Abstract
Background
Although the performance of immunocytology has been established in the surveillance of patients with urothelial carcinoma of the bladder (UCB), its value in the initial detection of UCB in patients with painless hematuria remains unclear.
Objective
To determine whether immunocytology improves our ability to predict the likelihood of UCB in patients with painless hematuria. Further, to test the clinical benefit of immunocytology in this setting using decision curve analysis.
Design, setting, and participants
The subjects were 1182 consecutive patients without a history of UCB presenting with painless hematuria and were enrolled at three centres.
Intervention
All patients underwent upper-tract imaging, cystourethroscopy, voided urine cytology, and immunocytology analysis. Bladder tumors were biopsied and histologically confirmed as UCB.
Measurements
Multivariable regression models were developed. Area under the curve was measured and compared using the DeLong test. A nomogram was constructed from the full multivariable model. Decision curve analysis was performed to evaluate the clinical benefit associated with use of the multivariable models including immunocytology.
Results and limitations
Immunocytology had the largest contribution to a multivariable model for the prediction of UCB (odds ratio: 18.3; p < 0.0001), which achieved a 90.8% predictive accuracy. Decision curve analysis revealed that models incorporating immunocytology achieved the highest net benefit at all threshold probabilities.
Conclusions
Immunocytology is a strong predictor of the presence of UCB in patients who present with painless hematuria. Incorporation of immunocytology into predictive models improves diagnostic accuracy by a statistically and clinically significant margin. The use of immunocytology in the diagnostic workup of patients with hematuria appears promising and should be further evaluated.
Keywords: Cystoscopy, Decision curve analysis, Early detection of cancer, Hematuria, Immunocytology, Nomograms, Urinary bladder neoplasms
1. Introduction
The diagnostic workup of patients with painless hematuria generally includes imaging of the upper urinary tract, urine cytology, and cystourethroscopy. Although cystourethroscopy is both sensitive and specific, its drawbacks include invasiveness and cost. Despite international guidelines suggesting urologic evaluation for most patients with hematuria [1,2], many patients are not appropriately referred to urologists [3,4]. Increased awareness of bladder cancer risk for patients with hematuria and appropriate use of urinary biomarkers may help to more accurately risk-stratify patients and ensure improved referral patterns.
Urine cytology has reasonable sensitivity for high-grade tumors but is not adequate for detecting low-grade tumors, which represent the most common type of urothelial carcinoma of the bladder (UCB) [5]. Moreover, it suffers from interrater variability, and its performance depends on cytopathologists’ expertise [6,7]. These limitations have generated a good deal of interest in the development and validation of other urinary biomarkers for UCB. One candidate biomarker is immunocytology, which uses three fluorescently labelled monoclonal antibodies directed against UCB-associated antigens (carcinoembryonic antigen and two bladder tumor–associated mucins). Previous multicentre studies have demonstrated that immunocytology has a higher sensitivity than urinary cytology in the surveillance of UCB patients, especially for low-grade tumors [5,8], leading to US Food and Drug Administration approval for its use in conjunction with cytology for surveillance. Several small single-institution studies have suggested that immunocytology may also perform well in the detection of UCB in patients with painless hematuria [9,10]. Therefore, we conducted a tri-institutional study to assess the performance of immunocytology in the detection setting. We used decision curve analysis to assess the benefit of prediction tools incorporating immunocytology for clinical decision making.
2. Materials and methods
2.1. Patients
The study was performed at three sites: EuromedClinic/Urologie24 (Fürth/Nürnberg, Germany), University of Tübingen (Tübingen, Germany), and General Hospital of Bolzano (Bolzano, Italy). Institutional review boards approved the study protocol for each site. Between 2000 (Fürth, n = 434), 2002 (Bolzano, n = 309), 2006 (Tübingen, n = 439), and 2010, 1216 consecutive patients with newly diagnosed painless hematuria (without voiding symptoms) and without a history of urothelial carcinoma (UC) were included. Patient age, gender, smoking history, and degree of hematuria were recorded. Microscopic hematuria was defined as three or more erythrocytes per high-power field under white-light microscopy from two of three properly collected urine specimens. Thirty-four patients were excluded from the analyses for inconclusive results, 14 for immunocytology, 11 for cytology, and 9 for absence of both. A retrospective analysis of prospectively collected data was performed.
