Abstract
Nutrition plays a minor role in psychiatric practice which is currently dominated by a pharmacological treatment algorithm. An accumulating body of evidence has implicated deficits in the dietary essential long-chain omega-3 (LCn-3) fatty acids, eicosapenaenoic acid (EPA) and docosahexaenoic acid (DHA), in the pathophysiology of several major psychiatric disorders. LCn-3 fatty acids have an established long-term safety record in the general population, and existing evidence suggests that increasing LCn-3 fatty acid status may reduce the risk for cardiovascular disease morbidity and mortality. LCn-3 fatty acid supplementation has been shown to augment the therapeutic efficacy of antidepressant, mood-stabilizer, and second generation antipsychotic medications, and may additionally mitigate adverse cardiometabolic side-effects. Preliminary evidence also suggests that LCn-3 fatty acid supplementation may be efficacious as monotherapy for primary and early secondary prevention and for perinatal symptoms. The overall cost-benefit ratio endorses the incorporation of LCn-3 fatty acids into psychiatric treatment algorithms. The recent availability of laboratory facilities that specialize in determining blood LCn-3 fatty acid status and emerging evidence-based consensus guidelines regarding safe and efficacious LCn-3 fatty acid dose ranges provide the infrastructure necessary for implementation. This article outlines the rationale for incorporating LCn-3 fatty acid treatment into psychiatric practice.
Keywords: Long-chain omega-3 fatty acids, Eicosapenaenoic acid (EPA), Docosahexaenoic acid (DHA), Bipolar disorder, Major depressive disorder, Schizophrenia, ADHD, Anxiety, Suicide, Cardiovascular disease, Primary prevention, Clinical staging
1. Introduction
Common psychiatric disorders including major depressive disorder, bipolar disorder, schizophrenia, attention deficit hyperactivity disorder (ADHD), and anxiety disorders are chronic and typically recurring illnesses associated with significant psychosocial morbidity. Outcomes data indicate that mood and psychotic disorders are associated with excess premature mortality attributable primarily to suicide and cardiovascular-related diseases [1–4]. The initial onset of these disorders frequently occurs during adolescence or early adulthood [5–10], and psychiatric symptoms are typically initially treated with pharmacological agents including antidepressant, mood-stabilizer, and/or second generation antipsychotic medications. Symptomatic relapse following medication discontinuation is common [11–13], and patients typically require long-term prophylactic treatment per consensus guidelines. However, long-term treatment with some medications may be associated with adverse cardiometabolic side-effects [14] as well as other significant side effects [15,16] in a subset of vulnerable patients. These data highlight the need for safer and better tolerated treatments, or adjunctive treatments, to improve long-term outcomes for psychiatric patients.
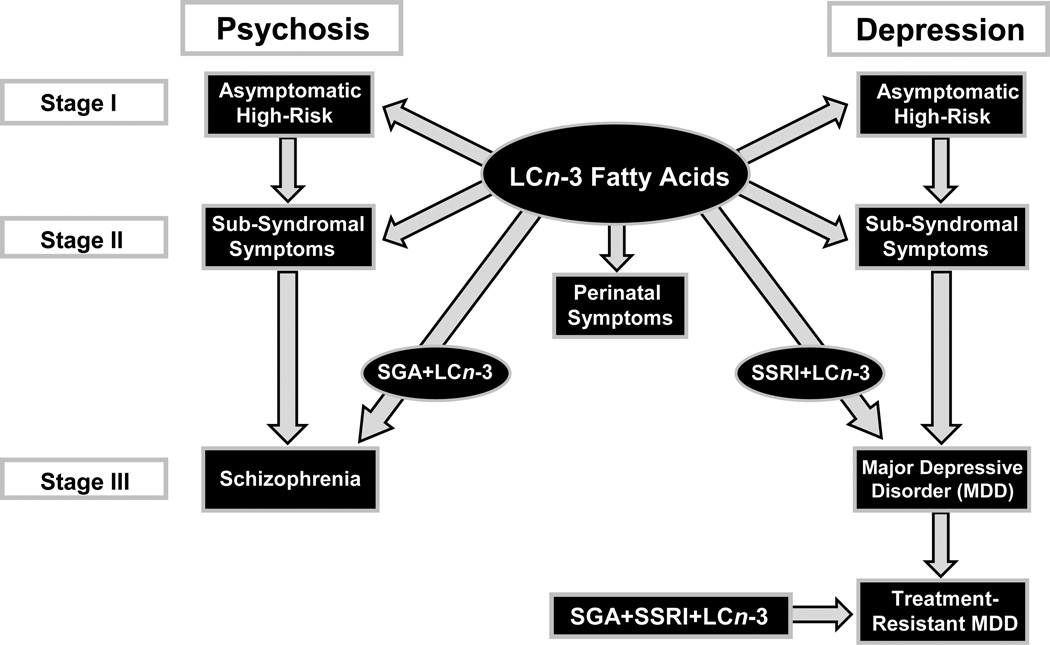
While nutrition remains a largely neglected aspect of psychiatric treatment practice, a substantial body of evidence has emerged over the past two decades which has implicated a deficiency in dietary essential long-chain omega-3 (LCn-3) fatty acids, eicosapenaenoic acid (EPA) and docosahexaenoic acid (DHA), in the pathophysiology of several major psychiatric disorders [17–20]. This is supported by evidence from cross-national and cross-sectional epidemiological surveys, case-control LCn-3 fatty acid composition studies, prospective observational and LCn-3 fatty acid intervention studies, and neurobiological studies in rodents. Additionally, accumulating evidence suggests that LCn-3 fatty acid deficiency may increase the risk of suicide and cardiovascular disease, two primary causes of excess premature mortality in patients with mood and psychotic disorders [1–4]. Based on the established long-term safety record of LCn-3 fatty acids in the general population, and multiple health benefits associated with increasing LCn-3 fatty acid status, the American Psychiatric Association has endorsed adjunctive treatment with LCn-3 fatty acids for the treatment of major depressive disorder [17]. However, the use of LCn-3 fatty acids in psychiatric practice remains limited and may be due to a lack of awareness of the evidence base supporting their beneficial role. This paper reviews evidence implicating LCn-3 fatty acid deficiency in the pathophysiology of psychiatric disorders and explores new roles for LCn-3 fatty acids in psychiatric treatment algorithms (Fig. 1).
Figure 1.
Proposed clinical staging model for LCn-3 fatty acid treatment of patients with or at high risk for developing schizophrenia or major depressive disorder. Stage I (primary prevention) proposes LCn-3 fatty acid monotherapy for subjects that are asymptomatic but are at high risk for developing a psychotic or mood disorder (i.e., they have a family history of psychiatric illness). For perinatal depression, LCn-3 fatty acid monotherapy may be proposed for treating symptoms when standard medications are contraindicated due to potential fetal teratogenic effects. Stage II (early secondary prevention) proposes LCn-3 fatty acid monotherapy for subjects exhibiting attenuated symptoms that do not meet criteria for a formal DSM-IV Axis I diagnosis (i.e., sub-syndromal). Stage III proposes adjunctive LCn-3 fatty acid treatment for patients with a formal DSM-IV diagnosis in conjunction with standard second generation antipsychotic (SGA) and/or selective serotonin reuptake inhibitor (SSRI) medications to augment efficacy and mitigate adverse cardiometabolic side effects. For treatment resistant depression, adjunctive LCn-3 fatty acid treatment in combination with SSRI and SGA medications may be considered.
2. LCn-3 fatty acid biosynthesis and status
As background, mammals require a dietary source of n-3 fatty acids to procure and maintain adequate LCn-3 fatty acid concentrations in peripheral and central tissues. Principal dietary sources of the vegetable short-chain n-3 fatty acid α-linolenic acid (ALA, 18:3n-3) include flaxseed, linseed, canola, soy, and perilla oils. The biosynthesis of LCn-3 fatty acids, including eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3), from ALA requires a series of microsomal desaturation and elongation reactions, and the final synthesis of DHA requires β-oxidation within peroxisomes. In healthy adults residing in western countries, ALA→EPA biosynthesis is limited and ALA→DHA and EPA→DHA biosynthesis is negligible [21]. Accordingly, bypassing microsomal- and peroxisomal-mediated biosynthesis with preformed dietary LCn-3 fatty acids is significantly more effective than ALA for increasing EPA+DHA levels in peripheral and central tissues [22–25]. Principal dietary sources of preformed EPA+DHA include fatty cold water fish, including salmon, trout, tuna, as well as fish oil supplements [26].
An individual’s LCn-3 fatty acid ‘status’ can be determined by evaluating the fatty acid composition of blood (plasma phospholipids, erythrocytes, platelets), breast milk, and peripheral (e.g., adipose and cardiac biopsies) or central (i.e., postmortem brain) tissues by gas chromatography. Erythrocyte (red blood cell, RBC) membrane EPA+DHA composition has been found to provide a valid and relatively non-invasive index of habitual (1–2 months) dietary LCn-3 fatty acid intake [27–29]. Additionally, RBC EPA+DHA composition is positively correlated with blood plasma, platelet, immune cell [30,31], adipose [32,33], breast milk [34], myocardial [35], and brain gray matter [36, 37] EPA+DHA composition. Because RBC LCn-3 fatty acid levels may be influenced by other factors in addition to dietary intake, including age, genes, gender, and alcohol intake [38], predicting ones LCn-3 fatty acid status based on diet alone may not be accurate. Analogous to routine cholesterol testing, there has been a recent emergence of laboratory facilities that specialize in determining blood LCn-3 fatty acid levels, and there is emerging evidence-based consensus regarding optimal levels in the context of cardiovascular disease risk [39]. This infrastructure is anticipated to play an important role in other fields of medicine including psychiatry.
