Abstract
We have previously demonstrated that centrally administered vasotocin (VT) inhibits social approach toward same-sex conspecifics in male and female goldfish, and that this behavioral effect is dependent upon VT projections to the hindbrain. We now show that there are no sex differences in sensitivity to the behavioral effects of VT, though differences do exist in responsiveness across seasons in both sexes. A central dose of 1 µg, but not 200 ng, inhibited social approach in goldfish in non-reproductive condition, whereas a dose as low as 40 ng inhibited social approach in fish in full reproductive condition. In males and females in full reproductive condition, social approach behavior was facilitated by central administration of 500 ng of a V1A specific antagonist. In addition, the behavioral effects of exogenously administered central VT were blocked by central administration of 1 µg of a V1A antagonist. These results demonstrate that the propensity to approach a conspecific, a simple behavior underlying many social interactions, is controlled by a V1A-like receptor, and that VT’s behavioral effects depend on reproductive context. Quantitative real-time PCR showed that the seasonal changes in behavioral responsiveness to VT are associated with changes in the expression of a V1A-like receptor in the hindbrain, but not the mid- or forebrain, indicating that the seasonal regulation of social approach behavior likely depends on the local modulation of the expression of this receptor within a primitive peptide circuit in this species.
Keywords: Vasopressin, Vasotocin, V1A, Receptor, Social behavior, Teleost
1. Introduction
Vasotocin (VT) and its mammalian homologue, vasopressin (VP), influence a variety of social behaviors in vertebrate animals, particularly in reproductive contexts (reviewed in Goodson and Bass, 2001; Rose and Moore, 2002). A major focus of research has therefore been to elucidate the molecular mechanisms and neural circuitry that underlie peptide influences, which are often sexually dimorphic, on sexual and aggressive behaviors associated with reproduction. However, few studies have tried to determine if and how these peptides influence simple approach behaviors that typically precede such interactions and that may play an important role in determining how social organisms are, in and out of reproductive contexts.
In goldfish, VT inhibits the tendency to approach conspecifics (Thompson and Walton, 2004). This inhibition is mediated by one of the most pronounced VT projections in goldfish brains, the projection from VT cells in the preoptic area to the hindbrain (Thompson and Walton, 2009), as determined by experiments showing that VT infusions into the 4th ventricle, near the hindbrain, inhibit the behavior more potently than do infusions into the 3rd ventricle (Thompson et al., 2008b). The VT fibers in the hindbrain appear to induce this effect through a peripheral feedback mechanism initiated by interactions with substance P cells in the dorsal motor vagus (DMV), which the VT fibers encapsulate. These substance P cells project to the periphery, and VT no longer inhibits social approach if tachykinin receptors that mediate substance P’s peripheral effects are blocked, suggesting that the activation of those cells by VT induces a change in the physiological state of the animal that stimulates ascending pathways that ultimately inhibit social approach responses.
Although our previous studies thus indicate that a simple social behavior is mediated by VT and elucidate the neural circuitry associated with that effect, we know little about the social contexts associated with VT’s behavioral effects. No sex differences in VT producing cells or projection patterns have been identified in goldfish brains (Parhar et al., 2001; Thompson and Walton, 2009), and we have observed that high doses of VT can similarly inhibit social approach in males and females (Thompson et al., 2008b), which suggests that VT may have similar functions in males and females in this species. However, we do not yet know if both sexes are equally sensitive to VT, if endogenous VT similarly inhibits social approach in both sexes, or whether VT exerts its effects in reproductive contexts. To answer these questions, we performed dose-response and vasopressin receptor antagonist studies in males and females in various reproductive states.
Additionally, we know very little about the receptor mechanisms that mediate VT’s behavioral effects. In mammals, the behavioral effects of VP are mediated primarily by the V1A and V1B receptors, though V1A-like receptors appear to mediate most social responses to VT in non-mammalian species (Blanchard et al., 2005; Goodson and Bass, 2001;Wersinger et al., 2002; Young et al., 2001). Although variations in V1A and V1A-like receptor expression patterns in the forebrain are correlated with species-specific and sexually dimorphic behavioral effects of VT and VP across vertebrate groups (reviewed in Goodson and Bass, 2001; Young et al., 2002; Goodson, 2008), nothing is known about if and how hindbrain V1A-like receptors may contribute to behavioral regulation, or if their expression can be affected by factors that influence social behavior. To address the latter question, we sequenced a V1A-like receptor from goldfish and determined if its expression changes in hindbrain circuits involved in the regulation of social approach in goldfish in association with seasonal changes in behavioral sensitivity to VT.
2. Methods
2.1. Subjects
Reproductively mature comet goldfish (Carassius auratus; 12–15 cm, 25–50 g) were purchased from commercial hatcheries in March just prior to spawning season. Fish were sorted by sex and held in same-sex group housing in 340 L tanks at 18–20 °C in long photoperiod (14:10 L:D) for a minimum of 3 weeks prior to surgeries and behavioral testing. For animals in non-reproductive winter conditions, fish were received from suppliers and housed as above. Over the following 6 months temperatures and photoperiod were gradually reduced to 15 °C and 10:14 h L:D to mimic natural seasonal cycles. This allowed the use of fish of known sex during a time of year when they cannot be reliably sexed due to lack of secondary sexual characteristics. All subjects were verified for sex and reproductive condition at the conclusion of behavioral testing. All surgical methods, behavioral protocols, and methods of sacrifice were in accordance with guidelines for the use of vertebrate animals established by the Research Oversight Committee (IACUC) at Bowdoin College.
2.2. Surgery
Each fish was removed from its home tank and implanted with a 5-mm single guide cannula (Plastics One, Roanoke VA) extending into the third ventricle, as previously described (Thompson and Walton, 2004). Briefly, fish were anesthetized in 0.1% MS-222 (Sigma–Aldrich, St. Louis, MO) and a hole was drilled through the skull above the juncture of the optic tectum and telencephalon. A micromanipulator was then used to lower and hold the cannula 1.0 mm below the brain surface into the third ventricle. The cavity around the cannula was filled with Gelfoam (Pharmacia, Kalamazoo, MI), and two surgical screws were inserted into the skull. Dental cement (A-M Systems, Carlsbourg, WA) was then applied to cover the surgical site and to anchor the cannula in place. Fish were returned their home tank and allowed to recover for 3 days before behavioral testing. Cannula placement was verified in all fish by an injection of ink after all behavioral testing was complete.
2.3. Behavioral testing
2.3.1. VT dose-response
For each test, fish were placed into the central compartment of a 70 L rectangular tank with two 5 L stimulus compartments on each end, separated by sealed Plexiglas to prevent chemical communication. Time spent within 2.5 cm of each partition during a 15 min baseline was recorded with a video tracking system (Limelight; Coulbourn Instruments, Whitehall, PA, USA). Fish were then captured, infused with 1 µl of the appropriate dose of VT (5, 40, or 200 ng) or vehicle, counterbalanced across days with 24 h between tests, and then placed back into the central test tank. Five minutes later, a stimulus fish was placed in the side compartment behind the partition where the fish spent the least amount of time during the baseline period, and time within 2.5 cm of that partition was again recorded for 15 min. Corrected proximity scores were calculated by subtracting the baseline time in proximity to that partition from the time in proximity during the 15 min while the stimulus fish was present. After the last day of testing, 1 µl India ink was infused through the cannula. Fish were sacrificed 10 min later and the brains removed to evaluate the spread of ink through the ventricular system. Any fish with no ink in the ventricles was excluded from the analysis.