Midstream urine specimens were collected, immediately processed, and subsequently examined cytologically and immunocytologically. Urine cytology was considered positive when malignant cells were present. All patients underwent clinical examination, including upper-tract imaging and cystourethroscopy, with biopsy of any suspicious lesions; they were considered positive for malignancy if histologically confirmed UCB was detected during initial cystourethroscopy or within the subsequent 3 mo. Histology and urine cytology slides were reviewed by genitourinary pathologists at each institution who had no knowledge of the clinical data. Pathologic stage and tumor grade were assigned according to the 2002 American Joint Cancer Committee TNM staging system and the 1998 World Health Organisation/International Society of Urologic Pathology grading system [11–14].
2.2. Immunocytology
uCyt+/ImmunoCyt (Scimedx, Denville, NJ, USA), a commercially available assay, was performed according to the manufacturer’s protocol, as previously described [9]. Positive and negative controls were performed with each test run. Specimens with more than one green or red urothelial cell were considered immunocytologically positive. A minimum of 500 cells needed to be analysed before a test was considered negative. All samples were processed and analysed by experienced staff members with no knowledge of the clinical data. More than 1000 immunocytology analyses are performed annually at each of the three study sites.
2.3. Statistical analysis
Descriptive statistics were calculated. Logistic regression analyses evaluated the associations between UCB and predictor variables. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated from the models. The area under the curve (AUC) method was used to quantify the predictive accuracy of each individual variable and of the combined multivariable model. All AUC estimates were internally validated using 200 bootstrap samples [15]. The DeLong test was used to evaluate the increments in AUC achieved by successively adding cytology and immunocytology results to a multivariable model. Regression coefficients from the multivariable model were used to generate a predictive nomogram [16]. A calibration plot was fitted to evaluate the extent of over- or underestimation of the observed UCB rate from the multivariable model.
Decision curve analysis was used to explore the clinical value of the multivariable models [17]. Decision curve analysis is a method for evaluating the clinical “net benefit” of prediction models; one sums the benefits (true positives) and subtracts the harms (false positives). Because the value of a true positive (eg, early detection of UCB) may differ from the disadvantages resulting from a false positive (eg, avoidable cystourethroscopy), the net benefit differentially weights true and false positives by using the threshold probability at which a patient (or provider) would opt for cystourethroscopy. For example, if a patient (or provider) would opt for cystourethroscopy with a 10% risk of UCB but would forgo cystourethroscopy with only a 9% risk, then the threshold probability is 10%. The best model displays higher net benefits throughout the clinically applicable range of threshold probabilities.
All p values are two-sided, with statistical significance evaluated at the 0.05 alpha level. Analyses were performed in SAS v.9.2 statistical software (SAS Institute, Cary, NC, USA). The nomogram and calibration plot were constructed in the R v.2.10.1 software environment (The R Foundation for Statistical Computing). Decision curve analysis was performed in the Stata v.11.0 statistical package (StataCorp, College Station, TX, USA).
3. Results
The cohort included 919 men and 263 women (male-to-female ratio: 3.5) whose median age was 65 yr (range: 18–93). Sixty-eight percent of subjects presented with microscopic hematuria and 32% with gross hematuria. Forty-eight percent of subjects were current or past smokers. Overall, 245 of 1182 (20.7%) subjects had UCB; 138 (58.7%) had low-grade tumors, and 97 (41.3%) had high-grade tumors. Stage distribution of the tumors was as follows: 160 (65.8%) pTa, 13 (5.3%) pTis, 44 (18.1%) pT1, 20 (8.2%) pT2, 3 (1.2%) pT3, and 3 (1.2%) pT4. Other aetiologies for hematuria included cystitis or urinary tract infection (UTI; n = 142), urolithiasis (n = 122), locally invasive or metastatic nonurothelial malignancies (n = 51), medical renal disease (n = 39), upper-tract UC (n = 16), prostatitis (n = 16), and renal cell carcinoma (n = 6).
