Abstract
Background
Apolipoprotein E ε4 (ApoE4 carrier) status, sex and cognitive impairment may interact to affect all-cause and cause-specific mortality risk.
Objectives
To confirm associations of ApoE4 carrier status, sex and time-dependent cognitive status with mortality risk, and investigate these associations' joint effects in a cohort of community-dwelling US adults.
Design & Setting
Data from the Baltimore Longitudinal Study of Aging were used.
Participants
Of n=3,047 (First-visit Age:17–98y, 60.1% men), we selected a sample with complete genetic data and with ≥1 visit at age≥50y (n=1,461).
Measurements
Time-to-death from all, cardiovascular or non-cardiovascular causes.
Results
Survival probability was lower for ApoE4 carriers, particularly at oldest ages. Cox proportional hazards model for all-cause mortality yielded a hazard ratio (HR) for ApoE4 carrier vs. non-carriers of 1.31,95%CI:1.02–1.68. This association was also found for cardiovascular mortality. Time-dependent all-cause dementia (HR=1.73, 95%CI:1.33–2.26) and mild cognitive impairment (HR=1.95,95%CI:1.42–2.67) increased all-cause mortality risk, associations also detected for non-cardiovascular mortality. When individuals were free of cognitive impairment, a dose-response relationship with ε4 alleles was found for all-cause mortality (HR=1.40,95%CI:0.94–2.07 for 1 ε4, and HR=2.61; 95%CI:1.12–6.07 for 2 ε4). After Alzheimer's Disease-type (AD) dementia onset, carrying only 1 ε4 allele increased all-cause mortality risk by ~77% compared to non-carriers. ApoE4 carrier status increased all-cause mortality risk in men and interacted with time-dependent AD to increase the risk of this outcome (RERI=2.15; 95% CI:1.22–3.07).
Conclusion
We found that ApoE4 carrier status increased all-cause and cardiovascular mortality risks, while interacting with sex and time-dependent AD status to affect all-cause mortality.
Keywords: Apolipoprotein E genotype, dementia, mild cognitive impairment, mortality, cardiovascular disease
INTRODUCTION
The ApoE ε4 allele is the most robust genetic risk factor for late-onset Alzheimer's Disease (AD), conferring more than a 3-fold increase in risk.1 In an ecological study, ApoE allele frequency differences explained 12%–17% of country-level mortality variations and 1%–2% of variation in older people's life span.2 Moreover, in large cohort studies and meta-analyses, carrying at least 1 ε4 allele (ApoE4 carrier) was related to increased risks of all-cause3–14 and cardiovascular15–18 mortality and higher incidence of coronary heart disease and stroke.18–19 Other studies found no clear association between ApoE4 carrier status and mortality.20–23 It is well-known that cardiovascular disease is the leading cause of death in the United States.24 Nevertheless, evidence is still scarce as to whether ApoE genotypes (including ApoE4 carrier status) are putative risk factors for cardiovascular mortality.15–18, 20–21
Importantly, although cognitive impairment25 and being male24 both consistently increase all-cause mortality risk, no study to our knowledge has assessed whether those two factors exhibit a joint effect with ApoE carrier status to increase all-cause and cause-specific mortality risk. This study analyzed data from a large and long-term cohort of community-dwelling US adults with the following primary objectives: (A) In separate analyses, we replicated associations of ApoE4 carrier status, time-dependent cognitive status and sex with mortality risk; (B) We extended prior studies by systematically examining whether associations between ApoE4 carrier status and mortality risk differed by sex and time-dependent cognitive status; (C) We assessed separately joint effects of ApoE4 carrier status and sex, and those of ApoE4 carrier status and time-dependent cognitive status in their associations with mortality risk.
METHODS
Study Design and participants
We analyzed data from the Baltimore Longitudinal Study of Aging (BLSA), an ongoing prospective open cohort study of community-dwelling adults initiated in 1958.26 Exclusionary criteria are summarized elsewhere.27 Physical, medical history, neurological and neuropsychological examinations were conducted and participants gave informed consent as approved by the Institutional Review Board of Medstar Health Research Institute. Of 3,047 BLSA participants (N1=3,047, First-visit Age: 17–98y, 60.1% men), we included those with complete ApoE genotype data (N2=1,704) of whom participants with ≥1 visit with age≥50y were eligible (N3=1,461). It is worth noting that the main mechanism for missing data on the ApoE genotype was cost-related, whereby only a sub-sample of the BLSA participants was selected at two separate time points for genotyping as described in the “ApoE4 carrier status and dosage” section. By end of follow-up, 1,251 deaths occurred (967 men, 284 women) of N1=3,047, and 355 deaths (233 men, 122 women) of N3=1,461. Mean age ± SD at death were: Men, 85.6y ± 8.6; Women, 87.7y±9.3 (P=0.033, t-test). Mean follow-up time was ~13.7y, with dates of visits ranging between 06 February 1958 and 11 November 2009. Censoring accounted for 9.6% of the BLSA eligible sample (3.6% failed contact, 4.7% withdrew, 0.6% lost to follow-up, 0.1% dropped, and 0.6% unknown reasons) and were excluded from follow-up by last-visit. As a sensitivity analysis, informative censoring was assessed by considering censored individuals to be alternatively assumed dead (scenario A) or alive (scenario B) at last-visit.28 Because the second alternative is the more likely scenario, only scenario B is presented.
Outcome assessment
Participants entered the risk set at age ≥50y, when they became at risk for cognitive impairment, and were followed to three main endpoints (all-cause, cardiovascular or non-cardiovascular mortality) or until censored. All BLSA participants were followed up for vital status. A consensus of 3 physicians determined underlying cause of death and date of death using death certificates, hospital and physician records, and autopsy data, as available. Cardiovascular mortality was ascertained using ICD-9 (390–459)29 and ICD-10 (I11.0–I80.3)30 for underlying causes of death.
ApoE and cognitive status variables
ApoE4 carrier status and dosage
ApoE genotype was determined in ~50% of our sample by polymerase chain reaction amplification of leukocyte DNA followed by HhaI digestion and product characterization at an earlier time point of the study31, and by the TaqMan assay systems in the remaining half relying on several single nucleotide polymorphisms around the ApoE gene at a later time point.32 We focused on the ε4 allele: carriers of 1 or 2 ε4 alleles were labeled ApoE4 carriers and were compared to non-carriers. Dosage of ε4 alleles (0, 1 or 2) was another exposure of interest in part of these analyses.
Cognitive status
All BLSA participants were followed annually and reviewed at a consensus conference if they screened positive on the Blessed Information Memory Concentration score33 (score ≥4), if their Clinical Dementia Rating34 score was ≥0.5 using subject or informant report, or if they screened “abnormal” on the Dementia Questionnaire.35 Irrespective of findings, participants were evaluated by case conference upon death or withdrawal. Dementia diagnoses were determined using DSM-III-R36 criteria. Dementia diagnoses by subtype were formulated during multidisciplinary evaluations with prospectively collected evidence using National Institute of Neurological and Communication Disorders—Alzheimer's Disease and Related Disorders Association criteria for diagnosis of possible, probable and definite AD.37
Mild cognitive impairment (MCI) was diagnosed when (1) cognitive impairment (usually memory) was evident for a single domain or (2) cognitive impairment in multiple domains occurred without any significant functional loss in activities of daily living (ADLs), based on the Petersen criteria38. Onset years for dementia or MCI were determined from the most recent case conference findings. We treated cognitive status as a time-dependent variable, whereby a participant was “cognitively normal” for part of the follow-up and then “all-cause dementia” (AD or non-AD) or MCI after the determined onset age.
Covariates
Potential confounders in the main associations of interest were classified as follows: (1) socio-demographic factors such as individual age at first visit, sex, race (white, Black, other ethnic group), completed years of schooling, and one time-dependent lifestyle factor (never, former or current smoker) and (2) measured first visit body mass index (BMI in kg/m2). Covariates were 83%–100% complete.
