Abstract
In the process of matrix assembly, multivalent extracellular matrix (ECM) proteins are induced to self-associate and to interact with other ECM proteins to form fibrillar networks. Matrix assembly is usually initiated by ECM glycoproteins binding to cell surface receptors, such as fibronectin (FN) dimers binding to α5β1 integrin. Receptor binding stimulates FN self-association mediated by the N-terminal assembly domain and organizes the actin cytoskeleton to promote cell contractility. FN conformational changes expose additional binding sites that participate in fibril formation and in conversion of fibrils into a stabilized, insoluble form. Once assembled, the FN matrix impacts tissue organization by contributing to the assembly of other ECM proteins. Here, we describe the major steps, molecular interactions, and cellular mechanisms involved in assembling FN dimers into fibrillar matrix while highlighting important issues and major questions that require further investigation.
Keywords: integrins, conformational change, insolubility, fibrillar, type I collagen, microfibrils
INTRODUCTION
The extracellular matrix (ECM) has been recognized as an essential structural component in multicellular organisms for millennia (Plato trans. 1965). However, the old view of ECM as an inert scaffold is clearly incorrect. ECM is a dynamic network, a reservoir for growth factors and fluids, and an essential organizer of tissues, cellular microenvironments, and stem cell niches. It shows exquisite tissue specificity and adapts to changes in age, development, and disease. Even so, the molecular events that assemble secreted ECM proteins into complex networks are still not completely understood. Why do we care about ECM assembly? Loss of assembly stops embryogenesis. Deranged assembly promotes scarring, tumorigenesis, and fibrotic disease. Delayed assembly underlies birth defects, chronic wounds, and skeletal malformations.
Using scanning electron microscopy (EM), two major structural forms of ECM are visible within tissues (Alberts et al. 2008). The interstitial matrix or stroma is composed of threadlike fibrils that form a fibrous and porous network surrounding cells, whereas the basement membrane (basal lamina) has a sheet-like structure that serves as a platform for cells and a boundary between tissue compartments. Although quite different structurally, interstitial matrices and basement membranes are assembled from similar types of proteins (collagens, proteoglycans, cell adhesive glycoproteins). In addition, the initial steps of assembly are quite similar for these two types of matrices (Mao & Schwarzbauer 2005a, Wierzbicka-Patynowski & Schwarzbauer 2003, Yurchenco & Patton 2009): ECM protein processing and secretion, binding to cell surface receptors, ECM protein self-association, and fibril growth. Thus, understanding the mechanisms of matrix formation in the interstitium can shed light on basement membrane assembly and vice versa.
This review will focus on our current understanding of fibronectin (FN) matrix assembly. FN is a ubiquitous ECM glycoprotein that is assembled into a fibrillar matrix in all tissues and throughout all stages of life. Its assembly is a cell-mediated process (McDonald 1988) and is essential for life (George et al. 1993). FN fibrils form linear and branched meshworks around cells and connect neighboring cells. EM images of cultured fibroblasts show long, interconnected fibrils ranging from more than 25 nm down to approximately 5 nm in diameter (Chen et al. 1978), which is approximately the size of the FN molecule itself at ~3 nm wide (Engel et al. 1981, Erickson & Carrell 1983, Leahy et al. 1996). Thin fibrils predominate in early fibroblast cultures, and as the matrix matures, these fibrils are clustered together into thicker fibril bundles (Chen et al. 1978, Singer 1979). What are the mechanisms and interactions that convert FN molecules into fibrils, networks, and bundles?
It has been more than 20 years since the last Annual Review article on ECM assembly (McDonald 1988). Since then, the amount of information about ECM organization and cell-ECM interactions has grown significantly with the identification of ECM protein receptors, new ECM components, signal transduction downstream of ECM, and mechanical properties of ECM (see, for example, Aszódi et al. 2006, Bershadsky et al. 2003, Hynes 1990, Schwartz et al. 1995). This information has led to new insights into the process of FN matrix assembly and how FN matrix affects assembly of other ECM proteins.
FIBRONECTIN DOMAIN ORGANIZATION
Domains and Binding Activities
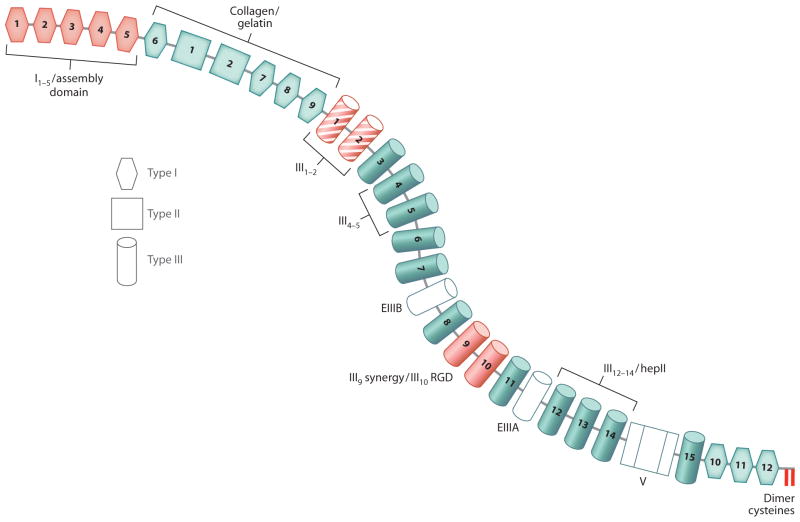
Many ECM proteins are modular and multidomain. This is well illustrated in the diagram of a FN subunit (Figure 1). FN has domains for interacting with other ECM proteins, cell surface receptors, glycosaminoglycans (GAGs), and other FN molecules (Hynes 1990, Mao & Schwarzbauer 2005a). This combination of domains allows FNs to bind simultaneously to cells and to molecules within the surrounding matrix.
Figure 1.
Diagram of a fibronectin (FN) subunit. Each FN subunit consists of three types of repeats: type I (hexagon), type II (square) and type III (cylinder). Based on rotary shadowing electron microscopy images, the two subunits of FN are curved with a contour similar to the shape in this diagram (Engel et al. 1981). Domains required to initiate assembly (red ) include the cell-binding domain (RGD site in III10 + synergy site in III9), the N-terminal assembly domain (I1–5), and the intermolecular dimer cysteines at the C terminus. The 70-kDa fragment extends from I1 through I9, including the assembly and the collagen/gelatin binding domains. The III1–2 domain (red with stripes) has two FN-binding sites and participates in conformational changes that promote assembly. Other FN-binding sites are located in the III4–5 domain and in the III12–14/hepII domain that also binds to heparin and syndecans. Alternatively spliced extra domains EIIIA, EIIIB, and the variable region (V) are shown in white.
FN is encoded by an ~8-kb mRNA yielding FN subunits that range in size from 230–270 kDa depending on alternative splicing (Hynes 1990). FN is a modular protein composed of types I, II, and III repeating units (Figure 1). Two intramolecular disulfide bonds form within each type I and type II module to stabilize the folded structure. Type III modules are seven-stranded β-barrel structures that lack disulfides (Leahy et al. 1996, Potts & Campbell 1994). Modules are organized into binding sites for collagen/gelatin, integrins, heparin, FN, and other extracellular molecules (Figure 1). The ~500-kDa FN dimer forms through a pair of antiparallel disulfide bonds at the C terminus.
FN exists in multiple isoforms generated by alternative splicing. The single FN gene transcript encodes 12 isoforms in rodents and cows and 20 isoforms in humans. Alternative splicing occurs by exon skipping at EIIIA/EDA and EIIIB/EDB and by exon subdivision at the V region/IIICS (Schwarzbauer 1991a; Figure 1). Roles have been identified for the V region in FN secretion (Schwarzbauer et al. 1989) and integrin binding (Guan & Hynes 1990, Wayner et al. 1989). EIIIA and EIIIB functions have been difficult to decipher using in vitro systems, and mice expressing EIIIA− or EIIIB−FN are viable and fertile (Muro et al. 2003, Tan et al. 2004). EIIIA/EIIIB double-null mice exhibit a requirement for these domains in vascular development during embryogenesis, but matrix incorporation of mutant FN was not affected (Astrof et al. 2007). In cell culture experiments, recombinant FN containing either EIIIA or EIIIB was somewhat more efficiently incorporated into an existing matrix (Guan et al. 1990), and EIIIB-null mouse embryo fibroblasts showed a modest reduction in FN matrix levels (Fukuda et al. 2002). From these results, it seems that these alternative exons are not required for matrix assembly but may affect matrix levels.
Activities that play critical roles in assembly include dimerization of FN subunits, cell-binding activity that localizes FN to the cell surface, and FN-binding activity that associates FN dimers into fibrils. However, each FN dimer has multiple integrin- and FN-binding sites, which raises questions about which sites directly participate in the assembly process and which carry out nonessential activities or function in post-assembly processes. Studies of matrix assembly have provided answers to some of these questions.
Domains Required for Fibronectin Matrix Assembly
The gold standard assay for demonstrating FN matrix assembly is conversion from deoxycholate detergent (DOC) solubility to DOC insolubility as originally defined by McKeown-Longo & Mosher (1983). This conversion is an irreversible process that stabilizes FN interactions within matrix fibrils to yield a mature fibrillar network. As visualized by fluorescence microscopy, FN matrix is a cell-associated fibrillar network extending between adjacent cells (Figure 2). Binding sites involved in assembly have been identified using these microscopic and biochemical assays combined with blocking reagents (antibodies or peptides), mutant FNs lacking specific sites or domains, and protein-binding assays.
Figure 2.
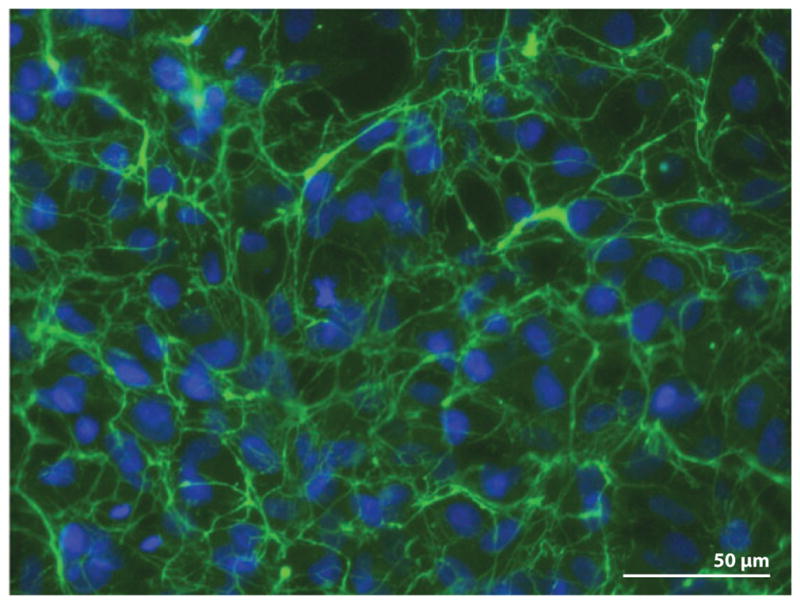
Fibronectin (FN) fibrillar matrix surrounds cells in culture. HT1080 cells were grown on a glass coverslip for 20 hours in medium supplemented with 0.1 μM dexamethasone and 25 μg ml−1 rat plasma FN as described in Brenner et al. (2000). Cells were fixed and stained with anti-FN monoclonal antibody (IC3) followed by fluorescein-tagged goat antimouse immunoglobulin G. Image shows FN fibrils ( green) around cells with 4′,6-diamidino-2-phenylindole (DAPI)-stained nuclei (blue).
