Abstract
There is growing interest in the diverse signaling pathways that regulate and affect breast tumorigenesis, including the role of phytochemicals and the emerging role of microRNAs (miRNAs). Recent studies demonstrate that miRNAs regulate fundamental cellular and developmental processes at the transcriptional and translational level under normal and disease conditions. While there is growing evidence to support the role of phytoalexin-mediated miRNA regulation of cancer, few reports address this role in breast cancer. Recent reports by our group and others demonstrate that natural products, including stilbenes, curcumin, and glyceollins, could alter the expression of specific miRNAs, which may lead to increased sensitivity of cancer cells to conventional anti-cancer agents and, therefore, hormone-dependent and hormone-independent tumor growth inhibition. This review will discuss how dietary intake of natural products, by regulating specific miRNAs, contribute to the prevention and treatment of breast cancer.
Keywords: Phytoalexins, microRNA, breast cancer, estrogen
Phytoalexins consist of a class of low molecular weight compounds that accumulate in plants in response to biological stress including wounding, freezing, pathogens, UV light, and exposure to agricultural chemicals. They are thought to be involved in providing plants with resistance to microbial invasion and colonization. Phytoalexins have been identified in at least 75 plant species including alfalfa, blueberries, celery, eggplant, garlic, grapes, peanuts, pepper, potato, rice, soybeans and tomatoes. Preclinical data suggest phytoalexins modulate cellular processes that impinge on cancer including proliferation, apoptosis, invasion and metastasis, hormonal regulation, and expression/activity of Phase I and II metabolizing enzymes. Phytoalexins have also been demonstrated to target signal transduction pathways,1 nuclear receptors,2–4 apoptosis, and cell cycle pathways.5,6 While multiple epidemiological and animal studies have shown that consumption of foods rich in fruits and vegetables decreased the occurrence of cancers,7–10 a better understanding of the molecular mechanisms by which phytoalexins regulate these processes is critical towards an improved understanding of their potential use as preventative and/or adjunctive agents in the clinical setting.
A specific class of endogenous, non-coding RNAs, classified as microRNAs (miRNAs), has been identified. It has been found that most miRNAs are conserved across species,11 indicating that they participate in normal biological processes. They are single-stranded RNAs of 21–25 nucleotides in length12 and found to regulate mRNA stability and translation by targeting the 3′ UTR of target mRNAs and either degrade mRNA transcripts or induce translational silencing13 (Figure 1). More than 1000 miRNAs have been identified in the human genome, and over one-third of all human protein coding genes are potentially regulated by miRNAs,14 MiRNAs regulate the expression of genes involved in development, growth, proliferation and apoptosis. The inhibition of the miRNA biogenesis pathway results in severe developmental defects and lethality in many organisms.15 They are associated with many biological processes and disease states16,17 including all stages of cancer18 from initiation to tumor promotion and progression. Several miRNAs have been functionally classified as oncogenes or tumor suppressers or act to regulate transcription factors (Table 1). Recent studies demonstrated that knock-down or the re-expression of specific miRNAs could induce drug sensitivity, inhibit the proliferation of cancer cells, and suppress cancer cell invasion and metastasis.19–21 Experimental evidence demonstrates that correction of specific miRNA alterations using miRNA mimics or antagomirs can normalize gene regulatory networks and signaling pathways and reverse the phenotype of cancerous cells.22
Figure 1.
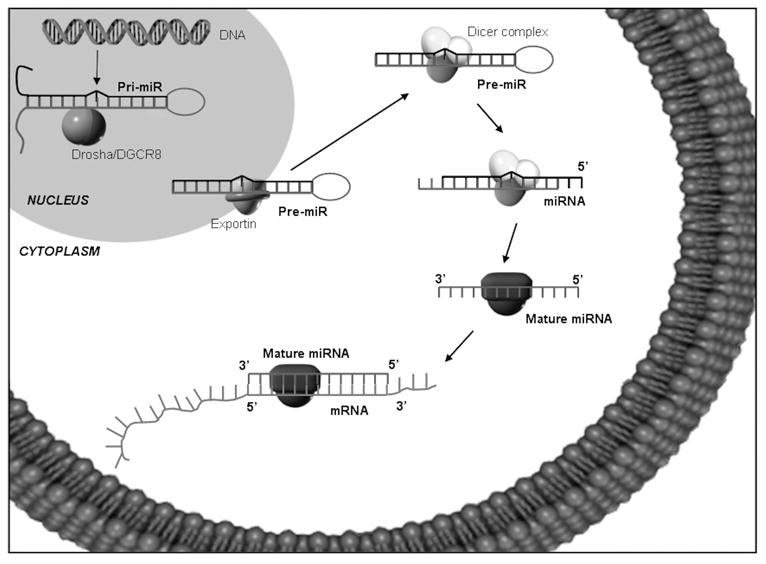
MicroRNAs (miRNAs).