2.3.2. Blocking the effects of exogenous VT
Female goldfish in reproductive condition were surgically implanted with cannula, as described above. On the day of testing, fish were captured in their home tank, infused with either 1 µg of the V1A specific antagonist ([β-Mercapto-β,β-cyclopentamethylenepropionyl1, O-me-Tyr2, Arg8]-Vasopressin; Manning compound) or vehicle, counterbalanced across days with 48 h between tests, and returned to their home tank for 30 min. Fish were then recaptured and placed in the social approach testing tank, as described above. After a 15 min baseline, fish were removed from the testing tank, rapidly infused with 40 ng of VT, and returned to the tank. Five minutes later they were exposed to a stimulus female, as described above, and behavior was recorded for 15 min.
2.3.3. Blocking the effects of endogenous VT
Male and female goldfish in reproductive condition were surgically implanted with cannula, as described above. On the day of testing, fish were captured from their home tank, infused with either 500 ng of V1A specific antagonist or vehicle, counterbalanced across days with 48 h between tests, and placed in the test tank. After 30 min, baseline behavior was recorded for 15 min. A same-sex stimulus fish was then added to the less preferred side and behavior was recorded for 15 min.
2.4. Gene sequencing
2.4.1. cDNA synthesis
RNA was extracted from seven adult goldfish (3 males and 4 females) in spring breeding conditions and in fall non-breeding conditions. Fish were deeply anesthetized in 0.1% MS-222, rapidly decapitated, and their brains (~0.15 g tissue per brain) were removed intact. Total goldfish RNA was isolated from brain tissue using the Ambion RNAqueous-Midi kit for cellular RNA isolation (Ambion, Austin, TX). For standard and 3′ RACE PCR reactions, cDNA was synthesized using Superscript II according to the manufacturer’s instructions (Invitrogen 3’RACE System for Rapid Amplification of cDNA Ends; Invitrogen, Carlsbad, CA). For 5′ RACE reactions, cDNA was synthesized with BD PowerScript Reverse Transcriptase according to the kit protocol (BD SMART RACE cDNA Amplification Kit; Clontech, Mountain View, CA). All cDNA was stored at −80 °C.
2.4.2. PCR amplification of VTR fragments
An initial set of degenerate primers based on highly conserved amino acid sequences for the second transmembrane domain and a region 5′ of the sixth transmembrane domain were used in initial PCR runs to amplify a fragment of the goldfish VTR. A series of gene-specific upstream primers were then designed from that fragment and used in subsequent 3′ RACE reactions with downstream primers (Universal and Abridged Amplification Primers) complimentary to an anchor sequence attached to the Poly-A tail during cDNA synthesis. Similarly, a series of gene-specific downstream primers were designed from the initially sequenced fragments and used in 5′ RACE reactions according to the BD SMART 5′ RACE protocol (Clontech, Mountain View, CA).
2.4.3. Cloning
All PCR products were run on 1% agarose gels and visualized with ethidium bromide. Products were inserted into pCR-II-TOPO vectors and transformed into TOP10 chemically competent Escherichia coli according to the TOPO TA Cloning kit protocol (Invitrogen). Bacteria were grown overnight on LB plates containing 50 µg/ml kanamycin and 80 µg/ml 5-bromo-4-chloro-3-indolyl-beta-d-galactopyranoside (X-gal) in dimethyl formamide (DMF). Selected colonies were then grown overnight in Luria broth containing 50 µg/ml kanamycin, and vectors were isolated for sequencing using either the Wizard Plus SV Minipreps DNA Purification System (Promega, Madison, WI) or the QIAprep Spin Miniprep Kit (QIAGEN, QIAGEN Sciences, MD).
2.4.4. Gene sequencing and analysis
All sequencing reactions were performed by the Mount Desert Island Biological Laboratory (Salisbury Cove, ME). Sequence traces were analyzed using either Chromas (Version 2.31, Technelysium Pty Lt) or Finch TV 1.4 chromatogram viewer (Geospiza, www.geospiza.com/finchtv). NCBI BLAST database and ORF Finder (National Center for Biotechnology Information, Bethesda, MD) were used for sequence analysis and sequence translation. Sequence alignments were performed using the alignment software ClustalW. All percent identity calculations were done by JalView.
2.5. qPCR analysis
2.5.1. RNA isolation and cDNA synthesis
Brains were removed from male goldfish in spring, reproductive condition and fall, non-reproductive conditions and immediately frozen and stored at −80 °C. Males in reproductive condition had tubercles, expressed milt, and had enlarged testes; males out of reproductive condition did not display secondary sexual characteristics and had regressed testes. Brains from fish in reproductive condition were removed first and so were stored for approximately 6 months longer than brains taken from fish that were not in reproductive condition.
The hindbrains were separated from the mid- and forebrains of fish from each group using a razor blade to cut between the cerebellum and vagal lobes. Hindbrain and mid-/forebrain samples were treated independently and identically from this point forward. Two brains were typically processed at a time, one each from fish sacrificed during the different seasons. All samples were homogenized, and total RNA was isolated using the RNApure protocol (Genhunter, Nashville, TN). Remaining DNA was digested with DNase I (Roche Applied Science, Basel, Switzerland), and RNA was purified using CHROMA SPIN Columns (Clontech, Mountain View, CA). Concentrations of purified total RNA from every sample were measured in triplicate on a NanoDrop 1000 Spectrophotometer (Thermo Scientific, Wilmington, DE). To control for any differences of efficiency during cDNA synthesis, all RNA samples were spiked with equal amounts (105) of Alien RNA transcript following the Alien QRTPCR Inhibitor Alert protocol (Stratagene, Cedar Creek, TX). For each sample, cDNA was reverse transcribed from 400 ng of total RNA according to the SuperScript III First-Strand Synthesis SuperMix using nonspecific oligo(dT)20 primers according to the qRT-PCR protocol (Invitrogen, Carlsbad, CA), though we reduced the 2nd hearting stage from 50° to 42°. Samples were thus normalized to total starting RNA quantity prior to cDNA synthesis rather than to housekeeping genes, which can vary systematically along with target genes (see Trainor and Hofmann, 2007, for a comparison of RNA normalization methods to the use of housekeeping genes). By spiking all initial RNA samples with Alien, we were also able to control for potential differences in the efficiency of the reverse transcription reaction (see below). Additionally, identical cDNA reactions were performed on all hindbrain samples, but without reverse transcriptase, so that we could test for potential genomic contamination.
2.5.2. qPCR reactions
All reactions were run in a StepOne Real-Time PCR System (Applied Biosystems, Foster City, CA) using the SYBR GreenER qPCR Supermix Universal kit (Invitrogen, Carlsbad, CA). Forward and reverse primers were 5′GCATCTCGTTTCCAAACCCAACCA3′ and 5′AGTGCATCCGTGAGCTCTTCTTCT3′, respectively (synthesized by Invitrogen (Carlsbad, CA)), which flank a 204 base pair segment of the VT receptor gene (see Fig. 5 for location of the primer sites on the gene). Primers were used at 10 µM concentration. The relative initial concentration of the VT receptor cDNA was measured by qPCR for 16 forebrain and 14 hindbrain samples, each in triplicate on a single plate for each brain region. Thus, all hindbrain samples from fish in and out of reproductive condition were run on a single plate, as were all forebrain samples from fish in and out of reproductive condition. Using the same batch of reagents and the same samples, additional qPCR runs were performed in duplicate to quantify Alien transcript levels according to the Alien qRT-PCR Inhibitor Alert protocol (Stratagene, Cedar Creek, TX). Finally, qPCR reactions were run using equal volumes of template from the cDNA synthesis reactions that did not contain reverse transcriptase. During all qPCR reactions, threshold cycle and melting temperature (MT) values were recorded by the associated software.
