Abstract
Hypothesis
Spiral ganglion neurons (SGN) in the Phex Hyp-Duk male mouse, a murine model of postnatal endolymphatic hydrops (ELH) undergo progressive deterioration reminiscent of human and other animal models of ELH with features suggesting apoptosis as an important mechanism.
Background
Histological analysis of the mutant's cochlea demonstrates ELH by postnatal day (P) 21 and SGN loss by P90. The SGN loss appears to occur in a consistent topographic pattern beginning at the cochlear apex.
Methods
SGN were counted at P60, P90 and P120. Semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR), quantitative PCR, and immunohistochemical analyses of activated caspases-3, -8 and -9 were performed on cochlear sections obtained from mutants and controls. Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling assay (TUNEL) was carried out on two mutants and two controls.
Results
Corrected SGN counts in control mice were greater in the apical turn of the cochleae at P90 and P120, respectively (P<0.01). Increased expression of activated caspase-3,-8 and -9 was seen in the mutant. At later time-points, activated caspase expression gradually declined in the apical turns and increased in basal turns of the cochlea. Quantitative and semi-quantitative PCR analysis confirmed increased expression of caspase-3, -8 and -9 at P21 and P40. TUNEL staining demonstrated apoptosis at P90 in the apical and basal turns of the mutant cochleae.
Conclusion
SGN degeneration in the Phex Hyp-Duk/Y mouse appear to mimic patterns observed in other animals with ELH. Apoptosis plays an important role in the degeneration of the SGN in the Phex Hyp-Duk male mouse.
Keywords: endolymphatic hydrops, Phex mouse, murine model of hydrops, apoptosis, spiral ganglion neurons
Introduction
Meniere's disease (MD) is characterized by fluctuating hearing loss, episodic vertigo, aural fullness, tinnitus and endolymphatic hydrops (ELH), an important histological hallmark. Despite description of the disease as early as 1861 (1), the etiology of MD remains largely unknown. Hallpike and Cairns (2), and Yamakawa (3) first confirmed ELH in MD in 1938. Many investigators have recognized the significance of ELH in the development of symptoms in patients with MD. In addition to ELH, degeneration of spiral ganglion cells and neuronal endings has been observed without corresponding severe inner hair cell loss in both human temporal bone studies and the experimental guinea pig model (4,5). The guinea pig model developed by Kimura in 1967 (6) has proven useful in the study of conditions exhibiting ELH, particularly the human MD condition. Although useful, this model suffers major limitations. Production of the model is time-consuming and cumbersome, does not reliably produce vestibular symptoms, requires post-operative animal care, and results in post-surgery survival rates less than 100%. Furthermore, induction of ELH by surgical obliteration of the endolymphatic duct deviates from the spontaneous development of ELH that occurs in MD. In addition, the resulting ELH requires postmortem histological confirmation because surgical induction does not reliably produce hydrops.
Many of these limitations have been overcome in a genetically altered mouse model carrying a mutant allele of the X-linked phosphate-regulating gene (Phex) designated the Phex Hyp-Duk gene (7). This allele contains an intragenic deletion of 30 kb in exons 13 and 14 of the Phex gene resulting in loss of functional Phex protein. A detailed description of the Phex Hyp-Duk mouse background, genotyping and histo-morphological analysis was previously published by Megerian et al (8). The Phex Hyp-Duk allele arose from a spontaneous mutation on the BALB/cAnBomUrd (abbreviated BALB/cUrd) background (7). The Phex Hyp-Duk mutation is currently maintained in our laboratory on the C57BL/6J (B6) background. The male mice carrying the Phex Hyp-Duk allele in the BALB/cUrd background were obtained by breeding BALB/cUrd (or B6) Phex Hyp-Duk carrier females (+/Phex Hyp-Duk) with BALB/cUrd (or B6) Phex wild-type (+/Y) mice.
The male mouse carrying the Phex Hyp-Duk gene (Phex Hyp-Duk/Y) on the BALB/cUrd background exhibits craniofacial and skeletal abnormalities, vestibular dysfunction, and hearing loss.
The cochleo-vestibular phenotype of the Phex mouse has been well characterized by Megerian et al (8). Affected mice undergo progressive bilateral fluctuating asymmetrical sensorineural hearing loss accompanied by progressive spiral ganglion degeneration. Histological analyses of cochleae in this model consistently demonstrated postnatal development of ELH by P21 and spiral ganglion neuron (SGN) loss by P90. Examination with light microscopy revealed cells with apoptotic features (condensed chromatin, contracted and convoluted cytoplasm, disintegrated nuclei) suggesting apoptosis as a potentially important mechanism of cell death (Figures 1). The SGN loss appears to follow a topographical distribution with loss beginning at the cochlear apex and proceeding to the basal turn by P90, but has not yet been quantified. The hair cells retain their normal histological morphology until advanced stages (>P300) (data not shown). The endolymphatic duct remains patent. These features were inconsistently present in some carrier females Phex Hyp-Duk /X and were absent in wild-type (+/Y) male mice.
Figure 1.
(A) shows a high-power photomicrograph (×100) of the SGN of a hematoxylin-eosin H&E stained section of the apex of the control (+/Y) mouse at P90 demonstrating preservation of the SGN. (B) In the Phex Hyp-Duk/Y, the neuronal population is decreased. Scale bar (applies to all panels) = 10 μm. (C, D) demonstrates SGNs with apoptotic features [A]: shrunken and convoluted cytoplasm, condensed chromatin and pyknosis, normal SGN [N], and Schwann cells [S].
This mouse model of ELH allows for a more a reliable production of ELH with the clinical and pathological findings reminiscent of those described in the human condition, most notably circling behavior which implies vestibular dysfunction. In addition, each litter generated by a breeding pair may contain several progeny, which consistently express the mutant phenotype. Thus, more mutant mice can be produced, over several cycles, than the limited number that can be produced through surgical induction one guinea pig at a time, which requires substantial time and expertise.
The purpose of this paper is to characterize the fate of the spiral ganglion neurons in the murine model of endolymphatic hydrops. Our hypothesis is that SGN in the Phex Hyp-Duk male undergo progressive deterioration in patterns that recapitulate human and other animals models of neural deterioration (apex to base) and have features that suggest apoptosis as a potential important mechanism in neuronal death.
