Abstract
Triple negative breast cancer (TNBC) is subtype of breast disease devoid of the estrogen, progesterone, and Her2/neu receptors which are targets for pharmacological intervention. There is a need for novel anti-breast cancer agents that target TNBC. Therefore, novel isochalcone DJ52 was evaluated using the alamar blue dye exclusion assay, the luciferase colony assay, and xenograft models to determine its efficacy and potency. DJ52 significantly decreased proliferation of cells measured by using the alamar blue dye method and produced IC50 values of DJ52, DJ56, and DJ82 at 10-6M, 10-5M, and 10-5M, respectively. In vivo studies were conducted by injecting MDA-MB-231 cells into SCID mice to determine tumor regression was measured over 20 days. DJ52 at 50mg/kg caused significant decrease in tumor volume (p value <.05) by nearly 50% compared with the control with vehicle alone. These data suggest that DJ52 has merit for further evaluation as a novel anticancer agent.
Keywords: Triple negative breast cancer, isochalcone, chalcone
Breast cancer is the leading cause of cancer death in the United States and the most common form of malignant cancer diagnosed in women.1 At least five subtypes (luminal A, luminal B, basal-like, ERBB2, and normal-like) have been identified on the basis of the pattern of expression.2 The luminal A and luminal B subtypes express ER+ tumours, while the basal-like, ERBB2 and normal-like subclasses express ER− tumours. Interestingly, the luminal subtype A exhibits a more favorable prognosis, while the luminal B tumours present a worse prognosis. The basal-like and ERBB2 subsets show the worst clinical outcome.3,4 The triple negative breast cancer (TNBC) is defined as breast epithelial cancer cells which lack the HER-2/neu (HER2) receptor over-expression, the estrogen receptor (ER) and the progesterone receptor (PR). These patients do not respond to either the typical hormonal treatments tailored to the ER and PR or the inhibition of the EGFR.5 TNBC comprises between 7%–20% of all breast cancers and patients typically have a very poor prognosis. Chemotherapy benefits some patients, but the relapse rate in these patients is very high and the survival rate is extremely low. Patients that present with these tumor types have a higher mortality rate than any other ethnic group.1 According to reports from the Americana Cancer Society, Although breast cancer is found to occur in all races, the rate of TNBC is higher in incidence within African American women than in other races, creating a disparity among this population.6 Therefore there is a need for novel pharmacological intervention and novel targets for this subclass of breast disease.
Chalcones are a group of drugs that are known as phytochemicals and have demonstrated anticancer activity, such as blueberry extracts, soy, garcinol, and curcumin.6 There have been several recent studies that various chalcones possess antiproliferative activity against various types of human breast cancers.6,7 Therefore, a series of novel chalcones, chemicals derived from natural products, were developed. These agents were screened in TNBC cell lines MDA-MB-231 and MDA-MB-468 for antiproliferative activity. Therefore, this study was to determine if these novel isochalcones have antiproliferative effects in triple negative breast cancer cells by to evaluating DJ compounds for growth inhibition in breast cancer cell lines MDA-MB231 and MDA-MB-468; and 2) measuring the effect of novel isochalcones in decreasing the tumor volume of MDA-MB-231 cells injected into SCID xenograft mouse models.
Methods
Cell lines and cell culture
Breast cancer cell lines MDA-MB-231 and MDA-MB-468 were purchased from American Type Culture Collection. All cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 250 mg/ml Amphotericin B and 10,000 I.U./ml Penicillin/Streptomycin. Cells were incubated at 37° C, 5% CO2 in a humidified incubator Cells were passaged with 0.05% Trypsin-EDTA every 2–3 days.
Colon assay
MDA-MB 231 and MDA-MB 468 cells were placed in phenol red containing DMEM supplemented with 10% FBS. 1×103 cells/well were plated in 6-well plates and treated with 10−12 M, 10−9 M and 10−6 M DJ52 or vehicle (DMSO) stimulation. Cells were incubated at 37° C, 5% CO2 in a humidified incubator for 10 to 14 days. Ten to 14 days later, cells were fixed with formaldehyde for 30 min and then stained with crystal violet 0.1% in methanol 20% for 30 min. The number of colonies were counted using ImageJ software. The data were reported as percentage of control ± standard error of the mean (SEM).
