Abstract
The concept of biased agonism has recently come to the fore with the realization that seven-transmembrane receptors (7TMRs, also known as G protein–coupled receptors, or GPCRs) activate complex signaling networks and can adopt multiple active conformations upon agonist binding. As a consequence, the “efficacy” of receptors, which was classically considered linear, is now recognized as pluridimensional. Biased agonists selectively stabilize only a subset of receptor conformations induced by the natural “unbiased” ligand, thus preferentially activating certain signaling mechanisms. Such agonists thus reveal the intriguing possibility that one can direct cellular signaling with unprecedented precision and specificity and support the notion that biased agonists may identify new classes of therapeutic agents that have fewer side effects. This review focuses on one particular class of biased ligands that has the ability to alter the balance between G protein–dependent and β-arrestin-dependent signal transduction.
Keywords: GRKs, pharmacological bias, drug discovery, efficacy
1. INTRODUCTION
Seven-transmembrane receptors (7TMRs), also termed G protein–coupled receptors (GPCRs), represent the largest class of membrane receptors, with more than 800 members identified in the human genome (1). 7TMRs can bind a wide diversity of ligands that regulate most physiological processes and are involved in a plethora of diseases. As such, they have long been targets for drug discovery, accounting for the majority of the currently marketed drugs (2). Upon ligand binding, 7TMRs undergo a conformational change that imparts heterotrimeric G protein coupling and activation. Activated G proteins then promote the generation of second messengers such as cyclic adenosine monophosphate (cAMP), calcium, or phosphoinositides.
However, it has been increasingly appreciated that, rather than signaling through linear second messenger–dependent cascades, 7TMRs activate ensembles of signaling pathways organized as integrated networks (3, 4). For instance, some 7TMRs couple to different G protein subtypes (5, 6), whereas some others directly interact with non–G protein signaling effectors through specific protein-protein interaction domains such as PDZ domains (7). Aside from heterotrimeric G proteins, two protein families specifically interact with the majority of 7TMRs in their activated conformation: G protein–coupled receptor kinases (GRKs) and β-arrestins (8). Until recently, the role of GRKs and β-arrestins was limited to their ability to control the desensitization, internalization, and recycling of 7TMRs (9). Over the past decade, new information has accrued regarding their functions, such that GRKs and β-arrestins are now considered to be G protein–independent signal transducers (8, 10, 11). In particular, β-arrestins act as multifunctional scaffolds that interact with many protein partners (12) and protein kinases, thereby leading to the phosphorylation of numerous intracellular targets (13). These β-arrestin-mediated signaling mechanisms include RhoA-dependent stress fiber formation (14); inhibition of nuclear factor κB (NF-κB)-targeted gene expression through IκB stabilization (15, 16); protein phosphatase 2A (PP2A)-mediated dephosphorylation of Akt, which leads to the activation of glycogen synthase kinase 3 and dopaminergic behavior (17); extracellular signal-regulated kinase (ERK)-dependent induction of protein translation (18) and antiapoptotic effects (19); phosphatidylinositol 3-kinase (PI3K)-mediated phospholipase A2 (PLA2) activation and increased vasodilation through GPR109A (20); and Kif3A-dependent trafficking and activation of the protein Smoothened in the primary cilium (21) (Figure 1).
Figure 1.
Pluridimensionality of β-arrestin-dependent signaling at seven-transmembrane receptor (7TMRs). Some of the best-characterized β-arrestin-induced signaling mechanisms are schematically represented. They include RhoA-dependent stress fiber formation (14); inhibition of nuclear factor κB (NF-κB)-targeted gene expression through IκB stabilization (15, 16); protein phosphatase 2A (PP2A)-mediated dephosphorylation of Akt, which leads to the activation of glycogen synthase kinase 3 (GSK3) and dopaminergic behavior (17); extracellular signal-regulated kinase (ERK)-dependent induction of protein translation and antiapoptosis (18, 19); phosphatidylinositol 3-kinase (PI3K)-mediated phospholipase A2 (PLA2) induction and increased vasodilation through GPR109A activation (20); and Kif3A-dependent relocalization and activation of the protein Smoothened (Smo) in the primary cilium (21). Other abbreviations: AT1A R, angiotensin type 1A receptor; β1AR, β1 adrenergic receptor; BAD, Bcl-2-associated death promoter; D2R, dopamine receptor D2; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; EIF4E, eukaryotic translation initiation factor 4E; HB-EGF, heparin-binding EGF-like growth factor; MEK, mitogen-activated protein kinase/extracellular signal signal-regulated kinase kinase; MMP, matrix metalloproteinase; PGD2, prostaglandin D2; Ptc, Patched; ROCK, Rho-associated protein kinase; Shh, sonic hedgehog homolog.
Overall, these numerous types of β-arrestin-mediated actions facilitate the integration of the multiple extracellular cues to which cells are exposed into context-adapted biological outcomes. Beyond its fundamental importance in cellular functioning, this newly appreciated complexity of 7TMR-associated signaling also provides a promising conceptual framework that may facilitate the development of new types of drugs. Indeed, it is possible to selectively enhance or dampen discrete aspects of signaling pathways within 7TMR-induced networks with particular ligands that can be termed biased agonists or antagonists.
2. THE GENERAL CONCEPT OF PHARMACOLOGICAL BIAS
Classically, a ligand is defined by two distinct parameters: its affinity and its intrinsic efficacy. Affinity accounts for the tightness of the ligand/receptor interaction, whereas intrinsic efficacy characterizes the ability of a receptor-bound ligand to elicit a biological response (22). Ligand potency depends on both affinity and efficacy. Potency of a given ligand is measured by comparison with a reference ligand acting at the same receptor. Thus, two agonists can be equipotent but have different intrinsic efficacies with compensating differences in affinity. On the basis of this simple concept, three classes of ligands have been delineated and widely used: (a) full agonists, (b) partial agonists, and (c) neutral antagonists. A fourth class of ligands—inverse agonists—was recognized later with the discovery of constitutive activity of receptors and the ensuing extended ternary complex model to help explain such activity (23). Consistent with this view, agonists are considered to have linear efficacy (24, 25), as predicted by receptor occupancy theory that identifies a receptor as existing in either an inactive or an active conformation (26, 27). In this paradigm, full and partial agonists stabilize an active conformation, whereas inverse agonists stabilize an inactive one; by contrast, neutral antagonists do not affect the equilibrium between the two conformations. According to this theory, the magnitudes of responses of different signaling pathways activated by a 7TMR agonist are expected to always correlate with one another, being proportional to the intrinsic efficacy of the agonist.
However, a growing number of experimental observations cannot be explained by this theory. Indeed, some ligands display “imbalanced efficacies” in their activation of distinct intracellular signaling pathways. For example, 7TMR-interacting proteins, such as the receptor activity–modifying proteins, have the ability to modify receptor activity in terms of activation by agonists and thus produce differences in ligand efficacy (28, 29). Accordingly, ligand efficacy can be affected by the cellular expression of these auxiliary proteins, a phenomenon referred to as conditional efficacy (29). In addition, an increasing number of examples of imbalanced efficacies among ligands occur within the same cellular context. Such findings cannot be explained by conditional efficacy but are cell-autonomous and attributable to inherent properties of the ligands (25). In some cases, ligands can be antagonists or inverse agonists on one pathway, while simultaneously being agonists for another (30–32). These types of effects have received various names, which include stimulus trafficking, functional selectivity, collateral efficacy, or biased agonism (25, 26, 33).
