Abstract
Background
Left atrial volume index (LAVI) has recently emerged as a useful biomarker for risk stratification and risk monitoring in many clinical settings. Many hemodynamic factors such as preload and afterload have an effect on evaluating left atrium function.
This study was performed to investigate the relationship between LAVI and aortic stiffness index (ASI) and selected markers characterizing hemodynamic state in patients with type 2 diabetes mellitus (DM2).
Material/Methods
The study population consisted of 100 patients (56 men, 44 women), 67.2 (±10.9) years old DM2, scheduled for routine coronary angiography. Standard transthoracic echocardiography was used to measure parameters needed for calculation of LAVI and ASI. During invasive procedures, central pulse pressure (CPP) in the ascendens aorta and left ventricle end-diastolic pressure (LVEDP) were recorded. Selected laboratory parameters were obtained, including lipidogram, serum uric acid, hs-CRP, fibrinogen, cTnT, myoglobin, BNP, HbA1C, creatinine, and GFR.
Results
Both LAVI and ASI were greater and CPP and LVEDP were markedly elevated in DM2 patients compared to controls. The independent predictors of LAVI were ASI (β=0.331; p=0.011), CPP (β=0.312; p=0.020), LVEDP (β=0.381; p=0.006), HbA1C (β=0.379; p=0.008), and BNP (β=0,423; p<0,001).
Conclusions
The strong correlation between HbA1C and both LAVI and ASI is a sign of negative influence of poor glycemia control on the left ventricle diastolic function and compliance of the aorta. The independent correlation between LAVI and ASI, CPP, and LVEDP improved by association of LAVI and ASI with specific biochemical markers suggests an association between LAVI and elastic properties of the aorta, as well as CPP in DM2 patients.
Keywords: left atrium volume index, aortic stiffness, glycated hemoglobin, BNP, central pulse pressure, diabetes mellitus
Background
Type 2 diabetes mellitus (DM2) is associated with a marked increase in risk of cardiovascular mortality [1]; therefore, detection of early cardiovascular changes in diabetic patients plays an important role in prevention and treatment.
Atherosclerosis is regarded as a combination of 2 features: atherosis and sclerosis. Sclerosis depends on deterioration of aortic elastic properties and is termed aortic stiffness. In non-diabetic individuals, increased aortic stiffness is an important cause of cardiovascular disease because it leads to increased systolic pressure and ventricular mass and to decreased diastolic coronary perfusion [2]. A number of studies have identified abnormalities of arterial stiffness in subjects with DM2, and it has been recognized that aortic stiffness is highly predictive of cardiovascular mortality in subjects with DM2 [3,4]. Large artery stiffening has been demonstrated in DM2 by using several different methods, including measurement of central pulse wave velocity, or estimation of aortic compliance, a technically demanding technique requiring the simultaneous measurement of stroke volume and diastolic pressure decay [4].
Blood pressure differs along the vascular tree. Measurement of brachial artery cuff may not correspond to aortic pressure, the so-called central pressure, which is a better risk predictor of cardiovascular complications [5]. There are 2 main components of central aortic pressure: a pulsatile and a nonpulsatile component. The nonpulsatile component depends on mean aortic pressure and central aortic diastolic pressure. The pulsatile component, termed central pulse pressure (CPP), is the difference between central systolic aortic pressure and central aortic diastolic pressure and is determined by total peripheral resistance, wave reflections, and arterial compliance [6]. Different methods have been introduced to assess aortic stiffness, including invasive and non-invasive techniques [7]. CPP and aortic stiffness are both predictors of coronary artery disease. In fact, invasively measured aortic (but not noninvasively obtained brachial) mean pressure and pulse pressure are significantly associated with the extent of coronary artery disease [8]. Aortic stiffening and the subsequent increase in CPP are also associated with carotid intima-media thickening, atheroma formation, and plaque rupture [9,10].
It has recently been recognized that CPP is independently associated with diastolic function [11]. In patients with DM 2 there is also a close association between left ventricular diastolic function and aortic stiffness, even after the exclusion of coronary artery disease [12]. It has been reported that aortic stiffness influences left ventricular structure and function, independent of blood pressure [13].
Because the left atrium is exposed to left ventricle filling pressures through the open mitral orifice during diastole, its size is influenced by the same factors that determine diastolic filling pressure. Left atrial distension is linked to cardiovascular outcomes in high-risk, elderly, and hypertensive adults and has recently been linked with aortic stiffness in these groups [14]. Left atrium volume is adjusted to body surface area and is expressed as left atrium volume index (LAVI). The LAVI is a new echocardiographic index and is compared to the “glycated hemoglobin of diabetes mellitus”, as is a reflection of long-standing hemodynamic conditions in different clinical settings.
This study was performed to determine the relationship between LAVI and aortic stiffness index (ASI) in patients with DM2. We took into account not only aortic stiffness, but also selected parameters characterizing hemodynamic state, which are influenced by arterial stiffness. Moreover, the relationship between LAVI, ASI, and selected biochemical markers was assessed.
Material and Methods
Clinical characterization of studied groups
The study population consisted of 100 consecutive patients (56 men, 44 women), with a mean age of 67.2 (±10.9) years, with type 2 DM, scheduled for routine coronary angiography for evaluation of coronary artery disease. Patients with reduced EF (<50%), acute coronary syndrome, decompensate heart failure, atrial fibrillation, congenital/acquire heart disease, idiopathic dilated or hypertrophy cardiomyopathy, and end-stage renal disease were excluded. DM2 was diagnosed according to the recommendations of the American Diabetes Association [15].
Control group
The control group comprised of 50 subjects (27 men and 23 women) with a mean age of 65.4±9.4 years, comparable to the study group clinical characteristics, except that they did not have DM2.
The study protocol was approved by the local Ethics Committee and followed the guidelines of the Declaration of Helsinki. Each participant gave written informed consent accepting the study protocol.
Echocardiography
The day before the index cardiac catheterization in all patient and control subjects, standard transthoracic echocardiographic examination was performed using a 2.5–3.5 MHz transducer (HP Sonos 7500, Hewlett Packard, Bloomfield, CT, USA) by the cardiologist, who was blinded to the clinical data of the study subjects. All echocardiographic measurements were done according to the guidelines of the American Society of Echocardiography [16].
