Abstract
Objective
To create a comprehensive model of the comparative impact of various interventions on maternal, fetal, and neonatal (MFN) mortality.
Methods
The major conditions and sub-conditions contributing to MFN mortality in low-resource areas were identified, and the prevalence and case fatality rates documented. Available interventions were mapped to these conditions, and intervention coverage and efficacy were identified. Finally, a computer model developed by the Maternal and Neonatal Directed Assessment of Technology (MANDATE) initiative estimated the potential of current and new interventions to reduce mortality.
Results
For PPH, the sub-causes, prevalence, and MFN case fatality rates were calculated. Available interventions were mapped to these sub-causes. Most available interventions did not prevent or treat the overall condition of PPH, but rather sub-conditions associated with hemorrhage and thus prevented only a fraction of the associated deaths.
Conclusion
The majority of current interventions address sub-conditions that cause death, rather than the overall condition; thus, the potential number of lives saved is likely to be overestimated. Additionally, the location at which mother and infant receive care affects intervention effectiveness and, therefore, the potential to save lives. A comprehensive view of MFN conditions is needed to understand the impact of any potential intervention.
Keywords: Low-income countries, Maternal mortality, Model, Postpartum hemorrhage, Stillbirth
1. Introduction
Maternal, fetal, and neonatal (MFN) mortality rates are unacceptably high, especially in low- and middle-income countries (LMICs) [1-4]. Most MFN mortality arises from common conditions, often occurring around birth [5-7]. The majority of these deaths could be prevented with access to adequate care, especially emergency obstetric and neonatal care at delivery. More than half of births worldwide occur outside a health facility, without a skilled attendant or the life-saving interventions available in high-income settings [8]. Many other births occur in facilities without adequate equipment or trained staff. Currently available interventions are often too complex for unskilled workers and hinder the widespread adoption of interventions to reduce MFN mortality [9]. Thus, innovative solutions are necessary to adapt many interventions for LMIC settings.
Although studies have estimated the effects of interventions on MFN mortality [2-5], no quantitative process currently compares specific medical interventions based on the potential to save lives in low-resource settings, given their availability, utilization, and efficacy. Innovative interventions across the continuum of care—including interventions for use in homes, health centers, and hospitals—may significantly improve perinatal outcomes. Comprehensive analyses are needed regarding the relationships between the causes of MFN mortality, in addition to the impact of interventions on these mortalities. Such analyses are of particular importance for low-resource settings with the highest mortality burden.
Most estimates of global maternal and neonatal mortality include broad causes of death (e.g. hemorrhage for maternal mortality). These categories, however, provide little guidance for which interventions would reduce mortality because the interventions are often directed at specific causes of death (e.g. retained placenta for postpartum hemorrhage [PPH]). To evaluate interventions that are likely to reduce mortality, the specific sub-causes of each condition must be addressed. When considering PPH, for example, uterotonics may reduce maternal mortality from postpartum uterine atony but not from other causes of hemorrhage. Only rarely do interventions address the overall condition. The proportion of deaths associated with each sub-cause is crucial, and in the example of PPH the number of deaths from atonic uterus must first be assessed to estimate the potential of an intervention to reduce the component of death associated with PPH.
The Maternal and Neonatal Directed Assessment of Technology (MANDATE) initiative was developed by the authors to address these needs. The primary objective was to evaluate the potential of interventions to reduce MFN mortality, with emphasis on low-resource settings. MANDATE has developed a web-based model to estimate the number of MFN lives saved through various interventions in order to inform which interventions, either new or adapted for use in various settings, could significantly reduce MFN mortalities. The present paper describes the MANDATE methods, using PPH as an example.
2. Materials and methods
A comprehensive English-language literature review of MFN mortality and relevant websites (WHO, UN, Cochrane Database of Systematic reviews) since 1980 was performed. The major conditions associated with MFN mortality and the specific etiologies associated with each condition were determined. The proportion of mortality associated with each etiology was calculated. Only previously published data were used, so Institutional Review Board approval was not required.
Next, the literature on interventions to reduce MFN mortality was reviewed to determine the efficacy, utilization, and penetration rates. Because a range of interventions (e.g. economic improvement and improved infrastructure) can impact mortality, medical interventions shown to reduce MFN mortality were examined and then mapped to the relevant causes of death [9,10]. To illustrate the framework, the present paper describes this process in detail for PPH specifically, but discusses its applicability across all causes of MFN mortality.