3. Psychiatric patients exhibit LCn-3 fatty acid deficiency
Several cross-sectional studies have investigated the LCn-3 fatty acid (EPA+DHA) ‘status’ of psychiatric patients. These studies have found that patients with MDD [40–45], bipolar disorder [45–47], schizophrenia [47–50], anxiety disorders [51] and ADHD [52–55] exhibit significant EPA+DHA deficits compared with demographically similar healthy subjects. A recent meta-analysis of 14 case-control fatty acid composition studies found that MDD patients exhibit significant RBC EPA+DHA deficits, whereas RBC levels of LCn-6 fatty acids including arachidonic acid (20:4n-6) do not differ between patients and healthy subjects [56]. The principle LCn-3 fatty acid found in brain gray matter is DHA, which comprises approximately 15% of total fatty acids composition [36], and RBC DHA composition is positively correlated with brain gray matter DHA composition [36,37]. Emerging evidence from human neuroimaging studies suggest that blood DHA levels are associated with different aspects of cortical structural and functional integrity relevant to psychiatric disorders [57]. Evidence also suggests that RBC EPA+DHA deficits precede or coincide with the initial onset of psychopathology [50,58]. Together, these findings suggest that RBC LCn-3 fatty acid deficiency may represent a general risk biomarker or risk factor associated with psychopathology.
While the etiology of LCn-3 fatty acid deficiency exhibited by psychiatric patients is likely multifactorial, understanding the underlying causes will have important implications for guiding treatment and prevention strategies. A number of findings suggest that deficiencies in oxidative defenses (i.e., alpha-tocopherol) are associated with increased susceptibility of RBC EPA+DHA to oxidative degradation [59–63]. It is also relevant that some studies have found that cigarette smoking, which is highly prevalent among patients with mood and psychotic disorders, is associated with elevated indices of RBC lipid peroxidation and lower EPA+DHA levels [64–66]. However, other findings suggest that elevated lipid peroxidation secondary to cigarette smoking cannot uniformly account for LCn-3 fatty acid deficits observed in psychiatric patients [38,46]. Additionally, heritable polymorphisms in the biosynthesis genes that mediate LCn-3 fatty acid biosynthesis may also be an important determinant of LCn-3 fatty acid status [67,68], and preliminary gene expression studies have observed alterations in the expression of biosynthesis genes in psychiatric patients [69–71]. Additionally or alternatively, evidence suggests that the LCn-3 fatty acid deficits observed in psychiatric patients are attributable to dietary LCn-3 fatty acid insufficiency [41,72]. Indeed, dietary LCn-3 fatty acid supplementation increases and normalizes RBC EPA+DHA composition in psychiatric patients [73,74]. Therefore, although the RBC EPA+DHA deficits observed in psychiatric patients may have a multifactorial etiology, they can be corrected with dietary LCn-3 fatty acid supplementation.
4. Rationale for treating LCn-3 fatty acid deficits in psychiatric patients
While beyond the scope of this review, there are several plausible biological mechanisms that could potentially mediate low LCn-3 fatty acid status observed in psychiatric patients and pathogenic mechanisms implicated in psychopathology. These mechanisms include a dysregulation in membrane signal transduction pathways [75], hypothalamic-pituitary-adrenal axis dysregulation [76–78], impaired neurotrophic production, neurogenesis and synaptogenesis during perinatal development [79–81], reduced neuronal resilience to physiological stressors [82], neurodevelopmental perturbations in serotonin [83–85] and dopamine [86–88] neurotransmission, and elevated pro-inflammatory immune signaling [89–91]. These and other examples provide a biological foundation linking LCn-3 fatty acid deficiency with relevant pathogenic processes, and additional clinical studies are warranted to evaluate whether these mechanisms can be modified by increasing LCn-3 fatty acid status.
More central to patient treatment, different lines of evidence suggest that LCn-3 fatty acid deficiency may represent a modifiable risk factor for psychiatric symptoms. First, cross-national epidemiological data indicate that greater habitual intake of fish and seafood (i.e., good dietary sources of LCn-3 fatty acids) are associated with reduced lifetime prevalence rates of unipolar depression and bipolar disorders [92–95]. Second, some cross-sectional studies [96–99], but not all [100,101], have observed an independent inverse association between low fish/seafood or fish oil intake and risk for developing depressive symptoms, particularly in women. Third, some prospective longitudinal observational studies [102–104] but not others [101,105] have observed an inverse association between baseline LCn-3 fatty acid intake/status and the subsequent emergence of depressive symptoms during the follow-up observation period. Fourth, retrospective studies suggest that population-wide shifts in habitual diets from fish-based to low LCn-3 ‘western diets’ are associated with increased rates of seasonal affective disorder, depression, and suicide [106]. Fifth, some fatty acid composition studies have observed an inverse correlation between plasma and/or RBC LCn-3 fatty acid composition, or LCn-3 fatty acid composition relative to LCn-6 composition (i.e., AA/EPA), and depression or manic symptom severity [72,107,108]. Sixth, prospective surveillance studies have found that low baseline LCn-3 fatty acid status is a predictor of depression development in human hepatitis C patients receiving interferon-α treatment [109,110] and the development of psychosis in ultra-high risk adolescents [111]. Although addition research is needed, this body of evidence suggests that low LCn-3 fatty acid status may represent a modifiable risk factor for mechanisms implicated in the progression of mood and psychotic disorders.
Consistent with this notion, independent meta-analyses of several small controlled LCn-3 fatty acid intervention trials have found a significant advantage of LCn-3 fatty acids over placebo in the treatment of syndromal depression [17,112,113]. Controlled and open-label trials have also found that LCn-3 supplementation, but not short-chain n-3 fatty acid supplementation [114], significantly reduces manic and/or depression symptom severity in pediatric and adolescent patients [73,74,115]. Importantly, reductions in manic and depression symptom severity following LCn-3 fatty acid supplementation were associated with increases in RBC EPA+DHA composition [73,74]. Accumulating evidence also suggests that LCn-3 fatty acid treatment may have benefits for positive and negative symptoms of schizophrenia, particularly when administered early in the course of illness [58,63,116–119]. A meta-analysis of controlled trials also found that LCn-3 fatty acid treatment produces a small but significant benefit for ADHD symptoms [120], and a preliminary controlled trial found that LCn-3 fatty acid treatment reduces anxiety symptoms in healthy subjects [121]. Although additional research is required to confirm and extend these findings, this body of evidence suggests that increasing LCn-3 fatty acid status has beneficial effects on different psychiatric symptoms.
Mood and psychotic disorders are associated with excess premature mortality primarily attributable to suicide [1–4], and several lines of evidence suggest that increasing LCn-3 fatty acid status may reduce suicide risk. First, cross-sectional epidemiological surveys have observed an inverse correlation between dietary LCn-3 fatty acid intake and the prevalence of suicidality [122], and that seasonal variations in suicide rates coincide with seasonal variations in serum LCn-3 fatty acid levels [123]. In two case-control studies, RBC or plasma LCn-3 fatty acid composition was found to be significantly reduced in suicidal patients [124,125], and a prospective surveillance study found that low baseline plasma DHA composition was a significant predictor of future suicidal attempts in medication-free patients with MDD [126]. Lastly, two controlled trials have found that chronic (12 week) dietary LCn-3 fatty acid treatment reduced suicidality in MDD patients [127,128]. While the mechanisms mediating this effect are not fully understood, these data suggest that increasing LCn-3 fatty acid status may have the added benefit of reducing suicide risk.
Excess premature mortality in psychiatric patients is also attributable to cardiovascular-related diseases [1–4], and multiple lines of evidence now suggest that LCn-3 fatty acid deficiency increases risk for cardiovascular disease morbidity and mortality [39]. Specifically, cross-national epidemiological surveys have observed reduced prevalence rates of cardiovascular disease in populations whose habitual diets include foods rich in LCn-3 fatty acids [129], and fatty acid composition studies have found an inverse correlation between RBC LCn-3 fatty acid status and cardiovascular risk factors [39]. Moreover, preclinical evidence suggests that LCn-3 fatty acids are protective against cardiac arrhythmias [130,131], and prospective studies have found that low baseline RBC LCn-3 fatty acid status is associated with increased risk of sudden cardiac arrest [132,133]. Intervention studies have found that increasing dietary LCn-3 fatty acid status reduces cardiovascular events and the incidence of sudden cardiac mortality [134–136]. It is relevant, therefore, that the low RBC EPA+DHA levels observed in patients with mood and psychotic disorders are similar to those observed in patients suffering acute coronary syndrome [137], and are associated with cardiovascular risk factors including elevated serum triglyceride and C-reactive protein (CRP) levels [138]. Together, these findings suggest that low LCn-3 fatty acid status may be an important determinant of premature excess mortality associated with cardiovascular disease in patients with psychiatric illnesses, and provides an independent rationale for LCn-3 fatty acid treatment.
4.1. Adjunctive LCn-3 fatty acid treatment
In the majority of prior controlled LCn-3 fatty acid intervention studies observing benefits for depressive symptoms [112,113], patients were also receiving conventional antidepressant medications. These data suggest that adjunctive LCn-3 fatty acid treatment augments the therapeutic efficacy of antidepressant medications. This is directly supported by two studies that compared selective serotonin reuptake inhibitor (SSRI) treatment with or without adjunctive LCn-3 fatty acids in patients with MDD. In both studies, adjunctive LCn-3 fatty acid treatment augmented the therapeutic efficacy of fluoxetine [139] or citalopram [140]. Additionally, adjunctive LCn-3 fatty acid treatment was found to reduce depression symptom severity in MDD patients that were refractory to standard antidepressant treatment [128]. Adjunctive LCn-3 fatty acid treatment was also found to reduce relapse rates in predominantly medicated adult patients with bipolar disorder [134], and to reduce manic symptom severity in medicated pediatric patients with bipolar disorder [74]. In first-episode psychotic patients, adjunctive LCn-3 fatty acid treatment was found to accelerate treatment response, improve tolerability, and permit a 20 percent reduction in second-generation antipsychotic (SGA) dose [135]. These preliminary findings suggest that adjunctive LCn-3 fatty acid treatment augments the therapeutic efficacy of conventional antidepressant, mood-stabilizer, and antipsychotic medications.