Table 1.
miRNAs THAT HAVE BEEN FUNCTIONALLY CLASSI FIED AS ONCOGENES OR TUMOR SU PPRESERS, OR THAT ACT TO REGULATE TRA NSCRIPTION FACTORS
| Phytoalexin Agent | Altered miRNA [Reference] | Targets/Function |
|---|---|---|
| Curcumin | ↑miR-22 [50] | ER, Sp1 |
| ↑miR-15a/16 [53] | Bcl-2 | |
| ↓miR-196 [50] | Oncogenic | |
| ↓miR-186 [51] | Caspase 10 | |
| ↓miR-21 [52] | AP-1 | |
| Glyceollins | ↑miR-22 [61 | EVI-1, ERBB3, CDC25/Tumor suppression |
| ↑miR-29b/c [61] | MYBL2/Cell Senescence | |
| ↑miR-30d [61] | Tumor suppression | |
| ↑miR-34a [61] | Tumor suppression | |
| ↑miR-195 [61] | RAF1, CCND1, CCNE, BCL2 | |
| ↑miR-181c/d [61] | NOTCH4, KRAS | |
| ↓miR-21 [61] | PTEN, PDCD4, TIMP3, TIMP1/OncomiR | |
| ↓miR-193a-5p [61] | p73/Limits the effectiveness of chemotherapy | |
| ↓miR-224 [61] | PAK4, MMP-9, CD40/Cell migration and invasion | |
| ↓miR-486-5p [61] | CD40/Cell migration and invasion | |
| ↓miR-542-5p [61] | Maintenance of a mesenchymal phenotype | |
| Resveratrol | ↓miR-155 [42] | Oncogenic |
| ↑miR-663 [42] | AP-1 | |
| ↑miR-141 [45] | Tumor suppression | |
| ↑miR-200c [45] | Tumor suppression | |
| ↑miR-16 [45] | Tumor suppression | |
| ↑miR-143 [45] | Tumor suppression |
While the number of publications on miRNAs in humans has increased exponentially since the first reports in 2001,23–25 there are few studies on phytoalexin regulation of miRNAs. Since the initial discovery of miRNAs, there has been progress towards therapeutic applications, and several natural and synthetic chemoprevention agents have also been evaluated as modulators of miRNA expression in different cancer types.26–29 These studies demonstrate, natural products, including curcumin, isoflavones, indole-3-carbinol (I3C), diindolylmethane (DIM), and epigallocatechin gallate (EGCG), could alter the expression of specific miRNAs, which may lead to the increased sensitivity of cancer cells to conventional anti-cancer agents thereby inhibiting tumor growth. As such, it has been proposed that manipulating miRNA regulation may be a novel avenue for developing efficient therapies against cancer. As breast cancer is defined as a disease of health disparity it is becoming increasing important to understand how these dietary agents and miRNAs can play a role in prevention and treatment outcomes of underserved populations. Therefore, the objective of this review is to summarize research on phytoalexin regulation of miRNAs and how dietary intake of natural products contributes to the prevention and treatment of breast cancer by regulating specific miRNAs, with a focus on human breast cancer cell line studies.
