Abstract
BACKGROUND
The oncologic benefit of resecting liver metastases in breast cancer patients is unclear. This study was performed to identify predictors of survival after hepatectomy.
METHODS
From 1997-2010, 86 patients underwent resection of breast cancer liver metastases (BCLM). Clinicopathologic characteristics of the primary tumor, timing of metastasis development and treatment were recorded. Response to pre-hepatectomy chemotherapy was evaluated according to RECIST criteria, and the best response to chemotherapy during treatment and the response immediately before hepatectomy were noted. Univariate and multivariate analyses were performed to identify predictors of disease-free (DFS) and overall survival (OS).
RESULTS
Fifty nine patients (69%) had estrogen (ER) or progesterone receptor (PR) positive primary tumors. Fifty-three patients (62%) had a solitary BCLM, and 73 (85%) had BCLM ≤5 cm. Sixty five patients (76%) received pre-hepatectomy hormonal and/or chemotherapy. Four patients (6%) had progressive disease (PD) as best response and 19 patients (30%) had PD prior to hepatectomy (p < 0.001). 70% of patients who received pre-operative chemotherapy or hormonal therapy demonstrated either response or stable disease immediately prior to hepatectomy. No post-operative deaths were observed. At a 62 month median follow-up, the DFS and OS were 14 and 57 months, respectively. On univariate analysis, primary tumor ER/PR status, best radiographic response, and preoperative radiographic response were associated with OS. On multivariate analysis, ER-negative primary disease (p=.009, HR 3.3, 95% CI: 1.4-8.2) and preoperative PD (p=.003, HR 3.8, 95% CI: 1.6-9.2) were associated with decreased OS.
CONCLUSIONS
Resection of BCLM in patients with ER positive disease that is responding to chemotherapy is associated with improved survival. The timing of surgical intervention is critical; resection before progression is associated with better outcome.
INTRODUCTION
Breast cancer is one of the most common malignancies in women, and with nearly 40,000 deaths attributable to the disease in 2010, it represents the second highest cause of cancer mortality in women in the United States1. Because death is due primarily to metastatic spread, strategies to treat extra-mammary disease are of utmost importance and interest. Along with bone and lung, the liver represents a common site of metastatic disease. In contrast to hepatic colorectal metastases, surgery for liver metastases from breast cancer is often not considered as a therapeutic option due to systemic disease involving multiple sites. Therefore, the majority of patients with hepatic metastases are treated with systemic chemotherapy without surgical intervention. The evolution of chemotherapeutic agents has resulted in improved survival for many patients with metastatic disease2, 3. However, even in cases of significant response, definitive cure of breast cancer metastatic to the liver is rarely achieved using systemic chemotherapy alone.
Due to this lack of curative effectiveness by chemotherapeutic regimens, there continues to be interest in surgical intervention for appropriately selected patients. While extended survival in this patient population is unusual, surgical extirpation has been associated with long-term survival 4-6 and 5-year survivors of resection of breast cancer liver metastases (BCLM) have been reported7-12. The clinical challenge has been to discern preoperatively which patients will experience long-term survival following hepatic metastasectomy from those who will experience recurrence early in their post-operative course, mitigating the potential benefits of surgery.
Previous studies reporting on hepatic metastasectomy have suggested that patients with a long (>2 year) disease-free interval between treatment of the primary tumor and diagnosis of liver metastases, favorable tumor marker status, response to chemotherapy and negative margins at hepatectomy are all favorable prognostic factors in patients with metastases to the liver9, 13-16. These small studies included patients with various different primary malignancies, and in many cases the breast cancer cohort within the overall study group was quite modest. In 2004 we published The University of Texas MD Anderson Cancer Center experience with resection of liver metastases from breast cancer in 31 patients, and while we reported encouraging survival data, we were not able to define specific patient or tumor variables that predicted for improved outcomes17.
The current study was undertaken to update our institutional experience with resection for BCLM. The primary aims were to document overall and disease free-survival in our patient population and to identify predictors of survival that could be assessed pre-operatively to optimize patient counseling, risk/benefit analyses and outcome.
PATIENTS AND METHODS
Patient selection
Women undergoing hepatic resection for pathologically confirmed BCLM between 1997 and 2010 at MD Anderson were prospectively entered into a database. Patients with extra-hepatic disease that was treated, and deemed stable or improving, were included. Patients with hepatic disease deemed unresectable on the basis of pre-operative three-dimensional imaging or intra-operative evaluation were excluded. Radiofrequency ablation (RFA) was considered as definitive therapy, and these patients were included in the final analysis if RFA was used in conjunction with resection; cases of RFA only were not included. The study was approved by the MD Anderson Institutional Review Board.
