Abstract
Canadian Thoracic Society (CTS) clinical guidelines for asthma and chronic obstructive pulmonary disease (COPD) specify that spirometry should be used to diagnose these diseases. Given the burden of asthma and COPD, most people with these diseases will be diagnosed in the primary care setting. The present CTS position statement was developed to provide guidance on key factors affecting the quality of spirometry testing in the primary care setting. The present statement may also be used to inform and guide the accreditation process for spirometry in each province.
Although many of the principles discussed are equally applicable to pulmonary function laboratories and interpretation of tests by respirologists, they are held to a higher standard and are outside the scope of the present statement.
Keywords: Primary care, Pulmonary function, Quality control, Reference values, Spirometry
Abstract
Dans les lignes directrices cliniques sur l’asthme et la maladie pulmonaire obstructive chronique (MPOC) de la Société canadienne de thoracologie (SCT), il est précisé que la spirométrie devrait être utilisée pour diagnostiquer trois maladies. Étant donné le fardeau de l’asthme et de la MPOC, la plupart des personnes atteintes de ces maladies seront diagnostiquées en première ligne. Le présent document de principes de la SCT vise à fournir des directives sur les principaux facteurs qui touchent la qualité de la spirométrie en première ligne. Il peut également être utilisé pour étayer et orienter le processus d’agrément de la spirométrie dans chaque province.
Même si bon nombre des principes abordés peuvent s’appliquer aux laboratoires d’évaluation de la fonction pulmonaire et à l’interprétation des tests par des pneumologues, ceux-ci doivent respecter des normes plus élevées qui dépassent la portée du présent document.
POSITION STATEMENT DEVELOPMENT PROCESS
The present position statement was developed by the CTS Pulmonary Function Standards Committee, comprised of experts with experience in the development of international spirometry guidelines, the use of spirometry in asthma and COPD, the training of individuals to conduct quality spirometry, the training of physicians and nurse practitioners to interpret spirometry, the development of normal values for spirometry and the evaluation of spirometers. The position statement reflects the key issues found in spirometry training in primary care and interpretation of spirometry testing conducted in primary care.
Consensus process
The present position statement is based on the joint standards for lung function testing by the American Thoracic Society (ATS) and the European Respiratory Society (ERS) published in 2005 (1,2). Where there may be either controversial issues or changes based on new data, these are indicated.
The recommendations were agreed on by consensus from the full committee through extensive discussions, review of the evidence, and review of existing guidelines and accreditation standards.
Who can conduct spirometry
Spirometry is not controlled under medical services regulations and, therefore, there are no legal restrictions on who can perform spirometry testing.
Provincial accreditation guidelines are variable across the country. British Columbia, Alberta and Ontario have guidelines that specify that a suitably trained and qualified “Medical Director” or “Quality Advisor” oversee spirometry testing.
Because spirometry requires the very active participation of the patient, the technologist must have the skills and ability to proceed well beyond the usual level of patient interaction in medical tests and should have basic life-support training. Spirometry testing can be conducted by the following:
Trained health care personnel who are registered respiratory therapists (RRT) or registered cardiopulmonary function technologists (RCPT[P]);
Other health care professionals whose formal training included studies in the anatomy and physiology of the cardiorespiratory system, and who subsequently successfully completed a recognized spirometry training course; and
Other trained health care technologists who subsequently successfully completed a recognized spirometry training course. For all of the above, an additional requirement is one month of supervised training in the performance and quality control of spirometry testing (3).
Key Message:
Spirometry should be conducted by trained and qualified personnel in a setting with a regular quality assurance program.
Training courses for conducting quality spirometry testing are available; including the CTS-endorsed SpiroTrec®course which is offered by RESPTrec® (www.resptrec.org). SpiroTrec© teaches individuals how to conduct quality spirometry including knowledge of spirometers, understanding of the ATS/ERS standards for spirometry, quality control, patient instruction and basic interpretation of the results. This course includes 16 h of preworkshop learning and assignments, an 8 h workshop and a subsequent quality assurance review of five to 10 spirometry tests per month for three months.
A recent study (4) found that asthma educators who enrolled in a 4 h course in conducting spirometry were able to meet ATS/ERS spirometry testing standards (2) 77% of the time. Short-term follow-up and supplementary training is important to maintain the quality of spirometry testing (5).
Who can interpret spirometry
Unfortunately, some provincial legislation and regulations permit physicians and, in some jurisdictions, nurse practitioners, to interpret spirometry with no special qualifications. While they will have had training in the respiratory system, many primary care physicians have little specific training in the interpretation of spirometry data. There are spirometry interpretation courses available both through workshops and on-line training (eg, the Ontario Lung Association Provider Education Program [www.olapep.ca/spirometry]).
Key Messages:
Primary care physicians and nurse practitioners who interpret spirometry should have completed a spirometry interpretation course or specific training in spirometry interpretation.
PATIENT CONDITIONS AND TESTING ENVIRONMENT
Indications and contraindications
Spirometry is performed to objectively assess individuals’ pulmonary function. It enables measuring the effect of a disease on lung function, monitoring its course or the result of therapeutic interventions, assessing preoperative risk and prognosticating many pulmonary conditions. Conversely, spirometry may be contraindicated (6). International guidelines regarding contraindications for lung function tests have been proposed; however, their evidence base is generally founded only on expert opinion (1). Moreover, recent developments in patient management, surgical practice and technology have decreased the invasive nature of procedures; therefore, some of the contraindications may no longer be justified. Additionally, patients with contraindications that would prevent testing in the primary care setting can be tested in a pulmonary function laboratory where there is access to resuscitation personnel and equipment.