Of the 1182 patients, 162 (13.7%) had positive cytology, 328 (27.7%) had positive immunocytology, and 150 (12.7%) had positive results for both tests. Stage distribution of immunocytology-positive versus immunocytology-negative cases was 128 (63.7%) versus 32 (76.2%) pTa, 12 (6.0%) versus 1 (2.4%) pTis, 38 (18.9%) versus 6 (14.3%) pT1, and 17 (8.5%) versus 3 (7.1%) pT2, respectively. Stage distribution of cytology-positive versus cytology-negative cases was 56 (49.6%) versus 104 (80.0%) pTa, 9 (8.0%) versus 4 (3.1%) pTis, 25 (22.1%) versus 19 (14.6%) pT1, and 17 (15.0%) versus 3 (2.3%) pT2, respectively. All six pT3 and pT4 tumors were positive for both immunocytology and cytology.
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of urinary cytology for predicting UCB were 46.5%, 94.9%, 70.4%, and 87.2%, respectively. The sensitivity, specificity, PPV, and NPV of immunocytology for predicting UCB were 82.4%, 86.6%, 61.6%, and 95.0%, respectively. Subgroup analyses of immunocytology in gross (n = 378) versus microscopic (n = 804) hematuria yielded similar performance characteristics in univariable (sensitivity 84% vs 81%; specificity 84% vs 88%; NPV 92% vs 96%) and multivariable analyses (OR: 20.2 vs 16.8; both p values <0.0001).
Univariable logistic regression analyses identified older age, male gender, past or current smoking history, gross hematuria, positive urine cytology, and positive immunocytology as predictors of UCB presence (Table 1). Abnormal upper-tract imaging was not associated with UCB presence (OR: 0.56; p = 0.07), but it was strongly associated with the presence of upper-tract UC (abnormal imaging in 15 of 16 patients). Immunocytology had the highest AUC (84.5%), followed by urine cytology (70.8%) and age (64.5%).
Table 1.
Univariable logistic regression analyses assessing the association between predictor variables and the presence of bladder cancer in 1182 patients with painless hematuria*
| Predictors of UCB | OR | 95% CI | p value | AUC, % |
|---|---|---|---|---|
| Age (continuous) | 1.04 | (1.03–1.06) | <0.0001 | 64.5 |
| Gender (male vs female) | 1.49 | (1.04–2.15) | 0.03 | 52.3 |
| Smoker (past or current vs never) | 3.38 | (2.49–4.58) | <0.0001 | 64.4 |
| Hematuria (gross vs microscopic) | 2.47 | (1.85–3.30) | <0.0001 | 60.3 |
| Cytology (positive vs negative) | 16.12 | (10.98–23.66) | <0.0001 | 70.8 |
| Immunocytology (positive vs negative) | 30.24 | (20.70–44.17) | <0.0001 | 84.5 |
UCB = urothelial carcinoma of the bladder; OR = odds ratio; CI = confidence interval; AUC = area under the curve.
AUC estimates are based on internal validation using 200 bootstrap samples.
In addition to age, smoking history, and gross hematuria, immunocytology (OR: 18.26; p < 0.0001) and cytology (OR: 2.92; p < 0.0001) were independently associated with UCB in multivariable analyses (Table 2). We also analysed the data by centre. In multivariable analyses adjusting for the effects of age, gender, smoking status, type of hematuria (gross vs microscopic), and cytology, immunocytology was an independent predictor of UCB presence within each centre (Bolzano cohort: OR: 23.3; p < 0.0001; Fürth/Nürnberg cohort: OR: 16.3; p < 0.0001; Tübingen cohort: OR; 10.4; p < 0.0001).
Table 2.
Multivariable logistic regression analyses assessing the association between predictor variables and the presence of bladder cancer in 1182 patients with painless hematuria*
| Predictors | Base model
|
Model 1
|
Model 2
|
Model 3
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | OR | 95% CI | p value | OR | 95% CI | p value | |
| Age (continuous) | 1.04 | (1.03–1.06) | <0.0001 | 1.03 | (1.02–1.05) | <0.0001 | 1.03 | (1.02–1.05) | <0.0001 | 1.03 | (1.01–1.05) | <0.0001 |
| Gender (male vs female) | 1.22 | (0.83–1.80) | 0.31 | 1.10 | (0.72–1.68) | 0.66 | 1.00 | (0.62–1.62) | 0.99 | 0.97 | (0.60–1.57) | 0.89 |
| Smoker (past or current vs never) | 3.17 | (2.30–4.35) | <0.0001 | 3.72 | (2.58–5.37) | <0.0001 | 3.38 | (2.27–5.03) | <0.0001 | 3.66 | (2.43–5.53) | <0.0001 |
| Hematuria (gross vs microscopic) | 1.90 | (1.40–2.58) | <0.0001 | 1.71 | (1.21–2.41) | 0.0020 | 1.71 | (1.16–2.51) | 0.0070 | 1.63 | (1.10–2.42) | 0.014 |
| Cytology (positive vs negative) | – | – | – | 14.71 | (9.70–22.28) | <0.0001 | – | – | – | 2.92 | (1.81–4.73) | <0.0001 |
| Immunocytology (positive vs negative) | – | – | – | – | – | – | 27.71 | (18.59–41.30) | <0.0001 | 18.26 | (11.80–28.24) | <0.0001 |
| AUC, % | – | – | 73.8% | – | – | 83.1% | – | – | 90.4% | – | – | 90.8% |
| p value; base vs 1, 1 vs 2, 2 vs 3 | – | – | – | – | – | <0.0001 | – | – | <0.0001 | – | – | 0.057 |
OR = odds ratio; CI = confidence interval; AUC = area under the curve.