Analysis
Analyses were completed with Stata 11.0.39 Differences in continuous variables were assessed using Student's t-test and one-way ANOVA, and χ2 test was used for categorical variables. We defined time-to-event starting from any age≥50y since first-visit (i.e. delayed entry) until death or censoring, and constructed Kaplan-Meier survival curves, comparing exposure groups. Differences in survival were assessed using Wilcoxon-Breslow-Gehan tests for equality or trend in number of all-cause deaths across groups.40–42
We used Cox proportional hazards (PH) models43 to examine covariate-adjusted effects of various predictors (including ApoE4 carrier status) on hazard rates of all-cause mortality and competing risk regression when competing events were cardiovascular vs. non-cardiovascular mortality.28 We estimated hazard ratios and their 95% confidence intervals (CI) from these models. In the main part of the analysis, we first replicated associations of ApoE4 carrier status and dosage, time-dependent cognitive status, and sex with mortality risk (Objective A). Second, we conducted stratified analyses by time-dependent cognitive status and sex, separately, to further examine sex-specific and cognitive status-specific associations between ApoE4 carrier status (as well as dosage) and mortality risk (Objective B). Finally, we assessed joint effects on the additive scale between ApoE4 carrier status and sex and between ApoE4 carrier status and time-dependent cognitive status in their association with mortality risk (Objective C). This was done by adding 2-way interaction terms for sex and time-dependent cognitive status with ApoE4 carrier status in each of the models and computing the relative excess risk due to interaction (RERI)44–45 with its 95% CI. With the null hypothesis being exact additivity, a lower 95%CI bound for the RERI greater than zero was interpreted as indicating super-additive joint effects (or synergism), whereas a lower 95%CI bound for the RERI greater than 1 provided further evidence of synergism without requiring any monotonicity assumption (eMethods 1). A 2-stage Heckman selection procedure was used in all Cox PH and competing risk models to address selection bias due to exclusion of participants without genetic data.46–47
RESULTS
Study sample characteristics by ApoE4 carrier status and cognitive status
Homozygous ε3 (ε3/ ε3) was the most common genotype (59%) in this sample. Heterozygous genotypes with ε2 but no ε4 accounted for 14% and genotypes with ≥1 ε4 alleles accounted for the remaining 27%. There were no differences in the genotype distributions by sex. Participants diagnosed with dementia were younger at first-visit when ApoE4 carriers compared to ApoE4 non-carriers (68.6y vs. 72.5y; Table 1). Irrespective of ApoE4 carrier status, “cognitively normal” individuals were younger at first-visit compared with MCI and dementia groups. ApoE4 carriers had younger dementia onset ages than non-carriers (79.6y vs. 83.1y). Among the ApoE4 carriers, MCI participants had lower educational level compared with the “cognitively normal” group. Higher proportions of non-Hispanic whites were found in the two cognitively impaired groups regardless of carrier status. Among “cognitively normal” participants non-Hispanic blacks were more prevalent in ApoE4 carriers compared to non-carriers (36.8% vs. 22.0%). First-visit mean BMI was lower in dementia compared to the “cognitively normal” group, irrespective of ApoE4 carrier status. Among MCI participants, first-visit BMI was lower in ApoE4 carriers vs. non-carriers. Mean age of all-cause and non-CVD deaths was higher in cognitively impaired individuals in both ApoE4 carrier status groups. Incident AD status accounted for 10% of the overall sample and 78% of all-cause dementia. Sample selectivity was observed and is described in detail in eMethods 2.
Table 1.
Selected characteristics and mortality status of eligible study participants (with at least one visit above age 50y and available genetic data; N=1,461) by ApoE4 carrier status and cognitive status by end of follow-up; Baltimore Longitudinal Study of Aging
| Cognitively normal N=1,179 | Mild Cognitive Impairment N=101 | All-cause dementia N=173 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||
| All (N=1,461) | ApoE4 non-carrier (N=866) | ApoE4 carrier (N=313) | ApoE4 non-carrier (N=74) | ApoE4 carrier (N=27) | ApoE4 non-carrier (N=113) | ApoE4 carrier (N=60) | ||||||||
| %, Mean | (±SD) | %, Mean | (±SD) | %, Mean | (±SD) | %, Mean | (±SD) | %, Mean | (±SD) | %, Mean | (±SD) | %, Mea n | (±SD) | |
| Selected Characteristics | ||||||||||||||
| Age, visit 1 (years) | 59.0 | (±15.0) | 56.6 | (±15.0)b | 55.9 | (±12.7)b | 69.0 | (±12.4) | 66.8 | (±14.9) | 72.5 | (±10.9) | 68.6 | (±130a |
| Age at onset (years) | — | — | — | — | — | 81.4 | (±7.8) | 80.3 | (±8.0) | 83.1 | (±6.7) | 79.6 | (±6. 4)a | |
| Sex (% men) | 52.4 | 52.1 | 47.0 | 59.4 | 63.0 | 61.9 | 55.0 | |||||||
| Education (years) | 16.3 | (±2.7) | 16.3 | (±2.6) | 16.3 | (±2.5)a | 16.0 | (±2.9) | 15.1 | (±3.8) | 16.3 | (±2.8) | 16.1 | (±2.9) |
| Race/ethnicity, % | ||||||||||||||
| Non-Hispanic white | 74.3 | 73.4b | 58.9a,b | 90.5 | 93.6 | 95.6 | 96.7 | |||||||
| Non-Hispanic black | 22.0 | 22.0 | 36.8 | 9.5 | 3.7 | 4.4 | 3.3 | |||||||
| Other ethnicity | 3.7 | 4.6 | 4.3 | 0.0 | 3.7 | 0.0 | 0.0 | |||||||
| Smoking status, visit 1, % | ||||||||||||||
| Never | 38.9 | 36.2 | 38.5 | 43.2 | 66.7 | 44.7 | 40.3 | |||||||
| Former | 43.4 | 45.4 | 45.6 | 34.6 | 18.5 | 40.6 | 44.8 | |||||||
| Current | 17.7 | 18.4 | 15.9 | 22.2 | 14.8 | 14.6 | 14.9 | |||||||
| Body mass index (kg/m2) | 26.0 | (±4.2) | 26.0 | (±4.2)b | 26.4 | (±4.8)b | 26.2 | (±3.7) | 24.5 | (±3.0)a | 25.0 | (±3.3) | 25.1 | (±3.6) |
| Mortality status | ||||||||||||||
| All-cause deaths | ||||||||||||||
| N | 355 | 121 | 43 | 54 | 15 | 85 | 44 | |||||||
| Age at death (years) | 86.3 | (±8.9) | 83.5 | (±10.9)b | 80.6 | (±8.9)b | 88.8 | (±6.0) | 88.2 | (±5.1) | 90.0 | (±5.8) | 88.7 | (±5. 1) |
| Cardiovascular death | ||||||||||||||
| N | 110 | 35 | 14 | 11 | 6 | 26 | 16 | |||||||
| Age at death (years) | 88.1 | (±7.6) | 86.6 | (±10.8) | 86.0 | (±5.0) | 88.5 | (±5.6) | 86.5 | (±5.7) | 91.3 | (±5.1) | 87.1 | (±4.3)a |
| Non-cardiovascular deaths | ||||||||||||||
| N | 245 | 86 | 29 | 34 | 9 | 59 | 28 | |||||||
| Age at death (years) | 85.5 | (±9.3) | 82.2 | (±10.7)b | 78.0 | (±9.3)b | 88.9 | (±6.2) | 89.3 | (±4.6) | 89.4 | (±6.0) | 89.6 | (±5.3) |
| ApoE genotype | % | N | N | N | N | N | N | |||||||
| ε3/ε3 | 58.7 | 699 | 0 | 61 | 0 | 90 | 0 | |||||||
| ε2/ε3 | 13.6 | 163 | 0 | 13 | 0 | 22 | 0 | |||||||
| ε2/ε2 | 0.34 | 4 | 0 | 0 | 0 | 1 | 0 | |||||||
| ε2/ε4 | 2.4 | 0 | 27 | 0 | 3 | 0 | 5 | |||||||
| ε3/ε4 | 22.2 | 0 | 256 | 0 | 20 | 0 | 47 | |||||||
| ε4/ε4 | 2.9 | 0 | 30 | 0 | 4 | 0 | 8 | |||||||
| AD status, % | 10.4 | — | — | — | — | 78.8 | 78.3 | |||||||
Abbreviations: AD=Alzheimer's Disease; ApoE=Apolipoprotein E; ApoE4 carrier status=having an ε4 allele.