FN’s dimer structure is mediated by a pair of disulfide bonds at the C terminus of each subunit (Figure 1; Hynes 1990). This covalent link plays an essential role in multimerization of dimers into fibrils. Expression of recombinant FN lacking these cysteines ablated dimerization; the resulting monomeric FN was secreted but did not form fibrils (Schwarzbauer 1991b). Recombinant dimeric proteins containing an FN-binding site but no cell-binding site were efficiently coassembled with full-length FN whereas monomeric versions were not (Ichihara-Tanaka et al. 1992, Sottile & Wiley 1994), which indicates that the dimer structure is involved in matrix incorporation even in the absence of cell binding. Interestingly, FN has endogenous protein disulfide isomerase activity that is located near the C-terminal disulfide bonds (Langenbach & Sottile 1999), where it may be important in forming the antiparallel dimer structure in the endoplasmic reticulum. This activity is partially cryptic and is enhanced by proteolysis (Langenbach & Sottile 1999), so perhaps it has an extracellular role in stabilizing FN interactions through disulfide exchange during matrix remodeling.
Cells mediate FN matrix assembly through integrin binding to the RGD (Arg-Gly-Asp) cell-binding domain. The primary receptor for FN matrix assembly is α5β1 integrin, which binds to the RGD sequence in III10 (Ruoslahti & Obrink 1996) and the synergy site in III9 (Aota et al. 1994; Figure 1). Antibody blockade of integrin-cell binding domain interactions using anti-integrin or anti-FN antibodies prevents fibril formation (Fogerty et al. 1990, McDonald et al. 1987). RGD-dependent integrins including α5β1 can bind to FN that lacks the synergy site (Danen et al. 1995, Sechler et al. 1996). However, both the RGD and synergy sites are required to initiate fibril formation (Sechler et al. 1997).
The pioneering work of McKeown-Longo & Mosher (1983, 1985) showed that the N-terminal 70-kDa fragment binds to cells in monolayer culture and, when added in excess, blocks FN matrix assembly. Antibodies to this region also block assembly (McDonald et al. 1987), and recombinant FN lacking all or part of the first five type I repeats (I1–5, Figure 1) is unable to form fibrils (Schwarzbauer 1991b). Within the 70-kDa fragment, the 40-kDa collagen/gelatin binding portion does not appear to play a direct role in assembly, although FN binding activity of the 70-kDa fragment is enhanced over binding of a fragment containing only I1–5 (McKeown-Longo & Mosher 1985). Functional analyses using recombinant fragments with various mutations showed that I1–5 functions as a unit to form the primary FN-binding and matrix assembly domain (Sottile et al. 1991).
Other Fibronectin Binding Sites
Affinity chromatography of soluble FN fragments has been used extensively to map binding sites for collagen/gelatin, heparin, fibrinogen, and other molecules (Hynes 1990, Ingham et al. 1997). In contrast, most of the binding sites involved in FN self-association have been detected using solid-phase binding assays. Surface adsorption of FN induces conformational changes, as demonstrated by exposure of antibody-binding sites (Ugarova et al. 1995, 1996) or by EM analyses (Engel et al. 1981, Erickson & Carrell 1983), and may also expose sites for FN-FN interactions.
Essentially all reported FN-binding sites interact with the N-terminal assembly domain, and most of these have been identified using the 70-kDa fragment in binding studies. Binding sites in III2 (Aguirre et al. 1994), III4–5 (Maqueda et al. 2007), III12–14 (Bultmann et al. 1998), and heat-denatured III1 (Hocking et al. 1994) have been identified (Figure 1), as has formation of a ternary complex containing the 70-kDa fragment, III1, and heat-denatured III10 (Hocking et al. 1996). In addition to FN, I1–5 also binds to fibrinogen, heparin, bacterial proteins, and thrombospondin (Hynes 1990).
Accessibility of FN binding sites in III1, III5, and III10 is dependent on denaturation (Hocking et al. 1994, 1996), which suggests that these sites are cryptic in native FN. The site in III4–5 appears to be cryptic in larger FN fragments because III4–6 does not bind to FN (Maqueda et al. 2007). The cryptic nature of some of these sites has raised the hypothesis that type III unfolding exposes sites for FN-FN interactions during fibril formation. The β-barrel structures of type III modules are not stabilized by intramolecular disulfide bonds, thus giving β-strands some conformational flexibility. No deficiencies in matrices assembled from recombinant proteins lacking these sites (FNΔIII1 or FNΔIII4–5) were detected by microscopy or DOC insolubility (Sechler et al. 2001), which makes it unlikely that these cryptic sites are individually essential for FN fibril formation.
Other cryptic sites in FN include a site in I1–5 for tenascin-C binding (Ingham et al. 2004) and a site in III8 that is made available by inclusion of the adjacent alternatively spliced exon EIIIB (Figure 1; Ventura et al. 2010). In addition to exposure by conformational changes, cryptic sites might also be made accessible by proteolysis during matrix remodeling. For example, proteolysis of FN can promote α4β1 integrin interactions with FN matrix (Valenick et al. 2005).
Given the number of FN-binding sites, one wonders if they are equivalent. Does the assembly domain use all of its binding sites to form fibrils? Our current understanding of the requirements for all of these sites is limited because differences in experimental approach yield different interpretations of function. For example, FNΔIII1 (recombinant FN lacking only the III1 module) forms a normal matrix, but FNΔIII1–2 does not (Sechler et al. 2001). FNΔIII4–5 also forms a normal matrix (Sechler et al. 2001), but addition of the III4–5 fragment blocks fibril formation by cells (Maqueda et al. 2007). FNΔIII1–2 does not form DOC-insoluble matrix, but FNΔIII1–7 (which lacks a larger FN segment) forms DOC-insoluble fibrils at an enhanced rate (Sechler et al. 1996). Addition of fragments spanning III1, III2, or III12–14 have limited ability to block matrix assembly (Bultmann et al. 1998, Chernousov et al. 1991) compared with the 70-kDa fragment, which is an extremely effective inhibitor of fibril formation (McDonald et al. 1987, McKeown-Longo & Mosher 1985, Sechler & Schwarzbauer 1998). One interpretation of these differential effects is that temporal and spatial distributions of FN-binding sites within the matrix may determine which domains interact. Perhaps there is a hierarchical order to FN interactions, or some of these interactions may require formation of ternary complexes involving other ECM components.
A FIBRONECTIN MATRIX ASSEMBLY MODEL
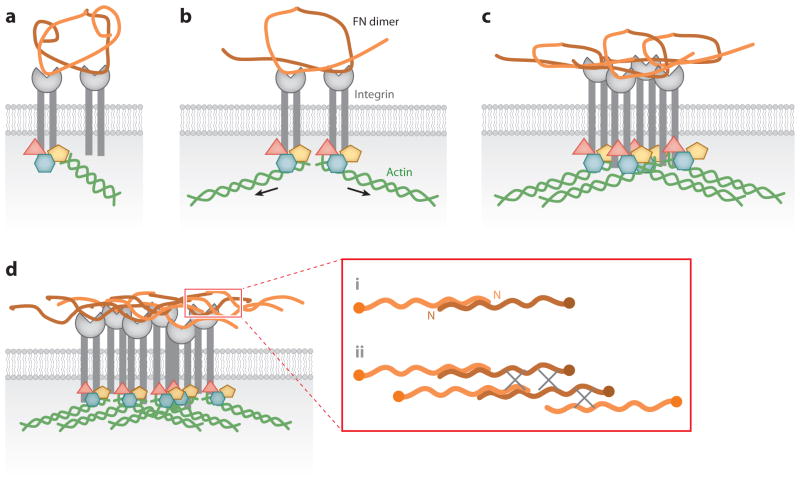
Using the key assays of DOC insolubility, microscopic visualization, and protein-binding assays, the basic steps of FN matrix assembly have been defined (Figure 3). Integrins tether FN dimers to initiate FN-FN interactions mediated by the N-terminal assembly domain. Conformational changes expose additional FN-binding sites to promote further FN interactions, associations between fibrils, and matrix insolubility. Evidence for these steps is described below.
Figure 3.
Major steps in fibronectin (FN) matrix assembly. Integrin-induced conversion of compact FN to extended fibrils is shown in four steps. (a) A compact FN dimer binds to integrins ( gray). FN subunits of a single dimer are shown in two shades of orange. (b) Intracellular proteins ( pink, yellow, blue) are recruited to integrin cytoplasmic domains and connected to the actin cytoskeleton ( green). Cytoskeletal connections increase cell contractility (arrows), which induces conformational changes in FN. (c) Integrin clustering and exposed FN-binding sites promote FN-FN interactions and further changes in FN conformation. (d ) Finally, these events trigger formation of stable insoluble fibrillar matrix. The inset (red box) shows interactions between single subunits of FN dimers. N indicates the N terminus of an FN subunit. Fibrils form through (i) end-to-end association of FN dimers, mediated by the N -terminal assembly domain, followed by (ii) lateral associations between fibrils that are likely to involve the other FN-binding sites in III1–2, III4–5, and III12–14. Gray X’s represent interactions between fibrils.
Fibronectin Dimer Secretion and Integrin Binding
FN in solution has a compact conformation, as detected by sedimentation, EM, and other measurements (Hynes 1990). FN in solution does not form fibrils even at extremely high concentrations, a property that is especially important in body fluids such as blood, where FN fibril formation could have life-threatening effects. Soluble FN exhibits selective binding to cell surface receptors and interacts with α5β1 but not with other RGD-dependent integrins (Huveneers et al. 2008). FN binds to α5β1 via its RGD and synergy sites, both of which are required for de novo FN fibril formation (Sechler et al. 1996, Sottile et al. 2000). However, full-length recombinant FN lacking only the RGD sequence as well as small dimeric FNs containing the N-terminal domain but lacking other parts of FN can coassemble with full-length FN (Ichihara-Tanaka et al. 1992, Sechler et al. 1996, Sottile & Wiley 1994). Incorporation of these recombinant proteins into fibrils must occur primarily through FN-FN interactions. Thus, not all FNs in a matrix must directly interact with integrins. Mouse embryos expressing FN with an inactive RGE sequence in place of RGD developed several days longer than FN-null embryos and were phenotypically quite similar to α5-null embryos (Takahashi et al. 2007, Yang & Hynes 1996). FN interactions with αv integrins have been shown to compensate for the absence of RGD or α5 integrin (Takahashi et al. 2007, Yang & Hynes 1996). These results indicate that, within tissues, some FN matrix can be assembled in the absence of RGD-α5β1 integrin interactions.