Using indicators of apoptosis, such as caspase expression and DNA fragmentation, and histological observation for SGN loss, we aim to demonstrate that SGNs in this ELH model do undergo apoptosis and that it is progressive with respect to both time and cochlear topography.
Materials and Methods
To characterize the fate of the SGN and validate our hypothesis we conducted corrected spiral ganglion cell counts, semi-quantitative RT-PCR and relative quantitative PCR analysis of apoptosis-related gene expression, immunostaining of activated caspases, and TUNEL (TdT-mediated dUTP nick-end labeling) staining method.
Animals and Genotyping
To test our hypothesis we compared the Phex Hyp-Duk /Y mouse to the wild type (+/Y) which was utilized as a control in all our experimental analyses. The Animal Care and Use Committee of Case Western Reserve University approved the care and use of the mice for this study.
In our experiment, both mutant and control male mice were confirmed by genotypic analysis. To identify the Phex Hyp-Duk /Y mice from their control littermates, the Phex gene was screened for the presence or absence of exon 14 using PCR amplification techniques. Briefly, DNA was isolated from tail biopsies using a Qiagen DNeasy Blood & Tissue Kit (Valencia, CA). Primers designed to amplify exons 10 and 14 were utilized. Exon 10 (positive control): forward primer KA 552 5'-TTGCCAACAGTTTTCCAAAGG-3'; reverse primer KA 553 5'-AAGCTCCCTACATCCCATCC-3' and exon 14 (deleted in mutant): forward primer KA 554 5'-ATAGCGTCTCTTCTGGTTGC-3'; reverse primer KA 555 5'-GCTGGCTACCCTGAGTTGAG-3'). The touchdown polymerase chain reaction (PCR) amplification using Taq Polymerase (Invitrogen) was previously described in detail (8). Expected product sizes for primer pairs 552/553 and 554/555 were 293 bp and 308 bp, respectively. The mutant allele Phex Hyp-Duk contains an intragenic deletion of 30 kb in exons 13 and 14. In the Phex Hyp-Duk /Y male the amplicon for exon 14 is absent. Wild-type females (+/+), carrier females (+/Phex Hyp-Duk) and wild-type male mice (+/Y) all demonstrate amplification of both exons 10 and 14. Detection of the male specific gene, Sry by PCR amplification as described by Kunieda et al (9), allows identification of males in young litters, and differentiation of wild type females (+/+), carrier females (+/Phex Hyp-Duk) and wild-type male mice (+/Y).
Spiral ganglion cell counts
At P60, P90 and P120, mice were anesthesized using an intraperitoneal injection of avertin (tribromoethanol stabilized in tertiary amyl hydrate) at a dose of 5 mg /10 g body weight and were perfused through the left ventricle of the heart with phosphate-buffered saline (PBS) followed by Bouin's fixative. At each time point, 5 controls and 5 Phex Hyp-Duk /Y mice were sacrificed. The inner ears from +/Y and Phex Hyp-Duk /Y mice were dissected, perfused with Bouin's fixative, immersed for 48 h, decalcified with Cal-EX solution for 6 h, and embedded in paraffin. Five-micrometer sections were cut, mounted on glass slides and counterstained in hematoxylin-eosin (H&E) (10,11). Spiral ganglion cells were observed under light microscopy (Leica DM4500 B, Leica Microsystems, Wetzlar, Germany).
Heterogeneity between the size of nuclei exists within a given treatment group and between the treatment groups themselves. This can result in a counting bias when using simple profile-base counts. To correct for this Abercrombie correction factors were applied to each of the treatment groups(12,13). The correction factor was determined by dividing the section thickness (5 μm) by the sum of the section thickness and the average diameter of the neuronal nucleus: T/T + H, where T is section thickness and H is the average nuclear diameter. The average nuclear diameter was determined separately for each group by measuring the diameter of numerous nuclei in midmodiolar sections of Rosenthal's canal selected randomly. The nuclear diameter was measured with the aid of Image J software.
Corrected cell densities (corrected number of cells per square millimeter) of spiral ganglion cells were then calculated. The average value in four sections from a cochlear turn of each animal was defined as the density for that turn. Data were analyzed by the ANOVA test. The criterion for statistical significance was set at p < 0.05 using the two-tailed test.
Semi-quantitative RT-PCR for measuring mRNA levels of apoptosis-related genes
A detailed description of the methodology was previously published by Tian C et al. (14,15). Four Phex Hyp-Duk/Y and four controls (+/Y mice) were sacrificed under avertin anesthesia: avertin (5 mg per 10 g) at P40. Cochleae were quickly isolated for total RNA and complementary DNA (cDNA) preparation. Total RNA (DNA-free) was prepared using the pure-Link™ Micro-to-Midi Total RNA Purification System (Invitrogen, Carlsbad, CA, USA). cDNA synthesis was carried out using the SuperScript First-Strand Synthesis System. 1.5 μg of total RNA from each sample were used as template for cDNA synthesis. The 20 μl reaction mixture contained 50 mM KCl, 10 mM Tris-HCl, pH 9.0 (at 25°C), 0.01% Triton X-100, 2 mM MgCl2, 250 nM of each primer (forward and reverse), 200 μM dNTPs, 1 μl of cDNA and 0.5 U of Taq DNA polymerase (New England BioLabs, Inc., Ipswich, MA, USA). PCR primers are listed in Table 1(16,17). PCR was performed in a Bio-Rad PTC-200 Peltier Thermal Cycler (Bio-Rad Laboratories, Inc. Hercules, CA, USA). Amplification conditions were 94°C for 2 min; followed by 30 cycles of 94°C for 30 s, 60°C for 40 s and 72°C for 50 s; followed by 5 min at 72°C. Ten μl of the PCR products were subject to agarose gel electrophoresis and the gray intensity of each band was digitized using ImageJ software (http://rsb.info.nih.gov/nih-image/NIH, Bethesda, MD, USA) and corrected by the glyceraldehyde 3-phosphate dehydrogenase mRNA level of the same sample.
Table 1.
