Abstract
Background
Growth hormone (GH) plays an incompletely understood role in the development of the central nervous system (CNS). In this study, we use transgenic mice expressing a growth hormone antagonist (GHA) to explore the role of GH in regulating postnatal brain, spinal cord and body growth into adulthood. The GHA transgene encodes a protein that inhibits the binding of GH to its receptor, specifically antagonizing the trophic effects of endogenous GH.
Results
Before 50 days of postnatal age, GHA reduces spinal cord weight more than brain weight, but less than body weight. Thereafter, GHA ceases to inhibit the increase in body weight, which approaches control levels by day 150. In contrast, GHA continues to act on the CNS after day 50, reducing spinal cord growth to a greater extent and for a longer duration than brain growth.
Conclusions
Judging from its inhibition by GHA, GH differentially affects the magnitude, velocity and duration of postnatal growth of the brain, spinal cord and body. GH promotes body enlargement more than CNS growth early in postnatal life. Later, its CNS effects are most obvious in the spinal cord, which continues to exhibit GH dependence well into adulthood. As normal CNS growth slows, so does its inhibition by GHA, suggesting that reduced trophic effects of GH contribute to the postnatal slowing of CNS growth. GHA is a highly useful tool for studying the role of endogenous GH on organ-specific growth during aging.
Background
The mammalian CNS acquires most of its mature size after birth. Human brain weight increases 3–4 fold between birth and maturity [1], while the spinal cord enlarges about 10 times postnatally [2]. After maturity is reached, the human brain declines in size by approximately 2% each decade [1], while spinal cord size declines approximately 3% per decade [3].
Growth hormone helps to drive postnatal CNS growth. Evidence for its role comes from several sources, including reduced brain size in human GH deficiency disorders and in experimental animals [4,5]. Transgenic mice have been especially useful in demonstrating the role of GH in CNS growth [6-9]. Strong evidence for a trophic role of GH in brain growth has come from transgenic mice whose somatotropes were selectively destroyed after birth by the expression of a GH promoter-driven diphtheria toxin transgene [6,10,11]. Additional evidence for trophic effects of GH on the CNS was obtained from transgenic mice that overexpress GH. Our laboratory previously found a 12% increase in mean brain weight in adult mice expressing excess GH (GH+) [7], consistent with other studies showing slight brain enlargement in such animals [8,9].
There is much less information about the effects of GH on the spinal cord than on the brain. We previously reported a mean increase of 35% in spinal cord weight in GH+ mice, indicating that the spinal cord has a much greater capacity for GH-dependent, supranormal growth than does the brain [7]. There is no information about the effects of reduced endogenous GH action on spinal cord size in transgenic mice. To examine further the role of GH in postnatal spinal cord, brain and body growth, we have utilized transgenic mice expressing GHA, an antagonist of human GH that inhibits the binding of endogenous GH to its receptor [12]. We find evidence for differential trophic effects of GH on the magnitude, rate and duration of postnatal growth of the body, brain and spinal cord in GHA transgenic mice, providing strong evidence for organ-specific dependence on GH during early postnatal life and adulthood.
Results
Postnatal body weight increase in WT(wild-type) mice
Body weights differed significantly (p < 0.05 or less) between males and females in WT mice at all ages. A period of early, rapid body growth was observed in WT mice until about day 50 (Fig. 1, solid lines). Between the age of weaning (day 21) and day 50, the rate of increase in body weight in WT males (0.51 ± 0.04 g/day) was about 50% faster than that in females (0.34 ± 0.03 g/day). By postnatal day 50, male and female mice had achieved slightly over three-quarters of the body weight achieved at 150 days (Fig. 1). Thereafter, the rate of body growth decreased 6- to 8-fold, but continued to the extent that at 150 days, body weights in WT mice of each gender in Fig. 1 were significantly higher (p < 0.01) than at 50 days of age. By 150 days of age, body weight was about 3.2 times higher than wean weight in WT males and 2.8 times in WT females. Body length reached a plateau in WT mice between postnatal days 75 and 100 (data not shown).
Figure 1.
