Abstract
Aims
Cardiac remodeling occurs in the infarcted heart (MI). The underlying regulatory mechanisms are under investigation. Platelet-derived growth factor (PDGF) is a family of growth factors that stimulates cell growth, differentiation and migration. Herein, we sought to determine whether PDGF is involved in cardiac repair/remodeling following MI.
Methods and Results
The temporal and spatial expression of PDGF isoforms (A, B, C and D) and PDGF receptor (PDGFR)-α and β as well as cell types expressing PDGF were examined in the infarcted rat heart. Sham-operated rats served as controls. We found that the normal myocardium expressed all PDGF isoforms, and cell types expressing PDGF were primarily interstitial cells. Following MI, PDGF-A and D were significantly increased in the infarcted myocardium during 6 weeks of the observation period and cells expressing PDGF-A and D were primarily endothelial cells, macrophages and myofibroblasts (myoFb). PDGF-B and C expression was, however, reduced in the infarcted heart. In the noninfarcted myocardium, PDGF-D expression was increased in the late stage of MI and cells expressing PDGF-D were predominantly fibroblasts. Both PDGFR-α and β were significantly increased in the infarcted myocardium in the early and late stages of MI and in the noninfarcted myocardium in the late stage of MI.
Conclusions
Enhanced PDGF-A, PDGF-D and PDGFR are coincident with angiogenesis, inflammatory and fibrogenic responses in the infarcted myocardium, suggesting their regulation on cardiac repair. Elevated PDGF-D in the noninfarcted myocardium suggests its involvement in the development of interstitial fibrosis that appears in the late stage of MI.
Keywords: Myocardial infarction, cardiac repair/remodeling, PDGF, PDGFR
INTRODUCTION
The family of PDGF is a group of multifunctional proteins with a wide variety of effects. PDGF is a key player in the processes of embryonic development and its signaling has been implicated in stimulating angiogenesis by promoting cellular proliferation and differentiation [1–3]. In addition, PDGF is a required element in cellular division for fibroblasts and contributes to the maintenance of connective tissue in adults [4].
The PDGF family is composed of four different polypeptide chains, the traditional PDGF-A and PDGF-B, and the more recently discovered PDGF-C and PDGF-D. The biologically active PDGF protein forms disulfide-bonded dimers, including four homodimers PDGF-AA, PDGF-BB, PDGF-CC and PDGF-DD, and one heterodimer, PDGF-AB. PDGFs exert their biological activities by activating two structurally related tyrosine kinase receptors, PDGFR-α and PDGFR-β, which activate overlapping signal transduction pathways including phosphatidylinositol 3 kinase, Ras-MAPK, Src family kinases and phospholipase Cγ [5] .
Excessive activity of PDGF has been associated with several human disorders, including atherosclerosis, organ fibrosis and tumorigenesis. A causal role for PDGF in atherosclerosis has been supported by in vivo and in vitro studies [6, 7]. Numerous studies have implicated PDGF working with transforming growth factor (TGF)-β in the development of organ fibrosis [8–10]. Fibrosis results from excessive deposition of extracellular matrix primarily by phenotypically transformed fibroblasts, termed myoFb [11]. PDGF plays a key role in expansion of myoFb by stimulating their proliferation, migration and survival [12, 13]. Elevated levels of PDGF have been consistently demonstrated in the fibrotic lesions of various organs. In pulmonary fibrosis, myoFb are the main source of PDGF-A [10, 14]. MyoFb also express elevated levels of PDGFR-α, suggesting that both paracrine and autocrine modes of signaling operate in these cells [14]. PDGF is also the most prominent mitogen contributing to fibrosis of the liver and kidney [15–17]. Additionally, PDGF has long been implicated in cancer and is known to be involved in many biological processes, particularly angiogenesis [2, 3, 18].
Following MI, extensive structural remodeling appears in the heart. Cardiac repair occurring at the site of myocyte loss preserves structural integrity and is essential to the heart’s recovery. Inflammation, fibrogenesis and angiogenesis are central to cardiac repair [19, 20]. Macrophages and myoFb are the major cells contributing to inflammatory and fibrogenic response in the infarcted myocardium [19, 21]. In addition, interstitial fibrosis is developed in the noninfarcted myocardium in the late stage of MI, which contributes to ventricular dysfunction [19, 22]. Factors regulating cardiac repair/remodeling have drawn considerable attention. Studies have shown that growth factors, such as fibroblast growth factors, TGF, vascular endothelial growth factor, etc. play an important role in cardiac repair/remodeling [23, 24]. However, the potential regulation of PDGF isoforms in the process of cardiac repair and remodeling remain to be elucidated. To determine whether PDGF is involved in cardiac repair and remodeling following MI, we tested the temporal and spatial gene and protein expression of PDGF-A, B, C and D, and PDGFR-α and β in the infarcted heart.
METHODS
Animal Model
The study was approved by University of Tennessee Health Science Center Animal Care and Use Committee. Left ventricular anterior transmural MI was created in 8-week-old male Sprague-Dawley rats (Harlan, Indianapolis, IN) via permanent ligation of the left coronary artery. Animals were anesthetized with 1.5% isoflurane, intubated and ventilated with a rodent respirator. The heart was exposed via a left thoracotomy and the left anterior descending artery was ligated with a 6-0 silk suture. The chest was then closed and lungs reinflated using positive end-expiratory pressure [25]. The surgery leads to free wall infarction of the left ventricle. Sham-operated rats served as controls. Rats with MI were sacrificed at postoperative day 1, 3, week 1, 2, 4 and 6 (n = 8 survived rats for each time point). Hearts were removed and kept frozen at −80 °C until use.