RT-PCR primers for detection of apoptosis-related genes
| ID | Sequence | Product Size (bp) | Reference |
|---|---|---|---|
| GAPDH-F | 5'-AACTTTGGCATTGTGGAAGG-3' | 351 | 1 |
| GAPDH-R | 5'-GGAGACAACCTGGTCCTCAG-3' | ||
| Caspase-3F | 5'-TGTCATCTCGCTCTGGTACG-3' | 201 | 1 |
| Caspase-3R | 5'-AAATGACCCCTTCATCACCA-3' | ||
| Caspase-8F | 5'-ATGGCGGAACTGTGTGACTCG-3' | 345 | 2 |
| Caspase-8R | 5'-GTCACCGTGGGATAGGATACAGCA-3' | ||
| Caspase-9F | 5'-CCTAGTGAGCGAGCTGCAAG-3' | 232 | |
| Caspase-9R | 5'-ACCGCTTTGCAAGAGTGAAG-3' |
Quantitative RT – PCR for measuring RNA levels of apoptosis related genes
RNA was extracted from mouse tissues with Total RNA (RNase-free DNase) and was prepared using the pure-LinkTM Micro-to-Midi Total RNA Purification System (Invitrogen, Carlsbad, CA, USA). At each time point, 6 controls and 6 mutants were used. cDNA synthesis was carried out using the SuperScript™ First-Strand Synthesis System (Catalog No. 11904-018). qRT – PCR reactions were performed in triplicate on a GeneAmp 7300 real-time thermal cycler (ABI) using Sybr Green chemistry. GAPDH was used as an endogenous control for all qRT – PCR reactions. Relative differences were calculated using the 2-ΔΔCt method (18). Primer sequences are available upon request.
Immunostaining for active caspases
A more detailed description of this technique is available in a previous publication by Han et al (15). In the following paragraph we provide a description of the technique utilized for immunostaining. A time course immunohistochemistry study of caspase expression was carried out for Phex Hyp-Duk/Y and +/Y mice (4 for each group at each time point). The time points selected were P14, P21, P40, P60 and P90. The following antibodies from Cell Signaling Technology, Inc. (Danvers, MA, USA) were used. Cleaved caspase-3 (Asp175) mouse-specific antibody detects endogenous levels of the large fragment (17/19 kDa) of activated caspase-3 resulting from cleavage adjacent to Asp175 (This antibody does not recognize full-length caspase-3 or other cleaved caspases.); caspase-8 antibody (mouse-specific) detects endogenous levels of the large 18 kDa subunit of active caspase-8; cleaved caspase-9 (Asp353) antibody (mouse-specific) detects endogenous levels of the 37 kDa subunit of mouse caspase-9 only after cleavage at aspartic acid 353. This antibody does not cross-react with full-length caspase-9 or with other caspases at endogenous levels. Mice were anesthetized and sacrificed. All inner ears were removed and cryosections were made and fixed in 4% PFA (diluted in 1×PBS) for 2 h. The sections were washed in 1 × PBS at room temperature twice for 5 min and permeabilized in 0.5% Triton X-100 for 30 min. After being washed twice in 1× PBS for 5 min and blocked in 5% BSA for 1 h, the samples were immersed in anti-active caspase-3, caspase-8 or caspase-9 (1:200 dilution) and incubated at 4°C overnight. After being washed twice in 1× PBS for 5 min, the samples were immersed in anti-rabbit secondary antibody Alexa 488 (1:500 dilution) for 1 h. The samples were also stained with propidium iodide (10 mg ml−1 in PBS) for 30 min at room temperature. The sample mounts were observed under immunofluorescent microscopy (Leica, DM4500 B, Leica Microsystems, Wetzlar, Germany).
TUNEL staining
TUNEL assay was carried out on the inner ears of Phex Hyp-Duk/Y and +/Y mice at P60 and P90 (four mice in each group) using an In Situ Cell Death Detection Kit (Roche Catalog # 11684795910). Briefly, the inner ear cryosections were fixed with freshly prepared 4% paraformaldehyde in PBS (pH 7.4) at 20 °C for 20 min. The sections were then washed for 30 min with PBS and incubated in blocking solution (3% H2O2 in methanol) for 10 min at 20 °C. After being rinsed with PBS, the slides were incubated in permeabilization solution (0.1% Triton X–100, 0.1% sodium citrate, freshly prepared) for 2 min on ice and then rinsed twice with PBS. 50 μl TUNEL reaction mixture obtained from the in situ kit was applied to the dry area around sample. The slides were then incubated for 60 min at 37°C in a humidified atmosphere in the dark, and rinsed 3 times with PBS. The samples were analyzed in a drop of PBS under a fluorescence microscope using an excitation wavelength in the range of 450–500 nm and detection in the range of 515–565 nm (green).
Results
Spiral ganglion cell density decreases progressively in mutants
To confirm that the experimental mice exhibited full penetration of the mutant phenotype of ELH and SGN loss, histological sections were examined as shown in figures 1. At P90 and P120 (Figure 2A and 2B, respectively), the mean corrected SGN density in the apical cochlear turn was significantly higher (P=0.003 and 0.0002, respectively) in the control mice than in the mutant mice. There was no difference between the middle and basal cochlear turns of the mutants and controls. There was no significant difference in the SGN counts in any of the three cochlear turns at P60 (data not shown)
Figure 2.

(A) corrected mean SGN densities in the cochlear turns of the mutant (Phex Hyp-Duk/Y, n=5) and control (+/Y, n=5) mice at P90. The density of SGNs in the apical cochlear turn in the Phex Hyp-Duk/Y mice was significantly lower than that of the +/Y mice (p=0.003). There were no significant differences for the ganglion cell densities in the middle or basal cochlear turns between the mutants and controls (P > 0.05).
(B) corrected mean SGN densities in the cochlear turns of mutant (Phex Hyp-Duk/Y, n=5) and control (+/Y, n=5) mice at P120. The densities of SGNs in the apical cochlear turn in the Phex Hyp-Duk/Y mice was significantly lower than that of the +/Y mice (P=0.0002). There were no significant differences in the mean density of the SGNs of the middle and basal cochlear turns between the mutants and controls (P > 0.05).
Error bars were generated by calculating the Standard Error of the Mean (SEM).