The following two-dimensionally guided M-mode echocardiographic parameters were recorded: interventricular end-systolic septum diameter (IVSSd [cm]), interventricular septum end-diastolic diameter (IVSDd [cm]), posterior wall end-systolic diameter (PWSd [cm]), posterior wall end-diastolic diameter (PWDd cm]), left ventricle end-diastolic diameter (LVEDd [cm]), left ventricle end-systolic diameter (LVESd [cm]), left atrium maximal diameter (LAmax [cm]), aortic maximal diameter (Aomax [cm), and aortic minimal diameter (Aomin [cm]). The dimensions of the aorta were recorded in M-mode 3 cm above the aortic valve from a parasternal long axis view. The Aomax (systolic diameter) was measured at the time of aortic valve maximal opening. The Aomin (diastolic diameter) was recorded at the peak of the QRS complex. Inner aortic diameters were measured with a calliper in systole and diastole as the distance between the trailing edge of the anterior aortic wall and the leading edge of the posterior aortic wall [17].
In 4-CH presentation additional parameters were recorded. These were: left atrium short diameter (LAshort cm]), left atrium longitudinal diameter (LAlong [cm]), left atrium surface (LAS [cm2]), and left atrium circumference (CAC [cm]). Consequently, several standard indices were calculated according to American Society of Echocardiography recommendations [18], such as: left ventricle stroke volume (SV[ml]), stroke index (SI [n]), cardiac output (CO [l/min]), cardiac index (CI [l/min/m2]), ejection fraction (EF [%]), and fractional shortening of the left ventricle (FS [%]). Using previously measured parameters, the most important indices were calculated using the formulas below:
left ventricle mass (LVM [g]): LVM=1.04×[(IVSDd+PWDd+LVEDd)3]–13.6 g,
left ventricle mass index (LVMI [g/m2]): LVMI=LVM/BSA,
end-systolic stress (ESS [103dyn/cm2]): ESS=0.334 ×SBP×LVESd/PWSd×(1+PWSd/LVESd),
midwall fractional shortening (mFS [n]): mFS= [(LVEDd+PWSd/2+IVSSd/2)–(LVESd+Hs/2)/(LVEDd+PWSD/2 +IVSSd/2)]×100; where Hs=IVSSd+PWSd,
ratio mFS/ESS [n],
left atrium volume (LAVmax [ml]): LAVmax=[π/6×(LAmax×LAshort×LAlong)] (Figure 1),
left atrium volume index (LAVmaxI [ml/m2]): LAVI=LAVmax/m2[18],
aortic stiffness index (ASI [n]): ASI=log [(SBP/DBP)/(Aomax–Aomin)]/Aomin (Figure 2) [17].
Figure 1.
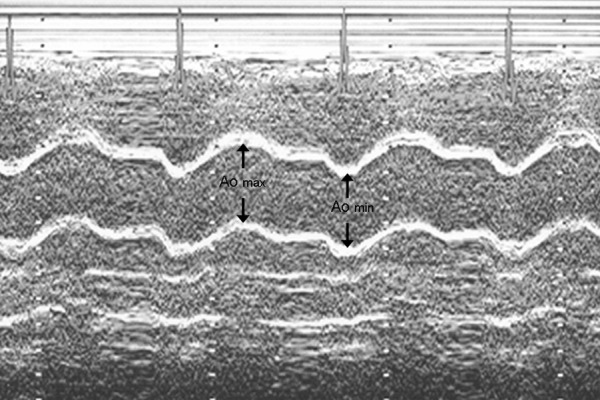
The measurement of diameters needed for ASI calculation (aortic maximal diameter (Aomax), aortic minimal diameter (Aomin).
Figure 2.
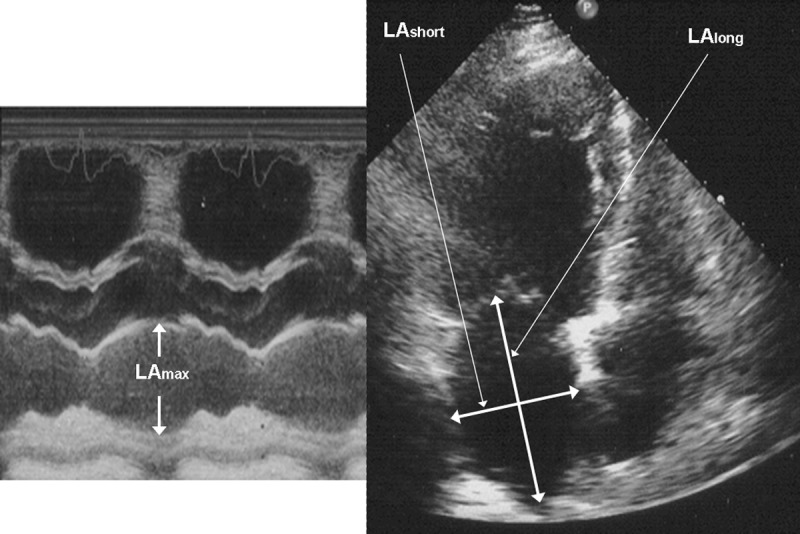
The measurement of diameters needed for LAVI calculation. Left atrium maximal diameter (LAmax), left atrium short diameter (LAshort), left atrium longitudinal diameter (LAlong).
Left ventricle hypertrophy was defined by an LVMI of >134 g/m2 in men and > 110 g/m2 in women.
Moreover, the following pulsed Doppler-derived parameters were measured: maximal velocity of early diastolic transmitral flow (E [cm/s]), maximal velocity of late diastolic transmitral flow (A [cm/s]), isovolumetric relaxation time (IVRT [ms]), maximal systolic velocity in pulmonary veins (S [cm/s]), and maximal diastolic velocity in pulmonary veins (D [cm/s]). The medians of measurements of 3 consecutive beats were used for statistical analysis. Consequently, ratios of E/A and S/D were calculated. Diastolic dysfunction was recognized according to recommendations of the Canadian Cardiovascular Society [19]. Patients with restrictive spectrum were not included to this study.
LAVI and ASI were the most important echocardiographic indices for this study. DM2 patients were divided into subgroups using mean values of the above indices as cut-off values and were compared (Figures 1 and 2).
Coronary angiography and invasive measurements
Briefly, before coronary angiography, ascending aortic and left ventricle pressures were obtained with catheter-manometer and recorded on a polygraph system (MAC-Lab Ex RAV catheterization system, Milwaukee, WI, USA). Ascending aortic and left ventricle pressures were measured as previously reported [20]. From the recorded pressure curves, the following variables were measured or calculated:
aortic systolic pressure (Aosystolic [mmHg]),
aortic diastolic pressure (Aodiastolic [mmHg]),
central pulse pressure (CPP [mmHg]): Aosystolic – Aodiastolic,
left ventricle end-diastolic pressure (LVEDP [mmHg]).