p<0.05 for null hypothesis that means or proportions are equal between ApoE4 carrier status groups within each cognitive status group, based on t- or χ2 test.
p<0.05 for null hypothesis that means or proportions are equal between cognitive status groups within each ApoE4 carrier status group, based on one-way ANOVA or χ2 test.
ApoE exposures (carrier status and dosage) and all-cause mortality
ApoE4 carriers had lower survival probability than non-carriers, particularly at ages 75 years and older (Wilcoxon-Breslow-Gehan test of equality=6.55, p=0.011). ApoE4 carrier status was associated with all-cause mortality (HR=1.31, 95%CI 1.02–1.68, p=0.032) after adjusting for sex, first-visit age, race, education, initial BMI, and time-dependent smoking status, dementia, and MCI status (Figure 1). This association was attenuated compared to a model without adjustment for time-dependent cognitive status (HR=1.44, 95%CI 1.13–1.83, p=0.003). ε4 dosage was also associated with all-cause mortality (eFigure 1). The Wilcoxon-Breslow-Gehan trend test indicated a potential dose-response of 8.36 (p=0.004). However, after adjustment of multiple covariates, the Cox PH model suggested that the association with all-cause mortality was mostly found among carriers of only 1 ε4 allele (1 ε4: HR=1.32, 95%CI 1.02–1.71, p=0.033; 2 ε4: HR=1.22, 95%CI 0.67–2.23, p=0.521).
FIG. 1. All-cause mortality vs. ApoE4 carrier status.
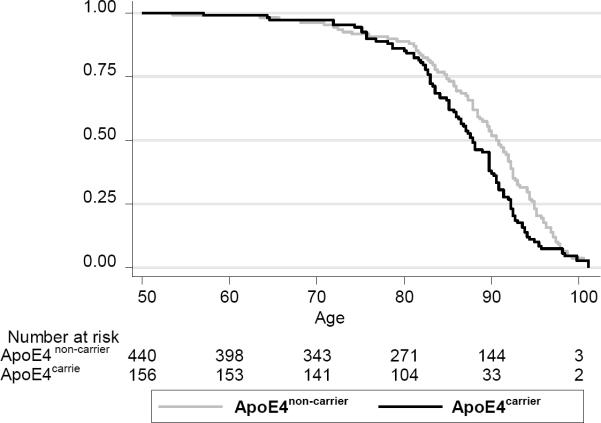
Wilcoxon (Breslow) test of equality of survivor function indicated significant differences: χ2 (d.f.=1)= 6.55, p=0.0105.
Cox PH model controlling for dementia and MCI status (time-dependent), sex, first-visit age, race, education, smoking status (time-dependent) and first-visit BMI yielded a hazard ratio for ApoE4 carrier vs. ApoE4 non-carrier status of 1.31 with a 95% CI: 1.02–1.68, p=0.032.
In sensitivity analyses, assuming that randomly censored participants survived after censoring, ApoE4 dosage was linked to increased risk of all-cause mortality (1 vs. no ε4: HR=1.23, 95%CI 0.96–1.59, p=0.095; 2 vs. no ε4: HR=1.96, 95%CI 1.12–3.45, p=0.019), with an apparent dose-response relationship.
Time-dependent cognitive status, sex and all-cause mortality
Participants with dementia and MCI had lower survival probabilities (eFigures 2.1–2.2). Both time-dependent all-cause dementia (HR=1.73, 95%CI 1.33–2.26, p<0.001) and time-dependent MCI (HR=1.95, 95% CI 1.42–2.67, p<0.001) were associated with an increased risk of all-cause mortality based on the model 1.1 (Table 2) after adjusting for ApoE4 carrier status and other covariates. AD and non-AD dementia had direct associations with all-cause mortality and lower survival probability (Model 1.2; eFigures 3.1–3.2). In particular, AD and non-AD diagnoses were associated with all-cause mortality (AD: HR=1.61, 95%CI 1.21–2.15, p<0.001; non-AD: HR=2.12, 95%CI 1.43–3.16, p<0.001) after adjusting for ApoE4 carrier status, MCI, BMI and time-dependent smoking status. Both AD and MCI diagnoses were linked to non-cardiovascular mortality (Model 3.2), whereas non-AD diagnosis was linked to cardiovascular mortality (Model 2.2). Overall, men had lower survival probability than women (χ2=6.02, p=0.0142, eFigure 4) and a Cox PH model with sole adjustment for ApoE4 carrier status indicated an increased risk of all-cause mortality in men (HR=1.41, 95%CI 1.12–1.77. p=0.003). After additionally controlling for demographic factors, smoking status, BMI and time-dependent cognitive status, the association between being male and all-cause mortality was attenuated to HR=1.29, 95%CI 0.99–1.63, p=0.056 (Model 1.2). However, men were at increased risk of non-cardiovascular mortality in the fully adjusted model (HR=1.41, 95%CI 1.04–1.90, p=0.025; (Model 3.2).
Table 2.
All-cause, cardiovascular and non-cardiovascular mortality by ApoE4 carrier status, sex and cognitive status (time-dependent)
| aHRa | 95% CI | p-value | |
|---|---|---|---|
| All-cause mortality | N=1,091 participants | n=336 deaths | |
| Model 1.1: All-cause dementia and MCI | |||
| ApoE4 (carrier vs. non-carrier) | 1.31 | (1.02;1.68) | 0.032 |
| Dementia(+ vs. −) | 1.73 | (1.33;2.26) | <0.001 |
| MCI (+ vs. −) | 1.95 | (1.41;2.67) | <0.001 |
| Male | 1.28 | (1.00;1.64) | 0.054 |
| Model 1.2: AD, non-AD and MCI | |||
| ApoE4 (carrier vs. non-carrier) | 1.30 | (1.01;1.66) | 0.038 |
| AD(+ vs. −) | 1.61 | (1.21;2.15) | 0.001 |
| Non-AD(+ vs. −) | 2.13 | (1.43;3.16) | <0.001 |
| MCI(+ vs. −) | 1.94 | (1.42;2.67) | <0.001 |
| Male | 1.28 | (0.99;1.64) | 0.056 |
| Cardiovascular Mortality | N=1,091 participants | n=105 deaths | |
| Model 2.1: All-cause dementia and MCI | |||
| ApoE4 (carrier vs. non-carrier) | 1.54 | (1.00;2.37) | 0.047 |
| Dementia(+ vs. −) | 1.45 | (0.90;2.33) | 0.125 |
| MCI (+ vs. −) | 1.38 | (0.76;2.50) | 0.288 |
| Male | 0.78 | (0.53;1.16) | 0.225 |
| Model 2.2: AD, non-AD and MCI | |||
| ApoE4 (carrier vs. non-carrier) | 1.51 | (0.98;2.33) | 0.063 |
| AD(+ vs. −) | 1.17 | (0.70;1.96) | 0.535 |
| Non-AD(+ vs. −) | 2.52 | (1.27;5.01) | 0.008 |
| MCI(+ vs. −) | 1.40 | (0.77;2.54) | 0.268 |
| Male | 0.76 | (0.51;1.13) | 0.182 |
|
| |||
| Non-cardiovascular | N=1,091 | n=231 | |
|
| |||
| Mortality | participants | deaths | |
| Model 3.1: All-cause dementia and MCI | |||
| ApoE4 (carrier vs. non-carrier) | 0.86 | (0.63;1.16) | 0.324 |
| Dementia(+ vs. −) | 2.12 | (1.53;2.93) | <0.001 |
| MCI (+ vs. −) | 2.27 | (1.56;3.31) | <0.001 |
| Male | 1.39 | (1.03;1.88) | 0.029 |
| Model 3.2: AD, non-AD and MCI | |||
| ApoE4 (carrier vs. non-carrier) | 0.86 | (0.64;1.16) | 0.330 |
| AD(+ vs. −) | 2.32 | (1.66;3.25) | <0.001 |
| Non-AD(+ vs. −) | 1.63 | (0.93;2.83) | 0.085 |
| MCI(+ vs. −) | 2.27 | (1.56;3.31) | 0.001 |
| Male | 1.41 | (1.04;1.90) | 0.025 |
Abbreviations: aHR=adjusted hazard ratio; ApoE=Apolipoprotein E; ApoE4 carrier status=having an ε4 allele; ApoE4 dosage=the number of ε4 alleles; BMI=Body Mass Index.