Phex Hyp-Duk/Y mice exhibit increased cochlear caspase mRNA expression
The relative mRNA levels of caspases-3, -8 and -9 in the cochleae were determined by semi-quantitative RT-PCR (Figure 3). The gray intensity of each band on the agarose gel was digitized using the ImageJ software. The relative mRNA level was calculated as the ratio of the gray intensities of the target gene and GAPDH of the same sample. The relative mRNA levels of caspase-3, caspase-8 and caspase-9 in the cochleae of the Phex Hyp-Duk/Y mice were significantly higher than those of the +/Y mice (P<0.05). The increase in the relative caspase mRNA expression has been shown to correlate with apoptotic activity(15).
Figure 3.
The relative mRNA levels of caspase genes in Phex Hyp-Duk/Y mice at P40. On the agarose gel electrophoresis of the RT-PCR products of caspase-3, -8 and -9 in the control (+/Y) and mutant (Phex Hyp-Duk/Y) mice, the gray intensity of each band was digitized using ImageJ software. The relative mRNA level was indicated by the ratio of the gray intensity of the target gene to that of GAPDH of the same sample. The mRNA levels of caspases in the cochlea of the Phex Hyp-Duk/Y mice were significantly higher than those of the control mice. *P<0.05
Quantitative real time PCR was then used to compare relative mRNA expression for caspases-3, -8 and -9 in mutant and control mice at P21 and P40. Relative expression was obtained by normalizing these levels to GAPDH from mRNA purified from dissected cochleas. At P21 (Figure 4A), the Phex Hyp-Duk/Y mice showed a trend toward increased expression of caspase-3 and -9 (P=0.1) and, a higher expression of caspase-8 (P=0.03) as compared to control mice. At P40 (Figure 4B), the relative expression of caspase-3, -8 and -9 was significantly higher in the Phex Hyp-Duk/Y mice. (P=0.008, 0.001 and 0.003, respectively). Caspase-8 expression was 6 fold higher in the mutant (P=0.001).
Figure 4.
qPCR was used to quantify the relative difference in caspase-3, -8 and -9 mRNA between control (+/Y) and mutant (Phex Hyp-Duk/Y) mice after normalizing to GAPDH. (A) shows the fold difference in caspase-3, -8 and -9 between the mutants and controls at P21. A trend towards increased expression of caspase-3 and -9 was present (P=0.1), and a higher expression of caspase-8 (P=0.03) was seen in the mutants compared to controls. (B) At P40, the relative expression of caspase-3, -8 and -9 was significantly higher in the mutants compared to controls (P=0.008, 0.001 and 0.003, respectively). Caspase-8 expression is 6 fold higher in the mutants. Error bars were generated by calculating the Standard Error of the Mean (SEM).
Expression of activated caspases-3, -8 and -9 in mutant mouse SGNs
Using in situ fluorescent immunohistochemical staining, caspase expression was assayed over a time course from P14 to P90 in the cochlear SGNs of the Phex Hyp-Duk/Y mice.
Beginning at P21, the staining intensity of all three antibodies in the SGNs was stronger in the Phex Hyp-Duk/Y mice than that in the +/Y mice (figure 5–8). Overall, caspases-3, -8 and -9 showed similar patterns of expression at each time point.
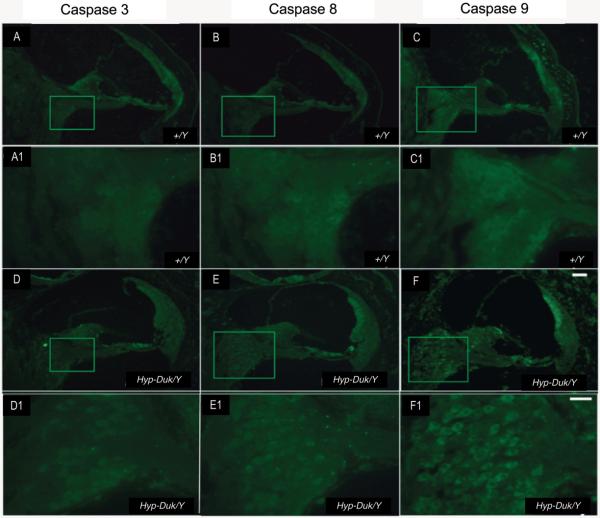
Figure 5.
Photomicrographs showing caspase expression in the ganglion cells of the PhexHyp-Duk/Y and control (+/Y) mice at 3 weeks by in situ fluorescent immunohistochemistry staining. Caspase-3 expression signal could be detected, and caspase-8 and -9 expression were obvious in the SGN of the apical cochlear turn of the PhexHyp-Duk/Y mice at 3 weeks (D, E and F, and D1, E1 and F1; D1, E1 and F1 are enlarged images from boxes in D, E and F, respectively). No significant staining or stained cell structures could be observed in the SGN of the +/Y mice (A, B and C, and A1, B1 and C1; A1, B1 and C1 are enlarged from A, B and C respectively). This suggests that apoptosis of the SGN begins at or around P21. Scale bar (A, B, C, D, E and F) = 100 μm,(A1, B1, C1, D1, E1 and F1) = 10 μm
Figure 8.
(A, C) high-powered photomicrograph showing the absence of significant expression on activated caspase-9 in the control (+/Y) mouse at P90. (B, D) shows increased expression of activated caspase-9 in the SGNs of the mutant (Phex Hyp-Duk/Y) mice at P90, respectively. SG; spiral ganglion. Scale bar (A and B) = 100 μm, (C and D)=10 μm.
All three caspases were detected in similar topographical distribution progressing from the apical to the basal cochlear turn at different time points (Figure 9A–D). In the Phex Hyp-Duk/Y mice, predominant staining of the apex at P40 confirmed the early apical expression of all three caspases; caspase-3 is shown (Figure 6B and 9B). At P60, staining was detected in all three turns of the cochlea; caspase-8 is shown (Figure 7B and 9C). At P90, more staining was noted in the basal turn compared to the apical and mid turns; caspase-8 is shown (Figure 7D and 9D). This pattern suggests that the apoptotic process progressed from the apex to the basal turn.
