Abstract
BACKGROUND:
Previous studies have indicated that oxidative stress plays an important role in the pathogenesis of chronic obstructive pulmonary disease (COPD).
OBJECTIVES:
To study local and systemic oxidative stress status in COPD patients, and to clarify the relationship between local and systemic oxidative stress.
METHODS:
Lipid peroxide malondialdehyde (MDA), glutathione (GSH), superoxide dismutase (SOD) and GSH peroxidase (GSH-PX) levels in induced sputum and plasma, as well as glucocorticoid receptor (GR) levels in peripheral blood leukocytes were examined in 43 acute exacerbation of COPD patients (group A), 35 patients with stable COPD (group B) and 28 healthy controls (14 smokers [group C]; 14 nonsmokers [group D]).
RESULTS:
MDA levels in induced sputum and plasma decreased progressively in groups A to D, with significant differences between any two groups (P<0.001). GSH, SOD and GSH-PX levels in both induced sputum and plasma increased progressively in groups A to D, with significant differences between any two groups (P<0.001). GR levels in peripheral blood leukocytes decreased progressively in groups D to A (all comparisons P<0.001). Pearson analysis revealed strong correlations between MDA, GSH, SOD and GSH-PX levels in plasma and induced sputum. The activity of SOD in plasma and sputum were both positively correlated with GR levels (partial correlation coefficients 0.522 and 0.574, respectively [P<0.001]).
CONCLUSIONS:
Oxidative stress levels were elevated in COPD patients. There was a correlation between local and systemic oxidative status in COPD, and between decreased SOD activity and decreased GR levels in COPD patients.
Keywords: Chronic obstructive pulmonary disease, Oxidants, Plasma, Sputum
Abstract
HISTORIQUE :
Selon des études antérieures, le stress oxydatif joue un rôle important dans la pathogenèse de la maladie pulmonaire obstructive chronique (MPOC).
OBJECTIFS :
Étudier l’état de stress oxydatif local et systémique chez les patients atteints d’une MPOC et clarifier le lien entre le stress oxydatif local et systémique.
MÉTHODOLOGIE :
Les chercheurs ont étudié les taux de formation de malondialdéhyde par le peroxyde lipidique (MDA), de glutathion (GSH), de superoxyde dismutase (SOD) et de GSH peroxydase (GSH-PX) dans les expectorations provoquées et le plasma, ainsi que les taux de récepteur des glucocorticoïdes (RG) dans les leucocytes du sang périphérique de 43 patients atteints d’une MPOC ayant une exacerbation aiguë (groupe A), de 35 patients ayant une MPOC stable (groupe B) et de 28 sujets témoins en santé (14 fumeurs [groupe C]; 14 non-fumeurs [groupe D]).
RÉSULTATS :
Les taux de MDA dans les expectorations provoquées et le plasma ont diminué progressivement dans les groupes A à D, et les différences sont significatives entre deux groupes, quels qu’ils soient (P<0,001). Les taux de GSH, de SOD et de GSH-PX dans les expectorations provoquées et le plasma ont augmenté progressivement dans les groupes A à D, et les différences sont significatives entre deux groupes, quels qu’ils soient (P<0,001). Les taux de RG dans les leucocytes du sang périphérique ont diminué progressivement dans les groupes D à A (toutes les comparaisons P<0,001). L’analyse de Pearson a révélé de fortes corrélations entre les taux de MDA, de GSH, de SOD et de GSH-PX dans le plasma et les expectorations provoquées. L’activité de la SOD dans le plasma et les expectorations étaient tous deux corrélés de manière positive avec les taux de RG (coefficients de corrélation partielle de 0,522 et de 0,574, respectivement [P<0,001]).
CONCLUSIONS :
Les patients atteints d’une MPOC présentaient un taux de stress oxydatif élevé. On constatait une corrélation entre le statut oxydatif local et systémique en cas de MPOC, et entre la diminution de l’activité de SOD et du taux de RG chez les patients atteints d’une MPOC.
Chronic obstructive pulmonary disease (COPD) is a disease state characterized by airflow limitation that is not fully reversible, and associated with an abnormal inflammatory response of the lungs to noxious particles or gases (1). Assessment of the severity of acute exacerbation of COPD (AECOPD) is based on the patient’s medical history, pre-existing comorbidities, symptoms, physical examination, arterial blood gas measurements and other laboratory tests (1). The lungs of COPD patients are continuously exposed to oxidants that are generated endogenously or exogenously, and not only directly injure lung tissue but also cause effusion of inflammatory cells and increased gene expression of proinflammatory mediators, thereby promoting the development and progression of COPD.
Some researchers have reported increased oxidative stress and altered levels of antioxidants in COPD patients (2–5), both locally and systemically (6). However, there are less robust data regarding oxidative stress status in AECOPD, and the relationship between systemic and local oxidative stress in COPD remains unclear. More attention is being devoted to corticosteroid treatment in COPD, especially in the acute exacerbation of severe or extremely severe COPD. However, corticosteroid therapy in COPD is much less effective than in asthma, which may be explained by the attenuated anti-inflammatory effect of corticosteroids in COPD arising from mechanisms such as decreased levels of glucocorticoid receptor (GR). Some researchers (7) reported that oxidative stress induced the downregulation of GR, thus weakening the anti-inflammatory effect of corticosteroids. However, whether there is a difference in GR levels between AECOPD and stable COPD patients is not clear. Therefore, to clarify the role of oxidative stress in patients with corticosteroid-resistant COPD, we examined indexes of oxidative stress in induced sputum and plasma, and the GR level in peripheral blood leukocytes to investigate the relationship between systemic and local oxidative stress status, and between oxidative stress and GR.