AUC estimates are based on internal validation using 200 bootstrap samples.
The base multivariable model, which included age, gender, smoking status, and type of hematuria, predicted UCB presence with an AUC of 73.8%. The addition of cytology improved the AUC to 83.1% (+9.3%; p < 0.0001), while adding immunocytology improved the AUC to 90.4% (+16.6%; p < 0.0001). The addition of immunocytology to the model with cytology (model 1 plus immunocytology) significantly improved the AUC (+7.7%; p < 0.0001). In contrast, the addition of cytology to the model with immunocytology (model 2 plus cytology) did not (+0.4%; p = 0.06). Including information on abnormalities of the lower urinary tract detected on standard imaging did not improve the predictive accuracy of the model by a prognostically or clinically significant margin.
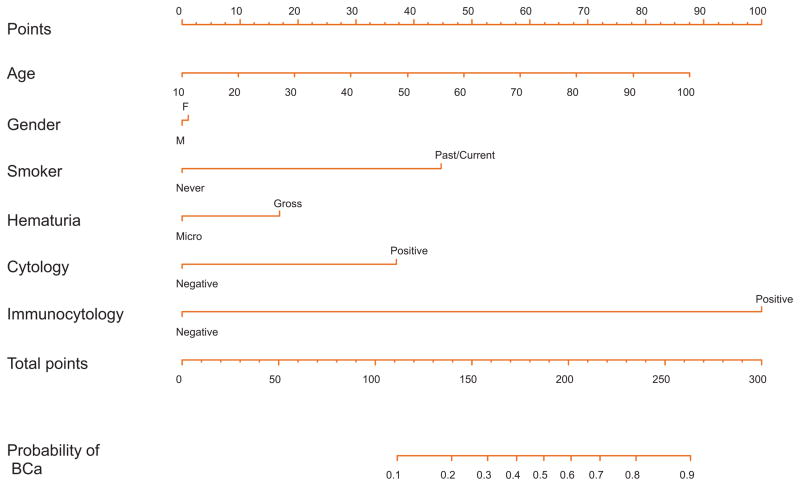
The nomogram for the full multivariable model, where age, gender, smoking status, type of hematuria, cytology, and immunocytology define the risk of having UCB, is shown in Figure 1. There is an approximately linear increase in the risk of UCB with advancing age. Immunocytology contributes the most risk points (100), followed by smoking history (45), urine cytology (37), and type of hematuria (17). The nomogram was well calibrated (Fig. 2), with minimal underestimation for individuals with a nomogram-predicted probability < 60% and slight overestimation for those with a nomogram-predicted probability >60%.
Fig. 1.
Nomogram for the prediction of bladder cancer (BCa) presence in patients with painless hematuria, where age, gender, smoking history, degree of hematuria, urine cytology, and immunocytology define the risk of BCa at cystourethroscopy.
Nomogram instructions: To obtain the nomogram-predicted probability of BCa at cystourethroscopy, locate patient values on each axis. Draw a vertical line to the Points axis to determine how many points are attributed for each variable value. Sum the points for all variables. Locate the sum on the Total Points line to assess the individual probability of BCa at cystourethroscopy on the Probability of BCa line.
BCa = bladder cancer.
Fig. 2.
