Abstract
Background
Individual patient meta-analysis to determine the analgesic efficacy and adverse effects of single-dose rofecoxib in acute postoperative pain.
Methods
Individual patient information was available from 14 trials; 13 in dental and one in postsurgical pain. For each patient the percentage of maximum possible pain relief (%maxTOTPAR) was determined at different time points. The proportion of patients with at least 50% maxTOTPAR, and number-needed-to-treat (NNT) for at least 50% maxTOTPAR, were then calculated, with time when 50% of patients had remedicated (TTR50) and number-needed-to-harm (NNH) for adverse effects.
Results
In dental pain, for rofecoxib 50 mg (1330 patients) compared with placebo (570 patients) the NNT was 1.9 (95% confidence interval 1.8 to 2.1) for six hours, 2.0 (1.8 to 2.1) at eight, 2.4 (2.2 to 2.6) at 12, and 2.8 (2.5 to 3.1) at 24 hours. The TTR50 was 15.5 hours. Adverse effects were uncommon, though post-extraction alveolitis (dry socket) occurred more often with rofecoxib 50 mg than with placebo, NNH 24 (14 to 80). For postsurgical pain in one trial (163 patients), the NNT for rofecoxib 50 mg for six hours was 3.9 (2.6 to 7.8), the TTR50 was 5.8 hours, and multiple-dose adverse effects over five days occurred at similar rates with rofecoxib 50 mg and placebo.
Conclusions
Single-dose rofecoxib 50 mg is an effective treatment with long-lasting analgesia and few adverse effects in dental pain. More information is required to confirm efficacy in postsurgical pain.
Background
Cox-2 selective inhibitors (coxibs), like rofecoxib, have been developed to provide better gastrointestinal tolerability than conventional non-steroidal anti-inflammatory drugs (NSAIDs). Relatively low rates of gastrointestinal adverse effects allow the use of higher doses of coxibs in the acute pain setting. These high doses may have the additional advantage of longer duration analgesia with extended dosing intervals.
Conventionally, trials in acute pain have been conducted over 4–6 hours because that was how long analgesia lasted for most drugs. Validated methods exist to allow the conversion of mean pain intensity or pain relief outcomes into dichotomous form (the proportion of patients with at least 50% pain relief) over 4–6 hours [1-4], but methods have not yet been developed to do this beyond six hours. For rofecoxib, a meta-analysis of mean pain outcome data from published trials in postoperative (dental or postsurgical) pain showed a good analgesic response with rofecoxib 50 mg over six hours, and relatively long duration of action (defined in the analysis by time to remedication) [5].
When information is available from individual patients in a trial we do not have to rely on mean values. Since actual pain measurements are available at all time points for each patient, it is possible to calculate for each patient the percent of maximum pain relief (%maxTOTPAR), the number of patients with at least 50% maxTOTPAR and, hence, the number-needed-to-treat (NNT) for at least 50% pain relief at different durations.
The previous analysis [5] was limited to information from five published trials and outcomes over six hours. The objectives of this individual patient meta-analysis were to extend the previous analysis to include more trials and patients, and to calculate NNTs for different durations up to 24 hours.
Methods
QUOROM guidelines for reporting meta-analyses were followed where appropriate [6]. Merck Research Laboratories, Rahway, New Jersey, provided individual patient data from 14 Phase III trials of rofecoxib in postoperative (dental or postsurgical) pain. All completed (July 2002) Phase III trials of rofecoxib meeting pre-specified criteria for inclusion in the meta-analysis were provided. This included information from five published trials in postoperative pain in a previous review [5], but excluded information from one Phase II study [7] which used a different formulation of rofecoxib. The Phase II trial had been included in a previous meta-analysis using published mean data [5].
Trials
The trials were randomised and double blind, and compared single, oral doses of rofecoxib with an active control and placebo in adults with moderate to severe postoperative pain. Dental studies were conducted over 24 hours. One postsurgical study was a multiple dose trial for which information for the first dose was available over 12 hours. In all trials, pain intensity and pain relief were measured using a standard four-point categorical pain intensity scale (0 none, 1 mild, 2 moderate, 3 severe) and a five-point point pain relief scale (0 none, 1 a little, 2 some, 3 a lot, 4 complete). Pain measurements were collected using patient diaries. Patients were assessed at baseline, then at least hourly for eight hours, and again at 12 and 24 hours. In some studies additional assessments were conducted between eight, 12 and 24 hours. The exact time at which a patient requested remedication (rescue analgesic), if required, was recorded. Adverse effects were recorded as the number of patients with any adverse effect(s), or of particular adverse effects. Table 1 shows the study treatments, dosing and number of patients for the individual trials.
Table 1.
