Abstract
Transient cerebral ischemia dramatically activates small ubiquitin-like modifier (SUMO2/3) conjugation. In cells exposed to 6 h of transient oxygen/glucose deprivation (OGD), a model of ischemia, SUMOylation increases profoundly between 0 and 30 min following re-oxygenation. To elucidate the effect of transient OGD on SUMO conjugation of target proteins, we exposed neuroblastoma B35 cells expressing HA-SUMO3 to transient OGD and used stable isotope labeling with amino acids in cell culture (SILAC) to quantify OGD-induced changes in levels of specific SUMOylated proteins. Lysates from control and OGD-treated cells were mixed equally, and HA-tagged proteins were immunoprecipitated and analyzed by 1D-SDS-PAGE-LC-MS/MS. We identified 188 putative SUMO3-conjugated proteins, including numerous transcription factors and coregulators, and PIAS2 and PIAS4 SUMO ligases, of which 22 were increased or decreased >±2-fold. In addition to SUMO3, the levels of protein-conjugated SUMO1 and SUMO2, as well as ubiquitin, were all increased. Importantly, protein ubiquitination induced by OGD was completely blocked by gene silencing of SUMO2/3. Collectively, these results suggest several mechanisms for OGD-modulated SUMOylation, point to a number of signaling pathways that may be targets of SUMO-based signaling and recovery from ischemic stress, as well as demonstrate a tightly controlled crosstalk between the SUMO and ubiquitin conjugation pathways.
Keywords: cerebral ischemia, oxygen/glucose deprivation, quantitative proteomics, SILAC, small ubiquitin-like modifier, stress response, SUMOylation, ubiquitin conjugation
INTRODUCTION
Small ubiquitin-like modifier (SUMO1–3) are ubiquitin-related proteins that post-translationally modify lysine residues of target proteins in a process similar to ubiquitin conjugation, which involves SUMO-specific activating, conjugating and ligating enzymes.1 SUMO2 and SUMO3 proteins share about 95% sequence identity. Since available antibodies cannot distinguish between SUMO2 and SUMO3, these SUMO paralogues are usually referred to as SUMO2/3. SUMOylation is a highly reversible process as SUMO-conjugated proteins are rapidly de-conjugated by sentrin-specific proteases (SENPs).2, 3 SUMO conjugation has been shown to modulate stability, activity and subcellular localization of proteins.1, 2, 4, 5 A large number of SUMOylated proteins are transcription factors and other nuclear proteins involved in gene expression and genome stability.6, 7 Furthermore, many SUMO target proteins have been identified in neurons that are cytosolic or cell membrane proteins.8 Therefore, any substantial change in levels of SUMO-conjugated proteins can be expected to have a major impact on the fate of cells.
SUMO conjugation is activated in various stress conditions, including hypoxia, hypo-/hyperthermia, and oxidative stress.9 The SUMOylation pathway is massively activated in hibernating animals during the torpor state when the body temperature drops to about 5°C.10 During hibernation torpor, cerebral blood flow is reduced to below detection levels, but neurons are not damaged as they would be by an episode of transient normothermic ischemia.11 Since protein synthesis is almost completely suppressed during hibernation torpor12, it is postulated that SUMO conjugation is a protective stress response shielding neurons from damage induced by transient ischemia.10 We and others have shown that SUMO2/3 conjugation is also sharply activated after global and focal cerebral ischemia and during deep hypothermic cardiopulmonary bypass13–16, further suggesting that SUMO2/3 conjugation is important for neuroprotective stress responses. After transient focal cerebral ischemia, levels of SUMO2/3 conjugated proteins are particularly high in neurons located at the border of the ischemic territory, and we have demonstrated that a short non-lethal duration of vascular occlusion is sufficient to activate this process.14 Furthermore, we found that SUMO2/3 conjugation protects neuronal cultures from transient oxygen/glucose deprivation (OGD)-induced damage.17 It is therefore of key clinical interest to identify proteins that are SUMOylated after ischemia in order to better understand the significance of this process for the fate of post-ischemic cells.
OGD is a widely used experimental approach to model the severe form of metabolic stress triggered by transient cerebral ischemia in vivo, and OGD has been previously used to investigate the role of SUMO conjugation in ischemic cell death.18, 19 Here, we sought a tractable system for investigating post-ischemic activation of SUMO conjugation of target proteins. Due to the low abundance and difficulty of enriching for endogenous SUMOylated proteins, we utilized neuroblastoma B35 cells stably expressing mouse HA-tagged SUMO3, and we took advantage of a stable isotope labeling with amino acids in cell culture (SILAC) approach to quantify OGD-dependent changes in SUMO conjugation. The result is the first proteomic study to investigate the changes in SUMO conjugation in cells exposed to ischemia-like conditions.
EXPERIMENTAL SECTION
Cell culture and transfection
Experiments were performed on neuroblastoma B35 cells (courtesy of Dr. P.F. Maness, University of North Carolina, Chapel Hill, USA). HA-tagged mouse SUMO3 expression vector was generated by cloning SUMO3 cDNA derived from mouse mRNA into pcDNA3-HA vector (Invitrogen). After verification of construct by DNA sequencing, B35 cells were stably transfected with HA-SUMO3 expression vector using Geneticin (500 μg/mL) for selection. For SILAC analyses, cells were cultured in lysine- and arginine-deficient DMEM (Pierce) containing 10% dialyzed FBS (Sigma) and 1% penicillin/streptomycin/fungizone (Invitrogen), supplemented with 10 mg/L L-Proline (Sigma) and either 50 mg/L L-Arg and L-Lys (Sigma) for light medium or 50 mg/L 15N4/13C6-Arg and 15N6/13C2-Lys (Sigma) for heavy medium. Cells were cultured for eight passages to allow complete incorporation of heavy amino acids, as verified by MS analysis.
Oxygen/glucose deprivation (OGD) and protein extraction
Cultures were exposed to OGD for 6 h or 8 h using an anoxic chamber (Forma Scientific Anaerobic System). Glucose-free balanced salt solution (BSS) (116 mM NaCl, 1.8 mM CaCl2, 0.8 mM MgSO4, 5.4 mM KCl, 1 mM NaH2PO4, 14.7 mM NaHCO3, and 10 mM HEPES, pH7.4) was equilibrated overnight in the anoxic chamber with the anoxic gas mixture (85% N2, 10% H2, 5% CO2, palladium catalyst turned on). Cultures were then transferred to the anoxic chamber, and cells were washed 3 times with anoxic BSS buffer. After OGD treatment, anoxic medium was replaced with growth medium and cells were returned to the incubator equilibrated with 95% air and 5% CO2 for reoxygenation. To prepare protein extracts, at the end of experiments, cells from control and post-OGD cultures were lysed with lysis buffer (50 mM β-glycerophosphate, 1 mM EDTA, 1 mM EGTA, 0.5 mM Na3VO4, 1% Triton X-100, pH 7.4) supplemented with 1% SDS to block SENP-induced desumoylation.20 To further reduce the risk of desumoylation during protein extraction, samples were homogenized by a short sonication for 10 seconds followed by heating to 95°C for 10 minutes. Protein concentration of extracts was analyzed using the BCA protein assay (Thermo Scientific).