The medians of measurements of 3 consecutive beats were used for statistical analysis. Consequently, standard coronary angiography in multiple views (GE Innova 2000 Medical System, Milwaukee, WI, USA) was done using Judkin’s and Sone’s technique according to the previously described principles [21]. Significant stenosis was defined by the presence of a stenosis ≥50% in at least 1 segment of the right coronary artery (RCA), left main (LM), left anterior descending (LAD), and circumflex (CX) branches of the left coronary artery. According to the number of involved vessels, the severity of coronary artery disease was classified as 1-vessel, 2-vessel and 3-vessel and/or LM disease [21].
Pressure and laboratory measurements
The systolic (SBP) and diastolic (DBP) pressures were obtained using an electronic sphygmomanometer. Mean blood pressure (MBP) was calculated by the formula: MBP=1/3 SBP+2/3 DBP. Additionally, the mean heart rate calculated from 10 consecutive beats was recorded. Body surface area was calculated from the Gehan and George formula as follows: BSA [m2]=0.0235 × (body mass [kg])0.51456 × height [cm]0.42246.
All the patients underwent fasting blood sampling prior to coronary angiography. The following standard parameters were measured by automated analyzer (ADVIA Centaur analyser, Bayer Health-Care Diagnostics, Tarrytown, NY, USA): hemoglobin [g/dl], sodium [mmol/l], potassium [mmol/l], creatinine [mg/dl], glomerular filtration rate (GFR) [ml/min/1.73 m2], urea [mg/l], uric acid [mg/dl], protein [g/l], albumin [g/l], high-sensitivity C-reactive protein (hs-CRP) [mg/ml], fibrinogen [g/l], total cholesterol (T-chol) [mg/dl], HDL-cholesterol (HDL-chol) [mg/dl], and triglycerides (TGC) [mg/dl]. LDL-cholesterol (LDL-chol) [mg/dl] was calculated using the Friedewald equation: LDL-chol=T-chol – HDL-chol – (TGC/5). GFR was calculated from the modification of diet in renal disease (MDRD) formula: GFR=186 × serum creatinine−1.154 × age−0.203 × F, where F=1 in men and F=0.742 in women. Cardiac troponin T (cTnT) [(μg/l] in plasma was measured by the electrochemiluminescence immunoassay (Elecsys 2010 analyser, Roche Diagnostics Gmb, Mannheim, Germany) with the detection limit of 0.01 μg/l. BNP [fmol/ml] in plasma was measured by ELISA (Biomedica, Bratislava, Slovakia) with the detection range between 0–640 fmol/ml. HbA1C [%] was analyzed by the chromatographic assay using an HPLC instrument and ion exchange or affinity column to separate HbA1C molecules from other hemoglobin molecules (Elecsys 2010 analyser, Roche Diagnostics Gmb, Mannheim, Germany) with the detection limit of 4–14%.
Statistical analysis
Statistical analysis was carried out on an PC using of a standard statistical package Statistica 6.0 Version (StatSoft Inc.). Results were tested for normality. Data are expressed as mean ±SD (parametrically distributed continuous variables) and percentage (categorical variables). The statistical significance of the differences between DM2 patients and control group means were compared by unpaired Student’s t-test, the Mann-Whitney test, or chi-square test with Yates correction. Differences between values before and after hemodialysis were determined using the paired Student’s t-test. Linear regression analysis was performed by using the Pearson test. Multiple stepwise regression analysis was performed to estimate the potential influence of various factors on LAVI and ASI. The following parameters were entered into the model: echocardiographic indices such as mFS/ESS, E/A, S/D, LVMI, hemodynamic measurements such as Aosystolic, Aodiastolic, CPP, LVEDP, and biochemical parameters including hemoglobin, sodium, potassium, creatinine, urea, total protein, albumin, hs-CRP, T-chol, LDL-chol, HDL-chol, TGC, cTnT, BNP, and HbA1c. Probability values of <0.05 were accepted as significant.
Results
Baseline comparison
Clinical characteristics and laboratory measurements of the study population and control group are listed in Table 1. Heart rate was significantly higher among DM2 patients compared to controls. DBP was markedly lower in the DM2 group, whereas SBP did not differ between study and control groups.
Table 1.
Clinical characteristics of the study and control groups.
| Parameter | DM2 n=100 |
Controls n=50 |
P |
|---|---|---|---|
| Age [years] | 67.2 (±10.9) | 65.4 (±9.4) | NS |
|
| |||
| Males [n (%)] | 56 | 27 | NS |
|
| |||
| Duration of diabetes [years] | 10.5 (±5.2) | 0 | <0.001 |
| Body mass [kg] | 86.2 (±15.3) | 85.2 (±15.7) | NS |
|
| |||
| Height [cm] | 167.9 (±7.4) | 168.3 (±7.7) | NS |
|
| |||
| Body mass index [kg/m2] | 30.7 (±6.3) | 30.2 (±6.0) | NS |
|
| |||
| Waist-to-hip ratio [n] | 0.98 (±0.12) | 0.95 (±0.14) | NS |
| Hypertension [n (%)] | 90 (90%) | 46 (92%) | NS |
|
| |||
| Heart failure [n (%)] | |||
| NYHA I | 13 (13%) | 7 (14%) | NS |
| NYHA II | 7 (7%) | 3 (6%) | NS |
|
| |||
| History of myocardial infarction [n (%)] | 45 (45%) | 22 (44%) | NS |
| Ischemic stroke [n (%)] | 4 (4%) | 2 (4%) | NS |
|
| |||
| Family history of cardiovascular disease [n (%)] | 31 (31%) | 15 (30%) | NS |
|
| |||
| Smoking [n (%)] | 27 (27%) | 14 (28%) | NS |
| Medication [n (%)] | |||
| ACE-i | 70 (70%) | 36 (72%) | NS |
| ARB | 22 (22%) | 11 (22%) | NS |
| CCB | 45 (45%) | 23 (46%) | NS |
| Beta-blocker | 80 (80%) | 45 (90%) | 0.04 |
| ASA | 95 (95%) | 48 (96%) | NS |
| Clopidogrel | 30 (30%) | 16 (32%) | NS |
| VKA | 5 (5%) | 2 (4%) | NS |
| Statins | 83 (83%) | 43 (86%) | NS |
| Insulin | 65 (65%) | 0 | <0.001 |
| Oral antidiabetic drugs | 35 (35%) | 0 | <0.001 |
ACE-I – angiotensin converting enzyme inhibitor; ARB – angiotensin receptor blocker; CCB – calcium channel blocker; ASA – acetylsalicylic acid; VKA – vitamin K antagonist; NS – not significant.