Potential risks associated with lung function testing primarily relate to the following: maximal pressures generated in the thorax and their impact on abdominal and thoracic organs; venous return and systemic blood pressure; expansion of the chest wall and lung; and active communicable diseases that pose a risk to staff and other patients (eg, tuberculosis). Spirometry and other forced expiratory manoeuvres will result in increased intrathoracic, intra-abdominal and intracranial pressures (7). Performing lung function tests can also be physically demanding for patients, thus increasing myocardial demand. This calls for prudence in patients with medical conditions that could be adversely affected by these physiological consequences (Table 1). Although there is no large-scale registry documenting the incidence of adverse events following pulmonary function testing, such risks are likely to be minimal in most patients. Importantly, the potential risks associated with testing should always be weighed against the benefit of obtaining information about lung function (6). When a spirometry test is unlikely to be of real clinical benefit to a compromised patient, it should be postponed until the patient has recovered from surgery, an infection or, for example, pneumothorax, hypo- or hypertension, or unstable arrhythmias. Conversely, a dogmatic approach of refusal to test patients with any contraindication must be tempered. For cases in which the benefit of obtaining objective measures of lung function may outweigh the risk of testing, referral to a specialist should be considered.
TABLE 1.
Relative contraindications for spirometry
| Relative contraindications | Mechanisms | Comments |
|---|---|---|
| Cerebral aneurysm Recent brain surgery Recent concussion Recent eye surgery Significant glaucoma |
Increases in intracranial/intraocular pressure due to decreased venous return | Increases in intraocular pressure during weightlifting (5) suggest spirometry testing may lead to clinically significant changes in intraocular pressures in most patients. Most experts suggest a three- to six-week recovery period following surgery before spirometry testing |
| Recent sinus surgery or middle ear surgery or infection | Increases in sinus and middle ear pressures | There is a risk that forced manoeuvres cause excessive pain or even ear drum rupture in cases of middle ear infection |
| Pneumothorax Significant aortic aneurysm* Recent thoracic surgery† Recent abdominal surgery Pregnancy‡ |
Increases in intrathoracic and intra-abdominal pressure | *Increases in intrathoracic or intra-abdominal pressures may increase blood pressure, but are not expected to increase aortic transmural pressure. †Postoperative physiotherapy including coughing is actually believed to be beneficial after cardiothoracic and abdominal surgery. Cough generally increases intrathoracic pressures up to 400 cmH2O, compared with 70 cmH2O – 200 cmH2O during spirometry. The risk is likely low in most patients ‡Lung function tests may increase the risk of early delivery in case of cervical incompetence |
| Systemic hypotension or severe hypertension (eg, >200/120 mmHg) Significant atrial/ventricular arrhythmia Noncompensated heart failure Recent myocardial infarction§ or pulmonary embolus History of syncope related to forced exhalation/cough |
Increases in myocardial demand or changes in blood pressure |
§Exercise testing one week after myocardial infarction appears to be safe. A shorter period could be appropriate following reperfusion therapy (eg, angioplasty), whereas caution is necessary in case of persistent myocardial ischemia Prudence is also called in many of these conditions with the use of ß2-sympathomimetics, although the risk of a single administration is likely to be minimal |
| Active tuberculosis Hepatitis B Hemoptysis or oral bleeding |
Infection control issues | General infection control measures should be adopted in accordance to local procedures |
| Inability to follow directions(eg, confusion, dementia, young age, language barrier) | In some cases, successful spirometry can be obtained with increased coaching and aid of an interpreter |
Although pre- and postbronchodilator spirometry testing is common for an initial visit, subsequent testing may not require postbronchodilator testing, depending on the reason for ordering spirometry. Some clinics may routinely conduct pre- and postbronchodilator testing to improve office efficiency and convenience for the patient (two tests in one session instead of two sessions) unless otherwise indicated.
Patient preparation
Contraindications should be listed on the spirometry requisition or checklist form. The requisition should clearly state the reason for requesting spirometry and include comments or questions by the referring physician or nurse practitioner. Spirometry may give rise to stress incontinence; therefore, appropriate precautions should be taken (see a sample form in Appendix A).
Testing should occur in a bright and comfortable environment. The patient should be seated. A chair with arms (to prevent falling sideways should syncope occur), without wheels and that can be adjusted so that the feet are flat on the floor is recommended.
Explanations:
Spirometry requires cooperation between the subject and the examiner; the results obtained will depend on technical and personal factors. The test should be fully explained to the patient, and delaying the test should be strongly considered in case of transient confusion or absence of cooperation (1). Videos that demonstrate the test procedure to help educate the patient are available from The Lung Association (www.youtube.com/watch?v=--7ORNHWVrY).
Details of smoking history and any recent illness that could influence the results should be recorded. Ideally, patients should avoid heavy exercise within 30 min, large meals within 2 h, alcohol consumption within 4 h and smoking within 1 h of testing (1).
Medications:
The technologist should record the name, dosage and the last administration of any medication that may alter lung function. The decision to avoid bronchodilators before testing is dependent on the reason for the test. If the study is performed to diagnose an underlying lung condition, then avoiding bronchodilators is useful. In this case, short-acting inhaled drugs should not be used within 4 h of testing, whereas long-acting bronchodilators should be stopped for longer periods (eg, 12 h for salmeterol and formoterol, 24 h for tiotropium, indacaterol and montelukast). Conversely, if the test is performed to determine a response to an existing therapeutic regimen, then the referring physician may choose not to withhold bronchodilator medications. The requisition form or checklist should state whether the patient should withhold medications before the test, and, if so, precisely which medications should be withheld and for how long.
Anthropometric measures:
Before testing, it is essential that height be measured accurately because height, ethnicity, age and sex determine the spirometry reference values. To properly measure height, the subject must be standing without shoes with his/her back flush against a hard surface, such as a wall, with a measuring tape attached to the wall with a right angle device making contact with top of the head and the tape. Ideally, this is accomplished with a proper stadiometer (8). The use of measuring devices attached to scales is inappropriate because it is exceedingly difficult to ensure that the back is held straight. In the presence of chest wall deformities or when height cannot be measured, arm span (middle finger tip to middle finger tip) can be used as an approximation of height (9).
Key Messages:
A checklist including all relative contraindications should be completed before testing
In the case of a contraindication, the risk and benefit of the test should be weighed by the treating physician or the medical director of the laboratory, and testing should be referred to a laboratory where emergency facilities are available.
Appropriate explanations and coaching are mandatory to obtain reliable pulmonary function tests. Precise anthropometric measurements are mandatory for determining reference values.
Infection control programs should be followed.