Consequently, to define a pharmacological agent properly, one has to consider the efficacies of all the biological responses it triggers at a given receptor. As mentioned above, ligand binding to 7TMRs provokes a plethora of functional responses. These include modulation of different signaling pathways, desensitization, internalization, recycling, and degradation, all of which contribute to the observed biological response. Theoretically, ligands that display imbalanced efficacies for any combination of the different facets of receptor behavior may exist. Examples of biased efficacy for different types of response (e.g., G protein coupling, β-arrestin-dependent signaling, internalization, desensitization) have been reported and support this view (see References 25 and 34 for detailed reviews). Such results support the general concept that efficacy is pluridimensional rather than linear.
However, it can be difficult to identify ligand bias in various experimental systems. For example, because of differences in receptor reserve and amplification that can occur in different assays, a partial agonist can lead to the same maximal response as a full agonist in an amplified assay (e.g., second messenger generation), whereas in a nonamplified assay (e.g., β-arrestin recruitment), the same partial agonist reaches a lower maximal response. Such data can be misinterpreted as reflecting a ligand bias (35). Multiple approaches that allow rigorous quantification of ligand bias have been recently validated with experimental data (36). These rely on pairwise comparisons of (a) magnitudes of responses at equimolar concentrations, (b) ligand concentrations that result in equiactive responses, or (c) estimates of efficacies derived from an operational model of agonism developed by Black & Leff (36, 37). The operational model was originally proposed as a means to help understand the action of agonists and to provide a systematic way to measure relative efficacy of agonists on the basis of an examination of dose-response curves (37). Each of these modes of calculation has advantages and limitations, and they therefore represent complementary approaches. However, there is an increasing need to compare ligands in more than two dimensions and to quantify their respective degrees of bias. Several theoretical representations have been proposed to illustrate the pluridimensional nature of efficacy (25, 35). However, owing to the large number of dimensions that have to be considered, none of them can readily be used to compare the pharmacological identities of ligands.
We propose a new approach to address this problem. As recently demonstrated by Rajagopal et al. (36), there are different ways to calculate a “bias factor” β for each pair of readouts (i.e., assays of responses) activated by a ligand. When more than two readouts are available, we propose that one should build a matrix for each ligand and then fill this matrix with the β factors corresponding to all the pairs of readouts. As shown in Figure 2, a heat map representation allows a graphical comparison of different ligands. Different matrices can be statistically analyzed to identify pluridimensional bias. The proposed approach can be adapted to any number of dimensions/readouts. With the development and diversification of screening methods, multiplexed high-throughput screening will likely be carried out in the future. To analyze data generated by such large-scale approaches, the matrix corresponding to each ligand can be linearized and then subjected to hierarchical clustering (Figure 2), in a manner akin to approaches used to analyze other types of data, such as data from studies of transcriptomes. It should be possible to identify families or clusters of ligands with similar “efficacy signatures.” This clustering should also help reveal information regarding the topology of the signaling networks activated by the target receptor (e.g., which readouts are in series and which are in parallel within the network).
Figure 2.
Comparison of pluridimensional efficacies for different ligands. (a) Matrix of pairwise-determined biased factors βof one ligand for n dimensions. Biased factors βcan be calculated from dose-response curves as recently proposed (36). Values of βcalculated for each pair of readouts are represented in a heat map in which red corresponds to positive bias and blue corresponds to negative bias. Matrices for two different ligands can be statistically tested for balance versus bias in the pluridimensional efficacies. (b) For multiple ligand comparisons (e.g., data from high-throughput multiplexed screening), the matrix corresponding to each ligand can be linearized and subsequently analyzed by hierarchical clustering. Clusters of compounds with similar efficacies or particular “signatures” can be identified. Information about the structure of the intracellular signaling network can potentially be inferred from these analyses (i.e., determination of independent versus co-regulated cellular outcomes).
The growing awareness of ligand bias and pluridimensional efficacies creates the opportunity to develop therapeutic agents that can control intracellular signaling with much greater precision while keeping the exquisite specificity (determined by receptor binding) associated with 7TMR ligands. It is therefore important to decipher the molecular basis of pharmacological bias of 7TMR ligands.
3. STRUCTURAL BASIS OF BIASED AGONISM AT THE RECEPTOR LEVEL
The discovery of biased ligands raises several fundamental issues about receptor activation that cannot be accommodated by the classical two-state model of receptor theory previously established with balanced agonists. A biased ligand of a given receptor induces selective coupling to only one portion of potential downstream signaling pathways, implying that it must induce and/or stabilize a receptor conformation that is distinct from the one induced by a balanced agonist. This conceptual framework negates the notion of a single active conformation of the receptor but instead suggests the existence of multiple active conformations. Consistent with this view, several lines of evidence strongly support the idea of multiple active conformations in 7TMRs stabilized as a function of chemical structure of ligands or their pharmacological profiles.
Fluorescence spectroscopy has been used to study the ability of pharmacologically different ligands to induce different conformations in the purified β2 adrenergic receptor (β2AR) via stabilization/destabilization of two molecular switches, namely the ionic lock [a hydrogen bond interaction between R131 of the DRY motif and E268 toward the end of TM6 (the sixth transmembrane region) in the structure of β2ARs] and the rotamer toggle switch (the rotamer conformation of W in the conserved CWXP motif in TM6 of β2ARs) (38–40). These studies demonstrated that ligands of different chemical structure can have similar pharmacological profiles but can affect the ionic lock and the rotamer toggle switch to different extents. Similarly, plasmon waveguide resonance spectroscopy has been used to study the conformational changes in purified and lipid-reconstituted human cannabinoid receptor (CB1R) upon binding of two chemically different but pharmacologically similar agonists, CP-55,940 and WIN 55,212-2. Binding of these two agonists to the receptor results in directionally opposite spectral shifts, suggesting that they stabilize different conformations (41). More recently, ligand-induced conformational changes in receptors have been investigated through the use of fluorescence resonance energy transfer (FRET)-based biosensors of various 7TMRs (42–46). Results from these studies have indicated that qualitatively distinct receptor conformations are stabilized by different ligands. For example, epinephrine and norepinephrine, endogenous agonists of the β2AR, exhibit different magnitudes and kinetics of FRET change even when tested at saturating concentrations (42).