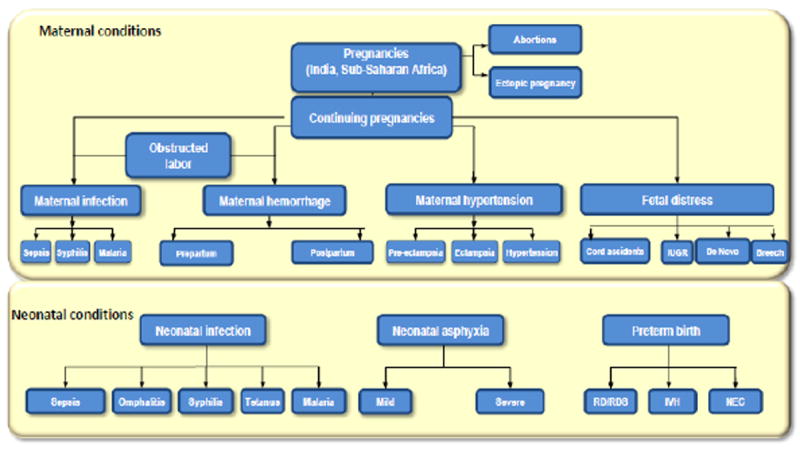
In summary, to evaluate conditions causing death, maps of the major conditions and specific etiologies within them were created (Figure 1). For each maternal and neonatal condition and sub-condition, MFN death rates were estimated. Additionally, the impact of maternal conditions on the prevalence of neonatal conditions was estimated. For example, placental abruption increases the prevalence of neonatal asphyxia and, subsequently, neonatal mortality. Once the flow through conditions leading to death was determined, an Excel-based (Microsoft, Redmond, WA, USA) computer model was used to estimate the impact of the interventions. The interventions were defined as prevention, diagnostic, or treatment (Box 1). An important concept is intervention coverage, which includes penetration, as defined by the availability of the technology and by actual utilization. Utilization also incorporates the skills of the user. The model quantifies less effective utilization in a given location or by certain levels of caregiver as a decrease in utilization. Using the concepts of coverage and efficacy, one can estimate the potential of an intervention to prevent a condition, to diagnose a condition, or to save MFN lives affected by that condition in different settings.
Figure 1.
MANDATE framework. Abbreviations: IUGR, intrauterine growth restriction; IVS, interventricular hemorrhage; MANDATE, Maternal and Neonatal Directed Assessment of Technology. NEC, necrotizing enterocolitis; RD/RDS, respiratory distress/respiratory distress syndrome.
Box 1. Maternal and Neonatal Directed Assessment of Technology (MANDATE) definitions.
No skilled providers present: a home or other setting at which no skilled healthcare provider is available.
Health clinic: a health facility with limited availability of skilled providers and lack of emergency obstetric and/or neonatal care (e.g. blood transfusion).
Hospital: a health facility with skilled providers.
Efficacy: the outcome of the technology under ideal conditions:
-
◆
For prevention, efficacy is the ability to prevent the onset of the condition in a mother/newborn at risk for that condition.
-
◆
For diagnostics, efficacy is the ability to identify a condition correctly if present.
-
◆
For treatment, efficacy is the ability to prevent death in someone with the condition.
Penetration: The availability of the technology intervention in a particular setting.
Utilization: The appropriate use of the technology intervention in the setting given its availability.
Coverage: For any technology, coverage equals penetration times utilization in the setting in which it is used.
Because interventions are administered at a location, within the model, an intervention can occur at home, in a clinic, or in a hospital. Certain interventions may be rationally used in some settings and not others; therefore, place of administration, defined in part by the skill of the practitioners generally available at that location, is crucial for understanding the potential impact of an intervention. A related feature is that mothers and/or newborns can and do move from one setting to another for medical care during the prenatal, delivery, and postpartum periods. Conceptually, improved or readily available diagnostics should lead to the use of effective treatments for a specific condition. However, a major impact of better or more available diagnostics might also be a change in the setting in which the treatment is administered.
In summary, the computer model simulates the relationships illustrated by the clinical conditions map. Each condition starts with the population at risk for that condition. The clinical condition (e.g. PPH) is divided into sub-causes of death, such as atonic uterus or retained placenta. The incidence and place of care (home, clinic, or hospital) are determined by sub-condition. The death rates associated with each sub-condition are determined and quantified, including the case fatality rates for mother, fetus, and neonate. Prevention, diagnostic, and treatment technologies, in addition to their coverage and efficacy, are estimated with regard to impact on mortality in the home, clinic, and hospital. Furthermore, the potential of an intervention to encourage patient movement between settings may be incorporated.
