Abstract
Autophagy is a cellular pathway involved in protein and organelle degradation. It is relevant to many types of cellular homeostasis and human diseases. High level of glucose is known to inflict podocyte injury, but little is reported about the relationship between high concentrations of glucose and autophagy in these cells. The present study demonstrates that high glucose promotes autophagy in podocytes. Rapamycin further enhances this effect, but 3-methyadenine inhibits it. The proautophagic effect of high glucose manifested in the form of enhanced podocyte expression of LC3-2 and beclin-1; interestingly, antioxidants such as NAC were found to inhibit high glucose-induced autophagy. High glucose induced the generation of ROS by podocytes in a time-dependent manner. High glucose also enhanced podocyte expression of MnSOD and catalase. These findings indicate that high glucose-induced autophagy is mediated through podocyte ROS generation.
Keywords: Autophagy, High glucose, Podocytes, Oxidative stress
Introduction
The glomerular filtration barrier (GFB) is a special structure characterized by a complex 3-dimensional framework of podocytes and endothelial cells. Podocytes form interdigitating foot processes, which envelop the glomerular capillaries [1,2]. Podocytes are highly differentiated cells and play an important role in the pathogenesis of certain renal diseases. Both podocyte injury and loss are key factors in the development of glomerular diseases and progression of renal failure. The podocyte is the primary glomerular target of toxic, immune, metabolic and oxidant stress. Recent studies have indicated that podocyte injury is a common trigger leading to virtually all forms of glomerulopathies in general, and their role in the development of diabetic nephropathy (DN) is pivotal in particular [3–8].
Podocytes, as an important component of the GFB, are often exposed to various damaging factors, which have the potential to induce oxidative stress and/or DNA damage [9,10]. If a cell is exposed to cytotoxic or phlogogenic macromolecules, it has tendency to form autophagosomes to contain damaged proteins and organelles; DNA injury is often associated with the stimulation of DNA synthesis—either to repair DNA molecules or to provide material for cell division [11,12]. Podocyte, as a terminally differentiated cell, is dependent for its survival on efficient sequestration of unwanted or damaged proteins and organelles into autophagosomes [13]. If the insult is overwhelming, it may undergo apoptosis without attempting autophagic support [14].
Eukaryote cells carry two major protein degradation pathways—ubiquitin-proteasome system (UPS) and autophagy [15]. Both of these pathways are responsible for the efficient degradation and turnover of proteins within the cell. Failure of either the UPS or autophagy has been associated with disease manifestation, while the upregulation of these processes has been shown to ameliorate certain disease entities [15,16]. The podocyte is one of the more long-lived post-mitotic cells. Highly differentiated cells, such as neurons and cardiomyocytes, rely on autophagy for quality control among proteins and organelles.
Autophagy and apoptosis are two processes through which injured and aged cells or organelles are eliminated [17–21]. The same stimuli can induce either autophagy or apoptosis depending on the threshold [17]. Autophagy not only plays a principal role in the supply of nutrients for cell survival, but also plays a constitutive role in cellular homeostasis, where it acts as a cytoplasmic quality control mechanism to eliminate old or unfolded proteins and damaged organelles [18,20,21]. Apoptosis also removes damaged or unwanted cells. Autophagy is a mode of stress adaptation that in general suppresses apoptosis. Under other conditions, autophagy provides another pathway to cell death and is described as programmed cell death (PCD) type II. Recent reports have suggested that autophagy is upregulated and plays a protective role in kidney disease [22]. One possible mechanism for how autophagy protects cells is that it may eliminate damaged mitochondria, which leads to mitochondrial outer membrane permeabilization and consequent apoptosis. Autophagy involves sequestration of proteins and cell organelles in autophagosomes, which directs them to lysosomes [23]. The formation of autophagosomes is dependent on the induction of several genes including LC3, beclin-1, and atgs [24].
The factors involved in the pathogenesis of diabetic nephropathy are multifaceted [25]. Some of these factors involve imbalances between pro- and anti-free-radical processes and the formation of excessive free radicals in the kidney [26]. The effect of high levels of glucose on the growth of podocytes has been evaluated previously in in vitro studies [10,27,28]. The relationship between ROS and autophagy is also well established. ROS are known to induce autophagy. Autophagy, in turn, affects ROS production. High levels of mitochondrial ROS damage the mitochondrial membrane and associated increased mitochondrial membrane permeabilization; the latter causes ROS leakage into the cytosol and damage to other organelles [29]. Autophagy selectively targets and removes these obsolete organelles (damaged mitochondria and ER proteins) and thus, limits ROS amplification [30].
In the present study, we evaluated the effects of high glucose on the induction of autophagy in mouse podocytes. We also studied the mechanisms involved in high-glucose-induced podocyte autophagy.
Materials and methods
Animals
All work with rats was approved by the Animal Ethics Committee of Wuhan University, Hubei, China and was performed in accordance with the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health. 16 male SD rats weighing between 170 and 200 g were purchased from Hubei Research Center of Experimental Animals and were maintained at a controlled temperature (23±1 °C) and humidity (55±5%) under an artificial light cycle, with a free access to tap water and standard rat chow. Rats were randomly divided into diabetic group and control group (with 8 rats per group). Diabetes was induced by a single dose of streptozotocin (STZ, 65 mg/kg, intraperitoneal) in rats. Age-matched control rats received an equal volume of vehicle (0.1 M citrate buffer, pH 4.5). 48 h after injection of STZ, the blood glucose level was measured from the tail vein. Rats with a blood glucose level over 16.7 mmol/L were considered as diabetic rats. Rats were kept in individual metabolic cages for 24 h urine collection at the end of 8 weeks after STZ. Urine was centrifuged (1000 rpm, 10 min) at 25 °C. Whole urine was stored at −70 °C and thawed just before use. At the end of 8 week after STZ, Urinary albumin excretion (UAE) was measured using an ELISA Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Blood hemoglobin A1c (HbA1c) levels were measured by the latex agglutination. At the end of the study, rats were anesthetized with pentobarbital sodium and the blood samples were taken through the abdominal aorta for measuring biochemical parameters, including blood urea nitrogen (BUN) and creatinine (Cr), by an automatic biochemistry analyzer (Hitachi Model 7600, Japan). Animals were then killed and the kidneys were harvested immediately. At sacrifice, the weight of left kidney was calculated, the relative weight (%) was calculated using body weight at sacrifice. One part of the kidney was fixed in 2% glutaraldehyde, followed by epoxy resin embedding for electron microscopic studies.