In addition to improving efficacy, adjunctive LCn-3 fatty acid treatment may also be protective against adverse cardiometabolic side effects associated with medication exposure. For example, SGA medications, including quetiapine, olanzapine, and risperidone, are associated with adverse cardiometabolic side-effects including elevated triglyceride levels and weight gain in the majority of first-episode patients [14]. This is a significant concern because these side-effects pose substantial long-term health risks in adulthood in an already vulnerable population [142–143]. Consistent with evidence that increasing LCn-3 fatty acid status is efficacious for lowering elevated triglyceride levels in the general population [144], studies have found that adjunctive LCn-3 fatty acid treatment significantly and dose-dependently decreased elevated fasting triglyceride levels in schizophrenic patients treated with clozapine [119,145]. Moreover, emerging evidence suggests that low LCn-3 fatty acid status is associated with increased waist circumference, overweight and obesity [146–149], and preliminary intervention studies have observed reductions in total fat mass, subcutaneous adipocyte diameter, and body mass index in adult overweight or obese patients following LCn-3 fatty acid supplementation [150–152]. Although prospective research is needed to confirm these protective effects, these preliminary findings suggests that adjunctive LCn-3 fatty acid treatment may mitigate adverse cardiometabolic side effects and weight gain following initiation of SGA treatment.
4.2. LCn-3 fatty acid monotherapy and clinical staging
The ‘clinical staging’ approach proposes that safer and better tolerated interventions be used for the treatment of symptoms in earlier stages of the illness, followed by more aggressive treatments that may pose greater health risks for non-responsive cases [153]. While this treatment model has been widely adopted by other fields of medicine including oncology, it is also heuristically valuable for guiding early interventions in children and adolescents with or at high risk for psychiatric disorders [154]. Although evidence from retrospective and prospective studies is beginning to elucidate prodromal criteria to identify individuals that are at ‘high risk’ for developing mood and psychotic disorders [154–156], prodromal symptoms may precede the onset of illness by many years necessitating that preventative interventions be safe and well tolerated with chronic exposure. Extant evidence further suggests that conventional medications may not be efficacious or well-tolerated for at risk youth [157]. Therefore, in view of the long-term safety and tolerability profile of LCn-3 fatty acids, they may be ideally suited for primary prevention in subjects meeting high risk criteria. As proof-of-concept, a double-blind trial randomized 81 patients at high risk for developing psychosis to 12-week treatment with 1.2 g/d of LCn-3 fatty acids or placebo, followed by a 40-week observation period after treatment cessation (12 months total) [58]. By the end of the 12-month treatment component, 2 of 41 individuals (4.9%) in the LCn-3 fatty acid arm and 11 of 40 (27.5%) in the placebo arm transitioned to threshold psychosis (p=0.007). It was concluded that LCn-3 fatty acids were efficacious for preventing or delaying psychosis transitioning in high risk patients. These preliminary data suggest that LCn-3 fatty acids may be efficacious and well-tolerated as a primary prevention intervention, and analogous trials are warranted in youth meeting high risk criteria for other psychiatric disorders.
In addition to primary prevention, LCn-3 fatty acid monotherapy may also have a role in early secondary prevention. Preliminary prospective intervention trials have found that LCn-3 fatty acid monotherapy significantly reduces symptom severity in pediatric and adolescent patients with MDD [115] or bipolar disorder [73]. For example, in a randomized, double-blind placebo-controlled trial conducted by Nemets et al., [115] children early in the course of depression received a placebo or LCn-3 fatty acid monotherapy for 16 weeks. The subjects treated with LCn-3 fatty acids exhibited significant reductions in depression symptom severity relative to subjects treated with placebo at week 8 which was sustained to week 16. Seventy percent of subjects treated with LCn-3 fatty acids, and none of the subjects treated with placebo, had a greater than 50% reduction in symptom severity, and 40% of subjects treated with LCn-3 fatty acids, and none of the subjects treated with placebo, exhibited symptomatic remission. In a separate study, LCn-3 fatty acid monotherapy was found to significantly reduce positive and negative symptom severity compared with placebo in patients at ultra-high risk for developing psychosis [58]. Although additional research is needed to confirm these preliminary findings, these data suggest that LCn-3 fatty acid monotherapy may be efficacious for the treatment of sub-syndromal as well as syndromal mood and psychotic symptoms in youth early in their illness course.
Additionally LCn-3 fatty acid monotherapy may also have a role in treating perinatal psychiatric symptoms when conventional medications may be contraindicated due to potential fetal teratogenic effects. For example, LCn-3 fatty acid monotherapy was found to reduce positive and negative symptom severity in a pregnant schizophrenic women following medication discontinuation [164]. Moreover, women experiencing postpartum depression exhibit plasma LCn-3 fatty acid deficits [158,159] and LCn-3 fatty acid treatment were found to reduce symptom severity in women with depression arising during pregnancy [160] or postpartum [161]. However, other studies did not observe preventative efficacy in women with [162] or without [163] a history of postpartum depression.
5. LCn-3 fatty acid safety and dosing considerations
Potential adverse events associated with LCn-3 fatty acid treatment include gastrointestinal disturbances, including nausea, diarrhea, gastroesophageal reflux, eructation, and less commonly emesis. In double-blind clinical trials of adult patients, the principal adverse events reported after chronic (8–12 weeks) treatment were gastrointestinal problems, and were considered mild and reported as frequently in patients receiving the olive oil placebo [17]. To minimize the gastrointestinal adverse events associated with LCn-3 fatty acids, patients should be instructed to take their pills with meals. Although taking fish oil at high doses (>3 g/d) has been associated in isolated cases with increased bleeding time in subjects also taking anticoagulant medications [165], controlled clinical trials have found that chronic high dose EPA+DHA alone or in combination with aspirin does not increase risk for clinically-significant increases in bleeding time [166–168]. Another safety consideration involves the potential threat of contamination of fish and seafood with methyl mercury, PCBs, and other environmental pollutants. However, most fish oil supplements are highly purified and do not exceed U.S. Food and Drug Administration (FDA) limits for methyl mercury and other environmental contaminants. As with all medications, patients should be informed of potential risks associated with LCn-3 fatty acids, and patients with an allergy to shellfish or seafood should be closely monitored.
The U.S. FDA considers doses up to 3 g/d of LCn-3 fatty acids to be ‘generally regarded as safe’, and the American Psychiatric Association has adopted the consensus recommendations of the American Heart Association for an EPA+DHA dose of 1 g/d [17]. The American Heart Association also recommends 3 g/d EPA+DHA for reducing elevated triglyceride levels. Prescription EPA+DHA (Lovaza® in the US, Omacor® in Europe, GlaxoSmithKline) and purified ethyl ester EPA containing no DHA (Vascepa™, Amarin Corporation) have been approved by the U.S. FDA for the treatment of hypertriglyceridemia (≥500 mg/dL) at a dose of 4 g/d. A free versus ethyl ester EPA+DHA formulation (Epanova, Omthera Pharmaceuticals Inc.) found to result in greater free fatty acid bioavailability is currently in development [169], and a vegetarian (i.e., algal-derived) EPA+DHA supplement option is also available. It is important to note, however, that LCn-3 fatty acids are not currently approved by the FDA for the treatment of any psychiatric disorder. Nevertheless, over-the-counter fish oil supplements containing similar capsule ethyl ester EPA+DHA concentrations are widely available to consumers at substantially lower costs.
Controlled intervention studies have found that doses in the range of 1–4 g/d of EPA+DHA in a 2:1 EPA to DHA ratio are efficacious for the treatment of mood symptoms [17,113]. Emerging evidence also suggests that a larger ratio of EPA to DHA may be more efficacious for treating depressive symptoms [170] as well as ADHD symptoms [120]. Furthermore, 1–4 g/d of EPA+DHA significantly increases RBC EPA+DHA composition to levels similar to those observed in healthy subjects in Japan [171], where the life-time prevalence rates of mood disorders are among the lowest in the world [95]. As with other medications, upward dose titration may be required as clinically indicated. In an open-label flexible dosing study, 8-week LCn-3 fatty acid monotherapy led to a statistically significant reduction in manic symptom severity scores in pediatric bipolar patients [73]. In this study the starting dose was 1.3 g/d of EPA+DHA, the maximum dose was 4.3 g/d, and the mean dose was 2.6 g/d. In this and other studies conducted in pediatric patients, no clinically-significant treatment-emergent adverse events were reported following long-term (8–16 weeks) EPA+DHA treatment at doses up to 4.3 g/d [74,115]. While there is a clear need for additional dose-ranging and dose-titrating secondary prevention trials to elucidate optimal LCn-3 fatty acid dosing strategies, existing evidence suggests that EPA+DHA doses in the range of 1–4 g/d are safe and well-tolerated in pediatric, adolescent, and adult psychiatric patients.
6. Clinical Vignette
Patient A., a 20 year old undergraduate student who met DSM-IV-TR criteria for major depressive disorder, recurrent, without psychotic features or generalized anxiety disorder, presented for treatment following a psychiatric hospitalization which was precipitated by increasing suicidal ideation. During his inpatient hospitalization, citalopram monotherapy was initiated at a dose of 20 mg daily and was continued following his discharge. Despite excellent compliance, A. continued to experience significant depressive symptoms, including depressed mood, guilt, anhedonia, weight gain, hyperphagia and hypersomnia. Following treatment with citalopram at a dose of 20 mg daily for approximately 4 weeks, citalopram was increased to 40 mg daily. However, A’s depressive symptoms were only mildly reduced and he continued to experience significant functional impairment. Augmentation with low-dose quetiapine (12.5 mg to 25 mg QHS) was attempted, though this was not tolerated secondary to excessive sedation and accentuation of weight gain and was discontinued. At that time, citalopram was increased to 60 mg daily. However, A. continued to report persistent depressed mood and a heavy neurovegetative burden, including anergia, hypersomnia and hyperphagia. Treatment with adjunctive LCn-3 fatty acids was initiated at a dose of 2.4 g daily (EPA: 1.6 g, DHA: 0.8 g, 4 capsules per day, OmegaRx®). Over the next 10 weeks, A’s score on the Children Depression Rating Scale-Revised (CDRS-R) decreased from 30 to 21 and he noted less depressed mood and moderate improvement in his neurovegetative symptoms. Moreover, his initially low red blood cell EPA+DHA composition (2.8% of total fatty acids) increased ~2-fold to 5.5% of total fatty acids following 10 week LCn-3 fatty acid treatment. He has continued to take adjunctive LCn-3 fatty acids for 12 months in addition to citalopram 60 mg daily. Importantly, A. has returned to school, is doing well academically and socially and his major depressive disorder has remained in remission. This case illustrates both the benefit and tolerability of adjunctive LCn-3 fatty acid treatment for patients exhibiting partial response to conventional antidepressant medications.