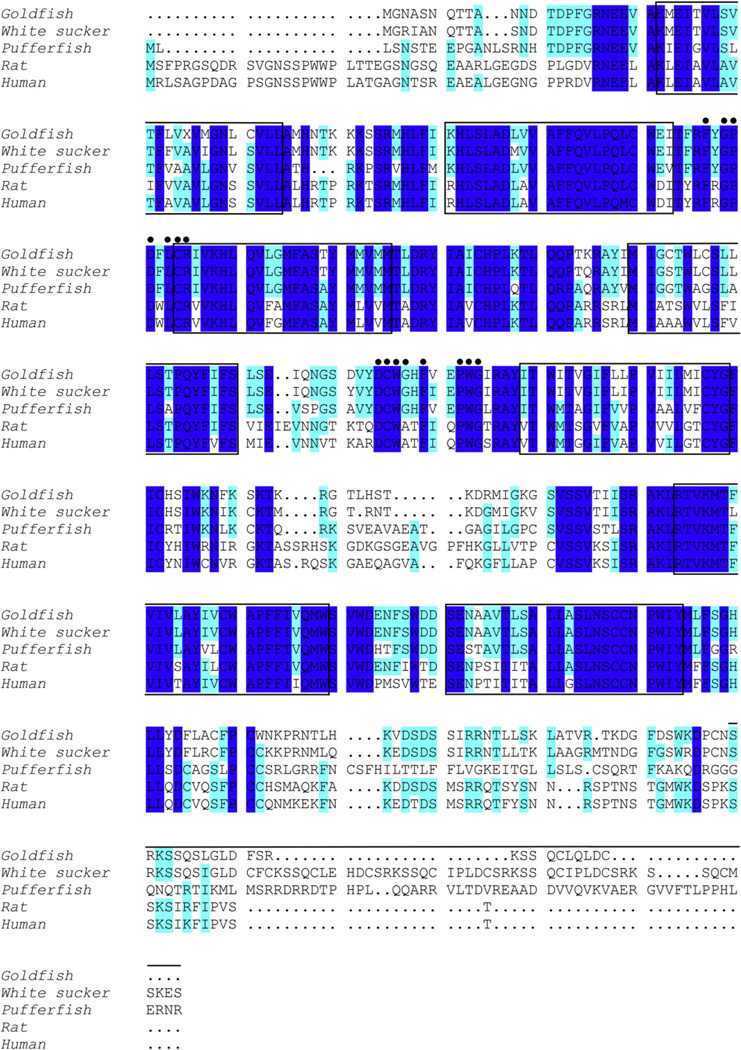
Fig. 5.
Alignment of the goldfish canonical VTR open reading frame (nucleotides 249–1445) with VTR/V1AR amino acid sequences for the white sucker (Catostomus commersoni), pufferfish (Takifugu rubripes), rat (Rattus norvegicus), and human (Homo sapiens). Shaded areas indicate conserved amino acid residues. Dots indicate conserved residues in vasopressin family receptors that may be involved in nonapeptide recognition (Mahlmann et al., 1994; Sharif and Hanley, 1992). The seven putative hydrophobic transmembrane domains are boxed. Solid line above sequence denotes C terminus repeated motifs in the white sucker sequence (Mahlmann et al., 1994).
2.5.3. Product verification
Melt curves were analyzed to determine that only one product was amplified during qPCR, as indicated by the detection of a single melting point at the temperature predicted for the size of the expected fragment. Additionally, the product was purified using the QIAquick PCR Purification Kit (QIAGEN, Valencia, CA) and sequenced by Mount Desert Island Biological Laboratory (Salisbury Cove, ME). This sequence was aligned with the goldfish VT receptor sequence using ClustalW2 software.
2.5.4. Data analysis
Behavior: Paired within-subjects t tests were used to compare responses within groups for behavioral tests. qPCR: Averages of triplicate CT values from the qPCR reactions amplifying the VT receptor and of the duplicate CT values for Alien were calculated for each subject. Each average was then raised to the −1 power, as described by Trainor and Hofmann (2007), and analyzed for normality. To control for potential differences in the efficiency of reverse transcription between the groups, analyses of variance (ANOVA)were performed for the forebrain and hindbrain samples using Alien 1/CT values as a covariate. Separate ANOVA’s for Alien 1/CT values were performed to see if there were any such group differences in reverse transcription efficiency. Fold differences between levels of VT receptor cDNA in the seasonal groups were calculated with the equation described by Pfaffl (2001): ratio(sample:control) = (Efficiencytarget)^Δ CTtarget(control − sample), substituting the in season group for the sample and the out of season group for the control. MT values were evaluated in each subject to ensure that the appropriate product was being amplified.