SPIROMETER SELECTION CONSIDERATIONS
Although some older spirometer systems use volume measurement devices, whereby all of the air that the patient exhales is collected in an expandable chamber, almost all spirometers used in physician office settings (and many pulmonary function laboratories) measure air flow and calculate the volume from this signal. Only flow measurement devices are considered here. Other guidelines to assist in spirometer selection are available (10,11).
ATS/ERS standards
The ATS/ERS Standardisation of Spirometry document (2) contains technical specifications for spirometers. All spirometers must meet the most current ATS/ERS standards, which at time of writing is the 2005 edition. Some spirometers are advertised as meeting ATS standards; however, the fine print will say state ‘meets ATS 1994 Standards’.
Exhalation-only versus full flow-volume loops
Some less expensive spirometers are designed for exhalation-only use. The patient inhales to maximal lung volume; then, while holding his/her breath, inserts the mouthpiece between the teeth, seals his/her lips around the mouthpiece and then forcibly exhales into the spirometer. This requires more coordination on the part of the patient and increases difficulty for the technologist conducting spirometry to assess test quality. Any leakage that occurs after the patient reaches maximal lung volume and before the mouthpiece is in place is lost and, therefore, not included in the calculation of forced vital capacity (FVC) or the back-extrapolated volume. Furthermore, some exhalation-only models are not equipped with real-time displays that permit proper coaching to obtain a best effort. The use of such spirometers is not recommended.
Select a spirometer that enables the patient to take tidal breaths while the technologist can observe that the mouthpiece and nose clip are functioning properly. The technologist then has better control of the manoeuvre and can better coach patients to reach maximal lung volume. Additionally, if any leakage is observed at maximal lung volume before the start of forced exhalation, it will be measured by the spirometer and used to determine whether it is within ATS/ERS standards for the back-extrapolated lung volume. Furthermore, the time spent at total lung capacity can be assessed to determine whether there is a pause at end inspiration and, if >1 s, the manoeuvre can be repeated (12).
Display
The spirometer display, whether using a built-in screen or via a connection to a computer screen, must show both the flow-volume loop and the volume-time graph with sufficient resolution for the technologist to distinguish whether end-of-test criteria are met, whether the shape of the flow volume curve is consistent with a maximal exhalation, and whether a cough or other artefact occurred in the first 1 s of exhalation. In most instances, the display will appear on a computer screen that is not supplied with the system. Most spirometers will have a USB cable to connect to a computer. Ensure that computer hardware and software are compatible with the spirometry system.
It must be possible to position the display so that the technologist can observe both the display and the patient simultaneously during the manoeuvres. This allows for instant coaching and also enables the technologist to terminate a poor effort early, rather than to push the patient to continue a tiring forced expiration.
Warning messages and suggested corrections:
The spirometer must analyze each manoeuvre to determine whether ATS/ERS requirements were met and provide warning messages for any condition that is not met. Examples include “false start – don’t hesitate” and “end of test criteria not met – blow out longer”. The spirometer must also analyze the set of manoeuvres to determine whether ATS/ERS repeatability criteria are met. Some systems offer real-time messages to indicate when 6 s of exhalation has been achieved, which can aid the technologist in coaching to achieve a good quality test. Most of the ATS/ERS requirements are based on adults rather than children, and many children can meet these requirements with submaximal efforts and poor quality tests. A good technologist recognizes this and repeats the effort even if the computerized system has accepted the test.
Calibration
A calibrating syringe is a required accessory for spirometers. ATS/ERS standards specify that a 3 L calibrating syringe be used for checking and calibrating the spirometer and additional specify accuracy requirements, including scheduled accuracy checks of the syringe. If filters are used for patient testing, calibration must be performed with a filter inline. Simple leak tests should be conducted monthly using a stopper in the syringe and checking for changes in syringe volume with pushing or pulling excursions. It may be necessary to purchase a special adaptor to connect the calibration syringe to your spirometer. Some calibrating syringes fit directly onto a disposable mouthpiece or filter.
Some spirometers are equipped with precalibrated disposable breathing-tube inserts that cannot be calibrated by the user. Spirometers using such precalibrated inserts must still be checked daily for accuracy use a 3 L calibrating syringe.
Some spirometers require environmental information such as room temperature, barometric pressure and/or altitude. If so, these parameters should be measured, not estimated. If a barometer is not available, pressure reported from a nearby weather station (refer to the Environment Canada website) must be corrected for altitude (table available at www.engineeringtoolbox.com/air-altitude-pressure-d_462.html).
Normal reference values:
To determine appropriate sets of reference values, refer to the following section in the present document. Select a spirometer that has these specific sets of normal reference values, both adult and pediatric, preprogrammed into its software. If these reference value sets are not included in the defaults provided by the spirometer, insist that they be installed before purchase.
Reports
The spirometry system must be capable of producing a report as described in the next section of the present document. When selecting a spirometer, consider whether it is compatible with the electronic medical record (EMR) system used in the facility. Some systems will permit customized EMR reports, other require reports to be printed to a portable document format (ie, PDF) file for attachment to an EMR.
Disposables
In the selection process, consider the cost and availability of mouthpieces, which usually incorporate a filter. Some models have different mouthpieces for adult and pediatric use. Some models have a mouthpiece incorporated into a disposable breathing tube, making a filter unnecessary. Although filters are not required by ATS/ERS standards, many health regions specify their use for infection control. Nose clips are recommended whenever conducting spirometry.
Robustness
The spirometer must be sufficiently robust to be unaffected by incidental bumps or drops. If the spirometer has been dropped or jarred significantly, a calibration check is recommended before continuing the test.
Technical support
The vendor from whom the spirometer is purchased should provide initial training on the use of the spirometer. It should also provide technical support for addressing problems with the operation of the spirometer. Request a ‘loaner’ device in the event that the spirometer needs to be returned to the company for servicing. There should be regular notification of software upgrades or patches to fix problems. The spirometer should be thoroughly checked following any updates to the software.
Key Messages:
A good quality spirometer is essential for reliable results.
ATS/ERS technical specifications must be met.
Choose a spirometer that can display large screen flow-volume and volume-time graphs in real time.
The software must be able to check for ATS/ERS acceptability and repeatability criteria and provide appropriate warning messages.