RT-PCR
Total RNA was extracted from cardiac tissue using Trizol Reagent (Invitrogen, Carlsbad, CA). The RNA was treated with DNase by using TURBO DNA-free kit (Ambion, Austin, TX), and purified with RNeasy Mini Kit (Qiagen Inc. USA, Valencia, CA). The purification, concentration and integrity of the RNA were examined with NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE), and Angilent Bioanalyzer (Angilent Technologies, Foster City, CA), respectively. cDNA was prepared from total RNA using a High Capacity cDNA reverse transcriptase kit (Applied Biosystems Foster City, CA). The gene-specific probes and primer sets for PDGF-A, B, C and D, PDGFR-α and β were deduced using Universal ProbeLibrary Assay Design software (https://www.roche-applied-science.com). PDGF-A, B, C and D, PDGFR-α and β mRNA levels were detected and analyzed on an LightCycler 480 System (Roche, Indianapolis, IN) under the following cycling conditions: 1 cycle at 95ºC for 5 min and then 45 cycles at 95ºC for 10 seconds, 60ºC for 30 seconds, and 72ºC for 10 seconds. The PCR mix contained 0.2μl of 10μM primers, 0.1μl of 10μM Universal library probe, 5μl of LC 480 master mix (2X), 2μl of template cDNA and RNase-free water to 10μl. TATA box-binding protein has been selected as the endogenous quantity control. Fold change was used to compare the different between groups [24].
Western Blotting
PDGF-A, B, C and D protein levels were measured by western blot. Briefly, the normal, noninfarcted left myocardium (septum), border zone and infarcted myocardium were dissected and homogenized. The supernatant was collected and separated by 10% SDS-PAGE. After electrophoresis, samples were transferred to PVDF membranes and incubated with antibody against PDGF-A, B (Santa Cruz Biotechnology, Santa Cruz, CA), PDGF-C (R&D, Minneapolis, MN) and PDGF-D (Invitrogen, Carlsbad, CA). Blots were subsequently incubated with peroxidase-conjugated secondary antibody (Sigma, St. Louis, MO). After washing, the blots were developed with an enhanced chemoluminescence method. The amount of protein detected was assessed by means of quantitative densitometry analysis with a computer image analyzing system [24].
Immunohistochemistry
Cells expressing PDGF isoforms were detected by immunohistochemical labeling. Cryostat coronal sections (6μm) were air-dried, fixed in 10% buffered formalin for 10 min, and washed in phosphate-buffered saline (PBS). Endogenous peroxidase activity was blocked by immersion of sections in 0.3% H2O2 for 10 min at room temperature. Sections were blocked with 5% goat serum in PBS. Tissues were incubated with primary antibodies against PDGF-A, B, C and D for 1 hr at room temperature. Sections were then incubated with peroxidase-conjugated secondary antibody for 1 hr at room temperature, washed in PBS for 10 min, and incubated with 0.5 mg/ml diaminobenzidine tetrahydrochloride 2-hydrate + H2O2 for 1 min. Negative control sections were incubated with secondary antibody alone. Sections were then dehydrated, mounted, and viewed by light microscopy [26].
Quantitative In Vitro Autoradiography
The localization and density of PDGFR were detected by quantitative in vitro autoradiography. Cryostat 16μm coronal cardiac sections were preincubated for 15 min in PBS buffer containing 0.2% bovine serum albumin, then incubated for 1 hr in a fresh volume of the same buffer containing 0.2μCi/mL 125I-PDGF-BB (PerkinElmer, Waltham, MA), the ligand for both PDGFR-α and β. Non-specific binding was measured in the presence of 1 μM unlabeled PDGF-BB. To characterize PDGFR subtypes, sections were incubated with 125I-PDGF-BB in the presence of 1μM unlabeled PDGF-AA. Unlabeled PDGF-AA displaces 125I PDGF-BB binding to PDGFR-α, but not PDGFR-β. After incubation, sections were washed, dried and exposed to Kodak NMB-6 film. Quantifications of PDGFR binding density and receptor subtypes were performed using a computer image analysis system. PFGFR binding is expressed as optical density [25].
Statistical Analysis
Statistical analysis of data obtained from RT-PCR, western blot and in vitro autoradiography was performed using analysis of variance. Values are expressed as mean±SEM with P<0.05 considered significant. Multiple group comparisons among controls and each group were made by Scheffés F-test.
RESULTS
Cardiac PDGF-A Expression
As detected by RT-PCR, PDGF-A mRNA was significantly increased in the border zone, i.e. the region between the infarcted and noninfarcted myocardium, in the first week postMI and then declined to normal levels in the following weeks (Figure 1, panel A). PDGF-A mRNA in the infarcted myocardium was unchanged in the first week postMI and then significantly increased at week 2 and remained elevated thereafter (Figure 1, panel B). In contrast, PDGF-A mRNA levels were unchanged in the noninfarcted myocardium compared to controls.