3. Results
3.1. Behavior
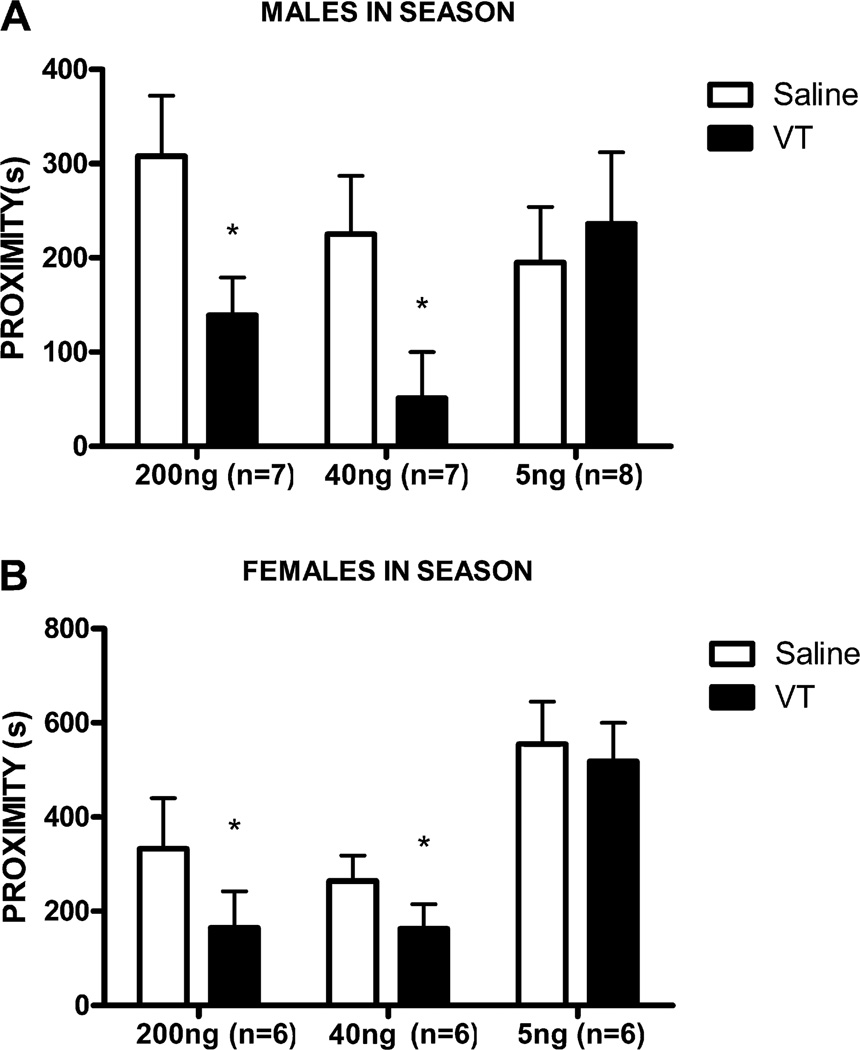
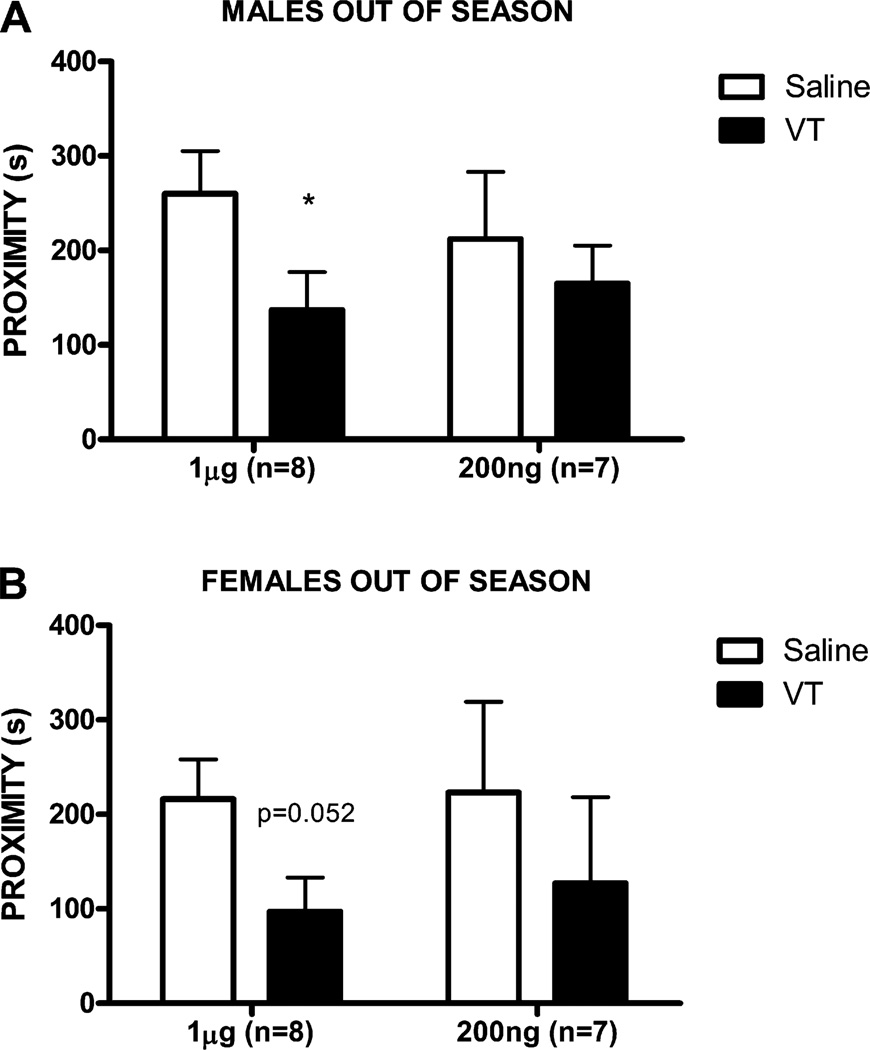
In male fish in full reproductive condition, social approach toward a conspecific male was inhibited by 200 ng VT (n = 7, t6 = 3.33, p = 0.02) and 40 ng VT (n = 7, t6 = 4.11, p = 0.006), but not by 5 ng VT (n = 8, t7 = 0.4, p = 0.70; Fig. 1A; all comparisons are with the same fish when infused with saline). For female fish in full reproductive condition, social investigation of a conspecific female was also inhibited by 200 ng VT (n = 6, t5 = 2.74, p = 0.04) and 40 ng VT (n = 6, t5 = 3.62, p = 0.01), but not by 5 ng VT (n = 6, t5 = 0.33, p = 0.76; Fig. 1B). In male fish in fall/winter conditions, social investigation of a conspecific male was inhibited by 1ug VT (n = 8, t7 = 2.83, p = 0.03), but not by 200 ng VT (n = 7, t6 = 0.56, p = 0.59; Fig. 2A). For female fish in fall/winter conditions, there was a marginal effect on social approach toward a conspecific female with 1ug VT (n = 8, t7 = 2.34, p = 0.052), but no effect on social approach with 200 ng VT (n = 7, t6 = 1.38, p = 0.22; Fig. 2B).
Fig. 1.
Mean (±SEM) time spent in proximity to a conspecific stimulus after ICV administration of saline (open bars) or VT at 3 doses (solid bars; 200 ng, 40 ng, 5 ng) for male goldfish (A) and female goldfish (B) in full reproductive condition. *p < 0.05.
Fig. 2.
Mean (±SEM) time spent in proximity to a conspecific stimulus after ICV administration of saline (open bars) or VT at 2 doses (solid bars; 1 µg, 200 ng) for male goldfish (A) and female goldfish (B) in late fall non-reproductive condition. *p < 0.05.