Cell culture
Conditionally immortalized murine podocytes
Conditionally immortalized murine podocytes (CIMPs) were provided by Dr. Peter Mundel (Mount Sinai School of Medicine, New York, NY, USA). The cells were maintained in RPMI 1640 medium (HyClone, USA) containing 10% heat-inactivated fetal calf serum (Gibco, USA), 100 U/ml penicillin G, and 100 μg/ml streptomycin in the presence of 5% CO2. To sustain podocyte proliferation, 10 U/ml recombinant murine interferon-γ (Sigma, USA) was added into the medium and the cells were maintained at 33 °C. To induce differentiation, podocytes were cultured at 37 °C without interferon-γ for 10–14 days. Podocytes from passages 15–25 were used in the present study. All experiments were performed on differentiated podocytes.
Evaluation of autophagy using electron microscopy
CIMPs treated with normal level of glucose (5 mM) and high level of glucose (30 mM) were washed and fixed with 2% glutaraldehyde, buffered with 0.05 M Na cacodylate (pH 7.3). After fixation, the cells were prepared for electron microscopy (EM). During EM studies, 10 cytoplasmic fields per grid were randomly captured per cell. The autophagosomes were labeled and measured using the ruler provided. The numbers of autophagic vacuoles were counted by two observers, and data were recorded. In estimating the size of the autophagic vacuoles, the measurement along the largest diameter was taken and recorded.
Detection of GFP-LC3 overexpression and autophagy
CIMPs were incubated to subconfluence on 6-well plates and then transfected with GFP-LC3 plasmid DNA (InvivoGen) for 36 h. Transfection was carried out with Lipofectamine 2000 (Invitrogen) per the manufacturer’s recommendation, and 4 μg/ml GFP-LC3 plasmid DNA was used for each plate. The GFP-LC3-transfected cells were treated with normal glucose (5 mM) and high glucose (30 mM) conditions. Microphotographs of GFP-LC3 labeled cells were taken using fluorescence microscopy.
Evaluation of autophagic vacuoles by monodansylcadaverine
CIMPs were subcultured in 6-well plates and were grown to 40% confluence. The cells were then treated with either normal glucose (5 mM) or high glucose (30 mM) for 24 h. The cells were then treated with monodansylcadaverine (MDC) at a concentration of 0.05 mmol/L for a period of 15 min. Then cells were washed with PBS (4 times for 5 min each) and immediately examined under a confocal microscope at excitation wavelength of 330 nm and emission wavelength of 515 nm.
Evaluation of autophagic vacuoles by acridine orange
CIMPs were grown to subconfluence in 6-well plates and incubated in medium containing either normal glucose (5 mM) or high glucose (30 mM) for various time periods. In parallel series of experiments, CIMPs were treated under control (normal glucose) and experimental conditions (high glucose) for 24 h. At the end of the incubation period, cells were treated with 5 μg/mL acridine orange (Sigma) in serum-free medium for 15 min. Then the cells were washed 4 times in PBS and examined under a confocal microscope. The occurrence of autophagy was graded semiquantitatively (1+ to 4+; number of orange vacuoles and intensity of orange fluorescence) by two observers unaware of the experimental conditions. A total of 200 cells were counted for each variable.
Determination of reactive oxygen species kinetics in CIMPs
The kinetics of reactive oxygen species (ROS) metabolism in CIMPs was determined by measuring the fluorescent signal from the redox-sensitive fluoroprobe 2′,7′-dichlorofluorescein diacetate (DCFDA) at multiple points in time. DCFDA is a non-fluorescent molecule that becomes fluorescent in the presence of a wide variety of ROS, including superoxide anion and hydroxyl radicals [31]. Briefly, CIMPs were grown in 6-well plates in phenol red-free DMEM. Cells were washed in PBS, and DCFDA (10 mM) in serum-free medium was added for 40 min at 37 °C. Then cells were washed with phenol red-free DMEM, followed by addition of either normal glucose (5 mM) or high glucose (30 mM), and DCF fluorescence was detected at the indicated points in time using a Fluorescence Multi-Well Plate Reader CytoFluor 4000 (PerSeptive Biosystems) set for excitation at 485 nm and emission at 525 nm. The intensity of the fluorescent signal was calculated with the equation (Ft−F0)/F0×100 [32]. Here Ft indicates fluorescence and F0 is baseline fluorescence.
Western blotting studies
Control and experimental CIMPs were washed at the end of the incubation period, and cells were harvested and lysed in RIPA buffer (150 mM sodium chloride, 1.0% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM Tris, pH 8.0) with protease and phosphatase inhibitors, and centrifuged at 12,000 rpm for 20 min at 4 °C. Then the protein samples were mixed with loading buffer and boiled at 95–100 °C for 5 min. Total protein extracts (20 μg/lane) were separated on a 15% polyacrylamide (PAGE) premade gel (Bio-Rad, Hercules, CA, USA) and transferred onto a nitrocellulose membrane with a Bio-Rad miniblot apparatus. Nitrocellulose membranes were then processed further for immunostaining with primary antibodies against beclin-1 (1:500, Cell Signaling Technology), LC3-2 (1:1000, sigma), MnSOD (1:1000, Stressgen) and catalase (1:1000, Epitomics) and then with the appropriate horseradish peroxidase-labeled secondary antibodies. The blots were developed with a chemiluminescence detection kit (Pierce) and exposed to X-ray film (Eastman Kodak, Rochester, NY, USA). Equal protein loading and the protein transfer were confirmed by immunoblotting for actin protein using a polyclonal B-actin antibody (1:1000, Santa Cruz Biotechnology) on the same Western blots.
Statistical analysis
Results are expressed as mean±SEM. Differences between two groups were analyzed by paired t-test, and multiple comparisons were evaluated by analysis of variance (ANOVA) and a Newman–Keuls multiple-range test was used to calculate the P value. Statistical significance was defined as P<0.05.