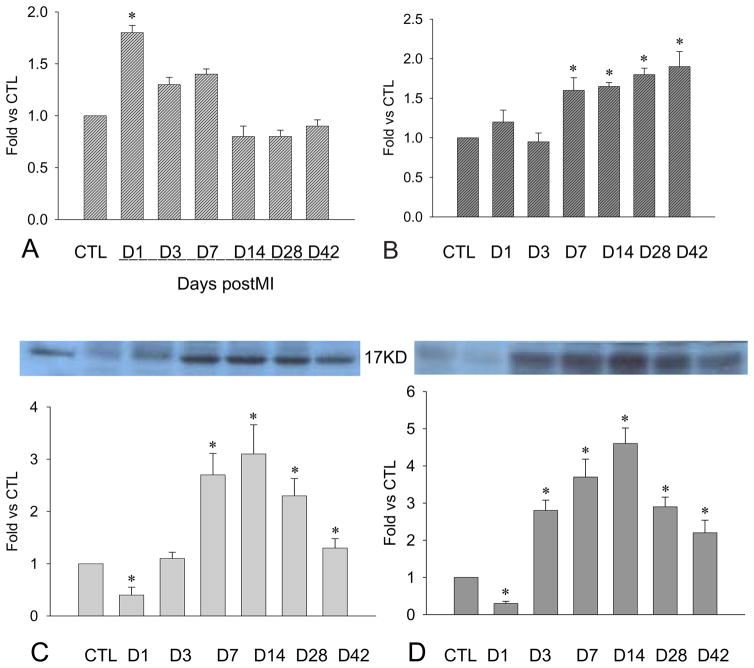
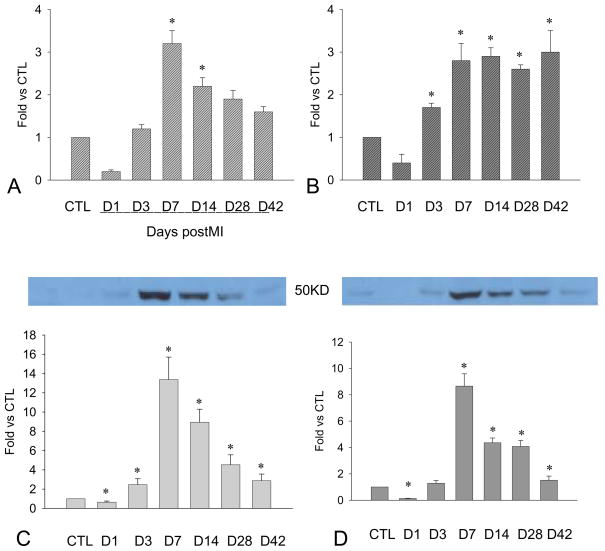
Figure 1.
Cardiac PDGF-A expression: Compared to controls, PDGFA mRNA was increased at the border zone within the first week postMI (panel A) and the infarcted myocardium from day 7 to day 42 (panel B). PDGF-A protein levels at the border zone (panel C) and infarcted myocardium (panel D) were significantly reduced at day 1, elevated at day 3, peaked at day 14, then declined but remained higher than in controls.
As detected by western blot, PDGF-A protein levels at the border zone (Figure 1, panel C) and infarcted myocardium (Figure 1, panel D) were significantly reduced at day 1, elevated at day 3, peaked at week 2, and declined thereafter, but remained elevated compared to controls.
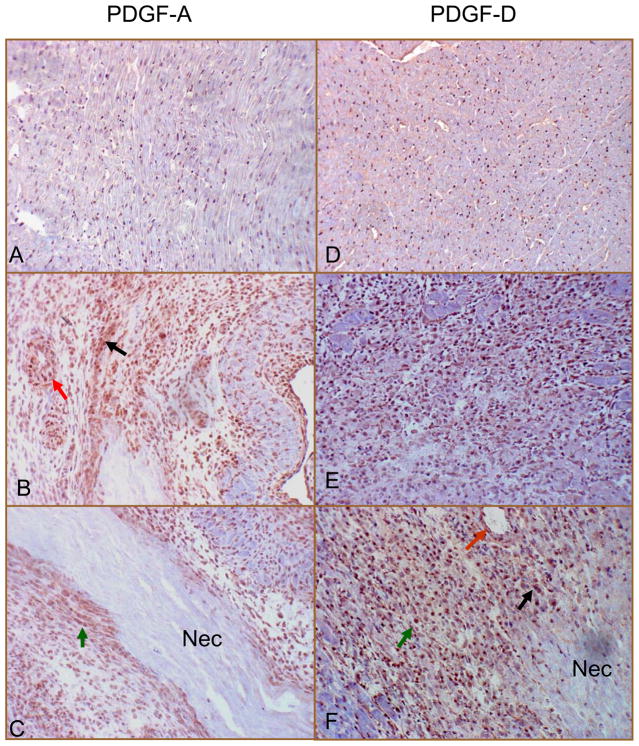
By immunohistochemistry, we observed positive PDGF-A staining in the normal heart, where cells expressing PDGF-A were primarily interstitial cells (Figure 2, panel A, brown spots). In the infarcted heart, strong staining was observed at the border zone (Figure 2, panel B) and infarcted myocardium (Figure 2, panel C), where cells expressing PDGF-A were primarily inflammatory cells, myoFb, and vascular endothelial and smooth muscle cells.
Figure 2.
Cells expressing PDGF-A and PDGF-D in the normal and infarcted heart: Immunohistochemistry showed positive PDGF-A (panel A) and PDGF-D (panel D) staining in the interstitial cells of the normal heart. Following MI, strong PDGF-A and PDGF-D staining was seen in the border zone (panels B and E, respectively) and infarcted myocardium (panels C and F, respectively) at day 7, where cells expressing PDGF-A and PDGF-D were primarily inflammatory cells (black arrow), myoFb (green arrow) and vascular smooth muscle cells and endothelial cells (red arrow). Nec: necrotic myocardium. X400
Cardiac PDGF-B Expression
Compared to controls, PDGF-B mRNA remained unchanged in the border zone (Figure 3, panel A), while it declined in the infarcted myocardium in the late stage of MI (Figure 3, panel B). PDGF-B mRNA remained unchanged in the noninfarcted myocardium in both early and late stages of MI.