Trials details
| Trial ID | Pain condition | Study drug and dose, number of patients | Design | Observations after 8 hrs | Quality, Validity scores |
| 27 | Dental | Rofecoxib 50 mg, 38 Naproxen 550 mg, 39 Placebo, 39 | Single oral dose, parallel | 12, 24 | Q 5 V 16 |
| 51 | Dental | Rofecoxib 50 mg, 72 Naproxen 550 mg, 49 Placebo, 48 | Single oral dose, parallel | 12, 24 | Q 5 V 16 |
| 66 | Dental | Rofecoxib 50 mg, 50 Ibuprofen 400 mg, 51 Placebo, 50 | Single oral dose, parallel | 12, 24 | Q 5 V 16 |
| 71 | Dental | Rofecoxib 50 mg, 50 Ibuprofen 400 mg, 52 Placebo, 50 | Single oral dose, parallel | 12, 24 | Q 5 V 16 |
| 84 | Dental | Rofecoxib 50 mg, 56 Ibuprofen 400 mg, 56 Placebo, 56 | Single oral dose, parallel | 12, 24 | Q 5 V 16 |
| 95 | Dental | Rofecoxib 50 mg, 90 Ibuprofen 400 mg, 46 Placebo, 45 | Single oral dose, parallel | 10, 12, 24 | Q 5 V 16 |
| 104 | Dental | Rofecoxib 50 mg, 151 Ibuprofen 400 mg, 46 Placebo, 45 | Single oral dose, parallel | 10, 12, 24 | Q 5 V 16 |
| 111 | Dental | Rofecoxib 50 mg, 159 Ibuprofen 400 mg, 53 Placebo, 52 | Single oral dose, parallel | 10, 12, 24 | Q 5 V 16 |
| 127 | Dental | Rofecoxib 50 mg, 180 Paracetamol 600 mg + Codeine 60 mg, 180 Placebo, 30 | Single oral dose, parallel | 12, 24 | Q 5 V 16 |
| 128 | Dental | Rofecoxib 50 mg, 182 Paracetamol 600 mg + Codeine 60 mg, 180 Placebo, 31 | Single oral dose, parallel | 12, 24 | Q 5 V 16 |
| 152 | Dental | Rofecoxib 50 mg, 90 Oxycodone 5 mg + Paracetamol 325 mg, 91 Placebo, 31 | Single oral dose, parallel | 12, 24 | Q 5 V 16 |
| 154 | Dental | Rofecoxib 50 mg, 91 Oxycodone 5 mg + Paracetamol 325 mg, 89 Placebo, 30 | Single oral dose, parallel | 12, 24 | Q 5 V 16 |
| 169 | Dental | Rofecoxib 50 mg, 121 Diclofenac 50 mg Q8 hr, 121 Placebo, 63 | Single oral dose, parallel | 10, 11, 12, 16, 20, 24 | Q 5 V 16 |
| 72 | Postsurgical | Rofecoxib 50 mg, 110 Naproxen 550 mg, 55 Placebo, 53 | Multiple with single dose efficacy data, AE 5 days, parallel | 12 hrs | Q 5 V 16 |
The quality of trials, in terms of their descriptions of randomisation, double blinding and withdrawals or drop-outs, was determined using a five point scale [8]. Study validity was determined using a 16-point pain validity scale [9]. These scales are described in Additional file 1.
Meta-analysis
Outcome data were pooled in an intention to treat (number of patients randomised) analysis. For each patient we calculated the area under the pain relief – time curve (TOTPAR) for six, eight, 12 and 24 hours. When a patient remedicated the pain relief score was set to zero for all remaining time points until the end of the observation period. For each patient we then calculated the percentage of the maximum possible TOTPAR for each time point (number of hours of observation multiplied by the maximum possible pain relief of 4; for example,24 for six hours, 32 for eight hours).
When making comparisons, each active analgesic was compared with placebo from those trials in which the active analgesic was used. Efficacy was defined as the number-needed-to-treat (NNT) for at least 50% pain relief. Duration of analgesia was defined as the time when 50% of patients with the same treatment had remedicated (TTR50).
Analyses for comparator treatments were based on information available only from the trials of rofecoxib mentioned in this report, but for most there was insufficient information to be confident of the result [10]. In acute pain studies we need information from at least 500 patients to be sure of an NNT ± 0.5 when the NNT is 2, and many more patients when the NNT is higher. Only ibuprofen 400 mg had sufficient information (601 patients in six trials). While data are provided for other comparators, only information from rofecoxib 50 mg and ibuprofen 400 mg will be discussed. The single postsurgical study was not pooled with dental trials for observations beyond six hours because the only outcome for which we know that these two pain models give the same result is NNT for at least 50% maxTOTPAR for 4–6 hours [11].
Relative benefit (or risk) was calculated using a fixed effects model [12], with no statistically significant difference between treatments assumed when the 95% confidence intervals included unity. Number-needed-to-treat (or harm) was calculated using the method of Cook and Sackett [13] using the pooled number of observations. NNT/H is the reciprocal of the absolute risk reduction or increase; for instance, if 75 out of 100 patients benefit with treatment and only 25 out of 100 benefit with placebo, the absolute risk increase is 0.75-0.25 = 0.5, and the NNT is 1/0.5 = 2.
The following terms were used to describe adverse outcomes in terms of harm or prevention of harm:
• When significantly fewer adverse effects occurred with active treatment than with placebo we used the term the number-needed-to-treat to prevent one event (NNTp).
• When significantly more adverse effects occurred with active treatment compared with placebo we used the term the number-needed-to-harm to cause one event (NNH).
Clinical homogeneity of trials was examined graphically [14] since heterogeneity tests and funnel plots have been shown to be unreliable [15,16]. The z test [17] was used to detect statistically significant differences between the NNTs derived for different treatments. Statistical significance was indicated by p < 0.01.
Results
All 14 trials scored the maximum of five points for quality and the maximum of 16 points for validity. In all 14 trials (2060 patients) the NNT for at least 50% pain relief over six hours was 2.0 (1.9 to 2.1) for rofecoxib 50 mg compared with placebo in dental plus postsurgical pain.
Dental pain
There were 13 trials in dental pain (studies 27, 51, 66, 71, 84, 95, 104, 111, 127, 128, 152, 154, 169). Patients underwent surgical removal of impacted third molars. Their mean age was 21 years, and 60% were women. Sixty-five percent of patients had moderate and 35% severe pain at baseline. Study treatments were rofecoxib 50 mg (1330 patients), placebo (570 patients), and active comparators of ibuprofen 400 mg in six trials (303 patients), enteric-coated diclofenac sodium 50 mg in one (121 patients), naproxen sodium 550 mg in two (88 patients), paracetamol 600 mg plus codeine 60 mg in two (360 patients), and oxycodone 5 mg plus paracetamol 325 mg in two (180 patients). Enteric-coated diclofenac sodium 50 mg was given every eight hours and efficacy estimates were therefore calculated only at six and eight hours for this drug.