METHODS
Subjects
From December 2009 to March 2011, 120 subjects were enrolled in the present study. Subjects included 90 COPD patients (79 male, 11 female; mean [± SD] age 72.2±6.2 years) (50 with AECOPD [group A] and 40 with stable COPD [group B]) from the inpatient respiratory unit of the First Affiliated Hospital of Sun Yat-sen University, Guangzhou, People’s Republic of China. Thirty healthy subjects (25 male, five female; mean age 68.6±8.7 years) recruited from an outpatient clinic represented the control group. COPD was defined according to the Global initiative for chronic Obstructive Lung Disease (GOLD) criteria and the AECOPD criteria established in 2007 (1) (forced expiratory volume in 1 s [FEV1] <80% predicted and FEV1/forced vital capacity [FVC] ratio [FEV1/FVC] <70% on postbronchodilator testing). AECOPD is defined as an event in the natural course of the disease characterized by a change in the patient’s baseline dyspnea, cough and/or sputum that is beyond normal day-to-day variations, is acute in onset and may warrant a change in regular medication in a patient with underlying COPD (1). The criteria for AECOPD are defined as follows:
New onset of at least two of the following major symptoms: apnea, purulent sputum or increased sputum;
New onset of one of the above major symptoms and at least two of the following minor symptoms: upper respiratory tract infection in the past five days, exacerbation of dyspnea, increased breath rates or heart rate increase 20% compared with baseline, exacerbation of cough, fever; and
Exacerbation of disease greater than two days’ duration (1).
The exclusion criteria were bronchial asthma, cancer, connective tissue disease, diabetes, hyperthyroidism, hypertension, stages 4 to 5 chronic kidney disease (categorized according to the classification system established by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative [8]) and severe heart failure. Severe heart failure was defined as New York Heart Association functional classification >III (9). Any AECOPD patient deemed to require treatment with systemic glucocorticosteroids (GCs) was excluded from the study. All patients could be treated with a bronchodilator regularly as required, including beta2-agonists and anticholinergic inhalation administered by spacers or air-driven nebulizers, oral theophylline tablets or intravenous aminophylline. For COPD patients who took inhaled surfactant GCs before recruitment, the doses and frequency were unchanged during the study. Antibiotics were given when signs of infection were present; controlled oxygen therapy was administered to maintain arterial partial pressure of oxygen ≥60 mmHg; and systemic GCs or antioxidant therapy were not administered four weeks before the study or during the study. Thirty age-matched (±2 years) outpatients with no history of respiratory disease were selected as controls (n=15 smokers in group C; n=15 nonsmokers in group D). Smokers were defined as having a smoking index (number of cigarettes daily × years of smoking) >10 packs. All subjects who met the criteria were informed of the study’s purpose and methods before signing informed consent. The present study was approved by the Medical Ethics Committee of the First Affiliated Hospital, Sun Yat-sen University.
Sputum induction and processing
Induced sputum samples were collected from all patients before starting treatment. Following administation of 200 μg of the beta2-sympathomimetic salbutamol, subjects inhaled hypertonic saline solution (3%) for 20 min from an ultrasonic nebulizer device (CSW-1, Institute of Photoelectric, China). To avoid contamination, saliva was expectorated into a separate container. Patients were encouraged to produce sputum samples at 5 min intervals. Peripheral oxygen saturation was continuously monitored and lung function was spirometrically verified every 5 min during induction to ensure the safety of the procedure. Induction was stopped when FEV1 decreased by more than 20% and could not be restored to more than 90% of the original FEV1 after inhalation of 200 μg salbutamol, or in subjects with cyanosis, dyspnea or distress. The first sputum sample was used for microbiological analysis. Subsequent samples were transported to the laboratory on ice for processing within 30 min. Cell division and cell counting were performed under light microscopy to determine possible contamination of sputum with saliva and squamous cells. The qualifying specimens were defined as having <20% squamous epithelial cells and a cell survival >50%. The sample was divided with two sterile, blunt forceps into small portions of approximately 0.2 g and nine times the volume of D-phosphate buffered saline was added. Samples were homogenized by gentle passage repeated 20 times through an 18-gauge needle. Homogenized samples were consecutively filtered through 100 μm and 40 μm cell strainers and centrifuged at 300 g at 4°C for 10 min. The supernatants were carefully transferred into other vials and centrifuged again at 10,000 g at 4°C for 5 min. The supernatants were pooled, divided into microfuge tubes and stored at −80°C until thawed specifically for further study (10).
Plasma collection
Blood samples were obtained at the same time as collection of sputum for measurement of GR, adrenocorticotropic hormone (ACTH) and cortisol. Samples (5 mL) were obtained in the early morning from the cubital vein, transferred into dry centrifuge tubes with edetic acid and subsequently centrifuged at 2500 rpm for 10 min. Separated plasma samples were stored at −80°C for future analysis.
Measurement of oxidative stress indexes
Malondialdehyde (MDA) levels were measured using the thiobarbital technique. Measurements of nonenzymatic antioxidant reduced glutathione (GSH), and enzymatic antioxidant superoxide dismutase (SOD) and GSH peroxidase (GSH-PX) activity were performed using a chemical colourimetry kit (Sigma-Aldrich, China). All measurements were conducted in strict accordance with manufacturer’s instructions. GR levels in peripheral blood leukocytes were evaluated using a radioligand binding assay (11). ACTH was measured by chemical colourimetry. Cortisol was measured by radioimmunoassay.