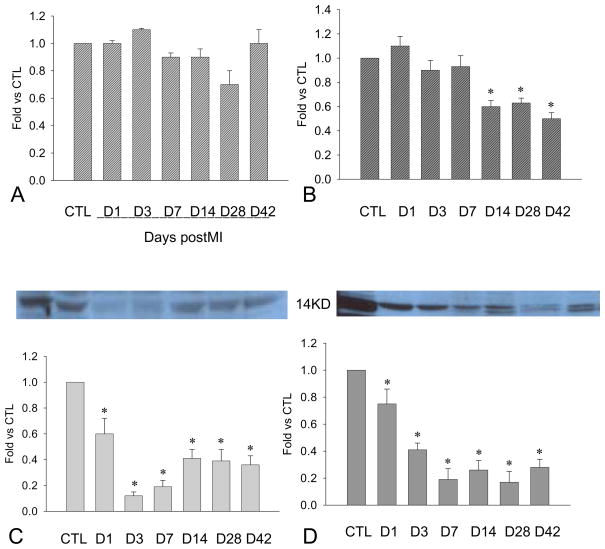
Figure 3.
Cardiac PDGF-B expression: Compared to controls, PDGF-B mRNA remained unchanged at the border zone (penal A), while levels decreased in the infarcted myocardium after week 2 (panel B). PDGF-B protein levels at the border zone (panel C) and infarcted myocardium (panel D) were significantly reduced for over the course of 6 weeks.
PDGF-B is normally expressed in the rat heart. Following MI, PDGF-B protein levels were significantly reduced in the border zone (Figure 3, panel C) and infarcted myocardium (Figure 3, panel D) in both early and late stages of MI.
Cardiac PDGF-C Expression
PDGF-C mRNA is detectable in the normal myocardium. Compared to controls, PDGF-C mRNA was reduced in the border zone (Figure 4, panel A) and infarcted myocardium (Figure 4, panel B) for over the course of six weeks postMI. PDGF-C mRNA levels in the noninfarcted myocardium were similar to controls.
Figure 4.
Cardiac PDGF-C expression: Compared to controls, PDGF-C mRNA was decreased at the border zone (panel A) and infarcted myocardium (panel B). PDGF-C protein levels at the border zone (panel C) and infarcted myocardium (panel D) were also reduced for over the course of 6 weeks of the observation period.
Compared to controls, PDGF-C protein levels were significantly reduced in the border zone (Figure 4, panel C) and infarcted myocardium (Figure 4, panel D) in both early and late stages of MI.
Cardiac PDGF-D Expression
We observed PDGF-D mRNA in the control heart. Following MI, PDGF-D mRNA levels in the border zone (Figure 5, panel A) and infarcted myocardium (Figure 5, panel B) were significantly reduced at day 1 postMI, elevated at day 3 and became significantly increased over the course of 6 weeks. Compared to controls (1±0.05), PDGF-D mRNA levels in the noninfarcted myocardium were unchanged at day 1 and 3 (0.87±0.09 and 0.89±0.05), up-regulated at week 1 (1.21±0.12) and remained elevated at week 2, 4 and 6 weeks (1.53±0.21, 1.47±0.21, and 1.48±0.14, respectively).
Figure 5.
Cardiac PDGF-D expression: Compared to controls, PDGF-D mRNA in the border zone (panel A) and infarcted myocardium (panel B) were decreased at day 1 postMI, significantly increased at day 3 and remained elevated thereafter. PDGF-D protein levels at the border zone (panel C) and infarcted myocardium (panel D) were significantly increased, peaking at day 7.
Compared to controls, PDGF-D protein levels at the border zone (Figure 5, panel C) and infarcted myocardium (Figure 5, panel D) were significantly reduced at day 1, elevated at day 3, peaked at week 1, and then gradually declined but remained significantly elevated compared to controls.
By immunohistochemistry, we found that cells expressing PDGF-D were primarily interstitial fibroblasts in the normal heart (Figure 2, panel D). Following MI, strong staining was observed at the border zone (Figure 2, panel E) and infarcted myocardium (Figure 2, panel F), where cells expressing PDGF-D were primarily inflammatory cells, myoFb and endothelial cells. Cells expressing PDGF-D in the noninfarcted myocardium were predominantly fibroblasts.
Cardiac PDGFR Expression
PDGFR-α and β mRNAs were expressed in the control heart. After MI, PDGFR-α mRNA in the border zone (Figure 6, penal A) and infarcted myocardium (Figure 6, penal B) was reduced at day 1 postMI, significantly increased at day 3 and remained elevated thereafter. PDGFR-α mRNA in the noninfarcted myocardium was unchanged in the early stage of MI, and then elevated in the late stage of MI (Figure 6, penal C). Compared to the controls, PDGFR-β mRNA levels were up-regulated in the border zone (Figure 6, panel D) and infarcted myocardium (Figure 6, panel E) for the course of 6 weeks, while it was elevated in the noninfarcted myocardium only in the late stage of MI (Figure 6, panel F).
