Abstract
Pharmacological studies suggest that A2B adenosine receptors mediate proinflammatory effects of adenosine in human mast cells in part by up-regulating production of Th2 cytokines and angiogenic factors. This concept has been recently challenged by the finding that mast cells cultured from bone marrow-derived mast cells (BMMCs) of A2B knockout mice display an enhanced degranulation in response to FcεRI stimulation. This finding was interpreted as evidence of anti-inflammatory functions of A2B receptors and it was suggested that antagonists with inverse agonist activity could promote activation of mast cells. In this report, we demonstrate that genetic ablation of the A2B receptor protein has two distinct effects on BMMCs, one is the previously reported enhancement of Ag-induced degranulation, which is unrelated to adenosine signaling; the other is the loss of adenosine signaling via this receptor subtype that up-regulates IL-13 and vascular endothelial growth factor secretion. Genetic ablation of A2B receptors had no effect on A3 adenosine receptor-dependent potentiation of Ag-induced degranulation in mouse BMMCs, but abrogated A2B adenosine receptor-dependent stimulation of IL-13 and vascular endothelial growth factor secretion. Adenosine receptor antagonists MRS1706 and DPCPX with known inverse agonist activity at the A2B subtype inhibited IL-13 secretion induced by the adenosine analog NECA, but did not mimic the enhanced Ag-induced degranulation observed in A2B knockout BMMCs. Thus, our study confirmed the proinflammatory role of adenosine signaling via A2B receptors and the anti-inflammatory actions of A2B antagonists in mouse BMMCs.
Adenosine is an intermediate product in the metabolism of ATP. Adenosine exerts its action by binding to G protein-coupled adenosine receptors. Four subtypes of adenosine receptors have been cloned and classified as A1, A2A, A2B, and A3 receptors (1). Among adenosine receptors, the A2B subtype has the lowest affinity to adenosine. A2B receptors are thought to remain silent under normal physiological conditions when adenosine concentrations are low and become active only when extracellular adenosine levels rise as a result of inflammation, ischemia, or cell damage (2).
There is growing evidence that adenosine plays an important role in chronic lung inflammation. Elevated concentrations of adenosine are found in bronchoalveolar lavage fluid (3) and exhaled breathe condensate (4) obtained from asthma patients. Studies in adenosine deaminase (ADA)3-deficient mice, characterized by elevated lung tissue levels of adenosine, strongly suggest a causal association between adenosine and an inflammatory phenotype (5, 6). These mice exhibit a lung phenotype with features of lung inflammation, bronchial hyperresponsiveness, enhanced mucus secretion, increased IgE synthesis, and elevated levels of proinflammatory cytokines and angiogenic factors that could be reversed by lowering adenosine levels with exogenous ADA (5). Even more striking are the similarities in lung inflammatory phenotypes found between ADA-deficient mice and mice overexpressing IL-13 (7). The A2B adenosine receptor subtype appears to play an important role in this model because pharmacological inhibition of A2B receptors significantly reduced elevations in proinflammatory cytokines as well as mediators of airway remodeling induced by high adenosine levels in the lungs of ADA-deficient mice (8).
Mast cell activation has been long recognized to be crucial to the pathophysiology of asthma (9) and adenosine has been shown to modulate mast cell functions (10). We have previously demonstrated that stimulation of A2B receptors in human mast cell line HMC-1 increases production of proinflammatory Th2 cytokines IL-4 and IL-13 (11, 12), as well as angiogenic factors IL-8 and vascular endothelial growth factor (VEGF) (13, 14). Furthermore, conditioned medium from these activated mast cells promoted capillary formation in an angiogenesis model (14) and induced IgE synthesis in naive B lymphocytes (11). For this and other reasons, mast cell A2B adenosine receptors have been suggested to promote pulmonary inflammation and airway remodeling associated with asthma.
Contrary to this body of evidence, a recent report by Hua et al. (15) on a mouse phenotype resulting from deletion of A2B receptor gene suggested that the A2B receptor functions as a negative regulator of mast cell activation. This conclusion was based on heightened susceptibility of the A2B adenosine receptor knockout (A2BKO) mice to Ag-induced anaphylaxis and mast cell degranulation, which was attributed to the loss of purportedly constitutive stimulation of adenylate cyclase by these receptors in mast cells. This results led the authors to propose that A2B antagonists with inverse agonist activity, binding to the same receptor site as agonists but exerting the opposite pharmacological effect, could enhance Ag-induced mast cell degranulation and proinflammatory cytokine production. However, the effect of direct agonist stimulation of adenosine receptors was not explored in that study, and in the absence of receptor stimulation, it is difficult to determine whether the enhanced degranulation of A2BKO mast cells indeed reflects the loss of adenosine-dependent signaling functions of A2B receptors or whether it could be due to the loss of a previously unrecognized function of the A2B receptor protein independent of adenosine ligation. Furthermore, the study of Hua et al. (15) did not address the previously suggested role of A2B receptors in stimulation of Th2 cytokines and angiogenic factors, nor did it sub-stantiate the claim of potential proinflammatory action of A2B antagonists on mast cells.