MDA levels were determined using the method of Wasowicz et al (12), using a spectrofluorometer (Model 4010, Hitachi, Japan). The amount of coloured complex obtained by the reaction of MDA and thiobarbituric acid was determined at wavelengths of 525 nm and 547 nm for excitation and emission, respectively. The concentration of MDA was expressed as μmol/L of plasma. The activity of plasma GSH-PX was measured spectrophotometrically as described by Paglia and Valentine (13). The enzymatic reaction was initiated by the addition of hydrogen peroxide and the rate of NADPH oxidation was followed at 340 nm. One unit of GSH-PX was expressed as the amount of enzyme that oxidizes 1 mmol NADPH/min. Results were expressed in units/g of hemoglobin. The activity of SOD was measured spectrophotometrically as described by Sun et al (14). In summary, xanthine-xanthine oxidase was used to generate a superoxide flux. Reduction of nitroblue tetrazolium by superoxide anion to blue formazan was determined at 560 nm. One unit of enzyme activity was defined as the amount of protein causing 50% inhibition of nitroblue tetrazolium reduction by SOD. Results were expressed in units/g of hemoglobin.
Pulmonary function testing
A spirometer (Sensor Medics Ltd, USA) was used for bronchodilator testing and to diagnose COPD. During sputum induction, lung functions were monitored with a portable spirometer. FEV1 values were obtained by means of a spirometer and FEV1 % predicted was calculated.
Statistical analysis
Data were expressed as mean ± SD. Comparisons of means among groups were performed using one-way ANOVA and the q test. The correlation between oxidative stress indexes and GR level was performed using partial correlated analysis. Correlations between MDA, GSH and GSH-PX levels in sputum and plasma and GR was performed after control of the SOD level in sputum and plasma. Correlation between SOD levels in sputum and plasma and GR was performed after control of smoking indexes. Analysis of the correlation between sputum and plasma oxidative stress indexes was performed using Pearson correlation analysis. Statistical analysis of the data was conducted using SPSS version 13.0 (IBM Corporation, USA); P<0.05 was considered to be statistically significant.
RESULTS
Clinical characteristics and pulmonary function
All 90 COPD patients met the criteria of grade III/IV COPD; 78 of these subjects completed sputum induction and their specimens were acceptable. There were 43 subjects in the AECOPD group (two current smokers, 27 patients were on chronic inhaled corticosteroids [ICS] and 35 subjects in the stable COPD group (three current smokers, 18 patients were on chronic ICS). Twelve patients and two controls were excluded because of intolerance to sputum induction and nonqualifying sputum samples. Twenty-eight controls were included (14 smokers [group C] and 14 nonsmokers [group D]). There was no statistical difference in sex or age among the four groups (P>0.05). There was no statistical difference in smoking index among groups A, B and C (P=0.182). There was no statistical difference in current smoking rates between groups A and B (P=0.652). There was no statistical difference in the usage rates of ICS among groups A and B (P=0.362). With respect to lung function parameters, there were significant differences among AECOPD group, stable COPD group and the control groups (P<0.001 for all comparisons). Nonsmokers had better lung function than smokers (P<0.001) (Table 1).
TABLE 1.
Clinical characteristics of subjects in chronic obstructive pulmonary disease (COPD) and control groups
| Characteristic |
Group
|
χ2 | P | |||
|---|---|---|---|---|---|---|
| AECOPD (A) | Stable COPD (B) | Smokers (C) | Nonsmokers (D) | |||
| Age, years | 72.6±7.3 | 70.9±8.5 | 72.2±3.9 | 64.9±10.6 | 0.626 | 0.089 |
| Cases, n (male, n/female, n) | 43 (38/5) | 35 (31/4) | 14 (12/2) | 14 (11/3) | 0.009 | 1.000 |
| Smoking status | ||||||
| Current smoker, n | 2 | 3 | Reference | Reference | 0.494 | 0.652 |
| Smoking index | 869±501 | 707±329 | 671±228 | Reference | 3.403 | 0.182 |
| FEV1 after bronchodilator use | ||||||
| L | 0.93±0.27 | 1.53±0.41* | 1.89±0.37† | 2.16±0.43‡ | 82.274 | <0.001 |
| % predicted | 39.3±6.3 | 51.2±8.3* | 74.9±5.7† | 93.6±4.5‡ | 290.292 | <0.001 |
| FEV1/FVC ratio | 55.4±9.9 | 62.2±8.8* | 74.4±1.9† | 82.8±6.3‡ | 28.187 | <0.001 |
| Inhaled glucocorticoids, n | 27 | 18 | Reference | Reference | 1.021 | 0.362 |
Data presented as mean ± SD unless otherwise indicated.
P<0.05 compared with group A;
P<0.05 compared with groups A and B;
P<0.05 compared with groups A, B and C. AECOPD Acute exacerbation of COPD; FEV1Forced expiratory volume in 1 s; FVC Forced vital capacity
Local (induced sputum) oxidative stress indexes
MDA levels in induced sputum of groups A, B, C and D were 6.98±1.39 nmol/L, 5.45±1.00 nmol/L, 4.12±0.63 nmol/L and 3.21±0.49 nmol/L, respectively. The differences between any two groups were statistically significant (all P<0.001). GSH levels in induced sputum in groups A, B, C and D were 199.65±36.30 mg/L, 305.12±9.17 mg/L, 358.34±8.74 mg/L and 402.46±11.46 mg/L, respectively. SOD levels in induced sputum in groups A, B, C and D were 42.4±7.64 U/mL, 59.72±11.96 U/mL, 85.56±2.55 U/mL and 104.79±7.54 U/mL, respectively. GSH-PX levels in induced sputum in groups A, B, C and D were 122.69±20.58 U, 175.01±28.58 U, 180.81±7.52 U and 203.03±10.91 U, respectively. GSH, SOD and GSH-PX levels in induced sputum increased progressively in groups A to D. The differences between any two groups were statistically significant (all P<0.001) (Figures 1 to 4).