All Cox proportional hazards and competing risk models additionally adjusted for first-visit age, race, education, smoking status (time-dependent) and first-visit BMI.
ApoE4 exposures (carrier status and dosage), sex and mortality, stratified by time-dependent cognitive status
We further examined whether associations of ApoE exposures (i.e. carrier status and dosage) and sex with mortality risk differed according to time-dependent cognitive status: “cognitively normal,” “MCI or dementia” or “AD” group (Figure 2.1 and eTable 1). Using similar adjustment procedures as before, we observed a clear dose-response relationship between ε4 allele and all-cause mortality (1 ε4: HR=1.40, 95%CI 0.94–2.07; and 1 ε4: HR=2.61, 95%CI 1.12–6.07) during the period while individuals were cognitively normal. This pattern was also detected for non-cardiovascular mortality. In contrast, for cardiovascular mortality and ApoE4 dosage 1 ε4 vs. no ε4 was associated with HR=2.14 (95% CI 1.07–4.31), but there was no association for 2 ε4 alleles. In contrast to the associations between ApoE and mortality while individuals were cognitively normal, there were no associations between ApoE and mortality risk after participants were diagnosed with MCI or dementia (Figure 2.1 and eTable 1). When restricting the sample to visits after AD onset (Figure 2.1 and eTable 1, ApoE4 dosage), 1 ε4 allele increased the risk of all-cause mortality by around 77% compared to the “No ε4” alleles genotype.
FIG. 2.1. Adjusted hazard ratios (with 95%CI) for all-cause mortality by ApoE4 carrier status (M1) and dosage(M2), stratified by time-dependent cognitive status (Cognitively normal, MCI+dementia, AD)a.
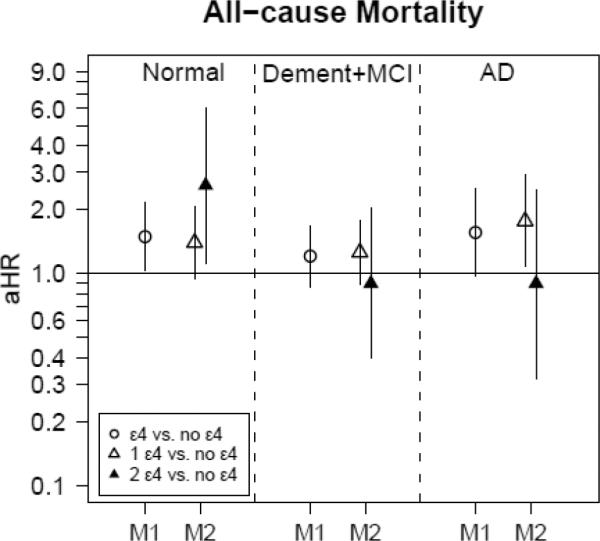
Abbreviations: AD=Alzheimer's Disease; aHR=adjusted hazard ratio;
ApoE=Apolipoprotein E; ApoE4 carrier status=having an ε4 allele; ApoE4 dosage=the number of ε4 alleles; BMI=Body Mass Index, M1: Model 1 with ApoE4carrier status as main exposure (referent category is ApoE4non-carrier), M2: Model 2 with ApoE4 dosage (1 or 2 ε4 alleles) as main exposure (referent category is ApoE4non-carrier); MCI=mild cognitive impairment.
a All models additionally adjusted for sex, first-visit age, race, education, smoking status (time-dependent) and first-visit BMI. Note that in this analysis, observations (rather than whole individuals) were restricted to the “cognitively normal” status, dementia/MCI status or AD status, thus the overlap in individuals between the three groups.
Men were at higher risk of non-cardiovascular mortality than women (eTable 1) while cognitively impaired “MCI or dementia” (Model 1: HR=1.57, 95%CI 1.07–2.29, p=0.020), whereas women were at higher risk of cardiovascular mortality within that same cognitive status group (Model 1: HR=0.51, 95%CI 0.30–0.88, p=0.016).
ApoE4 exposures (carrier status and dosage), time-dependent cognitive status and mortality, stratified by sex
There was a dose-response relationship between ApoE4 and all-cause mortality only in men (1 ε4 allele vs. none: HR=1.38, 95%CI 0.99–1.92; 2 ε4 alleles vs. none: HR=2.03, 95%CI 1.05–3.93), (Figure 2.2). There were no dose-response relationships with cause-specific mortality. Examining time-dependent cognitive status (eTable 2), all-cause dementia was positively associated with all-cause and non-cardiovascular mortality in both men and women, although MCI was associated with all-cause mortality in both sexes and non-cardiovascular mortality in men.
Joint effects (synergistic interaction) of ApoE4 carrier status with sex and cognitive status in relation to mortality
There was excess risk over and above ApoE4 carrier status and being male when both coexisted (Table 3). ApoE4 carrier status and sex interacted super-additively (Model 1: RERI=1.56, 95%CI 0.93–2.17, p<0.001 for null hypothesis that RERI=0) indicating that the joint additive effects of carrier status and sex augmented the risk for all-cause mortality beyond their individual effects. RERI also indicated a super-additive joint effect of ApoE4 and time-dependent cognitive status in most models for all-cause, cardiovascular and non-cardiovascular mortality. Importantly, ApoE4 carrier status interacted synergistically with time-dependent AD to increase the risk of all-cause mortality (Model 4: RERI=2.15, 95% CI 1.22–3.07), with the same pattern of RERI>1 observed for all-cause dementia (Model 3: RERI=2.18, 95%CI 1.35–3.01). A RERI>1 indicates synergism without needing to assume monotonicity of effects.44–45
Table 3.