PRINCIPLES FOR ROUTINE SPIROMETRY REPORTS
A sample spirometry report form is provided in Appendix B, which is based on the following principles:
Report on a minimal set of variables. Do not clutter the report with several extraneous variables.
Patient identification should include name, date of birth and institutional identification number.
Report observed values in the first column following the variable name. Avoid reporting reference values that can be confused with observed values and do not add any additional information.
Height should be reported in cm. Weight should be reported in kg to one decimal place.
Report the lower limit of normal (LLN). Per cent of reference values are reported because they are used to determine the degree of obstruction.
Report ratios as a decimal fraction so they are not confused with per cent of a reference value.
Print flow-volume and volume-time graphs with a sufficient size and scale them to make optimal use of the available plot area while maintaining the proper aspect ratio. For volume-time graphs, show the last second before start of maximal exhalation. Including all manoeuvres in the session rather than only the best pre- and post-curve is optional.
Include the source of the reference values on the report. If the age of the patient is outside of the age range for which the reference values were developed, flag the LLN values.
Report ethnicity and flag LLN values if the ethnicity does not match the reference value data set. Do not adjust the observed values or LLN for ethnicity by an arbitrary percentage.
Report the reason for the test.
Report technologist comments, which include an assessment of the quality of the spirometry session
Order of columns
1. Variable name with measurement units
Prebronchodilator
2. Observed value (using ATS/ERS selection criteria)
3. Lower limit of normal
4. Per cent of reference value
Postbronchodilator
5. Observed value
Percent of reference value
Volume change from prebronchodilator
Per cent change from prebronchodilator (the latter two are reported because a change ≥200 mL and ≥12% is required for a response to bronchodilators to be significant (2), although lower absolute volume change is acceptable for children or subjects with small lung volumes)
Spirometry variables to report
| Variable | Units |
|---|---|
| Forced vital capacity (FVC) | L |
| Forced expiratory volume in first 1 s (FEV1) | L |
| Ratio of FEV1 to FVC (FEV1/FVC) | – |
| Peak expiratory flow (PEF) | L/s |
| Forced expiratory time (FET) | s |
Optional variables:
The forced expiratory flow from 25% to 75% of exhalation (FEF25–75) is only recommended for children (13,14) and is used in settings primarily designed for children. Interpretation of FEF25–75 requires experience with its high degree of variability and its dependence on FVC.
Although not recommended for office spirometry, some advanced laboratories may report other indexes, particularly the ratio of inspiratory to expiratory flows at 50% vital capacity when upper airway obstruction is suspected.
Graphs
At a minimum, the best pre- and postbronchodilator tests should be displayed. The best manoeuvre is the one with the highest sum of FVC and FEV1. The individual graphs should be distinguishable by colour or line type (solid versus dashed), or by displacement along the x-axis. There should be an option to plot all acceptable manoeuvres for each of pre- and postbronchodilator tests and manoeuvres not meeting ATS/ERS criteria that the technologist has marked as acceptable or useable.
Flow-volume graph:
The graph must be present and must show both the expiratory and inspiratory limbs of the manoeuvre. The aspect ratio is such that 2 L/s on the flow axis equals 1 L on the volume axis. The graph should be scaled so that the maximum values are appropriate to the patient and the size of the graph must be adequate to show the detail of the flow-volume curve (eg, a fixed size of 16 L/s by 8 L is not acceptable).
Volume-time graph:
The graph must be present and must show the final 1 s of inhalation before the forced exhalation. The aspect ratio may be changed to accommodate lengthy expiratory times. The graph should be scaled so that the maximum values are appropriate to the patient and the size of the graph must be adequate to show the detail of the curve (eg, a fixed volume axis of 8 L is not acceptable).
Standard deviation graph (recommended):
The Global Lung Function Initiative (GLI) (15) suggests a visual plot of variables on a standard deviation (SD) scale, showing the reference value and the LLN (1.64 SDs below the mean value), which gives the interpreter a better sense of how the measured values compare with the normal range (see Figure 1 and Appendix B for examples).
Figure 1).
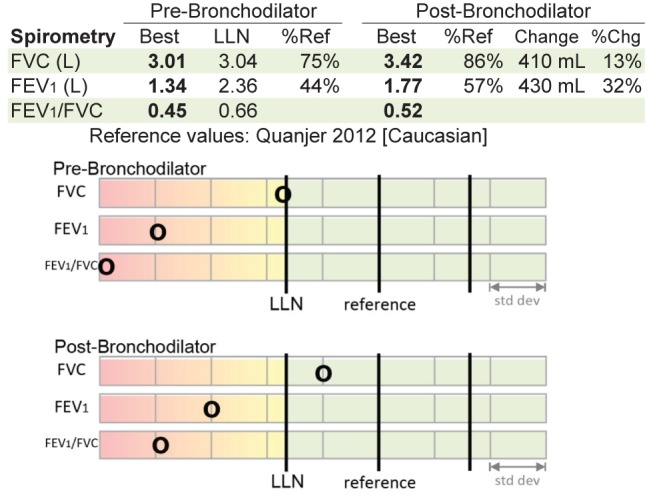
Standard deviation graph. This graph helps to visualize where the measured values lie in relation to the reference values and the lower limit of normal (LLN). Chg Change; FEV1Forced expiratory volume in 1 s; FVC Forced expiratory volume; Ref Reference
Technologist’s comments
The technologist’s comments provide information about the testing session that may affect the interpretation. Recent use of bronchodilators should be noted (drug, dose and time taken). The comments should include any deviation from the standardized ATS/ERS procedures. If the technologist believed that he/she was unable to obtain a maximal effort, it should be noted with an explanation of the problem. If the testing session was terminated prematurely, the reason for the termination should be provided. The number of acceptable and reproducible manoeuvres should be given.
On occasion, the technologist may override the computer software, which automatically classifies a manoeuvre as acceptable or unacceptable. For example, in cases where the technologist ends the expiratory phase of a manoeuvre after the preset limit (usually 12 s to 15 s), the technologist may reclassify it as acceptable. In such cases, the technologist should note that while the ATS/ERS end-of-test criterion was not met, the exhalation was terminated after 12 s and the test was deemed acceptable, which is often the case in COPD.