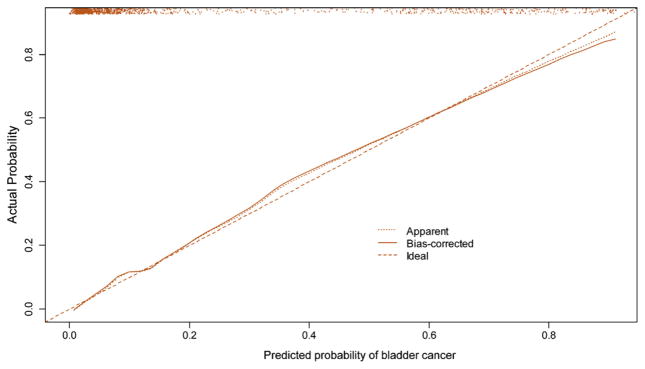
Calibration plot, where the x-axis represents the predicted probability and the y-axis represents the observed fraction of bladder cancer in 1182 patients with painless hematuria. The 45° dashed line represents ideal predictions, the solid line (bias-corrected) represents the internally validated predictions (using 200 bootstrap samples), and the dotted line (apparent) represents the uncorrected predictions. The scatter plot at the top of the figure shows the distribution of the individual nomogram-predicted probabilities.
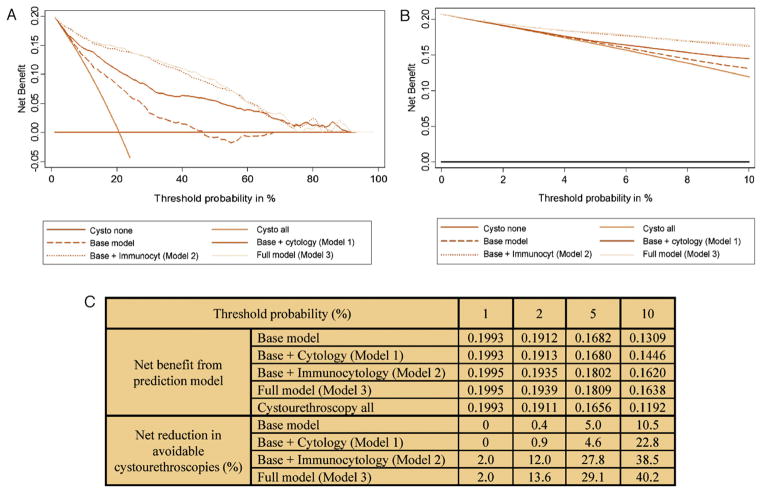
In the decision curve analysis, models containing immunocytology (model 2 and model 3) provide significantly higher net benefit than the model with cytology (model 1) up to threshold probabilities of 60% (Fig. 3a). The various prediction models offer net benefit over the “cystourethroscopy all” strategy at threshold probabilities >2% (Fig. 3b). At threshold probabilities of 2% and 5%, the full multivariable model, including immunocytology and cytology, resulted in a 13.6% and 29.1% net reduction in avoidable cystourethroscopies, respectively (Fig. 3c). The analyses in Table 3 show the clinical effects of using the prediction models with and without immunocytology. For example, using the full prediction model and only performing cystourethroscopy on patients with a predicted probability of UCB ≥2% could result in 211 fewer cystourethroscopies (18%) while missing only one cancer (0.4%).
Fig. 3.
(a) Decision curve analysis of the effect of prediction models for detection of urothelial carcinoma of the bladder (UCB) in 1182 patients with painless hematuria. Net benefit is plotted against threshold probabilities. (b) Expanded view of decision curves in the range of threshold probabilities from 1% to 10%. (c) Net benefit and reduction in avoidable cystourethroscopies for each model compared with the “cystourethroscopy all” strategy. Example of derivation of net benefit from prediction models in Figure 3c: At a 5% threshold, the value of 0.1809 (“net benefit from prediction model” for base model plus cytology plus immunocytology) is derived from the following 2 × 2 table. This table classifies patients with a predicted probability from the multivariable model ≥5% as positive for UCB and a predicted probability < 5% as negative for UCB (ie, the chosen threshold value). This predicted probability classification is then compared with the true UCB status for the patient.
| Model with cytology and immunocytology | BCa
|
Total | ||
|---|---|---|---|---|
| Absent | Present | |||
| Predicted probability | <5% (classified as negative) | 534 | 10 | 544 |
| ≥5% (classified as positive) | 403 | 235 | 638 | |
| Total | 937 | 245 | 1182 | |
Net benefit = true positive proportion − [false positive proportion × (0.05/0.95)] = 235/1182 − [403/1182 × 0.0526] = 0.1809
The reduction in the number of unnecessary cystourethroscopies per 100 patients is then calculated as follows: (net benefit of the model − net benefit of treat all)/(pt/(1 − pt)) × 100, where pt is the threshold probability. This value is net of false negatives and is therefore the equivalent of the reduction in unnecessary cystourethroscopies without a decrease in the number of patients with UCB who duly have cystourethroscopy.