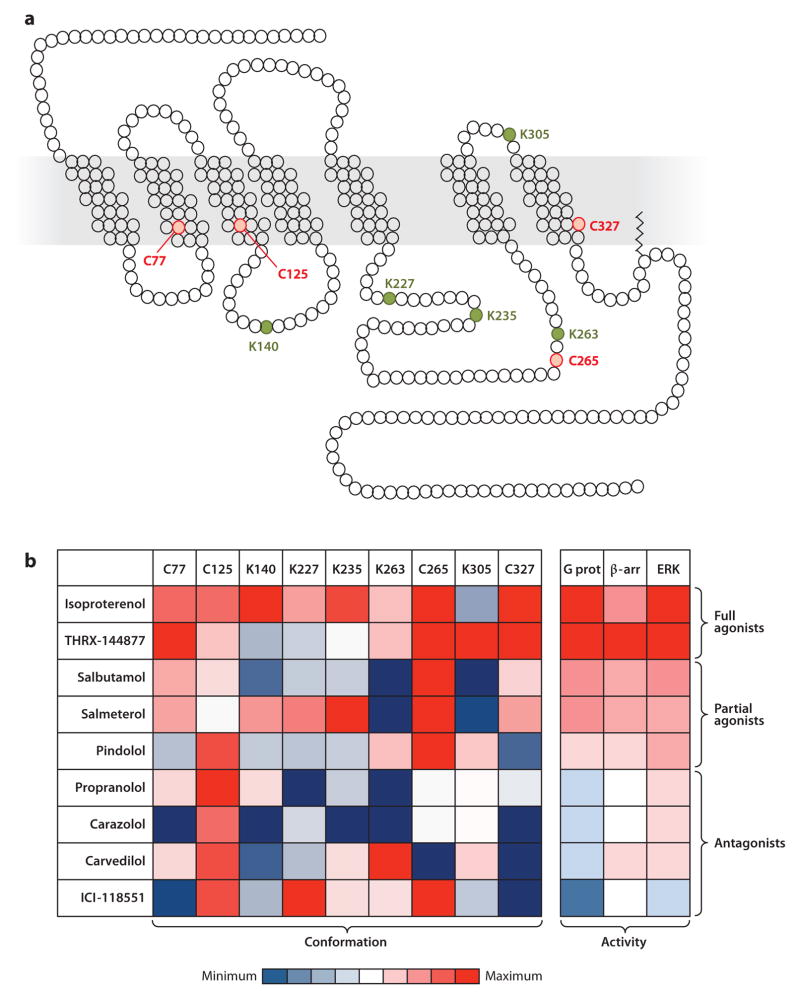
This concept of ligand-specific conformations has recently been assessed in a systematic fashion through the use of chemical labeling coupled to quantitative mass spectrometry with the purified human β2AR as a model system (47). This study used a panel of nine ligands that possess qualitatively and quantitatively different degrees of pharmacological efficacy at the β2AR. On the basis of their signaling outputs, these ligands can be grouped as antagonists of G protein and β-arrestin signaling, partial agonists for G protein and β-arrestin signaling, balanced full agonists for G protein and β-arrestin signaling, or as a selective agonist for β-arrestin signaling. Assessing conformational changes in the receptor subdomains was accomplished through the use of two chemical probes that react with cysteines or lysines exposed on the receptor surface. Chemical accessibility of four cysteines and five lysines distributed throughout the receptor was measured at different time points when the receptor was occupied with each of the ligands (Figure 3a). Because the chemical accessibility of these residues is sensitive to changes in their environment, the pattern of their reactivity reports both local and global conformational changes in the receptor. Out of nine residues that were studied, some—for example, Cys77 (located toward the end of the first intracellular loop) and Cys327 (adjacent to the NPXXY motif in the proximal C terminus)—exhibited a reactivity pattern that correlated fairly well with the pharmacological efficacy of the ligands (Figure 3b). Conversely, numerous residues—for example, Cys125 (located proximal to the DRY motif in the second intracellular loop) and Lys227 and Lys235 (both located in the third intracellular loop)—exhibited reactivity patterns that could not be rationalized in terms of the efficacy of the ligands, thus providing evidence of ligand-specific conformations in the receptor.
Figure 3.
Multiple ligand-specific conformations of the β2 adrenergic receptor (β2AR). Cysteines and lysines were labeled in the purified β2AR using isotope-coded N-ethylmaleimide and succinic anhydride, respectively. Mass spectrometry studies revealed that nine residues (four cysteines and five lysines) were suitable for quantifying site-specific conformational changes. (a) Schematic representation of the β2AR in single-letter amino acid code sequences showing the studied cysteines (C, red ) and lysines (K, green). (b) Labeling factors (i.e., negative log of relaxation times), measured at each of the nine cysteines or lysines upon receptor binding with nine different ligands, were plotted in a heat map (47). Values were calculated for each labeled residue upon treatment with each of the ligands; red corresponds to positive values and blue corresponds to negative values, relative to vehicle-treated β2AR. Relative activities of the nine ligands for G protein activation (G prot), β-arrestin recruitment (β-arr), and extracellular signal-regulated kinase (ERK) phosphorylation at the β2AR are represented in the heat map to facilitate direct comparison (right panel ) (47). The ligands were classified as full agonists, partial agonists, or antagonists on the basis of their pharmacological profiles.
Even more importantly, this study, for the first time, directly probed the conformational changes in the receptor induced by a well-defined β-arrestin-biased ligand. Carvedilol, one of the ligands in the panel, has unique properties: It is an inverse agonist for G protein–dependent signaling but a weak agonist for β-arrestin-dependent signaling. Interestingly, two residues, Lys263 and Cys265 (located in the third intracellular loop), exhibited a unique reactivity signature, i.e., strong increases or decreases compared with the unliganded reference, in the carvedilol-occupied conformation (Figure 3b). As carvedilol was the only ligand in the initial studies that is β-arrestin biased, it is tempting to speculate that these residues represent hot spots related to β-arrestin-coupled conformations of the receptor and that they may participate in activation switches critical for β-arrestin-dependent signaling. Together, these results support the view that more than one active conformation of the receptor exists and that different ligands are able to stabilize distinct conformations. These findings also challenge the widely held notion that all ligands of qualitatively similar pharmacological efficacy induce similar conformations of the receptor.
Another interesting idea that has been proposed on the basis of the architecture of ligand binding pockets, as visualized in the crystal structures of 7TMRs, postulates a minor binding pocket defined by transmembrane helices I, II, III, and VII (40, 48) in addition to the major ligand binding pocket located between transmembrane helices III, IV, V, VI, and VII. The conserved proline kink in TM2, an important feature of the minor binding pocket, has been proposed to play an important role in determining the qualitative nature of ligand bias (48). However, this hypothesis remains to be tested with rigorous experimentation, for example, by using receptor systems for which well-defined biased ligands and biased receptor mutants are available.
Although the aforementioned studies provide some interesting structural information about the β-arrestin-biased conformation of 7TMRs, detailed mapping of conformational differences between receptors occupied with balanced or biased agonists remains to be carried out. Such information will be critical both for understanding the mechanism of biased agonism at 7TMRs and for aiding in the design of pharmacological tools to selectively manipulate one or the other signaling pathway. There are at least three important aspects of a biased signaling conformation of receptors that need to be elucidated: (a) the structure of the ligand binding pocket with a balanced agonist versus a biased agonist (i.e., the binding mode), (b) the overall rearrangement of the central core of the receptor (i.e., the TM helices), and (c) the structural rearrangement of the intracellular surface of the receptor (i.e., the interface of the receptor-effector interaction). Although X-ray crystal structures of several 7TMRs have been reported over the past few years (49–56), none of these structures has revealed ligand-specific or biased signaling conformations of the receptors. To firmly establish the finer structural details of functionally coupled active conformations of 7TMRs, X-ray crystal structures of receptors bound with balanced and biased ligands will be required. A step toward this goal has been the determination of crystal structures of the β2AR in inactive (i.e., bound to an inverse agonist) and active (i.e., bound to a balanced agonist) conformations (50, 51). Such data have provided the first glimpse of the conformational changes that occur upon binding of a balanced agonist. Although there are only subtle changes in the binding pocket, a relatively large outward movement of the cytoplasmic end of TM6 and a substantial rearrangement of TM5 and TM7 are seen (50, 51). It will be interesting to see whether these large movements in the TM helices will be conserved in a biased signaling conformation of the receptor. Moreover, crystal structures of signaling complexes between the receptor and signal-transducing molecules, such as G proteins and β-arrestins, will also be extremely valuable in order to further our understanding of how the signal propagates from the ligand binding pocket to the central core of the receptor and ultimately to the effectors.