Dose response assay
Pharmacological treatments were performed in 96-well plates in assay medium containing each of the respective drugs. The alamar blue assay was done to treated cell lines to determine the IC50. The cancer cell lines MBA MB 468, MBA MB 231, and MCF-7 were harvested with trypsin and plated on to separate 96 well plates with 5000 cells per well. The cells were allowed 24 hours to recover from plating. After 24 hours, the cells were treated with novel agents in concentrations ranging from 10−3 M to 10−9 M and the alamar blue dye was added to each well at 10% its total volume and incubated for 48hrs. Biotek Synergy 4 Plate Reader was used to measure absorbance and background wavelengths at 550nm and 630nm to determine proliferation and calculated as a percent of the controls as follows:
Data are the mean ± SEM of triplicate determinations.
In vivo studies
Twenty female SCID mice 3–4 weeks old were purchased from Jackson Laboratories and housed five animals per cage with food and water ad libetum with 12 hour light/dark cycles. The density of MDA-MB-231 cells injected into the mammary fat pad was 5 × 105 cells and the tumors were allowed to form over 10–14 days. The tumor volumes were determined by caliper measurements and calculated as a percent of the untreated control tumors. Measurements were taken every day for 24 days.
EGF-stimulated inhibition of A431 cells
A431 cells were grown in RPMI with 10% FBS or in media containing 10% BSA. The cells were plated at a density of 5000 cells/well and were exposed to DJ56, the parent compound, genestein, and tyrophospstin for 48 hours in concentrations of 1–104 nM. Alamar blue was added to each well and measured as described above. Data are the mean ± SEM of triplicate determinations.
Results
DJ52, DJ53, and DJ56 demonstrated an IC50 at 10−6M in MDA-MB-468 cells. DJ82 demonstrated an IC50 at 10−3 M. Neither DJ64 nor control drug tamoxifen demonstrated to inhibit the growth of these cells by at least 50%. See Figure 1.
Figure 1.
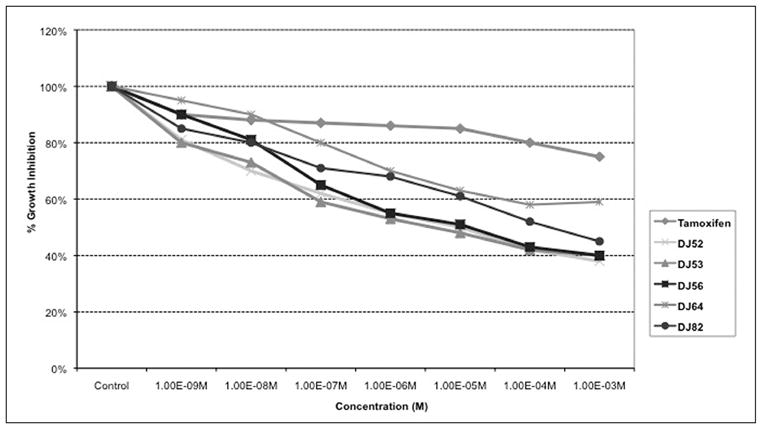
Dose response curve of metastatic human breast cancer MDA-MB-468 cells.
This graph (Figure 2) indicated that DJ52 caused an IC50 at 10−6 M. DJ56, DJ64, and DJ82 all demonstrate an IC50 at approximately the millimolar concentration. 5-fluorouracil demonstrated a drastic decrease in proliferation at the 10−3 M concentration.
Figure 2.
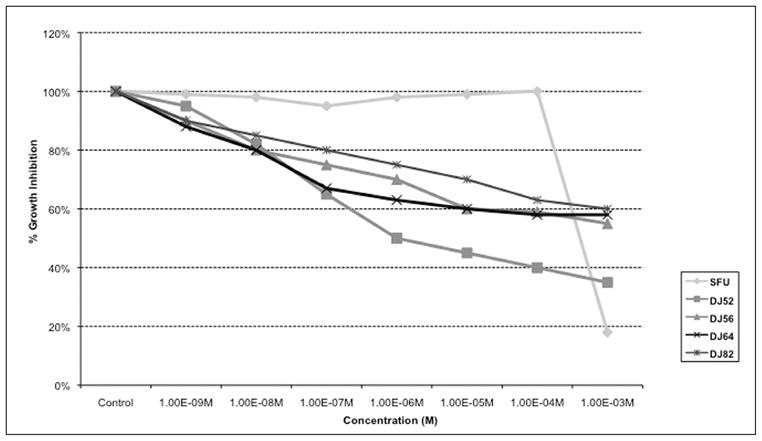
Dose response curve of human breast cancer MDA-MB-231 cells.