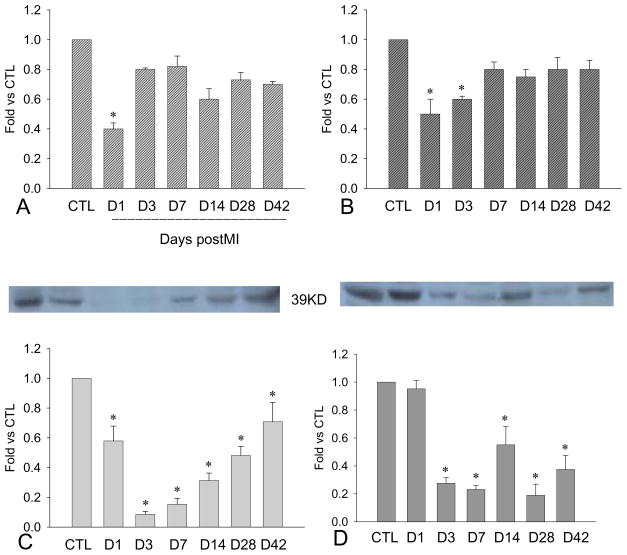
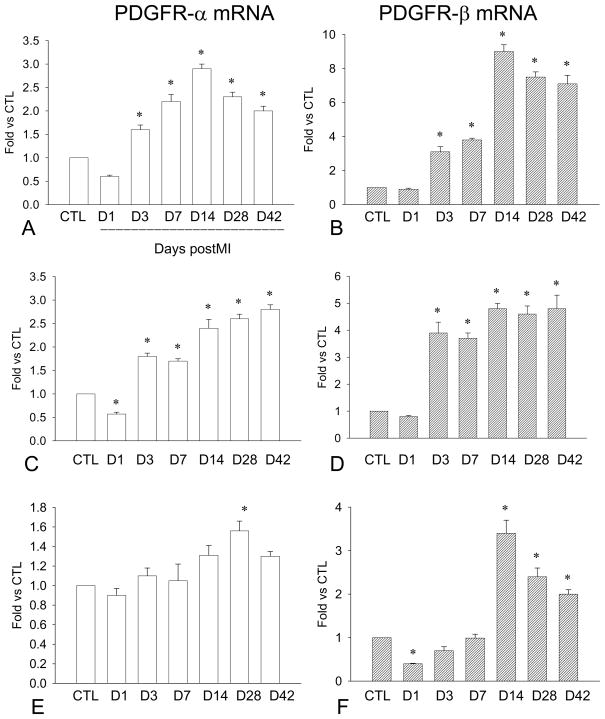
Figure 6.
Cardiac PDGFR gene expression: Compared to controls, PDGFR-α mRNA was significantly increased in the border zone (panel A) and infarcted myocardium (panel C) in both early and late stages of MI, whereas levels were only increased in the noninfarcted myocardium in the late state of MI (panel E). Similarly, PDGFR-β mRNA was increased in the border zone (panel B) and infarcted myocardium (panel D) in the early and late stage of MI. PDGF-β mRNA was elevated in the noninfarcted myocardium after week 2 (panel F).
The localization and density of cardiac PDGFR were detected by quantitative in vivo autoradiography. The normal heart expressed PDGFR (Figure 7, panel A). Following MI, PDGFR binding density was significantly increased in the infarcted myocardium at day 3 and remained elevated at week 1, 2, 4 and 6 (Figure 7, panels B–F). PDGFR binding density was unchanged in the noninfarcted myocardium at day 3 and significantly increased thereafter. PDGFR binding was partially displaced in the presence of unlabeled PDGF-AA in both sham-operated and infarcted heart (Figure 7, panels G and H, respectively).
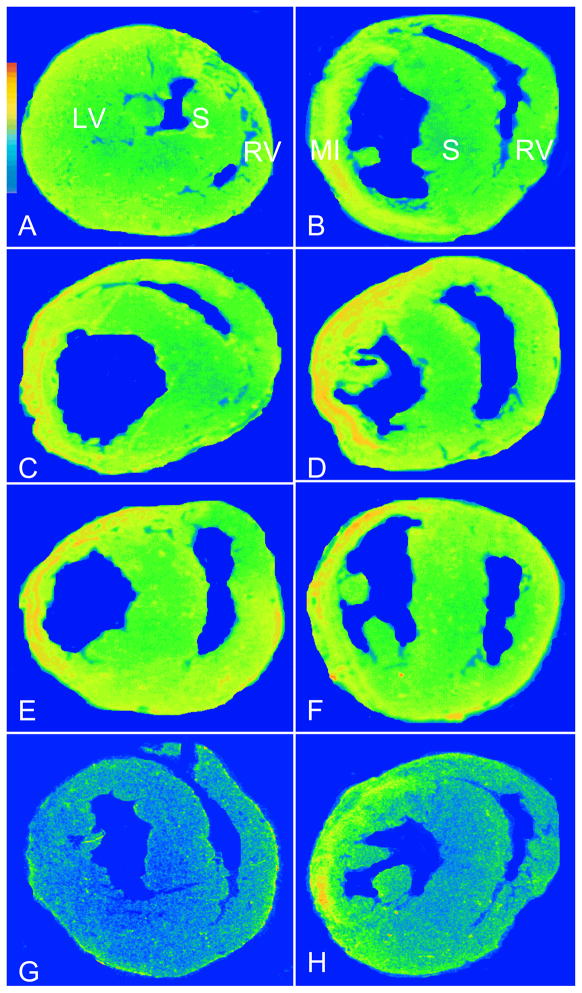
Figure 7.
Cardiac PDGFR binding density and subtypes: As detected by quantitative in vitro autoradiography, PDGFR was observed in the normal myocardium (panel A). Following MI, PDGFR levels were increased in the infarcted myocardium at day 3 (panel B) and remained elevated at day 7, 14, 28, and 42 (panels C–F). PDGFR in the noninfarcted myocardium remained unchanged at day 3 and 7 and then increased from day 14 to 42. PDGFR binding was partially displaced by PDGF-AA in the control (panel G) and infarcted heart (panel H).
The quantitative binding densities of PDGFR and receptor subtypes in the infarcted and noninfarcted myocardium are shown in Figure 8.