In addition to crystallography, other biophysical approaches should be used to study receptor dynamics. These studies should help capture the subtle but crucial changes in the receptor conformations that occur upon binding of ligands and effectors; such changes may not be visualized by X-ray crystallography but are critical for determining signaling efficacy. For example, nuclear magnetic resonance spectroscopy has been employed to investigate ligand-specific conformational changes around a salt bridge that links extracellular loops 2 (Asp192) and 3 (Lys305) in the β2AR (57). Although this study did not aim to uncover ligand-specific conformations, it established that in an agonist-stabilized active state, this salt bridge is weakened, probably as a result of outward movements in the cytoplasmic end of TM6 and lateral movement of the extracellular end of TM7. Similar studies carried out on the receptor occupied with biased ligands and/or complexed with their downstream effectors should provide novel information about the structural basis of biased agonism.
Although the majority of discussion on biased agonism thus far pertains to orthosteric biased ligands, another interesting area for further study will be to extend ideas that have followed the discovery of allosteric modulators of 7TMRs (58). These allosteric ligands can modulate receptor conformations in the presence of orthosteric ligands and therefore have the potential to fine-tune responses elicited by endogenous or synthetic ligands. The orthosteric site is the site on a receptor that binds the endogenous agonist. This domain is also recognized by classic competitive antagonists and inverse agonists. By contrast, the allosteric site on a receptor is one that is modulatory and topographically distinct from the binding site for the endogenous agonist. Positive and negative allosteric modulators have been described. Interestingly, a negative allosteric modulator of the follicle stimulating hormone receptor (FSH-R) has been reported to induce a biased steroidogenic response in primary granulosa cells (59). This result suggests that allosteric modulators are able to modulate the orthosteric ligand in a biased fashion, which further broadens the field of applications of pharmacological bias.
The discovery of novel features of ligand bias raises the question as to the number of active conformations of a receptor. Although the tremendous flexibility of 7TMRs in lipid bilayers (60, 61) implies that the conformational possibilities are enormous, these multiple active conformations are likely to share certain core features. In a simple conceptual scheme, a receptor in an unliganded state can be visualized as a continuum of conformations. Binding of a given ligand likely enriches one of these conformations by shifting the conformational equilibrium, thereby leading to a specific cellular response. At the same time, the population of other conformations is depleted. Supporting evidence for this hypothesis comes from a molecular dynamics simulation of the β2AR. Microsecond molecular dynamics simulations revealed that even when the receptor is unliganded, some receptor conformations have a formed ionic lock, whereas in others, the ionic lock is broken (62). Such a scenario is compatible with the idea that inverse agonists increase the population of a receptor conformation that has a formed ionic lock, whereas “traditional” agonists increase the population that have a conformation with a broken ionic lock. However, in-depth study of these multiple active conformations remains technically challenging and will probably be a major focus of the next phase of research in the area of 7TMR biased agonism.
4. FROM RECEPTOR CONFORMATION TO INTRACELLULAR SIGNALING: CENTRAL ROLE OF GRK-MEDIATED PHOSPHORYLATION “BAR CODES”
With the recognition of β-arrestin-mediated signaling, biased ligands that preferentially induce either G protein–dependent (G protein–biased) or β-arrestin-dependent (β-arrestin-biased) signaling have been identified (24, 26, 35). To date, G protein–biased and/or β-arrestin-biased ligands have been identified for dozens of 7TMRs (see Reference 63 for a detailed review), suggesting that they represent a general class of pharmacological ligands capable of selective modulation of most 7TMRs. Moreover, some of these biased ligands have already been proven to produce effects that are distinct from those of balanced compounds (26, 63, 64).
In this context, it is of particular interest to understand how ligand-induced stabilization of receptor conformations can be converted into selective G protein–dependent or β-arrestin-dependent signaling within the cell. It is clear that the molecular mechanisms that underlie the multiple functions of β-arrestins (i.e., desensitization of G protein signaling versus endocytosis and β-arrestin signaling) play a central role in this process. Early in vitro studies used the patterns of limited tryptic proteolysis to demonstrate that β-arrestin 1 and 2 undergo conformational changes upon interaction with phosphorylated peptides mimicking those produced by the vasopressin type 2 receptor (V2R) C terminus (65, 66). More recently, an intramolecular bioluminescence resonance energy transfer (BRET)-based biosensor has allowed the monitoring of conformational changes of β-arrestin 2 in living cells (67). Ligands of different 7TMRs have been compared through the use of this approach, and the results reveal that distinct receptor conformations, induced and/or stabilized by different ligands (i.e., balanced versus biased), can promote distinct and functionally specific conformations in β-arrestin (20, 68). These data thus support the notion that ligand-induced, functionally specific receptor conformations can be translated to downstream effectors such as β-arrestins, thereby directly impacting intracellular activities.
β-arrestin recruitment to 7TMRs is induced by two driving forces: agonist-induced modification of the receptor conformation (8, 69) and GRK-mediated phosphorylation of the ligand-occupied receptor (9). Interestingly, different GRK subtypes play specialized regulatory functions: Activation of the β-arrestin 2–dependent ERK pathway by the angiotensin type 1A receptor (AT1A R) (70), V2R (71), β2AR (72), and FSH-R (73) requires GRK5 and GRK6 action, whereas second messenger generation by V2R (71) and H1 histamine receptor (74) is negatively regulated by GRK2 but unaffected by GRK5 or GRK6. Furthermore, two endogenous C-C chemokine receptor 7 (CCR7) agonists, C-C chemokine ligands 19 and 21 (CCL19 and CCL21), selectively induce interaction of different GRKs with the CCR7 receptor (75). CCL19 induces receptor phosphorylation by GRK3 as well as GRK6, leading to receptor desensitization, endocytosis, and β-arrestin-mediated ERK activation. Conversely, CCL21 promotes phosphorylation of the receptor but only by GRK6, and this phosphorylation leads only to ERK activation. In light of these results, it has been hypothesized that there is a GRK-induced phosphorylation “bar code” at the C terminus of 7TMRs that regulates the nature of β-arrestin intracellular functions (10, 70, 72). This idea is consistent with previous results showing that the presence or absence of serine and threonine clusters in the receptor C terminus regulates the affinity of β-arrestin recruitment and the pattern of intracellular trafficking for a wide number of 7TMRs (76, 77).
However, until recently, little was known about the precise sites of phosphorylation on 7TMRs targeted by individual GRKs. A recent study combining tryptic phosphopeptide maps, mass spectrometry, and phosphospecific antibodies revealed the dynamically regulated site-specific phosphorylation of CXCR4 by multiple kinases, which leads to both positive and negative regulation of CXCR4 signaling (78). A separate study using similar techniques demonstrated that the M3-muscarinic receptor C terminus is differentially phosphorylated when stimulated with full versus partial agonists, phosphorylation being preferentially directed to specific sites (79). The detailed GRK-dependent phosphorylation bar code occurring at the β2 adrenergic receptor has also been deciphered (80). In the latter study, RNAi, mass spectrometry–based quantitative proteomics, and site-specific phosphoantibodies were used to define the phosphorylated serine and threonine residues in the intracellular portions of the β2AR. Isoproterenol, a full agonist at the β2AR, was compared with carvedilol, which, as described above, is a weak β-arrestin-biased ligand (81); studies were conducted in native cells or cells depleted of either GRK6 or GRK2 (80). From a functional standpoint, GRK2 sites (i.e., T360, S364, S396, S401, S407, and S411) are primarily responsible for receptor internalization, whereas GRK6 sites (i.e., S355 and S356) are required for β-arrestin-mediated ERK activation. Both GRK2 and GRK6 contributed to desensitization. Remarkably, carvedilol induced phosphorylation only on the GRK6 sites, whereas isoproterenol triggered phosphorylation on both GRK2 and GRK6 sites. In addition, phosphorylation on the GRK6 sites was increased in GRK2-depleted cells, thus implying a competition between the two GRKs for receptor phosphorylation. Together, these data provide compelling evidence that distinct GRKs can be preferentially recruited to, and can phosphorylate, a receptor as a function of its ligand-induced conformation. In this paradigm, different GRKs act as sensors that discriminate active receptor conformations stabilized by unbiased or β-arrestin-biased ligands. As predicted by the bar code hypothesis, this preferential recruitment of GRKs leads to distinct phosphorylation patterns in the receptor’s C terminus and imparts distinctive β-arrestin functions, presumably by stabilizing it in distinct conformations.