All-cause, cardiovascular and non-cardiovascular mortality: Interaction between ApoE4, sex and cognitive status on the multiplicative and additive scales: Cox PH and competing risk models; Baltimore Longitudinal Study of Aging
| All-cause mortality | Cardiovascular mortality | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| aHR | 95% CI | P-value | aHR | 95% CI | P-value | |
| N=1,091 particip ants | n=336 deaths | N=1,091 partici pants | n=105 deaths | |||
| Interaction with sex: model 1 | ||||||
| ApoE4 carrier status (+ vs. −) | 1.22 | (0.81;1.84) | 0.339 | 1.70 | (0.90;3.20) | 0.100 |
| Dementia(+ vs. −) | 1.73 | (1.33;2.26) | <0.001 | 1.45 | (0.90;2.32) | 0.126 |
| MCI (+ vs. −) | 1.94 | (1.41;2.67) | <0.001 | 1.39 | (0.76;2.53) | 0.280 |
| Male | 1.23 | (0.92;1.66) | 0.150 | 0.83 | (0.52;1.32) | 0.434 |
| Male×ApoE4 | 1.11 | (0.67;1.86) | 0.676 | 0.85 | (0.37;1.96) | 0.700 |
|
| ||||||
| RERI with 95% CI | 1.56 | (0.93;2.17) | <0.001 | 0.92 | (0.21 ;1.64) | 0.011 |
|
| ||||||
| Interaction with dementia: model 2 | ||||||
| ApoE4 carrier status (+ vs. −) | 1.31 | (0.96; 1.80) | 0.091 | 1.68 | (0.97;2.92) | 0.063 |
| Dementia(+ vs. −) | 1.73 | (1.28;2.34) | <0.001 | 1.56 | (0.91;2.66) | 0.105 |
| MCI (+ vs. −) | 1.94 | (1.41;2.67) | <0.001 | 1.38 | (0.76;2.50) | 0.294 |
| Male | 1.28 | (1.00;1.64) | 0.054 | 0.78 | (0.53;1.16) | 0.215 |
| Dementia×ApoE4 | 1.00 | (0.61;1.62) | 0.989 | 0.81 | (0.35;1.87) | 0.629 |
|
| ||||||
| RERI with 95% CI | 2.18 | (1.35;3.01) | <0.001 | 2.01 | (0.61;3.40) | 0.005 |
|
| ||||||
| Interaction with MCI: model 3 | ||||||
| ApoE4 carrier status (+ vs. −) | 1.37 | (1.05;1.80) | 0.019 | 1.48 | (0.94;2.35) | 0.093 |
| Dementia (+ vs. −) | 1.72 | (1.32;2.25) | <0.001 | 1.46 | (0.91;2.34) | 0.120 |
| MCI(+ vs. −) | 2.10 | (1.47;3.00) | <0.001 | 1.27 | (0.63;2.58) | 0.503 |
| Male | 1.28 | (1.00;1.65) | 0.049 | 0.78 | (0.52;1.15) | 0.211 |
| MCI×ApoE4 | 0.75 | (0.39;1.43) | 0.382 | 1.28 | (0.41;3.98) | 0.664 |
|
| ||||||
| RERI with 95% CI | 2.10 | (0.90;3.30) | 0.001 | 2.28 | (0.04;4.52) | 0.045 |
|
| ||||||
| Interaction with AD: model 4 | ||||||
| ApoE4 carrier status (+ vs. −) | 1.24 | (0.92;1.66) | 0.152 | 1.47 | (0.87;2.49) | 0.152 |
| AD(+ vs. −) | 1.54 | (1.10;2.14) | 0.011 | 1.13 | (0.62;2.06) | 0.687 |
| Non-AD(+ vs. −) | 2.15 | (1.45;3.19) | <0.001 | 2.53 | (1.28;5.03) | 0.008 |
| MCI (+ vs. −) | 1.95 | (1.42;2.68) | <0.001 | 1.40 | (0.77;2.54) | 0.267 |
| Male | 1.28 | (1.00;1.64) | 0.053 | 0.76 | (0.51;1.13) | 0.267 |
| AD×ApoE4 | 1.18 | (0.70;2.00) | 0.540 | 1.11 | (0.43 ;2.84) | 0.833 |
|
| ||||||
| RERI with 95% CI | 2.15 | (1.22;3.07) | <0.001 | 1.66 | (0.35;2.97) | 0.013 |
|
| ||||||
| Interaction with non-AD: model 5 | ||||||
| ApoE4 carrier status (+ vs. −) | 1.36 | (1.05;1.77) | 0.020 | 1.65 | (1.06;2.60) | 0.028 |
| AD(+ vs. −) | 1.60 | (1.20;2.14) | 0.001 | 1.16 | (0.69;1.93) | 0.576 |
| Non-AD(+ vs. −) | 2.48 | (1.55;3.96) | <0.001 | 3.16 | (1.48;6.76) | 0.003 |
| MCI (+ vs. −) | 1.93 | (1.41;2.66) | <0.001 | 1.39 | (0.77;2.53) | 0.279 |
| Male | 1.27 | (0.99;1.63) | 0.059 | 0.76 | (0.51;1.13) | 0.172 |
| Non-AD×ApoE4 | 0.66 | (0.31;1.42) | 0.291 | 0.54 | (0.15;1.98) | 0.356 |
|
| ||||||
| RERI with 95% CI | 2.20 | (0.83;3.57) | 0.002 | 2.84 | (−0.31;5.95) | 0.077 |
| Non-cardiovascular mortality |
|||
|---|---|---|---|
| aHRa | 95% CI | P-value | |
|
|
|||
| N=1,091 participants | N=231 deaths | ||
| Interaction with sex: model 1 | |||
| ApoE4 carrier status (+ vs. −) | 0.80 | (0.47;1.35) | 0.403 |
| Dementia(+ vs. −) | 2.12 | (1.53;2.93) | <0.001 |
| MCI (+ vs. −) | 2.27 | (1.56;3.31) | <0.001 |
| Male | 1.35 | (0.96;1.91) | 0.087 |
| Male×ApoE4 | 1.11 | (0.59;2.15) | 0.741 |
|
| |||
| RERI with 95% CI | 1.13 | (0.60;1.66) | <0.001 |
|
| |||
| Interaction with dementia: model 2 | |||
| ApoE4 carrier status (+ vs. −) | 0.85 | (0.57;1.26) | 0.418 |
| Dementia(+ vs. −) | 2.10 | (1.46;3.02) | <0.001 |
| MCI (+ vs. −) | 2.28 | (1.56;3.32) | <0.001 |
| Male | 1.40 | (1.03;1.88) | 0.029 |
| Dementia×ApoE4 | 1.03 | (0.56;1.90) | 0.918 |
|
| |||
| RERI with 95% CI | 1.80 | (0.98;2.62) | <0.001 |
|
| |||
| Interaction with MCI: model 3 | |||
| ApoE4 carrier status (+ vs. −) | 0.97 | (0.70;1.34) | 0.832 |
| Dementia (+ vs. −) | 2.10 | (1.52;2.90) | <0.001 |
| MCI(+ vs. −) | 2.68 | (1.79;4.00) | <0.001 |
| Male | 1.41 | (1.05;1.91) | 0.024 |
| MCI×ApoE4 | 0.52 | (0.23;1.19) | 0.122 |
|
| |||
| RERI with 95% CI | 1.32 | (0.34;2.40) | 0.008 |
|
| |||
| Interaction with AD: model 4 | |||
| ApoE4 carrier status (+ vs. −) | 0.86 | (0.60;1.25) | 0.437 |
| AD(+ vs. −) | 2.33 | (1.59;3.40) | <0.001 |
| Non-AD(+ vs. −) | 1.62 | (0.93;2.83) | 0.086 |
| MCI (+ vs. −) | 2.27 | (1.56;3.31) | <0.001 |
| Male | 1.41 | (1.04;1.90) | 0.025 |
| AD×ApoE4 | 0.99 | (0.52;1.86) | 0.974 |
|
| |||
| RERI with 95% CI | 1.96 | (0.98;2.93) | <0.001 |
|
| |||
| Interaction with non-AD: model 5 | |||
| ApoE4 carrier status (+ vs. −) | 0.85 | (0.62;1.17) | 0.311 |
| AD(+ vs. −) | 2.33 | (1.66;3.26) | <0.001 |
| Non-AD(+ vs. −) | 1.55 | (0.773;3.10) | 0.217 |
| MCI (+ vs. −) | 2.27 | (1.56;3.31) | <0.001 |
| Male | 1.41 | (1.04;1.90) | 0.025 |
| Non-AD×ApoE4 | 1.16 | (0.41;3.27) | 0.781 |
|
| |||
| RERI with 95% CI | 1.45 | (0.25;2.66) | 0.018 |
Abbreviations: AD=Alzheimer's Disease; ApoE=Apolipoprotein E; ApoE4 carrier status=having an ε4 allele; BMI=Body Mass Index; MCI=Mild Cognitive Impairment; RERI=Relative Excess Risk due to Interaction.