Analysis of cell death
The extent of OGD-induced cell death was evaluated by measuring the release of LDH from cells using the LDH Cytotoxicity Detection Kit (Clontech). The extent of cell death was calculated by relating LDH released from cells to total LDH activity.
Western blotting analysis
Western blotting was performed using SDS-PAGE gels (Bio-Rad). Proteins were transferred to PVDF membranes (Bio-Rad), and membranes were blocked for 1 hour in Tris-buffered saline solution supplemented with 0.1% Tween 20 (TBST) and 5% skim milk powder, and incubated with the first antibody for 16 h at 4°C. Membranes were then washed and incubated with goat anti-rabbit or -mouse horseradish peroxidase conjugates (Santa Cruz Biotechnology) for 1 h at room temperature. Proteins were visualized using the ECL Western blot analysis system (GE Healthcare). Antibodies used in this study include anti-SUMO2/3 polyclonal antibody (Covance), anti-HA polyclonal antibody (Cell Signaling), anti-ubiquitin monoclonal antibody (Cell Signaling), anti-TIF1β polyclonal antibody (Cell Signaling), and anti-RUNX1 polyclonal antibody (Novus Biologicals). A monoclonal antibody against β-actin (dilution 1:5000; Sigma) was used as loading control.
Proteomic analysis
Five 10 cm dishes of cells were used per group. Fifteen mg of protein from control (light) and post-OGD (heavy) cultures were mixed, and HA-SUMO3-conjugated proteins were immunoprecipitated using monoclonal anti-HA agarose (Sigma). Beads were washed and HA-tagged SUMO3 conjugated proteins eluted by incubating beads with PBS buffer containing 100 μg/mL HA peptide (Sigma). Proteins were precipitated with acetone, and precipitates were dissolved in SDS loading buffer and separated approximately 1 cm on a SDS-PAGE 4%–12% gel (Invitrogen). The gel was stained briefly with colloidal Coomassie (Invitrogen), and the protein-containing region was dissected into 5 adjacent slices. The excised gel slices were destained, and the proteins in the slices were reduced, alkylated, and digested with trypsin according to the “In-Gel Tryptic Digestion Protocol” available at (http://www.genome.duke.edu/cores/proteomics/sample-preparation/). Briefly, slices were destained with 1:1 MeCN:water, then dehydrated in MeCN and swelled in 50 mM ammonium bicarbonate (AmBic) containing 10 mM dithiothreitol (Sigma) for reduction at 80°C for 30 min, followed by alkylation with 20 mM iodoacetamide (Sigma) at room temperature for 20 min in the dark. Gel pieces were then taken through two shrink/swell cycles alternating acetonitrile (Fisher Scientific) and AmBic, and finally swelled in AmBic containing 10 ng/μl trypsin (Promega). Digestion was carried out overnight at 37°C and was quenched and peptides extracted using 0.1% vol/vol TFA in 1:1 MeCN:water. Samples were dried and reconstituted in 10 μL 1:2:97 vol:vol:vol TFA:MeCN:water for mass spectrometry analysis.
Five microliters of each sample were injected onto a 75 μm × 250 mm BEC C18 column (Waters) and separated using a gradient of 5% to 40% (vol:vol) acetonitrile with 0.1% (vol:vol) formic acid, with flow rate of 0.3 μL/min for 90 min on a nanoAcquity liquid chromatograph (Waters). The eluent was introduced to an LTQ-Orbitrap hybrid mass spectrometer (Thermo) using a nanoelectrospray interface. The Orbitrap MS/MS method used collisionally-induced dissociation (CID) fragmentation for peptide identification with both precursor and product ions being measured in the Orbitrap. Briefly, the precursor scan method used profile mode and 60000 resolution with AGC target of 1e6 and 1 microscan. MS/MS acquisition was performed on the top three precursor ions above a 5000-count threshold using CID with a 3 Da isolation window, normalized collision energy of 35%, and 1 microscan. Product ion spectra were collected in profile mode with a resolution of 7500 and AGC target setting of 2e5. Dynamic exclusion settings were: repeat count = 3, repeat duration = 30 sec, exclusion list = 250, and exclusion time = 120 seconds.
All data was imported into Rosetta Elucidator v3.3 (Rosetta Biosoftware, Inc) for quantitative analysis using the multidimensional labeled-pair pipeline. Feature identification and quantification was performed using the PeakTeller algorithm, and SILAC labeled-pairs were located with 15 ppm m/z tolerance and 0.2 min retention time tolerance allowing a maximum of 3 labels per peptide, either Lys + 8.014 Da or Arg + 10.008 Da. Database searching was initiated from Rosetta Elucidator with Mascot v2.2 (Matrix Sciences, Inc) against the NCBI RefSeq Rattus database (October 2010, 25278 entries). The database was first deduplicated and reversed using Protein Digestion Simulator v2.238 (http://ncrr.pnnl.gov/software/). Mascot searching was performed with the following search parameters: 10 ppm precursor and 0.02 Da product ion mass accuracy, tryptic enzyme specificity, a maximum of two missed cleavages, carbamidomethyl (C) as a fixed modification and oxidized (M), deamidated (NQ), 13C2 15N6 label (K), and 13C6 15N4 label (R) as variable modifications. Peptide annotations were performed at a 1% FDR after forward/reverse decoy database validation using the PeptideProphet implementation in Elucidator.21, 22 SILAC quantification at the peptide level was performed by calculating the peak heights of the most abundant ion in the isotope cluster and rationing between heavy and light pairs. Protein-level quantification was performed at the individual gel band level or at the aggregate experiment level by summing up the light or heavy intensities for each peptide to a protein, then using these summed values to obtain the protein heavy to light ratio for the specific band or the aggregate. P-values were calculated according to an error model as previously described.23, 24 Peptide and protein identifications and their respective quantitative values are included in Supplementary Table S1
Gene Ontology (GO) and Pathway Analyses
Gene Ontology (GO) terms for the proteins isolated via SUMO3 pulldown were annotated with the db2db tool in BioDBnet (http://biodbnet.abcc.ncifcrf.gov/), using the protein GI number as the input. We utilized Ingenuity Pathway Analysis v9.0 (https://analysis.ingenuity.com/) to assist with biological contextualization of the upregulated, putatively SUMOylated proteins as a function of OGD. All proteins in Table S1 which were upregulated at least 2-fold in one or more molecular weight fractions were input, with fold-changes, into IPA software.
Data deposition
All tandem mass spectra along with identifications are available as a Scaffold V3.0 (www.proteomesoftware.com) file at the following link: https://discovery.genome.duke.edu/express/resources/2153/OGD_SILAC_upload_021611.sf3.
SUMO Protease treatment
To identify bona fide SUMO-conjugated proteins, HA-tagged SUMO3 conjugated proteins from the same amount of extracts of light control and heavy OGD cultures (6 h OGD with 30 min reoxygenation) were separately immunoprecipitated using monoclonal anti-HA agarose (Sigma). Eluates from individual OGD experiment were incubated with or without SUMO protease 2 (LifeSensors) in PBS buffer pH 7.4 for 1 h at 30 °C and analyzed by Western blotting. Verifications were confirmed using biological triplicates.