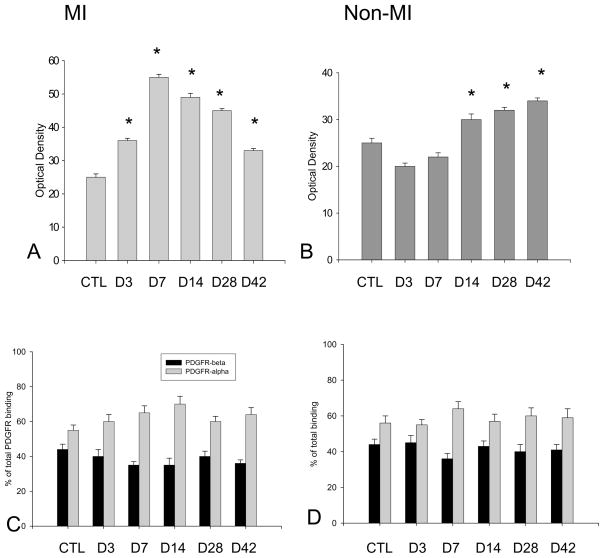
Figure 8.
Quantitative PDGFR binding density and subtypes: PDGFR binding in the infarcted and noninfarcted myocardium is shown in panels A and B, respectively. PDGFR subtypes assessed by displacement assay are shown in panels C and D, respectively.
DISSCUSSION
This is the first study exploring the expression of PDGF isoforms and their receptors in the normal and infarcted heart and their potential regulation on cardiac repair/remodeling.
Our results have shown that all of four PDGF isoforms are normally expressed in the myocardium, primarily expressed by interstitial fibroblasts. This observation indicates that PDGF plays a role in the growth and function of fibroblasts and contributes to the maintenance of connective tissue in the adult heart.
Following MI, cardiac repair occurs in the infarcted myocardium. Inflammatory response starts at the border zone a few hours after MI and then extends into the infarcted myocardium [26]. Monocyte-derived macrophages are the primary inflammatory cells involved in cardiac repair [21, 27]. These cells serve as the major source of inflammatory/fibrogenic cytokines and growth factors [27]. The inflammatory response is accompanied by angiogenesis and fibrogenesis. Cardiac inflammatory and angiogenic responses are most apparent in the first two weeks postMI [21, 26]. In addition, fibrous tissue formation is activated in the infarcted myocardium. The fibrogenic component substitutes for lost myocytes in the infarcted myocardium. Cells responsible for fibrous tissue formation at the site of MI consist principally of myoFb, phenotypically-differentiated fibroblasts, which contain the features of both smooth muscle cells and fibroblasts [28]. MyoFb are not residential cells in the normal myocardium. They appear at the border zone and the infarct site during the first week postMI, rapidly proliferate and are highly active in fibrous tissue formation via their expression of type I and III fibrillar collagens. Collagen fibers are morphologically evident at week 1 postMI, while an organized assembly of these fibers in the form of scar tissue becomes evident at week 2. This assembly continues to accumulate over 8 weeks [29].
Our study has shown that the expression of PDGF was changed in the infarcted heart compared to controls. PDGF-A and PDGF-D were significantly increased in the border zone and infarcted myocardium. Our immunohistochemical study has further revealed that inflammatory, endothelial cells and myoFb were the cells responsible for the elevated expression of cardiac PDGF-A and PDGF-D. The temporal and spatial activation of PDGF-A and PDGF-D is coincident with inflammation, angiogenesis and fibrogenesis. These findings suggest that PDGF-A and PDGF-D are involved in multiple responses during cardiac repair.
The regulation of PDGF in inflammation, angiogenesis and fibrosis has been explored in other pathological conditions. PDGF is a potent leukocyte chemoattractant and promotes inflammatory cell infiltration during wound healing [30]. Sustained angiogenesis is considered to be one of central hallmarks of cancer and PDGF serves as the predominant regulator of the pathological angiogenic process. The anti-PDGF signaling system has been considered an attractive therapeutic target for cancers [31]. PDGF have been also reported to stimulate myoFb differentiation and proliferation, contributing to the development of fibrosis in lung, kidney, liver and skin [10]. Factors activating PDGF expression have been documented. The expression of PDGF is stimulated by proinflammatory cytokines and its action is modulated by extracellular binding proteins and matrix molecules [10].
The differential expression of PDGF isoforms is observed in the infarcted heart - unlike PDGF-A and PDGF-D, the expression of PDGF-B and PDGF-C is decreased in the infarcted myocardium. The differential expression of PDGF isoforms have been also seen in other pathological conditions. In mice with obstructive uropathy [32], expressions of PDGF-D and PDGF-B were increased in the kidney, while PDGF-A or PDGF-C proteins remained unchanged. The possible meaning of having different isoforms vary in their expressions remains to be elucidated. Reduced PDGF-B and PDGF-C expression in the infarcted heart suggest that these PDGF isoforms may be the least effective of the four isoforms in cardiac repair.
It has been reported that other growth factor isoforms are differentially expressed in certain pathological condition. In myoFb of Crohn’s disease, TGF-β2 is enhanced, but TGF-β3 is reduced [33]. Thus, although the actions of the different isoforms of growth factors on cells are qualitatively similar in most cases, there might be distinct activities among isoforms.
Cardiac remodeling, characterized as interstitial fibrosis, is often developed in the noninfarcted myocardium in the late stage of MI, particularly in the heart with large infarction, and contributes to ventricular dysfunction. Our study has shown that PDGF-D expression was elevated at week 2 postMI and remained elevated thereafter. Cells expressing PDGF-D were primarily interstitial fibroblasts. These observations suggest the regulation of PDGF-D on fibrous tissue formation in the noninfarcted myocardium.