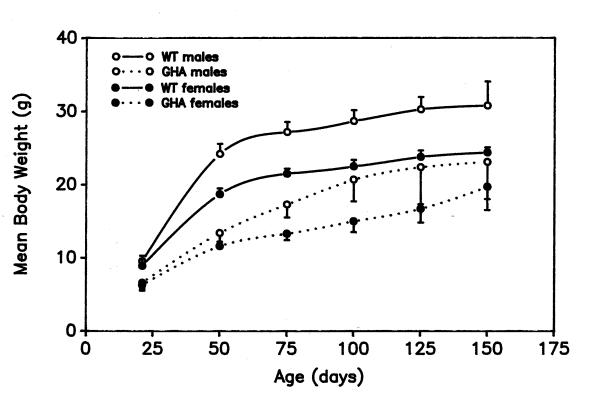
Body weights of male and female GHA and WT mice between 21 and 150 days of age Mean body weight (± SD) are shown for an average of 12 animals from different litters at each time point. Differences between GHA and WT mice of each gender were significant to at least p < 0.05 at each age analyzed.
GHA strongly inhibits early postnatal body weight
At the time of weaning, GHA mice were already 30% smaller (p < 0.01) than gender-matched, WT mice (Fig. 1, day 21). At each successive age analyzed, differences in body weight between GHA and control mice remained significant (p < 0.01), except for 150 day-old males. Body weight increased rapidly in GHA mice between weaning and day 50, as it did in WT mice. GHA strongly inhibited the rate of increase in body weight during this period of early, rapid growth, with body weight increasing 0.23 ± 0.02 g/day in GHA males and 0.18 ± 0.2 g/day in GHA females, about one-half as fast as in WT mice. At day 50, GHA males weighed 56% less than WT males (Fig. 1), and GHA females weighed 62% less than WT females. By day 50, male and female transgenic mice had reached only 58.0% and 50.0%, respectively, of the body weight they were to achieve by day 150 (Fig. 1). By 150 days of age, GHA and females had attained 3.5 and 3.1 times their wean weight, respectively.
GHA does not inhibit body weight increases after day 50
Figure 2 shows the body weights of GHA mice expressed as a percentage of gender-matched, littermate controls. Intriguingly, the growth inhibitory effect of the GHA transgene on body weight was lost after day postnatal 50, and GHA mice grew at a faster rate than WT mice until day 150. By day 150, body weights in GHA males and females had increased to 93.0 ± 5.4% and 78.9 ± 3.9%, respectively, of WT littermates. In contrast, body length in GHA mice remained almost constant in relation to WT mice (males: 84 ± 3%; females: 83 ± 2%) between postnatal days 25 and 150. These findings highlight the period between days 21 and 50, a period of rapid increase in postnatal body weight, as the time when GHA has its most potent inhibitory effect on body mass. After that period, GHA fails to inhibit body growth and GHA mice exhibit catch-up growth.
Figure 2.
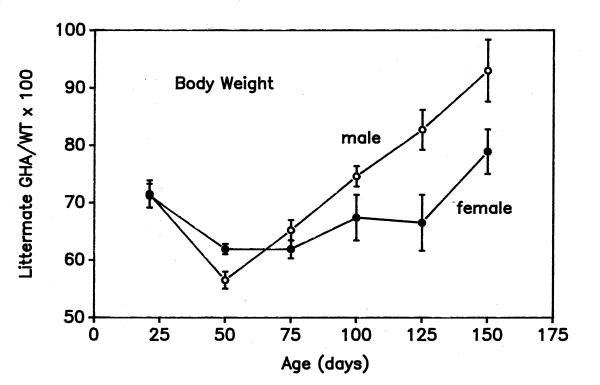
Age-dependent changes in body weight of GHA mice relative to WT littermate controls Data are expressed as a mean percent of WT values ± SEM. Note the loss of effective inhibition of body growth by GHA after 50 days of age in both male and female mice.