Because all these unresolved issues may have important implications for the understanding of the role of A2B receptors in regulation of inflammation and hence for development of new therapeutic approaches to diseases associated with inflammation, we addressed these questions in the current work by studying the effects of genetic A2B receptor ablation on adenosine receptor agonist-induced potentiation of mast cell degranulation, stimulation of IL-13, and VEGF secretion and by comparing these effects of A2B receptor gene disruption to the action of A2B receptor antagonists with known inverse agonist activity.
Materials and Methods
Reagents
NECA (5′-N-ethylcarboxamidoadenosine), CGS21680 (2-p(2-carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine), DPCPX (8-cyclopentyl-1,3-dipropylxanthine), and forskolin were purchased from Sigma-Aldrich. MRS1706 (N-(4-acetylphenyl)-2-(-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)phenoxy) acetamide) was obtained from Tocris. Adenylate cyclase inhibitor 2′,5′-dideoxyadenosine, MEK1/2-specific inhibitor UO126 (1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenyl-thio) butadiene) and its inactive analog UO124 (1,4-diamino-2,3-dicyano-1,4-bis(methylthio)butadiene), selective inhibitor of p38 MAPK SB202190 (4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)1H-imidazole) and its inactive analog SB202474 (4-Ethyl-2(p-methoxyphenyl)-5-(4′-pyridyl)-1H-imidazole) were purchased from Calbiochem. DMSO was purchased from Sigma-Aldrich. When used as a solvent, final DMSO concentrations in all assays did not exceed 0.1% and the same DMSO concentrations were used in vehicle controls.
Mice and bone marrow-derived mast cell (BMMC) culture
All studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. Animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of Vanderbilt University (Nashville, TN). Six- to 8-week-old, age- and sex-matched mice were used. A2BKO mice were obtained from Deltagen and wild-type (WT) C57BL/6 mice were purchased from Harlan World Headquarters. Genotyping protocols for A2BKO have been previously described (16). All of the A2BKO mice used in these studies were backcrossed to the C57BL/6 genetic background for 10 generations.
BMMCs were cultured as previously described (15). In brief, bone marrow was flushed from the femurs and tibias of each mouse with RPMI 1640 medium, supplemented with 2% FBS and 10 U/μl heparin. After centrifugation and washing with RPMI 1640, cells were resuspended at 0.5 × 106 cells/ml in RPMI 1640 medium supplemented with 10% FBS, 2 mM L-glutamine, nonessential amino acids, 50 μM 2-ME, 1× antibiotic-antimycotic mixture (Sigma-Aldrich), 20 ng/ml mouse IL-3 (PeproTech), and 20 ng/ml stem cell factor (United States Biologicals). Cells were maintained in suspension culture under humidified atmosphere of air/CO2 (19:1) at 37°C. The medium was renewed every week and 5- to 6-wk cultures were used for experiments.
Expression of FcεRI and c-kit on BMMC surface was determined by flow cytometry. After treatment with Fc Receptor Blocking reagent (Miltenyi Biotec), BMMCs (106 cells) were incubated with 10 μg/ml mouse anti-DNP IgE (clone SPE-7; Sigma-Aldrich) for 30 min on ice. After brief centrifugation to remove unbound IgE, cells were resuspended and labeled using FITC-conjugated anti-IgE (clone 23G3; eBioscience) and anti-CD117-PE (clone 2B8; BD Pharmingen) Abs for 20 min. Data acquisition was performed on a FACSCalibur flow cytometer (BD Immunocytometry Systems) and the data were analyzed with WinList 5.0 software. Nonviable cells were excluded by using 7-aminoactinomycin D. Ag negativity was defined as having the same fluorescent intensity as the isotype control.
Human mast cells
Human mast cell line HMC-1 was a gift from Dr. J. H. Butterfield (Mayo Clinic, Rochester, MN). HMC-1 cells were maintained in suspension culture at a density between 3 and 6 × 105 cells/ml by dilution with Iscove’s medium supplemented with 10% FBS, 2 mM glutamine, 1.2 mM α-thio-glycerol, and 1× antibiotic-antimycotic mixture. Cells were kept under humidified atmosphere of air/CO2 (19:1) at 37°C.