Results
Metabolic and physical parameters of rats in two groups are presented in Table 1.
Table 1.
Metabolic and physical parameters.
| Group | BG (mmol/L) | HbA1c (%) | BUN (mmol/L) | Scr (μmol/L) | KW/BW (mg/g) | UAE (μlg/24 h) |
|---|---|---|---|---|---|---|
| NC | 4.9±1.2 | 4.37±0.33 | 6.46±1.56 | 48.2±7.7 | 4.79±0.78 | 41.8±7.7 |
| DN | 29.4±3.6** | 10.1±1.89** | 7.19±1.97 | 53.6±5.2 | 5.61±0.62* | 1244.1±186.3** |
BG: blood glucose; HbA1c: hemoglobin A1c; BUN: blood urea nitrogen; Scr: serum creatinine; KW: kidney weight; BW: body weight; UAE: urinary albumin excretion; NC: normal control rats; DN: STZ-induced diabetic rats.
P<0.05 vs. NC group.
P<0.01 vs. NC group.
High glucose promotes autophagy
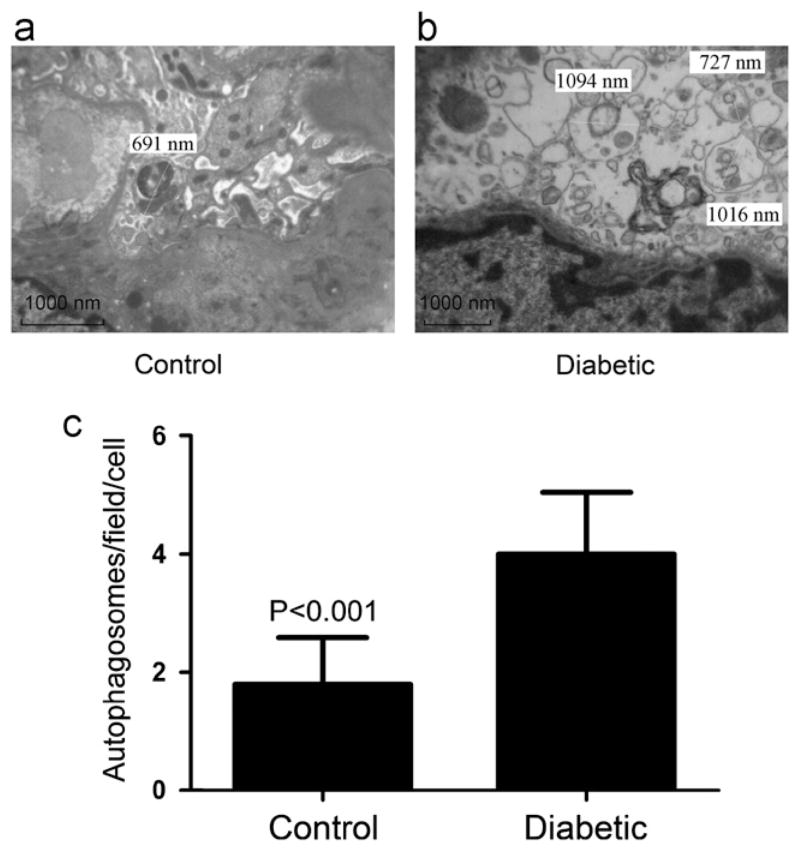
Renal cortical sections of two groups of rats were studied using EM. Representative electron microphotographs of podocytes in the control group and in the diabetic group are shown in Fig. 1a and b. The cumulative data of six sets of experiments are shown in Fig. 1c. Diabetic rats not only showed increased number of autophagosomes but also displayed larger size of autophagosomes when compared to control rats (P<0.001).
Fig. 1.
Electron microscopic evaluation of autophagy in podocytes of control rats and diabetic rats. (a) A representative electron microphotograph of a cell in a control rat. (b) A representative microphotograph of a cell in a diabetic rat. Numbers of autophagosomes were counted in 10 random fields; the diameter of each autophagosome was measured. (c) Cumulative data (means±SD) of 6 sets of experiments (10 podocytes in control rats and 10 cells in diabetic rats).
To determine whether high glucose promotes autophagy in podocytes, we incubated CIMPs in medium containing normal glucose (5 mM) and high glucose (30 mM) for 24 h (n=6). Subsequently, cells were studied using EM. Representative electron microphotographs of cells treated with normal glucose and those treated with high glucose are shown in Fig. 2a and b. The cumulative data of six sets of experiments are shown in Fig. 2c. Cells treated with high glucose not only showed larger autophagosomes but also presented a higher number of autophagosomes (P<0.001).
Fig. 2.
Electron microscopic evaluation of autophagy in CIMPs. Equal numbers of conditionally immortalized murine podocytes (CIMPs) were incubated in medium containing either normal glucose (5 mM) or high glucose (30 mM) for 24 h (n=6). Subsequently, cells were prepared for electron microscopic studies. (a) A representative electron microphotograph of a cell treated with normal glucose. (b) A representative microphotograph of a cell treated with high glucose. Number of autophagosomes was counted in 10 random fields; the diameter of each autophagosome was measured. (c) Cumulative data (means±SD) of 6 sets of experiments (50 cells treated with normal glucose and 50 cells with high levels of glucose).
To demonstrate the effect of high glucose on podocyte autophagy, CIMPs were transfected with GFP-LC3 and then incubated in medium containing either normal glucose (5mM) or high glucose (30mM) for 24 h. At the end of the incubation period, cells were examined under a fluorescence microscope. Representative microphotographs are shown in Fig. 3a. Cells treated with high glucose showed larger numbers of bright fluorescent particles indicating the presence of GFP-LC3-labeled autophagosomes.
Fig. 3.

High glucose enhances autophagy. (a) Representative microphotographs of GFP-LC3-transfected CIMPs incubated in medium containing either normal glucose (5 mM) or high glucose (30 mM) for 24 h. High-glucose-treated cells displayed brighter fluorescent, indicating the enhanced number of GFP-LC3-labeled autophagosomes. (b) Representative microphotographs of CIMPs incubated in medium containing either normal glucose (5 mM) or high glucose (30 mM) for 24 h, followed by treatment with monodansylcadaverine (MDC) for 15 min. Cells were washed and examined under a confocal microscope.