If the spirometry system does not follow current ATS/ERS guidelines, this information should be included in the comments. This will impact the application of the acceptability criteria because items, such as the back extrapolated volume, may not be measured.
Test quality grading (optional)
While various authors have recommended spirometry grading, such as the scholastic grading system (A, B, C, D and F [16]), there is no ATS or ERS standard. Furthermore, there is no uniform agreement regarding whether the grading is performed for each manoeuvre as a whole, or for the FEV1 and FVC separately (17). Some manufacturers have implemented one or other of the proposed systems. In general, A indicates that ATS/ERS standards were exceeded; B that standards were met; C and D that the data are useable but fall short of ATS/ERS standards (eg, only two acceptable manoeuvres but they are repeatable); and F that the test is not useable for interpretation. These can be used as a guide for technologist comments, but should not be used as a substitute for technologist comments. If the spirometry grade is included in the report, it is important for both the technologist and the interpreter to be familiar with the particular grading system that is being used by the spirometry software.
Note:
Grading systems were developed to determine which manoeuvres should be used in studies designed using healthy subjects to obtain normal reference values for spirometry. Patients with COPD can usually meet all criteria except for the end-of-test criteria. If a patient has met all other criteria but has exhaled for 12 s to 15 s and still failed to reach a plateau (<25 mL in the final 1 s of exhalation), then the technologist should indicate that the test is acceptable in spite of a grade less than B.
Key Message:
The report form must be designed to provide all required information without extraneous values in a format that is easily understood with graphic data to support the numerical data.
REFERENCE VALUES
The expected lung volume is most affected by height – the taller the individual, the larger the lung volume. The variation of lung volume with age is more complex. Lung volume increases as the lungs grow from birth to 18 to 20 years of age in females, and 20 to 24 years of age in males. Thereafter, lung volume declines with age because the lungs lose elastic recoil with reduced expiratory flows (18). Adult males have higher lung volumes than adult females of the same age and height. African Americans have lung volumes approximately 10% lower than Caucasians. Asians have lung volumes 2% to 8% lower than Caucasians (15).
Reference values for FEV1, FVC and FEV1/FVC are generated from population studies of healthy, asymptomatic individuals. For most biological measurements, the standard assumption is that for data with a normal distribution, values within 2 SDs of the mean value represent 95% of the population and are considered to be normal. This implies that 5% of the population (2.5% above and 2.5% below 2 SDs) are ‘abnormal’. For spirometry variables, values that are higher than the reference value are not considered to be abnormal. The LLN is defined as the 5th percentile (ie, the value that marks the lower 5% of the normal population). In a normal distribution, LLN is 1.64 SDs below the mean value (Figure 2) (19). Clearly, when using the LLN, 5% of the healthy, asymptomatic population will be classified as below normal. On the other hand, some people with lung disease will have values greater than LLN and will be classified as normal. The SDs graph on the report form helps to determine the likelihood and degree of abnormality (Figure 1). However, spirometry results are but one finding and must be considered in the context of history, symptoms and physical findings to make the diagnosis.
Figure 2).
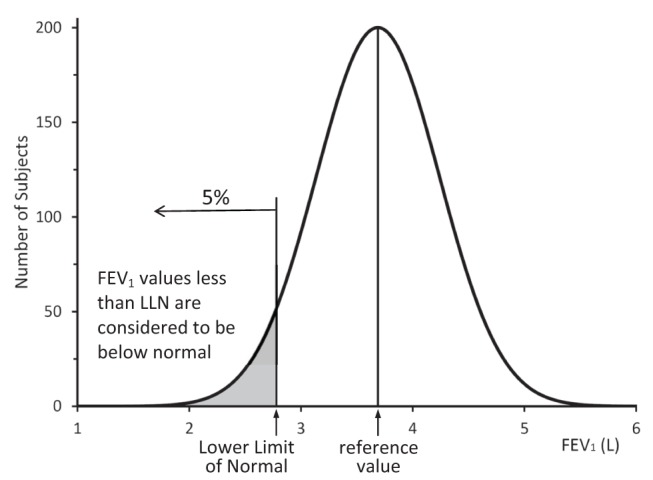
Lower limit of normal (LLN). A plot of the forced expiratory volume in 1 s (FEV1) values from a theoretical population of normal men of age 60 years of age and height 180 cm. The mean FEV1is 3.69 L (the reference value) and the LLN is 2.78 L using Quanjer et al (15) 2012 Caucasian reference values
While some studies have produced reference values for adults and others for children, it is recommended that a continuous set of reference values that applies to all ages be used. Errors often arise when trying to extrapolate reference values developed for adults to the adolescent range, or when trying to extrapolate children’s values above 18 or below the age range of the reference equations. The GLI recommends the all-age spirometry values developed by Quanjer et al (15), which are a composite of spirometry measures worldwide. These reference values are divided into Caucasians, African Americans, Southeast Asians and Northeast Asians.
Some spirometry systems have implemented the reference values from Stanojevic et al (20 [2008]) for ages six to 70 years. The Quanjer et al (15) 2012 reference values are the same over this age range but expand the age range to 3.5 to 90 years. Most major spirometer manufacturers have committed to implementing the Quanjer et al (15) reference values in their newest or forthcoming models (www.lungfunction.org/93-manufacturers.html).
Another choice of reference equations is the National Health and Nutrition Examination Survey (NHANES) III series (Hankinson et al [21]), with values for Caucasian, African Americans and Hispanics between eight and 80 years of age. These equations should not be used outside their age range, particularly for children younger than eight years of age (22). This set is contained in almost all current spirometry systems.
In terms of a ‘Made in Canada’ solution, recently published reference values for Caucasian Canadian adults 20 to 90 years of age (23) supplement those of Gutierrez et al (24) for adults 20 to 80 years of age. The subjects are sufficiently numerous to enable an accurate LLN and agree well with other large series such as NHANES III. Its greatest disadvantage is the problem of discontinuity between paediatric and adult equations because a separate pediatric set is required. The discontinuity occurs when the pediatric and adult reference equations produce significantly different values in the transition period.