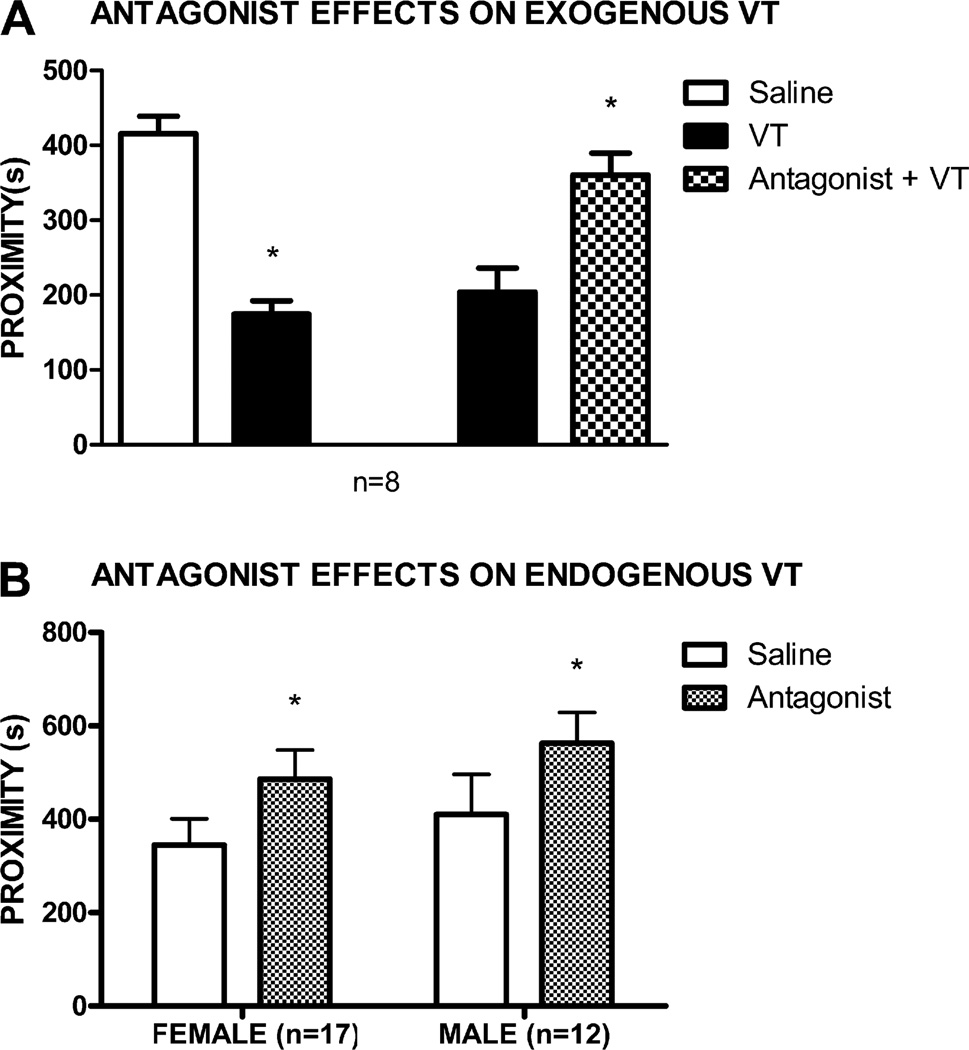
The behavioral effects of 40 ng of exogenously administered central VT in female fish in reproductive condition was blocked by 1 µg of V1A antagonist, as fish spent significantly more time near stimulus fish when VT was infused after saline than when VT after antagonist infusions (n = 8, t7 = 3.04, p = 0.02; Fig. 3A). For fish in full reproductive condition, social investigation of same-sex conspecifics was facilitated by central infusion of 500 ng V1A antagonist: males (n = 12, t11 = 2.22, p = 0.05) and females (n = 17, t16 = 2.21, p = 0.04) spent significantly more time near stimulus fish after infusions of antagonist than after infusions of saline (Fig. 3B).
Fig. 3.
(A) Mean (±SEM) time spent in proximity to a conspecific stimulus after ICV administration of saline followed by saline (open bar), saline followed by 40 ng VT (solid bars), or 500 ng of the V1A specific antagonist followed by 40 ng VT (checkered bar) in female goldfish in full reproductive condition. (B) Mean (±SEM) time spent in proximity to a conspecific stimulus after ICV administration of saline (open bars) or 500 ng of the V1A specific antagonist (checkered bars) for both male and female fish in full reproductive condition. *p < 0.05.
3.2. VTR mRNA sequence
PCR fragments with BLAST matches most similar to vasotocin receptor sequences in other species were combined, resulting in a 1981-base pair sequence that has a 74 percent identity (% ID) with the white sucker (Catostomus commersoni) VTR sequence (Fig. 4). This nucleotide sequence, which is the consensus sequence from seven fish from which the gene was independently sequenced, has an open reading frame of 398 amino acids spanning nucleotides 249–1445 that is highly conserved with the amino acid sequences for several teleost VT receptors and mammalian V1A receptors, particularly within the seven putative transmembrane domains (Fig. 5).
Fig. 4.
The nucleotide sequence of the goldfish vasotocin receptor mRNA transcript. The beginning and end of the open reading frame are highlighted and the region amplified by qPCR is underlined in italics.
3.3. qPCR gene expression analysis
Melt curves at the completion of qPCR showed single peaks at the predicted annealing temperature, indicating the goldfish VTR was the only product amplified by qPCR. This was confirmed by the direct sequencing of one of the qPCR products. Sequencing results showed 96% similarity to the consensus VTR mRNA (the regions immediately adjacent to the primers were difficult to resolve and accounted for the differences observed in the sequence). Seven standard curves generated by 2 fold serial dilutions of cDNA showed correlation values (r2) ranging from 0.75 to 0.97, with an average efficiency of 117%.
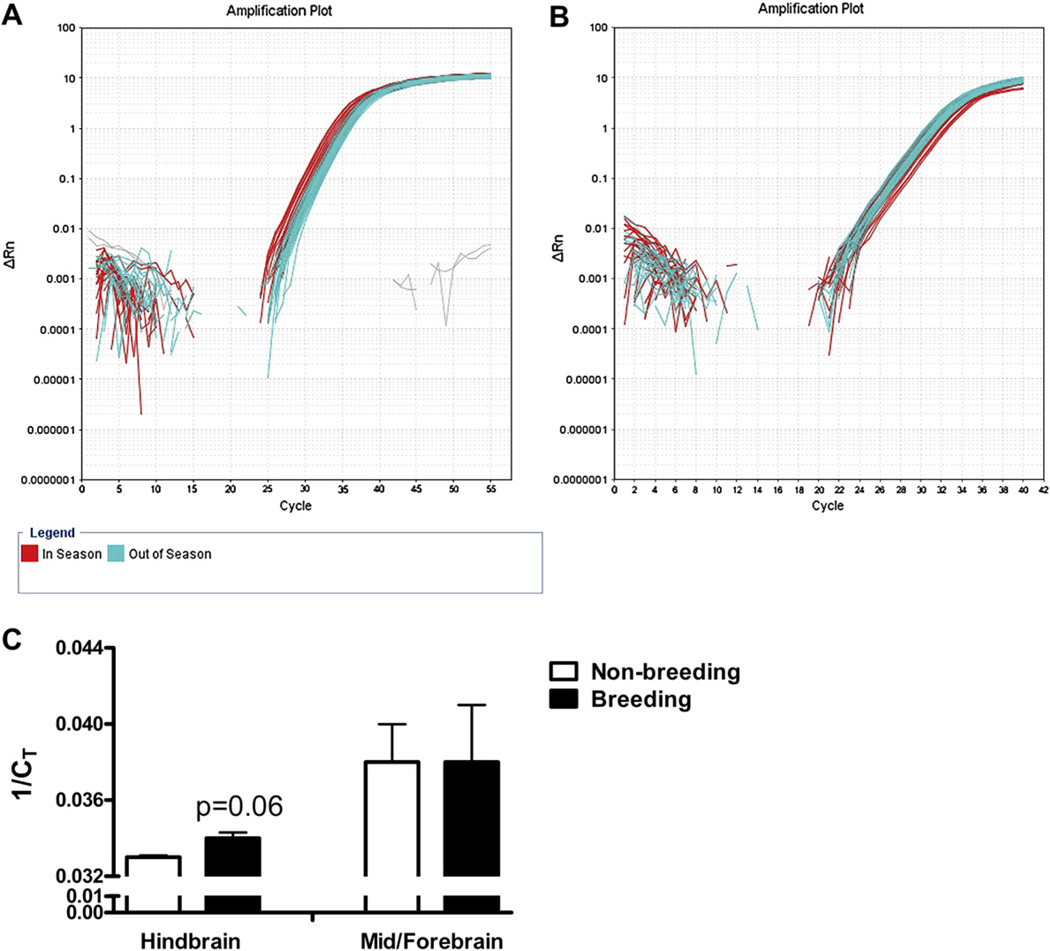
As predicted, there was a higher starting quantity of VT receptor cDNA in fish sacrificed during the breeding season than in fish sacrificed during the fall, as indicated by significantly higher 1/CT (F1,11 = 5.45, p = 0.04; Fig. 6). The 1/CT values for the Alien gene did not differ between groups (F1,12 = 0.27, p = 0.61). Levels of VT receptor expression in the hindbrain were 1.8 fold greater in spring, breeding fish than in the fall, non-breeding fish. However, there was amplification off the control sample that had not been treated with reverse transcriptase during the cDNA synthesis reaction from one fish, indicating potential genomic contamination. Without that sample, the same trend was present, with VT receptor expression being 1.7 fold higher in spring than in fall fish, though the difference just failed to reach significance (F1,10 = 4.32, p = 0.06). On the other hand, the starting quantity of VT receptor cDNA within the mid- and forebrain did not differ between spring, breeding and fall, non-breeding groups; there was not a significant difference in VT receptor 1/CT values, with Alien values as a covariate, between the groups (F1,13 = 0.5, p = 0.5). Again, Alien 1/CT values were not significantly different either (F1,14 = 0.16, p = 0.7).