SUMO2/3 gene silencing
B35 cells stably transfected with constructs expressing control miRNA (miR-Neg; a miRNA sequence not related to any mammalian gene) or SUMO2/3 miRNA (miR-SUMO2/3) were used as described previously.25
RESULTS
Oxygen/glucose deprivation activates SUMO2/3 conjugation
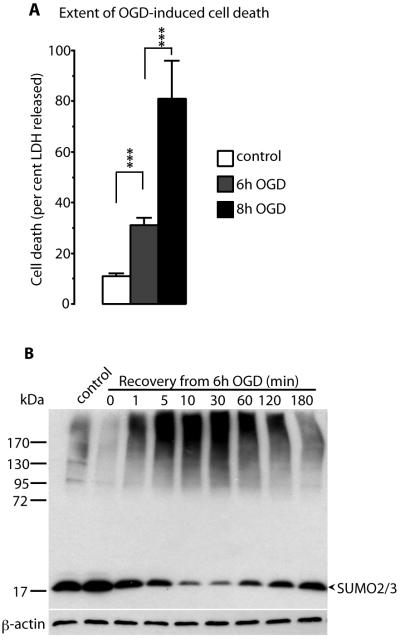
We first examined optimal conditions for investigating oxygen/glucose deprivation (OGD)-induced protein SUMOylation in a rat neuroblastoma cell line. B35 cells were exposed to 6 or 8 h of OGD, and the extent of OGD-induced cell death was evaluated after 22 h of recovery by measuring the release of LDH from cells/total LDH activity (fractional LDH release). In control cultures not exposed to OGD, fractional LDH release amounted to 11±1%, and it increased to 31±3 and 81±15% when cells were exposed to 6 h or 8 h of OGD, respectively (Figure 1A). We chose the 6 h time point for further studies because it induced only minor cell damage. To determine whether a rise in levels of SUMO2/3-conjugated proteins occurred following OGD, cells were exposed to 6 h of OGD and up to 180 min of recovery, and changes in levels of SUMOylated proteins were evaluated by Western blotting analysis (Figure 1B). As expected, levels of SUMO2/3 conjugated proteins declined during OGD, because SUMO conjugation is an energy-requiring process. During recovery from OGD, SUMO2/3 conjugation was markedly activated, as indicated by a massive increase in the smear of bands at high molecular weight and a considerable decrease in levels of free SUMO2/3 (Figure 1B, band at about 17 kDa). This is a pattern similar to that found after transient cerebral ischemia.14, 15 Since activation of SUMO2/3 conjugation was most pronounced after 30 min of re-oxygenation in B35 cells, we decided to employ this time point for proteomic analysis.
Figure 1.
Transient oxygen/glucose deprivation (OGD) activates SUMO2/3 conjugation and induces cell death. (A) B35 cells were exposed to 6 h or 8 h of OGD and 22 h of recovery. Cell death was evaluated by measuring the release of LDH from cells and relating LDH released from cells to total LDH activity. Data are presented as means ± SD (n=3). Statistically significant differences between groups were evaluated by ANOVA followed by Fisher's PLSD test; *** p≤0.001. (B) B35 neuroblastoma cells were exposed to 6 h of OGD and 0–180 min of recovery. Proteins were extracted as described in Experimental Section, and OGD-induced changes in levels of SUMO2/3 conjugated proteins were evaluated by Western blotting. Free SUMO2/3 is indicated by arrowhead.
Proteomic analysis identified modulation of numerous SUMO substrates by OGD
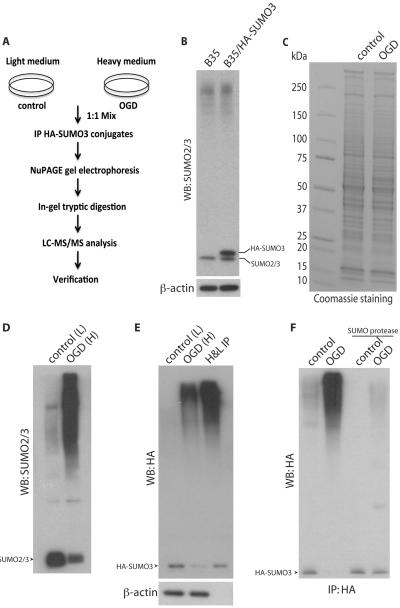
The proteomic approach to analyze OGD-induced changes in SUMO3 conjugation is summarized in Figure 2A. A stable B35 cell line expressing HA-SUMO3 in which overexpression of HA-SUMO3 produced the similar pattern of SUMO2/3 conjugation compared to untransfected B35 cells was selected for this study (Figure 2B). B35 cells stably expressing HA-SUMO3 were grown in light or heavy Lys- and Arg-supplemented DMEM. Cells were first cultured for 8 passages, and mass spectrometry (MS) analysis revealed complete incorporation of heavy lysine and arginine and no evidence of proline conversion in the heavy cells (data not shown). Extracts were prepared from control (light) and post-OGD (heavy; 6 h OGD with 30 min reoxygenation) cultures, which included lysis in buffer containing 1% SDS as well as heating at 95 °C to both inhibit endogenous SUMO proteases and to disrupt protein-protein interactions that might otherwise result in the adventitious precipitation of non-sumoylated proteins. Protein concentrations were measured and adjusted to identical concentrations, and both extracts were mixed for immunoprecipitation of HA-tagged SUMO3 conjugated proteins and subsequent quantitative proteomic analysis. We confirmed the measured protein concentrations of control and post-OGD extracts by SDS-PAGE electrophoresis and Coomassie staining (Figure 2C) and examined SUMO2/3 levels in control and OGD extracts from these HA-SUMO3-expressing cells. As observed previously, transient OGD resulted in a decrease in free SUMO2/3 and a robust increase in high molecular weight (MW) SUMO2/3 (Figure 2D); and as expected, the marked increase in conjugation of HA-tagged SUMO3 following transient OGD was evident in Western blot using anti-HA antibody and anti-HA immunoprecipitates were enriched for high MW HA-tagged proteins indicative of protein sumoylation (Figure 2E). The presence of SUMO3-conjugated proteins in immunoprecipates of control and OGD extracts were also verified by incubation with SUMO protease 2 to cleave SUMO3-protein conjugates (Figure 2F). After incubation with SUMO protease 2, the anti-HA-reactive high MW smear almost completely disappeared. Levels of SUMO2/3 conjugated proteins were not changed in cells expressing HA-SUMO3 compared to parent B35 cells (Figure 2F).
Figure 2.
SUMO proteomic analysis. (A) Scheme of experimental procedures. Cells were cultured in light (control) or heavy (OGD) medium, proteins extracted and identical amounts mixed. HA-tagged SUMO3 conjugated proteins were immunoprecipitated and separated by SDS-PAGE. After in-gel tryptic digestion, samples were analyzed using LC-MS/MS. Proteomics data were verified for individual proteins by immunoprecipitation of control and OGD extracts followed by SUMO protease exposure and immunoblotting using the appropriate antibodies. (B) In the B35/HA-SUMO3 stable cell line used in this study, overexpression of HA-SUMO3 did not result in a global increase in levels of SUMO conjugated proteins. To verify that identical protein levels of extracts from light and heavy cultures were mixed, samples were loaded onto a SDS-PAGE gel and proteins were visualized by Coomassie staining (C). Extracts from control (L) and OGD (H) samples and anti-HA immuoprecipitates from equal amounts of combined extracts were separated by SDS-PAGE and detected with anti-SUMO2/3 antibody (D) and anti-HA antibody (E) by Western blotting (WB). (F) HA-SUMO3 conjugated proteins were purified from control and OGD extracts separately and incubated with or without SUMO protease. Samples were subjected to Western blot analysis with anti-HA antibody. Free SUMO is indicated by arrowhead. IP, immunoprecipitation.