Efficacy in dental pain
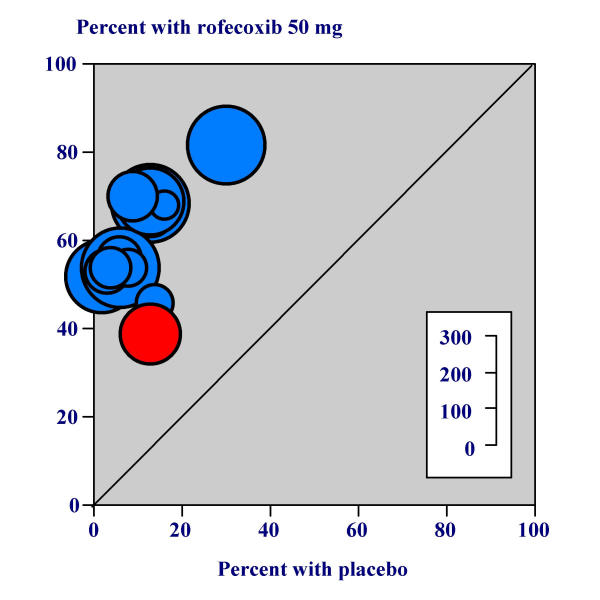
Table 2 shows the efficacy results for all active treatments compared with placebo over six, eight, 12 and 24 hours in dental pain. Using pooled data from 13 trials, the proportion of patients with at least 50% pain relief with rofecoxib 50 mg was 63% (832/1330 patients) over six hours (Figure 1), 61% (814/1330) over eight hours, 50% (662/1330) over 12 hours and 45% (595/1330) over 24 hours. The proportions of patients with at least 50% pain relief with placebo at six, eight, 12 and 24 hours were 10% (58/570; Figure 1), 11% (62/570), 8% (46/570), and 9% (49/570). Rofecoxib 50 mg was significantly more effective than placebo at all time points. For rofecoxib 50 mg compared with placebo, the NNT for at least 50% pain relief was 1.9 (1.8 to 2.1) over six hours, 2.0 (1.9 to 2.1) over eight hours, 2.4 (2.2 to 2.6) over 12 hours and 2.8 (2.5 to 3.1) over 24 hours. Rofecoxib 50 mg was statistically superior to ibuprofen 400 mg at eight (z = 3.75, p = 0.0002), 12 (z = 7.16, p = <0.00006) and 24 hours (z = 9.43, p = <0.00006).
Table 2.
NNT for at least 50% pain relief in dental pain
| Improved with | Mean percent improved with | |||||||
| Number of trials | Drug and dose (mg) | Active | Placebo | Relative risk (95% CI) | NNT (95% CI) | Placebo | Active | |
| Six hours | ||||||||
| 13 | Rofecoxib 50 mg | 832/1330 | 58/570 | 5.9 (4.6 to 7.5) | 1.9 (1.8 to 2.1) | 10 | 63 | |
| 6 | Ibuprofen 400 mg | 159/303 | 25/298 | 6.3 (4.2 to 9.2) | 2.3 (2.0 to 2.7) | 9 | 53 | |
| 1 | Diclofenac 50 mg | 32/121 | 8/63 | 2.1 (1.02 to 4.2) | 7.3 (4.0 to 42) | 13 | 26 | |
| 2 | Naproxen 550 mg | 52/88 | 9/87 | 5.7 (3.0 to 11) | 2.1 (1.6 to 2.7) | 11 | 59 | |
| 2 | Oxycodone 5 mg + paracetamol 325 mg | 30/180 | 5/61 | 2.0 (0.8 to 5.0) | nc | 8 | 17 | |
| 2 | Paracetamol 600 mg + codeine 60 mg | 102/360 | 11/61 | 1.6 (0.9 to 2.7) | nc | 18 | 28 | |
| Eight hours | ||||||||
| 13 | Rofecoxib 50 mg | 814/1330 | 62/570 | 5.4 (4.3 to 6.9) | 2.0 (1.9 to 2.1) | 11 | 61 | |
| 6 | Ibuprofen 400 mg | 135/303 | 25/298 | 5.3 (3.6 to 7.9) | 2.8 (2.4 to 3.4) | 8 | 44 | |
| 1 | Diclofenac 50 mg | 32/121 | 11/63 | 1.5 (0.8 to 2.8) | nc | 17 | 26 | |
| 6 | Ibuprofen 400 mg | 135/303 | 25/298 | 5.3 (3.6 to 7.9) | 2.8 (2.4 to 3.4) | 8 | 44 | |
| 2 | Naproxen 550 mg | 45/88 | 8/87 | 5.6 (2.8 to 11) | 2.4 (1.9 to 3.4) | 10 | 51 | |
| 2 | Oxycodone 5 mg + paracetamol 325 mg | 21/180 | 4/61 | 1.8 (0.6 to 5.0) | nc | 6 | 12 | |
| 2 | Paracetamol 600 mg + codeine 60 mg | 70/360 | 10/61 | 1.2 (0.7 to 2.1) | nc | 16 | 20 | |
| Twelve hours | ||||||||
| 13 | Rofecoxib 50 mg | 662/1330 | 46/570 | 5.9 (4.5 to 7.8) | 2.4 (2.2 to 2.6) | 8 | 50 | |
| 6 | Ibuprofen 400 mg | 73/303 | 19/298 | 3.8 (2.4 to 6.1) | 5.6 (4.3 to 8.2) | 6 | 24 | |
| 1 | Diclofenac 50 mg | No data – doses repeated every 8 hours | ||||||
| 6 | Ibuprofen 400 mg | 73/303 | 19/298 | 3.8 (2.4 to 6.1) | 5.6 (4.3 to 8.2) | 6 | 24 | |
| 2 | Naproxen 550 mg | 30/88 | 7/87 | 4.2 (2.0 to 9.1) | 3.9 (2.7 to 7.0) | 8 | 34 | |
| 2 | Oxycodone 5 mg + paracetamol 325 mg | 15/180 | 2/61 | 2.5 (0.6 to 11) | nc | 3 | 8 | |
| 2 | Paracetamol 600 mg + codeine 60 mg | 30/360 | 8/61 | 0.6 (0.3 to 1.3) | nc | 13 | 8 | |
| Twenty-four hours | ||||||||
| 13 | Rofecoxib 50 mg | 595/1330 | 49/570 | 5.1 (3.9 to 6.7) | 2.8 (2.5 to 3.1) | 9 | 45 | |
| 6 | Ibuprofen 400 mg | 43/303 | 21/298 | 2.0 (1.2 to 3.3) | 14 (8.3 to 44) | 7 | 14 | |
| 1 | Diclofenac 50 mg | No data – doses repeated every 8 hours | ||||||
| 6 | Ibuprofen 400 mg | 43/303 | 21/298 | 2.0 (1.2 to 3.3) | 14 (8.3 to 44) | 7 | 14 | |
| 2 | Naproxen 550 mg | 24/88 | 8/87 | 3.0 (1.4 to 6.2) | 5.5 (3.4 to 14) | 9 | 27 | |
| 2 | Oxycodone 5 mg + paracetamol 325 mg | 18/1804 | 61 | 1.4 (0.5 to 3.6) | nc | 7 | 10 | |
| 2 | Paracetamol 600 mg + codeine 60 mg | 24/360 | 7/61 | 0.6 (0.3 to 1.2) | nc | 11 | 7 | |
nc: not calculated because relative risk showed no statistically significant difference between active and placebo. Naproxen was given as the sodium salt
Figure 1.