To confirm the occurrence of autophagy under control and experimental conditions, CIMPs were incubated in medium containing either normal glucose (5 mM) or high glucose (30 mM) for 24 h, They were then treated with MDC for 15 min. Subsequently, cells were washed and a fluorescence assay was performed using confocal microscopy. Representative microphotographs are shown in Fig. 3b. These findings indicate that high glucose induces autophagy in CIMPs.
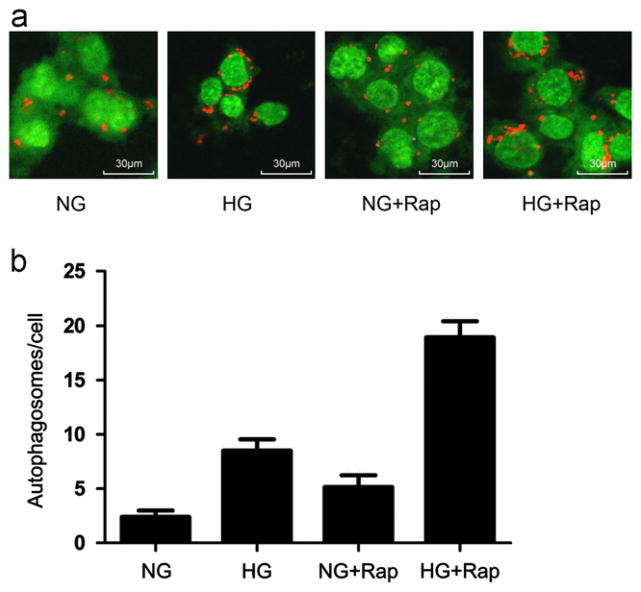
Rapamycin enhances high glucose-induced autophagy
Rapamycin has been reported to enhance autophagosome formation in a variety of cells, including podocytes [33,34]. We evaluated whether it could modulate high glucose-induced autophagy. CIMPs were incubated in medium containing either normal glucose (5 mM), high glucose (30mM), normal glucose+rapamycin (1 ng/mL), or high glucose+rapamycin (1 ng/mL) for 24 h (n=6). Subsequently, cells were stained with acridine orange and examined under a confocal microscope. Representative microphotographs of cells treated with normal glucose, high glucose, rapamycin+normal glucose, and rapamycin+high glucose are shown in Fig. 4a. Both high glucose and rapamycin promoted autophagosome formation in podocytes. However, rapamycin further enhanced high glucose-induced autophagosome formation. Cumulative data of six sets of experiments are shown in Fig. 4b.
Fig. 4.
Effect of rapamycin on high glucose-induced autophagosome formation. (a) Representative microphotographs of cells treated with normal glucose, high glucose, rapamycin+normal glucose and rapamycin+high glucose. Both high glucose and rapamycin promoted vacuoles formation (indicated by red staining). This effect of high glucose was further enhanced by rapamycin. (b) Equal numbers of CIMPs were incubated in medium containing normal glucose (5 mM) or high glucose (30 mM), rapamycin (1 ng/ml)+ normal glucose, or rapamycin+high glucose for 24 h (n=6). At the end of the incubation period, cells were stained with acridine orange and examined under a confocal microscope. Results (means±SD) are from 6 sets of experiments, each carried out in triplicate. P<0.05 for all groups compared to all other groups.
3-methyladenine inhibits high glucose-induced autophagy
3-methyladenine (3-MA) has been reported to inhibit autophagosome formation in podocytes [34]. To determine whether 3-MA can also inhibit high glucose-induced autophagy, CIMPs were incubated in medium containing normal glucose (5mM), high glucose (30mM), normal glucose+3-MA (2mM), or high glucose+3-MA (2 mM) for 24 h. Subsequently, cells were stained with acridine orange and examined under a confocal microscope. Representative microphotographs show the effects of 3-MA on high glucose-induced enhancement of autophagosome formation in Fig. 5a. Cumulative data of six sets of experiments are shown in Fig. 5b. Cells treated with high glucose showed an enhanced number of autophagosomes relative to control cells (P<0.05). However, this enhanced autophagic effect of high glucose was inhibited by 3-Ma.
Fig. 5.
Effect of 3-methyladenine (3-MA) on high glucose-induced autophagy. (a) Representative microphotographs of cells treated with normal glucose, high glucose, 3-MA+normal glucose, and 3-MA+high glucose, all stained with acridine orange. High levels of glucose promoted vacuole formation (indicated by red staining). This effect of high glucose was inhibited by 3-MA. (b) Equal numbers of CIMPs were incubated in medium containing normal glucose (5 mM), or high glucose (30 mM), 3-MA (2 mM)+ normal glucose, or 3-MA (2 mM)+high glucose for 24 h. Subsequently, cells were stained with acridine orange. Results (means±SD) are from 6 sets of experiments, each carried out in triplicate. P<0.05 for all groups compared to all other groups.
High glucose enhances podocyte LC3-2 expression
To determine the effect of high glucose on LC3-2 expression by podocytes, CIMPs were incubated in medium containing normal glucose (5mM), high glucose (30 mM), H2O2 (100 μM, positive control for autophagy), or normal glucose+L-glucose (normal glucose 5mM, L-Glucose 25mM, osmotic control for autophagy) for 24 h, followed by preparation of Western blotting and detection for LC3-2 or beclin-1. As shown in Fig. 6a and b, H2O2 was found to enhance the conversion of LC3-1 to LC3-2 in CIMPs. Similarly, high glucose was found to promote expression of LC3-2 in podocytes. As shown in Fig. 7a and b, high glucose was found to promote expression of beclin-1 in podocytes, but high levels of L-glucose have no significant difference with normal glucose in expression of beclin-1 by podocytes.
Fig. 6.