Example of net reduction in avoidable cystourethroscopies in Fig. 3c: For base model + cytology + immunocytology at 5% threshold probability, we would get: (0.1809 − 0.1656)/(0.05/0.95) × 100 = 29.1% (29.1 avoidable cystourethroscopies per 100 patients).
Table 3.
Reduction in cystourethroscopies and number of cancers missed, according to the threshold probabilities of 1%, 2%, and 5% for the different models in 1182 patients with painless hematuria
| Cystourethroscopies
|
BCa
|
|||
|---|---|---|---|---|
| Performed (%) | Avoided (%) | Found (%) | Missed (%) | |
| Cystourethroscopy all | 1182 (100) | – | 245 (100) | – |
| Cystourethroscopy those with ≥1% risk of cancer | ||||
| Base model | 1182 (100) | – | 245 (100) | 0 (0) |
| Base model plus cytology | 1182 (100) | – | 245 (100) | 0 (0) |
| Base model plus immunocytology | 1159 (98) | 23 (2) | 245 (100) | 0 (0) |
| Base model plus cytology plus immunocytology | 1159 (98) | 23 (2) | 245 (100) | 0 (0) |
| Cystourethroscopy those with ≥2% risk of cancer | ||||
| Base model | 1177 (99.6) | 5 (0.4) | 245 (100) | 0 (0) |
| Base model plus cytology | 1171 (99) | 11 (1) | 245 (100) | 0 (0) |
| Base model plus immunocytology | 990 (84) | 192 (16) | 244 (99.6) | 1 (0.4) |
| Base model plus cytology plus immunocytology | 971 (82) | 211 (18) | 244 (99.6) | 1 (0.4) |
| Cystourethroscopy those with ≥5% risk of cancer | ||||
| Base model | 1103 (93) | 79 (7) | 244 (99.6) | 1 (0.4) |
| Base model plus cytology | 968 (82) | 214 (18) | 237 (97) | 8 (3) |
| Base model plus immunocytology | 634 (54) | 548 (46) | 234 (96) | 11 (4) |
| Base model plus cytology plus immunocytology | 638 (54) | 544 (46) | 235 (96) | 10 (4) |
BCa = bladder cancer.
4. Discussion
In this analysis of prospectively collected multicentre data, immunocytology was a strong predictor of UCB in patients with painless hematuria. This was true within each centre, despite differences in disease prevalence and severity. Immunocytology outperformed all other predictor variables, including cytology. Previous reports demonstrated a high sensitivity for immunocytology in the surveillance of patients with recurrent UCB [5,8,18], but there are only a few small studies of immunocytology for the initial detection of UCB [9,10]. In agreement with these prior studies, we demonstrated that immunocytology has good sensitivity and specificity. In addition, previous studies have only reported the sensitivity and specificity of cytology and immunocytology rather than combining the results with clinical risk factors.
Immunocytology improved our ability to predict UCB presence by a statistically significant margin. The multivariable models we developed demonstrate the increase in AUC obtained by adding immunocytology to urine cytology results and readily available clinical risk factors. In fact, the full multivariable model achieved an extremely high level of accuracy (bootstrap-corrected AUC: 90.8%) in predicting UCB. Therefore, we developed a nomogram that urologists and other health care providers can use to predict an individual’s risk of UCB. Prediction tools such as nomograms have been shown to perform better than clinical judgment [19,20]. That being said, physician input is obviously essential in medical decision-making, both for the measurement of predictive variables and for the interpretation and application of prediction tools in clinical practice.