Fibronectin-Fibronectin Interactions
FN binding induces integrin clustering, which brings receptors together along with their bound FNs (Figure 3). These clusters provide locally high concentrations of FN at the cell surface, which is probably important to promote FN-FN interactions. The diameter of thin FN fibrils approximates the dimensions of individual type III modules (Dzamba & Peters 1991, Engel et al. 1981, Leahy et al. 1996), which indicates that compact FN in solution is extended during fibril polymerization. Intramolecular interactions between III2–3 and III12–14 maintain the compact form of FN ( Johnson et al. 1999). These regions overlap with FN-binding sites, which suggests that disrupting these interactions to extend compact FN dimers frees these sites to participate in intermolecular interactions (Figure 3).
A key insight into FN conformational changes came from studies of the stimulatory effects of Rho GTPase on FN assembly. Binding of lysophosphatidic acid (LPA) or sphingosine-1-phosphate (S-1-P) to receptors activates Rho (Anliker & Chun 2004). Rho-GTP then stimulates Rho kinases to enhance cell contractility by inducing actin-myosin interactions and actin rearrangement into stress fibers (Hall 2005). Rho activation also stimulates FN incorporation into matrix (Yoneda et al. 2007; Zhang et al. 1994, 1999), and FN matrix levels are reduced with blockade of Rho and loss of contractility (Zhong et al. 1998). Mechanistic insights into the role of contractility came from studies with the monoclonal antibody (MAb) L8 (Chernousov et al. 1987), which binds to the I9-III1 region of FN (Chernousov et al. 1991). Inhibition of Rho in cells with an established matrix reduced binding of MAb L8, which indicates that accessibility of this epitope depends on the contractile effects of cells on the matrix (Zhong et al. 1998). They also showed that binding of FN, the 70-kDa fragment, or MAb L8 to FN attached to a rubber surface was enhanced by stretching. Therefore, contractility and stretching affect availability of a III1 FN binding site through effects on FN conformation.
Another key discovery in FN binding was identification of a fragment of the III1 module (III1-C) that could induce FN aggregation into complexes that resemble collapsed fibrils. This III1-C fragment, also known as anastellin, encompasses the C-terminal two-thirds of the III1 module and has FN-binding activity (Morla & Ruoslahti 1992). FN treated with this fragment forms aggregates that can be stretched into structures that appear fibrillar (Morla et al. 1994). Interestingly, this so-called superfibronectin has enhanced cell adhesion activity, possibly because the aggregates bring multiple cell-binding domains into close proximity. Anastellin reportedly can cause loss of FN matrix leading to changes in cell morphology, cell signaling, and proliferation (Bourdoulous et al. 1998). However, whether FN matrix is actually lost with anastellin treatment has been brought into question by data showing loss of epitope accessibility without loss of FN fibrils upon addition of anastellin to cells in culture (Klein et al. 2003). These observations indicate that anastellin affects FN conformation in solution and within fibrils.
Only recently has the interaction of anastellin with FN been thoroughly investigated. It binds with a stoichiometry of 4:1; three anastellins bind within III1–3, and one binds at III11 (Ohashi & Erickson 2005). Anastellin induces aggregation of FN or FN fragments that contain III1–2 or III1–3 and enhances proteolytic sensitivity of FN, thus demonstrating that it affects FN conformation (Ohashi & Erickson 2005, Ohashi et al. 2009). The authors propose that spontaneous unfolding or “breathing” of type III modules allows anastellin binding, which then prevents refolding and allows exposed hydrophobic regions in the module β-strands to interact to form FN aggregates. β-strand swapping has been demonstrated for an individual type III module, III9, which formed amyloid-like fibrils upon temperature-induced partial unfolding (Litvinovich et al. 1998). It seems likely that β-strand exchange between FN molecules contributes to DOC insolubility, but this remains to be demonstrated.
GAG and proteoglycan interactions with FN have been implicated in matrix assembly (Chung & Erickson 1997, Galante & Schwarzbauer 2007, Klass et al. 2000, Morla & Ruoslahti 1992). Both syndecan transmembrane proteoglycans (Woods 2001) and ECM proteoglycans such as perlecan (Chung & Erickson 1997) can participate in this process. Interestingly, many of the FN-binding sites also have heparin-binding activity, which raises the possibility that proteoglycans may contribute to ternary complexes and mediate interactions between FN molecules or between FN and other ECM proteins or receptors.
Tethering of Fibronectin at the Cell Surface
An FN dimer bound to two integrins provides a way for cell contractility to apply forces that could affect FN conformation. Tethering at the cell-binding domains of an FN dimer would likely impact the C-terminal regions of each subunit and might also dissociate the interactions between III2–3 and III12–14 in the compact form (Figure 3a,b). But can integrin binding cause long-range conformational changes that affect the N-terminal assembly domain and the FN-binding sites in III1–2? The finding that the 70-kDa fragment is able to bind to cell layers and to block assembly raised the possibility of an FN matrix assembly receptor (McKeown-Longo & Mosher 1985). Molecules that have been implicated in 70-kDa fragment-cell interactions include FN itself, heparan sulfate proteoglycans, collagen (Colombi et al. 2003), molecules with large apparent molecular mass (Zhang & Mosher 1996), molecules associated with a laminin receptor (Bae et al. 2004), and integrins α5β1 (Hocking et al. 1998) and αvβ3 (Takahashi et al. 2007). Despite much investigation, no distinct molecule has emerged as the FN matrix assembly receptor, which suggests that it is not a single entity but is instead an activity and that many different molecules can serve in this role.
Evidence that multiple molecules affect assembly is supported by observations implicating substrate composition in the assembly process. Fibroblasts grown on surfaces coated with FN will rearrange the FN into fibrillar structures at matrix assembly sites that can be detected by 70-kDa fragment binding (Christopher et al. 1997, Wierzbicka-Patynowski & Schwarzbauer 2002). Using FN fragments as substrates, Mosher and colleagues (Xu et al. 2009) showed that assembly was limited when cells were grown on FN’s cell-binding domain alone and that optimal assembly occurred on fragments containing III1 plus C-terminal FN domains. Matrix assembly is enhanced with cells on a 3D fibrillar FN matrix (Mao & Schwarzbauer 2005b). Matrix assembly by cultured cells is facilitated by a rigid substrate and hampered by growth on soft materials (Halliday & Tomasek 1995; C. Carraher and J.E. Schwarzbauer, unpublished observations). Other ECM protein substrates negatively impact assembly, as observed with cells growing on vitronectin (Bae et al. 2004, Hocking et al. 1999). One possible interpretation of the substrate effects is that FN-binding activity within the protein on the substrate provides a tethering site for FN, which can then be pulled on to cause FN extension through conformational changes. This would explain why fragments containing the III1–2 domain support assembly when attached to a surface (Xu et al. 2009).
Integrin binding to FN induces receptor clustering, which brings together cytoplasmic molecules such as focal adhesion kinase (FAK), Src kinase, paxillin, and others to form protein-rich focal complexes that activate polymerization of actin filaments and intracellular signaling through kinase cascades (Geiger et al. 2001). Differential effects of substrate on FN matrix assembly may be sensed through formation of focal contacts and fibrillar adhesions. By following integrin movements over time, Yamada and colleagues (Pankov et al. 2000) showed that fibroblasts on an FN substrate maintained αvβ3 integrins in focal contacts at the cell periphery whereas α5β1 integrins moved centripetally in parallel with actin filaments. Focal contacts and fibrillar adhesions differ not only in location and movement but also in the proteins associated with the integrin cytoplasmic domains, i.e., paxillin at focal contacts and tensin at fibrillar adhesions moving with α5β1 (Geiger et al. 2001, Pankov et al. 2000, Zamir et al. 1999). Formation of FN fibrils by the movement of fibrillar adhesions is directional. Ohashi et al. (2002) observed that the ends of fibrils toward the cell periphery were stationary while elongation occurred at the proximal ends, which suggests that fibrillar adhesions are pulling against the substrate. Thus, cell-substrate attachment can promote separation of adhesion sites from sites of FN assembly. This provides a mechanism for extending compact FN dimers by applying tension through pulling against substrate-attached protein and may explain some of the observed effects of substrate on FN assembly.
Does Fibronectin Unfold?
The III1–2 domain has been the focus of many studies into FN conformational changes. It contains two FN-binding sites, one of them cryptic, and its activity is affected by stretch (Aguirre et al. 1994, Hocking et al. 1994, Zhong et al. 1998). Anastellin, which induces FN aggregation, is derived from this region (Morla et al. 1994). In addition, the III1–2 domain is unique among type III module pairs in that there is a flexible 17-residue linker between the modules that extends into the first β-strand of III2 (Vakonakis et al. 2007). This linker might increase the potential for domain extension. Fluorescence resonance energy transfer (FRET) has been used to follow changes in conformation of a FRET sensor of III1–2 tagged at opposite ends with cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP). A significant change in FRET signal was detected with mutation of interfacial residues predicted to form a salt bridge interaction between III1 and III2 (Karuri et al. 2009). Mutant III1–2 was further affected by 70-kDa fragment binding, which eliminated FRET (Karuri et al. 2009). These results support the idea that a salt bridge between III1 and III2 stabilizes this region and controls FN binding. Disruption of the salt bridge would then allow separation of the modules, making FN binding sites more accessible and allowing intermolecular interactions to occur.
The crystal structures of FN fragments show zigzag orientation of type III repeats with tilt angles of up to 62° between adjacent modules (Leahy et al. 1996, Sharma et al. 1999). Flattening of the zigzag into a more linear arrangement is one way to extend FN during fibril formation. Erickson (1994) proposed a model for more dramatic conformational changes of type III modules: Application of force might unravel a β-barrel into a random coil conformation. FN fibrils can stretch to four times their original length when pulled by cells (Ohashi et al. 1999), and fibril elasticity is easily visualized by video microscopy (Mao & Schwarzbauer 2006) (Figure 4 and Supplemental Video 1, follow the Supplemental Material link from the Annual Reviews home page at http://www.annualreviews.org). Both flattening of tilt angles and unraveling of β-barrels would increase the length of an FN dimer and provide some elasticity to fibrils. Single-molecule force spectroscopy measurements show that type III modules can unfold in vitro (Oberhauser et al. 2002). Furthermore, fluorescence microscopy with FRET spectroscopy has provided evidence for conformational changes in individual FN molecules during fibril formation (Baneyx et al. 2001) that have been interpreted as domain unfolding (Smith et al. 2007). Breathing of β-strands likely can briefly expose sites normally buried inside a β-barrel. However, complete unfolding of modules may be problematic, as the conformational changes could be irreversible, with the protein prevented from refolding by intermolecular interactions within fibrils or by aggregation, and such changes may also generate immunogenic sites that are not normally present in native structures. There is a debate as to whether FN conformational changes and fibril elasticity result from straightening of the zigzag orientation of type III modules, from unfolding, partial unraveling, or breathing of type III β structures, or from some combination of these events (Erickson 2002, Ohashi et al. 2009, Smith et al. 2007). These questions are likely to generate interest for the foreseeable future.
Figure 4.