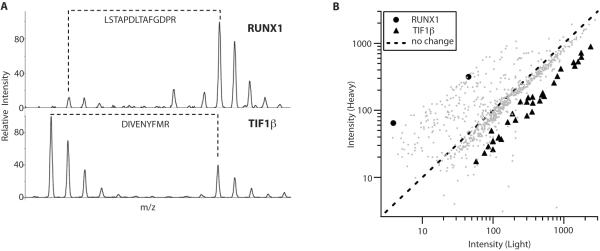
To improve depth of coverage and, in particular, to resolve free SUMO and SUMO-conjugated proteins, we separated the SILAC-encoded immunoprecipate by a short SDS-PAGE separation and excised five contiguous gel slices for in-gel trypsinization. 1D-LC-MS/MS analysis was performed on peptides recovered from each of these five gel slices, and putative SUMO-conjugated proteins were identified and quantified using Rosetta Elucidator (Figure 3 and Methods). Overall, 939 peptides to 240 unique proteins were identified. After peptide identification and quantification, 880 of these peptides were found to belong to a total of 624 SILAC pairs. These 624 peptide ratios resulted in the quantification of 188 proteins in control versus OGD immunoprecipitates (Table 1 and supplementary Table S1), including runt-related transcription factor 1 (RUNX1), and transcription intermediary factor 1β (TIF1β/TRIM28/KAP1), which were quantified by 2 and 27 SILAC pairs, respectively (Figure 3). The numbers of proteins quantified from Slice 1 (high molecular weight) to Slice 5 (low molecular weight) were 35, 81, 81, 81, and 16, respectively.
Figure 3.
Mass spectrometry-based quantification of SUMO conjugation via SILAC approach. . (A) Representative SILAC peptide pairs for an upregulated protein (RUNX1) and a downregulated protein (TIF1β). Peptide identifications are included in the figure, and associated m/z and intensity information in Table S1. (B) Non-normalized ratio plot including all quantified peptides in the experiment, with 2 peptides from RUNX1 and 31 peptides from TIF1β in black and peptides from the other 186 proteins in grey.
Table 1.
Most up- and down- regulated putative SUMO3 targets after OGD treatment.
| GI Number | Protein Description | Peptides | Ratios | Overall Fold Change (Heavy/Light) | P-value | Ratio by MW |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Slice 1 | Slice 2 | Slice 3 | Slice 4 | Slice 5 | ||||||
| 8392900 | runt-related transcription factor 1 | 2 | 2 | 7.8 | 1.0E−01 | 7.8 | ||||
| 13540673 | DNA ligase 1 isoform 1 | 3 | 2 | 7.1 | 4.4E−02 | 8.1 | 5.9 | |||
| 158186698 | heterogeneous nuclear ribonucleoprotein M isoform a | 25 | 17 | 6.2 | 0.0E+00 | 1.1 | 6.4 | 5.0 | 6.6 | |
| 197387226 | helicase ARIP4 | 4 | 4 | 6.1 | 4.4E−06 | −1.2 | 3.9 | |||
| 158533978 | myelin expression factor 2 | 6 | 4 | 6.1 | 6.3E−11 | −2.6 | 6.2 | |||
| 199560324 | Ngfi-A binding protein 2 | 8 | 5 | 5.5 | 3.0E−20 | −1.0 | 5.6 | 5.0 | ||
| 198278571 | E3 SUMO-protein ligase PIAS4 | 3 | 3 | 5.4 | 5.2E-03 | 1.4 | 4.4 | |||
| 157819153 | interferon regulatory factor 2-binding protein 1 | 3 | 3 | 4.0 | 7.6E−02 | 4.0 | ||||
| 293354431 | PREDICTED: heterogeneous nuclear ribonucleoprotein A0 | 3 | 3 | 3.7 | 5.8E−03 | 6.7 | 3.9 | 2.2 | ||
| 16758050 | E3 SUMO-protein ligase PIAS2 | 4 | 3 | 3.6 | 5.3E−03 | 2.2 | 4.1 | 1.6 | ||
| 109459149 | PREDICTED: nuclear mitotic apparatus protein 1 isoform 2 | 9 | 7 | 3.3 | 5.3E−04 | 1.1 | 1.7 | |||
| 62078769 | heterogeneous nuclear ribonucleoprotein H2 | 2 | 2 | 3.1 | 3.0E−02 | 5.1 | 2.5 | 1.1 | ||
| 19705547 | nucleus accumbens-associated protein 1 | 6 | 2 | 3.1 | 1.1E−01 | 2.4 | 3.8 | 2.4 | ||
| 67078510 | transcription factor SOX-6 | 2 | 2 | 3.0 | 2.2E−01 | 3.0 | ||||
| 57528279 | small ubiquitin-related modifier 1 precursor | 4 | 2 | 2.8 | 2.5E−02 | −18.8 | 2.2 | |||
| 61889073 | matrin-3 | 3 | 3 | 2.5 | 2.4E−01 | 1.6 | 2.3 | |||
| 58865526 | TAR DNA binding protein | 4 | 2 | 2.5 | 1.4E−01 | 5.6 | 2.6 | 1.3 | ||
| 109483564 | PREDICTED: promyelocytic leukemia-like | 12 | 8 | 2.4 | 7.9E−18 | −1.8 | 2.5 | 2.1 | ||
| 209870077 | transcription intermediary factor 1-beta | 31 | 27 | −2.8 | 0.0E+00 | −1.0 | −2.8 | −3.2 | ||
| 293356056 | PREDICTED: remodeling and spacing factor 1 | 7 | 6 | −3.6 | 4.4E−03 | −3.6 | ||||
| 56606080 | bromodomain-containing protein 8 | 3 | 3 | −4.0 | 1.2E−01 | −4.0 | ||||
| 293342765 | PREDICTED: histone cluster 1, H2ae-like | 5 | 3 | −4.7 | 7.5E−02 | −5.6 | −5.4 | −1.6 | ||
Proteins with at least 2 quantitative ratios and an absolute fold change ≥2, with number of identified peptides, number of quantitative ratios used to calculate the fold change. The ratios at the aggregate and per-slice level (Slice 1: high molecular weight; Slice 5: low molecular weight), green/red coding for fold decrease/increase, respectively.
Gene ontology (GO) annotation demonstrated that a large fraction (92/174 annotated proteins) of quantified proteins were predicted to have nuclear localization, which is consistent with previous observations that sumoylation is predominantly a nuclear event.6, 7 In addition to SUMO proteins themselves (see below), many quantified proteins were previously characterized SUMO substrates, including TIF1β,26 LIG1,27 PIAS2 and PIAS4,28 and the transcription factors SOX-6 and SOX-10.29, 30 There were also several potentially novel SUMO substrates, including RUNX1, atrophin-1 and matrin-3.