Comparison of ionic concentration has demonstrated no difference in sodium and potassium levels among DM2 and control groups. Biochemical measurements characterizing renal function were higher among DM2 patients than in the control group. Concentration of uric acid was significantly higher in the DM2 group compared to the control group. The total protein concentration and albumin levels were slightly depressed in DM2 subjects compared to normal subjects. Hs-CRP was several-fold higher among DM2 patients than in controls. Fibrinogen concentration was significantly higher in the DM2 group than in the control group. Lipidogram was higher in concentration of T-chol, LDL-chol, whereas HDL-chol was significantly depressed in DM2 patients. The TGC concentration was extremely elevated in DM2 as compared to controls. In DM2 patients, levels of cardiac markers such as cTnT and BNP were high, and in controls the level of cTnT was 0.0. Concentration of HbA1C was obviously higher among DM2 patients than in the control group (Table 1).
Baseline echocardiographic characteristic of the studied patients and controls are shown in Table 2. Most of the diameters derived from a two-dimensionally guided M-mode echocardiography were significantly greater in DM2 patients when compared to the control group. Similarly, several parameters of left ventricle systolic and diastolic function markedly differed between DM2 patients and control subjects. EDV was greater in DM2 patients but SV was similar in the DM2 and control groups; therefore, EF was significantly lower in the DM2 group. CO and CI did not differ between DM2 patients and controls. LVM and LVMI were significantly higher in DM2 patients. Other indices characterizing left ventricle contractility such as mFS and mFS/ESS were markedly depressed among patients with DM2. There were no significant differences in E between the normal subjects and DM2 patients. However, A was higher in the DM2 group; consequently mean E/A ratio was markedly decreased in this group. The DT and the IVRT time were longer in the DM2 group. Finally, S/D ratio derived from pulmonary venous flow was higher in DM2 patients. The prevalence of relaxation abnormalities was extremely common among DM2 subjects.
Table 2.
Pressure, heart rate and biochemical measurements collected in studied patients.
| Parameter | DM2 | Controls | P |
|---|---|---|---|
| Mean heart rate[beat/min] | 78.9 (±11.2) | 68.3 (±9.4) | 0.034 |
| Systolic blood pressure[mmHg] | 148.4 (±24.2) | 141.3 (±29.7) | NS |
| Diastolic blood pressure[mmHg] | 71.2 (±13.4) | 85.2 (±9.5) | 0.024 |
| Haemoglobin[g/dl] | 14.4 (±1.6) | 13.9 (±0.9) | NS |
| Sodium[mmol/l] | 137.3 (±2.6) | 138.2 (±2.1) | NS |
| Potassium[mmol/l] | 4.5 (±0.7) | 4.3 (±0.4) | NS |
| Creatynine [mg/dl] | 1.3 (±0.5) | 0.9 (±0.4) | 0.042 |
| GFR [ml/min/1.73 m2] | 63.2 (±13.2) | 92.3 (±16.4) | 0.001 |
| Urea[mg/l] | 54.8 (±7.2) | 44.6 (±7.8) | NS |
| Uric acid [mg/dl] | 7.1 (±1.8) | 5.4 (±1.7) | 0.023 |
| Total protein[g/l] | 67.6 (±1.5) | 70.1 (±1.8) | NS |
| Albumin[g/l] | 4.3 (±0.3) | 4.6 (±0.4) | NS |
| hs-CRP[mg/ml] | 5.4 (±1.3) | 1.7 (±0.9) | <0.001 |
| Fibrinogen [g/l] | 4.9 (±0.5) | 3.4 (±0.4) | 0.016 |
| Fasting glucose [mg/dl] | 138.3 (±45.2) | 93.2 (±28.2) | <0.001 |
| Total chol [mg/dl] | 227.7 (±47.5) | 192.7 (±27.2) | 0.048 |
| LDL-chol [mg/dl] | 144.1 (±35.1) | 119.0 (±22.6) | 0.034 |
| HDL-chol [mg/dl] | 43.1 (±14.9) | 49.2 (±13.4) | 0.042 |
| TGC [mg/dl] | 242.8 (±64.9) | 154.2 (±45.3) | <0.001 |
| cTnT [(μg/l] | 0.057 (±0.015) | 0.00 | <0.001 |
| BNP [fmol/ml] | 173.2 (±91.2) | 36.7 (±7.8) | <0.001 |
| HbA1C [%] | 7.51 (±3.5) | 4.3 (±1.3) | <0.001 |
NS – not significant.
All planimetric left atrium parameters were also greater in the DM2 group than in controls; consequently LAV as well LAVI were greater in this group. Patients with DM2 also exhibited significantly greater aortic stiffness expressed by higher ASI value than in the control group.
The angiographic data had similar prevalence and severity of coronary artery disease among study and control groups. However, when hemodynamic measurements were analyzed, groups differed significantly. Aosystolic was similar in both groups, but Aodiastolic pressure was markedly depressed among DM2 patients; in consequence, CPP was also markedly higher in this group. LVEDP was also increased in DM2 patients compared to control subjects (Table 3).
Table 3.
Echocardiographic data in studied patients.