The addition of immunocytology also improved our ability to predict UCB presence by a clinically (not only statistically) significant margin. To examine the potential clinical impact that the use of immunocytology provides, we performed a decision curve analysis [17]—a technique by which the consequences of clinical decisions are evaluated. For example, if a provider considers not performing cystourethroscopy on a patient with painless hematuria, the clinical consequences of performing a potentially unnecessary cystourethroscopy must be weighed against the risk of missing a tumor or other pathology. The threshold probability—the probability at which a physician (or patient) would opt for cystourethroscopy—is informative of how the physician weighs these relative harms. The addition of immunocytology to multivariable predictive models for UCB presence increased the net benefit for patients in the range of clinically relevant threshold probabilities. For example, if one decided to perform cystourethroscopy if the risk of UCB would be ≥2% based on the full model, one would spare 211 (18%) patients cystourethroscopy while missing only one low-grade, low-stage UCB (data not shown).
Immunocytology outperformed cytology in the detection of UCB, regardless of disease severity (data not shown). The performance of urine cytology, the most widely used biomarker in UCB, is limited in low-grade tumors and remains dependent on the skill of the cytopathologist [6,7]. The cytopathologists at the three centres in the current study are highly experienced. Nevertheless, immunocytology was more sensitive than cytology for the detection of UCB (82.4% vs 46.5%). Moreover, ruling out cancer is the most desired characteristic of an early detection biomarker. The NPV of immunocytology was higher than that of cytology (95.0% vs 87.2%). In addition, in univariable analyses, the OR and AUC of immunocytology were higher than those for cytology (30 vs 16 and 84.5% vs 70.8%, respectively). Furthermore, the addition of immunocytology to the base multivariable model improved its AUC by 17%, while the addition of cytology only resulted in a 9% improvement. Finally, the addition of immunocytology to a model, including clinical factors and cytology, significantly improved its AUC.
The current study has some potential limitations. The diagnostic performance of both cytology and immunocytology are dependent on pathologists’ skill and experience. This study was performed at high-volume cytology and immunocytology centres; therefore, the results might not be immediately generalisable. However, a previous study of immunocytology reported high interobserver concordance among pathologists after only 1 d of training [5]. Moreover, our nomogram was internally validated using a bootstrapping method [21]. External validation in a different population at other centres should be performed to confirm our findings before inclusion in daily clinical practice.
5. Conclusions
Immunocytology is a strong predictor of UCB presence in patients who present with painless hematuria, outperforming cytology. Incorporating immunocytology into multivariable prediction models for the detection of UCB dramatically increases their predictive accuracy by a statistically significant margin. This increment in predictive accuracy is clinically significant as evaluated by decision curve analysis. We developed highly accurate, well-calibrated nomograms to help in the clinical decision-making process regarding patient counseling, referral prioritisation, and possibly the extent of diagnostic workup for patients with painless hematuria. Further studies on the performance of immunocytology at different centres are needed before widespread utilisation in the early detection setting.
Acknowledgments
Funding/Support and role of the sponsor: None.
This research was performed under the auspices of the International Bladder Cancer Network (IBCN). We would like to thank Dr Andrew J. Vickers for his guidance regarding decision curve analysis, Dr Madhu Mazumdar for her supervision of the statistical analysis, and Kristin M Saunders for her editorial assistance.
Footnotes
Author contributions: Shahrokh F. Shariat had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Shariat, Schmitz-Drager, Stenzl, Pycha.
Acquisition of data: Tirsar, Schwentner, Mian, Hennenlotter, Martini.
Analysis and interpretation of data: Cha, Christos, Shariat, Schmitz-Drager.
Drafting of the manuscript: Cha, Shariat.
Critical revision of the manuscript for important intellectual content: Cha, Tirsar, Schwentner, Christos, Mian, Hennenlotter, Martini, Stenzl, Pycha, Shariat, Schmitz-Drager.
Statistical analysis: Cha, Christos, Shariat.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Stenzl, Pycha, Shariat, Schmitz-Drager.
Other (specify): None.
Financial disclosures: I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Dr. Paul J. Christos is a member of the Weill Cornell Cancer Center and was partially supported by the following grant: Clinical Translational Science Center (CTSC) (UL1-RR024996).