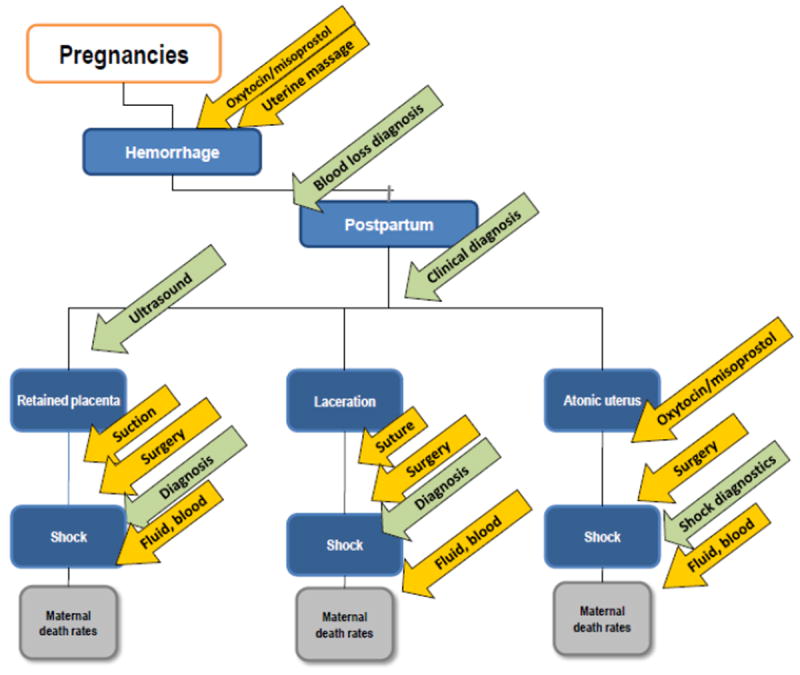
To analyze potential interventions to reduce obstetric hemorrhage, which is the leading cause of maternal death, PPH was examined. In this example, prepartum hemorrhage—which generally results from placental abruption or placenta previa and is associated with fewer maternal deaths, but with many stillbirths and neonatal deaths [11]—was not considered. Postpartum hemorrhage is usually caused by an atonic uterus; retained placental fragments; or cervical, vaginal, and perineal lacerations. Interventions include diagnostics to measure blood loss or hemoglobin levels, determining whether the mother is in shock, and lifesaving interventions such as blood or fluid replacement applied to hemorrhage cases, regardless of specific etiology. More commonly, however, each etiology of PPH has 1 or more specific potential interventions for prevention, diagnosis, and/or treatment, as included in the model (Figure 2).
Figure 2.
Map of obstetric hemorrhage and sub-conditions, and representative technologies that may reduce mortality.
As baseline care in the model, estimates for the penetration, utilization, and efficacy of preventions, diagnostics (including clinical recognition of bleeding), and treatments were evaluated and included. Because women with PPH may die of blood loss within several hours of initiation of the condition, timely treatment or movement to a health facility with appropriate support is often needed to prevent maternal death. Uterine atony is the most common etiology of PPH; manual compression and uterine massage may be reasonable preventive treatments for an atonic uterus and, although there are limited data regarding their ability to reduce mortality, these strategies are recommended by professional organizations for most deliveries to reduce PPH, so they were included in the model [12-14]. Another prevention included in the model was active management of the third stage of labor (AMTSL)—including use of oxytocics (e.g. misoprostol in community settings or oxytocin, which requires temperature control, in health facilities), uterine massage, and controlled cord traction—which has been associated with reduced risk for severe PPH [15,16]. Of the components of AMTSL, uterotonics, which are used to induce uterine contractions, have been studied most often. For example, randomized clinical trials have evaluated oxytocin and misoprostol for efficacy in reducing PPH [17-19]. Generally, these trials indicate that, although both oxytocin and misoprostol reduce PPH, oxytocin may be more effective at reducing PPH mortality [18].
Uterotonics, even if widely used, will address only a portion of the maternal deaths related to PPH: those associated with uterine atony. Uterine suction, or dilation and curettage may be necessary to address hemorrhage from a retained placenta. Surgical interventions may be necessary to stop bleeding from a uterine rupture or from cervical or vaginal lacerations. The treatments for major or uncontrolled hemorrhage (uterine surgery and blood transfusion) are usually available only in referral facilities [8]. The unavailability of blood transfusion is of particular concern in low-resource settings, where a limited number of facilities—inaccessible to many women—have such capability. Blood transfusion may be unavailable owing to, for example, lack of blood banking, reliable electricity, or blood typing capabilities [20]. Thus, the baseline model included relatively low estimated availability of blood transfusion at facilities, and none in the community (home or clinic).