Similar conclusions were obtained in a recent study that combined experimental approaches with computational modeling to decipher the molecular mechanisms as well as the dynamics governing ERK activation by the AT1A R in HEK293 cells (82). It was proposed that GRK2/3 and GRK5/6 regulate switching between the G protein–dependent and β-arrestin-dependent pathways as well as their distinct dynamics by phosphorylating distinct, as-yet unidentified, residues in the C-terminal region of the receptor. In addition, GRK2/3 was found not only to mediate desensitization of G protein activation but also to exert strong restraint on β-arrestin signaling. The dynamical model proposed for the AT1A R by Heitzler et al. (82) is remarkably consistent with the data reported for the β2AR (80). More generally, its most salient features (e.g., dual role of the GRK/β-arrestin system in desensitization and signaling, different dynamics of ERK activation by G protein–dependent and β-arrestin-dependent mechanisms, functional specialization of the different GRK subtypes) appear to be conserved across multiple 7TMRs, such as the β2AR (72), V2R (71), parathyroid hormone receptor (PTH-R) (31), FSH-R (73), and serotonin 5HT2C receptor (83). Therefore, it seems likely that the main features of the model developed for the AT1A R, including the central regulatory role played by the bar code, may be generally applicable to many other 7TMRs (see Figure 4 for a schematic representation).
Figure 4.
G protein–coupled receptor kinase (GRK)-mediated phosphorylation “bar code” at the C terminus of seven-transmembrane receptors (7TMRs) converts ligand-induced conformation of the receptor into selective β-arrestin intracellular functions. A general model for β-arrestin-biased ligand mechanism of action is proposed (78, 80, 82). GRK2/3 and GRK5/6 exert qualitatively different actions. GRK2/3 require Gβ for membrane recruitment and activation and phosphorylate specific serines and threonines in the receptor’s C-tail; the GRK2/3-mediated phosphorylation “bar code” then leads to desensitization and internalization upon β-arrestin recruitment. GRK5/6 do not require G proteins for their activation; the GRK5/6-dependent phosphorylation “bar code” then creates a signaling platform in which β-arrestin bridges the activated receptor to partners involved in intracellular signaling such as the mitogen-activated protein (MAP) kinase extracellular signal-regulated kinase (ERK) module. Other abbreviation: MEK, MAP kinase/extracellular signal-regulated kinase kinase.
Taken together, these findings provide a possible basis for explaining how biased agonists can directly recruit β-arrestin toward distinct, sometimes antagonistic, intracellular functions. Ligand efficacy, which, as discussed above, occurs from the stabilization of distinct receptor conformations, subsequently leads to specific phosphorylation patterns involving distinct GRKs. The phosphorylation bar code then directs β-arrestin conformation, thereby controlling its interaction partners and related functions.
5. POTENTIAL THERAPEUTIC APPLICATIONS OF β-ARRESTIN-BIASED LIGANDS
Paralleling the rapid rise in the number of G protein–biased or β-arrestin-biased ligands identified in vitro and the accumulation of mechanistic knowledge about them is the increasing recognition of the therapeutic potential of biased ligands that has been suggested in a variety of animal models of diseases. A wide array of physiological and pathophysiological systems and situations encountered in vivo appear to involve β-arrestin-mediated processes. The current list includes the cardiovascular and gastrointestinal systems, renal and pulmonary function, autoimmune conditions and Toll-like receptor signaling and inflammation, metabolism, bone mineral homeostasis, reproduction, the central nervous system, and cancer (see Reference 63 for a recent detailed review on this topic).
In some instances, β-arrestin bias confers positive effects, whereas G protein–dependent signaling may cause side effects. An example of beneficial β-arrestin-dependent effects is provided by the β-blocker carvedilol, a β-arrestin-biased ligand acting at both the β1AR and β2AR subtypes. Carvedilol stimulates epidermal growth factor receptor transactivation and ERK phosphorylation in a G protein–independent manner (81, 84, 85). Interestingly, chronic β-AR coupling to Gs is thought to be cardiotoxic (86, 87), whereas epidermal growth factor receptor transactivation has been reported to confer cardioprotection (85). Together, these observations suggest that carvedilol, which acts as an antagonist of G protein signaling and simultaneously engages cardioprotective β-arrestin signaling, might provide an added therapeutic benefit in the treatment of heart failure compared with other antagonists that block all βAR signaling. The AT1A R also plays an important regulatory role in the cardiovascular system. The recently identified β-arrestin-biased ligand Sar1,D-Ala8 angiotensin II (TRV120027) reduces mean arterial pressure while increasing cardiac performance and preserving stroke volume in rats, whereas classical angiotensin receptor antagonists reduce overall cardiac performance (64). Another example of a positive effect of a β-arrestin-biased ligand comes from the role of PTH in bone formation and homeostasis. Binding of PTH(1–34) results in full activation of Gαs and Gαq/11 at the PTH1 receptor. PTH-βarr, an inverse agonist of PTH1 receptor-mediated G protein–mediated signaling, induces β-arrestin-dependent ERK activation and therefore exhibits a β-arrestin bias (31). Interestingly, PTH-βarr induces anabolic bone formation in mice, as does PTH(1–34). Moreover, in β-arrestin 2 knockout mice, the increase in bone mineral density evoked by PTH(1–34) is attenuated, whereas that stimulated by PTH-βarr is abrogated (88). The biochemical differences between the G protein–dependent and the β-arrestin-dependent pathways to anabolic bone formation have yet to be fully elucidated, and the full physiological impact of biased signaling at this receptor remains to be defined.
β-arrestin-dependent signaling can also be responsible for adverse effects, as has been shown for GPR109A. A ligand of GPR109A, niacin (also known as nicotinic acid or vitamin B3), induces its coupling to Gαi/Gαo. This G protein–dependent pathway leads to a decrease in triglyceride levels, an increase in high-density lipoprotein, and a decrease in low-density lipoproteins. However, the clinical use of niacin has long been limited by cutaneous flushing (89). As a consequence of GPR109A coupling to Gαi, stimulation by niacin decreases cAMP levels. Conversely, niacin also leads to β-arrestin-dependent ERK activation and binding to activated cytosolic phospholipase A2 (cPLA2) (20). The interaction of β-arrestin 1 with cPLA2 generates arachidonate, which is responsible for the flushing response via its conversion to prostaglandin D2. Strikingly, a recently identified partial agonist of GPR109A exhibits the antilipolytic activity without the cutaneous flushing and seems to work via a G protein–biased signaling mechanism (20, 90).