Figure 1).
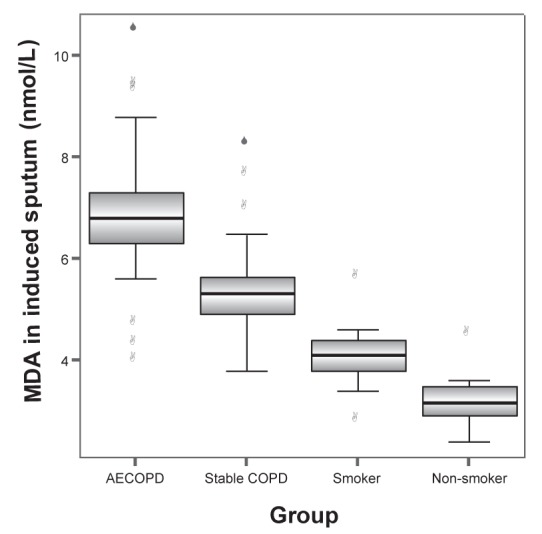
Malondialdehyde (MDA) levels in induced sputum in groups A (acute exacerbation of chronic obstructive pulmonary disease [AECOPD]), B (stable chronic obstructive pulmonary disease [COPD]), C (smoker) and D (nonsmoker) were 6.98±1.39 nmol/L, 5.45±1.00 nmol/L, 4.12±0.63 nmol/L and 3.21±0.49 nmol/L, respectively. The differences between any two groups were statistically significant (all P<0.001)
Figure 4).
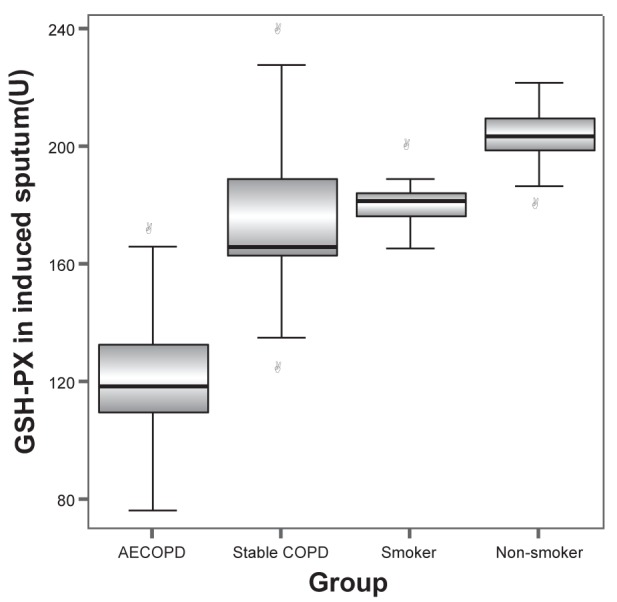
Glutathione peroxidase (GSH-PX) levels in induced sputum in groups A (acute exacerbation of chronic obstructive pulmonary disease [AECOPD]), B (stable chronic obstructive pulmonary disease [COPD]), C (smoker) and D (nonsmoker) were 122.69±20.58 U, 175.01±28.58 U, 180.81±7.52 U and 203.03±10.91 U, respectively. The differences between any two groups were statistically significant (all P<0.001)
Systemic (plasma) oxidative stress indexes
Plasma MDA levels in groups A, B, C and D were 8.82±0.43 nmol/L, 8.61±1.52 nmol/L, 6.12±0.63 nmol/L and 5.20±0.44 nmol/L, respectively. MDA levels in induced sputum decreased progressively in groups A to D. The differences between any two groups were statistically significant (all P<0.001). GSH levels in plasma in groups A, B, C and D were 255.38±47.69 mg/L, 351.99±43.73 mg/L, 402.01±8.88 mg/L and 424.21±8.02 mg/L, respectively. SOD levels in plasma in groups A, B, C and D were 52.03±7.43 U/mL, 82.21±6.21 U/mL, 102.76±8.36 U/mL and 123.26±5.90 U/mL, respectively. GSH-PX levels in plasma in groups A, B, C and D were 143.58±28.14 U, 182.97±4.59 U, 204.20±6.03 U and 224.23±7.86 U, respectively. Plasma GSH, SOD and GSH-PX levels increased progressively in groups A to D. The differences between any two groups were statistically significant (all P<0.001) (Table 2).
TABLE 2.
Comparison of malondialdehyde (MDA), glutathione (GSH), superoxide dismutase (SOD) and GSH-peroxidase (GSH-PX) levels in plasma in chronic obstructive pulmonary disease (COPD) patients and control groups
| Group | n | MDA, nmol/L | GSH, mg/L | SOD, U/mL | GSH-PX, U |
|---|---|---|---|---|---|
| Acute exacerbation of COPD (A) | 43 | 8.82±0.43 | 255.38±47.69 | 52.03±7.43 | 143.58±28.14 |
| Stable COPD (B) | 35 | 8.61±1.52* | 351.99±43.73* | 82.21±6.21* | 182.97±4.59* |
| Smokers (C) | 14 | 6.12±0.63† | 402.01±8.88† | 102.76±8.36† | 204.20±6.03† |
| Nonsmoker (D) | 14 | 5.20±0.44‡ | 424.21±8.02‡ | 123.26±5.90‡ | 224.23±7.86‡ |
| F | – | 76.541 | 88.094 | 402.419 | 81.716 |
| P | – | <0.001 | <0.001 | <0.001 | <0.001 |
Data presented as mean ± SD unless otherwise indicated.
