish tectum. Neuron. 2005;45:941–951. doi: 10.1016/j.neuron.2005.01.047. [DOI] [PubMed] [Google Scholar]
- Nikolaev A, McLaughlin T, O’Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pittman AJ, Law MY, Chien CB. Pathfinding in a large vertebrate axon tract: isotypic interactions guide retinotectal axons at multiple choice points. Development. 2008;135:2865–2871. doi: 10.1242/dev.025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plas DT, Dhande OS, Lopez JE, Murali D, Thaller C, Henkemeyer M, Furuta Y, Overbeek P, Crair MC. Bone morphogenetic proteins, eye patterning, and retinocollicular map formation in the mouse. J. Neurosci. 2008;28:7057–7067. doi: 10.1523/JNEUROSCI.3598-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plas DT, Lopez JE, Crair MC. Pretarget sorting of retinocollicular axons in the mouse. J. Comp. Neurol. 2005;491:305–319. doi: 10.1002/cne.20694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain FE, Gaynes JA, Stacher Horndli C, Law MY, Chien CB. Analyzing retinal axon guidance in zebrafish. Methods Cell Biol. 2010;100:3–26. doi: 10.1016/B978-0-12-384892-5.00001-3. [DOI] [PubMed] [Google Scholar]
- Reh TA, Pitts E, Constantine-Paton M. The organization of the fibers in the optic nerve of normal and tectum-less Rana pipiens. J. Comp. Neurol. 1983;218:282–296. doi: 10.1002/cne.902180305. [DOI] [PubMed] [Google Scholar]
- Rhiner C, Gysi S, Frohli E, Hengartner MO, Hajnal A. Syndecan regulates cell migration and axon guidance in C. elegans. Development. 2005;132:4621–4633. doi: 10.1242/dev.02042. [DOI] [PubMed] [Google Scholar]
- Satoda M, Takagi S, Ohta K, Hirata T, Fujisawa H. Differential expression of two cell surface proteins, neuropilin and plexin, in Xenopus olfactory axon subclasses. J. Neurosci. 1995;15:942–955. doi: 10.1523/JNEUROSCI.15-01-00942.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes JH. Nerve fibre topography in the retinal projection to the tectum. Nature. 1979;278:620–624. doi: 10.1038/278620a0. [DOI] [PubMed] [Google Scholar]
- St John JA, Clarris HJ, McKeown S, Royal S, Key B. Sorting and convergence of primary olfactory axons are independent of the olfactory bulb. J. Comp. Neurol. 2003;464:131–140. doi: 10.1002/cne.10777. [DOI] [PubMed] [Google Scholar]
- Steigemann P, Molitor A, Fellert S, Jackle H, Vorbruggen G. Heparan sulfate proteoglycan syndecan promotes axonal and myotube guidance by slit/robo signaling. Curr Biol. 2004;14:225–230. doi: 10.1016/j.cub.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Stuermer CA. Retinotopic organization of the developing retinotectal projection in the zebrafish embryo. J. Neurosci. 1988;12:4513–4530. doi: 10.1523/JNEUROSCI.08-12-04513.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowe T, Klostermann S, Baier H, Granato M, Crawford AD, Grunewald B, Hoffmann H, Karlstrom RO, Meyer SU, Müller B, Richter S, Nüsslein-Volhard C, Bonhoeffer F. Mutations disrupting the ordering and topographic mapping of axons in the retinotectal projection of the zebrafish, Danio rerio. Development. 1996;123:439–450. doi: 10.1242/dev.123.1.439. [DOI] [PubMed] [Google Scholar]
- Whitmore AV, Lindsten T, Raff MC, Thompson CB. The proapoptotic proteins Bax and Bak are not involved in Wallerian degeneration. Cell Death Differ. 2003;10:260–261. doi: 10.1038/sj.cdd.4401147. [DOI] [PubMed] [Google Scholar]
- Yan T, Feng Y, Zhai Q. Axon degeneration: Mechanisms and implications of a distinct program from cell death. Neurochem Int. 2010;56:529–534. doi: 10.1016/j.neuint.2010.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.