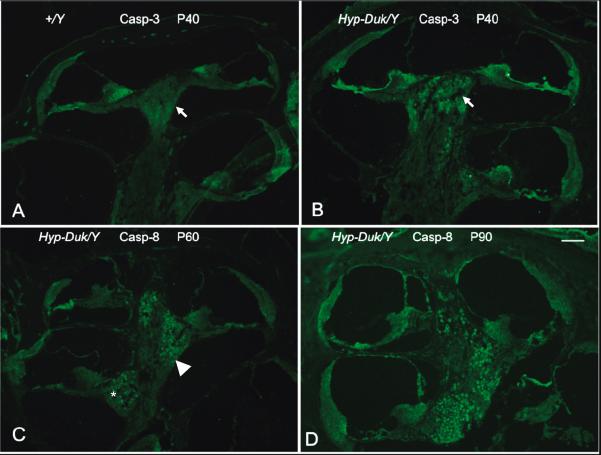
Figure 9.
The topographic progression of caspase expression in the PhexHyp-Duk/Y mutant. (A) Depicts a low-power photomicrograph of an immunostained mid-modiolar section of a control +/Y mouse at P40. The white arrow indicates the SGN population of the apex. No significant expression is seen. (B) Shows the expression of caspase-3 in the SGN population of the apex (white arrow) from a PhexHyp-Duk/Y mouse at P40. No significant expression is seen in the middle and basal turn. (C) Shows the expression of caspase-8 in all turns of the cochlea in the PhexHyp-Duk/Y mouse at P60 (white arrowhead and asterisk indicate SGN population of middle and basal turns respectively). (D) Shows the expression of caspase-8 in the PhexHyp-Duk/Y mouse at P90. Note the relative progression of expression from the apex to the basal turn (B, C and D). Scale bar (applies to all panels) = 100 μm. For publication purpose, photomicrographs with better staining were used. This explains the use of two different antibodies in this figure.
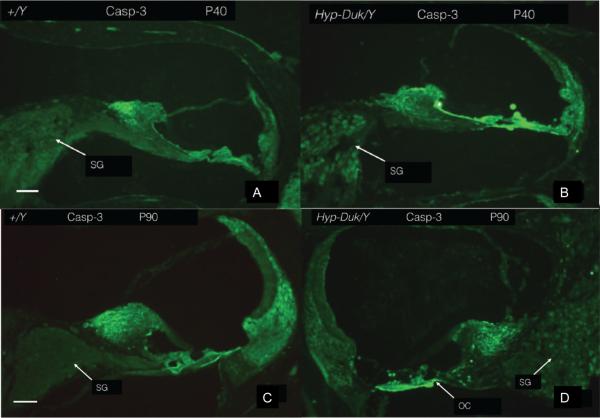
Figure 6.
(A, C) high-powered photomicrograph showing the absence of significant expression of activated caspase-3 in the control (+/Y) mouse at P40 and P90, respectively. (B, D) shows increased expression of activated caspase-3 in the SGNs of the mutant (PhexHyp-Duk/Y) mice at P40 and P90, respectively. SG; spiral ganglion. Scale bar (A to D) = 100 μm.
Figure 7.
(A, C) high-powered photomicrograph showing the absence of significant expression on activated caspase-8 in the control (+/Y) mouse at P60 and P90, respectively. (B, D) shows increased expression of activated caspase-8 in the SGNs of the mutant (PhexHyp-Duk/Y) mice at P60 and P90, respectively. SG; spiral ganglion. Scale bar (A to D) = 100 μm.
Apoptosis detected by TUNEL assay in mutant SGNs
To demonstrate DNA fragmentation, TUNEL assay was performed (Figure 10). At P90, no TUNEL signal was detected in the SGNs of the control +/Y mice (Figure 10A). In the Phex Hyp-Duk/Y mice a positive TUNEL signal was detected in the SGNs (Figure 10D). Counterstaining using propidium iodide (PI) confirmed the nuclear localization (Figure 10E and F). PI detects DNA termini 3P/5OH whereas TUNEL does not. Its fluorescence is acquired in a logarithmic mode for nuclei and in a linear mode for other parts of the cell (19). When both stains overlay, PI staining is a sensitive indicator of apoptosis. At earlier time points, the TUNEL assay appeared negative in SGNs in both the experimental or control animals (data not shown).
Figure 10.
TUNEL assay on the apical turn of cochleae from +/Y and PhexHyp-Duk/Y mice at P90. No TUNEL signal was detected in the SGN of the cochleae of +/Y mice (A); whereas, a positive signal was detected in the ganglion cells of the cochleae of PhexHyp-Duk/Y mice (circled in D). (B) and (E) are propidium iodide (PI)-stained ganglion cells from +/Y and PhexHyp-Duk/Y mice, respectively. (C) and (F) are merged images of the TUNEL and PI from (A and B), or (D and E), respectively. Scale bar = 25 μm and applies to all panels.
Discussion
The Phex Hyp-Duk male mouse was shown to be a reliable and easily reproducible murine model of postnatal ELH (8). Histomorphological analysis demonstrated the development of ELH by P21. This histological finding occurs in the absence of endolymphatic ductal obstruction. At time points prior to P90, the SGN population was intact and appeared normal. At P90, histological analysis of cochlear sections from Phex Hyp-Duk/Y mice demonstrated a partial loss of the SGN population. This loss exhibited a topographic organization with a chronological progression from the apex of the cochlea to the basal turn. Interestingly, the hair cells maintained normal morphology in the mutant mice until advanced age (>P300).
By demonstrating pro-apoptotic caspase expression at both the mRNA and protein levels over a time course, and by direct demonstration of apoptosis using the TUNEL assay, we have confirmed the hypothesis that apoptosis plays a role in progressive SGN deterioration exhibited in the PhexHyp-Duk murine model of ELH. This evidence for apoptotic cell death provides cytochemical characterization of the fate of at least a part of the SGN in the PhexHyp-Duk mouse and offers an explanation for the cellular loss seen upon histological analysis.
Reduced neuronal cell population in the PhexHyp-Duk/Y mice was confirmed using corrected spiral ganglion cell counts. In a previous publication (8), we showed that in younger mice (P21–25), the size and number of spiral ganglion neurons was comparable between the control (+/Y) and the PhexHyp-Duk/Y mice. To avoid a potential counting bias caused by a disparity in the nuclear size, a correction factor was applied to the SGN cell counts. Therefore, the decreased density in the older mutant is indicative of a reduction in the actual number of surviving neurons rather than a decrease in their relative individual size. Cells counts confirm an apical to base trajectory of neuronal deterioration that appears to follow other models of ELH (20,21)
Although the semi-quantitative RT-PCR method used in this study was validated to show the relative mRNA levels of panels of genes to GAPDH, the same panel of genes (caspase-3, -8 and -9) was then analyzed using relative qPCR which is a more sensitive method. The generated data was compared using the 2−ΔΔCt method, which is a standard analytical method.