Effect of high glucose on podocyte LC3-2 expression. Equal numbers of CIMPs were incubated in medium containing normal glucose (5 mM), high glucose (30 mM), or H2O2 (100 μM, positive control for autophagy) for 24 h. At the end of the incubation period, cells were harvested and Western blots were prepared and probed for LC3-2 and actin. (a) The upper lane: expression of LC3-2 by podocytes treated with normal glucose, high glucose, and H2O2. The bottom lane: podocyte expression of actin under the same conditions. (b) n=6, *P<0.05 high glucose compared to normal glucose. **P<0.05 H2O2 compared to normal glucose.
Fig. 7.
Effect of high glucose on podocyte beclin-1 expression. Equal numbers of CIMPs were incubated in medium containing normal glucose (5 mM), high glucose (30 mM), or normal glucose+L-glucose (normal glucose 5 mM, L-Glucose 25 mM, osmotic control for autophagy) for 24 h. At the end of the incubation period, cells were harvested and Western blots were prepared and probed for beclin-1 and actin. (a) The upper lane: expression of beclin-1 by podocytes treated with normal glucose, high glucose, and normal glucose+L-glucose. The bottom lane: podocyte expression of actin under the same conditions. (b) n=6, *P<0.05 high glucose compared to all other groups.
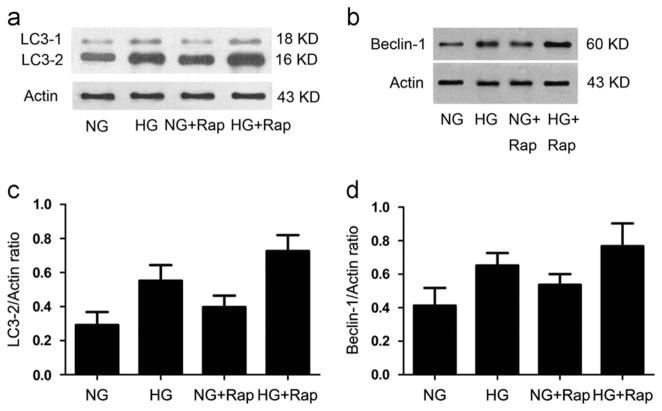
Rapamycin enhances high glucose-induced LC3-2 and beclin-1 expression by podocytes
Once we established that rapamycin promotes high glucose-induced autophagy, we next determined whether the effect of rapamycin is associated with LC3-2 and beclin-1 expression by CIMPs. CIMPs were incubated in medium, containing either normal glucose (5mM) or high glucose (30 mM), rapamycin (1 ng/ml)+normal glucose, or rapamycin (1 ng/ml)+high glucose for 24 h, followed by preparation of Western blotting and probing for LC3-2 and beclin-1. As shown in Fig. 8a and b, both high glucose and rapamycin enhanced conversion of LC3-1 to LC3-2 and beclin-1 by CIMPs. However, rapamycin further enhanced the effects of high glucose.
Fig. 8.
Effect of rapamycin on high glucose-induced podocyte LC3-2 and beclin-1 expression. Equal numbers of CIMPs were incubated in medium containing normal glucose (5 mM), high glucose (30 mM), rapamycin (1 ng/ml)+normal glucose, or rapamycin (1 ng/ml)+high glucose for 24 h. At the end of the incubation period, cells were harvested and Western blots were prepared and probed for LC3-2, beclin-1 and actin. (a and b) The top lane: LC3-2 and beclin-1 expression by control and experimental cells. The bottom lane: actin expression by cells treated under the same conditions. (c and d) n=6, P<0.05 for all groups compared to all other groups.
3-methyladenine inhibits high glucose-induced LC3-2 and beclin-1 expression by podocytes
3-methyladenine has been reported to inhibit autophagosome formation in podocytes. We asked whether the effect of 3-methyladenine is associated with LC3-2 and beclin-1 expression by CIMPs. CIMPs were incubated in medium, containing either normal glucose (5 mM) or high glucose (30 mM), normal glucose+3-MA (2 mM), or high glucose+3-MA (2 mM) for 24 h, followed by preparation of Western blotting and probing for LC3-2 and beclin-1. As shown in Fig. 9a and b. 3-methyladenine inhibited conversion of LC3-1 to LC3-2 and beclin-1 by CIMPs. The enhanced effect of high glucose was inhibited by 3-MA.
Fig. 9.
Effect of 3-MA on high glucose-induced podocyte LC3-2 and beclin-1 expression. Equal numbers of CIMPs were incubated in medium containing normal glucose (5 mM) or high glucose (30 mM), 3-MA (2 mM)+normal glucose, or 3-MA (2 mM)+high glucose for 24 h. At the end of the incubation period, cells were harvested and Western blots were prepared and probed for LC3-2, beclin-1 and actin. (a and b) The top lane: LC3-2 and beclin-1 expression by control and experimental cells. The bottom lane: actin expression by cells treated under the same conditions. (c and d) n=6, P<0.05 for all groups compared to all other groups.
Role of oxidative stress in induction of CIMPs autophagy
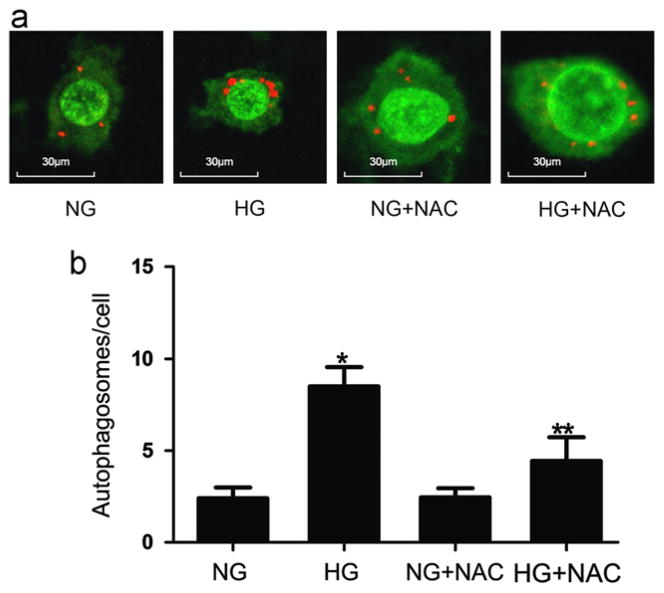
To determine whether oxidative stress plays a role in the induction of autophagy, CIMPs were treated with either normal glucose (5 mM) or high glucose (30 mM) in the presence or absence of N-acetylcysteine (NAC, 50 μM) for 24 h. Subsequently, cells were stained with acridine orange and examined under a confocal microscope. Representative microphotographs of experiments are shown in Fig. 10a and cumulative data of experiments are shown in Fig. 10b. These results indicate that oxidative stress plays a role in high glucose-induced autophagy by CIMPs.