Percent with at least 50% pain relief with rofecoxib 50 mg over six hours
Time to remedication in dental pain
Results for time to remedication are shown in Table 3. Duration of analgesia was longer with rofecoxib 50 mg than with other active comparators used in the trials. The TTR50 was 15.5 hours with rofecoxib 50 mg, 1.6 hours with placebo, and 7.1 hours with ibuprofen 400 mg.
Table 3.
Time to remedication
| Pain model | Drug & dose (mg) | Number of trials | Number of patients | TRR50(hrs) |
| Dental | Rofecoxib 50 mg | 13 | 1330 | 15.5 |
| Ibuprofen 400 mg | 6 | 303 | 7.1 | |
| Diclofenac 50 mg | 1 | 121 | 1.6 | |
| Ibuprofen 400 mg | 6 | 303 | 7.1 | |
| Naproxen 550 mg | 2 | 88 | 9.3 | |
| Oxycodone 5 mg + paracetamol 325 mg | 2 | 180 | 3.1 | |
| Paracetamol 600 mg + codeine 60 mg | 2 | 360 | 3.8 | |
| Placebo | 13 | 570 | 1.6 | |
| Postsurgical | Rofecoxib 50 mg | 1 | 110 | 5.8 |
| Naproxen 550 mg | 1 | 55 | 5.9 | |
| Placebo | 1 | 53 | 2.8 | |
Naproxen was given as the sodium salt
Adverse effects in dental pain
Results for adverse effects are shown in Table 4. There was no statistically significant difference in the number of patients reporting any adverse effects with rofecoxib 50 mg (35%, 459/1330 patients) compared with placebo (231/570, 41%), relative risk 0.9 (0.8 to 1.0).
Table 4.
Adverse effects in dental pain
| Adverse effects with active | Adverse effects with placebo | ||||||
| Number of trials | Drug and dose | Number | Percent | Number | Percent | Relative risk (95% CI) | NNH/NNTp (95% CI) |
| Any adverse event | |||||||
| 13 | Rofecoxib 50 mg | 459/1330 | 35 | 231/570 | 41 | 0.9 (0.8 to 1.0) | nc |
| 6 | Ibuprofen 400 mg | 102/303 | 34 | 122/298 | 41 | 0.8 (0.7 to 1.0) | nc |
| 1 | Diclofenac 50 mg* | 72/121 | 60 | 31/63 | 41 | 1.2 (0.9 to 1.6) | nc |
| 6 | Ibuprofen 400 mg | 102/303 | 34 | 122/298 | 41 | 0.8 (0.7 to 1.0) | nc |
| 2 | Naproxen 550 mg | 27/88 | 31 | 25/87 | 29 | 1.1 (0.7 to 1.7) | nc |
| 2 | Oxycodone 5 mg + paracetamol 325 mg | 116/180 | 64 | 29/61 | 48 | 1.4 (1.02 to 1.8) | 5.9 (3.2 to 39) |
| 2 | paracetamol 600 mg + codeine 60 mg | 146/360 | 41 | 24/61 | 39 | 1.0 (0.7 to 1.4) | nc |
| Post-extraction alveolitis | |||||||
| 13 | Rofecoxib 50 mg | 179/1330 | 13 | 52/570 | 9 | 1.5 (1.1 to 2.1) | 24 (14 to 80) |
| 6 | Ibuprofen 400 mg | 23/304 | 8 | 31/298 | 10 | 0.7 (0.4 to 1.2) | nc |
| 1 | Diclofenac 50 mg* | 9/180 | 5 | 3/63 | 5 | 1.0 (0.3 to 3.8) | nc |
| 6 | Ibuprofen 400 mg | 23/304 | 8 | 31/298 | 10 | 0.7 (0.4 to 1.2) | nc |
| 2 | Naproxen 550 mg | 13/88 | 15 | 8/87 | 10 | 1.6 (0.7 to 3.4) | nc |
| 2 | Oxycodone 5 mg + paracetamol 325 mg | 20/180 | 11 | 7/61 | 11 | 0.9 (0.4 to 2.2) | nc |
| 2 | Paracetamol 600 mg + codeine 60 mg | 27/360 | 8 | 3/61 | 6 | 1.3 (0.5 to 3.9) | nc |
| Dizziness | |||||||
| 13 | Rofecoxib 50 mg | 30/1330 | 2 | 25/570 | 5 | 0.5 (0.3 to 0.9) | 43 (24 to 253) |
| 6 | Ibuprofen 400 mg | 7/304 | 3 | 13/298 | 5 | 0.6 (0.3 to 1.4) | nc |
| 1 | Diclofenac 50 mg* | 9/180 | 8 | 5/63 | 8 | 0.6 (0.2 to 1.8) | nc |
| 6 | Ibuprofen 400 mg | 7/304 | 3 | 13/298 | 5 | 0.6 (0.3 to 1.4) | nc |
| 2 | Naproxen 550 mg | 1/88 | 2 | 1/87 | 1 | 0.9 (0.2 to 9.3) | nc |
| 2 | Oxycodone 5 mg + paracetamol 325 mg | 44/180 | 24 | 6/61 | 10 | 2.5 (1.1 to 5.6) | 6.9 (4.1 to 21) |
| 2 | Paracetamol 600 mg + codeine 60 mg | 19/360 | 1 | 1/61 | 2 | 3.3 (0.5 to 24) | nc |