P<0.05 compared with group A;
P<0.05 compared with groups A and B;
P<0.05 compared with groups A, B and C
ACTH, cortisol and GR levels in peripheral blood leukocytes
According to ANOVA, no statistical differences were found in ACTH (F=1.03, P=0.383) or cortisol (F=0.204, P=0.893) levels among the four groups.
GR levels in peripheral blood leukocytes in group A were significantly lower than in groups B, C and D (all P<0.001). GR levels in group B were also significantly lower than in groups C and D (all P<0.001), and GR levels in group C were significantly lower than in group D (P<0.001) (Table 3).
TABLE 3.
Comparison of adrenocorticotropic hormone (ACTH), cortisol and glucocorticoid receptor (GR) levels among the four groups
| Group | n | ACTH, pmol/L | Cortisol, nmol/L | GR (site/cell) |
|---|---|---|---|---|
| AECOPD (A) | 43 | 5.13±3.79 | 370.23±158.14 | 1565±719 |
| Stable COPD (B) | 35 | 4.39±2.19 | 350.34±128.81 | 2069±488* |
| Smoker (C) | 14 | 4.33±1.46 | 382.73±139.01 | 2739±926† |
| Nonsmoker (D) | 14 | 3.67±1.32 | 372.39±94.98 | 4793±1415‡ |
| F | – | 1.03 | 0.204 | 50.32 |
| P | – | 0.383 | 0.893 | <0.001 |
P<0.05 compared with group A;
P<0.05 compared with group B;
P<0.05 compared with group C. AECOPD Acute exacerbation of chronic obstructive pulmonary disease; COPD Chronic obstructive pulmonary disease
Analysis of correlation between plasma and induced sputum oxidative stress indexes
According to Pearson correlation analysis, the MDA level in sputum was positively correlated with MDA in plasma, and negatively correlated with GSH, SOD and GSH-PX levels in plasma (all P<0.001). Similarly, MDA levels in plasma was negatively correlated with GSH, SOD and GSH-PX levels in sputum (all P<0.001). GSH, SOD and GSH-PX levels in sputum were all positively correlated with plasma GSH, SOD and GSH-PX levels (all P<0.001) (Table 4).
TABLE 4.
Pearson correlation analysis of oxidative stress indexes between plasma and induced sputum
| Plasma |
Induced sputum
|
P | ||||||
|---|---|---|---|---|---|---|---|---|
| MDA | P | GSH | P | SOD | P | GSH-PX | ||
| MDA | 0.7565 | <0.001 | −0.8256 | <0.001 | −0.8255 | <0.001 | −0.7790 | <0.001 |
| GSH | −0.7980 | <0.001 | 0.8893 | <0.001 | 0.8378 | <0.001 | 0.8296 | <0.001 |
| SOD | −0.8243 | <0.001 | 0.9380 | <0.001 | 0.9279 | <0.001 | 0.8206 | <0.001 |
| GSH-PX | −0.8252 | <0.001 | 0.8947 | <0.001 | 0.8439 | <0.001 | 0.7997 | <0.001 |
GSH Glutathione; GSH-PX GSH peroxidase; MDA Malondialdehyde; SOD Superoxide dismutase
Analysis of correlation between oxidative stress and GR
GR was positively correlated with GSH, SOD and GSH-PX levels in both sputum and plasma according to Pearson correlated analysis (sputum r=0.532, r=0.566 and r=0.586, respectively [all P<0.001]; plasma r=0.341, r=0.514 and r=0.387, respectively [all P<0.001]). No correlation was found between GR and MDA levels. Partial correlation coefficients between GR and plasma MDA, GSH and GSH-PX were −0.177, 0.125 and 0.168, respectively, after controlling for plasma SOD levels (P>0.05). Partial correlation coefficients between GR and sputum MDA, GSH and GSH-PX were −0.189, 0.217 and 0.244, respectively, after controlling for sputum SOD levels (P>0.05). The correlation coefficients of GR and SOD in plasma and induced sputum were 0.512 and 0.574, respectively, after controlling for smoking index (both P<0.001) (Figures 3 and 4).
Figure 3).
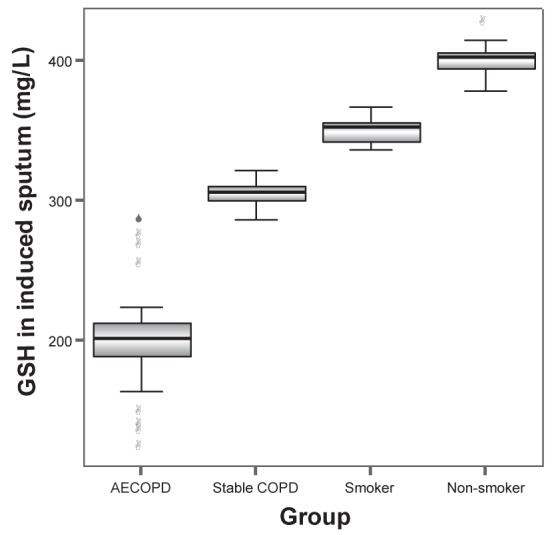
Glutathione (GSH) levels in induced sputum in groups A (acute exacerbation of chronic obstructive pulmonary disease [AECOPD]), B (stable chronic obstructive pulmonary disease [COPD]), C (smoker) and D (nonsmoker) were 199.65±36.30 mg/L, 305.12±9.17 mg/L, 358.34±8.74 mg/L and 402.46±11.46 mg/L, respectively. The differences between any two groups were statistically significant (all P<0.001)
DISCUSSION
Noninvasive methods, such as exhaled air, exhaled breath condensate and induced sputum, have been widely used in the indirect assessment of COPD and its progression. Of these techniques, exhaled air and induced sputum are relatively well standardized, while exhaled breath condensate, although promising, remains under evaluation. Bronchoalveolar lavage is also used in the study of COPD, but its use is restricted because it is invasive and complicated to perform. The safety and feasibility of the sputum induction technique in COPD, especially in the exacerbation stage, has been confirmed (15,16). Induced sputum primarily originates from secretions in the peripheral and central airways. There are several advantages of using induced sputum in the study of respiratory diseases, including being economical, noninvasive, accurate and reproducible (17–20). This method can reliably reflect the concentration of airway secretions and provides a good correlation with pathological changes measured by lung biopsy or bronchoalveolar lavage (21). For patients with stable symptoms, the measurement of soluble mediators in sputum and sputum cellular classification are reproducible. The induced sputum technique was used in our research to study local oxidative stress in COPD. The novel mechanical sputum-processing method used in the present study was reported by Hector et al (10) to provide a yield of cells and fluids similar to the conventional dithiothreitol method, but with the advantage of not affecting the oxidant assay by omitting the introduction of reducing agents.