GHA inhibits the magnitude and velocity of postnatal brain growth
There was no statistically significant difference in brain weight between males and females in either the WT or GHA mice, so data from both genders were pooled. Brain weight was significantly smaller in GHA than WT mice at every age studied (Fig. 3). At an early age, GHA inhibited brain weight less than body weight. For example, in 25 day-old littermates, average brain weight in GHA mice was 89.8 ± 4.3% of WT brain weight, while body weight in GHA mice was 60.2 ± 1.1% that of WT animals in females and 49.4 ± 6.4% in males. Consistent with the early inhibition of body weight by GHA, brain weight in 25 day-old littermates was 2.2 ± 0.1% of body weight in WT and 4.0 ± 0.2% in GHA males and 2.5 ± 0.04% and 3.8 ± 0.1%, respectively, in females. By day 50, after a period of relatively rapid growth (Fig. 3), the brain reached 96% of its 150-day size in WT mice and 94% in GHA mice. Thereafter, the brain grew more slowly in both WT and GHA mice, being significantly larger (p < 0.05 or less) than at 50 day by day 150 in WT animals and by day 100 in GHA animals. Thus, most brain growth had occurred by day 50 in both the control and GHA mice.
Figure 3.
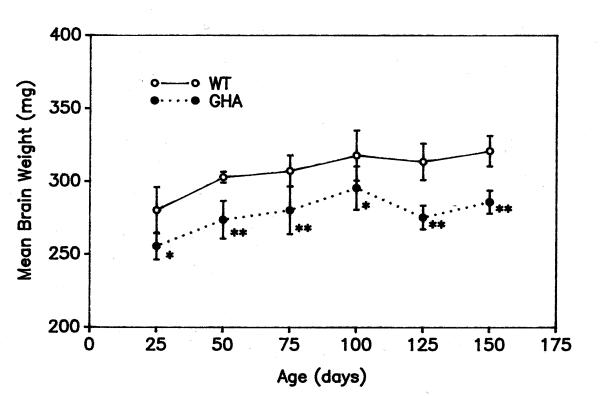
Brain weights in GHA and WT mice between 21 and 150 days of age. Mean brain weights (± SD) are shown for an average of 7 animals (sexes combined) from different litters at each time point. *p < 0.05; **p < 0.01
GHA inhibits the magnitude and velocity of postnatal spinal cord growth
As with the brain, there was no significant gender difference in spinal cord weight in either control or GHA mice. GHA mice had significantly smaller spinal cords than WT mice at each time point analyzed (Fig. 4). In 25 day-old male and female littermates, average spinal cord weight in GHA mice was 86.1 ± 1.2% of WT spinal cord weight. At that early age, spinal cord weight in littermate mice was 0.39 ± 0.04 % of body weight in WT and 0.66 ± 0.04% in GHA males, and 0.43 ± 0.0% and 0.60 ± 0.02%, respectively, in females. Spinal cord enlargement, like that of the brain, was more rapid before postnatal day 50 than afterwards in both WT and GHA mice. On the other hand, by day 50 the spinal cord had achieved only 70.7% of its weight at 150 days in WT mice and 75.4% in GHA mice (Fig. 4). Thus, after postnatal day 50, there was considerably more growth of spinal cord than the brain in both WT and GHA mice.
Figure 4.
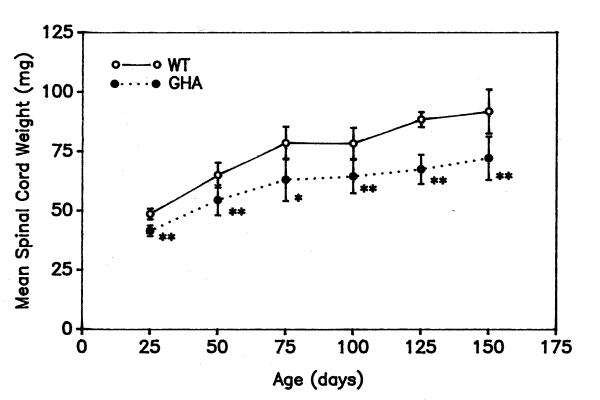
Spinal cord weights in GHA and WT mice between 21 and 150 days of age. Mean spinal cord weights (± SD) are shown for an average of 7 animals (sexes combined) from different litters at each time point. *p < 0.05; **p < 0.01
GHA has more potent and prolonged inhibitory effects on spinal cord than brain growth
Fig. 5 shows brain and spinal cord weight in GHA mice as a percentage of littermate controls. Of particular interest was the duration of GHA inhibition on postnatal growth, one measure of which is the age when the ratio of GHA to WT values no longer declines. Most inhibition of brain weight by GHA had occurred by the time of weaning and little further reduction was observed thereafter, with the difference between brain weight in the GHA and control mice remaining constant at about 10%. In contrast, GHA progressively inhibited spinal cord weight until postnatal days 125–150, when spinal cord weight in GHA mice had become 24.5 ± 6.5% less than that of their WT littermates (Fig. 5). Thus, GHA inhibition of spinal cord growth relative to littermate controls persisted after day 50, while brain growth and its inhibition by GHA were minimal after day 50.