6. CONCLUSION
Biased ligands represent an opportunity for the discovery of new drugs, potentially ones associated with fewer side effects. Such drugs may substantially expand and diversify the panel of pharmacological tools available to researchers and clinicians. However, to meet this ambitious challenge, numerous hurdles will have to be surmounted. Indeed, the increasing complexity of signaling mechanisms triggered by 7TMRs will likely require integrated systems biology approaches, such as ones that combine high-throughput generation of quantitative data and mathematical modeling, in order to predict how extracellular signals acting at 7TMRs engender specific, potentially biased responses. A systems-level understanding of 7TMR-mediated signaling networks may help further understanding and accelerate the discovery of new biased ligands. New multiplexed screening approaches, capable of monitoring multiple facets of 7TMR biological activities, will be required. Finally, more information about the structural basis of 7TMR activation, in all its dimensions, is sorely needed. These approaches have the potential to ultimately make the rational design of biased ligands a reality.
SUMMARY POINTS.
Pharmacological efficacy is pluridimensional rather than linear.
Activated 7TMRs can adopt multiple ligand-specific conformations.
Biased ligands have the ability to stabilize only a subset of the receptor conformations induced by a balanced ligand and thereby selectively activate intracellular signaling pathways.
G protein–biased or β-arrestin-biased ligands have been identified for a large number of 7TMRs and might therefore be considered general classes of pharmacological compounds.
A GRK-mediated phosphorylation “bar code” at the receptor C terminus may convert ligand-induced conformations of the receptor into β-arrestin-selective intracellular functions.
G protein–biased and β-arrestin-biased ligands have substantial therapeutic potential.
Multiplexed high-throughput screening may allow the discovery of biased ligands at many 7TMRs.
A proposed methodology allows the quantification of pharmacological bias simultaneously in multiple dimensions and for a large number of ligands.
Acknowledgments
We thank S. Rajagopal, A.W. Kashai, D. Heitzler, and A. Poupon for helpful discussions. We also thank Donna Addison and Quivetta Lennon for excellent secretarial assistance. This work was supported by grants RO1 HL16037 and HL 70631 from the National Institutes of Health (to R.J.L.) and in part by a Région Centre RASTA research grant and the Institut National de la Recherche Agronomique (to E.R.). R.J.L. is an investigator of the Howard Hughes Medical Institute.
Glossary
- 7TMR
seven-transmembrane receptor
- GPCR
G protein–coupled receptor
- GRK
G protein–coupled receptor kinase
- ERK
extracellular signal-regulated kinase
- β2AR
β2 adrenergic receptor
- FSH-R
follicle stimulating hormone receptor
- V2R
vasopressin type 2 receptor
- AT1AR
angiotensin type 1A receptor
- PTH-R
parathyroid hormone receptor
Footnotes
DISCLOSURE STATEMENT
R.J.L. is a founder of Trevena, Inc., a biotechnology company that is developing biased agonists as drugs.
LITERATURE CITED
- 1.Lagerström MC, Schiöth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–57. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- 2.Ma P, Zemmel R. Value of novelty? Nat Rev Drug Discov. 2002;1:571–72. doi: 10.1038/nrd884. [DOI] [PubMed] [Google Scholar]
- 3.Luttrell LM. Transmembrane signaling by G protein-coupled receptors. Methods Mol Biol. 2006;332:3–49. doi: 10.1385/1-59745-048-0:1. [DOI] [PubMed] [Google Scholar]
- 4.Jean-Alphonse F, Hanyaloglu AC. Regulation of GPCR signal networks via membrane trafficking. Mol Cell Endocrinol. 2011;331:205–14. doi: 10.1016/j.mce.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Hermans E. Biochemical and pharmacological control of the multiplicity of coupling at G-protein-coupled receptors. Pharmacol Ther. 2003;99:25–44. doi: 10.1016/s0163-7258(03)00051-2. [DOI] [PubMed] [Google Scholar]
- 6.Lefkowitz RJ, Pierce KL, Luttrell LM. Dancing with different partners: protein kinase A phosphorylation of seven membrane-spanning receptors regulates their G protein-coupling specificity. Mol Pharmacol. 2002;62:971–74. doi: 10.1124/mol.62.5.971. [DOI] [PubMed] [Google Scholar]
- 7.Bockaert J, Dumuis A, Fagni L, Marin P. GPCR-GIP networks: a first step in the discovery of new therapeutic drugs? Curr Opin Drug Discov Dev. 2004;7:649–57. [PubMed] [Google Scholar]
- 8.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–17. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 9.Lefkowitz RJ. G protein-coupled receptors: III. New roles for receptor kinases and β-arrestins in receptor signaling and desensitization. J Biol Chem. 1998;273:18677–80. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 10.Reiter E, Lefkowitz RJ. GRKs and β-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159–65. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Penela P, Murga C, Ribas C, Lafarga V, Mayor F., Jr The complex G protein-coupled receptor kinase 2 (GRK2) interactome unveils new physiopathological targets. Br J Pharmacol. 2010;160:821–32. doi: 10.1111/j.1476-5381.2010.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao K, McClatchy DB, Shukla AK, Zhao Y, Chen M, et al. Functional specialization of β-arrestin interactions revealed by proteomic analysis. Proc Natl Acad Sci USA. 2007;104:12011–16. doi: 10.1073/pnas.0704849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao K, Sun J, Kim J, Rajagopal S, Zhai B, et al. Global phosphorylation analysis of β-arrestin-mediated signaling downstream of a seven transmembrane receptor (7TMR) Proc Natl Acad Sci USA. 2010;107:15299–304. doi: 10.1073/pnas.1008461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes WG, Reiter E, Violin JD, Ren XR, Milligan G, Lefkowitz RJ. β-Arrestin 1 and Galphaq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J Biol Chem. 2005;280:8041–50. doi: 10.1074/jbc.M412924200. [DOI] [PubMed] [Google Scholar]
- 15.Gao H, Sun Y, Wu Y, Luan B, Wang Y, et al. Identification of β-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-κB pathways. Mol Cell. 2004;14:303–17. doi: 10.1016/s1097-2765(04)00216-3. [DOI] [PubMed] [Google Scholar]
- 16.Witherow DS, Garrison TR, Miller WE, Lefkowitz RJ. β-Arrestin inhibits NF-κB activity by means of its interaction with the NF-κB inhibitor IκBα. Proc Natl Acad Sci USA. 2004;101:8603–7. doi: 10.1073/pnas.0402851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/β-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–73. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 18.DeWire SM, Kim J, Whalen EJ, Ahn S, Chen M, Lefkowitz RJ. β-arrestin-mediated signaling regulates protein synthesis. J Biol Chem. 2008;283:10611–20. doi: 10.1074/jbc.M710515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahn S, Kim J, Hara MR, Ren XR, Lefkowitz RJ. β-Arrestin-2 mediates anti-apoptotic signaling through regulation of BAD phosphorylation. J Biol Chem. 2009;284:8855–65. doi: 10.1074/jbc.M808463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walters RW, Shukla AK, Kovacs JJ, Violin JD, DeWire SM, et al. β-Arrestin1 mediates nicotinic acid–induced flushing, but not its antilipolytic effect, in mice. J Clin Investig. 2009;119:1312–21. doi: 10.1172/JCI36806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovacs JJ, Whalen EJ, Liu R, Xiao K, Kim J, et al. β-arrestin-mediated localization of Smoothened to the primary cilium. Science. 2008;320:1777–81. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephenson RP. A modification of receptor theory. Br J Pharmacol Chemother. 1956;11:379–93. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samama P, Cotecchia S, Costa T, Lefkowitz RJ. A mutation-induced activated state of the β2-adrenergic receptor: extending the ternary complex model. J Biol Chem. 1993;268:4625–36. [PubMed] [Google Scholar]