Real-time RT-PCR
Real-time RT-PCR analysis was performed as previously described (17). Total RNA was isolated from cells using the RNeasy Mini kit (Qiagen). Real-time RT-PCR was conducted on ABI PRISM 7900HT Sequence Detection System (PE Applied Biosystems). Primer pairs and 6-carboxy-fluorescein-labeled probes for murine adenosine receptors and β-actin were provided by Applied Biosystems. RT-PCR using 1 μg of DNase-treated total RNA was performed under conditions recommended by the manufacturer. A standard curve for each amplicon was obtained using serial dilutions of total RNA. The results from triplicate PCR for a given gene at each time point were used to determine mRNA quantity relative to the corresponding standard curve. The relative mRNA quantity for a given gene measured from a single reverse transcription reaction was divided by the value obtained for β-actin to correct for fluctuations in input RNA levels and varying efficiencies of RT-PCR.
Measurement of cAMP accumulation
Cyclic AMP accumulation was measured as previously described (18). BMMCs (5 × 106 cells/ml) were preincubated in Tyrode’s buffer (150 mM NaCl, 2.7 mM KCl, 0.37 mM NaH2PO4, 1 mM MgSO4, 1 mM CaCl2, 5 g/L D-glucose, 10 mM HEPES-NaOH (pH 7.4)) containing 1 U/ml ADA and the cAMP phosphodiesterase inhibitor papaverine (1 mM) for 15 min at 37°C. To test the effect of adenylate cyclase inhibition on cAMP accumulation, HMC-1 cells (2 × 106 cells/ml) were preincubated in the same buffer containing increasing concentrations of 2′,5′-dideoxyadenosine for 30 min at 37°C. Adenosine receptor agonists, antagonists, forskolin or their vehicle (DMSO) were added to cells, and the incubation was allowed to proceed for 5 min at 37°C. The reaction was stopped by the addition of 1/5 volume of 25% TCA. The extracts were washed five times with 10 volumes of water-saturated ether. Cyclic AMP concentrations were determined using a cAMP assay kit (GE Healthcare).
Mast cell degranulation assay
Mast cell degranulation was determined by measuring β-hexosaminidase activity and histamine concentrations. BMMCs were incubated 1 h at 37°C with a monoclonal IgE directed against DNP-human serum albumin (DNP-HSA, clone SPE-7; Sigma-Aldrich) at a concentration of 10 μg/ml/106 cells in Tyrode’s buffer lacking Ca2+. After removal of unbound IgE by washing twice with Tyrode’s buffer, BMMCs were resuspended at a concentration of 106 cells/ml in Tyrode’s buffer containing 1 mM CaCl2 and 1 U/ml ADA. Cells were incubated in the presence or absence of DNP-HSA (Sigma-Aldrich) and in the presence or absence of NECA at 37°C. In some experiments, A2B antagonists or their vehicle were included in Tyrode’s buffer at this and all prior steps (see Fig. 5B). Reactions were terminated after 20 min by placing tubes on ice. The release of β-hexosaminidase was quantified in cell supernatants and pellets by the hydrolysis of p-nitrophenyl-N-acetyl-βD-glucosaminide (Sigma-Aldrich), as detailed elsewhere (19). Histamine concentrations were determined using an enzyme immunoassay kit (Immunotech Histamine kit; Beckman Coulter).
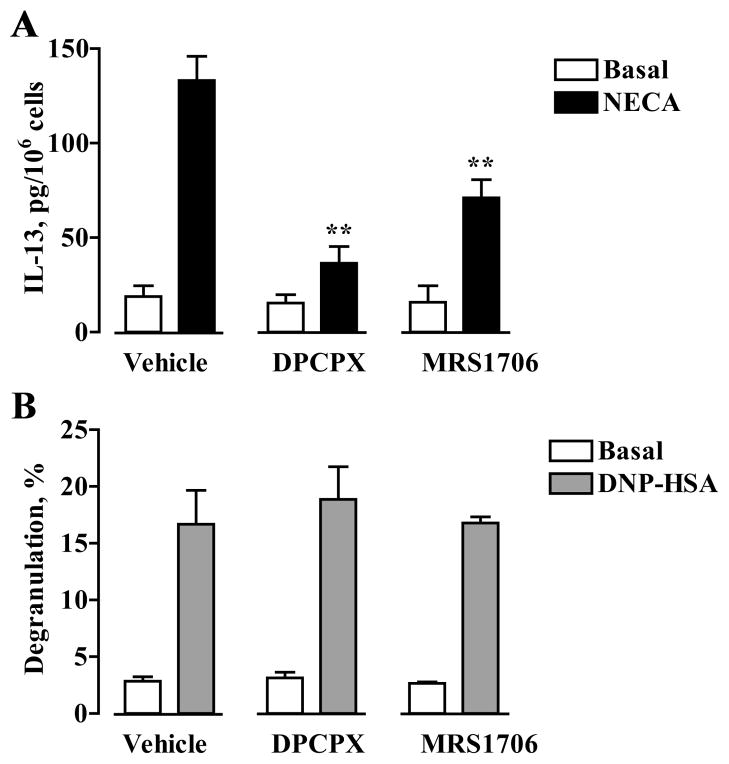
FIGURE 5.