Oxidative stress can be defined as an imbalance between oxidants/antioxidants because of excess oxidant and/or reduced antioxidant capacities, and causes tissue or organ injuries. The lungs of COPD patients are continuously exposed to oxidants that are generated either endogenously (eg, during activation of phagocytes) or exogenously (eg, air pollutants and cigarette smoke), and at the same time enzymatic antioxidant activities and nonenzymatic antioxidant levels decrease, which in combination not only directly injure lung tissue but also cause oxidation and inactivation of antiproteases, effusion of inflammatory cells and increased gene expression of proinflammatory mediators, thus promoting the development and progression of COPD. Oxidative stress in COPD involves local and systemic changes in oxidative stress levels (22). The present study showed that levels of the oxidation product MDA progressively decreased in AECOPD, stable COPD, healthy smokers and healthy nonsmokers, while the levels of the antioxidants GSH, SOD and GSH-PX increased in the reverse order. The data suggested that when COPD worsened, systemic and local oxidation products increased while antioxidant levels and the corresponding antioxidant activity decreased. In addition, when acute exacerbation was relieved, oxidant levels decreased while antioxidant levels increased. Patients with AECOPD and stable COPD showed correlations between the systematic and local imbalance of oxidation/antioxidation and had an increased oxidative stress level, especially in AECOPD. Our results were in accordance with those of Nadeem et al (6). We suggest that in AECOPD, inflammation causes an increase in airway and systemic oxidative stress levels. Harju et al (2) found that levels of GSH in alveolar macrophages, extracellular fluids and airway secretions were all significantly decreased in COPD patients; however, the influence of inflammation in AECOPD on local and systemic oxidative stress was not clarified. Rytila et al (3) and Kinnula et al (4) found that local oxidative stress was significantly increased in asymptomatic current chronic smokers and in patients with different stages of COPD compared with never-smokers and ex-smokers, and the oxidative stress level was correlated with the severity of disease. Altuntas et al (5) found that, compared with healthy nonsmokers, systemic oxidative stress levels were increased in COPD patients and healthy smokers. However, our study found that there were no significant differences between healthy smokers and nonsmokers in most local or systemic oxidative stress indexes, except for SOD activity, which contradicted studies by Rytila et al (3), Kinnula et al (4) and Altuntas et al (5). Therefore, we could not determine the influence of smoking on systemic and local oxidative stress levels in healthy individuals, possibly due to an insufficient sample size.
The GR is an intracellular soluble protein that binds GCs with high affinity and specificity, and is the important mediator of the physiological and pharmacological action of GCs. GCs are used in many respiratory diseases such as asthma, COPD, etc. However, the effect of GCs among different diseases or among different individuals is not consistent. Some studies found a correlation between the changes in the levels of GR and the curative effect (7). The predetermined condition for a cellular response to GCs is the existence of GRs. A decreased number of GRs is an important reason for an attenuated effect of GCs. In our study, there were no differences in plasma cortisol and ACTH levels among nonsmokers, healthy smokers, stable COPD and AECOPD patients; however, a decreasing trend in GR level was found. The GR level was lower in AECOPD than in stable COPD, which demonstrated the different degrees of GR changes in different stages of COPD. Compared with nonsmokers, the GR level in smokers significantly decreased, which reflected the effect of smoking on GR level in healthy people. Our study was in agreement with the study by Marwick et al (7), which reported decreased GR-alpha expression in rats exposed to smoke, in smokers with normal lung function and in COPD patients.
Our study found that decreased SOD activity was correlated with downregulation of GR in peripheral blood leukocytes, while other oxidative stress indexes were not found to be correlated with the GR level after controlling for SOD activity. We conclude that a strong correlation exists between SOD activity and GR level, and SOD activity remained positively correlated with GR after controlling for smoking, which suggests that decreased SOD activity partly explains the decreased GR level in COPD patients. There are no studies reporting a correlation between oxidative stress and GR level, and our study found that only SOD was correlated with the GR level. The mechanism by which oxidative stress caused a decrease in GR level was not clear. In a study of asthma, it was found that defective GR nuclear translocation (23), overexpression of nuclear factor-kappa B (24) and activated protein-1 (25), and increased release of inflammatory mediators (26,27), may cause the decreased expression of GR. It was also possible that oxidative injury directly led to a decreased GR level, or it was related to decreased histone deacetylase activity. However, these mechanisms were not clear, and additional research is needed.
