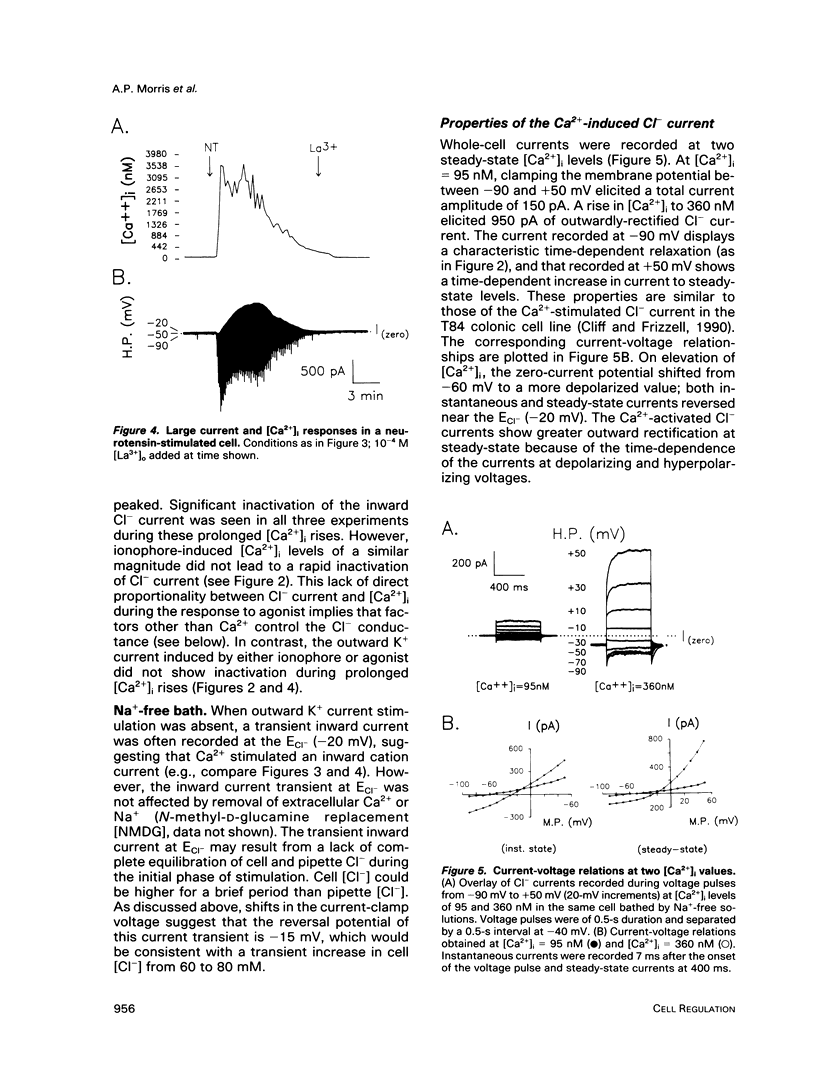
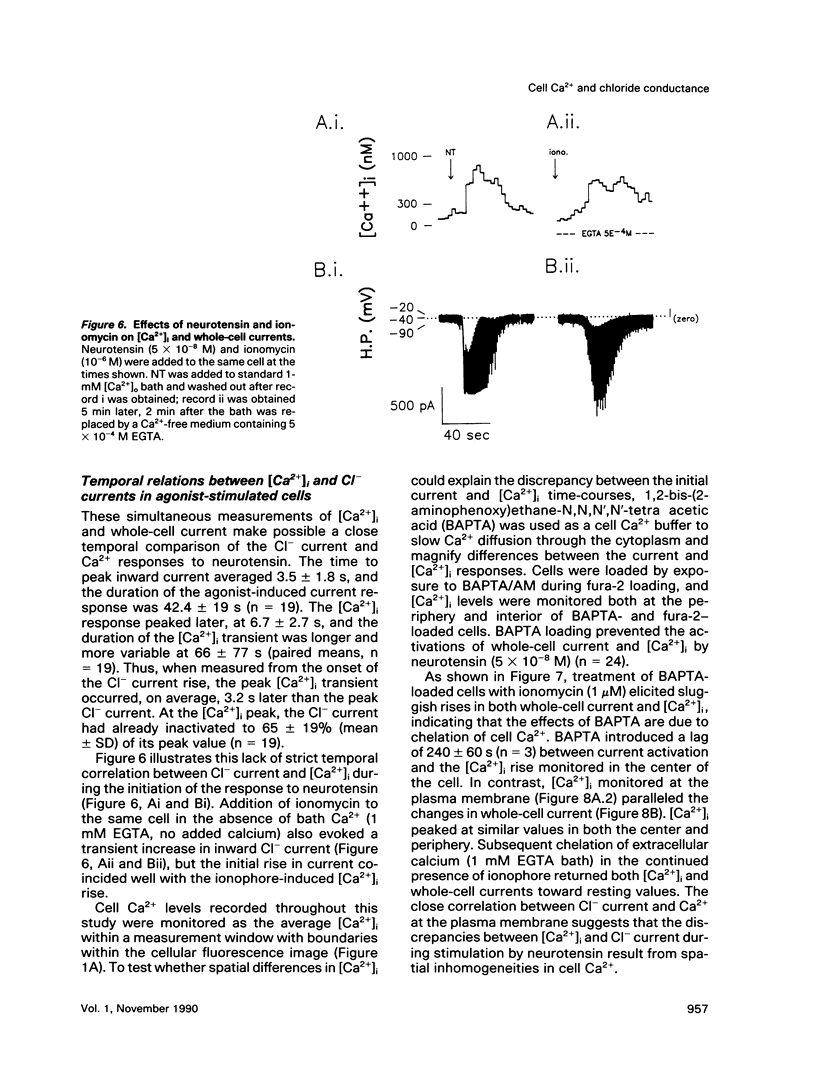
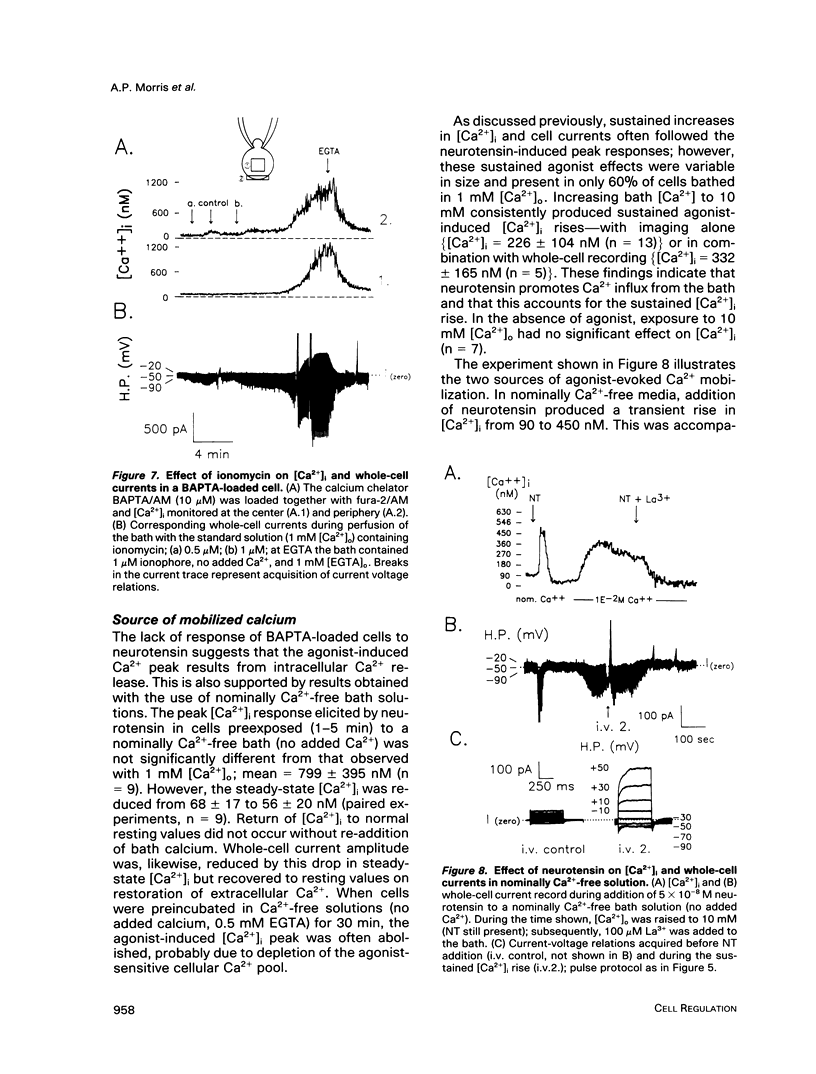
Abstract
We used perforated patch, whole-cell current recordings and video-based fluorescence ratio imaging to monitor the relation of plasma membrane ionic conductances to intracellular free Ca2+ within individual colonic epithelial cells (HT-29). The Ca2(+)-mediated agonist, neurotensin, activated K+ and Cl- conductances that showed different sensitivities to [Ca2+]i. The Cl- conductance was sensitive to increases or decreases in [Ca2+]i around the resting value of 76 +/- 32 (mean +/- SD) nM (n = 46), whereas activation of the K+ conductance required at least a 10-fold rise in [Ca2+]i. Neurotensin increased [Ca2+]i by stimulating a transient intracellular Ca2+ release, which was followed by a sustained rise in [Ca2+]i due to Ca2+ influx from the bath. The onset of the initial [Ca2+]i transient, monitored at a measurement window over the cell interior, lagged behind the rise in Cl- current during agonist stimulation. This lag was not present when the [Ca2+]i rise was due to Ca2+ entry from the bath, induced either by the agonist or by the Ca2+ ionophore ionomycin. The temporal differences in [Ca2+]i and Cl- current during the agonist-induced [Ca2+]i transient can be explained by a localized Ca2+ release from intracellular stores in the vicinity of the plasma membrane Cl- channel. Chloride currents recover toward basal values more rapidly than [Ca2+]i after the agonist-induced [Ca2+]i transient, and, during a sustained neurotensin-induced [Ca2+]i rise, Cl- currents inactivate. These findings suggest that an inhibitory pathway limits the increase in Cl- conductance that can be evoked by agonist. Because this Cl- current inhibition is not observed during a sustained [Ca2+]i rise induced by ionomycin, the inhibitory pathway may be mediated by another agonist-induced messenger, such as diacylglycerol.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amar S., Kitabgi P., Vincent J. P. Activation of phosphatidylinositol turnover by neurotensin receptors in the human colonic adenocarcinoma cell line HT29. FEBS Lett. 1986 May 26;201(1):31–36. doi: 10.1016/0014-5793(86)80565-8. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Boton R., Singer D., Dascal N. Inactivation of calcium-activated chloride conductance in Xenopus oocytes: roles of calcium and protein kinase C. Pflugers Arch. 1990 Apr;416(1-2):1–6. doi: 10.1007/BF00370214. [DOI] [PubMed] [Google Scholar]
- Busa W. B., Ferguson J. E., Joseph S. K., Williamson J. R., Nuccitelli R. Activation of frog (Xenopus laevis) eggs by inositol trisphosphate. I. Characterization of Ca2+ release from intracellular stores. J Cell Biol. 1985 Aug;101(2):677–682. doi: 10.1083/jcb.101.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff W. H., Frizzell R. A. Separate Cl- conductances activated by cAMP and Ca2+ in Cl(-)-secreting epithelial cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4956–4960. doi: 10.1073/pnas.87.13.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett J. K., Gunter-Smith P. J., Melvin J. E., Turner R. J. Physiological localization of an agonist-sensitive pool of Ca2+ in parotid acinar cells. Proc Natl Acad Sci U S A. 1989 Jan;86(1):167–171. doi: 10.1073/pnas.86.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzell R. A., Field M., Schultz S. G. Sodium-coupled chloride transport by epithelial tissues. Am J Physiol. 1979 Jan;236(1):F1–F8. doi: 10.1152/ajprenal.1979.236.1.F1. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Tawada Y., Shigekawa M. Protein kinase C activation stimulates plasma membrane Ca2+ pump in cultured vascular smooth muscle cells. J Biol Chem. 1989 Mar 25;264(9):4844–4849. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Guillemette G., Balla T., Baukal A. J., Catt K. J. Characterization of inositol 1,4,5-trisphosphate receptors and calcium mobilization in a hepatic plasma membrane fraction. J Biol Chem. 1988 Apr 5;263(10):4541–4548. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Henne V., Söling H. D. Guanosine 5'-triphosphate releases calcium from rat liver and guinea pig parotid gland endoplasmic reticulum independently of inositol 1,4,5-trisphosphate. FEBS Lett. 1986 Jul 7;202(2):267–273. doi: 10.1016/0014-5793(86)80699-8. [DOI] [PubMed] [Google Scholar]