Effects of DPCPX and MRS1706 on IL-13 secretion and β-hexosaminidase release from mast cells. A, BMMCs were incubated in the absence (Basal) or presence of 10 μM NECA and in the presence of 1 μM DPCPX, 100 nM MRS1706, or their vehicle for 6 h. IL-13 was measured in culture medium and expressed as picograms per 106 cells. Data are presented as mean ± SEM of five separate cell preparations. **, p < 0.01, significant difference from vehicle determined by one-way ANOVA with Dunnett’s post-test. B, Degranulation of BMMCs was assessed by measuring β-hexosaminidase release from cells preincubated with anti-DNP IgE after stimulation with 10 ng/ml DNP-HSA Ag or from nonstimulated cells (Basal) in the presence of 1 μM DPCPX, 100 nM MRS1706, or their vehicle. Values are expressed as mean ± SEM of three separate cell preparations.
Analysis of IL-6, IL-13, and VEGF secretion
When indicated, BMMCs were preincubated with anti-DNP-HSA as described. To determine IL-6 and IL-13 release, BMMCs (5 × 106 cells/ml) were incubated in the presence or absence of DNP-HSA, and in the presence or absence of NECA and A2B receptor antagonists in RPMI 1640 medium with 10% calf serum, 1× antibiotic-antimycotic mixture and 1 U/ml ADA for 6 h at 37°C. To determine VEGF release, BMMCs were incubated in the same medium for 16 h at 37°C.
HMC-1 cells were resuspended at a concentration of 2 × 106 cells/ml in Iscove’s medium containing 2 mM glutamine, 1.2 mM α-thioglycerol, and 1 U/ml ADA in the absence or presence of tested reagents. Reactions were started by addition of NECA or its vehicle and continued for 6 h under humidified atmosphere of air/CO2 (19:1) at 37°C. IL-6, IL-13, and VEGF concentrations in supernatants were measured using ELISA kits (R&D Systems).
To determine the effects of inhibitors of intracellular signaling pathways, BMMCs or HMC-1 cells were preincubated in the presence of tested compounds for 30 min at 37°C before addition of NECA.
Transfections and luciferase reporter assay
HMC-1 cells were transfected using Fugene 6 transfection reagent (Roche). A total of 2 μg of plasmid DNA (0.5 μg/μl) was mixed with 84 μl of serum-free Iscove’s medium containing 6 μl of Fugene 6. After 20 min of incubation at room temperature, the transfection mixture was added to 1 × 106 cells suspended in 1 ml of growth medium. The ratio 10:1 was used for VEGF or IL-13 firefly luciferase reporter to control Renilla luciferase reporter combinations. VEGF promoter-driven luciferase reporter, a firefly luciferase reporter plasmid comprising 5′ flanking −1005 to +379 bp of the human VEGF gene (20), was provided by Dr. G. L. Semenza (Johns Hopkins Hospital, Baltimore, MD). IL-13 promoter-driven luciferase reporter pIL13-939/+48-luc, a firefly luciferase reporter plasmid comprising 5′ flanking −939 to +48 bp of the human IL-13 gene (21), was gifted by Drs. H. Young, O. Shimozato, and D. Derse (National Cancer Institute, Frederick, MD). A control constitutively active Renilla luciferase plasmid pRL-SV40 was purchased from Promega. Twenty-four hours after transfection, cells were incubated in the presence or absence of 10 μM NECA for an additional 6 h. Reporter activity was then measured using a Dual-Luciferase Reporter Assay System (Promega). Firefly luciferase reporter activities were normalized against Renilla luciferase activities and expressed as relative luciferase activities over basal (set as 1).