Response to pre-operative systemic therapy
Demographic information, tumor specific variables, clinical outcomes, and imaging characteristics from each patient were reviewed. Synchronous disease was defined as hepatic metastases diagnosed at the time of the primary breast tumor. Metachronous disease was defined as hepatic metastases diagnosed after completion of therapy for the primary breast tumor. When chemotherapy was administered prior to hepatic resection, computed tomography or magnetic resonance imaging was performed every 3-4 cycles and immediately prior to surgical intervention. Radiographic responses of liver metastases were measured, not of the primary tumor or extra-hepatic disease. The Response Criteria in Solid Tumors (RECIST) criteria as outlined by the World Health Organization was used to characterize radiologic response to chemotherapy. RECIST definitions are as follows: complete response (CR) is 100% decrease in maximum diameter of lesion, partial response (PR) is ≥30% decrease in maximum diameter of lesion, progressive disease (SD) is ≥ 20% increase from maximum response in lesion, and stable disease (SD) is neither PR or PD18. Best RECIST response was defined as the best interval radiographic response between any two 3-dimensional imaging studies, while preoperative RECIST response was defined as the radiographic response observed immediately prior to resection of BCLM.
Surgical procedure
All operations were performed with curative intent. Radiofrequency ablation was rarely used, but employed in conjunction with hepatic resection for small tumors not otherwise resectable (i.e. tumor in a small liver remnant). Intra-operative ultrasound was carried out to define the portal and hepatic vein anatomy and its relationship to the metastases. Major hepatic resections were defined as those in which 3 or more Couinaud segments were removed. Surgical specimens were evaluated for size, grade, margin, and hormone receptor status (both the primary tumor and the metastasis were assessed when possible). Post-operative mortality was defined as death within 30 days of hepatectomy. Complications were divided into minor (requiring no invasive intervention) and major (invasive maneuver required for resolution).
Statistical analysis
Continuous variables were expressed as mean (standard deviation) or median (range) and categorical variables as number and frequency. SPSS statistical software v17.0 (SPSS, Inc., Chicago, IL) was used for all statistical analyses. Overall survival (OS) and disease-free survival (DFS) were calculated from the date of surgery to date of death or recurrence, respectively. OS and DFS curves were calculated using the Kaplan-Meier method and differences between patient subgroups were compared using the log-rank test. Univariate analysis was performed to assess the following variables for their association with OS and DFS: age>50, administration of neoadjuvant therapy for breast cancer, breast surgery type, pre-hepatectomy hormonal and chemotherapy, post-hepatectomy hormonal and chemotherapy, administration of trastuzumab as adjuvant therapy following breast surgery or before metastasectomy, tumor histology, primary tumor status, nodal status, metastasis sites and number, tumor grade, hormone receptor status, timing of detection of liver metastasis, number of liver metastases pre-operatively, maximal metastasis size >5 cm, hormone receptor status of metastasis, hepatic resection type, hepatectomy margin, best RECIST response to chemotherapy, immediate pre-operative RECIST response to chemotherapy, and sites of extra-hepatic disease. All patients receiving trastuzumab were treated after FDA approval in 1998. A p-value of <.05 was considered statistically significant. Clinicopathologic variables that were unknown were not included in the analysis. Clinically and/or statistically significant variables were included in multivariate analysis and evaluated by backward stepwise Cox regression analysis for OS.
RESULTS
Breast tumor characteristics
Eighty six patients were identified that underwent hepatic resection for BCLM from 1997-2010. All 86 patients were female, and a majority of the patients, 52(60%), were 50 years of age or younger (Table 1). Mastectomy was performed in 60 (70%) patients and 26 (30%) underwent breast-conserving surgery for treatment of their primary tumor. The majority of the primary tumors were T1 or T2 (76%) and N0 or N1 (62%). There were 27 (31%) patients who had M1 disease at the time of diagnosis of their primary breast tumor. Nearly 90% of the primary tumors were infiltrating ductal carcinoma. Most tumors were poorly differentiated (53%), and most tumors were positive for estrogen receptor (ER) and/or progesterone receptor (PR) (69%). A total of 32 (37%) patients had tumors that were identified as HER-2/-neu (HER2) positive; 15 of these patients had synchronous BCLM, while 17 had metachronous disease. Of the 15 patients with HER2-positive tumors and synchronous BCLM, 14 received neoadjuvant chemotherapy with trastuzumab. Of the 17 patients diagnosed with metachronous BCLM, 13 received chemotherapy prior to hepatectomy, and trastuzumab was included in the regimen in 10 (77%) patients.