Fig. 10.
Effect of NAC on high glucose-induced autophagosome formation. (a) Representative microphotographs of cells treated with normal glucose (5 mM), high glucose (30 mM), NAC (50 μM)+normal glucose, and NAC (50 μM)+high glucose. High glucose promoted vacuoles formation (indicated by red staining). This effect of high glucose was inhibited by NAC. (b) Equal numbers of CIMPs were incubated in medium containing normal glucose (5 mM) or high glucose (30 mM), NAC(50 μM)+normal glucose, or NAC (50 μM)+high glucose for 24 h (n=6). At the end of the incubation period, cells were stained with acridine orange and examined under a confocal microscope. Results (means±SD) are from 6 sets of experiments, each carried out in triplicate. *P<0.05 high glucose relative to all other variables, **P<0.05 high level of glucose+NAC relative to normal glucose and normal glucose+NAC.
To confirm the role of oxidative stress in the induction of CIMPs autophagy, CIMPs were treated with either normal glucose (5 mM) or high glucose (30 mM) in the presence or absence of N-acetylcysteine (NAC, 50 μM) for 24 h. Subsequently, Western blots were probed for LC3-2, beclin-1 and actin. As shown in Fig. 11a and b, high glucose enhanced LC3-2 and beclin-1 expression by CIMPs. However, this effect of high glucose was partly inhibited by NAC. These findings confirmed that oxidative stress plays a role in high glucose-induced autophagy-associated gene expression.
Fig. 11.

Effects of N-acetylcysteine on high glucose-induced podocyte LC3-2 and beclin-1 expression. Equal numbers of CIMPs were treated with either normal glucose or high glucose in the presence or absence of N-acetylcysteine (NAC, 50 μM) for 24 h. Subsequently, cells were harvested and Western blots were prepared and probed for LC3-2 and beclin-1. (a and b) The upper lane: LC3-2 and beclin-1 expression by control and experimental cells. The bottom lane: actin expression by cells treated under same conditions. (c and d) n=6, *P<0.05 high glucose compared to all other groups. **P<0.05 high glucose+NAC compared to normal glucose and normal glucose+NAC.
High glucose enhances ROS generation by podocytes
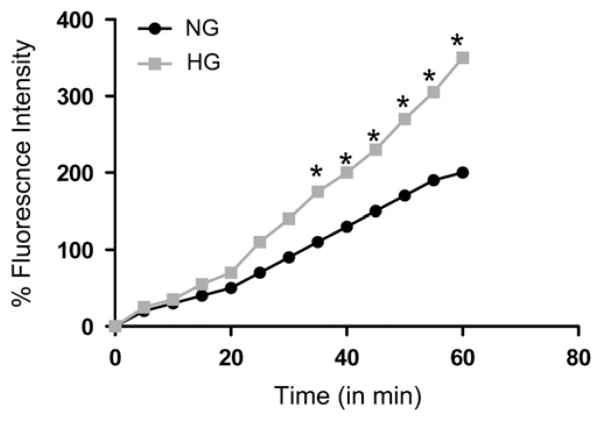
To determine the effect of high glucose on podocyte ROS generation, CIMPs were incubated in serum-free medium containing DCFDA for 40 min, followed by incubation in medium containing either normal glucose (5 mM) or high glucose (30 mM) for 60 min, and ROS generation was recorded at the indicated time periods. As shown in Fig. 12, high glucose enhanced ROS generation by CIMPs in a time-dependent manner.
Fig. 12.
Effects of high glucose on the generation of reactive oxygen species (ROS) by podocytes. Equal numbers of CIMPs were incubated in serum-free medium containing 2′,7′-dichlorofluorescein diacetate (DCFDA, 10 mM) for 40 min, followed by incubation in medium containing either normal glucose or high glucose for 60 min. ROS generation was recorded at the indicated time periods. *P<0.05 compared with respective control.
High glucose enhances MnSOD and catalase expression in CIMPs
To confirm whether high levels of glucose can induce oxidative stress on podocytes, we evaluated the effect of high glucose on podocyte expression of MnSOD and catalase. CIMPs were incubated in serum-free medium containing either normal glucose (5 mM) or high glucose (30 mM) for 24 h, followed by preparation of cells for Western blotting and probing for MnSOD and catalase. As shown in Fig. 13a and b, high glucose-treated CIMPs showed enhanced expression of both MnSOD and catalase.
Fig. 13.

Effect of high glucose on podocyte expression of MnSOD and catalase. Equal numbers of CIMPs were incubated in serum-free medium containing either normal levels of glucose or high levels of glucose for 24 h. At the end of the incubation period, cells were harvested and Western blots were prepared and probed for MnSOD, catalase, and actin. (a and b) Top: catalase and MnSOD expression by cells under control and experimental conditions. Bottom: actin expression by cells treated under the same conditions. (c and d) n=6, P<0.05 normal levels of glucose compared to high levels of glucose.
Discussion
Autophagy is a cellular pathway involved in protein and organelle degradation. It is connected to cellular homeostasis and human disease [15]. Autophagy was first described in the 1960s as a bulk degradation system that inactivates cell organelles, lipids, and protein components by the lysosomal pathway [35]. Autophagy has previously been shown to be essential to cell repair and turnover mechanisms for postmitotic cells such as neurons [15,36]. Strikingly, in the kidney, glomerular podocytes most prominently display autophagic activity. Recent studies have shown that mice lacking Atg5 in podocytes exhibited strongly increased susceptibility to models of glomerular diseases. These findings highlight the importance of induction of autophagy as a key to the maintenance of podocyte integrity [22].