Beginning at P21, immunostaining for activated caspases-3, -8 and -9 showed increased expression of all three in the SGNs of PhexHyp-Duk/Y mice compared to earlier time points and to control mice which, lacked SGN caspase expression at all time points. This reflects activation of both the intrinsic and extrinsic pathways of the apoptotic cascade. A significantly higher increase in the relative expression of caspase-8 may hint to the importance of the extrinsic pathway in the apoptosis of the SGNs in the Phex mouse. The extrinsic signaling pathways of apoptosis involve death receptors that are members of the tumor necrosis factor (TNF) receptor gene family (22). Several ligands (Fas-L/Fas-R) (23),TNF-∝/TNFR1 (24),Apo3L/DR3(25), Apo2L/DR4(26) and Apo2L/DR5 (27) have been shown to activate the “death domain” of the TNF receptor. Ligand binding results in the activation of cytoplasmic adapter proteins (FADD or TRADD), which lead to formation of a death inducing signaling complex (DISC). Once formed, DISC results in auto-catalytic activation of procaspase-8 into caspase-8 (28). Activation of the stress kinase pathway (Mitogen activated protein kinase (MAPK) and c-Jun N-Terminal Kinase (JNK)) has been shown to mediate apoptosis by transcriptional induction of the Fas-L/Fas-R and TNF-∝/TNFR1 expression. Conceivably, cellular stress induced by hydrops can cause preferential activation of the extrinsic pathway. Signaling cross talking clearly exist between the intrinsic and extrinsic pathways. The activation of pro-apoptotic proteins (Bad, Bid and Bax) can lead to initiation of the intrinsic pathway by modulating the release of cytochrome c from the mitochondria, which leads to activation of procaspase-9 into caspase-9. Both pathways converge to activate caspase-3. Activation of caspase-3 eventually triggers DNA fragmentation and cell death.
Commensurate to the topographical organization of ELH in the PhexHyp-Duk mouse is the progression of SGN loss and, as we have now demonstrated, apoptosis. At earlier time points, increased expression of activated caspases was predominantly seen in the apical turn of the cochleae. At P60, progressive involvement of the middle turn and later the basal turn was demonstrated using protein expression analysis. This organization is reminiscent of the SGN loss seen in the experimental guinea pig model (20,21) and archival human temporal bone specimens obtained from patients with MD (29).
At earlier time points (P40 and P60), the organ of Corti did not demonstrate an increase in expression of activated caspases-3, -8 or -9. The major apoptotic changes and cell loss occurred in the spiral ganglion cells. Delayed expression of caspase in the hair cell population and their supportive cells was not demonstrated in this study. In the histological cochlear sections from the PhexHyp-Duk/Y mouse, hair cell loss was not seen at P90 but was clearly apparent at P300. It is unknown whether hair cell loss results from a deficiency of essential neurotropic factors resulting from degeneration of the afferent dendrites of the SGN (30–32), or whether the loss is a more chronologically advanced response to injury that is preceded by degeneration of the more vulnerable SGN population.
The role of neurotoxicity has been highlighted in several neurodegenerative conditions affecting the SGN (33–35) and also in the guinea pig model of ELH (36,37). In fact, both in human temporal bone studies and in the experimental guinea pig model, degeneration of SGNs and of neuronal endings has been observed in the absence of corresponding severe hair cell loss (5,21). In the experimental guinea pig model, the diameter of the eighth cranial nerve was decreased in the hydropic ear compared to its control (36). Interestingly, this neuronal loss did not correlate with the severity of ELH. The combined neuronal cell and cochlear nerve degeneration, the modest alteration of the sensory epithelium, and the severity of ELH appear to highlight the role of neurotoxicity in the genesis of cochleo-vestibular dysfunction. While recent evidence supports a role for neurotoxicity in cochleo-vestibular dysfunction in the experimental guinea pig (37,38), to date there has been no definitive evidence to support this concept in the murine model.
In several experimental animal models of various otopathological conditions, apoptosis has been implicated in cell death (39–41). Is this fate a common response to injury of the inner ear sensory epithelium and/or supporting cells?
In various experimental designs, this programmed cell death was the ultimate outcome of various intrinsic and extrinsic factors acting on the cochlea (39,42,43). It appears that the genetic background of the PhexHyp-Duk/Y mouse creates some sort of a cytochemical perturbation that results in the myriad of histological findings not present in the control wild type (+/Y) mouse. In our experimental model, the BALB/cUrd genetic background may result in increased susceptibility or abnormal response to intrinsic or extrinsic factors that remain undetermined. Whether ELH is a direct factor leading to SGN loss or merely a correlated condition is unknown. The chronological appearance of ELH preceding SGN loss may suggest an etio-pathogenic role. A possible limitation of this study is the absence of abundant histological evidence of apoptosis on light microscopy. However, unlike experimental models of acute cellular injury, the apoptosis of the SGNs in this model is progressive and slow and may not be captured in large numbers. Nevertheless, we feel that the results of the semi-quantitative RT-PCR and the relative qPCR analysis, the protein expression analysis using in-situ immunostaining of active caspases and the TUNEL technique strongly suggest that apoptosis is at least in part responsible for the demise of the SGNs in the mutant. Certainly, other important mechanisms of cell death such as necrosis cannot be underestimated and may be responsible for some of the SGNs loss.
Although the analyses of caspase-3, -8, and -9 mRNA and activated protein expression were positive and well correlated at earlier time points, the TUNEL assay did not show robust staining at these time points. Conceivably, DNA fragmentation, which is a late event in the cascade leading to cellular demise, was not captured at these earlier time points. Because cellular loss was clearly evident at P90, the chosen time points may have missed a “critical window” where TUNEL assay may have shown more significant staining.
Another limitation of this study is the lack of clear characterization of the fate of the hair cell population. More experiments are needed to further elucidate the fate of the hair cells and their supporting structures.