- 24.Kenakin T. New concepts in drug discovery: collateral efficacy and permissive antagonism. Nat Rev Drug Discov. 2005;4:919–27. doi: 10.1038/nrd1875. [DOI] [PubMed] [Google Scholar]
- 25.Galandrin S, Oligny-Longpre G, Bouvier M. The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol Sci. 2007;28:423–30. doi: 10.1016/j.tips.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Violin JD, Lefkowitz RJ. β-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–22. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 28.Christopoulos A, Christopoulos G, Morfis M, Udawela M, Laburthe M, et al. Novel receptor partners and function of receptor activity-modifying proteins. J Biol Chem. 2003;278:3293–97. doi: 10.1074/jbc.C200629200. [DOI] [PubMed] [Google Scholar]
- 29.Kenakin T. Efficacy at G-protein-coupled receptors. Nat Rev Drug Discov. 2002;1:103–10. doi: 10.1038/nrd722. [DOI] [PubMed] [Google Scholar]
- 30.Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, et al. Independent β-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA. 2003;100:10782–87. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, et al. Distinct β-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem. 2006;281:10856–64. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- 32.Azzi M, Charest PG, Angers S, Rousseau G, Kohout T, et al. β-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc Natl Acad Sci USA. 2003;100:11406–11. doi: 10.1073/pnas.1936664100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kenakin T. Collateral efficacy in drug discovery: taking advantage of the good (allosteric) nature of 7TM receptors. Trends Pharmacol Sci. 2007;28:407–15. doi: 10.1016/j.tips.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Kenakin T. Ligand-selective receptor conformations revisited: the promise and the problem. Trends Pharmacol Sci. 2003;24:346–54. doi: 10.1016/S0165-6147(03)00167-6. [DOI] [PubMed] [Google Scholar]
- 35.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–86. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajagopal S, Ahn S, Rominger DH, Gowen-McDonald W, Lam CM, et al. Quantifying ligand bias at seven-transmembrane receptors. Mol Pharmacol. 2011 doi: 10.1124/mol.111.072801. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Black JW, Leff P. Operational models of pharmacological agonism. Proc R Soc Lond Ser B. 1983;220:141–62. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- 38.Swaminath G, Deupi X, Lee TW, Zhu W, Thian FS, et al. Probing the β2 adrenoceptor binding site with catechol reveals differences in binding and activation by agonists and partial agonists. J Biol Chem. 2005;280:22165–71. doi: 10.1074/jbc.M502352200. [DOI] [PubMed] [Google Scholar]
- 39.Yao X, Parnot C, Deupi X, Ratnala VR, Swaminath G, et al. Coupling ligand structure to specific conformational switches in the β2-adrenoceptor. Nat Chem Biol. 2006;2:417–22. doi: 10.1038/nchembio801. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz TW, Frimurer TM, Holst B, Rosenkilde MM, Elling CE. Molecular mechanism of 7TM receptor activation—a global toggle switch model. Annu Rev Pharmacol Toxicol. 2006;46:481–519. doi: 10.1146/annurev.pharmtox.46.120604.141218. [DOI] [PubMed] [Google Scholar]
- 41.Georgieva T, Devanathan S, Stropova D, Park CK, Salamon Z, et al. Unique agonist-bound cannabinoid CB1 receptor conformations indicate agonist specificity in signaling. Eur J Pharmacol. 2008;581:19–29. doi: 10.1016/j.ejphar.2007.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Granier S, Kim S, Shafer AM, Ratnala VR, Fung JJ, et al. Structure and conformational changes in the C-terminal domain of the β2-adrenoceptor: insights from fluorescence resonance energy transfer studies. J Biol Chem. 2007;282:13895–905. doi: 10.1074/jbc.M611904200. [DOI] [PubMed] [Google Scholar]
- 43.Reiner S, Ambrosio M, Hoffmann C, Lohse MJ. Differential signaling of the endogenous agonists at the β2-adrenergic receptor. J Biol Chem. 2010;285:36188–98. doi: 10.1074/jbc.M110.175604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zurn A, Zabel U, Vilardaga JP, Schindelin H, Lohse MJ, Hoffmann C. Fluorescence resonance energy transfer analysis of α2a-adrenergic receptor activation reveals distinct agonist-specific conformational changes. Mol Pharmacol. 2009;75:534–41. doi: 10.1124/mol.108.052399. [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann C, Zurn A, Bunemann M, Lohse MJ. Conformational changes in G-protein-coupled receptors—the quest for functionally selective conformations is open. Br J Pharmacol. 2008;153(Suppl 1):S358–66. doi: 10.1038/sj.bjp.0707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ziegler N, Batz J, Zabel U, Lohse MJ, Hoffmann C. FRET-based sensors for the human M1-, M3-, and M5-acetylcholine receptors. Bioorg Med Chem. 2010;19:1048–54. doi: 10.1016/j.bmc.2010.07.060. [DOI] [PubMed] [Google Scholar]
- 47.Kashai AW, Xiao K, Rajagopal S, Ahn S, Shukla AK, et al. Multiple ligand-specific conformations of the β2-adrenergic receptor. Nat Chem Biol. 2011 doi: 10.1038/nchembio.634. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenkilde MM, Benned-Jensen T, Frimurer TM, Schwartz TW. The minor binding pocket: a major player in 7TM receptor activation. Trends Pharmacol Sci. 2010;31:567–74. doi: 10.1016/j.tips.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 49.Rasmussen SG, Choi HJ, Fung JJ, Pardon E, Casarosa P, et al. Structure of a nanobody-stabilized active state of the β2 adrenoceptor. Nature. 2011;469:175–80. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenbaum DM, Zhang C, Lyons JA, Holl R, Aragao D, et al. Structure and function of an irreversible agonist-β2 adrenoceptor complex. Nature. 2011;469:236–40. doi: 10.1038/nature09665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, et al. High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–65. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, et al. GPCR engineering yields high-resolution structural insights into β2-adrenergic receptor function. Science. 2007;318:1266–73. doi: 10.1126/science.1150609. [DOI] [PubMed] [Google Scholar]
- 53.Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, et al. Crystal structure of the human β2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–87. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- 54.Warne T, Moukhametzianov R, Baker JG, Nehme R, Edwards PC, et al. The structural basis for agonist and partial agonist action on a β1-adrenergic receptor. Nature. 2011;469:241–44. doi: 10.1038/nature09746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, et al. Structure of a β1-adrenergic G-protein-coupled receptor. Nature. 2008;454:486–91. doi: 10.1038/nature07101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jaakola VP, Griffith MT, Hanson MA, Cherezov V, Chien EY, et al. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 2008;322:1211–17. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bokoch MP, Zou Y, Rasmussen SG, Liu CW, Nygaard R, et al. Ligand-specific regulation of the extracellular surface of a G-protein-coupled receptor. Nature. 2010;463:108–12. doi: 10.1038/nature08650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.May LT, Leach K, Sexton PM, Christopoulos A. Allosteric modulation of G protein–coupled receptors. Annu Rev Pharmacol Toxicol. 2007;47:1–51. doi: 10.1146/annurev.pharmtox.47.120505.105159. [DOI] [PubMed] [Google Scholar]