The Effect of Stilbene and Stilbene Derivatives on miRNA Regulation
Resveratrol (3, 5, 4′-trihydroxystilbene) is a polyphenolic, non-flavanoid bioactive compound that can be derived from fruits and plants, including the skin of red grapes, blueberries, and peanuts. It has been shown to exhibit a wide range of health-promoting benefits for the coronary, neurological, hepatic, and cardiovascular systems30–34 and to enhance stress resistance and extend the lifespan. It has been shown to inhibit inflammation, viral infection, oxidative stress, and platelet aggregation.35–37 Resveratrol has also been identified as a potent anti-cancer agent, exerting antiproliferative and proapoptotic properties in a variety of conditions, including cancer and has been shown to inhibit aromatase activity in aromatase transfected MCF-7 breast cancer cells (MCF-7aro).38 Numerous investigations utilizing various ER-positive (T47D and MCF-7) and ER-negative breast cancer cell lines (MDA-MB-231 and MDA-MB-468) demonstrated that resveratrol can upregulate p53, PTEN, and p27 while downregulating multiple targets involved in cell migration.39–41
Recent studies have shown that natural products, including curcumin, isoflavones, EGCG, I3C, and DIM, could alter the expression of specific miRNAs. For example, Tili et al., showed that resveratrol decreases the levels of miR-155 by upregulating miR-663, a tumor suppressor miRNA potentially targeting Jun B and JunD implicated in the immune response.42 Resveratrol also modulates the expression of a large number of miRNAs in prostate cancer, including that of the c-MYC activated miR-17-92 cluster (miR-17, miR-18b, miR-20a, miR-20b, and miR-92b) and miR-106ab clusters.43 Resveratrol treatment has also been shown to downregulate several oncogenic miRNAs and effectors of TGF-beta signaling pathways in human SW480 colon cancer cells.44 While the effect of resveratrol on miRNA regulation of various cancers has been demonstrated, the precise molecular mechanism of miRNA induction in breast cancer and the biological significance of resveratrol-induced miRNA have not been reported until recently.
In a recent report, Hagiwara et al., demonstrated that resveratrol upregulated tumor-suppressive miRNAs (i.e., miR-16, miR-141, miR-143, miR-200c, miR-340), in MDA-MB-231 breast cancer cells resulting in the induction of an anti-cancer effect against the cancer stem-like cells (CSC) phenotype in cancer cells.45 They also demonstrated that resveratrol inhibited the invasiveness of breast cancer cells as one of the CSC phenotypes by activating miR-141 and miR-200c. Treatment with, resveratrol also led to a reduction of malignancy by not only activating tumor-suppressive miRNA transcription but also by enhancing the RNAi activity mediated by the induction of Ago2 (a protein involved in posttranscriptional gene silencing). While resveratrol was effective in promoting an Ago2-dependent tumor-suppressive miRNA anti-cancer response in MDA-MB-231 cells, pterostilbene, an analogue of resveratrol suppressed cell growth more dramatically than resveratrol. This finding suggested resveratrol-induced tumor-suppressive miRNA expression and its anti-cancer activity in breast cancers are conserved among stilbene family members. Taken together, these results suggest a potential therapeutic role for resveratrol and its derivatives in the prevention of breast cancer by upregulating tumor suppressive miRNAs and regulating miRNA biogenesis.
The Effect of Curcumin on miRNA Regulation
Curcumin is a diferuloylmethane derived from the Indian spice tumeric (also called curry powder) and has been demonstrated to possess anti-inflammatory and antioxidant properties. Curcumin has been shown to exhibit therapeutic potential against a variety of cancers including leukemia and lymphoma, gastrointestinal cancers, genitourinary cancers, ovarian cancer, head and neck squamous cell carcinoma, lung cancer, melanoma, neurological cancers, and sarcoma. For example, in gastric cancer, curcumin suppresses proliferation and invasion pathways by repressing HER2 and G1/S phase transition, PAK1 activity and cyclin D1 expression.46 In prostate cancer, curcumin induces apoptosis47 and inhibits invasion in human laryngeal squamous carcinoma cells.48 Additionally, researchers have described the anticarcinogenic and antimetastatic properties of curcumin in a variety of breast cancer cell lines.49
As previously demonstrated, extensive research has been conducted establishing the complexity, involvement and alteration of multiple signal transduction pathways in cancer growth and progression. As our understanding of the complexities regulating cancer cell proliferation increases it is becoming more evident that targeting a single gene product or cell signaling pathway is unlikely to prevent or treat cancer. This suggests that drugs interacting with multiple targets will be more efficacious than current monotargeted anticancer drugs. As many plant-derived dietary agents have been demonstrated to exert multitargeted effects, increased interests in their use as therapeutic agents have ensued. More recently, curcumin has been demonstrated to possess an additional level of regulation, by altering miRNA expression in a variety of human cancers. For example, in human pancreatic cancer cells, curcumin upregulates miR-22, whose predicted targets are estrogen receptor α (ERα) and the transcription factor Sp1, while the oncogenic miR-196 was significantly downregulated.50 Curcumin also induces apoptosis in A549 lung cancer cells by downregulating miR-186, which targets caspase-1051 while in colorectal cancer cells, curcumin inhibited the transcriptional regulation of miR-21 via AP-1 and suppressed cell proliferation, tumor growth, invasion and metastasis.52 These data were therefore instrumental in providing a rationale for designing current and ongoing clinical trials for colorectal cancer prevention and pulmonary disease.