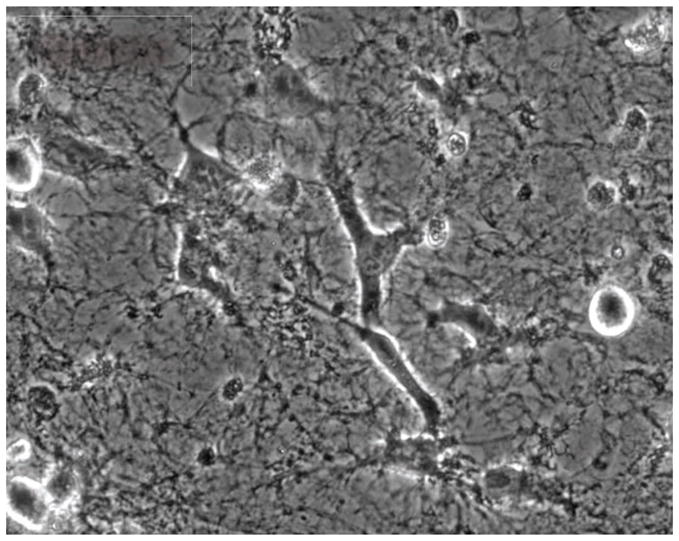
Migration of HT1080 cells on fibrillar fibronectin (FN) matrix. Extraction of a highly confluent fibroblast culture was used to prepare a cell-free fibrillar matrix in which FN is the major protein component. HT1080 human fibrosarcoma cells were allowed to attach to the matrix in serum-free medium for 2 h. Fetal bovine serum was then added to the medium to a final concentration of 10% to initiate migration. To watch the migration in action, please see Supplemental Video 1, which was originally published in Mao & Schwarzbauer (2006).
Molecular dynamics simulations and modeling have been applied to provide insights into potential conformational changes at the cell-binding site in FN. Simulated application of force to the III10 module suggests that stepwise unfolding of β-strands could regulate availability of the RGD sequence (Gee et al. 2008, Krammer et al. 1999) and may thus provide a novel mechanism for regulating interactions of cells with FN fibrils.
Fibronectin-Integrin Bond Strength and Signaling
Many FN receptors can support cell adhesion and migration on FN substrates but lack sufficient activity to assemble FN into fibrils. In contrast to α5β1, which is the primary integrin for FN matrix assembly, α4β1 (Sechler et al. 2000), αvβ1 (Zhang et al. 1993), αvβ3 (Wu et al. 1996), αIIbβ3 (Olorundare et al. 2001), and perhaps others are unable to assemble fibrils without treatments that increase their activities. These receptors also lack the ability to bind to soluble FN (Huveneers et al. 2008), which would likely impact recruitment of secreted FN to assembly sites on the cell surface. The distinction between integrins probably relates to FN-binding strength. α5β1-FN bond strength is dependent on engagement of FN’s synergy site, which leads downstream to increased FAK activation (Friedland et al. 2009). Both FAK activity (Ilic et al. 2004) and the synergy site (Sechler et al. 1997) are required for α5β1-mediated assembly. Integrins can bind to FN lacking the synergy site (Danen et al. 1995, Mao & Schwarzbauer 2006) but require stimulation using either Mn2+ or activating antibodies to assemble synergy-minus FN into fibrils. Taken together, we conclude that the strength of integrin binding to FN is a requisite part of initiating and propagating fibril formation and that the ability to achieve sufficient bond strength for assembly is a property of α5β1 that distinguishes it from other integrins.
Facilitating interactions between integrins and FN is one role of the actin cytoskeleton. Depolymerization of actin with drugs (Hynes 1990), deletion of the β integrin cytoplasmic domain (Wu et al. 1995), or actin rearrangement with inhibition of Rho GTPase (Zhong et al. 1998) causes loss of FN matrix, which demonstrates the importance of connections between ECM polymers outside cells with actin polymers inside. Integrin cytoplasmic domains also stimulate intracellular signaling, and certain pathways are critical for initiation and maintenance of matrix assembly. FAK plays a central role in integrin signaling and is essential for fibroblast assembly of FN fibrils (Ilic et al. 2004). Similarly, loss of talin binding to β1 integrin reduces integrin activity and FN assembly (Green et al. 2009). Other focal adhesion proteins participate in the assembly process, but their absence does not eliminate assembly. For example, members of the Src kinase family work with FAK in many cell types (Mitra & Schlaepfer 2006); assembly is delayed but not ablated in cells lacking these kinases (Wierzbicka-Patynowski & Schwarzbauer 2002) and is reduced in cells with enhanced Src activity (Huveneers & Danen 2009). Paxillin phosphorylation is a consequence of Src activation (Mitra & Schlaepfer 2006) and also contributes to FN matrix formation (Wierzbicka-Patynowski et al. 2007, Zaidel-Bar et al. 2007). Inhibition of Src kinase activity in cells after matrix accumulation caused a rapid loss of that matrix concomitant with a reduction in phosphopaxillin (Wierzbicka-Patynowski et al. 2007). Thus, not only are focal adhesion proteins involved in the initiation phase of assembly, but once matrix has been assembled, these proteins are needed to maintain matrix association with the cell surface.
Syndecan transmembrane proteoglycans have been implicated in many of the intracellular events that accompany integrin-FN binding. Syndecan-4 is involved in focal adhesion formation (Woods 2001), FAK and Rho activation (Wilcox-Adelman et al. 2002), and cell contractility (Yoneda et al. 2007). Although syndecan-4 small interfering RNA (siRNA) knockdown does not have a major effect on FN matrix, knockdown of syndecan-2 causes a significant reduction in DOC-insoluble FN fibrils (Galante & Schwarzbauer 2007), and deletion of the syndecan-2 cytoplasmic tail prevents assembly of FN and other matrix proteins (Klass et al. 2000). Syndecans bind to the hepII domain of FN (Woods et al. 2000) adjacent to the α5β1 integrin-binding domain (Figure 1), which may position them to contribute to cell-FN interactions. They also initiate syndecan-specific intracellular signaling cascades that involve protein kinase C (for syndecan-4) (Bass & Humphries 2002) and PDZ domain proteins (for syndecan-2) (Essner et al. 2006). Syndecans might act as coreceptors for ECM along with integrins, but more research is needed to determine the specific roles of syndecans in assembly.
Fibril Maturation and Conversion to Insolubility
Elegant experiments using an anti-FN MAb for immuno-EM analysis identified an 84-nm space between epitopes, which predicts a 20-nm overlap of the N termini and suggests that initially the fibrils form through end-to-end association of dimers (Dzamba & Peters 1991; Figure 3). Initial thin fibrils then grow in length and thickness as matrix matures. During fibril growth, FN matrix is converted to a DOC-insoluble form. This conversion follows soon after the initiation of assembly such that more than half of a given population of FN is DOC insoluble within 6 h (McKeown-Longo & Mosher 1983).
Insolubility is a critical property of the ECM and provides tissues with stability, rigidity, and shape. But how do FN fibrils become insoluble? For many years, disulfide exchange was assumed to account for the insolubility because multimeric FN is dissociated by reducing agents as detected by polyacrylamide gel electrophoresis, FN has protein disulfide isomerase activity, and FN has many intrachain disulfide bonds. However, a tour de force of fragmentation analysis failed to identify any disulfide-bonded fragments, which led to the conclusion that DOC insolubility arises from strong, but noncovalent, protein-protein interactions (Chen & Mosher 1996). These interactions could be related to the interactions that form type III modules into amyloid-like fibrils (Litvinovich et al. 1998). Recently, another mechanism was proposed to explain the requirement for reducing agents to dissociate FN matrix. Experiments show that FN can be trapped at the top of the stacking gel and appear multimeric simply by adding large ECM proteins to the gel samples (Ohashi & Erickson 2009). These results suggest that FN within fibrils exists as covalent dimers but that other interactions are noncovalent, again fitting with the strong protein-protein interactions model.
The combination of multiple FN-binding sites and noncovalent FN-FN interactions in multimeric fibrils may facilitate fibril elasticity. FN interactions initially appear to depend on N-terminal FN binding sites generating thin fibrils with end-to-end association of FN dimers (Dzamba & Peters 1991). FNs may be partially in the compact conformation, or type III modules may be in a zigzag arrangement in these fibrils (Erickson 2002). Stretching by cell contractility could then cause progressive extension by first unfolding the compact form and then straightening the zigzag of the modules as well as perhaps inducing module breathing. Binding sites exposed in extended fibrillar molecules would mediate lateral interactions between thin fibrils. Multiple FN-binding sites may be needed so that simultaneous interactions involving several sites along a fibril can stabilize relatively weak binding at individual sites (Figure 3). Thus, fibril alignment may depend on the sites that are available for interfibril interactions. Multiple binding sites might also contribute to fibril elasticity. For example, the length of a fibril bundle could be extended by slippage of individual fibrils along each other. Then, interactions between the N-terminal assembly domain and its different binding sites along FN could provide stopping points as fibrils move past each other.
Fibronectin Matrix Turnover
FN matrix assembly is a dynamic and continuous process. This point is well illustrated by experiments showing that FN matrix is lost when cells are deprived of FN (Sottile & Hocking 2002). FN fibrils are also dissociated when shear forces are applied to attached cells (Engler et al. 2009). Continuous FN polymerization is needed for matrix to be stabilized at the cell surface (Sottile & Hocking 2002, Wierzbicka-Patynowski et al. 2007). If FN polymerization is inhibited or FN expression is eliminated, the existing FN matrix subsides, which shows that a steady state exists between FN polymerization and turnover.
The α5β1 integrin, which carries out matrix assembly, may also be involved in its turnover. Caveolin-1 regulates α5β1 endocytosis, and β1 integrin is involved in the endocytosis of soluble FN (Shi & Sottile 2008, Sottile & Chandler 2005). Thus, our current understanding of turnover suggests that events that slow polymerization of FN into fibrils (such as reduced integrins or integrin activity, a reduced source of FN, or increased proteolysis) will increase integrin-FN endocytosis. Further investigation is needed to understand the mechanisms and regulation of this process.
FIBRONECTIN-DEPENDENT ASSEMBLY OF OTHER EXTRACELLULAR MATRIX PROTEINS
The list of ECM proteins that depend on FN for incorporation into the matrix is growing and includes collagens, fibrillin, fibulin, latent TGF-β binding protein (LTBP), and tenascin-C (Chung & Erickson 1997, Dallas et al. 2005, Kadler et al. 2008, Sabatier et al. 2009, Sottile & Hocking 2002, Twal et al. 2001). Some of these proteins associate directly with FN fibrils, whereas others appear to use FN matrix as a scaffold for deposition of independently structured fibers. Initiation of basement membrane assembly does not involve FN but instead relies on laminin-integrin interactions, connections to the actin cytoskeleton, and formation of laminin multimers, all steps that are mechanistically similar to the early stages of FN matrix assembly. This process has been recently reviewed by Yurchenco & Patton (2009).