Table 1.
Breast cancer specific clinicopathologic data of 86 patients undergoing hepatic resection for breast cancer liver metastases (BCLM); ER=estrogen receptor, PR=progesterone receptor)
| Variable | No. of patients (n=86) |
% | |
|---|---|---|---|
| Age, years | |||
| ≤50 | 52 | 60 | |
| >50 | 34 | 40 | |
| Tumor histology | |||
| Infiltrating ductal carcinoma | 73 | 89 | |
| Infiltrating lobular carcinoma | 6 | 7 | |
| Mixed tumor | 3 | 4 | |
| Primary tumor status | |||
| T1-2 | 61 | 76 | |
| T3-4 | 19 | 24 | |
| Nodal status | |||
| N0/1 | 52 | 62 | |
| N2/3 | 32 | 38 | |
| Metastasis status at breast cancer diagnosis | |||
| M0 | 58 | 68 | |
| M1 | 27 | 32 | |
| Timing of detection of liver metastasis | |||
| Synchronous | 25 | 29 | |
| Metachronous | 61 | 71 | |
| Grade | |||
| 1 | 4 | 6 | |
| 2 | 26 | 41 | |
| 3 | 34 | 53 | |
| Neoadjuvant therapy prior to breast surgery | |||
| Yes | 28 | 33 | |
| No | 58 | 67 | |
| Breast surgery type | |||
| Breast conserving surgery | 26 | 30 | |
| Mastectomy | 60 | 70 | |
| Hormone receptor status | |||
| ER and/or PR+ | 59 | 69 | |
| ER-/PR − | 21 | 24 | |
| Unknown | 6 | 7 | |
| Her-2/-neu status | |||
| Positive | 32 | 37 | |
| Negative | 40 | 47 | |
| Unknown | 14 | 16 |
Characteristics of the liver metastases and hepatic metastasectomy
The majority (62%) of the BCLM were solitary, and most (85%) were 5 cm or smaller. Sixty two percent of the hepatic resections were major (≥ 3 Couinaud segments). The mean estimated blood loss was 211.5 ml, and the mean length of hospital stay was 6.2 days (Table 2). There was no post-operative mortality. Complications occurred in 18 of 86 patients for an overall rate of 21%. Of these, two were related to anesthesia (pneumothorax after central line placement and cerebrospinal fluid leak following epidural removal). Thirteen complications were minor and required no invasive intervention. Three complications required invasive diagnostic or therapeutic maneuvers for definitive treatment (percutaneous drain/ERCP or chest tubes). Of the patients who had their liver metastases evaluated by immunohistochemistry, 74% had metastases positive for ER, 39% had metastases positive for PR, and 38% had metastases positive for HER2. The resection margins were microscopically negative (R0) in 90% of the patients and microscopically positive (R1) in 9%. There was 1 case of a macroscopically positive resection margin (R2). A total of 65 (76%) of the patients received chemotherapy and/or hormonal therapy prior to hepatectomy. Of the 32 patients with HER2 positive disease, 24 (75%) were treated with trastuzumab prior to hepatectomy.
Table 2.