All Cox proportional hazards and competing risk models additionally adjusted for first-visit age, race, education, smoking status (time-dependent) and first-visit BMI.
DISCUSSION
Survival probability was lower for ApoE4 carriers in our sample, particularly at oldest ages, for both all-cause mortality and cardiovascular mortality. Dementia and mild cognitive impairment increased the risks for all-cause mortality and for non-cardiovascular mortality. There was a dose-response relationship with ε4 alleles in individuals free of cognitive impairment for all-cause mortality. After Alzheimer's Disease-type (AD) dementia onset, carrying 1 ε4 allele increased all-cause mortality risk by ~77% compared to non-carriers. ApoE4 carrier status increased all-cause mortality risk in men and interacted with time-dependent AD to increase the risk of this outcome.
Accumulating evidence shows that ApoE4 carrier status is linked to increased all-cause3–14 and cardiovascular15–18 mortality risk. In one study, a dose-response relationship was detected near age 50, whereby the ε3/ε4 genotype was associated with 1.34-fold increase in mortality risk compared to ε3/ε3 (95% CI: 1.18–1.67); whereas the relative risk (RR) for ε4/ε4 was 1.81.5 This effect at younger ages was replicated by other studies9, 11, 13. However, a positive association between ApoE4 carrier status and mortality at older ages (75+y) was also found in other previous studies.4, 10, 12 Moreover, the ε4 allele was associated with increased risks of CHD mortality independently of CHD risk factors in men15–16 and with all-cause mortality and dementia-specific deaths among men and women12 in several cohort studies conducted in Finland. Other studies, however, failed to find an association in the general population.20–23 These inconsistencies may be attributed to differences in sample sizes, varying periods of follow-up and baseline age distributions as well as differences in ApoE genotype allele frequencies across populations. In our study, the association between ApoE4 carrier status and mortality was mostly detected at older ages, was restricted to men with an apparent dose-response relationship, and pertained mainly to cardiovascular mortality.
Potential factors that have been suggested to mediate the relationship between ApoE4 carrier status and cardiovascular mortality included an increased plasma level total cholesterol:High-density lipoprotein-cholesterol ratio48–49, increased levels of inflammatory markers, including plasma C-reactive proteins and adhesion molecules concentrations49, and increased carotid intimal medial thickness50.
Both sex and time-dependent cognitive status were strongly associated with mortality risk in our study. Sex differences in mortality is a well-known phenomenon worldwide, favoring higher survival probability in women.24 Putative explanations include differences in hormonal production, longer telomeres in women, and the protective effect of having an additional X-chromosome, though these factors could not fully explain survival differences by sex.51
In addition, cognitive impairment was consistently associated with an increased risk of all-cause mortality, with a pooled age-adjusted OR of 2.63, 95% CI: 2.17–3.21 for all-cause dementia based on a recent meta-analysis.25 Our study indicated that AD-type of dementia was specifically linked to increased risk in mortality of non-cardiovascular origin, while non-AD dementia (mostly vascular dementia) was associated with increased risk of cardiovascular mortality.
Sex differences in the association between ApoE genotype and mortality risk have been examined in a limited number of studies5, 8, 10, with one finding no difference5, and two finding inconsistent sex differences in the associations of ApoE4 and ApoE2 carrier status with mortality risk8, 10. In the first study (n=1,094, Age: ≥75y, length of follow-up~18y), a 49% elevated mortality risk in men was related to the ε4 allele, while this risk was reduced by 36% in women with the ε2 allele. This study found a significant ApoE4×sex interaction.10 In the second study (n= 4,701, Age: ≥75y, length of follow-up~7y), ApoE2 carrier status was associated with increased mortality risk in men only with a marked interaction on the additive scale whereby 43% of deaths in ε2/ε2 men were attributed to an interaction between sex and the ApoE genotype. Moreover, although a stronger positive association between ApoE4 carrier status and mortality risk was found in women, no interaction on the additive scale was observed.8 Both age distribution and follow-up time differences may explain those inconsistent findings. Our results were in line with Rosvall and colleagues10 whereby the positive association of ApoE4 carrier status with all-cause mortality risk was restricted to men, possibly due to the longer follow-up time (13.7y in our study, and 18y in Rosval and colleagues study10) compared to Hayden and colleagues.8 We additionally found a joint effect on the additive scale between being male and carrying an ε4 allele in relation to all-cause mortality risk (RERI>0). This suggests that the hazard rate of mortality for those exposed to both male sex and ApoE4 is greater than the sum of the two rates for each factor alone in the absence of the other. Thus, compounding the biological effects of being male and ApoE4 carrier status (possibly through gene-gene interactions between the ApoE gene and genes on the X chromosome51) lead to a greater than additive effect. However, further studies are needed to uncover the exact pathway that would explain this interaction.
Differences by cognitive status in ApoE genotype's association with mortality risk was assessed, to our knowledge, only in one study,10 whereby interaction on the multiplicative (rather than the additive) scale was tested for all-cause dementia. However, dementia and ApoE4 carrier status did not interact in relation to all-cause mortality, despite a significant 3-way interaction between ApoE4 carrier status, sex and coronary heart disease status. Our study indicated that in addition to super-additive joint effects observed with sex (RERI>0), ApoE4 carrier status interacted with all-cause dementia and AD to affect all-cause mortality risk, without needing to assume monotonicity of effects (RERI>1). Thus, hazard rate of mortality for those exposed to both AD and ApoE4 is greater than the sum of the two rates for each factor alone in the absence of the other. Because AD tended to affect the rate of non-cardiovascular mortality whereas ApoE4 carrier status was positively associated with cardiovascular mortality, this lack of overlap in the underlying cause of death may have lead to this type of observed synergism in hazard rates of all-cause mortality.
A number of other studies restricting their samples to AD cases found no association between ApoE4 carrier status and all-cause mortality. (e.g.52) We found a positive association between ApoE4 carrier status and all-cause mortality in both the “cognitively normal” and the AD group, but not among the “MCI and dementia” group. It is possible that the prospective nature of our follow-up enhanced our ability to detect associations for the more clearly defined cognitively normal and AD groups, whereas the MCI and dementia groups in our study were etiologically heterogeneous.
Our study has several strengths, including frequency and length of follow-up and use of multiple complementary statistical techniques by combining Kaplan-Meier survival curves, Cox PH and competing risk models, as well as testing for additive interaction in those models. We also included some time-dependent covariates in our main models, taking into account age of onset of cognitive outcomes such as dementia (AD vs. non-AD) and MCI.
Our study has also some limitations. First, the BLSA is an open cohort and a sample of convenience with continuous recruitment and dropout throughout follow-up. We used a 2-stage Heckman selection model46 to reduce selection bias and survival analysis methods to account for censoring (including informative censoring), unequal durations between visits and variations in first-visit age. Moreover, some positive findings may have been due to chance, residual confounding or selection bias, while negative findings may have resulted from inadequate power. The latter specifically precluded examining the association of ApoE2 carrier status with mortality. Thus, until replicated elsewhere, our findings should be interpreted with caution.