| Parameter | DM2 | Controls | P |
|---|---|---|---|
| Diameters of the heart | |||
| LVEDd [cm] | 5.54 (±0.76) | 4.93 (±0.51) | 0.033 |
| LVESd [cm] | 3.58 (±0.14) | 3.01 (±0.32) | 0.045 |
| PWDd [cm] | 1.27 (±0.28) | 0.95 (±0.04) | 0.031 |
| PWSd [cm] | 1.57 (±0.29) | 1.43 (±0.019) | NS |
| IVSDd [cm] | 1.39 (±0.27) | 0.98 (±0.07) | 0.006 |
| IVSSd [cm] | 1.61 (±0.31) | 1.48 (±0.09) | NS |
| Parameters of LV mass | |||
| LVM [g] | 231.3 (±84.59) | 150.3 (±30.3) | 0.001 |
| LVMI [g/m2] | 133.3 (±40.83) | 89.7 (±25.48) | 0.002 |
| LVH [%] | 54.5 | 0 | <0.001 |
| Parameters of heart stroke | |||
| SV [ml] | 79.31 (±14.41) | 81.87 (±12.43) | NS |
| SI [ml/beat/m2] | 45.32 (±10.91) | 46.84 (±11.84) | NS |
| CO [l/min] | 5.69 (±0.74) | 5.97 (±0.58) | NS |
| CI [l/min/m2] | 3.29 (±0.61) | 3.54 (±0.52) | NS |
| Parameters of systolic function | |||
| EF [%] | 54.72 (±6.54) | 63.72 (±3.37) | 0.007 |
| FS [%] | 27.14 (±5.17) | 32.02 (±4.21) | 0.013 |
| mFS [%] | 17.56 (±4.03) | 21.33 (±2.32) | 0.017 |
| mFS/ESS [n] | 0.171 (±0.051) | 0.222 (±0.051) | 0.009 |
| Parameters of diastolic function | |||
| E [cm/s] | 45.4 (±9.21) | 66.9 (±13.4) | NS |
| A [cm/s] | 68.5 (±12.3) | 57.4 (±9.4) | 0.04 |
| E/A [n] | 0.66 (±0.32) | 1.16 (±0.17) | 0.006 |
| IVRT [ms] | 111.1 (±25.5) | 77.69 (±15.19) | 0.001 |
| DT [ms] | 180.9 (±23.54) | 155.8 (±29.64) | 0.008 |
| S/D [n] | 2.05 (±0.31) | 1.32 (±0.25) | 0.003 |
| Relaxation abnormalities [%] | 65.3 | 1.75 | <0.001 |
| Parameters of left atrium | |||
| LAS [cm2] | 30.27 (±6.41) | 22.42 (±4.03) | 0.002 |
| LAC [cm] | 24.53 (±4.98) | 20.12 (±3.32) | 0.034 |
| LAmax [cm] | 4.79 (±0.48) | 4.09 (±0.31) | 0.006 |
| LAV [ml] | 58.2 (±17.02) | 45.02 (±10.64) | 0.014 |
| LAVI [ml/m2] | 33.99 (±9.84) | 26.39 (±5.45) | 0.021 |
| Parameters of aorta | |||
| ASI [n] | 5.87 (±1.54) | 3.44 (±1.35) | <0.001 |
NS – not significant.
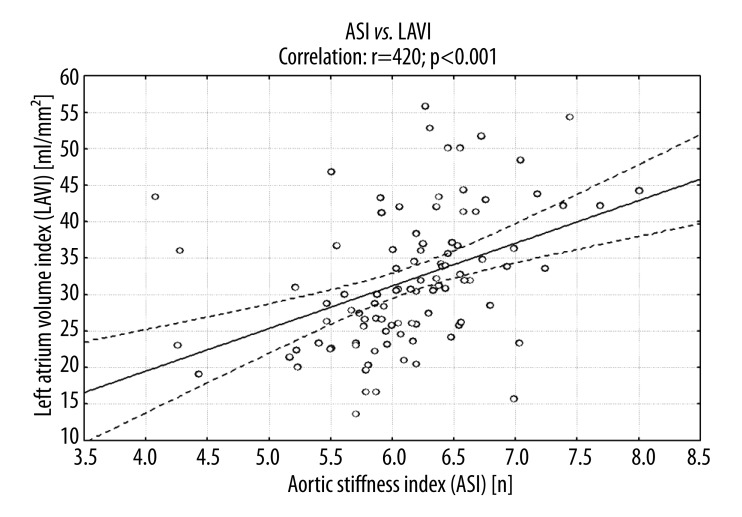
The study showed a significant correlation between LAVI and ASI (Figure 3). There was also a significant relationship between LAVI and the indices of left ventricular systolic function as mFS/ESS, ESS. The strong association between LAVI and left ventricular diastolic function markers was noted: positive with IVRT and negative with E/A ratio and S/D ratio. LAVI also positively correlated with indices of left ventricular hypertrophy: LVM and LVMI. Moreover, a significant association between LAVI and CPP and LVEDP was present (Table 4).
Figure 3.
The association between ASI and LAVI in patients with DM2.
Table 4.
Angiographic and invasive hemodynamic data in studied patients.
| Parameter | DM2 | Controls | p |
|---|---|---|---|
| Angiography | |||
| 1-vessel disease [n(%)] | 23 (23%) | 12 (24%) | NS |
| 2-vessel disease [n(%)] | 21 (21%) | 10 (20%) | NS |
| 3-vessel disease and/or LM [n(%)] | 28 (28%) | 14 (28%) | NS |
| No changes [n(%)] | 28 (28%) | 14 (28%) | NS |
| Hemodynamic parameters | |||
| Aosystolic [mmHg] | 142.3 (±28.7) | 140.9 (±29.4) | NS |
| Aodiastolic [mmHg] | 65.5 (±19.2) | 83.3 (±18.4) | 0.03 |
| CPP [mmHg] | 74.5 (±14.8) | 59.4 (±9.8) | 0.003 |
| LVEDP [mmHg] | 11.9 (±2.1) | 6.1 (±2.7) | 0.001 |
NS – not significant.
Cut-off values of selected echocardiographic parameters
Patients were divided into subgroups using a mean LAVI value of 33.99 ml/m2, calculated for the whole DM2 group as the cut-off value. There were 54 patients (LAVI=31.12±8.51 ml/m2) in the subgroup of LAVI < cut-off value, and 46 persons (LAVI 37.42±8.35 ml/m2) in subgroup of LAVI > cut-off value. Likewise, the study group was divided into 2 subgroups due to a mean calculated value ASI of 5.87 as the cut-off value. The first of these subgroups (ASI < cut-off value) consisted of 51 patients with ASI 4.32±1.42, and in the second subgroup (ASI > cut-off value) there were 49 subjects with ASI 7.34±1.12. The comparison of subgroups created by cut-off values of LAVI and ASI with regard to selected hemodynamic and laboratory data are shown in Table 5.
Table 5.
Significant correlations between LAVI, ASI and echocardiographic and hemodynamic parameters in patients with DM2.
| Parameter | LAVI | ASI | ||
|---|---|---|---|---|
| R | p | R | P | |
| Echocardiographic | ||||
| mFS | 0.351 | 0.039 | 0.395 | 0.014 |
| mFS/ESS | −0.362 | 0.023 | −0.432 | 0.033 |
| E/A | −0.346 | 0.021 | −0.335 | 0.043 |
| IVRT | 0.321 | 0.05 | 0.337 | 0.045 |
| S/D | −0.406 | 0.01 | −0.308 | 0.037 |
| LVM | 0.375 | 0.022 | 0.401 | 0.002 |
| LVMI | 0.336 | 0.031 | 0.452 | <0.001 |
| Hemodynamic | ||||
| Aodiastolic | −0.132 | 0.326 | −0.323 | 0.043 |
| CPP | 0.394 | 0.005 | 0.404 | 0.001 |
| LVEDP | 0.501 | 0.001 | 0.428 | 0.008 |
Multiple stepwise regression analysis
There were several factors influencing LAVI estimated by multivariate stepwise regression analysis, including: BNP, HbA1C, LVEDP, CPP and ASI. Multiple stepwise regression analysis showed that the ASI was independently associated with BNP, HbA1C, LDL-chol, CRP, and CPP (Table 6).