Fibronectin in Microfibril and Elastic Fiber Assembly
Microfibrils assemble through head-to-tail interactions of fibrillin that yield a linear fibril that resembles beads on a string (Ramirez & Sakai 2010). Assembly of fibrillin-1 into microfibrils occurs in cell culture but requires an extended time of several days for significant material to accumulate. Using human dermal fibroblasts, two groups have applied different approaches to show that FN matrix is required for microfibril assembly. In one study, knockdown of FN expression with siRNAs or blockade of α5β1 integrin with antibodies delayed accumulation of fibrillin-1 in the matrix (Kinsey et al. 2008). Similar reductions were observed in β1 integrin–null cells or with inhibition of Rho GTPase, which acts on the actin cytoskeleton downstream of integrins. In the other report, fibrillin-1 and FN were shown to colocalize by immunofluorescence and, importantly, by immuno-EM (Sabatier et al. 2009). Binding to FN depended on formation of fibrillin multimers, which led to the proposal that fibrillin multimers act as assembly intermediates that ultimately depend on FN matrix for organization into microfibrils (Sabatier et al. 2009).
Microfibrils have an important role in elastic fiber assembly. Tropoelastin is secreted and subsequently formed into aggregates through association with either microfibrils or cells. These elastin aggregates may nucleate further assembly. Live cell imaging studies using tagged tropoelastin showed that elastin aggregates first appeared on the cell surface and then, through cell motility, the small aggregates moved to and associated with elastin fibers (Czirok et al. 2006, Kozel et al. 2006). The link to cells may involve FN. Antibody staining of cultures over time detected FN fibrils first, but at later times fibrils positive for fibrillin, microfibril-associated glycoprotein-1 (MAGP-1), fibulin-5, and finally elastin appeared in the same location as FN (Wagenseil & Mecham 2007). Interestingly, lysyl oxidase (LOX), the enzyme that forms covalent cross-links in elastin fibers, binds to FN, and this interaction stimulates LOX activity (Fogelgren et al. 2005). Together these observations suggest a model in which tropoelastin aggregates are cross-linked by LOX at the cell surface, perhaps facilitated by LOX-FN interactions. These aggregates are associated with fibulins, which may facilitate rearrangements of aggregates into fibers through interactions with microfibrils (Wagenseil & Mecham 2007).
Type I Collagen Assembly
Triple-helical type I collagen molecules are assembled into ~1.5-nm diameter fibrils that have a repeating pattern with 67-nm periodicity resulting from the head-to-tail and lateral associations of individual collagen molecules (Alberts et al. 2008, Kadler et al. 2008). Collagen chains assemble into triple-helical molecules in the secretory pathway but are prevented from further assembly by the presence of N- and C-terminal procollagen domains. Activation of collagen for assembly into fibrils occurs by proteolytic cleavage of procollagen domains.
Cells play an important role in type I collagen fiber assembly. As shown elegantly by EM studies of cornea and tendon, there is close association of collagen molecules within cellular compartments that surround the forming fibrils (Birk & Trelstad 1984, 1986). These compartments facilitate end-to-end association of collagen molecules and lateral interactions between fibrils. Narrow channels appear to merge to generate larger compartments in which fibrils are assembled into fibers, perhaps through retraction of membrane protrusions (Banos et al. 2008, Birk & Trelstad 1986, Zhang et al. 2005). Application of image reconstruction techniques further defined these compartments and, because of their role in collagen fibril deposition, the name “fibripositor” has been coined (Kadler et al. 2008).
One obvious advantage of such compartments is that they concentrate collagen molecules in close proximity to promote interactions required for fibrillogenesis. In many cases, these assembly compartments are oriented parallel to the long axis of the cell, which would serve to orient nascent fibrils and may therefore play a critical role in directional deposition of collagen fibers. FN and type I collagen fibers are frequently found together in tissues and have been colocalized in the secretory pathway of fibroblasts (Ledger et al. 1980). Many groups have shown that collagen fibrils do not accumulate in the absence of FN matrix (Dallas et al. 2005, McDonald 1988, Sottile & Hocking 2002). In vivo, FN is found in developing tissues prior to the deposition of collagen. Because collagen can bind to FN, the temporal and spatial connections between FN and collagen suggest that FN acts as a scaffold that may aid in alignment of collagen fibrils. Perhaps FN matrix orients cells so that collagen fibrils are aligned and thus facilitates formation of parallel collagen fibers. FN matrix might also guide the retraction of cell processes so that collagen fibrils are deposited with parallel alignment.
Several reports have also indicated that collagen can enhance FN assembly. For example, FN matrix was significantly increased in cells from the MOV13 mouse (deficient in type I collagen) by expression of a collagen transgene (Dzamba et al. 1993). Presence of a 13–amino acid collagen-binding site was required for assembly of recombinant FNs in chick embryo fibroblasts (Colombi et al. 2003). One possible role for collagen in FN deposition might be to provide a rigid collagen network that increases tension in the matrix to facilitate FN assembly. This mechanism would be similar to the tensional effects of cell-cell interactions on FN assembly in Xenopus embryos (Dzamba et al. 2009). Although it is possible that collagen contributes to FN assembly, much more evidence shows that FN matrix is a critical scaffold for collagen assembly.
Fibronectin Matrix and Disease
Despite FN’s abundance and importance in the ECM, surprisingly few diseases are caused by defects in FN matrix assembly. One recently described example is glomerulopathy with FN deposits, a kidney disease characterized by non-fibrillar FN aggregates (Castelletti et al. 2008). Gene mapping and sequencing of samples from several pedigrees of affected patients identified mutations in III13 and III4. Interestingly, these mutations map to FN-binding domains (Figure 1), which suggests that the mutations impact FN interactions during matrix assembly. Another possibility is that III13 mutations destabilize the compact conformation of FN, which involves III12–14 (Figure 3), and prematurely expose other binding sites, which leads to dysregulation of FN-FN interactions.
The possibility exists that FN matrix contributes to other diseases. Defective or excessive FN matrix is present in fibrotic diseases, keloids, and hypertrophic scars (see, for example, Pozzi et al. 2009, Wolfram et al. 2009). Defects in FN matrix may underlie abnormalities attributed to other ECM proteins. For example, mutations in the diastrophic dysplasia sulfate transporter (DTDST) cause gross skeletal defects with undersulfated cartilage proteoglycans (Rossi et al. 1998). Loss of DTDST also causes a significant reduction in FN matrix assembly (Galante & Schwarzbauer 2007), which raises the possibility that the effects of DTDST mutations may extend to the organization or amount of FN matrix during chondrogenesis and lead downstream to defects in collagen deposition and cartilage formation. Whether FN matrix has a causal role or is an important contributing factor in these diseases remains to be determined.
Supplementary Material
Figure 5.
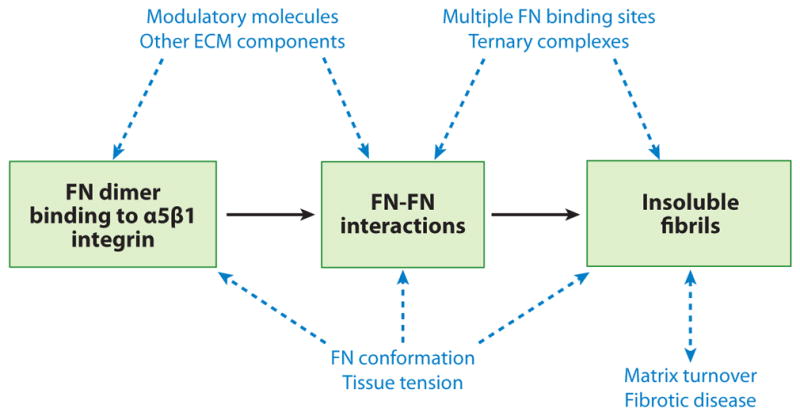
Future issues. The basic steps of fibronectin (FN) matrix assembly are boxed in green. Other proteins, interactions, processes, and mechanisms (blue) might impact various steps in matrix assembly as indicated (dashed blue arrows). Additional investigation is required to understand their effects. ECM, extracellular matrix.
SUMMARY POINTS.
Initiation of matrix assembly requires binding of FN dimers to integrin receptors followed by binding to other FN molecules via the N-terminal assembly domain.
Clustering of receptors bound to FN dimers generates a locally high FN concentration that promotes FN-FN interactions.
FN subunits change conformation from compact to extended. This process is mediated by integrins and depends on cell contractility through actin filaments and Rho GTPase activity.
Conformational changes increase FN-FN interactions by exposing FN-binding sites, some of which are cryptic in native FN.
Intracellular signaling downstream of integrins contributes to FN matrix formation; FAK plays a central role in initiating the signals that promote assembly.
FN fibrils are converted to a DOC detergent-insoluble form, which provides the matrix with stability and rigidity.
A steady state exists between FN matrix assembly and matrix turnover such that a decrease in fibril formation destabilizes the matrix and induces turnover.
Many questions remain about FN matrix assembly mechanisms and the role of FN matrix in tissue homeostasis and disease (Figure 5).
FUTURE ISSUES.
There are at least four FN-binding sites in each FN subunit. The N-terminal assembly domain has an essential role in fibril formation. Do the other sites have defined roles, for example, in staggering dimers within fibrils, in lateral interactions between fibrils, or in branching? Do several sites together form ternary complexes to stabilize FN-FN interactions?
What molecular interactions convert fibrils to DOC insolubility? Does β-strand exchange play a role, and are other ECM molecules involved in the process?
How does tissue tension regulate matrix assembly? Is it through integrin activity, cytoskeletal organization, FN conformation, or other mechanisms? Does deposition of collagen or other ECM proteins modulate tissue tension?
What events are involved in identifying fibrils for turnover and in converting insoluble fibrils into a form that can be endocytosed?
Acknowledgments
The authors wish to thank Dan Slone for help with images, NCI and NIGMS for funding (to J.E.S.), and NIH T32GM007388 for predoctoral support (to C.C.). We also acknowledge the contributions of current and former members of the Schwarzbauer lab and of all members of the FN matrix assembly community.