7. Summary and conclusions
There is now a substantial body of evidence that LCn-3 fatty acid deficiency is associated with pathophysiological mechanisms implicated in the progression of different psychiatric disorders, and may contribute risk to principle causes of excess premature mortality including suicide and cardiovascular disease. Emerging evidence suggests that LCn-3 fatty acids augment the therapeutic efficacy of antidepressant, mood-stabilizer, and SGA medications, and may additionally mitigate the adverse cardiometabolic side-effects associated with SGA medications. Preliminary evidence also suggests that LCn-3 fatty acids have efficacy as a primary prevention for high risk youth, for early secondary prevention within a ‘clinical staging’ framework, and for treating psychiatric symptoms during and following pregnancy. Additionally, LCn-3 fatty acids have an established long-term safety record and the overall cost-benefit ratio provides a strong rationale for incorporating LCn-3 fatty acid treatment into psychiatric treatment algorithms. The recent emergence of laboratory facilities that specialize in determining blood LCn-3 fatty acid status, emerging evidence-based consensus regarding optimal dosing regimens, and the availability of prescription and over-the-counter LCn-3 fatty acid supplements provide the infrastructure required for implementation.
Layperson's summary.
Nutrition plays a minor role in psychiatric practice which is currently dominated by a pharmacological treatment algorithm. An accumulating body of evidence has implicated deficits in the dietary essential long-chain omega-3 (LCn-3) fatty acids in the pathophysiology and treatment of several major psychiatric disorders. The overall cost-benefit ratio endorses the incorporation of LCn-3 fatty acids into psychiatric treatment algorithms.
ACKNOWLEDGEMENTS
This work was supported in part by National Institute of Health grants MH083924 and AG03617, and a NARSAD Independent Investigator Award to R.K.M. R.K.M. has received research support from Martek Biosciences Inc, Inflammation Research Foundation (IRF), Ortho-McNeil Janssen, AstraZeneca, Eli Lilly. R.K.M. is a member of the IRF scientific advisory board. J.R.S. has received research support from Eli Lilly, Shire and from the American Academy of Child and Adolescent Psychiatry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34–38 years. J Affect Disord. 2002;68:167–181. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- 2.Osby U, Brandt L, Correia N, Ekbom A, Sparén P. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58:844–850. doi: 10.1001/archpsyc.58.9.844. [DOI] [PubMed] [Google Scholar]
- 3.Brown S. Excess mortality of schizophrenia. A meta-analysis. Br J Psychiatry. 1997;171:502–508. doi: 10.1192/bjp.171.6.502. [DOI] [PubMed] [Google Scholar]
- 4.Osby U, Correia N, Brandt L, Ekbom A, Sparén P. Mortality and causes of death in schizophrenia in Stockholm county, Sweden. Schizophr. Res. 2000;45:21–28. doi: 10.1016/s0920-9964(99)00191-7. [DOI] [PubMed] [Google Scholar]
- 5.Burke KC, Burke JD, Jr, Rae DS, Regier DA. Comparing age at onset of major depression and other psychiatric disorders by birth cohorts in five US community populations. Arch Gen Psychiatry. 1991;48:789–795. doi: 10.1001/archpsyc.1991.01810330013002. [DOI] [PubMed] [Google Scholar]
- 6.Chengappa KN, Kupfer DJ, Frank E, Houck PR, Grochocinski VJ, Cluss PA, Stapf DA. Relationship of birth cohort and early age at onset of illness in a bipolar disorder case registry. Am J Psychiatry. 2003;160:1636–1642. doi: 10.1176/appi.ajp.160.9.1636. [DOI] [PubMed] [Google Scholar]
- 7.Perlis RH, Dennehy EB, Miklowitz DJ, Delbello MP, Ostacher M, Calabrese JR, Ametrano RM, Wisniewski SR, Bowden CL, Thase ME, Nierenberg AA, Sachs G. Retrospective age at onset of bipolar disorder and outcome during two-year follow-up: results from the STEP-BD study. Bipolar Disord. 2009;11:391–400. doi: 10.1111/j.1399-5618.2009.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Girolamo G, Dagani J, Purcell R, Cocchi A, McGorry PD. Age of onset of mental disorders and use of mental health services: needs, opportunities and obstacles. Epidemiol Psychiatr Sci. 2012;21:47–57. doi: 10.1017/s2045796011000746. [DOI] [PubMed] [Google Scholar]
- 9.Beesdo K, Pine DS, Lieb R, Wittchen HU. Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Arch Gen Psychiatry. 2010;67:47–57. doi: 10.1001/archgenpsychiatry.2009.177. [DOI] [PubMed] [Google Scholar]
- 10.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 11.Ayuso-Gutierrez JL, del Rio Vega JM. Factors influencing relapse in the long-term course of schizophrenia. Schizophr Res. 1997;28:199–206. doi: 10.1016/s0920-9964(97)00131-x. [DOI] [PubMed] [Google Scholar]
- 12.Cavanagh J, Smyth R, Goodwin GM. Relapse into mania or depression following lithium discontinuation: a 7-year follow-up. Acta Psychiatr Scand. 2004;109:91–95. doi: 10.1046/j.1600-0447.2003.00274.x. [DOI] [PubMed] [Google Scholar]
- 13.Geddes JR, Carney SM, Davies C, Furukawa TA, Kupfer DJ, Frank E, Goodwin GM. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361:653–661. doi: 10.1016/S0140-6736(03)12599-8. [DOI] [PubMed] [Google Scholar]
- 14.Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302:1765–1773. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63:332–339. doi: 10.1001/archpsyc.63.3.332. [DOI] [PubMed] [Google Scholar]
- 16.Martin A, Young C, Leckman JF, Mukonoweshuro C, Rosenheck R, Leslie D. Age effects on antidepressant-induced manic conversion. Arch Pediatr Adolesc Med. 2004;158:773–780. doi: 10.1001/archpedi.158.8.773. [DOI] [PubMed] [Google Scholar]
- 17.Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, Keck PE, Jr, Marangell LB, Richardson AJ, Lake J, Stoll AL. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J. Clin. Psychiatry. 2006;67:1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- 18.Hibbeln JR, Salem N., Jr Dietary polyunsaturated fatty acids and depression: when cholesterol does not satisfy. Am J Clin Nutr. 1995;62:1–9. doi: 10.1093/ajcn/62.1.1. [DOI] [PubMed] [Google Scholar]
- 19.McNamara RK. Evaluation of docosahexaenoic acid deficiency as a preventable risk factor for recurrent affective disorders: Current status, future directions, and dietary recommendations. Prostogland Leukotrienes Essential Fatty Acids. 2009;81:223–231. doi: 10.1016/j.plefa.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Parker G, Gibson NA, Brotchie H, Heruc G, Rees AM, Hadzi-Pavlovic D. Omega-3 fatty acids and mood disorders. Am J Psychiatry. 2006;163:969–978. doi: 10.1176/ajp.2006.163.6.969. [DOI] [PubMed] [Google Scholar]
- 21.Brenna JT, Salem N, Jr, Sinclair AJ, Cunnane SC. International Society for the Study of Fatty Acids and Lipids, ISSFAL. alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids. 2009;80:85–91. doi: 10.1016/j.plefa.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Barceló-Coblijn G, Murphy EJ, Othman R, Moghadasian MH, Kashour T, Friel JK. Flaxseed oil and fish-oil capsule consumption alters human red blood cell n-3 fatty acid composition: a multiple-dosing trial comparing 2 sources of n-3 fatty acid. Am J Clin Nutr. 2008;88:801–809. doi: 10.1093/ajcn/88.3.801. [DOI] [PubMed] [Google Scholar]
- 23.Francois CA, Connor SL, Bolewicz LC, Connor WE. Supplementing lactating women with flaxseed oil does not increase docosahexaenoic acid in their milk. Am J Clin Nutr. 2003;77:226–233. doi: 10.1093/ajcn/77.1.226. [DOI] [PubMed] [Google Scholar]
- 24.Jensen CL, Maude M, Anderson RE, Heird WC. Effect of docosahexaenoic acid supplementation of lactating women on the fatty acid composition of breast milk lipids and maternal and infant plasma phospholipids. Am J Clin Nutr. 2000;71:292S–299S. doi: 10.1093/ajcn/71.1.292s. [DOI] [PubMed] [Google Scholar]
- 25.Su HM, Bernardo L, Mirmiran M, Ma XH, Corso TN, Nathanielsz PW, Brenna JT. Bioequivalence of dietary alpha-linolenic and docosahexaenoic acids as sources of docosahexaenoate accretion in brain and associated organs of neonatal baboons. Pediatr Res. 1999;45:87–93. doi: 10.1203/00006450-199901000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 27.Fekete K, Marosvölgyi T, Jakobik V, Decsi T. Methods of assessment of n-3 long-chain polyunsaturated fatty acid status in humans: a systematic review. Am J Clin Nutr. 2009;89:2070S–2084S. doi: 10.3945/ajcn.2009.27230I. [DOI] [PubMed] [Google Scholar]
- 28.Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res. 1997;38:2012–2022. [PubMed] [Google Scholar]
- 29.Kuratko CN, Salem N., Jr Biomarkers of DHA status. Prostaglandins Leukot Essent Fatty Acids. 2009;81:111–118. doi: 10.1016/j.plefa.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Vidgren HM, Agren JJ, Schwab U, Rissanen T, Hänninen O, Uusitupa MI. Incorporation of n-3 fatty acids into plasma lipid fractions, and erythrocyte membranes and platelets during dietary supplementation with fish, fish oil, and docosahexaenoic acid-rich oil among healthy young men. Lipids. 1997;32:697–705. doi: 10.1007/s11745-997-0089-x. [DOI] [PubMed] [Google Scholar]