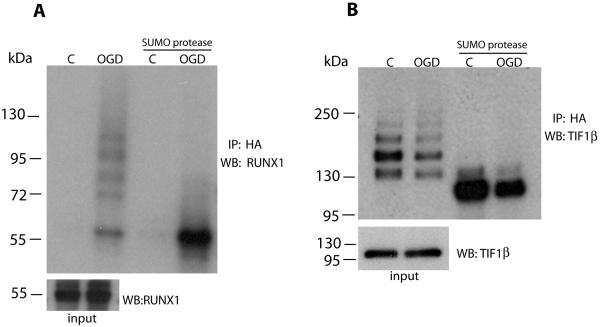
As expected based on immunoblotting of these samples (Figure 2) the overall distribution of all quantified SILAC pairs pointed to a significant increase in SUMO-conjugated proteins after OGD (Figure 3B). As criteria for determining which of the identified proteins were most robustly regulated following OGD, we required at least 2 quantitative ratios (thus at least two peptide identifications) and an absolute fold-change ≥2. Twenty-five proteins met these criteria, but the three keratins that were apparently “downregulated” (i.e. had light Lys and Arg only) were flagged as likely contaminants. Of the remaining 22 proteins (Table 1), 18 were increased and 4 decreased with OGD treatment. As an initial validation, we confirmed the increase in high MW RUNX1 and decrease in high MW TIF1β as a function of OGD (Figure 4) by Western blot analysis. In addition, the high MW forms of these proteins were abolished by treatment with a SUMO-specific protease, confirming SUMO conjugation.
Figure 4.
Verification of proteomic analysis. Proteomic analysis data were verified for RUNX1 (A) and TIF1β (B). HA-tagged SUMO3 conjugated proteins of extracts from control and OGD cultures were immunoprecipitated using anti-HA agarose and eluted with 100 μg/ml HA peptide. Eluates were left intact or incubated with SUMO protease to release SUMO from target proteins, loaded onto SDS-PAGE gels and immunoblotted using antibodies against RUNX1 (A) and TIF1β (B). WB, Western blotting; IP, immunoprecipitation.
We also retained quantitative information at a per-slice level (Table 1 and supplementary Table S1), which allowed us to potentially distinguish multiple SUMO-conjugated forms of the same protein and to differentiate free SUMO from protein-bound or polySUMO. Indeed, consistent with immunoblotting (Figure 2D, E) and our prior in vivo studies indicating decrease in levels of free SUMO2/3 after transient ischemia,14, 15 free SUMO3 appeared to be reduced after transient OGD, as evidenced by a ~2-fold decrease in SUMO3 in the lowest MW slice (Slice 5, Table S1). However, there was a gradual increase in SUMO3 with increase MW, including a greater than 4-fold increased in the highest MW fraction (Table S1). Thus, although there was only an apparent 1.3-fold increase in SUMO3 when the intensities of SILAC pairs were averaged across all fractions, an increase in high MW SUMO3 was clearly evident. Most of the proteins quantified were identified in more than one gel slice, consistent with heterogenous poly-SUMOylation (Table S1). Of the proteins that were overall >±2-fold changed post-OGD, several showed a greater degree of regulation in their high than low MW forms (Table 1). For example, the E3 SUMO-protein ligase PIAS2 was increased only 1.6-fold in slice 3 but was almost 7-fold increased in the highest MW slice. Thus, even the highly approximate MW data provided by SDS-PAGE fractionation can provide important information in the quantitative analysis of stimulus-induced SUMO conjugation.
Overall, HA-SUMO3 immunoprecipitates were enriched in SUMO paralogues and SUMO (E1, E2, E3) ligases. Despite SUMO3 being present at presumably much higher levels than the nearly identical SUMO2, or the less homologous (~50% identical) SUMO1, both of these SUMO paralogues were also identified in high MW fractions and were increased 2.5 to 3-fold after OGD, which is consistent with previous observations.7, 28 The quantity of immunoprecipitated SUMO E3 ligases PIAS2 and PIAS4 was also increased greatly after OGD. By analogy to PIAS1, which has been shown to be modified and activated by SUMO1, these modifications may further potentiate PIAS2- and PIAS4-dependent SUMOylation. On the other hand, OGD apparently decreased SUMO3-conjugation of the SUMO E1 subunit Sae1 and SUMO E2 ligase Ubc9 (Table S1). SUMO conjugation of Ubc9 on Lys14 has been shown to differentially affect target-specific sumoylation.31
Transcription factors (TFs), and co-repressors or enhancers, were among the putative SUMO substrates that were significantly modulated by OGD. The TFs RUNX1, NFAT5, SOX-6 and SOX-10 were all increased with OGD treatment, although SOX-10 and NFAT5 were identified by only one unique SILAC pair (both in two slices each; mean 5.8- and 4.5-fold increased with OGD, respectively). Of these, SUMO conjugation has been shown previously to represses the transcriptional activity of SOX-6 and SOX-10.29, 30 The apparent SUMOylation of numerous corepressors was modulated by OGD, including Ngfi-A binding protein 2, interferon regulatory factor 2-binding protein 1, nucleus accumbens-associated protein 1, TIF1β/TRIM28 and bromodomain-containing protein 8 (Table 1). Additional OGD-regulated SUMO substrates that did not meet strict significance criteria, included nuclear co-repressor 2, transcription enhancer factor 1, as well TIF1-α. SUMO conjugation of TIF1β is required for its transcriptional repression 26, and it is also worth noting that TIF1β itself is among TRIM proteins that possess SUMO E3 ligase activity.32
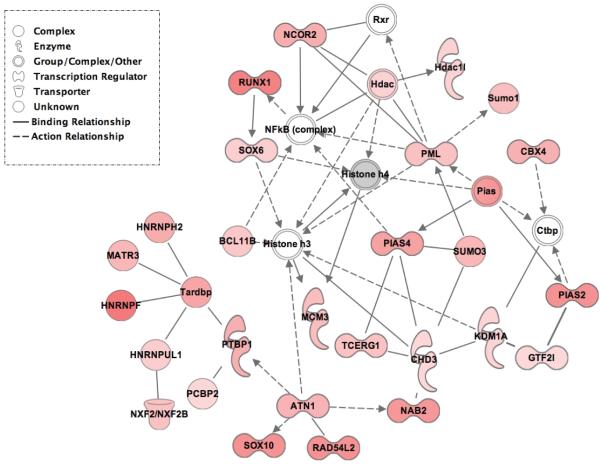
Ingenuity Pathway Analysis (IPA) was performed with proteins where SUMO3 conjugation was 2- or more fold up-regulated in any gel slice. The most significant pathway identified by IPA covers proteins playing key roles in post-translational protein modifications, gene expression and cell cycle (IPA score: 54; Figure 5). All of these proteins have predicted nuclear localization and include enzymes like histone deacetylase 1-like protein (Hdac1l; 2.74-fold), polypyrimidine tract binding protein (PTBP1; 4.9-fold), lysine-specific histone demethylase 1A (KDM1A; 2.67-fold) and transcription elongation regulator 1 (TCERG1; 3.758-fold), as well as transcription factors, such as runt-related transcription factor 1 (RUNX1; 7.8-fold), helicase ARIP4 (RAD54L2; 6.82-fold), Ngfi-A binding protein 2 (NAB2; 6.39-fold), transcription factors SOX-6 and SOX-10 (3.0- and 6.79-fold respectively), and nuclear receptor corepressor 2 (NCOR2; 5.1-fold). The observation that the most significant pathway identified by IPA covers a large proportion of the SUMO targets found in post-OGD cells and specifically involves nuclear proteins playing key roles in gene expression strongly implies that activation of SUMOylation modulates the fate of cells exposed to ischemia-like conditions. As expected, the most significant pathway identified by IPA did not cover proteins involved in cell damage, because we used a short period of OGD that induced only minor cell death.