Glossary
- ECM
extracellular matrix
- FN
fibronectin
- GAG
glycosaminoglycan
- DOC
deoxycholate detergent
- RGD
Arg-Gly-Asp cell-binding sequence
- FAK
focal adhesion kinase
- FRET
fluorescence resonance energy transfer
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Aguirre KM, McCormick RJ, Schwarzbauer JE. Fibronectin self-association is mediated by complementary sites within the amino-terminal one-third of the molecule. J Biol Chem. 1994;269:27863–68. [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. New York: Garland Science; 2008. p. 1268. [Google Scholar]
- Anliker B, Chun J. Cell surface receptors in lysophospholipid signaling. Semin Cell Dev Biol. 2004;15:457–65. doi: 10.1016/j.semcdb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Aota S, Nomizu M, Yamada K. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell adhesive function. J Biol Chem. 1994;269:24756–61. [PubMed] [Google Scholar]
- Astrof S, Crowley D, Hynes RO. Multiple cardiovascular defects caused by the absence of alternatively spliced segments of fibronectin. Dev Biol. 2007;311:11–24. doi: 10.1016/j.ydbio.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aszódi A, Legate KR, Nakchbandi I, Fässler R. What mouse mutants teach us about extracellular matrix function. Annu Rev Cell Dev Biol. 2006;22:591–621. doi: 10.1146/annurev.cellbio.22.010305.104258. [DOI] [PubMed] [Google Scholar]
- Bae E, Sakai T, Mosher DF. Assembly of exogenous fibronectin by fibronectin-null cells is dependent on the adhesive substrate. J Biol Chem. 2004;279:35749–59. doi: 10.1074/jbc.M406283200. [DOI] [PubMed] [Google Scholar]
- Baneyx G, Baugh L, Vogel V. Coexisting conformations of fibronectin in cell culture imaged using fluorescence resonance energy transfer. Proc Natl Acad Sci USA. 2001;98:14464–68. doi: 10.1073/pnas.251422998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banos CC, Thomas AH, Kuo CK. Collagen fibrillogenesis in tendon development: current models and regulation of fibril assembly. Birth Def Res C. 2008;84:228–44. doi: 10.1002/bdrc.20130. [DOI] [PubMed] [Google Scholar]
- Bass MD, Humphries MJ. Cytoplasmic interactions of syndecan-4 orchestrate adhesion receptor and growth factor receptor signalling. Biochem J. 2002;368:1–15. doi: 10.1042/BJ20021228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–95. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- Birk DE, Trelstad RL. Extracellular compartments in matrix morphogenesis: collagen fibril, bundle, and lamellar formation by corneal fibroblasts. J Cell Biol. 1984;99:2024–33. doi: 10.1083/jcb.99.6.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk DE, Trelstad RL. Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation. J Cell Biol. 1986;103:231–40. doi: 10.1083/jcb.103.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdoulous S, Orend G, MacKenna DA, Pasqualini R, Ruoslahti E. Fibronectin matrix regulates activation of RHO and CDC42 GTPases and cell cycle progression. J Cell Biol. 1998;143:267–76. doi: 10.1083/jcb.143.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner KA, Corbett SA, Schwarzbauer JE. Regulation of fibronectin matrix assembly by activated Ras in transformed cells. Oncogene. 2000;19:3156–63. doi: 10.1038/sj.onc.1203626. [DOI] [PubMed] [Google Scholar]
- Bultmann H, Santas AJ, Pesciotta Peters DM. Fibronectin fibrillogenesis involves the heparin II binding domain of fibronectin. J Biol Chem. 1998;273:2601–9. doi: 10.1074/jbc.273.5.2601. [DOI] [PubMed] [Google Scholar]
- Castelletti F, Donadelli R, Banteria F, Hildebrandt F, Zipfel PF, et al. Mutations in FN1 cause glomerulopathy with fibronectin deposits. Proc Natl Acad Sci USA. 2008;105:2538–43. doi: 10.1073/pnas.0707730105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Mosher DF. Formation of sodium dodecyl sulfate–stable fibronectin multimers. J Biol Chem. 1996;271:9084–89. doi: 10.1074/jbc.271.15.9084. [DOI] [PubMed] [Google Scholar]
- Chen LB, Murray A, Segal RA, Bushnell A, Walsh ML. Studies on intercellular LETS glycoprotein matrices. Cell. 1978;14:377–91. doi: 10.1016/0092-8674(78)90123-x. [DOI] [PubMed] [Google Scholar]
- Chernousov MA, Faerman AI, Frid MG, Printseva OY, Koteliansky VE. Monoclonal antibody to fibronectin which inhibits extracellular matrix assembly. FEBS Lett. 1987;217:124–28. doi: 10.1016/0014-5793(87)81255-3. [DOI] [PubMed] [Google Scholar]
- Chernousov MA, Fogerty FJ, Koteliansky VE, Mosher DF. Role of the I-9 and III-1 modules of fibronectin in the formation of an extracellular fibronectin matrix. J Biol Chem. 1991;266:10851–58. [PubMed] [Google Scholar]
- Christopher RA, Kowalczyk AP, McKeown-Longo PJ. Localization of fibronectin matrix assembly sites on fibroblasts and endothelial cells. J Cell Sci. 1997;110:569–81. doi: 10.1242/jcs.110.5.569. [DOI] [PubMed] [Google Scholar]
- Chung CY, Erickson HP. Glycosaminoglycans modulate fibronectin matrix assembly and are essential for matrix incorporation of tenascin-C. J Cell Sci. 1997;110:1413–19. doi: 10.1242/jcs.110.12.1413. [DOI] [PubMed] [Google Scholar]
- Colombi M, Zoppi N, De Petro G, Marchina E, Gardella R, et al. Matrix assembly induction and cell migration and invasion inhibition by a 13-amino acid fibronectin peptide. J Biol Chem. 2003;278:14346–55. doi: 10.1074/jbc.M211997200. [DOI] [PubMed] [Google Scholar]
- Czirok A, Zach J, Kozel BA, Mecham RP, Davis EC, Rongish BJ. Elastic fiber macro-assembly is a hierarchical, cell motion–mediated process. J Cell Physiol. 2006;207:97–106. doi: 10.1002/jcp.20573. [DOI] [PubMed] [Google Scholar]
- Dallas SL, Sivakumar P, Jones CJ, Chen Q, Peters DM, et al. Fibronectin regulates latent transforming growth factor-β (TGFβ) by controlling matrix assembly of latent TGFβ-binding protein-1. J Biol Chem. 2005;280:18871–80. doi: 10.1074/jbc.M410762200. [DOI] [PubMed] [Google Scholar]
- Danen EH, Aota S, van Kraats AA, Yamada KM, Ruiter DJ, van Muijen GN. Requirement for the synergy site for cell adhesion to fibronectin depends on the activation state of integrin α5β1. J Biol Chem. 1995;270:21612–18. doi: 10.1074/jbc.270.37.21612. [DOI] [PubMed] [Google Scholar]
- Dzamba BJ, Jakab KR, Marsden M, Schwartz MA, DeSimone DW. Cadherin adhesion, tissue tension, and noncanonical Wnt signaling regulate fibronectin matrix organization. Dev Cell. 2009;16:421–32. doi: 10.1016/j.devcel.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzamba BJ, Peters DM. Arrangement of cellular fibronectin in noncollagenous fibrils in human fibroblast cultures. J Cell Sci. 1991;100:605–12. doi: 10.1242/jcs.100.3.605. [DOI] [PubMed] [Google Scholar]
- Dzamba BJ, Wu H, Jaenisch R, Peters DM. Fibronectin binding site in type I collagen regulate fibronectin fibril formation. J Cell Biol. 1993;121:1165–72. doi: 10.1083/jcb.121.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Odermatt E, Engel A, Madri JA, Furthmayr H, et al. Shapes, domain organizations and flexibility of laminin and fibronectin, two multifunctional proteins of the extracellular matrix. J Mol Biol. 1981;150:97–120. doi: 10.1016/0022-2836(81)90326-0. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Chan M, Boettiger D, Schwarzbauer JE. A novel mode of cell detachment from fibrillar fibronectin matrix under shear. J Cell Sci. 2009;122:1647–53. doi: 10.1242/jcs.040824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson HP. Reversible unfolding of fibronectin type III and immunoglobulin domains provides the structural basis for stretch and elasticity of titin and fibronectin. Proc Natl Acad Sci USA. 1994;91:10114–18. doi: 10.1073/pnas.91.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson HP. Stretching fibronectin. J Musc Res Cell Motil. 2002;23:575–80. doi: 10.1023/a:1023427026818. [DOI] [PubMed] [Google Scholar]
- Erickson HP, Carrell NA. Fibronectin in extended and compact conformations. Electron microscopy and sedimentation analysis. J Biol Chem. 1983;258:14539–44. [PubMed] [Google Scholar]
- Essner JJ, Chen E, Ekker SC. Syndecan-2. Int J Biochem Cell Biol. 2006;38:152–56. doi: 10.1016/j.biocel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Fogelgren B, Polgár N, Szauter KM, Ujfaludi Z, Laczkó R, et al. Cellular fibronectin binds to lysyl oxidase with high affinity and is critical for its proteolytic activation. J Biol Chem. 2005;280:24690–97. doi: 10.1074/jbc.M412979200. [DOI] [PubMed] [Google Scholar]
- Fogerty FJ, Akiyama SK, Yamada KM, Mosher DF. Inhibition of binding of fibronectin to matrix assembly sites by anti-integrin (α5β1) antibodies. J Cell Biol. 1990;111:699–708. doi: 10.1083/jcb.111.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls α5β1 function. Science. 2009;323:642–44. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Yoshida N, Kataoka Y, Manabe R, Mizuno-Horikawa Y, et al. Mice lacking the EDB segment of fibronectin develop normally but exhibit reduced cell growth and fibronectin matrix assembly in vitro. Cancer Res. 2002;62:5603–10. [PubMed] [Google Scholar]
- Galante LL, Schwarzbauer JE. Requirements for sulfate transport and the diastrophic dysplasia sulfate transporter in fibronectin matrix assembly. J Cell Biol. 2007;179:999–1009. doi: 10.1083/jcb.200707150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee EP, Ingber DE, Stultz CM. Fibronectin unfolding revisited: modeling cell traction-mediated unfolding of the tenth type-III repeat. PLoS One. 2008;3:e2373. doi: 10.1371/journal.pone.0002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane extracellular matrix–cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–91. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Green JA, Berrier AL, Pankov R, Yamada KM. β1 integrin cytoplasmic domain residues selectively modulate fibronectin matrix assembly and cell spreading through talin and Akt-1. J Biol Chem. 2009;284:8148–59. doi: 10.1074/jbc.M805934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J-L, Hynes RO. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor α4β1. Cell. 1990;60:53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- Guan J-L, Trevithick JE, Hynes RO. Retroviral expression of alternatively spliced forms of rat fibronectin. J Cell Biol. 1990;110:833–47. doi: 10.1083/jcb.110.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–95. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- Halliday NL, Tomasek JJ. Mechanical properties of the extracellular matrix influence fibronectin fibril assembly in vitro. Exp Cell Res. 1995;217:109–17. doi: 10.1006/excr.1995.1069. [DOI] [PubMed] [Google Scholar]