Figure 5.
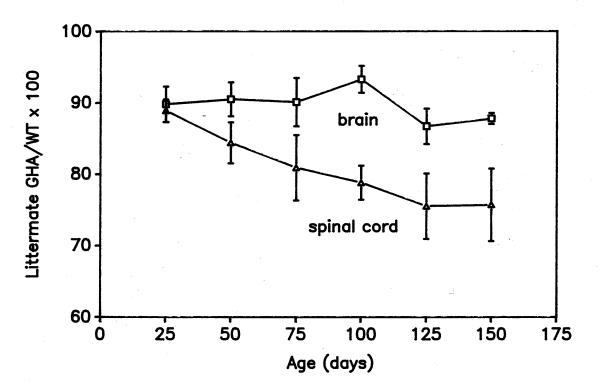
Age-dependent change in brain and spinal cord weight of GHA mice relative to WT littermate controls Data are expressed as a mean percent of WT values ± SEM. Note that GHA continues to reduce spinal cord after postnatal day 50, but has little, if any, further effect on brain size. First-order regression analyses showed a significant (p < 0.01) correlation between age and the y-axis values for the spinal cord (R = 0.97), but no significant correlation for the brain (R = 0.26).
Discussion
GHA differentially inhibits the postnatal growth of the body, brain and spinal cord. Because GHA antagonizes the action of GH at the GH receptor, we infer that endogenous GH also must differentially influence their growth. Much of the trophic action of GH on the CNS is probably mediated by IGF-1, which increases brain and spinal cord weight largely by increasing white matter [6,7,11,13]. Spinal motor neuron size is also increased postnatally by GH, but may not depend upon IGF-1 [7].
The contribution of GH to body, spinal cord and brain growth
The data presented here imply that GH action is responsible for at least 10% of the weight of the mature brain and at least 24% of the weight of the mature spinal cord in the mouse. These estimates are low, since there is evidence that GHA does not completely inhibit GH action. Mice made null for GH by toxin-mediated ablation of somatotropes [6,11] showed greater inhibition of brain and body growth than did GHA mice at the same age in our study (Fig. 6). Comparisons among GHA, GH null and GH+ mice [7] mice also provide strong evidence for differential effects of GH on body, brain and spinal cord growth. Fig. 6 compares the relative magnitude of GH effects on growth in these three different mutant models and includes published data from our laboratories [7,11] and others [6]. The relative effect of GH perturbation in all three types of mice in Fig. 6 is the same, being least on brain weight and most on body weight. While data are available for the spinal cord only in GHA and GH+ mice, our findings support a more potent requirement for GH as a mediator of postnatal spinal cord growth than brain growth.
Figure 6.
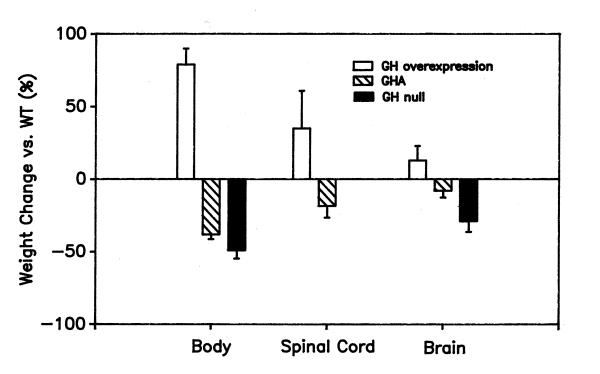
Comparison of body and CNS growth in transgenic mice with increased and decreased GH action Mean percent changes (± SD) reported for GH+ [7] and GH null mice [6,11] are compared with data from the present study. The average age of the mice was 65 ± 9 days. Note that the rank order of GH and GHA effects on growth is the same in all three experimental situations.