While several groups demonstrated the effect of curcumin on miRNA expression in various cancers, currently, few studies exist on the effect of curcumin-mediated miRNA regulation of breast cancer. While it is known that some antiapoptotic proteins such as Bcl-2 are overexpressed in a wide variety of cancers, recent evidence by Yang et al., demonstrate that curcumin caused a miR-15a and miR-16-mediated downregulation of bcl-2 induced apoptosis in MCF-7, SKBR-3 and Bcap-37 breast cancer cell lines.53 Interestingly a similar pattern of miR-15a/16 regulation of bcl-2 was also observed in leukemia.54 Taken together, these results indicate that the miR-15a/16 family can potentially serve as potential gene targets for bcl-2 overexpressing breast tumors.
The Effect of Glyceollins on miRNA
There is considerable evidence demonstrating potential beneficial effects of dietary phytoestrogens for many hormone-dependent conditions.55–57 Soy foods made from whole soybeans, or isolated soy proteins all contain relatively high concentrations of isoflavones. Isoflavones act as partial estrogen agonists and antagonists. Therefore, in addition to studying their use as chemotherapeutic agents, numerous researchers have studied the potential role of soy-based compounds as alternative therapeutic agents to conventional hormone replacement therapy in postmenopausal women.
Recently, our group identified the glyceollins as a novel group of phytoalexins isolated from activated soy demonstrated to be a novel antiestrogen that binds to the ER and inhibits estrogen-induced MCF-7 tumor xenograft progression.4,58 We reported that glyceollins, a novel groups of soy phytoalexins,59 inhibit the expression of estrogen dependent genes (i.e., Progesterone Receptor and stromal-cell derived factor-1), inhibit the ER transcriptional activity of MCF-7 cells, bind to the ER with high affinity, and inhibit the viability and proliferation of MCF-7 cells.4 We also demonstrated the antiproliferative effects of glyceollins in ER-positive BG-1 ovarian cancer cells4 as well as prostate cancer cells,60 suggesting alternative ER-independent mechanisms may be involved in glyceollins’ antitumorigenic activity.
While the effect of glyceollins has been demonstrated in breast cancer, until recently its hormonal role has been mostly limited to estrogen-dependent cells. To investigate the ER-independent antiproliferative mechanism of glyceollin, we performed xenograft studies using triple negative breast cancer cells (MDA-MB231 and MDA-MB468 cells). Interestingly, our results demonstrated that glyceollin treatment caused a modest reduction in tumorigenesis in both cell lines,61 a result of alterations in the miRnome and proteomic expression profile. Specifically, glyceollin-treated MDA-MB231 cells demonstrated a significant increase in miRNAs involved in EMT (miR-22, miR-29b, miR-29c, miR-30d, miR-34a and miR-195) and tumor suppressors (miR-181c and miR-181d). There was also a significant decrease in the expression of oncomiRs promoting tumorigenesis (miR-21and miR-193a-5p), oncomiRs promoting metastasis (miR-185 and miR- 224), miR-486-5p (involved in cell migration and invasion) and miR-542-5p (involved in maintenance of a mesenchymal phenotype). Upon further analysis, it was determined that the metastasis suppressor gene, non-metastatic cells 1 (NME1) demonstrated a 24.89-fold increase after glyceollin treatment and is targeted by both miR-486-5p and miR-542-5p. Proteomic analysis further demonstrated a (glyceollin-induced) 13.84-fold decrease in vimentin, a protein involved in epithelial to mesenchymal phenotype which is targeted by miR-30d. This further suggests that miRNAs involved in cell motility and maintenance of a mesenchymal phenotype are downregulated and therefore alter the proteome if the MDA-MB231 cells in a manner that is indicative of tumor suppressive effects. Taken together, these studies demonstrate for the first time the ability of the phytochemical, glyceollin, to inhibit the tumor growth of triple negative breast cancer cells. More importantly, the mechanism by which glyceollin exerts its antineoplastic effect is through dysregulation of miRNA expression which is a hallmark of numerous carcinomas including breast cancer and consistent with a less aggressive phenotype.