Fig. 6.
Logarithmic plots of the qPCR amplification results for hindbrain samples (A) and mid-/forebrain samples (B). There was a strong trend for higher VTR expression in hindbrain samples from fish killed during the reproductive season than from fish killed outside of the reproductive season, as evidenced by higher 1/Ct scores (C; p = 0.06). No similar trend was observed in the mid-/forebrain samples.
4. Discussion
The current results demonstrate that VT’s ability to inhibit social approach responses toward same-sex conspecifics, though not sexually dimorphic, does vary seasonally in goldfish, with fish of both sexes more sensitive to VT during the breeding season. Additionally, endogenous VT appears to influence this behavior only during the breeding season, as that is the only time we have been able to stimulate social approach responses with a V1A receptor antagonist. Thus, although VT does not specifically influence stereotypical sexual or aggressive behaviors in this species, as it does in many others (reviewed in Goodson and Bass, 2001), its effects are dependent on reproductive contexts, which is consistent with the idea that VT’s behavioral influences evolved to subserve reproductive functions. Furthermore, the increased sensitivity to VT during the breeding season appears paralleled, at least in males, by the up-regulation of a V1A-like receptor in the hindbrain, where we have previously shown VT acts to inhibit social approach behavior in this species (Thompson et al., 2008b; see Introduction).
However, we have not yet identified which preoptic cells project to the hindbrain and thus drive that circuit. In Astatotilapia burtoni, a cichlid, VT mRNA expression in the parvocellular neurons is positively correlated with subordinate avoidance behavior (Greenwood et al., 2008). It is therefore possible that those cells project to the hindbrain and induce flight responses through a VT mechanism similar to the one we have described in goldfish (Thompson et al., 2008b). There is a similar projection from the parvocellular VP neurons in the paraventricular nucleus to the hindbrain in mammals that could likewise influence withdrawal behaviors (Sawchenko and Swanson, 1982). It is also possible that other preoptic VT neuronal populations contribute to this circuit in teleosts. In Sergeant damselfish, VT immunoreactive fiber density in the DMV is positively correlated with gigantocellular VT somata number in both sexes (Maruska, 2009), suggesting that these cells, which are only found in teleosts, may give rise to the hindbrain projection in teleosts, or at least contribute to it. Additionally, fiber densities in the DMV vary seasonally in damselfish (Maruska, 2009), which, together with our finding that V1A-like expression in the hindbrain changes seasonally, suggests that seasonal regulation of this primitive circuit may contribute to seasonal social regulation in teleosts.
VT/VP influence many behaviors related to reproduction in vertebrates, including aggression related to territorial defense and/or mate guarding, courtship, and social recognition, particularly in males and often in the context of seasonal breeding cycles (reviewed in Goodson and Bass, 2001), as well as maternal behavior, at least in rats (Bosch and Neumann, 2008), all of which have consequences on reproductive success. On the other hand, we do not yet know what function VT-induced social withdrawal serves during the breeding season in goldfish. Goldfish are a highly social species that form mixed sex groups, or shoals (Kavaliers, 1989; Magurran and Pitcher, 1983), and they use a group “scramble competition” spawning strategy, during which a single female will ovulate and then spawn with many of the males within the group (Taborsky, 2001). It is possible that VT mediates social spacing during the breeding season, which is dependent on social signals indicative of whether a female is ovulating and ready to spawn. Indeed, social stimuli have been shown to activate VT/VP cells and/or drive peptide release in numerous species (Beiderbeck et al., 2007; Ebner et al., 2005; Gobrogge et al., 2007; Goodson and Evans, 2004; Goodson and Wang, 2006; Greenwood et al., 2008; Lim and Young, 2004; Thompson et al., 2008a). Male and female goldfish both excrete androstenedione (AD) at particular times during the reproductive season; males excrete high levels when exposed to female sexual stimuli (Sorensen et al., 2005), whereas females excrete high levels early in their ovulatory cycle before they are ready to spawn (Scott and Sorensen, 1994). AD promotes aggressive responses in some males (Poling et al., 2001) but, as we have recently observed, can also inhibit approach responses toward other males (A. Keeney, unpublished data). We suspect AD may stimulate aggression in dominant males or in contexts in which withdrawal is not possible, as was the case in the Poling et al. study, but promote flight/avoidance when it is possible, particularly in subordinates, by activating the VT hindbrain circuit. Females may secrete AD to prevent male approach and courtship before they are ready to spawn, but we have not yet determined if AD can also inhibit approach responses toward females, nor do we know how AD affects female behavior yet. AD could similarly inhibit social approach in both sexes by driving VT release within the hindbrain, or AD could activate the VT system in males, particularly subordinates, but other social stimuli that trigger withdrawal may activate the system in females.
We have also not yet determined if there are VT affiliative circuits in goldfish, as there are in voles and finches. In male prairie voles, VP projections to the ventral pallidum, most likely originating from the bed nucleus of the stria terminalis (BST), are critical for mating induced pair bond formation (Lim and Young, 2004). In several estrildid finches, VT neurons in the medial BST respond selectively to affiliative social stimuli, and species that live in large groups have more of these neurons than species that live in small groups (Goodson and Wang, 2006), suggesting that these neurons contribute to social cohesion. There are VT projections to limbic regions of the telencephalon in goldfish (Thompson and Walton, 2009), but those projections are sparse in relation to the VT hindbrain projection, so any affiliative functions associated with them may get masked by the simultaneous activation of the more developed hindbrain circuits induced by our infusions. Conversely, it is not yet known if there is an “avoidance” VT/VP circuit in finches, voles, or any other species like the one we have described in goldfish. However, Goodson (2008) has proposed that the stress-responsive PVN neurons in zebra finches may have such functions, and that an individual’s immediate social behavior depends upon the relative activation of affiliative and avoidance circuits.