There are no reference equations for the Canadian Aboriginal population. Not only is there a scarcity of data, but the various First Nations, Inuit and Métis cannot be considered a physiologically homogenous population. There are some suggestions that this group has larger lung volumes than Caucasians. Research is currently underway to address this issue.
Key Messages:
Reference values must be appropriate for the age and ethnicity of the population, and ideally capable of providing the LLN
The interpretation of spirometric tests should be based on the LLN
- Recommended reference values, in order of preference based on number of subjects studied and age range, are:
Spirometry tests involving Aboriginal Canadians and other ethnic groups should be interpreted with caution using Caucasian reference values
The GLI recommends the use of Quanjer et al GLI 2012 (15) reference values for all spirometry. The Caucasian values for the Stanojevic et al 2009 (20) reference set are the same as the Quanjer GLI 2012 set for ages six to 70 years. While some existing spirometer systems have the Stanojevic 2009 reference values implemented, which can be used as an interim measure, almost all manufacturers have agreed to implement the Quanjer GLI 2012 values on their spirometry systems. A reference value calculator for the Quanjer GLI 2012 reference values is available at <www.lungfunction.org/component/content/article/85-equations-and-tools/equations/151-excel-individual-calculator.html>
SPECIAL CONSIDERATIONS FOR SPIROMETRY IN YOUNG CHILDREN
Spirometry testing began mainly in adults as a means to diagnose and monitor the progress of asthma and COPD, and was subsequently used in children even though measurement techniques and principles are not always directly comparable or applicable. Forced expiratory flow depends heavily on the static elastic recoil of the lungs. Various studies involving adults have demonstrated that the FEV1 correlates well with the degree of disability from lung disease. Children have higher elastic recoil than adults with faster emptying of the lung, which means that the FEV1 can be relatively insensitive to early lung disease. Some children are able to exhale completely in 1 s.
While the ATS/ERS standards (2) for the minimum expiratory time allow for 3 s in children ≤10 years of age rather than 6 s in adults, the requirement for a plateau (<25 mL in the final 1 s of exhalation) remains. In young children who empty their lungs in <2 s, it can be very difficult to resist inspiration before the 3 s limit. In such cases, the technologist must override the automatic rejection of the test.
The ATS/ERS standards (2) set tolerances for repeatability of FEV1 and FVC between tests of 150 mL or 100 mL for FVC or FEV1 ≤1 L. The back-extrapolated volume used for the beginning of test criterion must be ≤150 mL or 5% of FVC, whichever is greater. This results in larger percentage errors in children’s spirometry being deemed acceptable by the spirometry software. In a child-friendly environment, a technologist experienced in working with children can frequently exceed these minimal standards and produce higher-quality spirometry.
Another challenge in testing children is the inspiratory manoeuvre. The ATS/ERS standards specify a rapid inspiration with no breath hold at end inspiration (2); however, knowing when the child has reached maximal inspiration can be challenging. Often, the technologist continues to encourage the child to breathe in, which translates to breath holding at total lung capacity. This problem is accentuated when using portable spirometers that are inserted in the mouth while at maximal lung volume (which are not recommended). The resulting FVC and FEV1 are lower after a slow inspiration and 4 s breath hold in the presence of any significant disease such as cystic fibrosis (12).
There was a brief spike in enthusiasm for the FEF25–75 in the adult literature; however, this rapidly waned when it was realized that the coefficient of variation of the test, particularly in those with COPD, was excessive because of the dependence of FEF25–75 on FVC. With COPD, where a plateau may not be reached, FVC may depend, in part, on the highly variable expiratory time. For children and young adults with greater elastic recoil, the variability of FVC is less and, hence, the FEF25–75 is more meaningful. Interpretation of this variable may still require an adjustment for the FVC (25).
Environment
It is helpful for the area where testing is taking place to have a bright and pleasant atmosphere (ie, pictures on the wall, children’s art and drawings) (26). Having toys and books on hand is also useful if the child needs to wait a few minutes before testing and while waiting 10 min to 15 min for postbronchodilator testing.
Incentive displays:
Some children are more willing and engaged in the spirometry test when an incentive is displayed on the computer screen such as a birthday cake with candles that extinguish during exhalation, a sailboat that moves across a pond or a balloon that expands and pops when exhalation is complete. However some technologists find this more distracting to the children than helpful.
Mouthpiece:
There is usually no need to use a mouthpiece in addition to the filter. Children are usually very adept at keeping their lips closed when reminded. An appropriately sized mouthpiece should be used if a better seal is needed.
Noseclips:
Children do not generally encounter problems with the noseclips. When noseclips are applied, children automatically start breathing through their mouths. Noseclips with ‘spongey’ tips will stay on a child’s small nose and are not so tight on their delicate noses. Furthermore, these types of noseclips are available in different colours. Letting the child choose the colour he or she wants makes them feel a part of the testing. Of course, some children will not allow application of the noseclip. Sometimes they may allow a parent to hold their nose. As a last resort, the spirometry test can then be performed without the noseclips but one should try to introduce them as testing proceeds.
Chairs:
Chairs should be adjustable to allow a small child to mount it. Ideally, they should also be able to sit straight with both feet on the floor.
Key Message:
Spirometry involving children requires technologists with specific skills working in a child-friendly environment to achieve optimal accuracy of the measurements and, hence, maximize the usefulness of spirometry in diagnosing and/or managing lung diseases in children.
QUALITY ASSURANCE AND ACCREDITATION
Any office, clinic or facility providing spirometry testing must be responsible for ensuring that the tests are performed to a high standard. Physicians and nurse practitioners who interpret the tests must first ensure that the tests are of adequate quality because test results are used for the diagnosis and management of patients with respiratory disease. This section outlines the main points to evaluate the quality of spirometry testing.
Documentation of quality assurance procedures is mandatory in all offices, clinics and facilities conducting spirometry testing (2). The requirements include the following:
Daily spirometer calibration checks, with additional calibrations as necessary, and monthly assessment of repeatability of the measures using biological controls and analysis of a log of calibration results.
Documentation of repairs or other modifications of the equipment.
Documentation of computer software and hardware updates or changes.
Qualification of all personnel conducting spirometry tests, including education and training, to assure that they are familiar with the theory and practical aspects of applied techniques, measurements, calibrations, hygiene, quality control and a basic background knowledge in lung physiology and pathology (8).