Conclusion
In conclusion, our data and that of others show that phytoalexins display unique activities that regulate miRNAs and the expression of multiple disease states, including cancer. It is clear these small, non-coding RNA molecules could inhibit target gene expression by binding the 3′UTR of target mRNA, resulting in either RNA degradation or inhibition of translation. To this end, aberrant miRNA expression has been correlated with tumor development, cancer progression, the formation of CSCs and the acquisition of EMT phenotype. For the first time, our group identified glyceollins as a novel phytoalexin with the ability to alter miRNAs regulating EMT and metastasis in breast cancer. In addition, the in vitro and in vivo data in this study may implicate a therapeutic potential for glyceollins in African American TNBC patients. Further results from others revealed the ability of resveratrol and curcumin to alter miRNAs involved in breast cancer prevention and apoptosis, respectively. These results indicate a mechanism by which dietary agents can modulate the activity of miRNAs crucial for cancer progression. MiRNAs have been characterized as the biomarkers for diagnosis and prognosis, and thus miRNAs are becoming attractive targets for cancer therapy. Emerging evidence is beginning to suggest that natural agents could be useful for targeting miRNAs, which in turn could enhance the efficacy of conventional cancer therapies. Therefore, targeting miRNAs by glyceollins, curcumin and resveratrol could be novel strategies toward designing combination approaches with conventional therapies for the prevention of tumor recurrence and achieving successful treatment outcome of patients diagnosed with cancer.
Acknowledgments
This publication was made possible by funding from the Louisiana Cancer Research Consortium and the NIH-RCMI grant #5G12RR026260 from the National Institute on Minority Health and Health Disparities. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the Louisiana Cancer Research Consortium or the NIH.
Notes
- 1.Pan MH, Lin YT, Lin CL, et al. Suppression of Heregulin-{beta}1/HER2-Modulated Invasive and Aggressive Phenotype of Breast Carcinoma by Pterostilbene via Inhibition of Matrix Metalloproteinase-9, p38 Kinase Cascade and Akt Activation. Evid Based Complement. Alternat Med. 2011;2011:562187. doi: 10.1093/ecam/nep093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowers JL, Tyulmenko VV, Jernigan SC, et al. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology. 2000 Oct;141(10):3657–67. doi: 10.1210/endo.141.10.7721. [DOI] [PubMed] [Google Scholar]
- 3.Payton-Stewart F, Khupse RS, Boué SM, et al. Glyceollin I enantiomers distinctly regulate ER-mediated gene expression. Steroids. 2010 Dec;75(12):870–8. doi: 10.1016/j.steroids.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerman MC, Tilghman SL, Boue SM, et al. Glyceollin I, a novel antiestrogenic phytoalexin isolated from activated soy. J Pharmacol Exp Ther. 2010 Jan;332(1):35–45. doi: 10.1124/jpet.109.160382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alkhalaf M. Resveratrol-induced apoptosis is associated with activation of p53 and inhibition of protein translation in T47D human breast cancer cells. Pharmacology. 2007;80(2–3):134–43. doi: 10.1159/000103253. [DOI] [PubMed] [Google Scholar]
- 6.Sakamoto T, Horiguchi H, Oguma E, et al. Effects of diverse dietary phytoestrogens on cell growth, cell cycle and apoptosis in estrogen-receptor-positive breast cancer cells. J Nutr Biochem. 2010 Sep;21(9):856–64. doi: 10.1016/j.jnutbio.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Reddy L, Odhav B, Bhoola KD. Natural products for cancer prevention: A global perspective. Pharmacol Ther. 2003 Jul;99(1):1–13. doi: 10.1016/s0163-7258(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 8.Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992;18(1):1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 9.Freedman ND, Park Y, Subar AF, et al. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int J Cancer. 2008 May 15;122(10):2330–6. doi: 10.1002/ijc.23319. [DOI] [PubMed] [Google Scholar]