In the present study, we observed the effects of high glucose on the induction of autophagy in podocytes. To quantitate the occurrence of autophagy, electron microscopic studies were carried out on podocytes of normal rats and diabetic rats and conditionally immortalized mouse podocytes (CIMPs) under normal and high glucose milieu. Podocytes of diabetic rats and CIMPs under high glucose milieu showed a higher numbers of autophagosomes per field than those of control groups. This enhanced effect of high glucose was further exacerbated by rapamycin and inhibited by pretreatment with 3-methyladenine, an inhibitor of autophagy. High glucose also enhanced podocyte expression of autophagic genes such as LC3-2 and beclin-1.
Podocytes are particularly susceptible to oxidative injury [37]. Because oxidative stress is often associated with autophagy induction [38–40], we examined the effects of high levels of glucose on the generation of podocyte reactive oxygen species (ROS). High glucose milieu promoted the generation of ROS by podocytes in a time-dependent manner. To determine the relationship between high glucose-induced oxidative stress and induction of autophagy, we tested the effects of antioxidants on high glucose-induced autophagy. As expected, the enhanced effect of high glucose-induced podocyte autophagy was inhibited by antioxidants. We therefore conclude that high glucose promotes podocyte autophagy through the generation of ROS.
High glucose milieu has been reported to induce oxidative stress in a variety of renal cells [41–44]. Several studies have shown the role of oxidative stress in the induction of autophagy [17–21]. Autophagy is a major homeostatic and quality control mechanism to maintain cellular integrity [15]. In the scenario of high glucose-induced oxidative stress, induction of autophagy may be required for the removal of damaged proteins and organelles. It shows that the induction of autophagy may be a default mechanism to prevent high glucose-induced podocyte injury.
Glucose has been reported to enhance the activation of renin angiotensin system (RAS) in podocytes [5,6]. We reported earlier that Ang II enhanced augtophagy in podocytes [34]. This effect of Ang II was mediated through podocyte ROS generation. Although we have not evaluated the role of Ang II in glucose-induced autophagy in the present study, but it will be important to explore this aspect in the future.
Beclin-1 is an autophagic gene that can intervene at every major step in the autophagic pathways from autophagosome formation to autophagosome/endosome maturation. It is also considered to be a tumor suppressor gene [45,46]. Its tumor-suppressive effects are associated with the induction of autophagy, which protects the cell from DNA damage. Reduced autophagy would lead to chromosomal aberrations and mutations. The DNA damage caused by excessive ROS may be another important inducer in carcinogenesis [47]. Accelerated mitochondrial ROS generation causes damage to mitochondrial membrane damage and ROS leakage into the cytosol, which, may damage other organelles [29]. Since autophagy selectively targets and removes these obsolete organelles (damaged mitochondrial and ER proteins), it will limit ROS amplification [30]. Therefore, oncogenic activation either by down regulation or inactivation of an autophagic gene may be considered a breakdown of this cellular protection machinery.
In summary, the present study shows that high glucose milieu promotes autophagy in podocytes. This effect of high glucose is mediated by mitochondrial ROS generation.
Acknowledgments
These studies were supported by the Grants from the National Science Foundation of China (81100519 to J.Z., 81100478 to W.L. and 81270762 to G.D.) and the National Institute of Health (RO1DK084910 and RO1DK083931 to P.C.S.)
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Huber TB, Benzing T. The slit diaphragm: a signaling platform to regulate podocyte function. Curr Opinion Nephrol Hypertension. 2005;14:211–216. doi: 10.1097/01.mnh.0000165885.85803.a8. [DOI] [PubMed] [Google Scholar]
- 2.Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 3.Li JJ, Kwak SJ, Jung DS, Kim JJ, Yoo TH, Ryu DR, Han SH, Choi HY, Lee JE, Moon SJ, Kim DK, Han DS, Kang SW. Podocyte biology in diabetic nephropathy. Kidney Int Suppl. 2007;106:S36–S42. doi: 10.1038/sj.ki.5002384. [DOI] [PubMed] [Google Scholar]
- 4.Marshall SM. The podocyte: a potential therapeutic target in diabetic nephropathy? Curr Pharm Des. 2007;13:2713–2720. doi: 10.2174/138161207781662957. [DOI] [PubMed] [Google Scholar]
- 5.Reddy GR, Kotlyarevska K, Ransom RF, Menon RK. The podocyte and diabetes mellitus: is the podocyte the key to the origins of diabetic nephropathy? Curr Opinion Nephrol Hypertension. 2008;17:32–36. doi: 10.1097/MNH.0b013e3282f2904d. [DOI] [PubMed] [Google Scholar]
- 6.Ziyadeh FN, Wolf G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev. 2008;4:39–45. doi: 10.2174/157339908783502370. [DOI] [PubMed] [Google Scholar]
- 7.Barisoni L, Schnaper HW, Kopp JB. Advances in the biology and genetics of the podocytopathies: implications for diagnosis and therapy. Arch Pathol Lab Med. 2009;133:201–216. doi: 10.1043/1543-2165-133.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lahdenkari AT, Lounatmaa K, Patrakka J, Holmberg C, Wartiovaara J, Kestila M, Koskimies O, Jalanko H. Podocytes are firmly attached to glomerular basement membrane in kidneys with heavy proteinuria. J Am Soc Nephrol. 2004;15:2611–2618. doi: 10.1097/01.ASN.0000139478.03463.D9. [DOI] [PubMed] [Google Scholar]