Clinicopathologic data of hepatic metastases and operative intervention (CR=complete response, PR=partial response, SD=stable disease, PD=progression of disease)
| Variable | No. of patients (n=86) |
% | |
|---|---|---|---|
| Number of liver metastases (prior to surgery) | |||
| Solitary | 53 | 62 | |
| Multiple | 33 | 38 | |
| Maximal liver metastasis size (final pathology) | |||
| ≤5 cm | 73 | 85 | |
| >5 cm | 13 | 15 | |
| Pre-hepatectomy hormonal and/or chemotherapy |
|||
| Yes | 65 | 76 | |
| No | 21 | 24 | |
| Pre-hepatectomy trastuzumab therapy (in HER2 positive patients) |
|||
| Yes | 24 | 75 | |
| No | 8 | 25 | |
| Post-hepatectomy chemotherapy | |||
| Yes | 30 | 35 | |
| No | 56 | 65 | |
| Hepatic resection | |||
| Minor (<3) | 33 | 38 | |
| Major (≥3) | 53 | 62 | |
| Pathology margin | |||
| R0 | 77 | 90 | |
| R1 | 8 | 9 | |
| R2 | 1 | 1 | |
| Best radiographic response to pre- hepatectomy chemotherapy and/or hormonal therapy |
|||
| CR | 10 | 16 | |
| PR | 39 | 62 | |
| SD | 10 | 16 | |
| PD | 4 | 6 | |
| Immediate pre-hepatectomy radiographic response to chemotherapy and/or hormonal therapy |
|||
| CR | 4 | 6 | |
| PR | 31 | 49 | |
| SD | 9 | 14 | |
| PD | 19 | 30 | |
| Sites of extra-hepatic disease at or prior to hepatectomy |
|||
| Bone | 11 | 46 | |
| Nodal disease | 7 | 29 | |
| Other | 6 | 25 | |
| Disease free interval | ≤ 2 years | 62 | 72 |
| >2 years | 24 | 28 | |
| Estimated blood loss, ml, mean | 211.5 | ||
| Length of hospital stay for hepatic resection, days, mean |
6.2 |
Imaging characteristics
For patients who received pre-hepatectomy chemotherapy, hepatic metastases were assessed radiographically following each course of treatment. Best response was complete response in 10 patients (16%), partial response in 39 (62%), stable disease in 10 (16%) and progressive disease in 4 (6%). To better characterize tumor response to chemotherapy just prior to surgery, RECIST response immediately preceding surgery was identified, regardless of RECIST responses recorded previously. Responses were generally less favorable when compared to best RECIST response. Only 4 patients (6%) had a complete response immediately before hepatectomy, while 19 (30%) had progressive disease.
Overall and disease-free survival data
The median follow-up time was 62 months (range 0.3-100). Median DFS for all patients was 14.2 months and median actuarial OS was 57 months. Factors found to be associated with DFS on univariate analysis are listed in table 3. Partial response as best response and partial response immediately before hepatectomy were associated with significantly extended DFS (31.1 and 48.8 months, respectively). Progressive disease immediately prior to hepatectomy portended shorter DFS (median 6.4 months versus 26.3 months for those without progressive disease before surgical intervention) (P=.001). Patients with ER positive primary tumors had significantly longer median DFS than those with ER-negative primary tumors (19.8 versus 7.8 months, P=.031).
Table 3.
Significant predictors of disease-free survival on univariate analysis (CR=complete response, PR=partial response, SD=stable disease, PD=progression of disease, ER=estrogen receptor)
| Variable | Disease-free survival (median, mo) |
P |
|---|---|---|
| All | 14.2 | |
| Best RECIST response CR | 18.5 vs. 4.1 | 0.02 |
| Best RECIST response PR | 31.1 vs. 6.4 | <.001 |
| Pre-operative RECIST response PR | 48.8 vs. 8.5 | .001 |
| Pre-operative RECIST response-- PD versus PR or SD |
6.4 vs. 26.3 | .001 |
| ER+ primary tumor | 19.8 vs. 7.8 | .031 |
| Metastasis size >5cm | 16.6 vs. 9.7 | .023 |
| Post-hepatectomy chemotherapy | 20.7 vs. 12.2 | .008 |
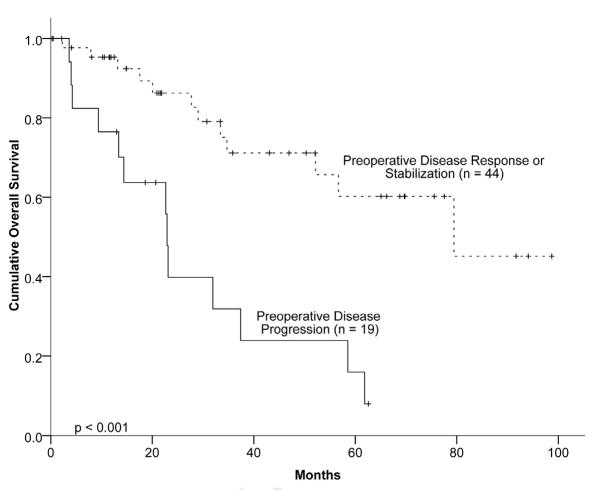
Factors determined to be associated with OS on univariate analysis are shown in table 4. Similar to DFS, patients with a partial response to chemotherapy as best response and a partial response immediately before hepatectomy were associated with improved OS. Patients with a partial response to chemotherapy had a median survival of nearly 80 months, compared to approximately 30 months for patients with less than a partial response. Patients with progressive disease immediately prior to hepatectomy had a median survival of 22.9 months compared to 79.4 months for those with at least a partial response (P<.001) (Figure 1). Likewise, patients with ER-positive and those with PR-positive primary tumors had median OS of 76.8 and 61.8 months, respectively, while patients with primary tumors negative for both ER and PR had a median OS of only 28.3 months. Figure 2 demonstrates the survival curves for patients based on ER status. Patients who underwent R0 resection had a median OS of 57 months, versus 33.6 months for those with R1 or R2 resections (P=.17). For patients with metachronous BCLM, a disease-free interval of greater than two years was associated with significantly longer OS (100.7 versus 47.5 months, p<.001). Finally, in the cohort ofHER2 positive patients, those treated with trastuzumab demonstrated a longer, but not statistically significant, OS of 74.2 versus 64.6 months (p=.17).