- 10.Steinmetz KA, Potter JD. Vegetables, fruit, and cancer prevention: a review. J Am Diet Assoc. 1996 Oct;96(10):1027–39. doi: 10.1016/S0002-8223(96)00273-8. [DOI] [PubMed] [Google Scholar]
- 11.Saxena S, Jonsson ZO, Dutta A. Small RNAs with imperfect match to endogenous mRNA repress translation. Implications for off-target activity of small inhibitory RNA in mammalian cells. J Biol Chem. 2003 Nov 7;278(45):44312–9. doi: 10.1074/jbc.M307089200. [DOI] [PubMed] [Google Scholar]
- 12.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004 Jan 23;116(2):281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 13.Cuellar TL, McManus MT. MicroRNAs and endocrine biology. J Endocrinol. 2005 Dec;187(3):327–32. doi: 10.1677/joe.1.06426. [DOI] [PubMed] [Google Scholar]
- 14.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010 Sep;11(9):597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 15.Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008 Mar;9(3):219–30. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- 16.Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009 Feb 20;136(4):642–55. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ventura A, Jacks T. MicroRNAs and cancer: short RNAs go a long way. Cell. 2009 Feb 20;136(4):586–91. doi: 10.1016/j.cell.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nana-Sinkam SP, Croce CM. MicroRNAs as therapeutic targets in cancer. Transl Res. 2011 Apr;157(4):216–25. doi: 10.1016/j.trsl.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Kong D, Li Y, Wang Z, et al. miR-200 regulates PDGF-D-mediated epithelial-mesenchymal transition, adhesion, and invasion of prostate cancer cells. Stem Cells. 2009 Aug;27(8):1712–21. doi: 10.1002/stem.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao JJ, Lin J, Yang H, et al. MicroRNA-221/222 negatively regulates estrogen receptor alpha and is associated with tamoxifen resistance in breast cancer. J Biol Chem. 2008 Nov 7;283(45):31079–86. doi: 10.1074/jbc.M806041200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Takeshita F, Patrawala L, Osaki M, et al. Systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors via downregulation of multiple cell-cycle genes. Mol Ther. 2010 Jan;18(1):181–7. doi: 10.1038/mt.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang V, Wu W. MicroRNA-based therapeutics for cancer. BioDrugs. 2009;23(1):15–23. doi: 10.2165/00063030-200923010-00002. [DOI] [PubMed] [Google Scholar]
- 23.Lee RC, Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science. 2001 Oct 26;294(5543):862–4. doi: 10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- 24.Lau NC, Lim LP, Weinstein EG, et al. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001 Oct 26;294(5543):858–62. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 25.Lagos-Quintana M, Rauhut R, Lendeckel W, et al. Identification of novel genes coding for small expressed RNAs. Science. 2001 Oct 26;294(5543):853–8. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 26.Sun M, Estrov Z, Ji Y, et al. Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol Cancer Ther. 2008 Mar;7(3):464–73. doi: 10.1158/1535-7163.MCT-07-2272. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, VandenBoom TG, 2nd, Kong D, et al. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine- resistant pancreatic cancer cells. Cancer Res. 2009 Aug 15;69(16):6704–12. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melkamu T, Zhang X, Tan J, et al. Alteration of microRNA expression in vinylcarbamate-induced mouse lung tumors and modulation by the chemopreventive agent indole-3-carbinol. Carcinogenesis. 2010 Feb;31(2):252–8. doi: 10.1093/carcin/bgp208. [DOI] [PubMed] [Google Scholar]