An intriguing finding from our study was that GHA mice exhibited catch-up growth after day 50 by increasing body growth rates to levels higher than in WT mice. During this period, body length showed a constant inhibition of 16.2 ± 0.03% in all GHA mice. Part of the catch-up growth may be due to greater increases in adipose tissue mass in GHA mice, as we have reported previously [14]. It is also possible that high levels of GHA and inhibition of GH action in the period of rapid growth between day 25 and 50 elicit compensatory increases in GH responsiveness either at the level of GH receptor expression or subsequent intracellular signal transduction systems.
Does GH play a role in the slowing of postnatal CNS growth?
Relative to its weight at birth, the spinal cord grows more postnatally than does the brain [1,2], and GH stimulates the enlargement of the spinal cord more than the brain [7]. Normal postnatal brain growth slows earlier than spinal cord growth (Figs. 3, 4), at the same time as GHA inhibition of their growth diminishes (Fig. 5). Taken together, these data suggest that cessation of GH action plays a role in the age-related slowing of brain and spinal cord enlargement. Three possible mechanisms by which GH might influence the slowing of CNS growth are: 1) a decrease in the concentration of circulating GH with age; 2) a decrease in GH responsiveness in the CNS at the level of the GH receptor or downstream events; or 3), hypothetically, an age-dependent increase in a growth antagonist that competes with the trophic action of GH on the CNS. GH responsiveness includes the GH-dependent synthesis and action of IGF-I, which has powerful trophic effects on postnatal growth of the brain [6,11,13] and spinal cord [7]. There is an inverse correlation between serum GHA and IGF-I concentrations among GHA mice [12].
An age-dependent decrease in the concentration of GH accessible to all parts of the CNS cannot account for the observation that GHA continued to inhibit spinal cord growth well after its action on brain growth was no longer apparent (Fig. 6). For the same reason, if either an age-related decrease in GH responsiveness or the action of some growth antagonist decreased the rate of brain growth, then such changes must have occurred more slowly in the spinal cord than in the brain. A decrease in GH responsiveness is consistent with reports of age-dependent decreases in GH binding in human brain [15] and in rat brain and spinal cord [16]. However, Zhai et al. [16] reported that GH binding began to decline earlier in the rat spinal cord than in the brain.
Does GH help to maintain CNS size in adult mice?
That GH is responsible for some of the postnatal growth of the brain and spinal cord suggests that GH could help to maintain CNS size with age. The dramatic decrease in circulating GH in older human beings [17] might contribute to the age-dependent atrophy observed in the spinal cord [3] and brain [1]. In order to investigate this possibility in experimental animals, one would ideally want to allow the CNS to reach its mature size and then to alter the action of endogenous GH. Transgenic mice expressing an inducible GHA gene could be used to investigate this possibility. Such transgenic mice would be useful in studying not only the effects of GH in the adult mouse CNS, but also a wide variety of other actions GH may have throughout the body.
Conclusions
GH plays a significant, but not an exclusive nor uniform role in postnatal CNS and body growth. Based upon the actions of GHA, we conclude that GH differentially affects the magnitude, velocity and duration of postnatal growth of the brain, spinal cord and body. Early in postnatal life, GH promotes body enlargement more than CNS growth. Later, its CNS effects are most obvious in the spinal cord, which continues to exhibit GH dependence well into adulthood. Studies using GHA transgenic mice could help to determine whether the somatotrophic system contributes to the age-related slowing of CNS growth and whether it helps to maintain CNS size in adulthood.
Methods
GHA mouse line
Mice hemizygous for the GHA transgene were obtained from the laboratory of Dr. John J. Kopchick, Edison Biotechnology Institute, Ohio University. We used line hGH G120R, which bears a human GH transgene that has an arginine substituted for the normal glycine at position 120, converting its product to a GH antagonist [12,18].