Evaluation of ERK and p38 MAPK activation
BMMCs were cultured in their growth medium without IL-3 and stem cell factor for 24 h. Three hours before experiment, BMMCs and HMC-1 cells were starved by culturing in their corresponding growth medium without serum. After starvation, cells were resuspended in Tyrode’s buffer at a concentration of 2 × 107 cells/ml and stimulated with 10 μM NECA for the indicated periods. Reactions were stopped by cell lysis in ice-cold RIPA buffer (Santa Cruz Biotechnology). Proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (NEN Life Science Products). Membranes were blocked and incubated consecutively with primary Ab and HRP-conjugated secondary Ab. Immunoreactive proteins were visualized by ECL reagents (NEN Life Science Products). Anti-phospho-ERK (sc-7976), anti-phospho-p38 (sc-7975), anti-ERK (sc-154), and anti-p38 (sc-7149) MAPKs were purchased from Santa Cruz Biotechnology.
Results
Expression of mast cell surface markers in A2BKO and WT BMMCs
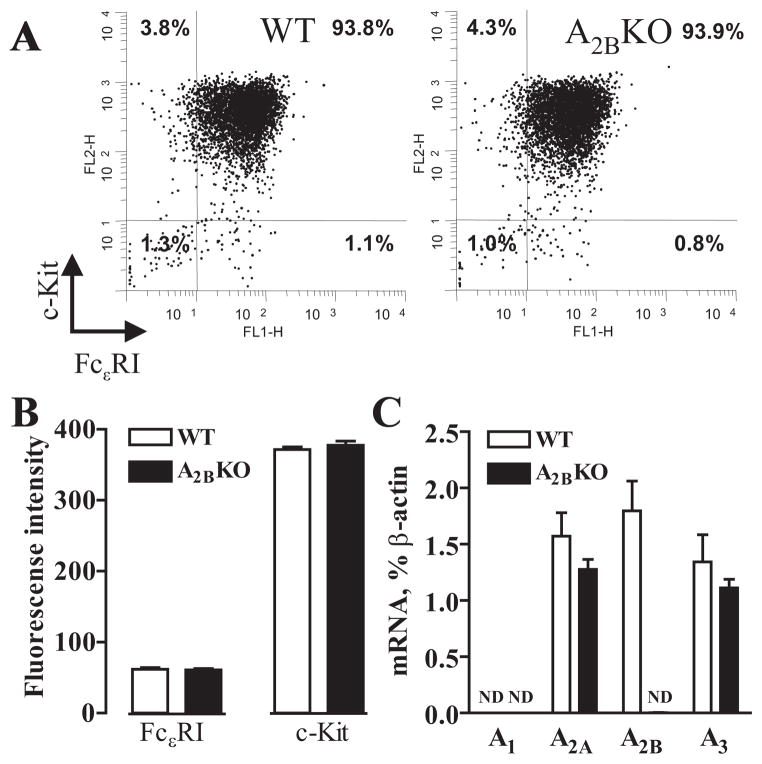
Mast cells obtained from A2BKO mice were morphologically similar to those obtained from WT animals. After 5 wk in culture, over 90% of A2BKO and WT BMMCs expressed both c-kit and FcεRI on their surface (Fig. 1A). The expression levels of these mast cell surface markers were the same in A2BKO and WT BMMCs as determined by flow cytometry (Fig. 1B).
FIGURE 1.
Expression of FcεRI, c-kit and adenosine receptors in WT and A2BKO BMMCs. A, Representative cytofluorographic dot plots of FcεRI and c-kit cell surface expression on mast cells derived from WT and A2BKO mice by culturing bone marrow cells in the presence of IL-3 and stem cell factor for 5 wk as described in Materials and Methods. B, Expression levels of FcεRI and c-kit on WT and A2BKO BMMCs are presented as mean fluorescence intensity above isotype control. Values are expressed as mean ± SEM of five separate cell preparations. C, Real-time RT-PCR analysis of mRNA encoding adenosine receptor subtypes was performed as described in Materials and Methods. Values are expressed as mean ± SEM of five separate cell preparations. Nd, No transcripts detected.
Effect of adenosine A2B receptor gene ablation on adenosine receptor mRNA expression in BMMCs
Real-time RT-PCR analysis of WT BMMCs revealed mRNA encoding A2A, A2B, and A3 receptor subtypes (1.57 ± 0.21%, 1.8 ± 0.27%, and 1.34 ± 0.24% of β-actin, respectively) and no detectable levels of A1 receptor transcripts (Fig. 1C). As expected, we did not detect the expression of A2B receptor mRNA in A2BKO BMMCs. We also documented that A2B receptor gene ablation had no significant effect on A2A and A3 receptor mRNA expression in A2BKO BMMCs (1.28 ± 0.09% and 1.11 ± 0.08% of β-actin, respectively).