Our study has many public health implications. First, identifying genotypes for increased mortality risk is gaining importance in clinical medicine. Here, ApoE4 carrier status was an important risk factor for all-cause and cardiovascular mortality. Stratified analyses by sex and time-dependent cognitive status indicated ApoE4 exposures had an important association with all-cause mortality only among men, both while individuals were cognitively normal and after AD onset. All-cause dementia and AD status interacted in a super-additive manner with ApoE4 carrier status to increase all-cause mortality risk, even in the absence of monotonic effects. The specific effects of AD and MCI on non-cardiovascular mortality should be studied carefully. Future studies should further examine mediating effects of time-dependent cognitive status and markers of cardiovascular morbidity that would establish the pathway by which ApoE4 carrier status is a risk factor for mortality.
Supplementary Material
FIG. 2.2. Adjusted hazard ratios (with 95%CI) for all-cause mortality by ApoE4 carrier status (M1) and dosage(M2), stratified by sexa.
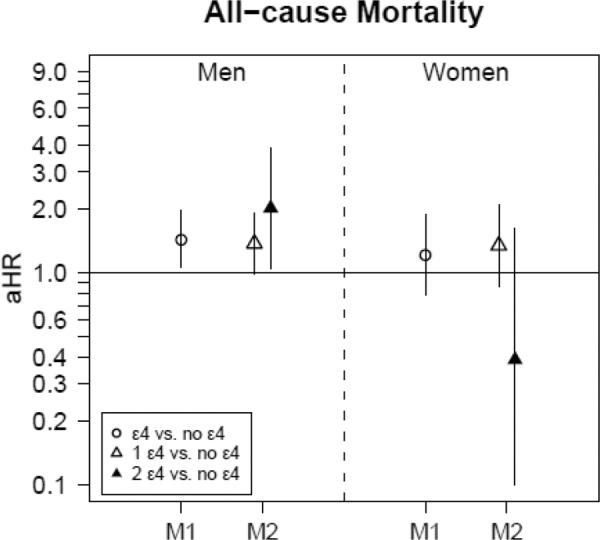
Abbreviations: aHR=adjusted hazard ratio; ApoE=Apolipoprotein E; ApoE4 carrier status=having an ε4 allele; ApoE4 dosage=the number of ε4 alleles; BMI=Body Mass Index, M1: Model 1 with ApoE4carrier status as main exposure (referent category is ApoE4non-carrier), M2: Model 2 with ApoE4 dosage (1 or 2 ε4 alleles) as main exposure (referent category is ApoE4non-carrier); MCI=mild cognitive impairment.
aAll models additionally adjusted for dementia and MCI status (time-dependent), first-visit age, race, education, smoking status (time-dependent) and first-visit BMI.
ACKNOWLEDGMENTS
The authors would like to thank Drs. Antonio Terracciano, PhD (NIA/NIH/IRP) and Alyssa Gamaldo, PhD (NIA/NIH/IRP) for their internal review of this manuscript.
This research was supported entirely by the Intramural Research Program of the NIH, National Institute on Aging (NIA/NIH/IRP).
Sponsor's Role: The sponsor had no role in the study, design of this paper.
Footnotes
Conflict of Interest The authors declare no conflict of interest.
Author Contributions: May A. Beydoun had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
May A. Beydoun: Write-up of manuscript, plan of analysis, data management, statistical analysis, interpretation of findings, literature review, revision of manuscript.
Hind A. Beydoun: Plan of analysis, literature search and review, write-up of parts of the manuscript, revision of manuscript.
Jay S. Kaufman: Plan of analysis, literature review, write-up of parts of the manuscript, revision of the manuscript.
Yang An: Statistical analysis, plan of analysis, revision of manuscript.
Susan M. Resnick: Data acquisition, plan of analysis, write-up of parts of the manuscript, revision of manuscript.
Richard O'Brien: Data acquisition, plan of analysis, revision of manuscript.
Luigi Ferrucci: Data acquisition, plan of analysis, revision of manuscript.
Alan B. Zonderman: Data acquisition, plan of analysis, write-up of parts of the manuscript, revision of manuscript.
REFERENCES
- 1.Bertram L, McQueen MB, Mullin K, et al. Systematic meta-analyses of Alzheimer disease genetic association studies: The AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 2.Ewbank DC. The APOE gene and differences in life expectancy in Europe. J Gerontol A Biol Sci Med Sci. 2004;59:16–20. doi: 10.1093/gerona/59.1.b16. [DOI] [PubMed] [Google Scholar]
- 3.Corder EH, Basun H, Lannfelt L, et al. Attenuation of apolipoprotein E Epsilon4 allele gene dose in late age. Lancet. 1996;347:542. doi: 10.1016/s0140-6736(96)91173-3. [DOI] [PubMed] [Google Scholar]
- 4.Corder EH, Lannfelt L, Viitanen M, et al. Apolipoprotein E genotype determines survival in the oldest old (85 years or older) who have good cognition. Arch Neurol. 1996;53:418–422. doi: 10.1001/archneur.1996.00550050048022. [DOI] [PubMed] [Google Scholar]
- 5.Ewbank DC. Mortality differences by APOE genotype estimated from demographic synthesis. Genet Epidemiol. 2002;22:146–155. doi: 10.1002/gepi.0164. [DOI] [PubMed] [Google Scholar]
- 6.Ewbank DC. Differences in the association between apolipoprotein E genotype and mortality across populations. J Gerontol A Biol Sci Med Sci. 2007;62:899–907. doi: 10.1093/gerona/62.8.899. [DOI] [PubMed] [Google Scholar]
- 7.Fillenbaum GG, Blazer DG, Burchett BM, et al. Apolipoprotein E epsilon4 and risk of mortality in African American and white older community residents. Gerontologist. 2002;42:381–386. doi: 10.1093/geront/42.3.381. [DOI] [PubMed] [Google Scholar]
- 8.Hayden KM, Zandi PP, Lyketsos CG, et al. Apolipoprotein E genotype and mortality: findings from the Cache County Study. J Am Geriatr Soc. 2005;53:935–942. doi: 10.1111/j.1532-5415.2005.53301.x. [DOI] [PubMed] [Google Scholar]
- 9.Lane KA, Gao S, Hui SL, et al. Apolipoprotein E and mortality in African-Americans and Yoruba. J Alzheimers Dis. 2003;5:383–390. doi: 10.3233/jad-2003-5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosvall L, Rizzuto D, Wang HX, et al. APOE-related mortality: Effect of dementia, cardiovascular disease and gender. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Raiha I, Marniemi J, Puukka P, et al. Effect of serum lipids, lipoproteins, and apolipoproteins on vascular and nonvascular mortality in the elderly. Arterioscler Thromb Vasc Biol. 1997;17:1224–1232. doi: 10.1161/01.atv.17.7.1224. [DOI] [PubMed] [Google Scholar]
- 12.Tilvis RS, Strandberg TE, Juva K. Apolipoprotein E phenotypes, dementia and mortality in a prospective population sample. J Am Geriatr Soc. 1998;46:712–715. doi: 10.1111/j.1532-5415.1998.tb03805.x. [DOI] [PubMed] [Google Scholar]
- 13.Vogt MT, Cauley JA, Kuller LH. Apolipoprotein E phenotype, arterial disease, and mortality among older women: The study of osteoporotic fractures. Genet Epidemiol. 1997;14:147–156. doi: 10.1002/(SICI)1098-2272(1997)14:2<147::AID-GEPI4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Fillenbaum GG, Burchett BM, Lee JH, et al. Mortality and apolipoprotein E in African-American, and White elders: An attempted replication. Am J Med Genet A. 2003;119A:141–146. doi: 10.1002/ajmg.a.20146. [DOI] [PubMed] [Google Scholar]
- 15.Stengard JH, Pekkanen J, Ehnholm C, et al. Genotypes with the apolipoprotein epsilon4 allele are predictors of coronary heart disease mortality in a longitudinal study of elderly Finnish men. Hum Genet. 1996;97:677–684. doi: 10.1007/BF02281882. [DOI] [PubMed] [Google Scholar]
- 16.Stengard JH, Zerba KE, Pekkanen J, et al. Apolipoprotein E polymorphism predicts death from coronary heart disease in a longitudinal study of elderly Finnish men. Circulation. 1995;91:265–269. doi: 10.1161/01.cir.91.2.265. [DOI] [PubMed] [Google Scholar]