| Drowsiness | |||||||
| 13 | Rofecoxib 50 mg | 8/1330 | 0.6 | 1/570 | 0.6 | 0.9 (0.3 to 3.1) | nc |
| 6 | Ibuprofen 400 mg | 1/304 | 0.7 | 0/298 | 0.5 | 1.3 (0.2 to 11) | nc |
| 1 | Diclofenac 50 mg* | 5/180 | 3 | 0/63 | 0 | 3.5 (0.2 to 64) | nc |
| 6 | Ibuprofen 400 mg | 1/304 | 0.7 | 0/298 | 0.5 | 1.3 (0.2 to 11) | nc |
| 2 | Naproxen 550 mg | 0/88 | 0 | 0/87 | 0 | 0.9 (0.0 to 16) | nc |
| 2 | Oxycodone 5 mg + paracetamol 325 mg | 6/180 | 4 | 1/61 | 2 | 2.2 (0.3 to 18) | nc |
| 2 | Paracetamol 600 mg + codeine 60 mg | 7/360 | 2 | 1/61 | 2 | 1.2 (0.2 to 9.2) | nc |
| Nausea | |||||||
| 13 | Rofecoxib 50 mg | 112/1330 | 8 | 80/570 | 14 | 0.6 (0.5 to 0.8) | 18 (11 to 41) |
| 6 | Ibuprofen 400 mg | 38/304 | 12 | 51/298 | 17 | 0.7 (0.5 to 1.08) | nc |
| 1 | Diclofenac 50 mg* | 21/180 | 12 | 10/63 | 16 | 0.7 (0.4 to 1.5) | nc |
| 6 | Ibuprofen 400 mg | 38/304 | 12 | 51/298 | 17 | 0.7 (0.5 to 1.08) | nc |
| 2 | Naproxen 550 mg | 4/88 | 5 | 3/87 | 3 | 1.3 (0.3 to 5.7) | nc |
| 2 | Oxycodone 5 mg + paracetamol 325 mg | 61/180 | 34 | 10/61 | 17 | 2.0 (1.1 to 3.5) | 6.0 (3.5 to 19) |
| 2 | Paracetamol 600 mg + codeine 60 mg | 70/360 | 19 | 6/61 | 10 | 2.0 (0.9 to 4.4) | 10 (5.5 to 92) |
| Vomiting | |||||||
| 13 | Rofecoxib 50 mg | 51/1330 | 4 | 51/570 | 9 | 0.4 (0.3 to 0.6) | 19 (13 to 38) |
| 6 | Ibuprofen 400 mg | 25/304 | 8 | 36/298 | 12 | 0.7 (0.4 to 1.1) | nc |
| 1 | Diclofenac 50 mg* | 6/180 | 3 | 7/63 | 11 | 0.3 (0.1 to 1.0) | nc |
| 2 | Naproxen 550 mg | 1/88 | 2 | 1/87 | 2 | 0.9 (0.1 to 9.3) | nc |
| 2 | Oxycodone 5 mg + paracetamol 325 mg | 35/180 | 19 | 3/61 | 6 | 3.4 (1.2 to 9.8) | 7.2 (4.6 to 18) |
| 2 | Paracetamol 600 mg + codeine 60 mg | 58/360 | 16 | 4/61 | 7 | 2.46 (0.9 to 6.5) | 10 (5.9 to 44) |
*NB: Diclofenac was taken every eight hours during the study. Naproxen was given as the sodium salt Bold type: NNTp to prevent one event (significantly fewer events occurred with rofecoxib 50 mg compared with placebo
Post-extraction alveolitis (dry socket) was reported significantly more often with rofecoxib 50 mg (179/1330, 13%) than with placebo (52/570, 9%), relative risk 1.5 (1.1 to 2.1). The NNH was 24 (14 to 80). The other most commonly reported adverse effects were dizziness, nausea, and vomiting. Significantly fewer events were reported with rofecoxib 50 mg compared with placebo for some adverse effects. The NNTps to prevent one event were 43 (24 to 253) for dizziness, 18 (11 to 41) for nausea, and 19 (13 to 38) for vomiting.
Ibuprofen 400 mg was not associated with significantly increased or decreased rates of patients with any adverse effect or particular adverse effects. One case of gastrointestinal bleeding was reported with ibuprofen 400 mg, otherwise no adverse effects were described as serious for any intervention.
Postsurgical pain
In one trial (study 72) following major orthopaedic surgery (total hip or knee replacement or femoral fracture repair with open reduction and internal fixation), the mean age of patients was 65 years, 58% were women, and 82% had moderate pain at baseline. Rofecoxib 50 mg (110 patients) was compared with naproxen sodium 550 mg (55 patients) or placebo (53 patients). Single-dose efficacy data were available from this five-day study; efficacy data were not available for multiple doses. Multiple dose adverse effects were collected over five days and are described below.