- 59.Dias JA, Bonnet B, Weaver BA, Watts J, Kluetzman K, et al. A negative allosteric modulator demonstrates biased antagonism of the follicle stimulating hormone receptor. Mol Cell Endocrinol. 2011;333:143–50. doi: 10.1016/j.mce.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kobilka BK, Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28(8):397–406. doi: 10.1016/j.tips.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 61.Vaidehi N. Dynamics and flexibility of G-protein-coupled receptor conformations and their relevance to drug design. Drug Discov Today. 2010;15(21–22):951–57. doi: 10.1016/j.drudis.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 62.Dror RO, Arlow DH, Borhani DW, Jensen MO, Piana S, Shaw DE. Identification of two distinct inactive conformations of the β2-adrenergic receptor reconciles structural and biochemical observations. Proc Natl Acad Sci USA. 2009;106:4689–94. doi: 10.1073/pnas.0811065106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of β-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17(3):126–39. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Violin JD, DeWire SM, Yamashita D, Rominger DH, Nguyen L, et al. Selectively engaging β-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. J Pharmacol Exp Ther. 2010;335:572–79. doi: 10.1124/jpet.110.173005. [DOI] [PubMed] [Google Scholar]
- 65.Xiao K, Shenoy SK, Nobles K, Lefkowitz RJ. Activation-dependent conformational changes in β-arrestin 2. J Biol Chem. 2004;279:55744–53. doi: 10.1074/jbc.M409785200. [DOI] [PubMed] [Google Scholar]
- 66.Nobles KN, Guan Z, Xiao K, Oas TG, Lefkowitz RJ. The active conformation of β-arrestin1: direct evidence for the phosphate sensor in the N-domain and conformational differences in the active states of β-arrestins1 and -2. J Biol Chem. 2007;282:21370–81. doi: 10.1074/jbc.M611483200. [DOI] [PubMed] [Google Scholar]
- 67.Charest PG, Terrillon S, Bouvier M. Monitoring agonist-promoted conformational changes of β-arrestin in living cells by intramolecular BRET. EMBO Rep. 2005;6:334–40. doi: 10.1038/sj.embor.7400373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shukla AK, Violin JD, Whalen EJ, Gesty-Palmer D, Shenoy SK, Lefkowitz RJ. Distinct conformational changes in β-arrestin report biased agonism at seven-transmembrane receptors. Proc Natl Acad Sci USA. 2008;105:9988–93. doi: 10.1073/pnas.0804246105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gurevich VV, Benovic JL. Visual arrestin interaction with rhodopsin: Sequential multisite binding ensures strict selectivity toward light-activated phosphorylated rhodopsin. J Biol Chem. 1993;268:11628–38. [PubMed] [Google Scholar]
- 70.Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, et al. Functional antagonism of different G protein-coupled receptor kinases for β-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci USA. 2005;102:1442–47. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ren XR, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ. Different G protein-coupled receptor kinases govern G protein and β-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci USA. 2005;102:1448–53. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, et al. β-Arrestin-dependent, G protein-independent ERK1/2 activation by the β2 adrenergic receptor. J Biol Chem. 2006;281:1261–73. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 73.Kara E, Crepieux P, Gauthier C, Martinat N, Piketty V, et al. A phosphorylation cluster of five serine and threonine residues in the C-terminus of the follicle-stimulating hormone receptor is important for desensitization but not for β-arrestin-mediated ERK activation. Mol Endocrinol. 2006;20:3014–26. doi: 10.1210/me.2006-0098. [DOI] [PubMed] [Google Scholar]
- 74.Iwata K, Luo J, Penn RB, Benovic JL. Bimodal regulation of the human H1 histamine receptor by G protein-coupled receptor kinase 2. J Biol Chem. 2005;280:2197–204. doi: 10.1074/jbc.M408834200. [DOI] [PubMed] [Google Scholar]
- 75.Zidar DA, Violin JD, Whalen EJ, Lefkowitz RJ. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc Natl Acad Sci USA. 2009;106:9649–54. doi: 10.1073/pnas.0904361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-β-arrestin complexes after receptor endocytosis. J Biol Chem. 2001;276:19452–60. doi: 10.1074/jbc.M101450200. [DOI] [PubMed] [Google Scholar]
- 77.Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, βarrestin1, and βarrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201–10. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 78.Busillo JM, Armando S, Sengupta R, Meucci O, Bouvier M, Benovic JL. Site-specific phosphorylation of CXCR4 is dynamically regulated by multiple kinases and results in differential modulation of CXCR4 signaling. J Biol Chem. 2010;285:7805–17. doi: 10.1074/jbc.M109.091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Butcher AJ, Prihandoko R, Kong KC, McWilliams P, Edwards JM, et al. Differential G-protein coupled receptor phosphorylation provides evidence for a signalling barcode. J Biol Chem. 2011;286:11506–18. doi: 10.1074/jbc.M110.154526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nobles KN, Xiao K, Ahn S, Shukla AK, Lam CM, et al. Distinct GRK phosphorylation sites on the β2-adrenergic receptor: a “bar code” which differentially encodes β-arrestin functions. Sci Signal. 2011 doi: 10.1126/scisignal.2001707. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, et al. A unique mechanism of β-blocker action: carvedilol stimulates β-arrestin signaling. Proc Natl Acad Sci USA. 2007;104:16657–62. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heitzler D, Durand G, Rizk A, Ahn S, Kim J, et al. GRKs: molecular switches controlling the balance between G protein and β-arrestin-mediated signaling by 7TMRs. 2011 Submitted. [Google Scholar]
- 83.Labasque M, Reiter E, Becamel C, Bockaert J, Marin P. Physical interaction of calmodulin with the 5-hydroxytryptamine2C receptor C-terminus is essential for G protein-independent, arrestin-dependent receptor signaling. Mol Biol Cell. 2008;19:4640–50. doi: 10.1091/mbc.E08-04-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim IM, Tilley DG, Chen J, Salazar NC, Whalen EJ, et al. β-Blockers alprenolol and carvedilol stimulate β-arrestin-mediated EGFR transactivation. Proc Natl Acad Sci USA. 2008;105:14555–60. doi: 10.1073/pnas.0804745105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, et al. β-arrestin-mediated β1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Investig. 2007;117:2445–58. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bristow MR. β-Adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–69. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- 87.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of β-adrenergic signaling in heart failure? Circ Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 88.Gesty-Palmer D, Flannery P, Yuan L, Corsino L, Spurney R, et al. A β-arrestin-biased agonist of the parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation. Sci Transl Med. 2009;1:1ra1. doi: 10.1126/scitranslmed.3000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bodor ET, Offermanns S. Nicotinic acid: an old drug with a promising future. Br J Pharmacol. 2008;153(Suppl 1):S68–75. doi: 10.1038/sj.bjp.0707528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Semple G, Skinner PJ, Gharbaoui T, Shin YJ, Jung JK, et al. 3-(1H-tetrazol-5-yl)-1,4,5,6-tetrahydro-cyclopentapyrazole (MK-0354): a partial agonist of the nicotinic acid receptor, G-protein coupled receptor 109a, with antilipolytic but no vasodilatory activity in mice. J Med Chem. 2008;51:5101–8. doi: 10.1021/jm800258p. [DOI] [PubMed] [Google Scholar]