Table 4.
Significant predictors of overall survival on univariate analysis (CR=complete response, PR=partial response, SD=stable disease, PD=progression of disease, ER=estrogen receptor, PR=progesterone receptor)
| Variable | Overall survival (median, mo) |
P |
|---|---|---|
| All | 57 | |
| Best RECIST response PR | 79.4 vs. 31.9 | 0.049 |
| Pre-operative RECIST response PR | 79.4 vs. 29.0 | 0.016 |
| ER+ primary tumor | 76.8 vs. 23.1 | <.001 |
| PR+ primary tumor | 61.8 vs. 31.9 | 0.026 |
| ER−/PR- primary tumor | 28.3 vs. 76.8 | <.001 |
| Disease free interval >2 years | 100.7 vs. 47.5 | <.001 |
Figure 1.
Overall actuarial survival based on immediate pre-operative progression of hepatic disease by RECIST criteria.
Figure 2.
Overall actuarial survival based on estrogen receptor (ER) status
On multivariate analysis (Table 5), two factors were significantly associated with decreased OS: ER-negative primary tumor (hazard ratio [HR] 3.3; 95% confidence interval [CI] 1.4-8.2, P=.009) and disease progression immediately before hepatectomy (HR 3.8; 95% CI 1.6-9.2, P=.003).
Table 5.
Significant predictors of overall survival on multivariate analysis (PR=partial response, PD=progression of disease, ER=estrogen receptor)
| Variable | Hazard ratio | P | 95% confidence interval |
|---|---|---|---|
| Best RECIST response PR | 1.63 | .47 | .4-6.2 |
| R1 or R2 hepatectomy | 2.53 | .123 | .8-8.3 |
| Pre-operative RECIST response PD | 3.8 | .003 | 1.6-9.2 |
| ER negative primary tumor | 3.3 | .009 | 1.4-8.2 |
DISCUSSION
To our knowledge this study represents the largest single-institution experience to date of hepatic resection for patients with BCLM, and our data demonstrate that metastasectomy for BCLM is safe and associated with prolonged survival in selected patients. There were no post-operative deaths in our series and the patients experienced an acceptable rate of complications (21%). Additionally, we found that assessment of tumor response in the hepatic metastases with cross-sectional imaging just prior to resection was the strongest predictor of survival.
In our study, women with BCLM undergoing hepatic resection demonstrated a 43.6% 5-year actuarial OS rate. Those with ER and/or PR-positive tumors had improved OS, confirming the findings of previous investigators5. This is consistent with other reports suggesting a survival advantage in hormone receptor positive breast cancer, due to both favorable tumor biology and the administration of hormonal therapy. Of note, a majority of the patients in our study cohort did not receive hormonal therapy prior to surgical intervention for their liver metastases even though a majority of patients (69%) were candidates for such an approach– i.e., their primary tumor and/or metastatic disease were positive for ER and/or PR. We would therefore hypothesize that a more liberal use of pre-operative hormonal therapy in these patients could further improve the survival outcomes in patients undergoing resection of BCLM.