Demonstration of apoptotic cell death in the SGN of PhexHyp-Duk/Y mice presents new horizons for research and treatment of ELH and, by extrapolation, MD. Several investigators have shown that blockade of mediators of apoptosis confers an otoprotective effect in various experimental models (44–47). The PhexHyp-Duk mouse model may serve as a physiologically relevant and cost-effective platform for testing downregulation of effectors of apoptosis. Such downregulation may promote survival of the SGN and, hence, offer otoprotective effects.
The optimal delivery method: topical, intratympanic injection, or intralabyrinthine perfusion (or injection), will need to be established. Furthermore, the timing of the intervention and the optimal therapeutic modality remain to be determined.
Conclusion
Although not exclusive, progressive apoptosis appears to be a significant mechanism of cell death in the SGNs of the PhexHyp-Duk male mouse. This programmed cell death appears to show a topographic organization with cell loss at the apex preceding loss at the basal turn. This murine model of endolymphatic hydrops may provide a reliable and reproducible experimental platform to study pharmacotherapeutic intervention targeting apoptotic gene expression.
Acknowledgments
This work was supported in part by a grant from the American Otologic Society to Dr Cliff A. Megerian, and by the NIH grants R01DC007392, R01DC009246 to Dr Qing Y. Zheng.
Footnotes
There are no financial disclosures
Conflict of interest: none
Accepted for oral presentation at the 144th AOS Annual Meeting in Chicago, IL, April 2011.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ménière P. Maladies de l'oreille interne offrant les symptomes de la congestion cérébrale apoplectiforme. Gaz Méd de Paris. 1861;16:88. [Google Scholar]
- 2.Hallpike CS, Cairns H. Observations on the Pathology of Meniere's Syndrome: (Section of Otology) Proc R Soc Med. 1938;31:1317–36. doi: 10.1177/003591573803101112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paparella MM, Morizono T, Matsunaga T. Kyoshiro Yamakawa, MD, and temporal bone histopathology of Meniere's patient reported in 1938. Commemoration of the centennial of his birth. Arch Otolaryngol Head Neck Surg. 1992;118:660–2. doi: 10.1001/archotol.1992.01880060110023. [DOI] [PubMed] [Google Scholar]
- 4.Nadol JB., Jr Degeneration of cochlear neurons as seen in the spiral ganglion of man. Hear Res. 1990;49:141–54. doi: 10.1016/0378-5955(90)90101-t. [DOI] [PubMed] [Google Scholar]
- 5.Nadol JB, Jr, Adams JC, Kim JR. Degenerative changes in the organ of Corti and lateral cochlear wall in experimental endolymphatic hydrops and human Meniere's disease. Acta Otolaryngol Suppl. 1995;519:47–59. doi: 10.3109/00016489509121870. [DOI] [PubMed] [Google Scholar]
- 6.Kimura RS. Experimental blockage of the endolymphatic duct and sac and its effect on the inner ear of the guinea pig. A study on endolymphatic hydrops. Ann Otol Rhinol Laryngol. 1967;76:664–87. doi: 10.1177/000348946707600311. [DOI] [PubMed] [Google Scholar]
- 7.Lorenz-Depiereux B, Guido VE, Johnson KR, et al. New intragenic deletions in the Phex gene clarify X-linked hypophosphatemia-related abnormalities in mice. Mamm Genome. 2004;15:151–61. doi: 10.1007/s00335-003-2310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Megerian CA, Semaan MT, Aftab S, et al. A mouse model with postnatal endolymphatic hydrops and hearing loss. Hear Res. 2008;237:90–105. doi: 10.1016/j.heares.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunieda T, Xian M, Kobayashi E, et al. Sexing of mouse preimplantation embryos by detection of Y chromosome-specific sequences using polymerase chain reaction. Biol Reprod. 1992;46:692–7. doi: 10.1095/biolreprod46.4.692. [DOI] [PubMed] [Google Scholar]
- 10.Jeong SW, Kim LS, Hur D, et al. Gentamicin-induced spiral ganglion cell death: apoptosis mediated by ROS and the JNK signaling pathway. Acta Otolaryngol. 130:670–8. doi: 10.3109/00016480903428200. [DOI] [PubMed] [Google Scholar]
- 11.Lee JE, Nakagawa T, Kim TS, et al. A novel model for rapid induction of apoptosis in spiral ganglions of mice. Laryngoscope. 2003;113:994–9. doi: 10.1097/00005537-200306000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Abercrombie M, Johnson ML. Quantitative histology of Wallerian degeneration; nuclear population in rabbit sciatic nerve. J Anat. 1946;80:37–50. [PubMed] [Google Scholar]
- 13.Guillery RW. On counting and counting errors. J Comp Neurol. 2002;447:1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- 14.Tian C, Liu XZ, Han F, et al. Ush1c gene expression levels in the ear and eye suggest different roles for Ush1c in neurosensory organs in a new Ush1c knockout mouse. Brain Res. 1328:57–70. doi: 10.1016/j.brainres.2010.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han F, Yu H, Tian C, et al. A new mouse mutant of the Cdh23 gene with early-onset hearing loss facilitates evaluation of otoprotection drugs. Pharmacogenomics J. doi: 10.1038/tpj.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen M, Ona VO, Li M, et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- 17.Zender L, Hutker S, Liedtke C, et al. Caspase 8 small interfering RNA prevents acute liver failure in mice. Proc Natl Acad Sci U S A. 2003;100:7797–802. doi: 10.1073/pnas.1330920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bookout AL, Mangelsdorf DJ. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl Recept Signal. 2003;1:e012. doi: 10.1621/nrs.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lecoeur H. Nuclear apoptosis detection by flow cytometry: influence of endogenous endonucleases. Exp Cell Res. 2002;277:1–14. doi: 10.1006/excr.2002.5537. [DOI] [PubMed] [Google Scholar]