3. Results
For PPH in Sub-Saharan Africa, the number of maternal deaths in the absence of treatment was estimated, followed by the number of maternal deaths given the current level of preventive, diagnostic, and treatment interventions. Impact on maternal mortality was then estimated for 2 alternate prevention/treatment scenarios. The first scenario examined the potential impact on maternal mortality of increased penetration and utilization of oxytocin for both prevention and treatment of PPH (increasing from an estimated 38% to 60% coverage in clinics and from 60% to 70% in hospitals). The second scenario evaluated the potential impact on maternal mortality of increased availability of blood products and improved transfer of women to health facilities with available blood products. In this case, the model estimated the effect of increased coverage of blood transfusion from 3% to 45% in clinics and from 36% to 85% in hospitals, with the majority of those diagnosed with hemorrhagic shock transferred to hospitals.
With no diagnostics, preventions, or treatments, there would be an estimated 93 150 hemorrhage-related maternal deaths per year in Sub-Saharan Africa, with 79 270 attributed to atonic uterus (Table 1). For the baseline, current level of care, a total of 67 590 hemorrhage-related deaths was estimated, with 54 730 attributed to atonic uterus. With expanded oxytocin use, the number of hemorrhage-related deaths was reduced to 57 370 (with 44 510 attributed to atonic uterus): a 15% reduction in mortality from the estimated current mortality. With blood products and increased transport to health facilities, maternal hemorrhage-related mortality was reduced to 42 030 deaths: a 38% reduction from the current maternal mortality.
Table 1.
Maternal mortality associated with PPH in Sub-Saharan Africa with scenarios a
| Scenario | No treatment | Baseline | Oxytocin: increased coverage in clinics and hospitals | Blood transfusion and transport: increased coverage and efficacy in hospitals | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| Home | Clinic | Hospital | Total | Home | Clinic | Hospital | Total | Home | Clinic | Hospital | Total | Home | Clinic | Hospital | Total | |
| Retained placenta | 4660 | 2280 | 420 | 7360 | 4470 | 2260 | 420 | 7150 | 4470 | 2260 | 420 | 7150 | 2055 | 817 | 1615 | 4486 |
| Laceration | 3260 | 2280 | 980 | 6520 | 3125 | 2000 | 585 | 5710 | 3125 | 2000 | 585 | 5710 | 1438 | 771 | 1251 | 3460 |
| Atonic uterus | 38 660 | 28 040 | 12 580 | 79 270 | 32 970 | 16 910 | 4850 | 54 740 | 25 945 | 14 080 | 4490 | 44 510 | 15 182 | 6599 | 12 302 | 34 082 |
| Total PPH- related deaths | 93 150 | 67 590 | 57 370 | 42 030 | ||||||||||||
Abbreviation: PPH, postpartum hemorrhage.
Using 2008 estimated birth rates from Demographic Health Surveys data.
4. Discussion
Using available data, maternal deaths associated with PPH—a major cause of maternal death—were estimated for several potential scenarios. In Sub-Saharan Africa, with current penetration and utilization of various interventions, only approximately one-quarter of maternal deaths that would probably occur with no treatment are being averted. Increasing coverage of oxytocin could reduce maternal mortality; however, more significant reductions could occur with increased availability of blood products and transfer to facilities with this capability. This is probably because of the fact that oxytocin addresses only hemorrhage-related mortality associated with atonic uterus, whereas blood products and transfer have the potential to manage hemorrhage overall.
One of the most important observations was that interventions are targeted to specific clinical sub-conditions and, thus, have the potential to reduce only a fraction of the deaths associated with that condition. Uterotonics such as oxytocin or misoprostol are one such example. Although these have been widely promoted to reduce death associated with hemorrhage, this intervention has the potential to reduce only the subset of maternal deaths associated with PPH due to atonic uterus. Thus, the map of how interventions may address both causes and sub-causes of death is necessary to estimate accurately the potential lives saved. A related advantage of this type of analysis, although not shown in the PPH example, is the ability to address the interconnectedness of the conditions associated with the majority of MFN deaths [21-24]. Because of the interrelated etiologies of these deaths, some interventions have the potential to reduce causes of death across conditions. As such, the detailed analysis of sub-conditions causing MFN death will be critical to evaluating the potential impact of various interventions.