Documented infection control procedures.
Accreditation questions
Has a suitably trained physician or nurse practitioner been appointed as Medical Director/Quality Advisor to oversee spirometry testing?
Is there a quality assurance program in place and does it monitor staff competency, equipment performance, laboratory technique and procedure reporting, safety and utilization?
Has the individual conducting spirometry testing been adequately trained?
Has the individual interpreting the spirometry tests been adequately trained?
Does the spirometry requisition form or checklist comply with CTS recommendations?
Do spirometry reports comply with CTS recommendations?
Are response times established for reporting spirometry test results?
Are the reference values used for the report appropriate for the patient population?
Are technologists’ comments available for the interpreter?
Is there a policy manual, and does the manual include organizational chart staff/office policies and procedures?
Are there protocols for frequency of calibration for biological control subjects?
Are records of quality control procedures kept and routinely reviewed?
Are tolerance limits well defined for quality control checks?
Is there a plan of action if tolerance limits are exceeded?
Are there manuals available for equipment, policies and procedures, and safety?
Are these manuals site specific and updated annually?
Do the procedures for spirometry testing define acceptable and reproducible criteria for spirometry tests?
Is there an equipment log that details any malfunctions with spirometry equipment and corrective actions taken and does the log provide a history of the maintenance performed and by whom?
Are there default guidelines for cleaning, disinfecting and sterilizing equipment?
Are there specific procedures for individuals with known infections?
Is there a procedure for medical emergencies?
The above represents a general outline. For more detailed outline of a specific questionnaire, see the College of Physicians and Surgeons of Alberta Questionnaire (www.cpsa.ab.ca/services/Quality_of_Care_Main/Accreditation_Facilities/Pulmonary_Functions/Pulmonary_Standards.aspx) or Diagnostic Accreditation Program of British Columbia (http://www.dap.org/Default.aspx?p=57) for examples of questionnaires in current use for different levels of pulmonary function testing.
Distances travelled in Canada can be extensive, which may preclude onsite inspections for all facilities. In that case, the questionnaire may be a remote way of quality control for the facilities. This could be accompanied by spirometry reports from the facilities to assess the final product.
Where provinces have an accreditation program that monitors the quality of diagnostic testing it is recommended that a questionnaire such as the one outlined above be part of that process.
Acknowledgments
The authors thank the Canadian Thoracic Society (CTS) and the Canadian Respiratory Guidelines Committee for guidance and support throughout the process. In particular, they acknowledge the very significant amount of editorial support and administrative guidance provided by Janet Sutherland, Director of the Canadian Thoracic Society. The authors also thank the expert peer reviewers: Doug Harrison, rrt (Kelowna, British Columbia), Dr Charles G Irvin (Burlington, Vermont, USA), Dr Mark D Montgomery (Calgary, Alberta), Dr Hans Pasterkamp (Winnipeg, Manitoba), Laura Seed rcpt(p) (Toronto, Ontario), Dr Gary W Smith (Orillia, Ontario) and Dr Itamar E Tamari (Toronto, Ontario).
APPENDIXES
A. Sample Spirometry requisition form or checklist

B. Sample Spirometry report form
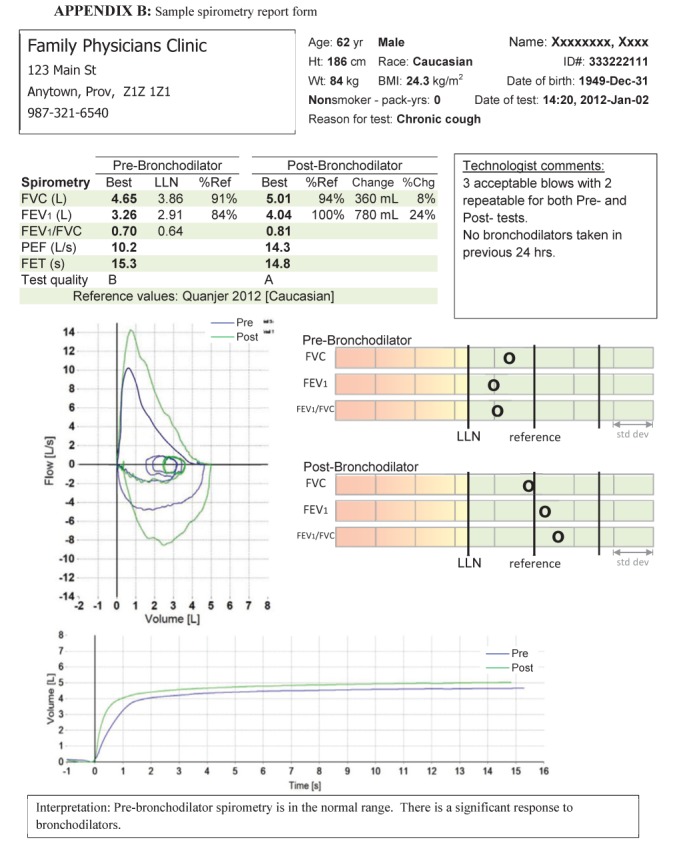
BMI Body mass index; Chg Change; FET Forced expiratory time; FEV1Forced expiratory volume in 1 s; FVC Forced vital capacity; LLN Lower limit of normal; PEF Peak expiratory flow; Ref Reference; Wt Weight
Footnotes
DISCLOSURE OF COMPETING INTERESTS: Members of the CTS Pulmonary Functions Standards committee declared potential conflicts of interest at the time of appointment and these were updated throughout the process, in accordance with accordance with the CTS Conflict of Interest Disclosure Policy. Individual member conflict of interest statements are posted at www.respiratoryguidelines.ca
EDITORIAL INDEPENDENCE: The CTS Pulmonary Function Standards Committee is accountable to the CTS Respiratory Guidelines Committee and the CTS Board of Directors, and is functionally and editorially independent from any funding sources of the CTS. The CTS receives unrestricted grants to facilitate the knowledge translation activities of CTS clinical assemblies. No funders played a role in the collection, review, analysis or interpretation of the scientific literature or in any decisions regarding the recommendations or key messages presented in this document.