Effect of adenosine A2B receptor gene ablation on cAMP levels in BMMCs
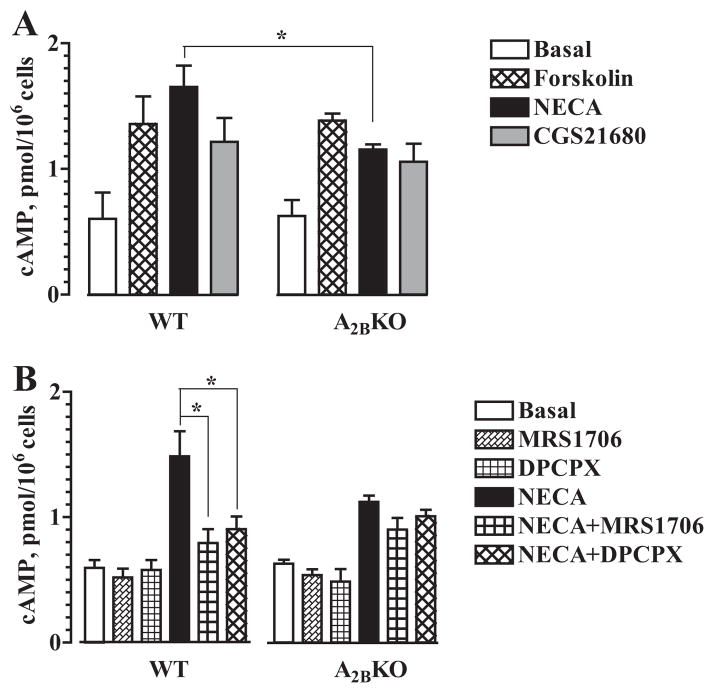
Adenosine receptor subtypes were initially characterized by their effects on adenylate cyclase activity; A2A and A2B receptors stimulate adenylate cyclase via coupling to Gs proteins, whereas A1 and A3 receptors inhibit stimulation of this enzyme via coupling to Gi proteins (1). Therefore, we determined whether A2B receptor gene ablation has any effect on cAMP levels in BMMCs. As seen in Fig. 2A, comparative analysis revealed no significant difference in basal cAMP levels between BMMCs isolated from WT and A2BKO mice (0.602 ± 0.210 and 0.625 ± 0.127 pmol/106 cells, respectively). Similarly, no significant difference between WT and A2BKO BMMCs was found in cAMP accumulation induced with the adenylate cyclase stimulator forskolin (10 μM). However, stimulation of adenosine receptors with the nonselective agonist NECA (10 μM) induced greater cAMP accumulation in WT cells compared with A2BKO BMMCs (1.65 ± 0.17 and 1.15 ± 0.04 pmol/106 cells, respectively; p < 0.05, n = 5 separate cell preparations). In contrast, we found no significant difference in cAMP accumulation between WT and A2BKO BMMCs stimulated with the selective A2A receptor agonist CGS21680 (1 μM). Thus, cAMP accumulation induced by activation of all adenosine receptors with NECA was significantly lower in A2BKO BMMCs due to the lack of A2B receptors, but genetic ablation of A2B receptors had no effect on basal cAMP levels and cAMP accumulation induced by stimulation of A2A receptors with CGS21680 or by receptor-independent stimulation of adenylate cyclase with forskolin. The selective A2B antagonist MRS1706 (100 nM) and the A1/A2B antagonist DPCPX (1 μM) had no significant effect on basal cAMP levels in both WT and A2BKO cells. However, these antagonists significantly attenuated NECA-induced cAMP accumulation in WT but not in A2BKO BMMCs (Fig. 2B).
FIGURE 2.
Intracellular cAMP levels in WT and A2BKO BMMCs. A, Cyclic AMP accumulation in WT and A2BKO mast cells incubated in the presence of 1 μM forskolin, 10 μM NECA, 1 μM CGS21680 or their vehicle (Basal). Values are expressed as mean ± SEM of five separate cell preparations. *, p < 0.05, significant difference between corresponding WT and A2BKO values determined using unpaired two-tail t test. B, Effects of 100 nM MRS1706, 1 μM DPCPX, or their vehicle on cAMP accumulation in the absence (MRS1706, DPCPX, or Basal, respectively) or presence (NECA+MRS1706, NECA+DPCPX, or NECA, respectively) of 10 μM NECA. Values are expressed as mean ± SEM of five separate cell preparations. *, p < 0.05, significant difference from vehicle in NECA-stimulated WT BMMCs determined using one-way ANOVA with Dunnett’s posttest.