Figure 5.
Ingenuity Pathway Analysis (IPA) was performed with proteins where SUMO3 conjugation was 2- or more fold up-regulated in any gel slice. The most significant pathway identified by IPA covers proteins playing key roles in post-translational protein modifications, gene expression and cell cycle control.
Ubiquitination is induced by OGD and depends on SUMO conjugation
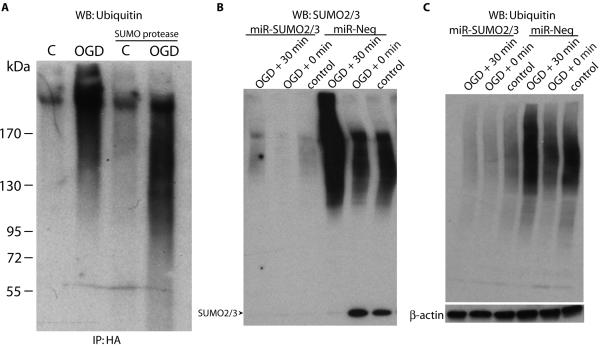
Ubiquitin peptides were quantified in almost all of the gel slices, and although ubiquitin was increased 1.7-fold overall with OGD treatment, it was >2-fold increased in several of the high MW gel slices. Indeed, a marked increase in ubiquitin-conjugated proteins was observed in HA immunoprecipitates by immunoblotting after OGD (Figure 6A). Furthermore, SUMO protease treatment increased the mobility of ubiquitinylated proteins by SDS-PAGE, indicative of a shift of these proteins to lower MW, although total ubiquitin did not appear to be substantially reduced. SUMO2/3 conjugates have been previously shown to be enriched for ubiquitin.33 Our data suggest that OGD-induced ubiquitination occurs primarily on the substrates themselves and not on protein-bound SUMO.
Figure 6.
Protein ubiquitination is increased after OGD and depends on SUMO expression. (A) Immunoprecipitates from control and OGD extracts were immunoblotted for ubiqutin, and SUMO protease was utilized to demonstrate SUMO conjugation as in Fig. 4. (B) SUMO2/3 and (C) ubiquitin levels in B35 cells stably expressing control or SUMO2/3 miRNA were analyzed by Western blotting in untreated cells and immediately following or 30 min after 6 h OGD. WB, Western blotting. IP, immunoprecipitation.
We further investigated a requirement for SUMO2/3 on ubiquitin conjugation in B35 cells expressing control miRNA (miR-Neg) or SUMO2/3 miRNA (miR-SUMO2/3). Expression of miR-SUMO2/3, but not miR-Neg, almost completely silenced SUMO2/3 expression in response to transient OGD (Figure 6B). In addition, the marked OGD-induced ubiquitination in miR-Neg-expressing cells was completely suppressed in cells expressing miR-SUMO2/3 (Figure 6C). This data demonstrates that SUMO2/3 is required for ubiquitination in response to transient OGD and suggests that SUMO2/3 conjugation precedes ubiquitination of SUMO substrates.
DISCUSSION
Accumulating evidence suggests that SUMO2/3 conjugation is important for neuroprotective stress responses under conditions of transient cerebral ischemia. However, the precise targets of SUMO2/3 under these conditions have not been previously identified. Here, we used a SILAC-based proteomic approach to identify SUMO3 targets and to quantify their modulation in an experimental model of transient ischemia. This study begins to address both the mechanisms by which OGD modulates protein SUMOylation and the potential functional consequences, including modulation of gene transcription, DNA repair and protein ubiquitination. While not novel, the advantages of a “GeLC” approach which preserves protein MW information, are particular evident for the analysis of SUMO (and other ubiquitin-related) modifications, as such an analysis can not only differentiate changes in free and protein-conjugated SUMO, but may in principal allow the resolution of stimulus/stress-coupled changes in polySUMO conjugation, either through alterations to SUMO chain length or the number of SUMO-modified Lys residues in a particular protein.
Although mechanisms for OGD-regulated SUMOylation are still unknown, we can speculate that it will require post-translational modification or differential expression of components of the SUMO conjugation machinery. For example, based on the identification of PIAS2 and PIAS4 as putative targets of OGD-induced SUMOylation, we hypothesize that the increased activity or expression of these E3 ligases may be important for global increases in SUMO conjugation. It will be interesting to determine the role of these PIAS isoforms in mediating OGD-dependent SUMO conjugation and whether they exhibit unique substrate specificities. Furthermore, since Ubc9 levels have been shown to positively correlate with the degree of SUMO conjugation,10 it would be surprising if the effects of OGD on Ubc9-SUMO were a consequence of reduced Ubc9 expression. Rather, SUMOylation of Ubc9 has been shown to regulate SUMO target discrimination and the attenuation of Ubc9-SUMO by OGD may therefore be important for target specificity.31 The reduction in SUMO3 conjugation of some proteins in response to OGD, including TIF1β, was somewhat unexpected given the overall global increase in SUMOylation. Phosphorylation of TIF1β on Ser824 has been shown to decrease SUMO-1 conjugation via the SUMO-specific protease SENP1.26 By analogy, transient OGD may induce similar phosphorylation and deSUMOylation of TIF1β. More generally, OGD-mediated phosphorylation may alter the association of SUMO ligases and proteases with their targets and might in part explain the modulation of SUMO3 conjugation by OGD.
It is well established that ubiquitin conjugation is markedly activated after both transient global or focal cerebral ischemia.34, 35 However, we have shown here for the first time that SUMO2/3 is required for OGD-induced protein ubiquitination. The interplay between the SUMO and ubiquitin systems is increasingly recognized: ubiquitin is identified in SUMO2/3 immunoprecipitates;36 ubiquitin-proteasome inhibition results in accumulation of SUMO2/3 conjugates;36 SUMO itself can be ubiquitinylated and SUMO E3 ligases can modulate ubiquitin conjugation.37 Furthermore, SUMO and ubiquitin can compete for the same lysine residue, as exemplified by the NFκB inhibitor protein IκBα, which can be either SUMO- or ubiquitin-conjugated at lysine K21.4 Although sumoylation can act as a signal for ubiquitination of proteins and degradation at the proteasome,37 we speculate that these modifications coordinate additional critical cellular processes post-OGD, including DNA damage repair, cell proliferation and apoptosis.38–40 However, further investigation is required to determine the significance of SUMO-dependent ubiquitination following transient OGD.