- 31.Browning LM, Walker CG, Mander AP, West AL, Madden J, Gambell JM, Young S, Wang L, Jebb SA, Calder PC. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am J Clin Nutr. 2012;96:748–758. doi: 10.3945/ajcn.112.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47:348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Ogura T, Takada H, Okuno M, Kitade H, Matsuura T, Kwon M, Arita S, Hamazaki K, Itomura M, Hamazaki T. Fatty acid composition of plasma, erythrocytes and adipose: their correlations and effects of age and sex. Lipids. 2010;45:137–144. doi: 10.1007/s11745-010-3386-3. [DOI] [PubMed] [Google Scholar]
- 34.Bergmann RL, Haschke-Becher E, Klassen-Wigger P, Bergmann KE, Richter R, Dudenhausen JW, Grathwohl D, Haschke F. Supplementation with 200 mg/day docosahexaenoic acid from mid-pregnancy through lactation improves the docosahexaenoic acid status of mothers with a habitually low fish intake and of their infants. Ann Nutr Metab. 2008;52:157–166. doi: 10.1159/000129651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, Porter CB, Borkon AM. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation. 2004;110:1645–1649. doi: 10.1161/01.CIR.0000142292.10048.B2. [DOI] [PubMed] [Google Scholar]
- 36.Carver JD, Benford VJ, Han B, Cantor AB. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res Bull. 2001;56:79–85. doi: 10.1016/s0361-9230(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 37.Connor WE, Neuringer M, Lin DS. Dietary effects on brain fatty acid composition: the reversibility of n-3 fatty acid deficiency and turnover of docosahexaenoic acid in the brain, erythrocytes, and plasma of rhesus monkeys. J Lipid Res. 1990;31:237–247. [PubMed] [Google Scholar]
- 38.Sands SA, Reid KJ, Windsor SL, Harris WS. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids. 2005;40:343–347. doi: 10.1007/s11745-006-1392-2. [DOI] [PubMed] [Google Scholar]
- 39.Harris WS. The omega-3 index as a risk factor for coronary heart disease. Am J Clin Nutr. 2008;87:1997S–2002S. doi: 10.1093/ajcn/87.6.1997S. [DOI] [PubMed] [Google Scholar]
- 40.Assies J, Pouwer F, Lok A, Mocking RJ, Bockting CL, Visser I, Abeling NG, Duran M, Schene AH. Plasma and erythrocyte fatty acid patterns in patients with recurrent depression: a matched case-control study. PLoS One. 2010;5(5):e10635. doi: 10.1371/journal.pone.0010635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord. 1998;48:149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- 42.Maes M, Smith R, Christophe A, Cosyns P, Desnyder R, Meltzer H. Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20: 4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J Affect Disord. 1996;38:35–46. doi: 10.1016/0165-0327(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 43.Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry. 1998;43:315–319. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- 44.Riemer S, Maes M, Christophe A, Rief W. Lowered omega-3 PUFAs are related to major depression, but not to somatization syndrome. J Affect Disord. 2010;123:173–180. doi: 10.1016/j.jad.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 45.McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J Affect Disord. 2010;126:303–311. doi: 10.1016/j.jad.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiu CC, Huang SY, Su KP, Lu ML, Huang MC, Chen CC, Shen WW. Polyunsaturated fatty acid deficit in patients with bipolar mania. Eur Neuropsychopharmacol. 2003;13:99–103. doi: 10.1016/s0924-977x(02)00130-x. [DOI] [PubMed] [Google Scholar]
- 47.Ranjekar PK, Hinge A, Hegde MV, Ghate M, Kale A, Sitasawad S, Wagh UV, Debsikdar VB, Mahadik SP. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res. 2003;121:109–122. doi: 10.1016/s0165-1781(03)00220-8. [DOI] [PubMed] [Google Scholar]
- 48.Evans DR, Parikh VV, Khan MM, Coussons C, Buckley PF, Mahadik SP. Red blood cell membrane essential fatty acid metabolism in early psychotic patients following antipsychotic drug treatment. Prostaglandins Leukot Essent Fatty Acids. 2003;69:393–399. doi: 10.1016/j.plefa.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 49.Khan MM, Evans DR, Gunna V, Scheffer RE, Parikh VV, Mahadik SP. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics. Schizophr. Res. 2002;58:1–10. doi: 10.1016/s0920-9964(01)00334-6. [DOI] [PubMed] [Google Scholar]
- 50.Reddy RD, Keshavan MS, Yao JK. Reduced red blood cell membrane essential polyunsaturated fatty acids in first episode schizophrenia at neuroleptic-naive baseline. Schizophr Bull. 2004;30:901–911. doi: 10.1093/oxfordjournals.schbul.a007140. [DOI] [PubMed] [Google Scholar]
- 51.Green P, Hermesh H, Monselise A, Marom S, Presburger G, Weizman A. Red cell membrane omega-3 fatty acids are decreased in nondepressed patients with social anxiety disorder. Eur Neuropsychopharmacol. 2006;16:107–113. doi: 10.1016/j.euroneuro.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 52.Chen JR, Hsu SF, Hsu CD, Hwang LH, Yang SC. Dietary patterns and blood fatty acid composition in children with attention-deficit hyperactivity disorder in Taiwan. J. Nutr. Biochem. 2004;15:467–472. doi: 10.1016/j.jnutbio.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 53.Colter AL, Cutler C, Meckling KA. Fatty acid status and behavioural symptoms of attention deficit hyperactivity disorder in adolescents: a case-control study. Nutr J. 2008;7:8. doi: 10.1186/1475-2891-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stevens LJ, Zentall SS, Deck JL, Abate ML, Watkins BA, Lipp SR, Burgess JR. Essential fatty acid metabolism in boys with attention-deficit hyperactivity disorder. Am. J. Clin. Nutr. 1995;62:761–768. doi: 10.1093/ajcn/62.4.761. [DOI] [PubMed] [Google Scholar]
- 55.Young GS, Maharaj NJ, Conquer JA. Blood phospholipid fatty acid analysis of adults with and without attention deficit/hyperactivity disorder. Lipids. 2004;39:117–123. doi: 10.1007/s11745-004-1209-3. [DOI] [PubMed] [Google Scholar]
- 56.Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68:140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 57.McNamara RK. Deciphering the role of docosahexaenoic acid in brain maturation and pathology with magnetic resonance imaging. Prostaglandins Leukot Essent Fatty Acids. 2012 doi: 10.1016/j.plefa.2012.03.011. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amminger GP, Schäfer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, Mackinnon A, McGorry PD, Berger GE. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 59.Palozza P, Sgarlata E, Luberto C, Piccioni E, Anti M, Marra G, Armelao F, Franceschelli P, Bartoli GM. n-3 fatty acids induce oxidative modifications in human erythrocytes depending on dose and duration of dietary supplementation. Am J Clin Nutr. 1996 Sep;64(3):297–304. doi: 10.1093/ajcn/64.3.297. [DOI] [PubMed] [Google Scholar]
- 60.Thorlaksdottir AY, Skuladottir GV, Petursdottir AL, Tryggvadottir L, Ogmundsdottir HM, Eyfjord JE, Jonsson JJ, Hardardottir I. Positive association between plasma antioxidant capacity and n-3 PUFA in red blood cells from women. Lipids. 2006;41:119–125. doi: 10.1007/s11745-006-5079-5. [DOI] [PubMed] [Google Scholar]
- 61.Maes M, De Vos N, Pioli R, Demedts P, Wauters A, Neels H, Christophe A. Lower serum vitamin E concentrations in major depression. Another marker of lowered antioxidant defenses in that illness. J Affect Disord. 2000;58:241–246. doi: 10.1016/s0165-0327(99)00121-4. [DOI] [PubMed] [Google Scholar]
- 62.Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum Psychopharmacol. 2007;22:67–73. doi: 10.1002/hup.829. [DOI] [PubMed] [Google Scholar]
- 63.Arvindakshan M, Ghate M, Ranjekar PK, Evans DR, Mahadik SP. Supplementation with a combination of omega-3 fatty acids and antioxidants (vitamins E and C) improves the outcome of schizophrenia. Schizophr Res. 2003;62:195–204. doi: 10.1016/s0920-9964(02)00284-0. [DOI] [PubMed] [Google Scholar]
- 64.Gokulakrishnan A, Ali AR. Cigarette smoke-induced biochemical perturbations in human erythrocytes and attenuation by epigallocatechin-3-gallate--tea catechin. Pharmacol Rep. 2010;62:891–899. doi: 10.1016/s1734-1140(10)70349-2. [DOI] [PubMed] [Google Scholar]
- 65.Padmavathi P, Reddy VD, Kavitha G, Paramahamsa M, Varadacharyulu N. Chronic cigarette smoking alters erythrocyte membrane lipid composition and properties in male human volunteers. Nitric Oxide. 2010;23:181–186. doi: 10.1016/j.niox.2010.05.287. [DOI] [PubMed] [Google Scholar]
- 66.Leng GC, Smith FB, Fowkes FG, Horrobin DF, Ells K, Morse-Fisher N, Lowe GD. Relationship between plasma essential fatty acids and smoking, serum lipids, blood pressure and haemostatic and rheological factors. Prostaglandins Leukot Essent Fatty Acids. 1994;51:101–108. doi: 10.1016/0952-3278(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 67.Moltó-Puigmartí C, Plat J, Mensink RP, Müller A, Jansen E, Zeegers MP, Thijs C. FADS1 FADS2 gene variants modify the association between fish intake and the docosahexaenoic acid proportions in human milk. Am J Clin Nutr. 2010;91:1368–1376. doi: 10.3945/ajcn.2009.28789. [DOI] [PubMed] [Google Scholar]