Colony formation assays were performed in both MDA-MB231 and MDA-MB468 triple negative breast cancer cells to further examine the mechanism of action of the isochalcone DJ52. (See Figure 3.) Results demonstrate that DJ52 caused a dose dependent decrease in the cellular viability of MDA-MB 231. At the 10−6 M concentration, cellular viability was 32% of the control value. Interestingly, the 10−6 M dose of DJ52 caused a modest decrease in the viability of MDA-MB 468 cells, which was approximately 67% of the control value. As the MDA-MB 231 cells were more responsive to the antiproliferative properties of DJ52, this may suggest that DJ52 has enhanced selectivity for MDA-MB 231 cells over MDA-MB 468 cells.
Figure 3.
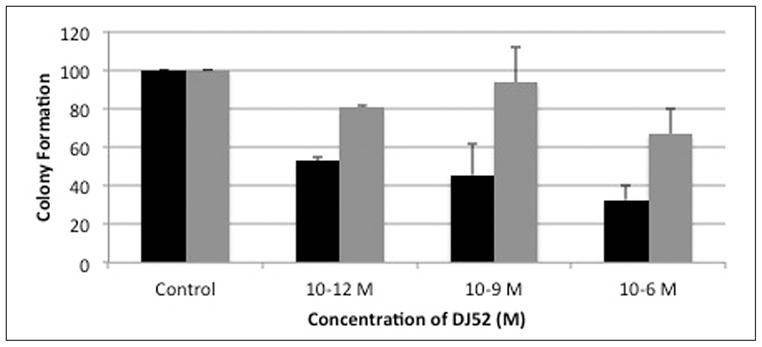
DJ 52 causes dose-dependent decrease in growth measure by colony assay.
Tumor volumes of MDA-MB-231 cells in the SCID mice treated with DJ52 demonstrated 50% tumor regression with the average tumor volume at 60 mm3 after 21 days of treatment (Figure 4). The tumor volume in the untreated control mice doubled over the same period when compared with the experimental group.
Figure 4.
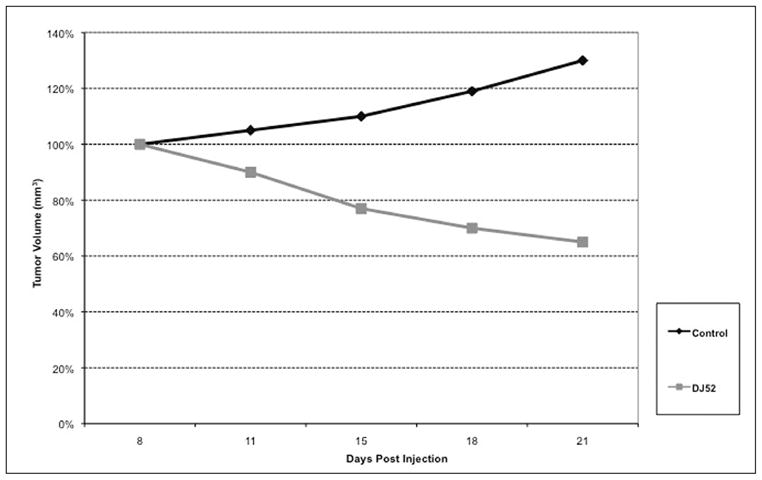
Effects of DJ52 on MDA-MB-231 tumors in SCID mice.
Parent novel compound DJ56 demonstrated significant decrease of tyrosine kinase activity in A431 cells with an IC50 at 10−3 nM concentration (Figure 5). Genestein demonstrated an IC50 at the same concentration. Tyrophostin demonstrated an IC50 at 10−4 nM. Data are the mean ± SEM of triplicate determinations.
Figure 5.
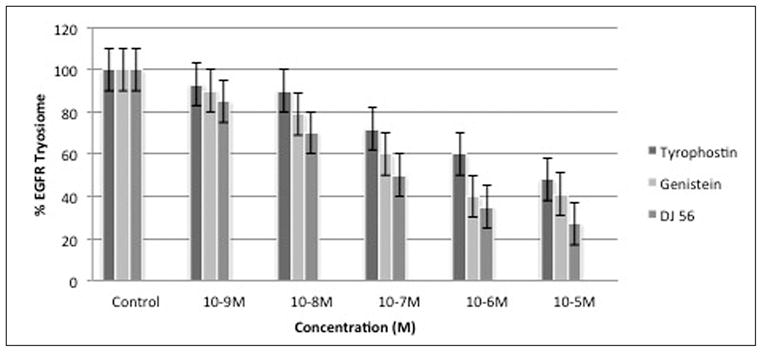
%EGF-receptor tyrosine kinase phosphorylation of serum-stimulated A431 cells.