- 9.Chuang PY, Yu Q, Fang W, Uribarri J, He JC. Advanced glycation end products induce podocyte apoptosis by activation of the FOXO4 transcription factor. Kidney Int. 2007;72:965–976. doi: 10.1038/sj.ki.5002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Susztak K, Raff AC, Schiffer M, Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 11.Pan M, Maitin V, Parathath S, Andreo U, Lin SX, StGermain C, Yao Z, Maxfield FR, Williams KJ, Fisher EA. Pre-secretory oxidation, aggregation, and autophagic destruction of apoprotein-B: a pathway for late-stage quality control. Proc Natl Acad Sci USA. 2008;105:5862–5867. doi: 10.1073/pnas.0707460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zierhut C, Diffley JF. Break dosage, cell cycle stage and DNA replication influence DNA double strand break response. EMBO J. 2008;27:1875–1885. doi: 10.1038/emboj.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asanuma K, Tanida I, Shirato I, Ueno T, Takahara H, Nishitani T, Kominami E, Tomino Y. MAP-LC3, a promising autophagosomal marker, is processed during the differentiation and recovery of podocytes from PAN nephrosis. FASEB J. 2003;17:1165–1167. doi: 10.1096/fj.02-0580fje. [DOI] [PubMed] [Google Scholar]
- 14.Galluzzi L, Morselli E, Vicencio JM, Kepp O, Joza N, Tajeddine N, Kroemer G. Life, death and burial: multifaceted impact of autophagy. Biochem Soc Trans. 2008;36:786–790. doi: 10.1042/BST0360786. [DOI] [PubMed] [Google Scholar]
- 15.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winslow AR, Rubinsztein DC. Autophagy in neurodegeneration and development. Biochim Biophys Acta. 2008;1782:723–729. doi: 10.1016/j.bbadis.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baehrecke EH. Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol. 2005;6:505–510. doi: 10.1038/nrm1666. [DOI] [PubMed] [Google Scholar]
- 18.Gozuacik D, Kimchi A. Autophagy and cell death. Curr Top Dev Biol. 2007;78:217–245. doi: 10.1016/S0070-2153(06)78006-1. [DOI] [PubMed] [Google Scholar]
- 19.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meijer AJ, Codogno P. Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol. 2004;36:2445–2462. doi: 10.1016/j.biocel.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 22.Hartleben B, Gödel M, Meyer-Schwesinger C, Liu S, Ulrich T, Köbler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstädt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. Clin Invest. 2010;120:1084–1096. doi: 10.1172/JCI39492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohsumi Y, Mizushima N. Two ubiquitin-like conjugation systems essential for autophagy. Semin Cell Dev Biol. 2004;15:231–236. doi: 10.1016/j.semcdb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 25.Blázquez-Medela AM, López-Novoa JM, Martínez-Salgado C. Mechanisms involved in the genesis of diabetic nephropathy. Curr Diabetes Rev. 2010;6:68–87. doi: 10.2174/157339910790909422. [DOI] [PubMed] [Google Scholar]
- 26.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 27.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signaling. 2006;8:1597–1607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 28.Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem. 2005;280:39616–39626. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 29.Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 30.Azad MB, Chen Y, Gibson SB. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signaling. 2009;11:777–790. doi: 10.1089/ars.2008.2270. [DOI] [PubMed] [Google Scholar]
- 31.Walrand S, Valeix S, Rodriguez C, Ligot P, Chassagne J, Vasson MP. Flow cytometry study of polymorphonuclear neutrophil oxidative burst: a comparison of three fluorescent probes. Clin Chim Acta. 2003;331:103–110. doi: 10.1016/s0009-8981(03)00086-x. [DOI] [PubMed] [Google Scholar]
- 32.van Kats JP, Schalekamp MA, Verdouw PD, Duncker DJ, Danser AH. Intrarenal angiotensin II: interstitial and cellular levels and site of production. Kidney Int. 2001;60:2311–2317. doi: 10.1046/j.1523-1755.2001.00049.x. [DOI] [PubMed] [Google Scholar]
- 33.Vicencio JM, Galluzzi L, Tajeddine N, Ortiz C, Criollo A, Tasdemir E, Morselli E, Ben Younes A, Maiuri MC, Lavandero S, Kroemer G. Senescence, apoptosis or autophagy? When a damaged cell must decide its path—a mini-review. Gerontology. 2008;54:92–99. doi: 10.1159/000129697. [DOI] [PubMed] [Google Scholar]
- 34.Yadav A, Vallabu S, Arora S, Tandon P, Slahan D, Teichberg S, Singhal PC. ANG II promotes autophagy in podocytes. Am J Physiol Cell Physiol. 2010;299:C488–C496. doi: 10.1152/ajpcell.00424.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining clean cells. Autophagy. 2005;1:131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 37.Kerjaschki D. Dysfunctions of cell biological mechanisms of visceral epithelial cell (podocytes) in glomerular diseases. Kidney Int. 1994;45:300–313. doi: 10.1038/ki.1994.39. [DOI] [PubMed] [Google Scholar]
- 38.Xue L, Fletcher GC, Tolkovsky AM. Autophagy is activated by apoptotic signalling in sympathetic neurons: an alternative mechanism of death execution. Mol Cell Neurosci. 1999;14(3):180–198. doi: 10.1006/mcne.1999.0780. [DOI] [PubMed] [Google Scholar]
- 39.Kirkland RA, Adibhatla RM, Hatcher JF, Franklin JL. Loss of cardiolipin and mitochondria during programmed neuronal death: evidence of a role for lipid peroxidation and autophagy. Neuroscience. 2002;115(2):587–602. doi: 10.1016/s0306-4522(02)00512-2. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Gibson SB. Is mitochondrial generation of reactive oxygen species a trigger for autophagy? Autophagy. 2008;4(2):246–248. doi: 10.4161/auto.5432. [DOI] [PubMed] [Google Scholar]
- 41.Ha H, Hwang IA, Park JH, Lee HB. Role of reactive oxygen species in the pathogenesis of diabetic nephropathy. Diabetes Res Clin Pract. 2008;82(Suppl 1):S42–S45. doi: 10.1016/j.diabres.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 42.Hakim FA, Pflueger A. Role of oxidative stress in diabetic kidney disease. Med Sci Monit. 2010;16:RA37–RA48. [PubMed] [Google Scholar]
- 43.Kashihara N, Haruna Y, Kondeti VK, Kanwar YS. Oxidative stress in diabetic nephropathy. Curr Med Chem. 2010;17:4256–4269. doi: 10.2174/092986710793348581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, Cattoretti G, Levine B. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang J, Lam GY, Brumell JH. Autophagy signaling through reactive oxygen species. Antioxid Redox Signaling. 2011;14:2215–2231. doi: 10.1089/ars.2010.3554. [DOI] [PubMed] [Google Scholar]