Efficacy in postsurgical pain
Results are shown in Table 5. No data were available at 24 hours. The mean event rates (proportion of patients with at least 50% pain relief) with rofecoxib 50 mg over six, eight, and 12 hours were 39% (43/110 patients, Figure 1), 40% (44/110), and 35% (38/110). With naproxen sodium 550 mg the mean event rates were 36% (20/55), 33% (18/55), and 25% (14/55) and with placebo were 13% (7/53) (Figure 1), 11% (6/53), and 4% (2/53).
Table 5.
NNT for at least 50% pain relief in postsurgical pain
| Improved with | Mean percent improved with | ||||||
| Time (hours) | Drug and dose (mg) | Active | Placebo | Relative risk (95% CI) | NNT (95% CI) | Placebo | Active |
| 6 | Rofecoxib 50 mg | 43/110 | 7/53 | 3.0 (1.4 to 6.1) | 3.9 (2.6 to 7.8) | 13 | 39 |
| Naproxen 550 mg | 20/55 | 7/53 | 2.8 (1.3 to 6.0) | 4.3 (2.6 to 13) | 13 | 36 | |
| 8 | Rofecoxib 50 mg | 44/110 | 6/53 | 3.5 (1.6 to 7.8) | 3.5 (2.4 to 6.9) | 11 | 40 |
| Naproxen 550 mg | 18/55 | 6/53 | 2.9 (1.2 to 6.7) | 4.7 (2.7 to 16) | 11 | 33 | |
| 12 | Rofecoxib 50 mg | 38/110 | 2/53 | 9.2 (2.3 to 37) | 3.3 (2.4 to 4.9) | 4 | 35 |
| Naproxen 550 mg | 14/55 | 2/53 | 6.8 (1.6 to 28) | 4.6 (2.9 to 11) | 4 | 25 | |
Naproxen was given as the sodium salt
Rofecoxib 50 mg was significantly more effective than placebo at all time points. For rofecoxib 50 mg compared with placebo, the NNT for at least 50% pain relief was 3.9 (2.6 to 7.8) over six hours, 3.5 (2.4 to 6.9) over eight hours, and 3.3 (2.4 to 4.9) over 12 hours. Naproxen sodium 550 mg was significantly more effective than placebo at all time points. For naproxen sodium 550 mg compared with placebo, the NNT for at least 50% pain relief was 4.3 (2.6 to 13) over six hours, 4.7 (2.7 to 16) over eight hours, and 4.6 (2.9 to 11) over 12 hours.
Time to remedication in postsurgical pain
TTR50 with rofecoxib 50 mg was similar to that of naproxen sodium 550 mg (Table 3). It was 5.8 hours with rofecoxib 50 mg, 5.9 hours with naproxen sodium 550 mg, and 2.8 hours with placebo.
Adverse effects in postsurgical pain
Information on adverse effects was collected over five days for multiple doses of study treatments; single dose information was not available. The most commonly reported adverse effects were fever, constipation, and nausea. No adverse effects were serious. There were too few patients to analyse reliably particular adverse effects like dizziness or nausea.
There was no statistically significant difference in the reported incidence of patients with any adverse effect with rofecoxib 50 mg (42/54 patients, 78%) compared with placebo (41/53, 77%), relative risk 1.0 (0.8 to 1.2). With naproxen sodium 550 mg 37/55 patients (67%) reported adverse effects, again with no significant difference compared with placebo, relative risk 0.9 (0.7 to 1.1).
Discussion
Individual patient data is the gold standard in meta-analysis and has been performed only rarely in acute pain, with tramadol [18], and tramadol plus paracetamol [19]. This review is therefore the first individual patient data analysis for NSAIDs and coxibs in acute pain. For rofecoxib, individual patient information was available from fourteen trials in postoperative pain. The trials were of high quality and validity, scoring the maximum for both. The same methods and outcomes were used in all trials, thus ensuring clinical homogeneity. Since trials were conducted over 24 hours in dental pain and 12 hours in postsurgical pain, and because only for six hour TOTPAR can we be confident that there is no difference between pain models [11], the prior decision was to analyse these two pain models separately.
Historically, single-dose analgesic trials for most treatments have been conducted over 4–6 hours since this is typically how long pain relief has lasted. Trials of coxibs, like rofecoxib and valdecoxib [20], have been conducted over 12–24 hours. Because of the long duration of analgesia at the doses given, the potential existed to use trials of rofecoxib to calculate single dose efficacy estimates over durations up to 24 hours in dental pain and 12 hours in postsurgical pain. This would not have been possible without individual patient information because methods to convert mean summary data into dichotomous form exist only for 4–6 hours [2-4]. Examination of efficacy estimates over longer durations allowed examination of the duration of analgesic effect, the caveat being that in some studies there was only one additional observation beyond 12 hours.
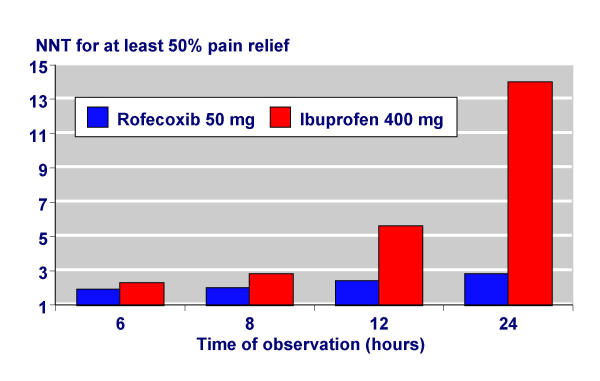
In dental pain the six-hour NNTs for rofecoxib 50 mg and ibuprofen 400 mg were similar at about two. This means that for every two patients treated one would obtain at least 50% pain relief with active treatment who would not have done with placebo. For rofecoxib 50 mg the NNTs were similar (all below 3) at six, eight, 12 and 24 hours, whereas for ibuprofen 400 mg the NNTs increased (drug less effective) with time (Figure 2). This comparison of rofecoxib 50 mg with ibuprofen 400 mg at longer times is the only one we have with adequate numbers of patients. External, or indirect, comparisons are only available over six hours. Table 6 shows a comparison for coxibs, NSAIDs, and simple analgesics over six hours after third molar surgery [20,21]. Oral rofecoxib 50 mg and valdecoxib 20 mg and 40 mg all have mean NNT values below 2, and have overlapping confidence intervals. Standard doses of diclofenac and ibuprofen have similar, if slightly higher, NNTs, while standard doses of paracetamol and aspirin have considerably higher (worse) NNTs.