- 17.Eichner JE, Kuller LH, Orchard TJ, et al. Relation of apolipoprotein E phenotype to myocardial infarction and mortality from coronary artery disease. Am J Cardiol. 1993;71:160–165. doi: 10.1016/0002-9149(93)90732-r. [DOI] [PubMed] [Google Scholar]
- 18.Bennet AM, Di Angelantonio E, Ye Z, et al. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA. 2007;298:1300–1311. doi: 10.1001/jama.298.11.1300. [DOI] [PubMed] [Google Scholar]
- 19.Eichner JE, Dunn ST, Perveen G, et al. Apolipoprotein E polymorphism and cardiovascular disease: a HuGE review. Am J Epidemiol. 2002;155:487–495. doi: 10.1093/aje/155.6.487. [DOI] [PubMed] [Google Scholar]
- 20.Boemi M, Sirolla C, Testa R, et al. Apolipoprotein E polymorphisms and mortality in Italian type 2 diabetic patients. Eur J Clin Invest. 2003;33:296–300. doi: 10.1046/j.1365-2362.2003.01141.x. [DOI] [PubMed] [Google Scholar]
- 21.Heijmans BT, Slagboom PE, Gussekloo J, et al. Association of APOE epsilon2/epsilon3/epsilon4 and promoter gene variants with dementia but not cardiovascular mortality in old age. Am J Med Genet. 2002;107:201–208. doi: 10.1002/ajmg.10142. [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Tang MX, Schupf N, et al. Mortality and apolipoprotein E in Hispanic, African-American, and Caucasian elders. Am J Med Genet. 2001;103:121–127. doi: 10.1002/ajmg.1528. [DOI] [PubMed] [Google Scholar]
- 23.Lima-Costa MF, Peixoto SV, Taufer M, et al. Apolipoprotein e genotype does not predict 9-year all-cause mortality in brazilian older adults: The Bambui Cohort Study. J Am Geriatr Soc. 2008;56:2366–2368. doi: 10.1111/j.1532-5415.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 24.Kochanek KD, Xu J, Murphy SL, et al. Deaths: Preliminary Data for 2009. 2011 http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_04.pdf. National Vital Statistics Reports, Volume 59: Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. [PubMed]
- 25.Dewey ME, Saz P. Dementia, cognitive impairment and mortality in persons aged 65 and over living in the community: A systematic review of the literature. Int J Geriatr Psychiatry. 2001;16:751–761. doi: 10.1002/gps.397. [DOI] [PubMed] [Google Scholar]
- 26.Shock N, Greulich RC, Andres R, et al. Normal Human Aging: The Baltimore Longitudinal Study of Aging. US Government Printing Office; Washington, DC: 1984. [Google Scholar]
- 27.Zonderman AB, Giambra LM, Arenberg D, et al. Changes in immediate visual memory predict cognitive impairment. Arch Clin Neuropsychol. 1995;10:111–123. [PubMed] [Google Scholar]
- 28.Cleves M, Gould WW, Gutierrez RG, et al. An Introduction to Survival Analysis Using Stata. 3rd Edition College Station; Texas: 2010. [Google Scholar]
- 29.World Health Organization . The ICD-9 Classification of Mental and Behavioral Disorders: Diagnostic Criteria for Research. World Health Organization; Geneva, Switzerland: 1979. [Google Scholar]
- 30.World Health Organization . The ICD-10 Classification of Mental and Behavioral Disorders: Diagnostic Criteria for Research. second edition World Health Organization; Geneva, Switzerland: 2004. [Google Scholar]
- 31.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 32.Koch W, Ehrenhaft A, Griesser K, et al. TaqMan systems for genotyping of disease-related polymorphisms present in the gene encoding apolipoprotein E. Clin Chem Lab Med. 2002;40:1123–1131. doi: 10.1515/CCLM.2002.197. [DOI] [PubMed] [Google Scholar]
- 33.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 34.Morris JC. Clinical dementia rating: A reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9(Suppl 1):173–176. doi: 10.1017/s1041610297004870. discussion 177-178. [DOI] [PubMed] [Google Scholar]
- 35.Kawas C, Segal J, Stewart WF, et al. A validation study of the Dementia Questionnaire. Arch Neurol. 1994;51:901–906. doi: 10.1001/archneur.1994.00540210073015. [DOI] [PubMed] [Google Scholar]
- 36.American Psychiatric Association . Third Edition. American Psychiatric Association; Washington, DC: 1987. Diagnostic and Statistical Manual of Mental Disorders. Revised. [Google Scholar]
- 37.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 38.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 39.STATA . Statistics/Data Analysis: Release 11.0. Stata Corporation; College Station, TX: 2009. [Google Scholar]
- 40.Wilcoxon F. Individual comparisons by ranking methods. Biometrics. 1945;1:80–83. [Google Scholar]
- 41.Breslow NE. A generalized Kruskal–Wallis test for comparing K samples subject to unequal patterns of censorship. Biometrika. 1970;57:579–594. [Google Scholar]
- 42.Gehan EA. A generalized Wilcoxon test for comparing arbitrarily singly-censored samples. Biometrika. 1965;52:203–223. [PubMed] [Google Scholar]
- 43.Cox DR. Regression Models and Life-Tables. J Royal Statistical Soc Series B (Methodological) 1972;34:187–220. [Google Scholar]
- 44.VanderWeele TJ. Causal interactions in the proportional hazards model. Epidemiology. 2011;22:713–717. doi: 10.1097/EDE.0b013e31821db503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol. 2007;17:227–236. doi: 10.1016/j.annepidem.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Heckman JJ. Sample selection bias as a specification error. Econometrica. 1979;47:153–161. [Google Scholar]
- 47.Beydoun MA, Boueiz A, Abougergi MS, et al. Sex differences in the association of the apolipoprotein E epsilon 4 allele with incidence of dementia, cognitive impairment, and decline. Neurobiol Aging. 2012;33:720–731. e724. doi: 10.1016/j.neurobiolaging.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ingelsson E, Schaefer EJ, Contois JH, et al. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA. 2007;298:776–785. doi: 10.1001/jama.298.7.776. [DOI] [PubMed] [Google Scholar]
- 49.Kofler BM, Miles EA, Curtis P, et al. Apolipoprotein E genotype and the cardiovascular disease risk phenotype: Impact of sex and adiposity (the FINGEN study) Atherosclerosis. 2012;221:467–470. doi: 10.1016/j.atherosclerosis.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 50.Paternoster L, Martinez Gonzalez NA, Lewis S, et al. Association between apolipoprotein E genotype and carotid intima-media thickness may suggest a specific effect on large artery atherothrombotic stroke. Stroke. 2008;39:48–54. doi: 10.1161/STROKEAHA.107.488866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eskes T, Haanen C. Why do women live longer than men? Eur J Obstet Gynecol Reprod Biol. 2007;133:126–133. doi: 10.1016/j.ejogrb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 52.Bonsignore M, Heun R. Mortality in Alzheimer's disease. Dement Geriatr Cogn Disord. 2003;15:231–236. doi: 10.1159/000068779. [DOI] [PubMed] [Google Scholar]
- 53.Skrondal A. Interaction as departure from additivity in case-control studies: A cautionary note. Am J Epidemiol. 2003;158:251–258. doi: 10.1093/aje/kwg113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