Discussion
Triple negative breast cancer is an aggressive subtype of breast disease that demonstrates poor prognosis in patients. Chalcones are derivatives from soy and have been reported to have both antiproliferative and chemopreventative properties.7,8 Isochalcones are precursors to the flavonoids that are synthesized by plants. Chalcones have been reported to inhibit proliferation in MCF-7 and MDA-MB231 cells by blocking cell cycle progression.9 Chalcones licochalcone-A, isoliquiritigenin and flavokawain A are found to induce apoptosis and cell cycle arrest in cancer cell lines.10,11 Some flavonoids have been reported to induce apoptosis or decrease COX-2 expression.12,13,14,15
DJ52 was reported to decrease both proliferation and COX-2 expression in androgen dependent prostate cancer cell line LNCaP, which suggest that the DJ compounds work through a mechanism other than as antiestrogens or anti-androgens because proliferation is decreased in cell line models from both hormone sensitive and hormone refractory disease.16 In Figure 1, DJ52 significantly decreased the proliferation of MDA-MB-468 cells when compared with DJ56, DJ82, DJ64, DJ53, and tamoxifen (p<.05). It is demonstrated in Figure 2 that indicates that DJ56 demonstrated an IC50 value at the millimolar concentration, as did 5-flurouracil (5FU). DJ52, however, indicated an IC50 value at the micromolar concentration. The colony assay in Figure 3 confirms the antiproliferative effects of DJ52 in both MDA-MB-231 and MDA-MB-468 cells. However, DJ52 was more selective for the MDA-MB-231 cells by causing a more significant antiprolifertive effect in this model (p<.05). Figure 4 demonstrates that by evaluating MDA-MB-231 tumors that developed when these cells were injected into the mammary fat pad of SCID mice, the tumor volumes decreased significantly over 21 days compared with the untreated control. DJ52 has demonstrated to both slow the growth of TNBC cells, but also to cause death in MDA-MB-231 cells as indicated in Figure 3, which is significant. Chalcones have been reported to cause cell death in MCF-7 cells, which are estrogen positive.17,18,19 There are some reports that indicated that chalcones may have a role in increasing ROS levels.20,21,22 Figure 5 demonstrated that when parental compound DJ56 was compared with potent inhibitors of tyrosine kinase stimulation, genestein and tyrophostin, DJ56 significantly decreased the binding of the EGF receptor to its ligand. These data suggest that receptor tyrosine kinase inhibition may be involved with the mechanism of action of these novel agents. However, the mechanism of action for these DJ compounds is not fully understood. The receptor tyrosine kinase pathway is a potential signaling pathway to evaluate for pharmacological intervention in patients with triple negative breast cancer. Although African American women, many of whom may be poor and have limited access to medical care, have a lower breast cancer incidence, their mortality rate from breast cancer is significantly higher that that of their White counterparts. Therefore, these data suggest that DJ52 has merit for further investigations for its antiproliferative effects in triple negative breast cancer and to evaluate DJ52 for its mechanism of action to identify potential biomarkers for pharmacological intervention to relieve the burden of disease in this population.
Acknowledgments
The authors wish to acknowledge funding from the Komen for the Cure grant # SG-06-0126-DR2, Office of Naval Research grant # N00014-10-1-0270 and the cell culture core facility supported by the Louisiana Cancer Research Consortium and RCMI grant #NIH 1G12RR026260.