The PDGF are inactive in their monomeric forms. They form dimers and stimulate cellular responses by binding to PDGFR on the cell surface. [34]. These two receptor isoforms dimerize upon binding the PDGF dimer, leading to three possible receptor combinations, namely -αα, -ββ and -αβ. PDGF-DD binds to PDGFR-ββ with high affinity. PDGF-AA binds only to PDGFR-αα, while PDGF-BB is the only PDGF that can bind all three receptor combinations with high affinity. Our results have shown the normal heart contains PDGFR. Our study has further shown that both PDGFR-α and PDGFR-β mRNA and binding density are elevated at the border zone and infarcted myocardium. Cardiac PDGFR binding is displaced by ∼60% in the presence of unlabeled PDGF-AA, suggesting that the heart contains ∼60% of PDGFR-α and ∼40% of PDGFR-β. The enhanced PDGFR is co-localized with PDGF-A and PDGF-D in the infarcted myocardium. These observations imply that PDGF-A and PDGF-D play a role in cardiac repair in an autocrine/paracrine manner. PDGFR-β mRNA and binding density are also significantly increased at the noninfarcted myocardium in the late stage of MI, which is correlated with elevated PDGF-D, further supporting the regulation of PDGF-D in the development of interstitial fibrosis.
In summary, the current study has explored the expression of PDGF isoforms and PDGFR in the infarcted rat heart. PDGF-A, PDGF-D and PDGFR were up-regulated in the infarcted myocardium, demonstrating the contribution of PDGF to cardiac repair and heart recovery. Increased PDGF-D and PDGFR in the noninfarcted myocardium suggests that PDGF-D is involved in the development of interstitial fibrosis. Further studies are required to determine whether blockade of PDGFR in the late stage of MI suppresses cardiac fibrosis and improves ventricular function.
Highlights.
Platelet-derived growth factor (PDGF) is a family of growth factors;
The study explored the involvement of PDGF in cardiac repair/remodeling postMI;
PDGF isofomes are differentially expressed in the infarcted heart;
PDGF-A/D and PDGFR were elevated in the infarcted heart;
We conclude that PDGF regulates myocardial remodeling.
Acknowledgments
This work was supported by NIH Heart, Blood, and Lung Institute (1RO1-HL096503, Yao Sun).
Footnotes
DISCLOSURES
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoch RV, Soriano P. Roles of PDGF in animal development. Development. 2003;130:4769–84. doi: 10.1242/dev.00721. [DOI] [PubMed] [Google Scholar]
- 2.Li H, Fredriksson L, Li X, Eriksson U. PDGF-D is a potent transforming and angiogenic growth factor. Oncogene. 2003;22:1501–10. doi: 10.1038/sj.onc.1206223. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Kong D, Banerjee S, Li Y, Adsay NV, Abbruzzese J, et al. Down-regulation of platelet-derived growth factor-D inhibits cell growth and angiogenesis through inactivation of Notch-1 and nuclear factor-kappaB signaling. Cancer Res. 2007;67:11377–85. doi: 10.1158/0008-5472.CAN-07-2803. [DOI] [PubMed] [Google Scholar]
- 4.Heldin CH, Westermark B. Platelet-derived growth factors: a family of isoforms that bind to two distinct receptors. Br Med Bull. 1989;45:453–64. doi: 10.1093/oxfordjournals.bmb.a072334. [DOI] [PubMed] [Google Scholar]
- 5.Taylor CC. Platelet-derived growth factor activates porcine thecal cell phosphatidylinositol-3-kinase-Akt/PKB and ras-extracellular signal-regulated kinase-1/2 kinase signaling pathways via the platelet-derived growth factor-beta receptor. Endocrinology. 2000;141:1545–53. doi: 10.1210/endo.141.4.7415. [DOI] [PubMed] [Google Scholar]
- 6.Karvinen H, Rutanen J, Leppanen O, Lach R, Levonen AL, Eriksson U, et al. PDGF-C and -D and their receptors PDGFR-alpha and PDGFR-beta in atherosclerotic human arteries. Eur J Clin Invest. 2009;39:320–7. doi: 10.1111/j.1365-2362.2009.02095.x. [DOI] [PubMed] [Google Scholar]
- 7.Perez J, Torres RA, Rocic P, Cismowski MJ, Weber DS, Darley-Usmar VM, et al. PYK2 Signaling is required for PDGF-dependent vascular smooth muscle cell proliferation. Am J Physiol Cell Physiol. doi: 10.1152/ajpcell.00315.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trojanowska M. Role of PDGF in fibrotic diseases and systemic sclerosis. Rheumatology (Oxford) 2008;47 (Suppl 5):v2–4. doi: 10.1093/rheumatology/ken265. [DOI] [PubMed] [Google Scholar]
- 9.Ross R, Bowen-Pope DF, Raines EW. Platelet-derived growth factor and its role in health and disease. Philos Trans R Soc Lond B Biol Sci. 1990;327:155–69. doi: 10.1098/rstb.1990.0051. [DOI] [PubMed] [Google Scholar]
- 10.Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004;15:255–73. doi: 10.1016/j.cytogfr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. [is this I correct?]Paracrine cells important in health and disease. Am J Physiol. 1999;277:C1–9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 12.Kinnman N, Francoz C, Barbu V, Wendum D, Rey C, Hultcrantz R, et al. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest. 2003;83:163–73. doi: 10.1097/01.lab.0000054178.01162.e4. [DOI] [PubMed] [Google Scholar]