CONCLUSIONS
Oxidative stress levels are increased in COPD patients. There is a correlation between local and systemic oxidative status in COPD. There is a correlation between increased oxidative stress levels or decreased SOD activity and decreased GR levels in patients with COPD. The mechanism linking oxidative stress and GR level is unknown and additional studies are needed to investigate the association.
Figure 2).
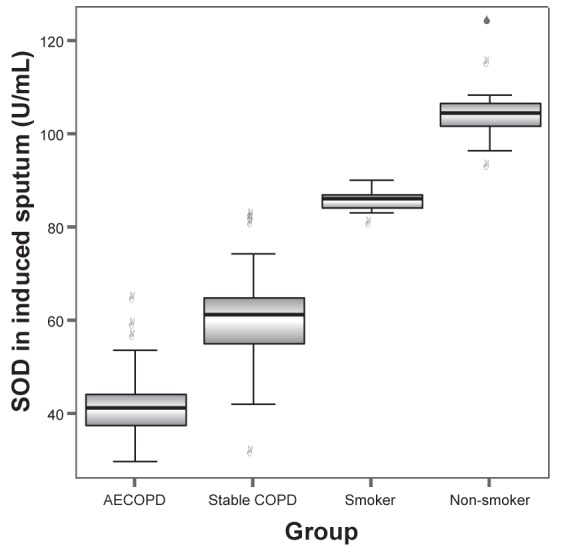
Superoxide dismutase (SOD) levels in induced sputum in groups A (acute exacerbation of chronic obstructive pulmonary disease [AECOPD]), B (stable chronic obstructive pulmonary disease [COPD]), C (smoker) and D (nonsmoker) were 42.4±7.64 U/mL, 59.72±11.96 U/mL, 85.56±2.55 U/mL and 104.79±7.54 U/mL, respectively. The differences between any two groups were statistically significant (all P<0.001)
Figure 5).
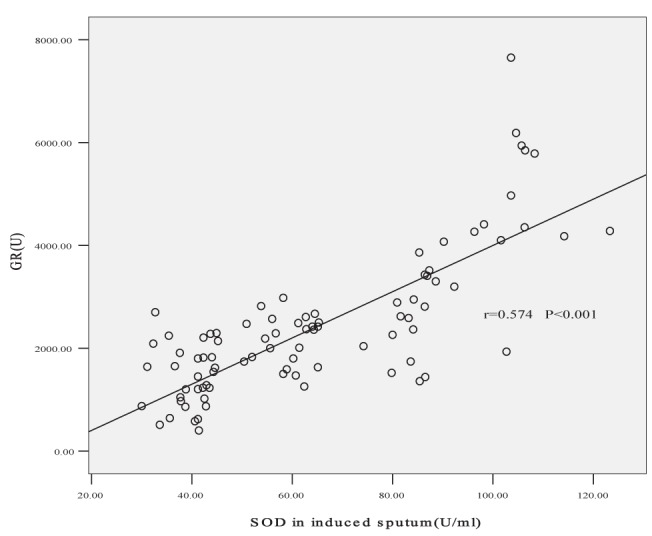
Correlation of superoxide dismutase (SOD) in induced sputum and glucocorticocoid receptor (GR) level. The partial correlation coefficient of GR and SOD in induced sputum was 0.574 after controlling for smoking index (P<0.001)
Figure 6).
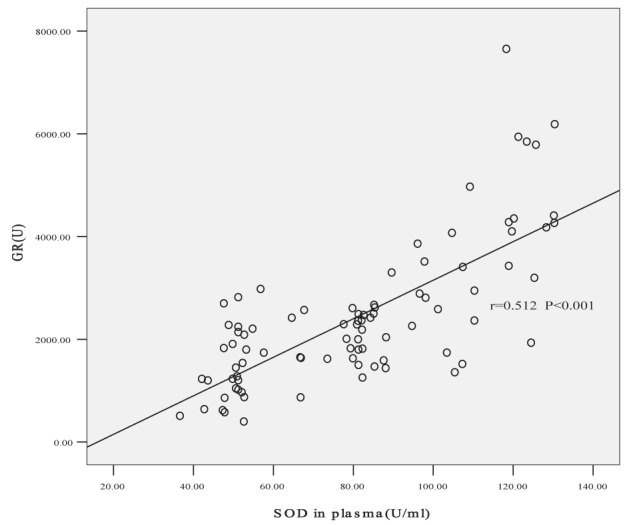
Correlation of superoxide dismutase (SOD) in plasma and glucocorticcoid receptor (GR) level. The partial correlation coefficient of GR and SOD in induced sputum was 0.512 after controlling for smoking index (P<0.001)
Acknowledgments
The authors thank all of the nurses of our department for blood sample collection and assistance in our study. The authors thank Professor Gao for assistance in assay of oxidative stress indexes.
Footnotes
DISCLOSURES: The authors have no financial disclosures or conflicts of interest to declare.
AUTHOR CONTRIBUTIONS: Mian Zeng was involved in the design, supervision and writing of the manuscript. Yue Li assisted with data collection and translation of the manuscript. Yujie Jiang assisted with collection of subjects and sputum induction. Guifang Lu performed blood sample collection. Xiaomei Huang was involved with the oxidative stress indexes assay, and Kaipan Guan with the GR assay. All authors read and approved the final manuscript.