- 20.Bixenstine PJ, Maniglia MP, Vasanji A, et al. Spiral ganglion degeneration patterns in endolymphatic hydrops. Laryngoscope. 2008;118:1217–23. doi: 10.1097/MLG.0b013e31816ba9cd. [DOI] [PubMed] [Google Scholar]
- 21.Momin SR, Melki SJ, Alagramam KN, et al. Spiral ganglion loss outpaces inner hair cell loss in endolymphatic hydrops. Laryngoscope. 120:159–65. doi: 10.1002/lary.20673. [DOI] [PubMed] [Google Scholar]
- 22.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 23.Chicheportiche Y, Bourdon PR, Xu H, et al. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem. 1997;272:32401–10. doi: 10.1074/jbc.272.51.32401. [DOI] [PubMed] [Google Scholar]
- 24.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 25.Marsters SA, Sheridan JP, Pitti RM, et al. Identification of a ligand for the death-domain-containing receptor Apo3. Curr Biol. 1998;8:525–8. doi: 10.1016/s0960-9822(98)70204-0. [DOI] [PubMed] [Google Scholar]
- 26.Suliman A, Lam A, Datta R, et al. Intracellular mechanisms of TRAIL: apoptosis through mitochondrial-dependent and -independent pathways. Oncogene. 2001;20:2122–33. doi: 10.1038/sj.onc.1204282. [DOI] [PubMed] [Google Scholar]
- 27.Gong B, Almasan A. Apo2 ligand/TNF-related apoptosis-inducing ligand and death receptor 5 mediate the apoptotic signaling induced by ionizing radiation in leukemic cells. Cancer Res. 2000;60:5754–60. [PubMed] [Google Scholar]
- 28.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuknecht H, Merchant S, Nadol J. Pathology of the ear. 2010:671–7. [Google Scholar]
- 30.Warnecke A, Wissel K, Hoffmann A, et al. The biological effects of cell-delivered brain-derived neurotrophic factor on cultured spiral ganglion cells. Neuroreport. 2007;18:1683–6. doi: 10.1097/WNR.0b013e3282f0b5d7. [DOI] [PubMed] [Google Scholar]
- 31.Richardson RT, O'Leary S, Wise A, et al. A single dose of neurotrophin-3 to the cochlea surrounds spiral ganglion neurons and provides trophic support. Hear Res. 2005;204:37–47. doi: 10.1016/j.heares.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Z, Liu Q, Davis RL. Complex regulation of spiral ganglion neuron firing patterns by neurotrophin-3. J Neurosci. 2005;25:7558–66. doi: 10.1523/JNEUROSCI.1735-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruel J, Chen C, Pujol R, et al. AMPA-preferring glutamate receptors in cochlear physiology of adult guinea-pig. J Physiol. 1999;518(Pt 3):667–80. doi: 10.1111/j.1469-7793.1999.0667p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puel JL, Ruel J, Guitton M, et al. The inner hair cell afferent/efferent synapses revisited: a basis for new therapeutic strategies. Adv Otorhinolaryngol. 2002;59:124–30. doi: 10.1159/000059250. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z, Peppi M, Kujawa SG, et al. Regulated expression of surface AMPA receptors reduces excitotoxicity in auditory neurons. J Neurophysiol. 2009;102:1152–9. doi: 10.1152/jn.00288.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Megerian CA. Diameter of the cochlear nerve in endolymphatic hydrops: implications for the etiology of hearing loss in Meniere's disease. Laryngoscope. 2005;115:1525–35. doi: 10.1097/01.mlg.0000167804.82950.9e. [DOI] [PubMed] [Google Scholar]
- 37.Anne S, Kisley LB, Tajuddin ST, et al. Molecular changes associated with the endolymphatic hydrops model. Otol Neurotol. 2007;28:834–41. doi: 10.1097/MAO.0b013e3180515381. [DOI] [PubMed] [Google Scholar]
- 38.Steinbach S, Lutz J. Glutamate induces apoptosis in cultured spiral ganglion explants. Biochem Biophys Res Commun. 2007;357:14–9. doi: 10.1016/j.bbrc.2007.03.098. [DOI] [PubMed] [Google Scholar]
- 39.Alam SA, Ikeda K, Oshima T, et al. Cisplatin-induced apoptotic cell death in Mongolian gerbil cochlea. Hear Res. 2000;141:28–38. doi: 10.1016/s0378-5955(99)00211-7. [DOI] [PubMed] [Google Scholar]
- 40.Feng H, Yin SH, Tang AZ, et al. Caspase-3 activation in the guinea pig cochlea exposed to salicylate. Neurosci Lett. 479:34–9. doi: 10.1016/j.neulet.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 41.Lang H, Liu C. Apoptosis and hair cell degeneration in the vestibular sensory epithelia of the guinea pig following a gentamicin insult. Hear Res. 1997;111:177–84. doi: 10.1016/s0378-5955(97)00098-1. [DOI] [PubMed] [Google Scholar]
- 42.Huang T, Cheng AG, Stupak H, et al. Oxidative stress-induced apoptosis of cochlear sensory cells: otoprotective strategies. Int J Dev Neurosci. 2000;18:259–70. doi: 10.1016/s0736-5748(99)00094-5. [DOI] [PubMed] [Google Scholar]
- 43.Nakagawa T, Yamane H, Takayama M, et al. Apoptosis of guinea pig cochlear hair cells following chronic aminoglycoside treatment. Eur Arch Otorhinolaryngol. 1998;255:127–31. doi: 10.1007/s004050050027. [DOI] [PubMed] [Google Scholar]
- 44.Ylikoski J, Xing-Qun L, Virkkala J, et al. Blockade of c-Jun N-terminal kinase pathway attenuates gentamicin-induced cochlear and vestibular hair cell death. Hear Res. 2002;163:71–81. doi: 10.1016/s0378-5955(01)00380-x. [DOI] [PubMed] [Google Scholar]
- 45.Matsui JI, Ogilvie JM, Warchol ME. Inhibition of caspases prevents ototoxic and ongoing hair cell death. J Neurosci. 2002;22:1218–27. doi: 10.1523/JNEUROSCI.22-04-01218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsui JI, Haque A, Huss D, et al. Caspase inhibitors promote vestibular hair cell survival and function after aminoglycoside treatment in vivo. J Neurosci. 2003;23:6111–22. doi: 10.1523/JNEUROSCI.23-14-06111.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staecker H, Liu W, Malgrange B, et al. Vector-mediated delivery of bcl-2 prevents degeneration of auditory hair cells and neurons after injury. ORL J Otorhinolaryngol Relat Spec. 2007;69:43–50. doi: 10.1159/000096716. [DOI] [PubMed] [Google Scholar]