During the timeframe of our study there was a change in management of patients with HER2-positive disease. Specifically, trastuzumab became used routinely for the treatment of metastatic disease in those with HER2-positive tumors in 200119. Of the 32 patients that had HER2-positive disease, 24 (75%) received trastuzumab therapy after diagnosis of BCLM and prior to hepatectomy. Of these 24 patients, 11 (46%) demonstrated a pathologic complete response (CR) in the liver. While this rate is not as high as the 60% pathologic CR rate reported for treatment of primary breast tumors with concurrent chemotherapy and trastuzumab in the neoadjuvant setting, the results are striking and confirm previous reports of the efficacy of trastuzumab for HER2-positive disease in the metastatic setting19-21. Additionally, we noted that these patients had longer survival on univariate analysis–79 versus 52 months, although this did not reach statistical significance (p=.17) This may be due in part to the relatively small number of patients with HER2-positive disease receiving trastuzumab that were included in the current study. We would expect that if more patients with HER2 positive disease were included in the analysis, this would result in a statistically significant survival advantage.
Importantly, the most reliable predictor of survival in patients undergoing resection of BCLM was radiologic response of liver metastases to chemotherapy immediately prior to surgical intervention. A majority of patients undergoing resection for BCLM do receive chemotherapy prior to resection, and many patients demonstrate an objective response. However, some patients develop chemoresistance and progress following their initial response 22, 23. In our series, the cohort of patients who underwent resection following progression of disease seen on imaging experienced shortened survival. The poor outcome observed in this group of patients indicates the need for careful evaluation of response to chemotherapy and consideration for alternate treatment strategies. The timing of hepatic resection is critical and it is important for the surgeon to choose the optimal window of opportunity for surgical resection, before evidence of disease progression in the liver. If patients demonstrate a radiographic response to chemotherapy and/or hormonal therapy, we recommend continuing pre-hepatectomy systemic therapy. When metastases no longer demonstrate radiographic response, hepatectomy should be considered.
Our study has several limitations that are inherent to a retrospective analysis of a prospectively collected database. This study was limited to a cohort of patients undergoing surgical intervention and therefore represents a highly selected population; 86 patients met selection criteria for this study, while approximately 2000 patients with stage IV breast cancer were treated at MD Anderson during the same time period. We did not compare this cohort who had operative intervention with a matched patient population receiving chemotherapy only. Such an approach is not feasible, as a majority of patients with limited, resectable hepatic disease will be referred for surgical resection, and finding a truly “matched” cohort of patients treated with only chemotherapy would be difficult. In the absence of a prospective, randomized trial–which is unlikely for this relatively rare and heterogeneous patient population– findings such as those presented in this large series serve as the best available data.
When evaluating patients with BCLM for hepatic resection, surgeons must use multiple criteria to determine appropriate operative candidates. In the setting of multi-modality treatment, surgical resection should be viewed as complementary to chemotherapy and/or hormonal therapy. To maximize survival and minimize unnecessary surgical morbidity, our study suggests that hormone receptor status, response to systemic therapy and timing of operative intervention are critical factors that must be taken into account.
Acknowledgments
This research is supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Cancer Facts and Figures. American Cancer Society; 2010. Accessed at http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-and-figures-2010. [Google Scholar]
- 2.Ernst MF, van de Poll-Franse LV, Roukema JA, et al. Trends in the prognosis of patients with primary metastatic breast cancer diagnosed between 1975 and 2002. Breast. 2007;16:344–51. doi: 10.1016/j.breast.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Imkampe AK, Bates T. Improvements in breast cancer survival over time, related to adjuvant treatment and node status. Eur J Surg Oncol. 2009;35:151–5. doi: 10.1016/j.ejso.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Lubrano J, Roman H, Tarrab S, Resch B, Marpeau L, Scotte M. Liver resection for breast cancer metastasis: does it improve survival? Surg Today. 2008;38:293–9. doi: 10.1007/s00595-007-3617-2. [DOI] [PubMed] [Google Scholar]