The likelihood that individual VT/VP circuits induce different, perhaps even opposite, effects on social approach behavior could complicate therapies that target the vasopressin system in humans. VP has anxiogenic actions in rodents (Bielsky et al., 2004; Landgraf et al.,1995; Liebsch et al., 1996) and humans, in which it specifically increases state anxiety (Thompson et al., 2006), indicating that VP receptor antagonists may be effective anxiolytics. However, if humans have affiliative forebrain VP circuits like those in prairie voles and finches, then such treatments could also inhibit social attachment processes. The presence of such circuits, at least in women, is suggested by VP’s ability, even as it increases anxiety, to promote affiliative responses toward other women (Thompson et al., 2006). Conversely, if VP hindbrain projections can, as in goldfish, induce social withdrawal in humans, and/or if other forebrain circuits promote aggression in humans, as they can in numerous other species (Ferris, 1992; Goodson and Adkins-Regan, 1999; Semsar et al., 2001; Winslow et al., 1993) and as is suggested by VP’s ability to stimulate agonistic facial responses in men (Thompson et al., 2004, 2006), then pharmacological manipulations intended to promote social attachment by selectively activating affiliative circuits will be difficult. The effectiveness of any such pharmacological manipulations could even differ between the sexes. Thus far, as in goldfish, no anatomical dimorphisms in the VP system have been observed in human brains (Fliers et al., 1986). However, unlike the similarity in behavioral responsiveness to VT within the aversive circuit in male and female goldfish, our human studies suggest that VP may induce differential effects on agonistic and affiliative responses in men and women (Thompson et al., 2006).
4.1. Molecular mechanisms
Our antagonist studies indicate that VT’s effects are mediated by a V1A -like receptor, and our sequencing indicates that the goldfish VT receptor has an open reading frame that is remarkably conserved with the V1A receptor sequences in mammals. As already mentioned, we have previously demonstrated that VT fiber projections to the hindbrain are dense in goldfish and that VT promotes social withdrawal through actions in the hindbrain (Thompson et al., 2008b; Thompson and Walton, 2009). We now report preliminary evidence that the seasonal increase in sensitivity to VT, at least in males, is associated with increased V1A-like receptor expression in the hindbrain. This primitive peptide circuit is thus likely modified by factors associated with seasonal breeding in this species. Interestingly, dose responsiveness increases 5–25 fold, while receptor expression only increases 1.8 fold in the hindbrain. This may reflect amplification associated with the inositol phosphate second messenger system, which V1A receptors are coupled to (Kirk et al., 1986), and/or the recycling of a limited pool of receptors following ligand binding and internalization (Lewis et al., 2005).
The most obvious mechanism for the seasonal regulation of V1A-like receptor expression in the hindbrain is the seasonal variation in sex steroid levels that occur in goldfish. VT/VP systems are sensitive to sex steroids, but steroids typically regulate VT/VP peptide production and/or release (reviewed in Goodson and Bass, 2001; DeVries, 2008). However, sex steroids, particularly androgens, do modulate VT/VP receptor binding in several species (Boyd and Moore, 1991; Delville et al., 1996; Young et al., 2000) and appear to account for the differences in receptor binding that occur across seasons in Syrian hamsters (Caldwell et al., 2008). Androgen receptors are not present in the hindbrain in goldfish, but aromatase and estrogen receptors are (Gelinas and Callard, 1997), though in seemingly more dorsolateral areas than where we observe dense VT-fiber terminals and thus where we would expect high receptor expression. Also, receptor expression did not change in forebrain areas that also have aromatase and steroid receptors, though local changes could have been masked by the inclusion of the entire fore and midbrains in those samples. Clearly, additional studies are needed to verify our preliminary qPCR results and to determine what may cause seasonal differences in V1A-like expression in the hindbrain.
5. Conclusions
VT neuromodulation similarly inhibits social approach behaviors toward conspecifics in male and female goldfish. There is, however, a difference in behavioral responsiveness across seasons, whereby fish in reproductive condition are most sensitive to VT, which is paralleled by an increase in V1A-like receptor expression in the hindbrain. These findings indicate that while VT has similar effects on a simple social approach response in both sexes, its endogenous influences nonetheless depend on reproductive context, and that the seasonal factors that influence these behaviors modulate sensitivity to VT in a primitive hindbrain circuit.
Acknowledgements
This work was supported by NSF grant # 0420938.
References
- Beiderbeck DI, Neumann ID, Veenema AH. Differences in intermale aggression are accompanied by opposite vasopressin release patterns within the septum in rats bred for low and high anxiety. Eur. J. Neurosci. 2007;26:3597–3605. doi: 10.1111/j.1460-9568.2007.05974.x. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Griebel G, Farrokhi C, Markham C, Yang M, Blanchard DC. AVP V1b selective antagonist SSR149415 blocks aggressive behaviors in hamsters. Pharmacol. Biochem. Behav. 2005;80:189–194. doi: 10.1016/j.pbb.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proc. Natl. Acad. Sci. U. S. A. 2008;105:17139–17144. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd SK, Moore FL. Gonadectomy reduces the concentrations of putative receptors for arginine vasotocin in the brain of an amphibian. Brain Res. 1991;541:193–197. doi: 10.1016/0006-8993(91)91018-v. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Smith DA, Albers HE. Photoperiodic mechanisms controlling scent marking: interactions of vasopressin and gonadal steroids. Eur. J. Neurosci. 2008;27:1189–1196. doi: 10.1111/j.1460-9568.2008.06071.x. [DOI] [PubMed] [Google Scholar]
- DeVries GJ. Sex differences in vasopressin and oxytocin innervation of the brain. Prog. Brain Res. 2008;170:17–27. doi: 10.1016/S0079-6123(08)00402-0. [DOI] [PubMed] [Google Scholar]
- Delville Y, Mansour KM, Ferris CF. Testosterone facilitates aggression by modulating vasopressin receptors in the hypothalamus. Physiol. Behav. 1996;60:25–29. doi: 10.1016/0031-9384(95)02246-5. [DOI] [PubMed] [Google Scholar]
- Ebner K, Wotjak CT, Landgraf R, Engelmann M. Neuroendocrine and behavioral response to social confrontation: residents versus intruders, active versus passive coping styles. Horm. Behav. 2005;47:14–21. doi: 10.1016/j.yhbeh.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Ferris C. Role of vasopressin in aggressive and dominant/subordinate behaviors. Ann. N. Y. Acad. Sci. 1992;652:212–226. doi: 10.1111/j.1749-6632.1992.tb34357.x. [DOI] [PubMed] [Google Scholar]
- Fliers E, Guldenaar SE, van de Wal N, Swaab DF. Extrahypothalamic vasopressin and oxytocin in the human brain; presence of vasopressin cells in the bed nucleus of the stria terminalis. Brain Res. 1986;375:363–367. doi: 10.1016/0006-8993(86)90759-6. [DOI] [PubMed] [Google Scholar]