- 29.Tsang WP, Kwok TT. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells. J Nutr Biochem. 2010 Feb;21(2):140–6. doi: 10.1016/j.jnutbio.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Jang M, Cai L, Udeani GO, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997 Jan 10;275(5297):218–20. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 31.Bradamante S, Barenghi L, Villa A. Cardiovascular protective effects of resveratrol. Cardiovasc Drug Rev. 2004 Fall;22(3):169–88. doi: 10.1111/j.1527-3466.2004.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang Q, Xu J, Rottinghaus GE, et al. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002 Dec 27;958(2):439–47. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- 33.Sinha K, Chaudhary G, Gupta YK. Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats. Life Sci. 2002 Jun 28;71(6):655–65. doi: 10.1016/s0024-3205(02)01691-0. [DOI] [PubMed] [Google Scholar]
- 34.Inoue H, Jiang XF, Katayama T, et al. Brain protection by resveratrol and fenofibrate against stroke requires peroxisome proliferator-activated receptor alpha in mice. Neurosci Lett. 2003 Dec 11;352(3):203–6. doi: 10.1016/j.neulet.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Pace-Asciak CR, Rounova O, Hahn SE, et al. Wines and grape juices as modulators of platelet aggregation in healthy human subjects. Clin Chim Acta. 1996 Mar 15;246(1–2):163–82. doi: 10.1016/0009-8981(96)06236-5. [DOI] [PubMed] [Google Scholar]
- 36.Fauconneau B, Waffo-Teguo P, Huguet F, et al. Comparative study of radical scavenger and antioxidant properties of phenolic compounds from Vitis vinifera cell cultures using in vitro tests. Life Sci. 1997;61(21):2103–10. doi: 10.1016/s0024-3205(97)00883-7. [DOI] [PubMed] [Google Scholar]
- 37.Jang DS, Kang BS, Ryu SY, et al. Inhibitory effects of resveratrol analogs on unopsonized zymosan-induced oxygen radical production. Biochem Pharmacol. 1999 Mar 15;57(6):705–12. doi: 10.1016/s0006-2952(98)00350-5. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Lee KW, Chan FL, et al. The red wine polyphenol resveratrol displays bilevel inhibition on aromatase in breast cancer cells. Toxicol Sci. 2006 Jul;92(1):71–7. doi: 10.1093/toxsci/kfj190. [DOI] [PubMed] [Google Scholar]
- 39.Alkhalaf M. Resveratrol-induced apoptosis is associated with activation of p53 and inhibition of protein translation in T47D human breast cancer cells. Pharmacology. 2007;80(2–3):134–43. doi: 10.1159/000103253. [DOI] [PubMed] [Google Scholar]
- 40.Pozo-Guisado E, Alvarez-Barrientos A, Mulero-Navarro S, et al. The antiproliferative activity of resveratrol results in apoptosis in MCF-7 but not in MDA-MB-231 human breast cancer cells: cell-specific alteration of the cell cycle. Biochem Pharmacol. 2002 Nov 1;64(9):1375–86. doi: 10.1016/s0006-2952(02)01296-0. [DOI] [PubMed] [Google Scholar]
- 41.Pozo-Guisado E, Merino JM, Mulero-Navarro S, et al. Resveratrol-induced apoptosis in MCF-7 human breast cancer cells involves a caspase-independent mechanism with downregulation of Bcl-2 and NF-kappaB. Int J Cancer. 2005 May 20;115(1):74–84. doi: 10.1002/ijc.20856. [DOI] [PubMed] [Google Scholar]
- 42.Tili E, Michaille JJ, Adair B, et al. Resveratrol decreases the levels of miR-155 by upregulating miR-663, a microRNA targeting JunB and JunD. Carcinogenesis. 2010 Sep;31(9):1561–6. doi: 10.1093/carcin/bgq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dhar S, Hicks C, Levenson AS. Resveratrol and prostate cancer: promising role for microRNAs. Mol Nutr Food Res. 2011 Aug;55(8):1219–29. doi: 10.1002/mnfr.201100141. [DOI] [PubMed] [Google Scholar]