- 68.Koletzko B, Lattka E, Zeilinger S, Illig T, Steer C. Genetic variants of the fatty acid desaturase gene cluster predict amounts of red blood cell docosahexaenoic and other polyunsaturated fatty acids in pregnant women: findings from the Avon Longitudinal Study of Parents and Children. Am J Clin Nutr. 2011;93:211–219. doi: 10.3945/ajcn.110.006189. [DOI] [PubMed] [Google Scholar]
- 69.Liu Y, McNamara RK. Elevated delta-6 desaturase (FADS2) gene expression in the prefrontal cortex of patients with bipolar disorder. J Psychiatry Res. 2011;45:269–272. doi: 10.1016/j.jpsychires.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McNamara RK, Liu Y. Reduced fatty acid biosynthesis gene expression in the prefrontal cortex of patients with major depressive disorder. J Affect Disord. 2011;129:359–363. doi: 10.1016/j.jad.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y, Jandacek R, Rider T, Tso P, McNamara RK. Elevated delta-6 desaturase (FADS2) gene expression in the postmortem prefrontal cortex of schizophrenic patients: Relationship with fatty acid composition. Schizophr Res. 2009;109:113–120. doi: 10.1016/j.schres.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Long-chain omega-3 polyunsaturated fatty acids in the blood of children and adolescents with juvenile bipolar disorder. Lipids. 2008;43:1031–1038. doi: 10.1007/s11745-008-3224-z. [DOI] [PubMed] [Google Scholar]
- 73.Wozniak J, Biederman J, Mick E, Waxmonsky J, Hantsoo L, Best C, Cluette-Brown JE, Laposata M. Omega-3 fatty acid monotherapy for pediatric bipolar disorder: a prospective open-label trial. Eur Neuropsychopharmacol. 2007;17:440–447. doi: 10.1016/j.euroneuro.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 74.Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. Eur J Clin Nutr. 2009;63:1037–1040. doi: 10.1038/ejcn.2008.81. [DOI] [PubMed] [Google Scholar]
- 75.McNamara RK, Ostrander M, Abplanalp W, Richtand NM, Benoit S, Clegg D. Modulation of phosphoinositide-protein kinase C signal transduction by omega-3 fatty acids: Implications for the pathophysiology and treatment of recurrent neuropsychiatric illness. Prostogland Leukotrienes Essential Fatty Acids. 2006;75:237–257. doi: 10.1016/j.plefa.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 76.Delarue J, Matzinger O, Binnert C, Schneiter P, Chioléro R, Tappy L. Fish oil prevents the adrenal activation elicited by mental stress in healthy men. Diabetes Metab. 2003;29:289–295. doi: 10.1016/s1262-3636(07)70039-3. [DOI] [PubMed] [Google Scholar]
- 77.Jazayeri S, Keshavarz SA, Tehrani-Doost M, Djalali M, Hosseini M, Amini H, Chamari M, Djazayery A. Effects of eicosapentaenoic acid and fluoxetine on plasma cortisol, serum interleukin-1beta and interleukin-6 concentrations in patients with major depressive disorder. Psychiatry Res. 2010;178:112–115. doi: 10.1016/j.psychres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 78.Michaeli B, Berger MM, Revelly JP, Tappy L, Chioléro R. Effects of fish oil on the neuroendocrine responses to an endotoxin challenge in healthy volunteers. Clin Nutr. 2007;26:70–77. doi: 10.1016/j.clnu.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 79.Rao JS, Ertley RN, Lee HJ, DeMar JC, Jr, Arnold JT, Rapoport SI, Bazinet RP. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry. 2007;12:36–46. doi: 10.1038/sj.mp.4001888. [DOI] [PubMed] [Google Scholar]
- 80.Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, Kim HY. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem. 2009;111:510–521. doi: 10.1111/j.1471-4159.2009.06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kawakita E, Hashimoto M, Shido O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience. 2006;139:991–997. doi: 10.1016/j.neuroscience.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 82.Bazan NG. Cellular and molecular events mediated by docosahexaenoic acid-derived neuroprotectin D1 signaling in photoreceptor cell survival and brain protection. Prostaglandins Leukot Essent Fatty Acids. 2009;81:205–211. doi: 10.1016/j.plefa.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Delion S, Chalon S, Guilloteau D, Besnard JC, Durand G. alpha-Linolenic acid dietary deficiency alters age-related changes of dopaminergic and serotoninergic neurotransmission in the rat frontal cortex. J Neurochem. 1996;66:1582–1591. doi: 10.1046/j.1471-4159.1996.66041582.x. [DOI] [PubMed] [Google Scholar]
- 84.Kodas E, Galineau L, Bodard S, Vancassel S, Guilloteau D, Besnard JC, Chalon S. Serotoninergic neurotransmission is affected by n-3 polyunsaturated fatty acids in the rat. J Neurochem. 2004;89:695–702. doi: 10.1111/j.1471-4159.2004.02401.x. [DOI] [PubMed] [Google Scholar]
- 85.McNamara RK, Able JA, Liu Y, Jandacek R, Rider T, Tso P, Lipton JW. Omega-3 fatty acid deficiency during perinatal development increases serotonin turnover in the prefrontal cortex and decreases midbrain tryptophan hydroxylase-2 expression in adult female rats: Dissociation from estrogenic effects. J Psychiatry Res. 2009;43:656–663. doi: 10.1016/j.jpsychires.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Levant B, Radel JD, Carlson SE. Decreased brain docosahexaenoic acid during development alters dopamine-related behaviors in adult rats that are differentially affected by dietary remediation. Behav Brain Res. 2004;152:49–57. doi: 10.1016/j.bbr.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 87.Zimmer L, Vancassel S, Cantagrel S, Breton P, Delamanche S, Guilloteau D, Durand G, Chalon S. The dopamine mesocorticolimbic pathway is affected by deficiency in n-3 polyunsaturated fatty acids. Am J Clin Nutr. 2002;75:662–667. doi: 10.1093/ajcn/75.4.662. [DOI] [PubMed] [Google Scholar]
- 88.Kodas E, Vancassel S, Lejeune B, Guilloteau D, Chalon S. Reversibility of n-3 fatty acid deficiency-induced changes in dopaminergic neurotransmission in rats: critical role of developmental stage. J Lipid Res. 2002;43:1209–1219. [PubMed] [Google Scholar]
- 89.Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids. 2008;79:101–108. doi: 10.1016/j.plefa.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 90.Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, Rudolph V, Freeman BA, Schopfer FJ. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol. 2010;6:433–441. doi: 10.1038/nchembio.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McNamara RK, Lotrich FE. Elevated immune-inflammatory signaling in mood disorders: A new therapeutic target? Expert Rev Neurother. 2012;12:1143–1161. doi: 10.1586/ern.12.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351:1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- 93.Hibbeln JR. Seafood consumption, the DHA content of mothers' milk and prevalence rates of postpartum depression: a cross-national, ecological analysis. J Affect Disord. 2002;69:15–29. doi: 10.1016/s0165-0327(01)00374-3. [DOI] [PubMed] [Google Scholar]
- 94.Peet M. International variations in the outcome of schizophrenia and the prevalence of depression in relation to national dietary practices: an ecological analysis. Br J Psychiatry. 2004;184:404–408. doi: 10.1192/bjp.184.5.404. [DOI] [PubMed] [Google Scholar]
- 95.Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. Am J Psychiatry. 2003;160:2222–2227. doi: 10.1176/appi.ajp.160.12.2222. [DOI] [PubMed] [Google Scholar]
- 96.Tanskanen A, Hibbeln JR, Tuomilehto J, Uutela A, Haukkala A, Viinamäki H, Lehtonen J, Vartiainen E. Fish consumption and depressive symptoms in the general population in Finland. Psychiatr Serv. 2001;52:529–531. doi: 10.1176/appi.ps.52.4.529. [DOI] [PubMed] [Google Scholar]
- 97.Timonen M, Horrobin D, Jokelainen J, Laitinen J, Herva A, Räsänen P. Fish consumption and depression: the Northern Finland 1966 birth cohort study. J Affect Disord. 2004;82:447–452. doi: 10.1016/j.jad.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 98.Raeder MB, Steen VM, Vollset SE, Bjelland I. Associations between cod liver oil use and symptoms of depression: the Hordaland Health Study. J Affect Disord. 2007;101:245–249. doi: 10.1016/j.jad.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 99.Kamphuis MH, Geerlings MI, Tijhuis MA, Kalmijn S, Grobbee DE, Kromhout D. Depression and cardiovascular mortality: a role for n-3 fatty acids? Am J Clin Nutr. 2006;84:1513–1517. doi: 10.1093/ajcn/84.6.1513. [DOI] [PubMed] [Google Scholar]
- 100.Appleton KM, Peters TJ, Hayward RC, Heatherley SV, McNaughton SA, Rogers PJ, Gunnell D, Ness AR, Kessler D. Depressed mood and n-3 polyunsaturated fatty acid intake from fish: non-linear or confounded association? Soc Psychiatry Psychiatr Epidemiol. 2007;42:100–104. doi: 10.1007/s00127-006-0142-3. [DOI] [PubMed] [Google Scholar]
- 101.Hakkarainen R, Partonen T, Haukka J, Virtamo J, Albanes D, Lönnqvist J. Is low dietary intake of omega-3 fatty acids associated with depression? Am J Psychiatry. 2004;161:567–569. doi: 10.1176/appi.ajp.161.3.567. [DOI] [PubMed] [Google Scholar]