Figure 2.
Comparison of NNTs of rofecoxib 50 mg and ibuprofen 400 mg over different times
Table 6.
Comparison of NNTs for oral analgesics in dental pain. NNT are for at least 50% pain relief compared with placebo over six hours
| Drug and dose | Number of patients in comparison | NNT (95% CI) |
| Rofecoxib 50 mg | 1900 | 1.9 (1.8 to 2.1) |
| Valdecoxib 40 mg1 | 473 | 1.6 (1.4 to 1.8) |
| Valdecoxib 20 mg1 | 204 | 1.7 (1.4 to 2.0) |
| Diclofenac 50 mg2 | 367 | 2.1 (1.8 to 2.6) |
| Ibuprofen 400 mg2 | 3402 | 2.2 (2.1 to 2.4) |
| Paracetamol 975/1000 mg2 | 1038 | 3.7 (3.1 to 4.7) |
| Paracetamol 600/650 mg2 | 1265 | 4.2 (3.6 to 5.2) |
| Aspirin 600/650 mg2 | 3635 | 4.7 (4.2 to 5.4) |
1. Barden et al. BMC Anesthesiol. 2003 3:1 2. Barden et al, BDJ, in press.
In this analysis, time for 50% of patients to have remedicated was calculated for each drug and for placebo from individual patients. The results showed that in dental pain the duration of analgesia was longer with a single dose of rofecoxib 50 mg than for other comparator drugs (at the doses used in the trials). In the postsurgical trial TTR50 was shorter at 5.8 hours. The difference in TTR50 values for dental compared with postsurgical pain may be due to the different pain context (more major orthopaedic than dental surgery), the more elderly population in the postsurgical study (mean age 65 years versus 21 years in the dental trials), and that results were simply not robust in the postsurgical study because they were based on too little information. Oral valdecoxib 20 mg or 40 mg has comparable duration of analgesia to rofecoxib 50 mg in dental studies [20], though in many fewer patients.
Overall, efficacy results for rofecoxib 50 mg from this individual patient meta-analysis were similar to those derived using published mean summary data [5]. There were minor differences. For instance, the NNT for at least 50% pain relief over 4–6 hours from five published trials with 675 patients in the comparison was 2.3 (2.0 to 2.6) in dental plus postsurgical pain. Here, in all 14 trials with 2060 patients it was 2.0 (1.9 to 2.1) in dental plus postsurgical pain. Again, the previous estimate for duration of analgesia using time to remedication calculated from weighted mean values was 13.6 hours, and here it was 15.5 hours measured from individual patients (though in dental pain only).
Adverse effects are also important, though in single dose trials they are both uncommon and inadequately reported [22]. Information from many patients is required to determine NNH reliably even for relatively common, minor, events like dizziness or nausea [23]. Adverse effects were uncommon with single doses of rofecoxib 50 mg, and rates of particular adverse effects were low. There is no obvious reason for post-extraction alveolitis with rofecoxib; the incidence with rofecoxib 50 mg (13%) was similar to that with comparator NSAIDs (5–15%, Table 4). Central nervous system and gastrointestinal effects would be expected with the opioid combination comparators, though results were based on few patients and NNHs were not robust.
Surveys have shown that acute pain is often not managed well [24]. Potential exists for better pain management since drugs with fast onset and long-lasting analgesic action are available. Information on remedication in trials may help demonstrate duration of analgesia with different treatments, since patients are meant to remedicate only when they have inadequate pain relief. Longer duration analgesics may be of importance, not only as part of a multimodal approach to analgesia in the perioperative period [25]. A recent survey of French general practitioners found acute pain at home after hospital discharge to be a major problem [26].
Conclusions
Single dose rofecoxib 50 mg in dental pain had comparable analgesia to ibuprofen 400 mg over six hours, but was superior at eight and 12 hours. Adverse effects were uncommon. Results for postsurgical pain were not robust because of limited patient numbers.
Competing interests
RAM has been a consultant for Merck Sharp and Dohme Ltd (MSD). RAM, HJM and JE have received lecture fees from pharmaceutical companies. The authors have received research support from charities and government sources at various times. This work was supported by an unrestricted, educational grant from MSD. The terms of the financial support from MSD included freedom for authors to reach their own conclusions, and an absolute right to publish the results of their research, irrespective of any conclusions reached. MSD did have the right to view the final manuscript before publication, and did so. No author has any direct stock holding in any pharmaceutical company.
Authors' contributions
JE performed the analysis and wrote the draft manuscript. RAM checked all analyses. JE, RAM and HJM contributed equally to the design, writing and reviewing of the paper.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Quality and validity scales. This file describes in detail the scales for trial quality and trial validity that have been used in the analysis.
Acknowledgments
Acknowledgements
Financial support was in the form of an unconditional, educational grant from Merck Sharp and Dohme Ltd, UK. Merck Research Laboratories, Rahway, New Jersey provided individual patient data for inclusion in this review. Additional support was provided by the Oxford Pain Relief Trust and Oxford Pain Research funds.
Contributor Information
Jayne E Edwards, Email: jayne.edwards@pru.ox.ac.uk.
R Andrew Moore, Email: andrew.moore@pru.ox.ac.uk.
Henry J McQuay, Email: henry.mcquay@pru.ox.ac.uk.