- 44.Tili E, Michaille JJ, Alder H, et al. Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGFβ signaling pathway in SW480 cells. Biochem Pharmacol. 2010 Dec 15;80(12):2057–65. doi: 10.1016/j.bcp.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hagiwara K, Kosaka N, Yoshioka Y, et al. Stilbene derivatives promote Ago2-dependent tumour-suppressive microRNA activity. Sci Rep. 2012;2:314. doi: 10.1038/srep00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai XZ, Wang J, Li XD, et al. Curcumin suppresses proliferation and invasion in human gastric cancer cells by downregulation of PAK1 activity and cyclin D1 expression. Cancer Biol Ther. 2009 Jul;8(14):1360–8. doi: 10.4161/cbt.8.14.8720. [DOI] [PubMed] [Google Scholar]
- 47.Shankar S, Srivastava RK. Involvement of Bcl-2 family members, phosphatidylinositol 3′-kinase/AKT and mitochondrial p53 in curcumin (diferuloylmethane)-induced apoptosis in prostate cancer. Int J Oncol. 2007 Apr;30(4):905–18. [PubMed] [Google Scholar]
- 48.Mitra A, Chakrabarti J, Banerji A, et al. Curcumin, a potential inhibitor of MMP-2 in human laryngeal squamous carcinoma cells HEp2. J Environ Pathol Toxicol Oncol. 2006;25(4):679–90. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i4.70. [DOI] [PubMed] [Google Scholar]
- 49.Anaund P, Sundaram C, Jhurani S, et al. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 2008 Aug 18;267(1):133–64. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 50.Sun M, Estrov Z, Ji Y, et al. Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol Cancer Ther. 2008 Mar;7(3):464–73. doi: 10.1158/1535-7163.MCT-07-2272. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Du Y, Wu C, et al. Curcumin promoted apoptosis in human lung adenocarcinoma cells through miR-186* signaling pathway. Oncol Rep. 2010 Nov;24(5):1217–23. doi: 10.3892/or_00000975. [DOI] [PubMed] [Google Scholar]
- 52.Mudduluru G, George-William JN, Muppala S, et al. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci Rep. 2011 Jun;31(3):185–97. doi: 10.1042/BSR20100065. [DOI] [PubMed] [Google Scholar]
- 53.Yang J, Cao Y, Sun J, et al. Curcumin reduces the expression of Bcl-2 by upregulating miR-15a and miR-16 in MCF-7 cells. Med Oncol. 2010 Dec;27(4):1114–8. doi: 10.1007/s12032-009-9344-3. [DOI] [PubMed] [Google Scholar]
- 54.Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005 Sep 27;102(39):13944–9. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Setchell KD. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr. 1998 Dec;68(6 Suppl):1333S–1346S. doi: 10.1093/ajcn/68.6.1333S. [DOI] [PubMed] [Google Scholar]
- 56.Murkies A. Phytoestrogens-what is the current knowledge? Aust Fam Physician. 1998 Jan;27( Suppl 1):S47–51. [PubMed] [Google Scholar]
- 57.Setchell KD, Cassidy A. Dietary isoflvones: biological effects and relevance to human health. J Nutr. 1999 Mar;129(3):758S–767S. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- 58.Salvo VA, Boué SM, Fonseca JP, et al. Antiestrogenic glyceollins suppress human breast and ovarian carcinoma tumorigenesis. Clin Cancer Res. 2006 Dec 1;12(23):7159–64. doi: 10.1158/1078-0432.CCR-06-1426. [DOI] [PubMed] [Google Scholar]
- 59.Tilghman SL, Boué SM, Burow ME. Glyceollins, a novel class of antiestrogenic phytoalexins. Mol Cell Pharmacol. 2010;2(4):155–60. [Google Scholar]
- 60.Payton-Stewart F, Schoene NW, Kim YS, et al. Molecular effects of soy phytoalexin glyceollins in human prostate cancer cells LNCaP. Mol Carcinog. 2009 Sep;48(9):862–71. doi: 10.1002/mc.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rhodes LV, Tilghman SL, Boue SM. Glyceollins as novel targeted therapeutic for the treatment of triple-negative breast cancer. Oncol Lett. 2012 Jan;3(1):163–71. doi: 10.3892/ol.2011.460. [DOI] [PMC free article] [PubMed] [Google Scholar]