- 102.Astorg P, Couthouis A, Bertrais S, Arnault N, Meneton P, Guesnet P, Alessandri JM, Galan P, Hercberg S. Association of fish and long-chain n-3 polyunsaturated fatty acid intakes with the occurrence of depressive episodes in middle-aged French men and women. Prostaglandins Leukot Essent Fatty Acids. 2008;78:171–182. doi: 10.1016/j.plefa.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 103.Colangelo LA, He K, Whooley MA, Daviglus ML, Liu K. Higher dietary intake of long-chain omega-3 polyunsaturated fatty acids is inversely associated with depressive symptoms in women. Nutrition. 2009;25:1011–1019. doi: 10.1016/j.nut.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sanchez-Villegas A, Henríquez P, Figueiras A, Ortuño F, Lahortiga F, Martínez-González MA. Long chain omega-3 fatty acids intake, fish consumption and mental disorders in the SUN cohort study. Eur J Nutr. 2007;46:337–346. doi: 10.1007/s00394-007-0671-x. [DOI] [PubMed] [Google Scholar]
- 105.Astorg P, Bertrais S, Alessandri JM, Guesnet P, Kesse-Guyot E, Linard A, Lallemand MS, Galan P, Hercberg S. Long-chain n-3 fatty acid levels in baseline serum phospholipids do not predict later occurrence of depressive episodes: a nested case-control study within a cohort of middle-aged French men and women. Prostaglandins Leukot Essent Fatty Acids. 2009;81:265–271. doi: 10.1016/j.plefa.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 106.McGrath-Hanna NK, Greene DM, Tavernier RJ, Bult-Ito A. Diet and mental health in the Arctic: is diet an important risk factor for mental health in circumpolar peoples?--a review. Int J Circumpolar Health. 2003;62:228–241. doi: 10.3402/ijch.v62i3.17560. [DOI] [PubMed] [Google Scholar]
- 107.Sublette ME, Bosetti F, DeMar JC, Ma K, Bell JM, Fagin-Jones S, Russ MJ, Rapoport SI. Plasma free polyunsaturated fatty acid levels are associated with symptom severity in acute mania. Bipolar Disord. 2007;9:759–765. doi: 10.1111/j.1399-5618.2007.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Adams PB, Lawson S, Sanigorski A, Sinclair AJ. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31:S157–S161. doi: 10.1007/BF02637069. [DOI] [PubMed] [Google Scholar]
- 109.Su KP, Huang SY, Peng CY, Lai HC, Huang CL, Chen YC, Aitchison KJ, Pariante CM. Phospholipase A2 and cyclooxygenase 2 genes influence the risk of interferon-alpha-induced depression by regulating polyunsaturated fatty acids levels. Biol Psychiatry. 2010;67:550–557. doi: 10.1016/j.biopsych.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lotrich FE, Sears B, McNamara RK. Elevated ratio of arachidonic acid to long-chain omega-3 fatty acids predicts depression development following interferon-alpha treatment: Relationship with interleukin-6. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.08.007. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Amminger GP, Schäfer MR, Klier CM, Slavik JM, Holzer I, Holub M, Goldstone S, Whitford TJ, McGorry PD, Berk M. Decreased nervonic acid levels in erythrocyte membranes predict psychosis in help-seeking ultra-high-risk individuals. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.167. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 112.Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr. 2010;91:757–770. doi: 10.3945/ajcn.2009.28313. [DOI] [PubMed] [Google Scholar]
- 113.Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68:1056–1061. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- 114.Gracious BL, Chirieac MC, Costescu S, Finucane TL, Youngstrom EA, Hibbeln JR. Randomized, placebo-controlled trial of flax oil in pediatric bipolar disorder. Bipolar Disord. 2010;12:142–154. doi: 10.1111/j.1399-5618.2010.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry. 2006;163:1098–1100. doi: 10.1176/ajp.2006.163.6.1098. [DOI] [PubMed] [Google Scholar]
- 116.Emsley R, Myburgh C, Oosthuizen P, van Rensburg SJ. Randomized, placebo-controlled study of ethyl-eicosapentaenoic acid as supplemental treatment in schizophrenia. Am. J. Psychiatry. 2002;159:1596–1598. doi: 10.1176/appi.ajp.159.9.1596. [DOI] [PubMed] [Google Scholar]
- 117.Mellor JE, Laugharne JD, Peet M. Schizophrenic symptoms and dietary intake of n-3 fatty acids. Schizoph. Res. 1995;18:85–86. doi: 10.1016/0920-9964(95)00084-4. [DOI] [PubMed] [Google Scholar]
- 118.Peet M, Brind J, Ramchand CN, Shah S, Vankar GK. Two double-blind placebo-controlled pilot studies of eicosapentaenoic acid in the treatment of schizophrenia. Schizophr Res. 2001;49:243–251. doi: 10.1016/s0920-9964(00)00083-9. [DOI] [PubMed] [Google Scholar]
- 119.Peet M, Horrobin DF E-E Multicentre Study Group. A dose-ranging exploratory study of the effects of ethyl-eicosapentaenoate in patients with persistent schizophrenic symptoms. J. Psychiatr. Res. 2002;36:7–18. doi: 10.1016/s0022-3956(01)00048-6. [DOI] [PubMed] [Google Scholar]
- 120.Bloch MH, Qawasmi A. Omega-3 fatty acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2011;50:991–1000. doi: 10.1016/j.jaac.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Glaser R. Omega-3 supplementation lowers inflammation and anxiety in medical students: a randomized controlled trial. Brain Behav Immun. 2011;25:1725–1734. doi: 10.1016/j.bbi.2011.07.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tanskanen A, Hibbeln JR, Hintikka J, Haatainen K, Honkalampi K, Viinamäki H. Fish consumption, depression, and suicidality in a general population. Arch Gen Psychiatry. 2001;58:512–513. doi: 10.1001/archpsyc.58.5.512. [DOI] [PubMed] [Google Scholar]
- 123.De Vriese SR, Christophe AB, Maes M. In humans, the seasonal variation in polyunsaturated fatty acids is related to the seasonal variation in violent suicide and serotonergic markers of violent suicide. Prostaglandins Leukot Essent Fatty Acids. 2004;71:13–18. doi: 10.1016/j.plefa.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 124.Garland MR, Hallahan B, McNamara M, Carney PA, Grimes H, Hibbeln JR, Harkin A, Conroy RM. Lipids and essential fatty acids in patients presenting with self-harm. Br J Psychiatry. 2007;190:112–117. doi: 10.1192/bjp.bp.105.019562. [DOI] [PubMed] [Google Scholar]
- 125.Huan M, Hamazaki K, Sun Y, Itomura M, Liu H, Kang W, Watanabe S, Terasawa K, Hamazaki T. Suicide attempt and n-3 fatty acid levels in red blood cells: a case control study in China. Biol Psychiatry. 2004;56:490–496. doi: 10.1016/j.biopsych.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 126.Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. Am J Psychiatry. 2006;163:1100–1102. doi: 10.1176/ajp.2006.163.6.1100. [DOI] [PubMed] [Google Scholar]
- 127.Hallahan B, Hibbeln JR, Davis JM, Garland MR. Omega-3 fatty acid supplementation in patients with recurrent self-harm. Single-centre double-blind randomised controlled trial. Br J Psychiatry. 2007;190:118–122. doi: 10.1192/bjp.bp.106.022707. [DOI] [PubMed] [Google Scholar]
- 128.Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002;59:913–919. doi: 10.1001/archpsyc.59.10.913. [DOI] [PubMed] [Google Scholar]
- 129.Whelton SP, He J, Whelton PK, Muntner P. Meta-analysis of observational studies on fish intake and coronary heart disease. Am J Cardiol. 2004;93:1119–1123. doi: 10.1016/j.amjcard.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 130.Billman GE, Kang JX, Leaf A. Prevention of sudden cardiac death by dietary pure omega-3 polyunsaturated fatty acids in dogs. Circulation. 1999;99:2452–2457. doi: 10.1161/01.cir.99.18.2452. [DOI] [PubMed] [Google Scholar]
- 131.Ninio DM, Murphy KJ, Howe PR, Saint DA. Dietary fish oil protects against stretch-induced vulnerability to atrial fibrillation in a rabbit model. J Cardiovasc Electrophysiol. 2005;16:1189–1194. doi: 10.1111/j.1540-8167.2005.50007.x. [DOI] [PubMed] [Google Scholar]
- 132.Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–1118. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 133.Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, Bovbjerg V, Arbogast P, Smith H, Kushi LH, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995;274:1363–1367. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- 134.Stoll AL, Severus WE, Freeman MP, Rueter S, Zboyan HA, Diamond E, Cress KK, Marangell LB. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56:407–412. doi: 10.1001/archpsyc.56.5.407. [DOI] [PubMed] [Google Scholar]
- 135.Berger GE, Proffitt TM, McConchie M, Yuen H, Wood SJ, Amminger GP, Brewer W, McGorry PD. Ethyl-eicosapentaenoic acid in first-episode psychosis: a randomized, placebo-controlled trial. J Clin Psychiatry. 2007;68:1867–1875. doi: 10.4088/jcp.v68n1206. [DOI] [PubMed] [Google Scholar]