Table 6.
The comparison of calculated cut-off value of ASI and LAVI to selected hemodynamic and laboratory data in patients with DM2.
| LAVI < cut-off value (n=54) | LAVI > cut-off value (n=46) | p | |
|---|---|---|---|
| Biochemical data | |||
| BNP [fmol/ml] | 141.3 (±57.9) | 202.5 (±87.6) | 0.001 |
| HbA1C [%] | 6.01 (±2.7) | 9.11 (±3.9) | 0.003 |
| cTnT [μg/l] | 0.058 (±0.014) | 0.061 (±0.018) | NS |
| Creatynine [mg/dl] | 1.1 (±0.4) | 1.5 (±0.4) | 0.046 |
| GFR [ml/min/1.73 m2] | 48.9 (±16.4) | 78.3 (±21.3) | 0.011 |
| Uric acid [mg/dl] | 5.8 (±1.5) | 8.4 (±3.2) | 0.036 |
| hs-CRP [mg/ml] | 3.5 (±1.4) | 7.4 (±1.9) | 0.012 |
| Fibrinogen [g/l] | 3.4 (±0.8) | 6.4 (±0.8) | 0.029 |
| T-chol [mg/dl] | 218.4 (±65.7) | 237.4 (±54.4) | NS |
| LDL-chol [mg/dl] | 131.2 (±37.8) | 159.3 (±36.8) | 0.041 |
| Hemodynamic data | |||
| CPP [mmHg] | 50.3 (±7.5) | 67.9 (±15.7) | 0.019 |
| LVEDP [mmHg] | 7.8 (±2.0) | 16.1 (±5.2) | <0.001 |
| ASI < cut-off value (n=51) | ASI > cut-off value (n=49) | p | |
| Biochemical data | |||
| BNP [fmol/ml] | 148.1 (±52.5) | 185.5 (±74.6) | 0.012 |
| HbA1C [%] | 6.31 (±2.9) | 8.81 (±3.3) | 0.022 |
| cTnT [μg/l] | 0.060 (±0.022) | 0.059 (±0.014) | NS |
| Creatynine [mg/dl] | 1.2 (±0.7) | 1.4 (±0.3) | NS |
| GFR [ml/min/1.73 m2] | 57.2 (±18.7) | 69.2 (±24.3) | 0.043 |
| Uric acid [mg/dl] | 5.5 (±1.8) | 8.6 (±3.7) | 0.016 |
| hs-CRP [mg/ml] | 2.7 (±1.0) | 8.1 (±2.2) | 0.002 |
| Fibrinogen [g/l] | 4.5 (±0.6) | 5.4 (±0.9) | NS |
| T-chol [mg/dl] | 207.1 (±65.7) | 246.3 (±78.4) | 0.025 |
| LDL-chol [mg/dl] | 125.6 (±33.6) | 166.1 (±37.2) | 0.011 |
| Hemodynamic data | |||
| CPP [mmHg] | 48.2 (±8.9) | 69.3 (±16.7) | 0.009 |
| LVEDP [mmHg] | 9.1 (±2.2) | 14.9 (±6.2) | 0.002 |
NS – not significant.
Discussion
The ascending aorta and its arch are mainly composed of elastic fibers that stretch during systole and contract to their original length during diastole. This allows the aorta to store energy during systole and gives it back during diastole, thus supporting the heart in its action of pumping blood. Loss of aortic wall elasticity results in systolic hypertension, which causes left ventricular myocardial hypertrophy, and increases oxygen demand, diastolic dysfunction, and valve incompetence [22].
Assessment of subclinical target organ damage, including arterial stiffness, has been considered essential for the evaluation of cardiovascular risk, the choice of treatment, and the follow up in different clinical settings. The present study showed that DM2 deteriorates elastic properties of the aorta and that there is a link, which is both hemodynamically and metabolically mediated, between aortic stiffness and left atrium enlargement. We, for the first time, have demonstrated that LAVI reflects the long-standing hemodynamic condition in DM2 patients.
The most important, and thus far unreported, finding of the present study is that there is a relationship between left atrium enlargement and both aortic stiffness and aortic CPP in patients with DM2. In this study DM2 individuals with higher LAVI had both increased ASI and aortic CPP. To our best knowledge this is the first study to report unfavorable simultaneous influence of both ASI and CPP on left atrium size expressed as LAVI. It was previously reported that pulse pressure, measured noninvasively, was the best determinant of left atrial enlargement in non-dippers with never-treated essential hypertension [23]. Pulse pressure influences atrial function because noninvasively obtained pulse pressure has emerged as an important predictor of incident atrial fibrillation, even after considering other clinical variables known to be associated with this arrhythmia (including left atrial size measured in M-mode, left ventricular mass, and left fractional shortening) [24]. Recently, it has been suggested that central aortic blood pressure has higher predictive risk for cardiovascular disease than does peripheral brachial artery blood pressure because the left ventricle pumps directly against the afterload in the central arteries [5]. Diabetes mellitus is a part of metabolic syndrome. There is strong association between body mass index and waist-to-hip ratio, components of metabolic syndrome, and both pulse pressure and central systolic blood pressure [25]. We found that ASI was significantly higher in patients with DM2 than in subjects without impaired glucose metabolism. There was also a strong correlation between ASI and CPP and central diastolic pressure. Moreover, in multivariate analysis, ASI contributed significantly and independently to CPP. Pulse pressure has been traditionally believed to increase cardiovascular risk because of an increase in afterload leading to left ventricular hypertrophy. It has also been emphasized that low diastolic blood pressure, being in part responsible for high CPP, leads to an impairment of myocardial perfusion with all its adverse consequences. Currently, the presence of a bidirectional link between atherosclerosis and CPP is commonly postulated, meaning that an increased CPP may be both a cause and an effect of atherosclerosis.