References
- 1.Grossfeld GD, Litwin MS, Wolf JS, Jr, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy—part II: patient evaluation, cytology, voided markers, imaging, cystoscopy, nephrology evaluation, and follow-up. Urology. 2001;57:604–10. doi: 10.1016/s0090-4295(01)00920-7. [DOI] [PubMed] [Google Scholar]
- 2.Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Böhle A, Palou-Redorta J. EAU guidelines on non–muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2008;54:303–14. doi: 10.1016/j.eururo.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 3.Johnson EK, Daignault S, Zhang Y, Lee CT. Patterns of hematuria referral to urologists: does a gender disparity exist? Urology. 2008;72:498–502. doi: 10.1016/j.urology.2008.01.086. discussion 502–3. [DOI] [PubMed] [Google Scholar]
- 4.Nieder AM, Lotan Y, Nuss GR, et al. Are patients with hematuria appropriately referred to urology? A multi-institutional questionnaire based survey. Urol Oncol. 2010;28:500–3. doi: 10.1016/j.urolonc.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Messing EM, Teot L, Korman H, et al. Performance of urine test in patients monitored for recurrence of bladder cancer: a multicenter study in the United States. J Urol. 2005;174:1238–41. doi: 10.1097/01.ju.0000173918.84006.4d. [DOI] [PubMed] [Google Scholar]
- 6.Karakiewicz PI, Benayoun S, Zippe C, et al. Institutional variability in the accuracy of urinary cytology for predicting recurrence of transitional cell carcinoma of the bladder. BJU Int. 2006;97:997–1001. doi: 10.1111/j.1464-410X.2006.06036.x. [DOI] [PubMed] [Google Scholar]
- 7.van Rhijn BWG, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol. 2005;47:736–48. doi: 10.1016/j.eururo.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Mian C, Maier K, Comploj E, et al. uCyt+/ImmunoCyt in the detection of recurrent urothelial carcinoma: an update on 1991 analyses. Cancer. 2006;108:60–5. doi: 10.1002/cncr.21712. [DOI] [PubMed] [Google Scholar]
- 9.Schmitz-Dräger BJ, Beiche B, Tirsar L-A, Schmitz-Dräger C, Bismarck E, Ebert T. Immunocytology in the assessment of patients with asymptomatic microhaematuria. Eur Urol. 2007;51:1582–8. doi: 10.1016/j.eururo.2006.10.046. discussion 1588. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz-Drager BJ, Tirsar LA, Schmitz-Drager C, et al. Immunocytology in the assessment of patients with asymptomatic hematuria. World J Urol. 2008;26:31–7. doi: 10.1007/s00345-007-0228-x. [DOI] [PubMed] [Google Scholar]
- 11.Greene FL. AJCC cancer staging manual. 6. NewYork: Springer-Verlag; 2002. American Joint Committee on Cancer, American Cancer Society. [Google Scholar]
- 12.Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22:1435–48. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Sauter G, Algaba F, Amin MB, et al. Noninvasive urothelial neoplasias: WHO classification of noninvasive papillary urothelial tumors. In: Eble JN, Sauter G, Epstein JI, editors. World Health Organization classification of tumors: pathology and genetics of tumors of the urinary system and male genital organs. Lyon: IARC Press; 2004. pp. 110–23. [Google Scholar]
- 14.Montironi R, Lopez-Beltran A, Scarpelli M, Mazzucchelli R, Cheng L. Morphological classification and definition of benign, preneoplastic and non-invasive neoplastic lesions of the urinary bladder. Histopathology. 2008;53:621–33. doi: 10.1111/j.1365-2559.2008.03025.x. [DOI] [PubMed] [Google Scholar]
- 15.Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 16.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565–74. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfister C, Chautard D, Devonec M, et al. Immunocyt test improves the diagnostic accuracy of urinary cytology: results of a French multicenter study. J Urol. 2003;169:921–4. doi: 10.1097/01.ju.0000048983.83079.4c. [DOI] [PubMed] [Google Scholar]
- 19.Ross PL, Gerigk C, Gonen M, et al. Comparisons of nomograms and urologists’ predictions in prostate cancer. Semin Urol Oncol. 2002;20:82–8. doi: 10.1053/suro.2002.32490. [DOI] [PubMed] [Google Scholar]
- 20.Walz J, Gallina A, Perrotte P, et al. Clinicians are poor raters of life-expectancy before radical prostatectomy or definitive radiotherapy for localized prostate cancer. BJU Int. 2007;100:1254–8. doi: 10.1111/j.1464-410X.2007.07130.x. [DOI] [PubMed] [Google Scholar]
- 21.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–81. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]