Additionally, the location at which mother and infant receive care is important in assessing the potential to save lives, as demonstrated when women were transferred in the PPH example. When evaluating interventions, the location can be viewed in terms of where the mother receives prenatal care and care at birth, and the potential role of the intervention in moving women and their infants to another location. Although many efforts are underway to promote birth in a health facility, the potential of a medical intervention to move women to these health facilities has not been well studied, despite being an important possible role. In the PPH example, if transfer to a hospital were not possible, interventions that facilitated blood transfusion at lower levels of care would reduce maternal deaths associated with hemorrhage.
An important limitation of evaluating the impact of interventions was the small number of quality studies documenting mortality rates, specifically the sub-causes of death, in geographic areas with the highest rates of mortality. Furthermore, the utilization and coverage of interventions, including barriers to their use, have not been widely studied. Thus, research into these areas would enhance the understanding of geographic-specific causes of mortality and the barriers to existing interventions. As development of the computer model continues, the populations affected by each of the conditions will be fully connected to enable complete analyses of the ability of interventions to impact mortality across conditions. Therefore, if placental abruptions were reduced or treated more expeditiously, the prevalence of neonatal asphyxia related to that condition would be expected to decrease. With decreasing prevalence, even if the case fatality rate associated with neonatal asphyxia remained unchanged, the total number of asphyxia-related neonatal deaths would decrease. The MANDATE model will be able to make these adjustments, and therefore it provides an important web-based tool for examining the potential of a wide range of current and possible interventions to reduce MFN mortality.
The MANDATE model is useful as more than simply an academic exercise. Several foundations, including the Bill and Melinda Gates Foundation and WHO, and agencies of several governments have funded projects exploring how the development of new technologies and the modification or expansion of coverage for existing technologies could reduce MFN mortality. Use of the MANDATE model has already enabled such organizations to focus on projects with the most potential to save MFN lives, and it will continue to do so in the future. Because users can adjust each parameter of the model based on their own assumptions, they have maximum flexibility to assess the potential value of their proposed intervention.
Acknowledgments
The present work was supported through a grant from the Bill and Melinda Gates Foundation to the Research Triangle Institute.
Footnotes
Conflict of interest
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cousens S, Blencowe H, Stanton C, Chou D, Ahmed S, Steinhardt L, et al. National, regional, and worldwide estimates of stillbirth rates in 2009 with trends since 1995: a systematic analysis. Lancet. 2011;377(9774):1319–30. doi: 10.1016/S0140-6736(10)62310-0. [DOI] [PubMed] [Google Scholar]
- 2.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–87. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 3.Hogan MC, Foreman KJ, Naghavi M, Ahn SY, Wang M, Makela SM, et al. Maternal mortality for 181 countries, 1980-2008: a systematic analysis of progress towards Millennium Development Goal 5. Lancet. 2010;375(9726):1609–23. doi: 10.1016/S0140-6736(10)60518-1. [DOI] [PubMed] [Google Scholar]
- 4.McClure EM, Pasha O, Goudar SS, Chomba E, Garces A, Tshefu A, et al. Epidemiology of stillbirth in low-middle income countries: a Global Network Study. Acta Obstet Gynecol Scand. 2011;90(12):1379–85. doi: 10.1111/j.1600-0412.2011.01275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldenberg RL, McClure EM, Bhutta ZA, Belizán JM, Reddy UM, Rubens CE, et al. Stillbirths: the vision for 2020. Lancet. 2011;377(9779):1798–805. doi: 10.1016/S0140-6736(10)62235-0. [DOI] [PubMed] [Google Scholar]