Effect of adenosine A2B receptor gene ablation on adenosine-dependent potentiation of BMMC degranulation
Stimulation of adenosine receptors per se does not produce the release of preformed mediators from BMMCs, but potentiates degranulation induced by clustering FcεRI (22). This adenosine action is mediated via A3 adenosine receptors because adenosine agonists do not potentiate Ag-induced degranulation of A3KO BMMCs (23). As seen in Fig. 3A, basal release of β-hexosaminidase, a granule-associated enzyme, from A2BKO BMMCs was similar to that observed in WT cells. Stimulation of adenosine receptors with 10 μM NECA had no significant effect on basal β-hexosaminidase release, but potentiated the release induced by 10 ng/ml DNP-HSA from both A2BKO and WT cells. Our results show that Ag-dependent clustering of FcεRI with DNP-HSA produced greater degranulation of A2BKO BMMCs compared with WT cells as determined by release of β-hexosaminidase (Fig. 3A) and histamine (data not shown). Ag-induced degranulation of A2BKO BMMCs was also greater in the presence of NECA (Fig. 3A). However, the degree of potentiation of DNP-HSA-induced degranulation by NECA was virtually the same in both WT and A2BKO cells (1.6 ± 0.1- and 1.7 ± 0.1-fold, respectively) (Fig. 3B), suggesting that A2B receptors do not play a role in this adenosine action on mouse mast cells.
FIGURE 3.
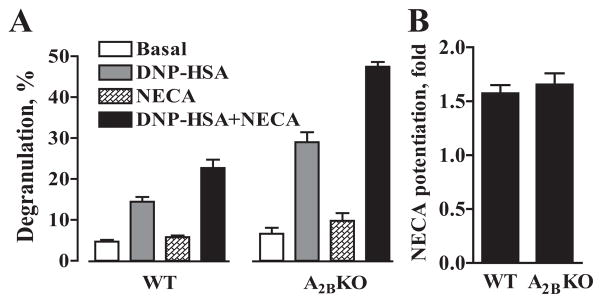
Potentiation of Ag-induced degranulation by NECA in WT and A2BKO mast cells. A, Degranulation of WT and A2BKO BMMCs was assessed by measuring β-hexosaminidase release from cells preincubated with anti-DNP IgE after stimulation with 10 ng/ml DNP-HSA Ag (DNP-HSA, DNP-HSA+NECA) or from nonstimulated cells (Basal, NECA) in the absence (Basal, DNP-HSA) or presence (NECA, DNP-HSA+NECA) of 10 μM NECA. Values are expressed as mean percentage ± SEM of three separate cell preparations. B, Potentiation of Ag-induced degranulation by NECA in WT and A2BKO BMMCs calculated as fold value from data presented in A.
Effect of adenosine A2B receptor gene ablation on IL-6, IL-13, and VEGF release from BMMCs
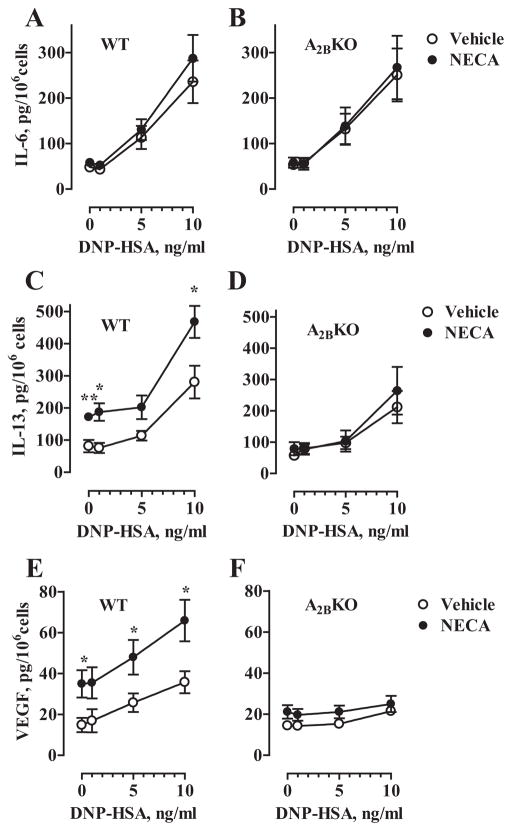
In addition to triggering the release of preformed mediators, FcεRI-dependent stimulation of BMMCs induces synthesis and secretion of several proinflammatory cytokines and cell growth factors including IL-6, IL-13, and VEGF (24 –26). As seen in Fig. 4A, activation of WT BMMCs with increasing concentrations of Ag significantly increased IL-6 in culture medium from basal levels from 48 ± 8 to 113 ± 25 pg/ml at 5 ng/ml DNP-HSA, and to 236 ± 46 pg/ml at 10 ng/ml DNP-HSA (p < 0.01, ANOVA with Dunnett’s posttests, n = 5 separate cell preparations). However, stimulation of adenosine receptors with NECA had no significant effect on basal or Ag-induced IL-6 release. Similar levels of basal and Ag-induced IL-6 release and the lack of NECA stimulation were observed in A2BKO BMMCs (Fig. 4B). Thus, our results indicate that adenosine receptors play no role in the regulation of IL-6 secretion in BMMCs.