References
- Cooper SA. Single-dose analgesic studies: the upside and downside of assay sensitivity. In: Max MB, Portenoy RK, Laska EM, editor. The design of analgesic clinical trials (Advances in Pain Research and Therapy) Vol. 18. New York Raven Press; 1991. pp. 117–124. [Google Scholar]
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics. Pain. 1996;66:229–237. doi: 10.1016/0304-3959(96)03032-1. [DOI] [PubMed] [Google Scholar]
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: Verification from independent data. Pain. 1997;69:127–130. doi: 10.1016/S0304-3959(96)03251-4. [DOI] [PubMed] [Google Scholar]
- Moore A, Moore O, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: Use of pain intensity and visual analogue scales. Pain. 1997;69:311–315. doi: 10.1016/S0304-3959(96)03306-4. [DOI] [PubMed] [Google Scholar]
- Barden J, Edwards JE, McQuay HJ, Moore RA. Single-dose rofecoxib for acute postoperative pain in adults: a quantitative systematic review. BMC Anesthesiol. 2002;2:4. doi: 10.1186/1471-2253-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled: the QUOROM statement. The Lancet. 1999;354:1896–1900. doi: 10.1016/S0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- Ehrich EW, Dallob A, De Lepeleire I, Van Hecken A, Riendeau D, Yuan W, Porras A, Wittreich J, Seibold JR, De Schepper P, Mehlisch DR, Gertz BJ. Characterization of rofecoxib as a cyclooxygenase-2 isoform inhibitor and demonstration of analgesia in the dental pain model. Clin Pharmacol Ther. 1999;65:336–347. doi: 10.1016/S0009-9236(99)70113-X. [DOI] [PubMed] [Google Scholar]
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Smith LA, Oldman AD, McQuay HJ, Moore RA. Teasing apart quality and validity in systematic reviews: an example from acupuncture trials in chronic neck and back pain. Pain. 2000;86:119–132. doi: 10.1016/S0304-3959(00)00234-7. [DOI] [PubMed] [Google Scholar]
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything – large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain. 1998;78:209–216. doi: 10.1016/S0304-3959(98)00140-7. [DOI] [PubMed] [Google Scholar]
- Barden J, Edwards JE, McQuay HJ, Moore RA. Pain and analgesic response after third molar extraction and other postsurgical pain. Pain. 2004;107:86–90. doi: 10.1016/j.pain.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratios and standardised ratios and rates. In: Gardner MJ, Altman DG, editor. Statistics with confidence – confidence intervals and statistical guidelines London British Medical Journal. 1995. pp. 50–63. [Google Scholar]
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. British Medical Journal. 1995;310:452–454. doi: 10.1136/bmj.310.6977.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Abbé KA, Detsky AS, O'Rourke K. Meta-analysis in clinical research. Ann Intern Med. 1987;107:224–233. doi: 10.7326/0003-4819-107-2-224. [DOI] [PubMed] [Google Scholar]
- Gavaghan DJ, Moore RA, McQuay HJ. An evaluation of homogeneity tests in meta-analyses in pain using simulations of individual patient data. Pain. 2000;85:415–424. doi: 10.1016/S0304-3959(99)00302-4. [DOI] [PubMed] [Google Scholar]
- Tang J-L, Liu JLY. Misleading funnel plot for detection of bias in meta-analysis. Journal of Clinical Epidemiology. 2000;53:477–484. doi: 10.1016/S0895-4356(99)00204-8. [DOI] [PubMed] [Google Scholar]
- Tramèr MR, Reynolds DJM, Moore RA, McQuay HJ. Impact of covert duplicate publication on meta-analysis: a case study. British Medical Journal. 1997;315:635–639. doi: 10.1136/bmj.315.7109.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RA, McQuay HJ. Single-patient data meta-analysis of 3453 postoperative patients: Oral tramadol versus placebo, codeine and combination analgesics. Pain. 1997;69:287–294. doi: 10.1016/S0304-3959(96)03291-5. [DOI] [PubMed] [Google Scholar]
- Edwards JE, McQuay HJ, Moore RA. Combination analgesic efficacy: individual patient data meta-analysis of single-dose oral tramadol plus acetaminophen in acute postoperative pain. J Pain Symptom Manage. 2002;23:121–130. doi: 10.1016/S0885-3924(01)00404-3. [DOI] [PubMed] [Google Scholar]
- Barden JE, Edwards JE, McQuay HJ, Moore RA. Oral valdecoxib and injected parecoxib for acute postoperative pain: a quantitative systematic review. BMC Anesthesiology. 2003;3:1–8. doi: 10.1186/1471-2253-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barden J, Edwards JE, McQuay HJ, Wiffen PJ, Moore RA. Relative efficacy of oral analgesics after third molar extraction. British Dental Journal. [DOI] [PubMed]
- Edwards JE, McQuay HJ, Moore RA, Collins S. Reporting of adverse effects in clinical trials should be improved. Lessons from acute postoperative pain. Journal of Pain and Symptom Management. 1999;81:289–297. doi: 10.1016/S0304-3959(99)00022-6. [DOI] [PubMed] [Google Scholar]
- Edwards JE. Determination of analgesic efficacy and harm in postoperative pain using systematic review methods. D Phil thesis, University of Oxford. 2001.
- Bruster S, Jarman B, Bosanquet N, Weston D, Erens R, Delbanco TL. National survey of hospital patients. British Medical Journal. 1994;309:1542–1546. doi: 10.1136/bmj.309.6968.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinatra R. Role of COX-2 inhibitors in the evolution of acute pain management. J Pain Symptom Management. 2002;24:S18–27. doi: 10.1016/S0885-3924(02)00410-4. [DOI] [PubMed] [Google Scholar]
- Robaux S, Bouaziz H, Cornet C, Boivin JM, Lefevre N, Laxenaire MC. Acute postoperative pain management at home after ambulatory surgery: a French pilot survey of general practitioners' views. Anesthesia & Analgesia. 2002;95:1258–1262. doi: 10.1097/00000539-200211000-00029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quality and validity scales. This file describes in detail the scales for trial quality and trial validity that have been used in the analysis.