REFERENCES
- 1.Miller MR, Crapo RO, Hankinson JL, et al. ATS/ERS Task Force General considerations for lung function testing. Eur Respir J. 2005;26:153–61. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 2.Miller MR, Hankinson JL, Brusasco V, et al. Standarisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 3.Gardner RM, Clausen JL, Epler GR, Hankinson JL, Permutt S, Plummer AL. Pulmonary function laboratory personnel qualifications. Am Rev Resp Dis. 1986;134:623–4. doi: 10.1164/arrd.1986.134.3.623. [DOI] [PubMed] [Google Scholar]
- 4.Licskai CJ, Sands TW, Paolatto L, Nicoletti I, Ferrone M. Spirometry in primary care: An analysis of spirometry test quality in a regional primary care asthma program. Can Respir J. 2012;19:249–54. doi: 10.1155/2012/653084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borg BM, Hartley MF, Fisher MT, Thompson BR. Spirometry training does not guarantee valid results. Respir Care. 2010;55:689–94. [PubMed] [Google Scholar]
- 6.Cooper BG. An update on contraindications for lung function testing. Thorax. 2011;66:714–23. doi: 10.1136/thx.2010.139881. [DOI] [PubMed] [Google Scholar]
- 7.Vieira GM, Oliveira HB, de Andrade DT, Bottaro M, Ritch R. Intraocular pressure variation during weight lifting. Arch Ophthalmol. 2006;124:1251–4. doi: 10.1001/archopht.124.9.1251. [DOI] [PubMed] [Google Scholar]
- 8.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 9.Parker JM, Dillard TA, Phillips VY. Arm span-height relationships in patients referred for spirometry. Am J Respir Crit Care Med. 1996;154:533–6. doi: 10.1164/ajrccm.154.2.8756834. [DOI] [PubMed] [Google Scholar]
- 10.NLHEP, USA. Checklist for Compliance with National Lung Health Education Program Guidelines for Office Spirometers. < www.nlhep.org/Documents/srp-checklist.pdf. 2012> (Accessed November 2, 2012)
- 11.Liistro G, Vanwelde C, Vincken W, Vandevoorde J, Verleden G, Buffels J. Technical and Functional Assessment of 10 Office Spirometers. Chest. 2006;130:657–65. doi: 10.1378/chest.130.3.657. [DOI] [PubMed] [Google Scholar]
- 12.Braggion C, Pradal U, Mastella G, Coates AL, Milic-Emili J. Effect of different inspiratory manoeuvres on FEV1 in patients with cystic fibrosis. Chest. 1996;110:642–7. doi: 10.1378/chest.110.3.642. [DOI] [PubMed] [Google Scholar]
- 13.Polgar G, Promadhat V, editors. Pulmonary Function Testing in Children: Techniques and Standards. Philadelphia: WB Saunders; 1971. pp. 87–212. [Google Scholar]
- 14.Lebecque P, Kiakulanda P, Coates AL. Spirometry in the asthmatic child: Is the FEF25–75 a more sensitive test than the FEV1/FVC. Pediatr Pulmonol. 1993;16:19–22. doi: 10.1002/ppul.1950160105. [DOI] [PubMed] [Google Scholar]
- 15.Quanjer PH, Stanojevic S, Cole TJ, et al. the ERS Global Lung Function Initiative Multi-ethnic reference values for spirometry for the 3–95 year age range: The global lung function 2012 equations. Eur Respir J. 2012;40:1324–43. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enright PL, Johanson LR, Connett JE, Voelker H, Buist AS. Spirometry in the lung health study. 1. Methods and quality control. Am Rev Respir Dis. 1991;143:1215–23. doi: 10.1164/ajrccm/143.6.1215. [DOI] [PubMed] [Google Scholar]
- 17.Centres for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES) 2009–2010 Data Documentation Codebook, and Frequencies < www.cdc.gov/nchs/nhanes/nhanes2009-2010/SPX_F.htm 2010> (Accessed November 2, 2012) [Google Scholar]
- 18.Mead J, Turner JM, Macklem PT, Little JB. Significance of the relationship between lung recoil and maximum expiratory flow. J Appl Physiol. 1967;22:95–108. doi: 10.1152/jappl.1967.22.1.95. [DOI] [PubMed] [Google Scholar]
- 19.Miller MR. Institute of Occupational & Environmental Medicine, University of Birmingham. < www.millermr.com/info.html> (Accessed October 29, 2012)
- 20.Stanojevic S, Wade A, Cole TJ, et al. Asthma UK Spirometry Collaborative Group Spirometry centile charts for young Caucasian children: The Asthma UK Collaborative Initiative. Am J Respir Crit Care Med. 2009;180:547–52. doi: 10.1164/rccm.200903-0323OC. [DOI] [PubMed] [Google Scholar]
- 21.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 22.Subbarao P, Lebecque P, Corey ML, Coates AL. Comparison of spirometric reference values. Ped Pulmonol. 2004;37:515–22. doi: 10.1002/ppul.20015. [DOI] [PubMed] [Google Scholar]
- 23.Tan WC, Bourbeau J, Hernandez P, et al. Canadian prediction equations of spirometric lung function for Caucasian adults 20 to 90 years of age: Results from the Canadian Obstructive Lung Disease (COLD) Study and the Lung Health Canadian Environment (LHCE) study. Can Respir J. 2011;18:321–6. doi: 10.1155/2011/540396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutierrez C, Ghezzo RH, Abboud RT, et al. Reference values of pulmonary function tests for Canadian Caucasians. Can Respir J. 2004;11:414–24. doi: 10.1155/2004/857476. [DOI] [PubMed] [Google Scholar]
- 25.Cockcroft DW, Berscheid BA. Volume adjustment of maximal midexpiratory flow. Importance of changes in total lung capacity. Chest. 1980;78:595–600. doi: 10.1378/chest.78.4.595. [DOI] [PubMed] [Google Scholar]
- 26.Seed L, Wilson D, Coates AL. Children should not be treated like little adults in the PFT lab. Respir Care. 2012;57:61–71. doi: 10.4187/respcare.01430. [DOI] [PubMed] [Google Scholar]