Although the mechanism by which DM2 leads to left atrium enlargement is unclear, there are 2 possible explanations. One is that in DM2 accumulation of some glycosides in the atrial wall may cause atrial cardiomyopathy, as it is reported to result in left ventricle diabetic cardiomyopathy [26]. We found a strong correlation between LAVI and HbA1C, supporting the concept of a specific atrial diabetic cardiomyopathy, which may be related to glycemia control. Chronic hyperglycemia can lead to nonenzymatic glycation of amino acids on tissue proteins leading to formation of advance glycation products [27]. This process leads to early reversible glycation products, followed by irreversible Amadori products and subsequent diabetic cardiomyopathy. An enlarged left atrium may also be part of the definition of diabetic cardiomyopathy as a specific atrial cardiomyopathy. The clinical significance of the glucose tolerance status for atrial function emphasized recently by Iguchi et al. [28] stated that presence of atrial fibrillation appears to be associated with the level of HbA1C, especially in patients with HbA1C <6.5%. The concept of diabetic atrial cardiomyopathy is also supported by 2 further important findings of our study. The first finding is the independent association between LAVI and BNP, as well as a correlation between LAVI and diastolic dysfunction, observed in our patients. BNP is a cardiac hormone predominantly released from the cardiac ventricle in response to left ventricular volume expansion and pressure overload. BNP can be reliably used to screen diabetic patients for the presence of left ventricular dysfunction [29]. During diastole, the left atrium is directly exposed to left ventricle pressure, which increases due to left ventricle dysfunction. Consequently, left atrium pressure increases in order to maintain adequate left ventricle filling. This causes an increase in left atrium wall tension, resulting in stretching and enlargement of the left atrium. Thus LAVI reflects chronic exposure of the left atrium to deteriorated left ventricle diastolic function and the resultant increased left atrium filling pressure. LAVI is recognized as a relatively load-independent marker of left ventricular filling pressures. In fact, in our diabetic patients, left ventricle diastolic pressure was significantly elevated compared to normal subjects and was independently influenced by LAVI. The second finding is the independent correlation between ASI and BNP observed in our study. Previous studies have also reported that left ventricular diastolic function in DM2 patients is linked to aortic stiffness [30]. The results of this study may provide mechanistic insight into the association between aortic CPP and aortic stiffness and end-organ damage, particularly left ventricle diastolic dysfunction [30].
It is well known that aortic elastic properties are affected by atherosclerosis risk factors, including diabetes mellitus. HbA1C, hs-CRP, and LDL-cholesterol were among laboratory independent predictors of ASI in multivariate analysis. The independent association between ASI and HbA1C suggests that poor glycemia control is independently associated with impaired aortic elastic properties and may lead to aortic stiffness. Our data is in agreement with the large, but non-invasive, Hoorn study, which showed that deteriorating glucose tolerance status was associated with increased arterial stiffening [31]. Although the mechanism by which DM2 leads to an increase in aortic stiffness is unclear, the following is a possible explanation. In DM2 accumulation of some glycosides in the arterial wall may cause the stiffness [26]. No enzymatic glycosylation of the elastin and collagen of the aortic wall may contribute to the functional abnormalities of the aorta in patients with DM2, but even high glycosylated hemoglobin concentrations considered within the normal range may influence aortic function in nondiabetic individuals with normal glucose tolerance [32]. Nonenzymatic glycosylation of proteins and formation of advanced glycation end-products may have adverse effects on tissue structure and function by generation of reactive oxygen species [33]. A similar action is attributed to 2 biochemical markers, CRP and LDL-chol, which markedly influenced ASI in the present study. Both of them, together with glycosylated hemoglobin, may lead to endothelial cell dysfunction and, in consequence, relaxation of the aortic wall; thus, the aortic stiffness and high aortic CPP may be cumulative and unfavorable effects of these metabolically active factors. Whatever the mechanism, aortic stiffness and aortic CPP are associated with high mortality in patients with DM2.
Conclusions
In conclusion, this is the first simultaneous hemodynamic and echocardiographic study, and it demonstrates for the first time that there is a link, hemodynamically- and metabolically-mediated, between aortic stiffness and left atrium enlargement. The elevation of either LVEDP or CPP suggests deterioration of hemodynamic performance in DM patients. The strong correlation between HbA1C and both LAVI and ASI is a sign of negative influence of poor glycemia control on left ventricle diastolic function and elastic properties of the aorta. The independent correlation between LAVI and ASI, CPP, and LVEDP, improved by association of LAVI and ASI with specific biochemical markers, suggests association between left atrium enlargement and elastic properties of the aorta, as well as CPP in DM2 patients. The clinical importance of these findings rests in the potential for improving diastolic function by lowering aortic stiffness and central pulse pressure, an untested hypothesis that requires further studies.
Table 7.
Factors influencing ASI and LAVI estimated by multivariate stepwise regression analysis in patients with DM2.
| DM | |||||
|---|---|---|---|---|---|
| Dependent variable | Independent variables | B | Standard error | β | P |
| LAVI | BNP | 1.27 | 0.425 | 0.423 | <0.001 |
| HbA1C | 0.645 | 0.053 | 0.379 | 0.008 | |
| LVEDP | 0.548 | 0.176 | 0.381 | 0.006 | |
| CPP | 0.342 | 0.067 | 0.312 | 0.020 | |
| ASI | 2.23 | 0.34 | 0.331 | 0.011 | |
| ASI | BNP | 0.516 | 0.153 | 0.308 | 0.016 |
| HbA1C | 1.845 | 0.365 | 0.401 | 0.004 | |
| LDL-chol | 0.221 | 0.083 | 0.301 | 0.024 | |
| hs-CRP | 4.167 | 1.525 | 0.372 | 0.005 | |
| CPP | 0.297 | 0.032 | 0.426 | 0.001 | |
Footnotes
Conflict of interest
None declared.