- Henson J. H., Begg D. A., Beaulieu S. M., Fishkind D. J., Bonder E. M., Terasaki M., Lebeche D., Kaminer B. A calsequestrin-like protein in the endoplasmic reticulum of the sea urchin: localization and dynamics in the egg and first cell cycle embryo. J Cell Biol. 1989 Jul;109(1):149–161. doi: 10.1083/jcb.109.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitabgi P., Poustis C., Granier C., Van Rietschoten J., Rivier J., Morgat J. L., Freychet P. Neurotensin binding to extraneural and neural receptors: comparison with biological activity and structure--activity relationships. Mol Pharmacol. 1980 Jul;18(1):11–19. [PubMed] [Google Scholar]
- Kopp R., Lambrecht G., Mutschler E., Moser U., Tacke R., Pfeiffer A. Human HT-29 colon carcinoma cells contain muscarinic M3 receptors coupled to phosphoinositide metabolism. Eur J Pharmacol. 1989 Oct 17;172(4-5):397–405. doi: 10.1016/0922-4106(89)90021-7. [DOI] [PubMed] [Google Scholar]
- Laburthe M., Rousset M., Boissard C., Chevalier G., Zweibaum A., Rosselin G. Vasoactive intestinal peptide: a potent stimulator of adenosine 3':5'-cyclic monophosphate accumulation in gut carcinoma cell lines in culture. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2772–2775. doi: 10.1073/pnas.75.6.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., McCann J. D., Anderson M. P., Clancy J. P., Liedtke C. M., Nairn A. C., Greengard P., Welsch M. J. Regulation of chloride channels by protein kinase C in normal and cystic fibrosis airway epithelia. Science. 1989 Jun 16;244(4910):1353–1356. doi: 10.1126/science.2472006. [DOI] [PubMed] [Google Scholar]
- Marty A., Tan Y. P., Trautmann A. Three types of calcium-dependent channel in rat lacrimal glands. J Physiol. 1984 Dec;357:293–325. doi: 10.1113/jphysiol.1984.sp015501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem S., Pandol S. J., Beeker T. G. Calcium mobilizing hormones activate the plasma membrane Ca2+ pump of pancreatic acinar cells. J Membr Biol. 1988 Nov;106(1):57–69. doi: 10.1007/BF01871767. [DOI] [PubMed] [Google Scholar]