REFERENCES
- 1.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of COPD: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 2.Harju TH, Peltoniemi MJ, Rytila PH, et al. Glutathione S-transferase omega in the lung and sputum supernatants of COPD patients. Respir Res. 2007;8:48. doi: 10.1186/1465-9921-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rytila P, Rehn T, Ilumets H, et al. Increased oxidative stress in asymptomatic current chronic smokers and GOLD stage 0 COPD. Respir Res. 2006;7:69. doi: 10.1186/1465-9921-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinnula VL, Ilumets H, Myllarniemi M, et al. 8-Isoprostane as a marker of oxidative stress in nonsymptomatic cigarette smokers and COPD. Eur Respir J. 2007;29:51–5. doi: 10.1183/09031936.00023606. [DOI] [PubMed] [Google Scholar]
- 5.Altuntas E, Turgut T, Ilhan N, et al. The levels of oxidant and antioxidant in patients with COPD. Tuberk Toraks. 2003;51:373–9. [PubMed] [Google Scholar]
- 6.Nadeem A, Raj HG, Chhabra SK. Increased oxidative stress and altered levels of antioxidants in chronic obstructive pulmonary disease. Inflammation. 2005;29:23–32. doi: 10.1007/s10753-006-8965-3. [DOI] [PubMed] [Google Scholar]
- 7.Marwick JA, Caramori G, Stevenson CS, et al. Inhibition of PI3Kδ restores glucocorticoid function in smoking-induced airway inflammation in mice. Am J Respir Crit Care Med. 2009;179:542–8. doi: 10.1164/rccm.200810-1570OC. [DOI] [PubMed] [Google Scholar]
- 8.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 9.The Criteria Committee of the New York Heart Association . Diseases of the Heart and Blood Vessels: Nomenclature and Criteria for Diagnosis. 6th edn. Boston: Little Brown; 1964. [Google Scholar]
- 10.Hector A, Jonas F, Kappler M, et al. Novel method to process cystic fibrosis sputum for determination of oxidative state. Respiration. 2010;80:393–400. doi: 10.1159/000271607. [DOI] [PubMed] [Google Scholar]
- 11.Gerald RC, Kendall AS, Allan M. Glucocorticoid receptors and sensitivity of isolated human leukemia and lymphoma cells. Cancer Res. 1978;38:4268–72. [PubMed] [Google Scholar]
- 12.Wasowicz W, Neve J, Peretz A. Optimized steps in fluorometric determination of thiobarbituric acidreactive substances in serum: Importance of extraction pH and influence of sample preservation and storage. Clin Chem. 1993;39:2522–6. [PubMed] [Google Scholar]
- 13.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 14.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 15.Zeng M, Wu JF, Xie CM, et al. Safety of sputum induction in subjects with acute exacerbations of chronic obstructive pulmonary disease. Chinese J Tuberculos Respir Dis. 2005;28:238–41. [PubMed] [Google Scholar]
- 16.Zeng M, Wen Y, Liu LY, et al. Role of TNF-α, sTNF-R55 and sTNF-R75 in inflammation of acute exacerbations of chronic obstructive pulmonary disease. Respiration. 2009;78:399–403. doi: 10.1159/000210263. [DOI] [PubMed] [Google Scholar]
- 17.Beeh KM, Beier J, Kornmann O, et al. Long-term repeatability of induced sputum cells and inflammatory markers in stable, moderately severe COPD. Chest. 2003;123:778–83. doi: 10.1378/chest.123.3.778. [DOI] [PubMed] [Google Scholar]
- 18.Brightling CE, Monterio W, Green RH, et al. Induced sputum and other outcome measures in chronic obstructive pulmonary disease: Safety and repeatability. Respir Med. 2001;95:999–1002. doi: 10.1053/rmed.2001.1195. [DOI] [PubMed] [Google Scholar]
- 19.Boorsma M, Lutter R, van de Pol MA, et al. Repeatability of inflammatory parameters in induced sputum of COPD patients. COPD. 2007;4:321–9. doi: 10.1080/15412550701597720. [DOI] [PubMed] [Google Scholar]
- 20.Beier J, Beeh KM, Kornmann O, et al. Stability of glutathione in induced sputum: Impact of freezing. Respiration. 2003;70:523–7. doi: 10.1159/000074211. [DOI] [PubMed] [Google Scholar]
- 21.Grootendorst DC, Sont JK, Willems LN, et al. Comparison of inflammatory cell counts in asthma: induced sputum vs bronchoalveolar lavage and bronchial biopsies. Clin Exp Allergy. 1997;27:769–79. [PubMed] [Google Scholar]
- 22.Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006;28:219–42. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- 23.Umland SP, Schleimer RP, Johnston SL. Review of the molecular and cellular mechanisms of action of glucocorticoids for use in asthma. Pulm Pharmacol Ther. 2002;15:35–50. doi: 10.1006/pupt.2001.0312. [DOI] [PubMed] [Google Scholar]
- 24.Matthews JG, Ito K, Barnes PJ, et al. Defective glucocorticoid receptor nuclear translocation and altered histone acetylation patterns in glucocorticoid-resistant patients. J Allergy Clin Immunol. 2004;113:1100–8. doi: 10.1016/j.jaci.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 25.Loke TK, Mallett KH, Ratoff J, et al. Systemic glucocorticoid reduces bronchial mucosal activation of activator protein 1 components in glucocorticoid-sensitive but not glucocorticoid-resistant asthmatic patients. J Allergy Clin Immunol. 2006;118:368–75. doi: 10.1016/j.jaci.2006.04.055. [DOI] [PubMed] [Google Scholar]
- 26.Corrigan CJ, Loke TK. Clinical and molecular aspects of glucocorticoid resistant asthma. Ther Clin Risk Manag. 2007;3:771–87. [PMC free article] [PubMed] [Google Scholar]
- 27.Berry MA, Hargadon B, Shelley M, et al. Evidence of a role of tumor necrosis factor α in refractory asthma. N Engl J Med. 2006;354:697–708. doi: 10.1056/NEJMoa050580. [DOI] [PubMed] [Google Scholar]