Source of support: Self financing
References
- 1.Pyorala K, Laakso M, Uusitupa M. Diabetes and atherosclerosis: an epidemiologic view. Diabetes Metab Rev. 1987;3:463–524. doi: 10.1002/dmr.5610030206. [DOI] [PubMed] [Google Scholar]
- 2.Westerhof N, O’Rourke MF. Hemodynamic basis for the development of left ventricular failure in systolic hypertension and for its logical therapy. J Hypertens. 1995;13:943–52. doi: 10.1097/00004872-199509000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Cruickshank K, Riste L, Anderson SG, et al. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–90. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 4.Woodman RJ, Watts GF. Measurement and application of arterial stiffness in clinical research: focus on new methodologies and diabetes mellitus. Med Sci Monit. 2003;9(5):RA81–89. [PubMed] [Google Scholar]
- 5.Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50:197–203. doi: 10.1161/HYPERTENSIONAHA.107.089078. [DOI] [PubMed] [Google Scholar]
- 6.Dart AM, Kingwell BA. Pulse pressure – a review of mechanisms and clinical relevance. J Am Coll Cardiol. 2011;37:975–84. doi: 10.1016/s0735-1097(01)01108-1. [DOI] [PubMed] [Google Scholar]
- 7.Woodman RJ, Kingwell BA, Beilin LJ, et al. Assessment of central and peripheral arterial stiffness. AHJ. 2005;18:249–60. doi: 10.1016/j.amjhyper.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 8.Philippe F, Chemaly E, Blacher J, et al. Aortic pulse pressure and extent of coronary artery disease in percutaneous transluminal coronary angioplasty candidates. AHJ. 2002;15:672–77. doi: 10.1016/s0895-7061(02)02961-8. [DOI] [PubMed] [Google Scholar]
- 9.Boutouyrie P, Bussy C, Lacolley P, et al. Association between local pulse pressure, mean blood pressure, and large-artery remodelling. Circulation. 1999;100:1387–93. doi: 10.1161/01.cir.100.13.1387. [DOI] [PubMed] [Google Scholar]
- 10.Tomori T, Keiko S, Shinkichi H, et al. Carotid atherosclerosis and arterial peripheral pulse wave velocity in cerebral thrombosis. J Clin Neurosci. 2006;13:45–49. doi: 10.1016/j.jocn.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Subherval S, de las Fuentes L, Waggoner AD, et al. Central aortic pressure is independently associated with diastolic function. Am Heart J. 2010;159:1081–88. doi: 10.1016/j.ahj.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eren M, Gorgulu S, Uslu N, et al. Relation between aortic stiffness and left ventricular diastolic function in patients with hypertension, diabetes, or both. Heart. 2004;90:37–43. doi: 10.1136/heart.90.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lartaud-Idjoiadiene I, Lompre AM, Kieffer P, et al. Cardiac consequences of prolonged exposure to an isolated increase in aortic stiffness. Hypertension. 1999;34:63–69. doi: 10.1161/01.hyp.34.1.63. [DOI] [PubMed] [Google Scholar]
- 14.Lantelme P, Laurent S, Besnard C, et al. Arterial stiffness is associated with left atrial size in hypertensive patients. Arch Cardiovasc Dis. 2008;101:35–40. doi: 10.1016/s1875-2136(08)70253-5. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2009;(Suppl 1):S62–67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel M, Spertus J, Brindis R, et al. ACCF proposed method for evaluating the appropriateness of cardiovascular imaging. J Am Coll Cardiol. 2005;46:1606–13. doi: 10.1016/j.jacc.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 17.Stefanadis C, Stratos C, Boudoulas H, et al. Distensibility of the ascending aorta: comparison of invasive and noninvasive techniques. Eur Heart J. 1990;11:990–96. doi: 10.1093/oxfordjournals.eurheartj.a059639. [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Rakowski H, Appleton C, Chan K. Canadian consensus recommendations for the measurement and reporting of diastolic dysfunction by echocardiography: from the Investigators of Consensus on Diastolic Dysfunction by Echocardiography. J Am Soc Echocardiogr. 1996;9:736–60. doi: 10.1016/s0894-7317(96)90076-0. [DOI] [PubMed] [Google Scholar]
- 20.Ohte N, Saeki T, Miyabe H, et al. Relationship between blood pressure obtained from the upper arm with a cuff-type sphygmomanometer and central-tipped micromanometer. Heart Vessels. 2007;22:410–15. doi: 10.1007/s00380-007-0998-5. [DOI] [PubMed] [Google Scholar]
- 21.Levin DC, Gardiner GA. Coronary angiography. In: Braunwald E, editor. Heart Disease: A Textbook of Cardiovascular Medicine. Philadelphia, Pa: WB Saunders Co; 1992. pp. 235–75. [Google Scholar]
- 22.Karwowski W, Naumik B, Szczepański M, Myśliwiec M. The mechanism of vascular calcification – a systematic review. Med Sci Monit. 2012;18(1):RA1–11. doi: 10.12659/MSM.882181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Triantafyllidi H, Ikonomidis I, Lekakis J, et al. Pulse pressure determines left atrial enlargement in non-dipper patients with never-treated essential hypertension. J Hum Hypertens. 2007;21:897–99. doi: 10.1038/sj.jhh.1002236. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell GF, Vasan RS, Keyes MJ, et al. Pulse pressure and risk of new-onset atrial fibrillation. JAMA. 2007;297:709–15. doi: 10.1001/jama.297.7.709. [DOI] [PubMed] [Google Scholar]
- 25.Budimir D, Jeroncic A, Gunjaca G, et al. Sex-specific association of anthropometric measures of body composition with arterial stiffness in a healthy population. Med Sci Monit. 2012;18(2):CR65–71. doi: 10.12659/MSM.882457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brownlee M. Glycation products and the pathogenesis of diabetic complications. Diabetes Care. 1992;15:1835–43. doi: 10.2337/diacare.15.12.1835. [DOI] [PubMed] [Google Scholar]
- 27.Avendano GF, Agarval RK, Bashey RI, et al. Effects of glucose intolerance on myocardial function and collage-linked glycation. Diabetes. 1999;48:1443–47. doi: 10.2337/diabetes.48.7.1443. [DOI] [PubMed] [Google Scholar]
- 28.Iguchi Y, Kimura K, Shibazaki K, et al. HbA1C and atrial fibrillation: A cross-sectional study in Japan. Int J Cardiol. 2012;156:156–59. doi: 10.1016/j.ijcard.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 29.Epshteyn V, Morrison K, Krishnaswamy P, et al. Utility of B-type natriuretic peptide (BNP) as a screen for left ventricular dysfunction in patients with diabetes. Diabetes Care. 2003;26:2081–87. doi: 10.2337/diacare.26.7.2081. [DOI] [PubMed] [Google Scholar]
- 30.Eren M, Gorgulu S, Uslu N, et al. Relation between aortic stiffness and left ventricular diastolic function in patients wit hypertension, diabetes, or both. Heart. 2004;90:37–43. doi: 10.1136/heart.90.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henry RMA, Kostense PJ, Spijkerman MW, et al. Arterial stiffness increases with deteriorating glucose tolerance status. The Horn Study. Circulation. 2003;107:2089–95. doi: 10.1161/01.CIR.0000065222.34933.FC. [DOI] [PubMed] [Google Scholar]
- 32.Stakos DA, Schuster DP, Sparks EA, et al. Association between glycosylated haemoglobin, left ventricular mass and aortic function in nondiabetic individuals with insulin resistance. Eur J Endocrinol. 2007;157:63–68. doi: 10.1530/EJE-07-0121. [DOI] [PubMed] [Google Scholar]
- 33.Wautier JL, Schmidt AM. Protein glycation: a firm link to endothelial cell dysfunction. Circ Res. 2004;95:233–38. doi: 10.1161/01.RES.0000137876.28454.64. [DOI] [PubMed] [Google Scholar]