Numerous lines of evidence suggest that the alteration of SUMO conjugation following OGD is a protective stress response that helps cells to better withstand a transient period of impaired energy metabolism. For example, in a mouse model of stroke, we found SUMO2/3 conjugation to be particularly activated in post-ischemic neurons located at the border of the ischemic territory. A short duration of vascular occlusion, which is not expected to cause major cell damage, is sufficient to activate this process,15 suggesting that SUMOylation is not an instigator of cell or tissue damage. SUMO2 and SUMO3 has been shown to promote cell survival after heat shock.28 Further work is needed to determine the significance of the OGD-modulated SUMO substrates that have been identified in the present study. Additional complementary `omic approaches, include analysis of transcriptome and global proteome analysis, should also provide additional clarity as to the physiological significance of SUMOylation in cellular and animal models of transient cerebral ischemia.
CONCLUSIONS
We demonstrate here for the first time how SUMO conjugation of target proteins is modified when cells are exposed to transient OGD, ischemia-like stress conditions, using SILAC-based quantitative proteomics analysis. Targets where the extent of SUMO conjugation was massively activated were predominantly nuclear proteins involved in gene expression. Once tools are avaible permitting proteomic analysis of endogenous SUMOylated proteins using brain tissue samples the results presented here will provide an important platform to investigate in detail the role of SUMO conjugation brains stressed by a transient interruption of blood supply. This will allow to designing new avenues of therapeutic intervention for patients suffering from stroke or cardiac arrest followed by resuscitation. Our observation that OGD-induced activation of ubiquitin conjugation was almost completely blocked when SUMO2/3 conjugation was suppressed by silencing their expression suggests that activation of SUMO2/3 conjugation is required for activation of ubiquitin conjugation in OGD-stressed cells. Considering the various signal transduction pathways that modulate ubiquitin and SUMO conjugation and control key cellular functions, including cell proliferation, apoptosis, and DNA damage repair, the interplay between ubiquitin and SUMO conjugation may play a more prominent role than previously anticipated.
Supplementary Material
ACKNOWLEDGEMENT
This research was supported by RO1 grant HL095552 from the National Institutes of Health and by funds from the Department of Anesthesiology, Duke University Medical Center. The excellent technical support of Meredith Turner and Pei Miao is gratefully acknowledged. We also gratefully acknowledge Prof. Robert Lefkowitz for the use of the LTQ-Orbitrap mass spectrometer.
ABBREVIATIONS
- CID
collisionally-induced dissociation
- HNRNP H2
heterogeneous nuclear ribonucleoprotein H2
- IPA
Ingenuity Pathway Analysis
- IP
immunoprecipitation
- LDH
lactate dehydrogenase
- miR
microRNA
- MS
mass spectrometry
- OGD
oxygen/glucose deprivation
- PIAS
protein inhibitor of activated signal transducer and activator of transcription
- PML
promyelotic leukemia protein
- RUNX1
runt-related transcription factor 1
- SENP
sentrin-specific protease
- SILAC
stable isotope labeling with amino acids in cell culture
- SOX
SRY-box containing gene
- SUMO
small ubiquitin-like modifier
- TIF1β
transcription intermediary factor 1-beta
- Ubc9
ubiquitin conjugating enzyme 9 (the only SUMO conjugating enzyme identified so far)
- WB
Western blotting
Footnotes
Supporting Information
List of all putative SUMO3 targets identified by SILAC-based quantitative proteomics including details for mass spectrometry analysis. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Hay RT. SUMO: a history of modification. Mol Cell. 2005;18(1):1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Hay RT. SUMO-specific proteases: a twist in the tail. Trends Cell Biol. 2007;17(8):370–6. doi: 10.1016/j.tcb.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Xu Z, Chan HY, Lam WL, Lam KH, Lam LS, Ng TB, Au SW. SUMO proteases: redox regulation and biological consequences. Antioxid Redox Signal. 2009;11(6):1453–84. doi: 10.1089/ars.2008.2182. [DOI] [PubMed] [Google Scholar]
- 4.Desterro JM, Rodriguez MS, Hay RT. SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation. Mol Cell. 1998;2(2):233–9. doi: 10.1016/s1097-2765(00)80133-1. [DOI] [PubMed] [Google Scholar]
- 5.Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–82. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- 6.Bruderer R, Tatham MH, Plechanovova A, Matic I, Garg AK, Hay RT. Purification and identification of endogenous polySUMO conjugates. EMBO Rep. 2011 doi: 10.1038/embor.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vertegaal AC, Ogg SC, Jaffray E, Rodriguez MS, Hay RT, Andersen JS, Mann M, Lamond AI. A proteomic study of SUMO-2 target proteins. J Biol Chem. 2004;279(32):33791–8. doi: 10.1074/jbc.M404201200. [DOI] [PubMed] [Google Scholar]
- 8.Martin S, Wilkinson KA, Nishimune A, Henley JM. Emerging extranuclear roles of protein SUMOylation in neuronal function and dysfunction. Nat Rev Neurosci. 2007;8(12):948–59. doi: 10.1038/nrn2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang W, Sheng H, Homi HM, Warner DS, Paschen W. Cerebral ischemia/stroke and small ubiquitin-like modifier (SUMO) conjugation--a new target for therapeutic intervention? J Neurochem. 2008;106(3):989–99. doi: 10.1111/j.1471-4159.2008.05404.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee YJ, Miyake S, Wakita H, McMullen DC, Azuma Y, Auh S, Hallenbeck JM. Protein SUMOylation is massively increased in hibernation torpor and is critical for the cytoprotection provided by ischemic preconditioning and hypothermia in SHSY5Y cells. J Cereb Blood Flow Metab. 2007;27(5):950–62. doi: 10.1038/sj.jcbfm.9600395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frerichs KU, Kennedy C, Sokoloff L, Hallenbeck JM. Local cerebral blood flow during hibernation, a model of natural tolerance to “cerebral ischemia”. J Cereb Blood Flow Metab. 1994;14(2):193–205. doi: 10.1038/jcbfm.1994.26. [DOI] [PubMed] [Google Scholar]
- 12.Frerichs KU, Smith CB, Brenner M, DeGracia DJ, Krause GS, Marrone L, Dever TE, Hallenbeck JM. Suppression of protein synthesis in brain during hibernation involves inhibition of protein initiation and elongation. Proc Natl Acad Sci U S A. 1998;95(24):14511–6. doi: 10.1073/pnas.95.24.14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang W, Ma Q, Mackensen GB, Paschen W. Deep hypothermia markedly activates the small ubiquitin-like modifier conjugation pathway; implications for the fate of cells exposed to transient deep hypothermic cardiopulmonary bypass. J Cereb Blood Flow Metab. 2009;29(5):886–90. doi: 10.1038/jcbfm.2009.16. [DOI] [PubMed] [Google Scholar]