- 6.Bhutta ZA, Chopra M, Axelson H, Berman P, Boerma T, Bryce J, et al. Countdown to 2015 decade report (2000-10): taking stock of maternal, newborn, and child survival. Lancet. 2010;375(9730):2032–44. doi: 10.1016/S0140-6736(10)60678-2. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg RL, McClure EM, Bann CM. The relationship of intrapartum and antepartum stillbirth rates to measures of obstetric care in developed and developing countries. Acta Obstet Gynecol Scand. 2007;86(11):1303–9. doi: 10.1080/00016340701644876. [DOI] [PubMed] [Google Scholar]
- 8.Darmstadt GL, Lee AC, Cousens S, Sibley L, Bhutta ZA, Donnay F, et al. 60 60 Million non-facility births: who can deliver in community settings to reduce intrapartum-related deaths? Int J Gynecol Obstet. 2009;107(Suppl 1):S89–112. doi: 10.1016/j.ijgo.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Free MJ. Achieving appropriate design and widespread use of health care technologies in the developing world. Overcoming obstacles that impede the adaptation and diffusion of priority technologies for primary health care. Int J Gynecol Obstet. 2004;85(Suppl 1):S3–13. doi: 10.1016/j.ijgo.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367(9516):1066–74. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 11.Karoshi M, Keith L. Challenges in managing postpartum hemorrhage in resource-poor countries. Clin Obstet Gynecol. 2009;52(2):285–98. doi: 10.1097/GRF.0b013e3181a4f737. [DOI] [PubMed] [Google Scholar]
- 12.Patel A, Goudar SS, Geller SE, Kodkany BS, Edlavitch SA, Wagh K, et al. Drape estimation vs. visual assessment for estimating postpartum hemorrhage. Int J Gynecol Obstet. 2006;93(3):220–4. doi: 10.1016/j.ijgo.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Hofmeyr GJ, Abdel-Aleem H, Abdel-Aleem MA. Uterine massage for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2008;(3) doi: 10.1002/14651858.CD006431.pub2. CD006431. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Aleem H, Singata M, Abdel-Aleem M, Mshweshwe N, Williams X, Hofmeyr GJ. Uterine massage to reduce postpartum hemorrhage after vaginal delivery. Int J Gynecol Obstet. 2010;111(1):32–6. doi: 10.1016/j.ijgo.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 15.Begley CM, Gyte GM, Murphy DJ, Devane D, McDonald SJ, McGuire W. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2010;(7) doi: 10.1002/14651858.CD007412.pub2. CD007412. [DOI] [PubMed] [Google Scholar]
- 16.Sosa CG, Althabe F, Belizan JM, Buekens P. Use of oxytocin during early stages of labor and its effect on active management of third stage of labor. Am J Obstet Gynecol. 2011;204(3):238.e1–5. doi: 10.1016/j.ajog.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derman RJ, Kodkany BS, Goudar SS, Geller SE, Naik VA, Bellad MB, et al. Oral misoprostol in preventing postpartum haemorrhage in resource-poor communities: a randomised controlled trial. Lancet. 2006;368(9543):1248–53. doi: 10.1016/S0140-6736(06)69522-6. [DOI] [PubMed] [Google Scholar]
- 18.Gülmezoglu AM, Villar J, Ngoc NT, Piaggio G, Carroli G, Adetoro L, et al. WHO multicentre randomised trial of misoprostol in the management of the third stage of labour. Lancet. 2001;358(9283):689–95. doi: 10.1016/s0140-6736(01)05835-4. [DOI] [PubMed] [Google Scholar]
- 19.Widmer M, Blum J, Hofmeyr GJ, Carroli G, Abdel-Aleem H, Lumbiganon P, et al. Misoprostol as an adjunct to standard uterotonics for treatment of post-partum haemorrhage: a multicentre, double-blind randomised trial. Lancet. 2010;375(9728):1808–13. doi: 10.1016/S0140-6736(10)60348-0. [DOI] [PubMed] [Google Scholar]
- 20.Soltani H, Hutchon DR, Poulose TA. Timing of prophylactic uterotonics for the third stage of labour after vaginal birth. Cochrane Database Syst Rev. 2010;(8) doi: 10.1002/14651858.CD006173.pub2. CD006173. [DOI] [PubMed] [Google Scholar]
- 21.Akinola OI, Fabamwo AO, Tayo AO, Rabiu KA, Oshodi YA, Onyekwere CA. Evaluation of blood reservation and use for caesarean sections in a tertiary maternity unit in south western Nigeria. BMC Pregnancy Childbirth. 2010;10:57. doi: 10.1186/1471-2393-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhutta ZA, Lassi ZS, Blanc A, Donnay F. Linkages among reproductive health, maternal health, and perinatal outcomes. Semin Perinatol. 2010;34(6):434–45. doi: 10.1053/j.semperi.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Goldenberg RL, McClure EM, Althabe F. Commentary: improving important pregnancy outcomes. Birth. 2009;36(1):51–3. doi: 10.1111/j.1523-536X.2008.00299.x. [DOI] [PubMed] [Google Scholar]
- 24.Goldenberg RL, McClure EM. Disparities in interventions for child and maternal mortality. Lancet. 2012;379(9822):1178–80. doi: 10.1016/S0140-6736(12)60474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