- Hocking DC, Smith RK, McKeown-Longo PJ. A novel role for the integrin-binding III-10 module in fibronectin matrix assembly. J Cell Biol. 1996;133:431–44. doi: 10.1083/jcb.133.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking DC, Sottile J, McKeown-Longo PJ. Fibronectin’s III-1 module contains a conformation-dependent binding site for the amino-terminal region of fibronectin. J Biol Chem. 1994;269:19183–91. [PubMed] [Google Scholar]
- Hocking DC, Sottile J, McKeown-Longo PJ. Activation of distinct α5β1-mediated signaling pathways by fibronectin’s cell adhesion and matrix assembly domains. J Cell Biol. 1998;141:241–53. doi: 10.1083/jcb.141.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking DC, Sottile J, Reho T, Fassler R, McKeown-Longo PJ. Inhibition of fibronectin matrix assembly by the heparin-binding domain of vitronectin. J Biol Chem. 1999;274:27257–64. doi: 10.1074/jbc.274.38.27257. [DOI] [PubMed] [Google Scholar]
- Huveneers S, Danen EH. Adhesion signaling—crosstalk between integrins, Src and Rho. J Cell Sci. 2009;122:1059–69. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- Huveneers S, Truong H, Fässler R, Sonnenberg A, Danen EH. Binding of soluble fibronectin to integrin α5β1—link to focal adhesion redistribution and contractile shape. J Cell Sci. 2008;121:2452–62. doi: 10.1242/jcs.033001. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Fibronectins. New York: Springer-Verlag; 1990. p. 544. [Google Scholar]
- Ichihara-Tanaka K, Maeda T, Titani K, Sekiguchi K. Matrix assembly of recombinant fibronectin polypeptide consisting of amino-terminal 70 kDa and carboxyl-terminal 37 kDa regions. FEBS Lett. 1992;299:155–58. doi: 10.1016/0014-5793(92)80236-a. [DOI] [PubMed] [Google Scholar]
- Ilic D, Kovacic B, Johkura K, Schlaepfer DD, Tomasevic N, et al. FAK promotes organization of fibronectin matrix and fibrillar adhesions. J Cell Sci. 2004;117:177–87. doi: 10.1242/jcs.00845. [DOI] [PubMed] [Google Scholar]
- Ingham KC, Brew SA, Erickson HP. Localization of a cryptic binding site for tenascin on fibronectin. J Biol Chem. 2004;279:28132–35. doi: 10.1074/jbc.M312785200. [DOI] [PubMed] [Google Scholar]
- Ingham KC, Brew SA, Huff S, Litvinovich SV. Cryptic self-association sites in type III modules of fibronectin. J Biol Chem. 1997;272:1718–24. doi: 10.1074/jbc.272.3.1718. [DOI] [PubMed] [Google Scholar]
- Johnson KJ, Sage H, Briscoe G, Erickson HP. The compact conformation of fibronectin is determined by intramolecular ionic interactions. J Biol Chem. 1999;274:15473–79. doi: 10.1074/jbc.274.22.15473. [DOI] [PubMed] [Google Scholar]
- Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karuri NW, Lin Z, Rye HS, Schwarzbauer JE. Probing the conformation of the fibronectin III1–2 domain by fluorescence resonance energy transfer. J Biol Chem. 2009;284:3445–52. doi: 10.1074/jbc.M805025200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey R, Williamson MR, Chaudhry S, Mellody KT, McGovern A, et al. Fibrillin-1 microfibril deposition is dependent on fibronectin assembly. J Cell Sci. 2008;121:2696–704. doi: 10.1242/jcs.029819. [DOI] [PubMed] [Google Scholar]
- Klass CM, Couchman JR, Woods A. Control of extracellular matrix assembly by syndecan-2 proteoglycan. J Cell Sci. 2000;113:493–506. doi: 10.1242/jcs.113.3.493. [DOI] [PubMed] [Google Scholar]
- Klein RM, Zheng M, Ambesi A, Van De Water L, McKeown-Longo PJ. Stimulation of extracellular matrix remodeling by the first type III repeat in fibronectin. J Cell Sci. 2003;116:4663–74. doi: 10.1242/jcs.00778. [DOI] [PubMed] [Google Scholar]
- Kozel BA, Rongish BJ, Czirok A, Zach J, Little CD, et al. Elastic fiber formation: a dynamic view of extracellular matrix assembly using timer reporters. J Cell Physiol. 2006;207:87–96. doi: 10.1002/jcp.20546. [DOI] [PubMed] [Google Scholar]
- Krammer A, Lu H, Isralewitz B, Schulten K, Vogel V. Forced unfolding of the fibronectin type III module reveals a tensile molecular recognition switch. Proc Natl Acad Sci USA. 1999;96:1351–56. doi: 10.1073/pnas.96.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenbach KJ, Sottile J. Identification of protein-disulfide isomerase activity in fibronectin. J Biol Chem. 1999;274:7032–38. doi: 10.1074/jbc.274.11.7032. [DOI] [PubMed] [Google Scholar]
- Leahy DJ, Aukhil I, Erickson HP. 2.0 Å crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell. 1996;84:155–64. doi: 10.1016/s0092-8674(00)81002-8. [DOI] [PubMed] [Google Scholar]
- Ledger PW, Uchida N, Tanzer ML. Immunocytochemical localization of procollagen and fibronectin in human fibroblasts: effects of the monovalent ionophore, monensin. J Cell Biol. 1980;87:663–71. doi: 10.1083/jcb.87.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinovich SV, Brew SA, Aota S, Akiyama SK, Haudenschild C, Ingham KC. Formation of amyloid-like fibrils by self-association of a partially unfolded fibronectin type III module. J Mol Biol. 1998;280:245–58. doi: 10.1006/jmbi.1998.1863. [DOI] [PubMed] [Google Scholar]
- Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005a;24:389–99. doi: 10.1016/j.matbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Mao Y, Schwarzbauer JE. Stimulatory effects of a three-dimensional microenvironment on cell-mediated fibronectin fibrillogenesis. J Cell Sci. 2005b;118:4427–36. doi: 10.1242/jcs.02566. [DOI] [PubMed] [Google Scholar]
- Mao Y, Schwarzbauer JE. Accessibility to the fibronectin synergy site in a 3D matrix regulates engagement of α5β1 versus αvβ3 integrin receptors. Cell Commun Adhes. 2006;13(5–6):267–77. doi: 10.1080/15419060601072215. [DOI] [PubMed] [Google Scholar]
- Maqueda A, Moyano JV, Hernández Del Cerro M, Peters DM, Garcia-Pardo A. The heparin III–binding domain of fibronectin (III4–5 repeats) binds to fibronectin and inhibits fibronectin matrix assembly. Matrix Biol. 2007;26:642–51. doi: 10.1016/j.matbio.2007.06.001. [DOI] [PubMed] [Google Scholar]
- McDonald JA. Extracellular matrix assembly. Annu Rev Cell Biol. 1988;4:183–207. doi: 10.1146/annurev.cb.04.110188.001151. [DOI] [PubMed] [Google Scholar]
- McDonald JA, Quade BJ, Broekelman TJ, LaChance R, Forsman K, et al. Fibronectin’s cell-adhesive domain and an amino-terminal matrix assembly domain participate in its assembly into fibroblast peri-cellular matrix. J Biol Chem. 1987;262:2957–67. [PubMed] [Google Scholar]
- McKeown-Longo PJ, Mosher DF. Binding of plasma fibronectin to cell layers of human skin fibroblasts. J Cell Biol. 1983;97:466–72. doi: 10.1083/jcb.97.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown-Longo PJ, Mosher DF. Interaction of the 70,000-mol. wt amino terminal fragment of fibronectin with matrix-assembly receptor of fibroblasts. J Cell Biol. 1985;100:364–74. doi: 10.1083/jcb.100.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–23. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Morla A, Ruoslahti E. A fibronectin self-assembly site involved in fibronectin matrix assembly: reconstruction in a synthetic peptide. J Cell Biol. 1992;118:421–29. doi: 10.1083/jcb.118.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morla A, Zhang Z, Ruoslahtl E. Superfibronectin is a functionally distinct form of fibronectin. Nature. 1994;367:193–96. doi: 10.1038/367193a0. [DOI] [PubMed] [Google Scholar]
- Muro AF, Chauhan AK, Gajovic S, Iaconcig A, Porro F, et al. Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J Cell Biol. 2003;162:149–60. doi: 10.1083/jcb.200212079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhauser AF, Badilla-Fernandez C, Carrion-Vazquez M, Fernandez JM. The mechanical hierarchies of fibronectin observed with single-molecule AFM. J Mol Biol. 2002;319:433–47. doi: 10.1016/S0022-2836(02)00306-6. [DOI] [PubMed] [Google Scholar]
- Ohashi T, Augustus AM, Erickson HP. Transient opening of fibronectin type III (FNIII) domains: the interaction of the third FNIII domain of FN with anastellin. Biochemistry. 2009;48:4189–97. doi: 10.1021/bi900001g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi T, Erickson HP. Domain unfolding plays a role in superfibronectin formation. J Biol Chem. 2005;280:39143–51. doi: 10.1074/jbc.M509082200. [DOI] [PubMed] [Google Scholar]
- Ohashi T, Erickson HP. Revisiting the mystery of fibronectin multimers: the fibronectin matrix is composed of fibronectin dimers cross-linked by non-covalent bonds. Matrix Biol. 2009;28:170–75. doi: 10.1016/j.matbio.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi T, Kiehart DP, Erickson HP. Dynamics and elasticity of the fibronectin matrix in living cell culture visualized by fibronectin-green fluorescent protein. Proc Natl Acad Sci USA. 1999;96:2153–58. doi: 10.1073/pnas.96.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi T, Kiehart DP, Erickson HP. Dual labeling of the fibronectin matrix and actin cytoskeleton with green fluorescent protein variants. J Cell Sci. 2002;115:1221–29. doi: 10.1242/jcs.115.6.1221. [DOI] [PubMed] [Google Scholar]
- Olorundare OE, Peyruchaud O, Albrecht RM, Mosher DF. Assembly of a fibronectin matrix by adherent platelets stimulated by lysophosphatidic acid and other agonists. Blood. 2001;98:117–24. doi: 10.1182/blood.v98.1.117. [DOI] [PubMed] [Google Scholar]
- Pankov R, Cukierman E, Katz BZ, Matsumoto K, Lin DC, et al. Integrin dynamics and matrix assembly: tensin-dependent translocation of α5β1 integrins promotes early fibronectin fibrillogenesis. J Cell Biol. 2000;148:1075–90. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plato. Timaeus (English translation) Baltimore: Penguin Books; 1965. p. 121. [Google Scholar]
- Potts JR, Campbell ID. Fibronectin structure and assembly. Curr Opin Cell Biol. 1994;6:648–55. doi: 10.1016/0955-0674(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Pozzi A, Voziyan PA, Hudson BG, Zent R. Regulation of matrix synthesis, remodeling and accumulation in glomerulosclerosis. Curr Pharm Des. 2009;15:1318–33. doi: 10.2174/138161209787846748. [DOI] [PubMed] [Google Scholar]
- Ramirez F, Sakai LY. Biogenesis and function of fibrillin assemblies. Cell Tissue Res. 2010;339:71–82. doi: 10.1007/s00441-009-0822-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Kaitila I, Wilcox WR, Rimoin DL, Steinmann B, et al. Proteoglycan sulfation in cartilage and cell cultures from patients with sulfate transporter chondrodysplasias: relationship to clinical severity and indications on the role of intracellular sulfate production. Matrix Biol. 1998;17:361–69. doi: 10.1016/s0945-053x(98)90088-9. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Obrink B. Common principles in cell adhesion. Exp Cell Res. 1996;227:1–11. doi: 10.1006/excr.1996.0243. [DOI] [PubMed] [Google Scholar]