When placed under the control of a mouse metallothionein 1 transcriptional control element, expression of the GHA transgene lowered serum levels of IGF-I by approximately 60% and lowered body weights to 60–70% of WT littermate control weights at three months of age [18]. Heterozygous transgenic mice on the original B6SJLF1/J (C57BL/6J × SJL/J) background were bred with B6SJLF1/J WT mice to provide the animals studied here. All mice were reared with their littermates and received normal mouse chow and water containing 25 mM zinc sulfate to maximize transgene expression. Transgenic mice were readily distinguished from their gender-matched, WT littermates between the time of weaning (21 days of age) and day 125. Figure 7 shows the distribution of body weights in male mice at 50 days of age. A strict bimodal distribution of body weights was observed for both males (Fig. 7) and females (data not shown). Each mouse in a litter was tagged at weaning and its weight and length recorded at 25-day intervals. Body length measured from the snout to the proximal end of the tail was used as an index of skeletal growth. Animal care and euthanasia were carried out in accordance with the standards of the University of North Carolina and the American Association of Veterinary Medicine.
Figure 7.
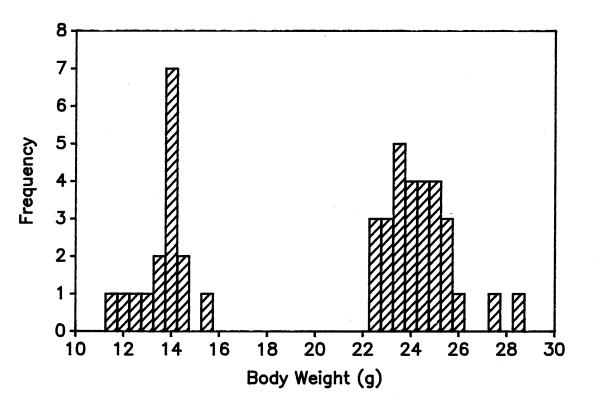
Body weight as a phenotypic marker for pooled litters of GHA and WT mice No overlap between the lower body weights of GHA (left) and heavier WT (right) mice was observed for either 50 day-old male (shown here) or female mice. The mean body weight (± SD) for GHA males at 50 days was 13.4 ± 1.2 g versus 24.2 ± 1.4 g for WT males.
Tissue collection
At 25-day intervals between ages 25 and 150 days, mice were injected i.p. with a mixture of xylazine (4 mg/kg) and ketamine (400 mg/kg), and when fully anesthetized, the animals were decapitated. The brain of each animal, including the cerebellum, but not the brainstem, was dissected and weighed on a Mettler semi-micro (P1210) or micro (H20) balance. The spinal cord was dissected under a stereomicroscope from the mid-cervical through the sacral segments, the cauda equina and all other spinal roots were removed, and the spinal weight was recorded.
Statistical analyses
Means ± s.e.m are presented in the text wherever possible, while percentages only are used when expressing the percent difference between two unpaired means. Tests of significance were performed using Student's paired or unpaired t-test. Where absolute weights are presented, values for WT and GHA mice are derived from all litters, including those containing only WT or GHA mice.
Authors' contributions
DLM designed and supervised the study and participated in the tissue collection and analyses with VBH. The laboratory of JJK created the GHA transgenic line and provided the founder mice and editorial assistance for this study. CRF maintained the mouse colony and participated in tissue collection under the direction of PKL, who helped to analyze and prepare the data for publication.
Acknowledgments
Acknowledgements
This study was supported by grants from the UNC University Research Council to DLM and by NIH grants DK40247 and AG09973 to PKL. JJK is supported in part by the state of Ohio's Eminent Scholars Program that includes a gift from Milton and Lawrence Goll and by DiAthegen LLC. Mouse facilities of the UNC Center for Gastrointestinal Biology and Disease aided this work.
Contributor Information
DL McIlwain, Email: dlmc@med.unc.edu.
VB Hoke, Email: vbhoke@med.unc.edu.
JJ Kopchick, Email: kopchick@mail.biotech.ohiou.edu.
CR Fuller, Email: scarhead@med.unc.edu.
PK Lund, Email: empk@med.unc.edu.