- Pandol S. J., Schoeffield M. S., Fimmel C. J., Muallem S. The agonist-sensitive calcium pool in the pancreatic acinar cell. Activation of plasma membrane Ca2+ influx mechanism. J Biol Chem. 1987 Dec 15;262(35):16963–16968. [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Reinlib L., Mikkelsen R., Zahniser D., Dharmsathaphorn K., Donowitz M. Carbachol-induced cytosolic free Ca2+ increases in T84 colonic cells seen by microfluorimetry. Am J Physiol. 1989 Dec;257(6 Pt 1):G950–G960. doi: 10.1152/ajpgi.1989.257.6.G950. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Sage S. O. Stimulated calcium efflux from fura-2-loaded human platelets. J Physiol. 1987 Dec;393:513–524. doi: 10.1113/jphysiol.1987.sp016837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C. A., Meldolesi J., Milner T. A., Satoh T., Supattapone S., Snyder S. H. Inositol 1,4,5-trisphosphate receptor localized to endoplasmic reticulum in cerebellar Purkinje neurons. Nature. 1989 Jun 8;339(6224):468–470. doi: 10.1038/339468a0. [DOI] [PubMed] [Google Scholar]
- Smallwood J. I., Gügi B., Rasmussen H. Regulation of erythrocyte Ca2+ pump activity by protein kinase C. J Biol Chem. 1988 Feb 15;263(5):2195–2202. [PubMed] [Google Scholar]
- Smith P. L., McCabe R. D. A23187-induced changes in colonic K and Cl transport are mediated by separate mechanisms. Am J Physiol. 1984 Dec;247(6 Pt 1):G695–G702. doi: 10.1152/ajpgi.1984.247.6.G695. [DOI] [PubMed] [Google Scholar]
- Supattapone S., Worley P. F., Baraban J. M., Snyder S. H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988 Jan 25;263(3):1530–1534. [PubMed] [Google Scholar]
- Takemura H., Putney J. W., Jr Capacitative calcium entry in parotid acinar cells. Biochem J. 1989 Mar 1;258(2):409–412. doi: 10.1042/bj2580409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. M., Lindeman R. P., Parangi S., Chase H. S., Jr Role of calcium in mediating action of carbachol in T84 cells. Am J Physiol. 1989 Nov;257(5 Pt 1):C976–C985. doi: 10.1152/ajpcell.1989.257.5.C976. [DOI] [PubMed] [Google Scholar]