- 134.Hu FB, Bronner L, Willett WC, Stampfer MJ, Rexrode KM, Albert CM, Hunter D, Manson JE. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287:1815–1821. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- 135.Jacobson TA. Secondary prevention of coronary artery disease with omega-3 fatty acids. Am. J. Cardiol. 2006;98:61–70. doi: 10.1016/j.amjcard.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 136.Psota TL, Gebauer SK, Kris-Etherton P. Dietary omega-3 fatty acid intake and cardiovascular risk. Am. J. Cardiol. 2006;98:3–18. doi: 10.1016/j.amjcard.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 137.Block RC, Harris WS, Reid KJ, Sands SA, Spertus JA. EPA and DHA in blood cell membranes from acute coronary syndrome patients and controls. Atherosclerosis. 2008;197:821–828. doi: 10.1016/j.atherosclerosis.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 138.Baghai TC, Varallo-Bedarida G, Born C, Häfner S, Schüle C, Eser D, Rupprecht R, Bondy B, von Schacky C. Major depressive disorder is associated with cardiovascular risk factors and low Omega-3 Index. J Clin Psychiatry. 2011;72:1242–1247. doi: 10.4088/JCP.09m05895blu. [DOI] [PubMed] [Google Scholar]
- 139.Jazayeri S, Tehrani-Doost M, Keshavarz SA, Hosseini M, Djazayery A, Amini H, Jalali M, Peet M. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42:192–198. doi: 10.1080/00048670701827275. [DOI] [PubMed] [Google Scholar]
- 140.Gertsik L, Poland RE, Bresee C, Rapaport MH. Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. J Clin Psychopharmacol. 2012;32:61–64. doi: 10.1097/JCP.0b013e31823f3b5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr. 2002;76:653–658. doi: 10.1093/ajcn/76.3.653. [DOI] [PubMed] [Google Scholar]
- 142.Must A, Strauss RS. Risks and consequences of childhood and adolescent obesity. Int J Obes Relat Metab Disord. 1999;23(Suppl 2):S2–S11. doi: 10.1038/sj.ijo.0800852. [DOI] [PubMed] [Google Scholar]
- 143.Sinaiko AR, Donahue RP, Jacobs DR, Jr, Prineas RJ. Relation of weight and rate of increase in weight during childhood and adolescence to body size, blood pressure, fasting insulin, and lipids in young adults. The Minneapolis Children's Blood Pressure Study. Circulation. 1999;99:1471–1476. doi: 10.1161/01.cir.99.11.1471. [DOI] [PubMed] [Google Scholar]
- 144.McKenney JM, Sica D. Role of prescription omega-3 fatty acids in the treatment of hypertriglyceridemia. Pharmacotherapy. 2007;27:715–728. doi: 10.1592/phco.27.5.715. [DOI] [PubMed] [Google Scholar]
- 145.Caniato RN, Alvarenga ME, Garcia-Alcaraz MA. Effect of omega-3 fatty acids on the lipid profile of patients taking clozapine. Aust N Z J Psychiatry. 2006;40:691–697. doi: 10.1080/j.1440-1614.2006.01869.x. [DOI] [PubMed] [Google Scholar]
- 146.Micallef M, Munro I, Phang M, Garg M. Plasma n-3 polyunsaturated fatty acids are negatively associated with obesity. Br J Nutr. 2009;102:1370–1374. doi: 10.1017/S0007114509382173. [DOI] [PubMed] [Google Scholar]
- 147.Burrows T, Collins CE, Garg ML. Omega-3 index, obesity and insulin resistance in children. Int J Pediatr Obes. 2011;6:532–539. doi: 10.3109/17477166.2010.549489. [DOI] [PubMed] [Google Scholar]
- 148.Klein-Platat C, Drai J, Oujaa M, Schlienger JL, Simon C. Plasma fatty acid composition is associated with the metabolic syndrome and low-grade inflammation in overweight adolescents. Am J Clin Nutr. 2005;82:1178–1184. doi: 10.1093/ajcn/82.6.1178. [DOI] [PubMed] [Google Scholar]
- 149.Karlsson M, Mårild S, Brandberg J, Lönn L, Friberg P, Strandvik B. Serum phospholipid fatty acids, adipose tissue, and metabolic markers in obese adolescents. Obesity. 2006;14:1931–1939. doi: 10.1038/oby.2006.225. [DOI] [PubMed] [Google Scholar]
- 150.Kabir M, Skurnik G, Naour N, Pechtner V, Meugnier E, Rome S, Quignard-Boulangé A, Vidal H, Slama G, Clément K, Guerre-Millo M, Rizkalla SW. Treatment for 2 mo with n-3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr. 2007;86:1670–1679. doi: 10.1093/ajcn/86.5.1670. [DOI] [PubMed] [Google Scholar]
- 151.Couet C, Delarue J, Ritz P, Antoine JM, Lamisse F. Effect of dietary fish oil on body fat mass and basal fat oxidation in healthy adults. Int J Obes Relat Metab Disord. 1997;21:637–643. doi: 10.1038/sj.ijo.0800451. [DOI] [PubMed] [Google Scholar]
- 152.Kunesová M, Braunerová R, Hlavatý P, Tvrzická E, Stanková B, Skrha J, Hilgertová J, Hill M, Kopecký J, Wagenknecht M, Hainer V, Matoulek M, Parízková J, Zák A, Svacina S. The influence of n-3 polyunsaturated fatty acids and very low calorie diet during a short-term weight reducing regimen on weight loss and serum fatty acid composition in severely obese women. Physiol Res. 2006;55:63–72. doi: 10.33549/physiolres.930770. [DOI] [PubMed] [Google Scholar]
- 153.McGorry PD, Hickie IB, Yung AR, Pantelis C, Jackson HJ. Clinical staging of psychiatric disorders: a heuristic framework for choosing earlier, safer and more effective interventions. Aust N Z J Psychiatry. 2006;40:616–622. doi: 10.1080/j.1440-1614.2006.01860.x. [DOI] [PubMed] [Google Scholar]
- 154.McNamara RK, Nandagopal JJ, Strakowski SM, DelBello MP. Preventative strategies for early-onset bipolar disorder: Towards a clinical staging model. CNS Drugs. 2010;24:983–996. doi: 10.2165/11539700-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 155.Conus P, Ward J, Hallam KT, Lucas N, Macneil C, McGorry PD, Berk M. The proximal prodrome to first episode mania--a new target for early intervention. Bipolar Disord. 2008;10:555–565. doi: 10.1111/j.1399-5618.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 156.Yung AR, Phillips LJ, Yuen HP, Francey SM, McFarlane CA, Hallgren M, McGorry PD. Psychosis prediction: 12-month follow up of a high-risk ("prodromal") group. Schizophr Res. 2003;60:21–32. doi: 10.1016/s0920-9964(02)00167-6. [DOI] [PubMed] [Google Scholar]
- 157.McNamara RK, Strawn JR, Chang KD, Delbello MP. Interventions for youth at high risk for bipolar disorder and schizophrenia. Child Adolesc Psychiatr Clin N Am. 2012;21:739–751. doi: 10.1016/j.chc.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.De Vriese SR, Christophe AB, Maes M. Lowered serum n-3 polyunsaturated fatty acid (PUFA) levels predict the occurrence of postpartum depression: further evidence that lowered n-PUFAs are related to major depression. Life Sci. 2003;73:3181–3187. doi: 10.1016/j.lfs.2003.02.001. [DOI] [PubMed] [Google Scholar]
- 159.Otto SJ, de Groot RH, Hornstra G. Increased risk of postpartum depressive symptoms is associated with slower normalization after pregnancy of the functional docosahexaenoic acid status. Prostaglandins Leukot Essent Fatty Acids. 2003;69:237–243. doi: 10.1016/s0952-3278(03)00090-5. [DOI] [PubMed] [Google Scholar]
- 160.Su KP, Huang SY, Chiu TH, Huang KC, Huang CL, Chang HC, Pariante CM. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69:644–651. doi: 10.4088/jcp.v69n0418. [DOI] [PubMed] [Google Scholar]
- 161.Freeman MP, Hibbeln JR, Wisner KL, Brumbach BH, Watchman M, Gelenberg AJ. Randomized dose-ranging pilot trial of omega-3 fatty acids for postpartum depression. Acta Psychiatr. Scand. 2006;113:31–35. doi: 10.1111/j.1600-0447.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- 162.Marangell LB, Martinez JM, Zboyan HA, Chong H, Puryear LJ. Omega-3 fatty acids for the prevention of postpartum depression: negative data from a preliminary, open-label pilot study. Depress Anxiety. 2004;19:20–23. doi: 10.1002/da.10148. [DOI] [PubMed] [Google Scholar]
- 163.Browne JC, Scott KM, Silvers KM. Fish consumption in pregnancy and omega-3 status after birth are not associated with postnatal depression. J. Affect Disord. 2006;90:131–139. doi: 10.1016/j.jad.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 164.Su KP, Shen WW, Huang SY. Omega-3 fatty acids as a psychotherapeutic agent for a pregnant schizophrenic patient. Eur. Neuropsychopharmacol. 2001;11:295–299. doi: 10.1016/s0924-977x(01)00098-0. [DOI] [PubMed] [Google Scholar]
- 165.Buckley MS, Goff AD, Knapp WE. Fish oil interaction with warfarin. Ann Pharmacother. 2004;38:50–52. doi: 10.1345/aph.1D007. [DOI] [PubMed] [Google Scholar]
- 166.Eritsland J, Arnesen H, Seljeflot I, Kierulf P. Long-term effects of n-3 polyunsaturated fatty acids on haemostatic variables and bleeding episodes in patients with coronary artery disease. Blood Coagul Fibrinolysis. 1995;6:17–22. doi: 10.1097/00001721-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 167.Mueller BA, Talbert RL, Tegeler CH, Prihoda TJ. The bleeding time effects of a single dose of aspirin in subjects receiving omega-3 fatty acid dietary supplementation. J Clin Pharmacol. 1991;31:185–190. doi: 10.1002/j.1552-4604.1991.tb03706.x. [DOI] [PubMed] [Google Scholar]
- 168.Harris WS. Expert opinion: omega-3 fatty acids and bleeding-cause for concern? Am J Cardiol. 2007;99(6A):44C–46C. doi: 10.1016/j.amjcard.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 169.Davidson MH, Kling D, Maki KC. Novel developments in omega-3 fatty acid-based strategies. Curr Opin Lipidol. 2011;22:437–444. doi: 10.1097/MOL.0b013e32834bd642. [DOI] [PubMed] [Google Scholar]
- 170.Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry. 2011;72:1577–1584. doi: 10.4088/JCP.10m06634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Itomura M, Fujioka S, Hamazaki K, Kobayashi K, Nagasawa T, Sawazaki S, Kirihara Y, Hamazaki T. Factors influencing EPA+DHA levels in red blood cells in Japan. In Vivo. 2008;22:131–135. [PubMed] [Google Scholar]