- Gelinas D, Callard GV. Immunolocalization of aromatase- and androgen receptor-positive neurons in the goldfish brain. Gen. Comp. Endocrinol. 1997;106:155–168. doi: 10.1006/gcen.1997.6891. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Jia X, Wang Z. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J. Comp. Neurol. 2007;502:1109–1122. doi: 10.1002/cne.21364. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Nonapeptides and the evolutionary patterning of sociality. Prog. Brain Res. 2008;170:3–15. doi: 10.1016/S0079-6123(08)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Adkins-Regan E. Effect of intraseptal vasotocin and vasoactive intestinal polypeptide infusions on courtship song and aggression in the male zebra finch (Taeniopygia guttata) J. Neuroendocrinol. 1999;11:19–25. doi: 10.1046/j.1365-2826.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Horm. Behav. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK. Neural responses to territorial challenge and nonsocial stress in male song sparrows: segregation, integration, and modulation by a vasopressin V1 antagonist. Horm. Behav. 2004;46:371–381. doi: 10.1016/j.yhbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Wang Y. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17013–17017. doi: 10.1073/pnas.0606278103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood AK, Wark AR, Fernald RD, Hofmann HA. Expression of arginine vasotocin in distinct preoptic regions is associated with dominant and subordinate behaviour in an African cichlid fish. Proc. Biol. Sci. 2008;275:2393–2402. doi: 10.1098/rspb.2008.0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavaliers M. Day–night rhythms of shoaling behavior in goldfish: opioid and pineal involvement. Physiol. Behav. 1989;46:167–172. doi: 10.1016/0031-9384(89)90250-3. [DOI] [PubMed] [Google Scholar]
- Kirk CJ, Guillon G, Balestre MN, Jard S. Stimulation, by vasopressin and other agonists, of inositol-lipid breakdown and inositol phosphate accumulation in WRK 1 cells. Biochem. J. 1986;240:197–204. doi: 10.1042/bj2400197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holsboer F, Engelmann M. V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J. Neurosci. 1995;15:4250–4258. doi: 10.1523/JNEUROSCI.15-06-04250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CM, Dolence EK, Hubbard CS, Rose JD. Identification of roughskin newt medullary vasotocin target neurons with a fluorescent vasotocin conjugate. J. Comp. Neurol. 2005;491:381–389. doi: 10.1002/cne.20701. [DOI] [PubMed] [Google Scholar]
- Liebsch G, Wotjak CT, Landgraf R, Engelmann M. Septal vasopressin modulates anxiety-related behaviour in rats. J. Hered. 1996;217:101–104. [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125:35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Magurran AE, Pitcher TJ. Foraging, timidity and shoal size in minnows and goldfish. Behav. Ecol. Sociobiol. 1983;12:147–152. [Google Scholar]
- Mahlmann S, Meyerhof W, Hausmann H, Heierhorst J, Schonrock C, Zwiers H, Lederis K, Richter D. Structure, function, and phylogeny of [Arg8]vasotocin receptors from teleost fish and toad. Proc. Natl. Acad. Sci. U. S. A. 1994;91:1342–1345. doi: 10.1073/pnas.91.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruska KP. Sex and temporal variations of the vasotocin neuronal system in the damselfish brain. Gen. Comp. Endocrinol. 2009;160:194–204. doi: 10.1016/j.ygcen.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Parhar IS, Tosaki H, Sakuma Y, Kobayashi M. Sex differences in the brain of goldfish: gonadotropin-releasing hormone and vasotocinergic neurons. Neuroscience. 2001;104:1099–1110. doi: 10.1016/s0306-4522(01)00153-1. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poling KR, Fraser EJ, Sorensen PW. The three steroidal components of the goldfish preovulatory pheromone signal evoke different behaviors in males. Comp. Biochem. Physiol. B., Biochem. Mol. Biol. 2001;129:645–651. doi: 10.1016/s1096-4959(01)00361-x. [DOI] [PubMed] [Google Scholar]
- Rose JD, Moore FL. Behavioral neuroendocrinology of vasotocin and vasopressin and the sensorimotor processing hypothesis. Front. Neuroendocrinol. 2002;23:317–341. doi: 10.1016/s0091-3022(02)00004-3. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J. Comp. Neurol. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- Scott AP, Sorensen PW. Time course of release of pheromonally active gonadal steroids and their conjugates by ovulatory goldfish. Gen. Comp. Endocrinol. 1994;96:309–323. doi: 10.1006/gcen.1994.1186. [DOI] [PubMed] [Google Scholar]
- Semsar K, Kandel FL, Godwin J. Manipulations of the AVT system shift social status and related courtship and aggressive behavior in the bluehead wrasse. Horm. Behav. 2001;40:21–31. doi: 10.1006/hbeh.2001.1663. [DOI] [PubMed] [Google Scholar]
- Sharif M, Hanley MR. Peptide receptors. Stepping up the pressure. Nature. 1992;357:279–280. doi: 10.1038/357279a0. [DOI] [PubMed] [Google Scholar]
- Sorensen PW, Pinillos M, Scott AP. Sexually mature male goldfish release large quantities of androstenedione into the water where it functions as a pheromone. Behav. Ecol. Sociobiol. 2005;140:164–175. doi: 10.1016/j.ygcen.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Taborsky M. The evolution of bourgeois, parasitic, and cooperative reproductive behaviors in fishes. J. Hered. 2001;92:100–110. doi: 10.1093/jhered/92.2.100. [DOI] [PubMed] [Google Scholar]
- Thompson R, Gupta S, Miller K, Mills S, Orr S. The effects of vasopressin on human facial responses related to social communication. Psychoneuroendocrinology. 2004;29:35–48. doi: 10.1016/s0306-4530(02)00133-6. [DOI] [PubMed] [Google Scholar]
- Thompson RR, Dickinson PS, Rose JD, Dakin KA, Civiello GM, Segerdahl A, Bartlett R. Pheromones enhance somatosensory processing in newt brains through a vasotocin-dependent mechanism. Proc. Biol. Sci. 2008a;275:1685–1693. doi: 10.1098/rspb.2008.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RR, George K, Walton JC, Orr SP, Benson J. Sex-specific influences of vasopressin on human social communication. Proc. Natl. Acad. Sci. U. S. A. 2006;103:7889–7894. doi: 10.1073/pnas.0600406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RR, Walton JC. Peptide effects on social behavior: effects of vasotocin and isotocin on social approach behavior in male goldfish (Carassius auratus) Behav. Neurosci. 2004;118:620–626. doi: 10.1037/0735-7044.118.3.620. [DOI] [PubMed] [Google Scholar]
- Thompson RR, Walton JC. Vasotocin immunoreactivity in goldfish brains: characterizing primitive circuits associated with social regulation. Brain Behav. Evol. 2009;73:153–164. doi: 10.1159/000219485. [DOI] [PubMed] [Google Scholar]
- Thompson RR, Walton JC, Bhalla R, George KC, Beth EH. A primitive social circuit: vasotocin-substance P interactions modulate social behavior through a peripheral feedback mechanism in goldfish. Eur. J. Neurosci. 2008b;27:2285–2293. doi: 10.1111/j.1460-9568.2008.06210.x. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Hofmann HA. Somatostatin and somatostatin receptor gene expression in dominant and subordinate males of an African cichlid fish. Behav. Brain Res. 2007;179:314–320. doi: 10.1016/j.bbr.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS., 3rd Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol. Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm. Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Young LJ, Pitkow LJ, Ferguson JN. Neuropeptides and social behavior: animal models relevant to autism. Mol. Psychiatry. 2002;7:S38–S39. doi: 10.1038/sj.mp.4001175. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z, Cooper TT, Albers HE. Vasopressin (V1a) receptor binding, mRNA expression and transcriptional regulation by androgen in the Syrian hamster brain. J. Neuroendocrinol. 2000;12:1179–1185. doi: 10.1046/j.1365-2826.2000.00573.x. [DOI] [PubMed] [Google Scholar]