- 14.Yang W, Sheng H, Warner DS, Paschen W. Transient focal cerebral ischemia induces a dramatic activation of small ubiquitin-like modifier conjugation. J Cereb Blood Flow Metab. 2008;28(5):892–6. doi: 10.1038/sj.jcbfm.9600601. [DOI] [PubMed] [Google Scholar]
- 15.Yang W, Sheng H, Warner DS, Paschen W. Transient global cerebral ischemia induces a massive increase in protein sumoylation. J Cereb Blood Flow Metab. 2008;28(2):269–79. doi: 10.1038/sj.jcbfm.9600523. [DOI] [PubMed] [Google Scholar]
- 16.Cimarosti H, Lindberg C, Bomholt SF, Ronn LC, Henley JM. Increased protein SUMOylation following focal cerebral ischemia. Neuropharmacology. 2008;54(2):280–9. doi: 10.1016/j.neuropharm.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Datwyler AL, Lattig-Tunnemann G, Yang W, Paschen W, Lee SL, Dirnagl U, Endres M, Harms C. SUMO2/3 conjugation is an endogenous neuroprotective mechanism. J Cereb Blood Flow Metabol. doi: 10.1038/jcbfm.2011.112. doi: 10.1038/jcbfm.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YJ, Castri P, Bembry J, Maric D, Auh S, Hallenbeck JM. SUMOylation participates in induction of ischemic tolerance. J Neurochem. 2009;109(1):257–67. doi: 10.1111/j.1471-4159.2009.05957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loftus LT, Gala R, Yang T, Jessick VJ, Ashley MD, Ordonez AN, Thompson SJ, Simon RP, Meller R. Sumo-2/3-ylation following in vitro modeled ischemia is reduced in delayed ischemic tolerance. Brain Res. 2009;1272:71–80. doi: 10.1016/j.brainres.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malakhov MP, Mattern MR, Malakhova OA, Drinker M, Weeks SD, Butt TR. SUMO fusions and SUMO-specific protease for efficient expression and purification of proteins. J Struct Funct Genomics. 2004;5(1–2):75–86. doi: 10.1023/B:JSFG.0000029237.70316.52. [DOI] [PubMed] [Google Scholar]
- 21.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Analytical chemistry. 2002;74(20):5383–92. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 22.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Analytical chemistry. 2003;75(17):4646–58. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 23.Nittis T, Guittat L, LeDuc RD, Dao B, Duxin JP, Rohrs H, Townsend RR, Stewart SA. Revealing novel telomere proteins using in vivo cross-linking, tandem affinity purification, and label-free quantitative LC-FTICR-MS. Molecular & cellular proteomics : MCP. 2010;9(6):1144–56. doi: 10.1074/mcp.M900490-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weng L, Dai H, Zhan Y, He Y, Stepaniants SB, Bassett DE. Rosetta error model for gene expression analysis. Bioinformatics. 2006;22(9):1111–21. doi: 10.1093/bioinformatics/btl045. [DOI] [PubMed] [Google Scholar]
- 25.Yang W, Paschen W. Gene expression and cell growth are modified by silencing SUMO2 and SUMO3 expression. Biochem Biophys Res Commun. 2009;382(1):215–8. doi: 10.1016/j.bbrc.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Mascle XH, Germain-Desprez D, Huynh P, Estephan P, Aubry M. Sumoylation of the transcriptional intermediary factor 1beta (TIF1beta), the Co-repressor of the KRAB Multifinger proteins, is required for its transcriptional activity and is modulated by the KRAB domain. J Biol Chem. 2007;282(14):10190–202. doi: 10.1074/jbc.M611429200. [DOI] [PubMed] [Google Scholar]
- 27.Miller MJ, Barrett-Wilt GA, Hua Z, Vierstra RD. Proteomic analyses identify a diverse array of nuclear processes affected by small ubiquitin-like modifier conjugation in Arabidopsis. Proc Natl Acad Sci U S A. 2010;107(38):16512–7. doi: 10.1073/pnas.1004181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golebiowski F, Matic I, Tatham MH, Cole C, Yin Y, Nakamura A, Cox J, Barton GJ, Mann M, Hay RT. System-wide changes to SUMO modifications in response to heat shock. Sci Signal. 2009;2(72):ra24. doi: 10.1126/scisignal.2000282. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Lloris R, Osses N, Jaffray E, Shen LN, Vaughan OA, Girwood D, Bartrons R, Rosa JL, Hay RT, Ventura F. Repression of SOX6 transcriptional activity by SUMO modification. FEBS Lett. 2006;580(5):1215–21. doi: 10.1016/j.febslet.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 30.Taylor KM, Labonne C. SoxE factors function equivalently during neural crest and inner ear development and their activity is regulated by SUMOylation. Dev Cell. 2005;9(5):593–603. doi: 10.1016/j.devcel.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Knipscheer P, Flotho A, Klug H, Olsen JV, van Dijk WJ, Fish A, Johnson ES, Mann M, Sixma TK, Pichler A. Ubc9 sumoylation regulates SUMO target discrimination. Mol Cell. 2008;31(3):371–82. doi: 10.1016/j.molcel.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 32.Ivanov AV, Peng H, Yurchenko V, Yap KL, Negorev DG, Schultz DC, Psulkowski E, Fredericks WJ, White DE, Maul GG, Sadofsky MJ, Zhou MM, Rauscher FJ., 3rd PHD domain-mediated E3 ligase activity directs intramolecular sumoylation of an adjacent bromodomain required for gene silencing. Mol Cell. 2007;28(5):823–37. doi: 10.1016/j.molcel.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tatham MH, Matic I, Mann M, Hay RT. Comparative Proteomic Analysis Identifies a Role for SUMO in Protein Quality Control. Science signaling. 2011;4(178):rs4. doi: 10.1126/scisignal.2001484. [DOI] [PubMed] [Google Scholar]
- 34.Hu BR, Janelidze S, Ginsberg MD, Busto R, Perez-Pinzon M, Sick TJ, Siesjo BK, Liu CL. Protein aggregation after focal brain ischemia and reperfusion. J Cereb Blood Flow Metab. 2001;21(7):865–75. doi: 10.1097/00004647-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Liu CL, Martone ME, Hu BR. Protein ubiquitination in postsynaptic densities after transient cerebral ischemia. J Cereb Blood Flow Metab. 2004;24(11):1219–25. doi: 10.1097/01.WCB.0000136706.77918.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schimmel J, Larsen KM, Matic I, van Hagen M, Cox J, Mann M, Andersen JS, Vertegaal AC. The ubiquitin-proteasome system is a key component of the SUMO-2/3 cycle. Mol Cell Proteomics. 2008;7(11):2107–22. doi: 10.1074/mcp.M800025-MCP200. [DOI] [PubMed] [Google Scholar]
- 37.Geoffroy MC, Hay RT. An additional role for SUMO in ubiquitin-mediated proteolysis. Nat Rev Mol Cell Biol. 2009;10(8):564–8. doi: 10.1038/nrm2707. [DOI] [PubMed] [Google Scholar]
- 38.Bergink S, Jentsch S. Principles of ubiquitin and SUMO modifications in DNA repair. Nature. 2009;458(7237):461–7. doi: 10.1038/nature07963. [DOI] [PubMed] [Google Scholar]
- 39.Broemer M, Meier P. Ubiquitin-mediated regulation of apoptosis. Trends in cell biology. 2009;19(3):130–40. doi: 10.1016/j.tcb.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Hoeller D, Crosetto N, Blagoev B, Raiborg C, Tikkanen R, Wagner S, Kowanetz K, Breitling R, Mann M, Stenmark H, Dikic I. Regulation of ubiquitin-binding proteins by monoubiquitination. Nature cell biology. 2006;8(2):163–9. doi: 10.1038/ncb1354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.