References
- Dekaban AS, Sadowsky D. Changes in brain weights during the span of human life. Ann Neurol. 1978;4:345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- Lassek AM, Rasmussen GL. A quantitative study of newborn and adult spinal cords of man. J Comp Neurol. 1938;69:371–379. [Google Scholar]
- Kameyama T, Hashizume Y, Ando T, Takahashi A. Morphometry of the normal cadaveric cervical spinal cord. Spine. 1994;19:2077–2081. doi: 10.1097/00007632-199409150-00013. [DOI] [PubMed] [Google Scholar]
- Baumann G. Mutations in the growth hormone releasing hormone receptor: a new form of dwarfism in humans. Growth Hormone & IGF Res Suppl B. 1999;9:24–29. doi: 10.1016/s1096-6374(99)80077-x. [DOI] [PubMed] [Google Scholar]
- Noguchi T. Retarded cerebral growth of hormone-deficient mice. Comp Biochem Physiol. 1991;98:239–248. [PubMed] [Google Scholar]
- Behringer RR, Lewin TM, Quaife CJ, Palmiter RD, Brinster RL, D'Ercole AJ. Expression of insulin-like growth factor I stimulates normal somatic growth in growth hormone-deficient transgenic mice. Endocrinol. 1990;127:1033–1040. doi: 10.1210/endo-127-3-1033. [DOI] [PubMed] [Google Scholar]
- Chen L, Lund PK, Burgess SB, Rudisch BE, McIlwain DL. Growth hormone, insulin-like growth factor I, and motoneuron size. J Neurobiol. 1997;32:202–212. doi: 10.1002/(SICI)1097-4695(199702)32:2<202::AID-NEU5>3.3.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Hammer RE, Brinster RL, Palmiter RD. Use of gene transfer to increase animal growth. Cold Spring Harbor Symp Quant Biol. 1985;50:379–387. doi: 10.1101/sqb.1985.050.01.048. [DOI] [PubMed] [Google Scholar]
- Shea BT, Hammer RE, Brinster RL. Growth allometry of the organs in giant transgenic mice. Endocrinol. 1987;121:1924–1930. doi: 10.1210/endo-121-6-1924. [DOI] [PubMed] [Google Scholar]
- Behringer RR, Mathews LS, Palmiter RD, Brinster RL. Dwarf mice produced by genetic ablation of growth hormone-expressing cells. Genes Devel. 1988;2:453–461. doi: 10.1101/gad.2.4.453. [DOI] [PubMed] [Google Scholar]
- Hepler JE, Lund PK. Molecular biology of the insulin-like growth factors. Molec Neurobiol. 1990;4:93–127. doi: 10.1007/BF02935586. [DOI] [PubMed] [Google Scholar]
- Chen WY, Chen N, Yun J, Wagner TE, Kopchick JJ. In vitro and in vivo studies of antagonistic effects of human growth hormone analogs. J Biol Chem. 1994;269:15892–15897. [PubMed] [Google Scholar]
- D'Ercole AJ. Expression of insulin-like growth factor-I in transgenic mice. Ann NY Acad Sci. 1993;692:149–160. doi: 10.1111/j.1749-6632.1993.tb26213.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Knapp JR, Kopchick JJ. Enlargement of interscapular brown adipose tissue in growth hormone antagonist transgenic and in growth hormone receptor gene-disrupted dwarf mice. Exp Biol Med (Maywood) 2003;228:207–215. doi: 10.1177/153537020322800212. [DOI] [PubMed] [Google Scholar]
- Lai Z, Roos P, Zhai Q, Olsson Y, Fholenhag K, Larsson C, Nyberg F. Age-related reduction in human growth hormone binding sites in the human brain. Brain Res. 1993;621:260–266. doi: 10.1016/0006-8993(93)90114-3. [DOI] [PubMed] [Google Scholar]
- Zhai Q, Lai Z, Roos P, Nyberg F. Characterization of growth hormone binding sites in rat brain. Acta Paediatr Suppl. 1994;406:92–95. doi: 10.1111/j.1651-2227.1994.tb13433.x. [DOI] [PubMed] [Google Scholar]
- Hammerman MR. Insulin-like growth factors and aging. Endocrinol Metab Clinics. 1987;16:995–1011. [PubMed] [Google Scholar]
- Chen N, Chen WY, Stiker LJ, Stiker GE, Kopchick JJ. Co-expression of bovine growth hormone (GH) and human GH antagonist genes in transgenic mice. Endocrinol. 1997;138:851–854. doi: 10.1210/en.138.2.851. [DOI] [PubMed] [Google Scholar]