- Sabatier L, Chen D, Fagotto-Kaufmann C, Hubmacher D, McKee MD, et al. Fibrillin assembly requires fibronectin. Mol Biol Cell. 2009;20:846–58. doi: 10.1091/mbc.E08-08-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–99. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer JE. Fibronectin: from gene to protein. Curr Opin Cell Biol. 1991a;3:786–91. doi: 10.1016/0955-0674(91)90051-y. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer JE. Identification of the fibronectin sequences required for assembly of a fibrillar matrix. J Cell Biol. 1991b;113:1463–73. doi: 10.1083/jcb.113.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer JE, Spencer CS, Wilson CL. Selective secretion of alternatively spliced fibronectin variants. J Cell Biol. 1989;109:3445–53. doi: 10.1083/jcb.109.6.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechler JL, Corbett SA, Schwarzbauer JE. Modulatory roles for integrin activation and the synergy site of fibronectin during matrix assembly. Mol Biol Cell. 1997;8:2563–73. doi: 10.1091/mbc.8.12.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechler JL, Cumiskey AM, Gazzola DM, Schwarzbauer JE. A novel RGD-independent fibronectin assembly pathway initiated by α4β1 integrin binding to the alternatively spliced V region. J Cell Sci. 2000;113:1491–98. doi: 10.1242/jcs.113.8.1491. [DOI] [PubMed] [Google Scholar]
- Sechler JL, Rao H, Cumiskey AM, Vega-Colon I, Smith MS, et al. A novel fibronectin binding site required for fibronectin fibril growth during matrix assembly. J Cell Biol. 2001;154:1081–88. doi: 10.1083/jcb.200102034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechler JL, Schwarzbauer JE. Control of cell cycle progression by fibronectin matrix architecture. J Biol Chem. 1998;273:25533–36. doi: 10.1074/jbc.273.40.25533. [DOI] [PubMed] [Google Scholar]
- Sechler JL, Takada Y, Schwarzbauer JE. Altered rate of fibronectin matrix assembly by deletion of the first type III repeats. J Cell Biol. 1996;134:573–83. doi: 10.1083/jcb.134.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Askari JA, Humphries MJ, Jones EY, Stuart DI. Crystal structure of a heparin- and integrin-binding segment of human fibronectin. EMBO J. 1999;18:1468–79. doi: 10.1093/emboj/18.6.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Sottile J. Caveolin-1-dependent β1 integrin endocytosis is a critical regulator of fibronectin turnover. J Cell Sci. 2008;121:2360–71. doi: 10.1242/jcs.014977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer II. The fibronexus: a transmembrane association of fibronectin-containing fibers and bundles of 5 nm microfilaments in hamster and human fibroblasts. Cell. 1979;16:675–85. doi: 10.1016/0092-8674(79)90040-0. [DOI] [PubMed] [Google Scholar]
- Smith ML, Gourdon D, Little WC, Kubow KE, Eguiluz RA, et al. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol. 2007;5:e268. doi: 10.1371/journal.pbio.0050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottile J, Chandler J. Fibronectin matrix turnover occurs through a caveolin-1-dependent process. Mol Biol Cell. 2005;16:757–68. doi: 10.1091/mbc.E04-08-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell. 2002;13:3546–59. doi: 10.1091/mbc.E02-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottile J, Hocking DC, Langenbach KJ. Fibronectin polymerization stimulates cell growth by RGD-dependent and -independent mechanisms. J Cell Sci. 2000;113:4287–99. doi: 10.1242/jcs.113.23.4287. [DOI] [PubMed] [Google Scholar]
- Sottile J, Schwarzbauer JE, Selegue J, Mosher DF. Five type I modules of fibronectin form a functional unit that binds to fibroblasts and Staphylococcus aureus. J Biol Chem. 1991;266:12840–43. [PubMed] [Google Scholar]
- Sottile J, Wiley S. Assembly of amino-terminal fibronectin dimers into the extracellular matrix. J Biol Chem. 1994;269:17192–98. [PubMed] [Google Scholar]
- Takahashi S, Leiss M, Moser M, Ohashi T, Kitao T, et al. The RGD motif in fibronectin is essential for development but dispensable for fibril assembly. J Cell Biol. 2007;178:167–78. doi: 10.1083/jcb.200703021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MH, Sun Z, Opitz SL, Schmidt TE, Peters JH, George EL. Deletion of the alternatively spliced fibronectin EIIIA domain in mice reduces atherosclerosis. Blood. 2004;104:11–18. doi: 10.1182/blood-2003-09-3363. [DOI] [PubMed] [Google Scholar]
- Twal WO, Czirok A, Hegedus B, Knaak C, Chintalapudi MR, et al. Fibulin-1 suppression of fibronectin-regulated cell adhesion and motility. J Cell Sci. 2001;114:4587–98. doi: 10.1242/jcs.114.24.4587. [DOI] [PubMed] [Google Scholar]
- Ugarova TP, Ljubimov AV, Deng L, Plow EF. Proteolysis regulates exposure of the IIICS-1 adhesive sequence in plasma fibronectin. Biochemistry. 1996;35:10913–21. doi: 10.1021/bi960717s. [DOI] [PubMed] [Google Scholar]
- Ugarova TP, Zamaroon C, Veklich Y, Bowditch RD, Ginsberg MH, et al. Conformational transitions in the cell binding domain of fibronectin. Biochemistry. 1995;34:4457–66. doi: 10.1021/bi00013a039. [DOI] [PubMed] [Google Scholar]
- Vakonakis I, Staunton D, Rooney LM, Campbell ID. Interdomain association in fibronectin: insight into cryptic sites and fibrillogenesis. EMBO J. 2007;26:2575–83. doi: 10.1038/sj.emboj.7601694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenick LV, Hsia HC, Schwarzbauer JE. Fibronectin fragmentation promotes α4β1 integrin-mediated contraction of a fibrin-fibronectin provisional matrix. Exp Cell Res. 2005;309:48–55. doi: 10.1016/j.yexcr.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Ventura E, Sassi F, Parodi A, Balza E, Borsi L, et al. Alternative splicing of the angiogenesis associated extra-domain B of fibronectin regulates the accessibility of the B-C loop of the type III repeat 8. PLoS One. 2010;5:e9145. doi: 10.1371/journal.pone.0009145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenseil JE, Mecham RP. New insights into elastic fiber assembly. Birth Def Res C. 2007;81:229–40. doi: 10.1002/bdrc.20111. [DOI] [PubMed] [Google Scholar]
- Wayner EA, Garcia-Pardo A, Humphries MJ, McDonald JA, Carter WG. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989;109:1321–30. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicka-Patynowski I, Mao Y, Schwarzbauer JE. Continuous requirement for pp60-Src and phosphopaxillin during fibronectin matrix assembly by transformed cells. J Cell Physiol. 2007;210:750–56. doi: 10.1002/jcp.20886. [DOI] [PubMed] [Google Scholar]
- Wierzbicka-Patynowski I, Schwarzbauer JE. Regulatory role for Src and phosphatidylinositol 3-kinase in initiation of fibronectin matrix assembly. J Biol Chem. 2002;277:19703–8. doi: 10.1074/jbc.M200270200. [DOI] [PubMed] [Google Scholar]
- Wierzbicka-Patynowski I, Schwarzbauer JE. The ins and outs of fibronectin matrix assembly. J Cell Sci. 2003;116:3269–76. doi: 10.1242/jcs.00670. [DOI] [PubMed] [Google Scholar]
- Wilcox-Adelman SA, Denhez F, Goetinck PF. Syndecan-4 modulates focal adhesion kinase phosphorylation. J Biol Chem. 2002;277:32970–77. doi: 10.1074/jbc.M201283200. [DOI] [PubMed] [Google Scholar]
- Wolfram D, Tzankov A, Pülzl P, Piza-Katzer H. Hypertrophic scars and keloids—a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg. 2009;35:171–81. doi: 10.1111/j.1524-4725.2008.34406.x. [DOI] [PubMed] [Google Scholar]
- Woods A. Syndecans: transmembrane modulators of adhesion and matrix assembly. J Clin Invest. 2001;107:935–41. doi: 10.1172/JCI12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Longley RL, Tumova S, Couchman JR. Syndecan-4 binding to the high affinity heparin-binding domain of fibronectin drives focal adhesion formation in fibroblasts. Arch Biochem Biophys. 2000;374:66–72. doi: 10.1006/abbi.1999.1607. [DOI] [PubMed] [Google Scholar]
- Wu C, Hughes PE, Ginsberg MH, McDonald JA. Identification of a new biological function for the integrin αvβ3: initiation of fibronectin matrix assembly. Cell Adhes Commun. 1996;4:149–58. doi: 10.3109/15419069609014219. [DOI] [PubMed] [Google Scholar]
- Wu C, Keivenst VM, O’Toole VTE, McDonald JA, Ginsberg MH. Integrin activation and cytoskeletal interaction are critical steps in the assembly of a fibronectin matrix. Cell. 1995;83:715–24. doi: 10.1016/0092-8674(95)90184-1. [DOI] [PubMed] [Google Scholar]
- Xu J, Bae E, Zhang Q, Annis DS, Erickson HP, Mosher DF. Display of cell surface sites for fibronectin assembly is modulated by cell adherence to 1F3 and C-terminal modules of fibronectin. PLoS One. 2009;4:e4113. doi: 10.1371/journal.pone.0004113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JT, Hynes RO. Fibronectin receptor functions in embryonic cells deficient in α5β1 integrin can be replaced by αv integrins. Mol Biol Cell. 1996;7:1737–48. doi: 10.1091/mbc.7.11.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda A, Ushakov D, Multhaupt HAB, Couchman JR. Fibronectin matrix assembly requires distinct contributions from Rho kinases I and -II. Mol Biol Cell. 2007;18:66–75. doi: 10.1091/mbc.E06-08-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD, Patton BL. Developmental and pathogenic mechanisms of basement membrane assembly. Curr Pharm Des. 2009;15:1277–94. doi: 10.2174/138161209787846766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Milo R, Kam Z, Geiger B. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J Cell Sci. 2007;120:137–48. doi: 10.1242/jcs.03314. [DOI] [PubMed] [Google Scholar]
- Zamir E, Katz BZ, Aota S, Yamada KM, Geiger B, Kam Z. Molecular diversity of cell-matrix adhesions. J Cell Sci. 1999;112:1655–69. doi: 10.1242/jcs.112.11.1655. [DOI] [PubMed] [Google Scholar]
- Zhang G, Young BB, Ezura Y, Favata M, Soslowsky LJ, et al. Development of tendon structure and function: regulation of collagen fibrillogenesis. J Musculoskelet Neuronal Interact. 2005;5:5–21. [PubMed] [Google Scholar]
- Zhang Q, Checovich WJ, Peters DM, Albrecht RM, Mosher DF. Modulation of cell surface fibronectin assembly sites by lysophosphatidic acid. J Cell Biol. 1994;127:1447–59. doi: 10.1083/jcb.127.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Mosher DF. Cross-linking of the NH2-terminal region of fibronectin to molecules of large apparent molecular mass. J Biol Chem. 1996;271:33284–92. doi: 10.1074/jbc.271.52.33284. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Peyruchaud O, French KJ, Magnusson MK, Mosher DF. Sphingosine 1-phosphate stimulates fibronectin matrix assembly through a Rho-dependent signal pathway. Blood. 1999;93:2984–90. [PubMed] [Google Scholar]
- Zhang Z, Morla AO, Vuori K, Bauer JS, Juliano RL, Ruoslahti E. The αvβ1 integrin functions as a fibronectin receptor but does not support fibronectin matrix assembly and cell migration on fibronectin. J Cell Biol. 1993;122:235–42. doi: 10.1083/jcb.122.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–51. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.