- 13.Tangkijvanich P, Santiskulvong C, Melton AC, Rozengurt E, Yee HF., Jr p38 MAP kinase mediates platelet-derived growth factor-stimulated migration of hepatic myofibroblasts. J Cell Physiol. 2002;191:351–61. doi: 10.1002/jcp.10112. [DOI] [PubMed] [Google Scholar]
- 14.McGowan SE, Grossmann RE, Kimani PW, Holmes AJ. Platelet-derived growth factor receptor-alpha-expressing cells localize to the alveolar entry ring and have characteristics of myofibroblasts during pulmonary alveolar septal formation. Anat Rec (Hoboken) 2008;291:1649–61. doi: 10.1002/ar.20764. [DOI] [PubMed] [Google Scholar]
- 15.Borkham-Kamphorst E, van Roeyen CR, Ostendorf T, Floege J, Gressner AM, Weiskirchen R. Pro-fibrogenic potential of PDGF-D in liver fibrosis. J Hepatol. 2007;46:1064–74. doi: 10.1016/j.jhep.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Czochra P, Klopcic B, Meyer E, Herkel J, Garcia-Lazaro JF, Thieringer F, et al. Liver fibrosis induced by hepatic overexpression of PDGF-B in transgenic mice. J Hepatol. 2006;45:419–28. doi: 10.1016/j.jhep.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Floege J, Eitner F, Alpers CE. A new look at platelet-derived growth factor in renal disease. J Am Soc Nephrol. 2008;19:12–23. doi: 10.1681/ASN.2007050532. [DOI] [PubMed] [Google Scholar]
- 18.Shih AH, Holland EC. Platelet-derived growth factor (PDGF) and glial tumorigenesis. Cancer Lett. 2006;232:139–47. doi: 10.1016/j.canlet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Cleutjens JP, Diaz-Arias AA, Weber KT. Cardiac angiotensin converting enzyme and myocardial fibrosis in the rat. Cardiovasc Res. 1994;28:1423–32. doi: 10.1093/cvr/28.9.1423. [DOI] [PubMed] [Google Scholar]
- 20.Weber KT, Sun Y, Cleutjens JP. Structural remodeling of the infarcted rat heart. Exs. 1996;76:489–99. doi: 10.1007/978-3-0348-8988-9_30. [DOI] [PubMed] [Google Scholar]
- 21.Zhao W, Zhao D, Yan R, Sun Y. Cardiac oxidative stress and remodeling following infarction: role of NADPH oxidase. Cardiovasc Pathol. 2009;18:156–66. doi: 10.1016/j.carpath.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez Rosas M. Cardiac remodeling and inflammation. Arch Cardiol Mex. 2006;76 (Suppl 4):S58–66. [PubMed] [Google Scholar]
- 23.Nah DY, Rhee MY. The inflammatory response and cardiac repair after myocardial infarction. Korean Circ J. 2009;39:393–8. doi: 10.4070/kcj.2009.39.10.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao T, Zhao W, Chen Y, Ahokas RA, Sun Y. Acidic and basic fibroblast growth factors involved in cardiac angiogenesis following infarction. Int J Cardiol. doi: 10.1016/j.ijcard.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Y, Weber KT. Angiotensin II receptor binding following myocardial infarction in the rat. Cardiovasc Res. 1994;28:1623–8. doi: 10.1093/cvr/28.11.1623. [DOI] [PubMed] [Google Scholar]
- 26.Zhao W, Zhao T, Chen Y, Ahokas RA, Sun Y. Reactive oxygen species promote angiogenesis in the infarcted rat heart. Int J Exp Pathol. 2009;90:621–9. doi: 10.1111/j.1365-2613.2009.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 28.Sun Y, Weber KT. Infarct scar: a dynamic tissue. Cardiovasc Res. 2000;46:250–6. doi: 10.1016/s0008-6363(00)00032-8. [DOI] [PubMed] [Google Scholar]
- 29.Weber KT, Sun Y, Tyagi SC, Cleutjens JP. Collagen network of the myocardium: function, structural remodeling and regulatory mechanisms. J Mol Cell Cardiol. 1994;26:279–92. doi: 10.1006/jmcc.1994.1036. [DOI] [PubMed] [Google Scholar]
- 30.Kumagai S, Ohtani H, Nagai T, Funa K, Hiwatashi NO, Shimosegawa, et al. Platelet-derived growth factor and its receptors are expressed in areas of both active inflammation and active fibrosis in inflammatory bowel disease. Tohoku J Exp Med. 2001;195:21–33. doi: 10.1620/tjem.195.21. [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi H, Kanzawa T, Kondo Y, Kondo S. Inhibition of platelet-derived growth factor signalling induces autophagy in malignant glioma cells. Br J Cancer. 2004;90:1069–75. doi: 10.1038/sj.bjc.6601605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taneda S, Hudkins KL, Topouzis S, Gilbertson DG, Ophascharoensuk V, Truong L, et al. Obstructive uropathy in mice and humans: potential role for PDGF-D in the progression of tubulointerstitial injury. J Am Soc Nephrol. 2003;14:2544–55. doi: 10.1097/01.asn.0000089828.73014.c8. [DOI] [PubMed] [Google Scholar]
- 33.McKaig BC, Hughes K, Tighe PJ, Mahida YR. Differential expression of TGF-beta isoforms by normal and inflammatory bowel disease intestinal myofibroblasts. Am J Physiol Cell Physiol. 2002;282:C172–82. doi: 10.1152/ajpcell.00048.2001. [DOI] [PubMed] [Google Scholar]
- 34.Shim AH, Liu H, Focia PJ, Chen X, Lin PC, He X. Structures of a platelet-derived growth factor/propeptide complex and a platelet-derived growth factor/receptor complex. Proc Natl Acad Sci U S A. 107:11307–12. doi: 10.1073/pnas.1000806107. [DOI] [PMC free article] [PubMed] [Google Scholar]