Notes
- 1.National Cancer Institute. Surveillance epidemiology and end results: SEER stat fact sheets—breast. Bethesda, MD: National Cancer Institute; 2012. [Google Scholar]
- 2.Bertucci F, Finetti P, Cervera N, et al. How basal are triple-negative breast cancers? Int J Cancer. 2008 Jul;123(1):236–40. doi: 10.1002/ijc.23518. [DOI] [PubMed] [Google Scholar]
- 3.Bhargava R, Gerald WL, Li AR, et al. EGFR gene amplification in breast cancer: correlation with epidermal growth factor receptor mRNA and protein expression and HER-2 status and absence of EGFR-activating mutations. Mod Pathol. 2005 Aug;18(8):1027–33. doi: 10.1038/modpathol.3800438. [DOI] [PubMed] [Google Scholar]
- 4.Calza S, Hall P, Auer G, et al. Intrinsic molecular signature of breast cancer in a population-based cohort of 412 patients. Breast Cancer Res. 2006;8(4):R34. doi: 10.1186/bcr1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007 Aug;13(15 Pt 1):4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 6.Padhye S, Ahmad A, Oswal N, et al. Fluorinated 2′-hydroxychalcones as garcinol analogs with enhanced antioxidant and anticancer activities. Bioorg Med Chem Lett. 2010 Oct 1;20(19):5818–21. doi: 10.1016/j.bmcl.2010.07.128. [DOI] [PubMed] [Google Scholar]
- 7.Syam S, Abdelwahab SI, Al-Mamary MA, et al. Synthesis of chalcones with anticancer activities. Molecules. 2012 May 25;17(6):6179–95. doi: 10.3390/molecules17066179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacquemier J, Padovani L, Rabayrol L, et al. Typical medullary breast carcinomas have a basal/myoepithelial phenotype. J Pathol. 2005 Nov;207(3):260–8. doi: 10.1002/path.1845. [DOI] [PubMed] [Google Scholar]
- 9.Jemal A, Center MM, Ward E, et al. Cancer occurrence. Methods Mol Biol. 2009;471:3–29. doi: 10.1007/978-1-59745-416-2_1. [DOI] [PubMed] [Google Scholar]
- 10.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 11.Livasy CA, Karaca G, Nanda R, et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006 Feb;19(2):264–71. doi: 10.1038/modpathol.3800528. [DOI] [PubMed] [Google Scholar]
- 12.Lau GT, Ye L, Leung LK. The licorice flavonoid isoliquiritigenin suppresses phorbol ester-induced cyclooxygenase-2 expression in the non-tumorigenic MCF-10A breast cell line. Planta Med. 2010 May;76(8):780–5. doi: 10.1055/s-0029-1240699. [DOI] [PubMed] [Google Scholar]
- 13.Minami CA, Chung DU, Chang HR. Management options in triple-negative breast cancer. Breast Cancer (Auckl) 2011;5:175–99. doi: 10.4137/BCBCR.S6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perou CM, Borresen-Dale AL. Systems biology and genomics of breast cancer. Cold Spring Harb Perspect Biol. 2011 Feb;3(2) doi: 10.1101/cshperspect.a003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rakha EA, El-Rehim DA, Paish C, et al. Basal phenotype identifies a poor prognostic subgroup of breast cancer of clinical importance. Eur J Cancer. 2006 Dec;42(18):3149–56. doi: 10.1016/j.ejca.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Rakha EA, El-Sayed ME, Green AR, et al. Prognostic markers in triple-negative breast cancer. Cancer. 2007 Jan;109(1):25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 17.Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008 Jan;52(1):108–18. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 18.Johnson KP, Rowe GC, Jackson BA, et al. Novel antineoplastic isochalcones inhibit the expression of cyclooxygenase 1,2 and EGF in human prostate cancer cell line LNCaP. Cell Mol Biol (Noisy-le-grand) 2001 Sep;47(6):1039–45. [PubMed] [Google Scholar]
- 19.Adams LS, Phung S, Yee N, et al. Blueberry phytochemicals inhibit growth and metastatic potential of MDA-MB-231 breast cancer cells through modulation of the phosphatidylinositol 3-kinase pathway. Cancer Res. 2010 May 1;70(9):3594–605. doi: 10.1158/0008-5472.CAN-09-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mercader AG, Pomilio AB. (Iso)flav(an)ones, chalcones, catechins, and theaflavins as anticarcinogens: mechanisms, anti-multidrug resistance and QSAR Studies. Curr Med Chem. 2012;19(25):4324–47. doi: 10.2174/092986712802884277. [DOI] [PubMed] [Google Scholar]
- 21.Solomon VR, Lee H. Anti-breast cancer activity of heteroaryl chalcone derivatives. Biomed Pharmacother. 2012 Apr;66(3):213–20. doi: 10.1016/j.biopha.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Jainey P, Bhat I. Antitumor, analgesic, and anti-inflammatory activities of synthesized pyrazolines. J Young Pharm. 2012 Apr;4(2):82–7. doi: 10.4103/0975-1483.96621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahu NK, Balbhadra SS, Choudhary J, et al. Exploring pharmacological significance of chalcone scaffold: a review. Curr Med Chem. 2012;19(2):209–25. doi: 10.2174/092986712803414132. [DOI] [PubMed] [Google Scholar]
- 24.Spatafora C, Tringali C. Natural-derived polyphenols as potential anticancer agents. Anticancer Agents Med Chem. 2012 Oct 1;12(8):902–18. doi: 10.2174/187152012802649996. [DOI] [PubMed] [Google Scholar]