- 5.Adam R, Aloia T, Krissat J, et al. Is liver resection justified for patients with hepatic metastases from breast cancer? Ann Surg. 2006;244:897–907. doi: 10.1097/01.sla.0000246847.02058.1b. discussion -8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selzner M, Morse MA, Vredenburgh JJ, Meyers WC, Clavien PA. Liver metastases from breast cancer: long-term survival after curative resection. Surgery. 2000;127:383–9. doi: 10.1067/msy.2000.103883. [DOI] [PubMed] [Google Scholar]
- 7.Harrison LE, Brennan MF, Newman E, et al. Hepatic resection for noncolorectal, nonneuroendocrine metastases: a fifteen-year experience with ninety-six patients. Surgery. 1997;121:625–32. doi: 10.1016/s0039-6060(97)90050-7. [DOI] [PubMed] [Google Scholar]
- 8.Caralt M, Bilbao I, Cortes J, et al. Hepatic resection for liver metastases as part of the “oncosurgical” treatment of metastatic breast cancer. Ann Surg Oncol. 2008;15:2804–10. doi: 10.1245/s10434-008-0072-2. [DOI] [PubMed] [Google Scholar]
- 9.Elias D, Maisonnette F, Druet-Cabanac M, et al. An attempt to clarify indications for hepatectomy for liver metastases from breast cancer. Am J Surg. 2003;185:158–64. doi: 10.1016/s0002-9610(02)01204-7. [DOI] [PubMed] [Google Scholar]
- 10.Elias D, Lasser PH, Montrucolli D, Bonvallot S, Spielmann M. Hepatectomy for liver metastases from breast cancer. Eur J Surg Oncol. 1995;21:510–3. doi: 10.1016/s0748-7983(95)96972-1. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimoto M, Tada T, Saito M, Takahashi K, Uchida Y, Kasumi F. Surgical treatment of hepatic metastases from breast cancer. Breast Cancer Res Treat. 2000;59:177–84. doi: 10.1023/a:1006398401352. [DOI] [PubMed] [Google Scholar]
- 12.Pocard M, Pouillart P, Asselain B, Falcou MC, Salmon RJ. Hepatic resection for breast cancer metastases: results and prognosis (65 cases) Ann Chir. 2001;126:413–20. doi: 10.1016/s0003-3944(01)00526-0. [DOI] [PubMed] [Google Scholar]
- 13.Weitz J, Blumgart LH, Fong Y, et al. Partial hepatectomy for metastases from noncolorectal, nonneuroendocrine carcinoma. Ann Surg. 2005;241:269–76. doi: 10.1097/01.sla.0000150244.72285.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy SK, Barbas AS, Marroquin CE, Morse MA, Kuo PC, Clary BM. Resection of noncolorectal nonneuroendocrine liver metastases: a comparative analysis. J Am Coll Surg. 2007;204:372–82. doi: 10.1016/j.jamcollsurg.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann K, Franz C, Hinz U, et al. Liver resection for multimodal treatment of breast cancer metastases: identification of prognostic factors. Ann Surg Oncol. 17:1546–54. doi: 10.1245/s10434-010-0931-5. [DOI] [PubMed] [Google Scholar]
- 16.Adam R, Chiche L, Aloia T, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg. 2006;244:524–35. doi: 10.1097/01.sla.0000239036.46827.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vlastos G, Smith DL, Singletary SE, et al. Long-term survival after an aggressive surgical approach in patients with breast cancer hepatic metastases. Ann Surg Oncol. 2004;11:869–74. doi: 10.1245/ASO.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Riaz A, Memon K, Miller FH, et al. Role of the EASL, RECIST, and WHO response guidelines alone or in combination for hepatocellular carcinoma: radiologic-pathologic correlation. J Hepatol. 54:695–704. doi: 10.1016/j.jhep.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 20.Hurley J, Doliny P, Reis I, et al. Docetaxel, cisplatin, and trastuzumab as primary systemic therapy for human epidermal growth factor receptor 2-positive locally advanced breast cancer. J Clin Oncol. 2006;24:1831–8. doi: 10.1200/JCO.2005.02.8886. [DOI] [PubMed] [Google Scholar]
- 21.Buzdar AU, Valero V, Ibrahim NK, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13:228–33. doi: 10.1158/1078-0432.CCR-06-1345. [DOI] [PubMed] [Google Scholar]
- 22.Er O, Frye DK, Kau SW, et al. Clinical course of breast cancer patients with metastases limited to the liver treated with chemotherapy. Cancer J. 2008;14:62–8. doi: 10.1097/PPO.0b013e3181629a7b. [DOI] [PubMed] [Google Scholar]
- 23.Zinser JW, Hortobagyi GN, Buzdar AU, Smith TL, Fraschini G. Clinical course of breast cancer patients with liver metastases. J Clin Oncol. 1987;5:773–82. doi: 10.1200/JCO.1987.5.5.773. [DOI] [PubMed] [Google Scholar]