FIGURE 4.
Effect of A2B receptor gene ablation on IL-6, IL-13, and VEGF secretion from BMMCs. WT (A, C, and E) and A2BKO (B, D, and F) BMMCs were preincubated with anti-DNP IgE and stimulated with 0, 1, 5, or 10 ng/ml DNP-HSA in the presence of 10 μM NECA (●) or its vehicle (○) for 6 h (A–D), or for 16 h (E and F). IL-6, IL-13, and VEGF were measured in culture medium and expressed as picograms per 106 cells. Data are presented as mean ± SEM of five separate cell preparations. *, p < 0.05 and **, p < 0.01, statistical differences of secretion in the presence of NECA from corresponding values obtained in the presence of vehicle determined by unpaired two-tail t test.
In contrast, stimulation of adenosine receptors with 10 μM NECA significantly increased IL-13 secretion in WT BMMCs from basal levels from 81 ± 19 to 172 ± 9 pg/ml in the absence of Ag and from 280 ± 51 to 468 ± 50 pg/ml in the presence of 10 ng/ml DNP-HSA (Fig. 4C). Because this effect of NECA was not seen in A2BKO cells (Fig. 4D), our data suggest that A2B receptors mediate adenosine-dependent stimulation of IL-13 in BMMCs.
Similarly, stimulation of adenosine receptors with 10 μM NECA significantly increased VEGF secretion in WT BMMCs for basal levels from 14 ± 3 to 35 ± 6 pg/ml in the absence of Ag and from 35 ± 5 to 66 ± 10 pg/ml in the presence of 10 ng/ml DNP-HSA (Fig. 4E). As in the case of IL-13 secretion, NECA had no significant effect on VEGF secretion in A2BKO BMMCs (Fig. 4F), indicating that adenosine-dependent release of both IL-13 and VEGF is regulated via A2B receptors.
Effects of inverse A2B receptor agonists on BMMC degranulation
It has been recently speculated, although not tested, that A2B antagonists with inverse agonist activity could enhance Ag-induced degranulation in BMMCs similarly to the effect of genetic A2B receptor ablation (15). To test this hypothesis, we chose adenosine receptor antagonists MRS1706 and DPCPX because they were recently characterized as inverse agonists at the A2B receptor (27). We initially confirmed that these compounds antagonize an A2B receptor-mediated process in these cells. As seen in Fig. 5A, 1 μM DPCPX and 100 nM MRS1706 significantly inhibited NECA-induced IL-13 secretion by 87 ± 8 and 53 ± 8%, respectively (p < 0.01, ANOVA with Dunnett’s posttests, n = 5 separate cell preparations). However, these compounds used at the same concentrations had no effect on Ag-induced BMMC degranulation (Fig. 5B).
HMC-1 as a model to study A2B receptor-dependent regulation of mast cell cytokine and growth factor production
Although HMC-1 cells express very low levels of functional FcεRI (28), this human mast cell line has proven useful to uncover the A2B receptor-dependent up-regulation of Th2 cytokines and angiogenic factors (11, 14), a process independent from FcεRI stimulation as we show in the current study in BMMCs. Because the use of an established cell line for detailed analysis of intracellular signaling pathways could be advantageous due to availability of plentiful material and better reproducibility of results, we next sought to determine whether HMC-1 can serve as an appropriate model to study A2B receptor-dependent regulation of mast cell cytokine and growth factor production, by comparing IL-13 and VEGF regulation in BMMCs and HMC-1 cells.
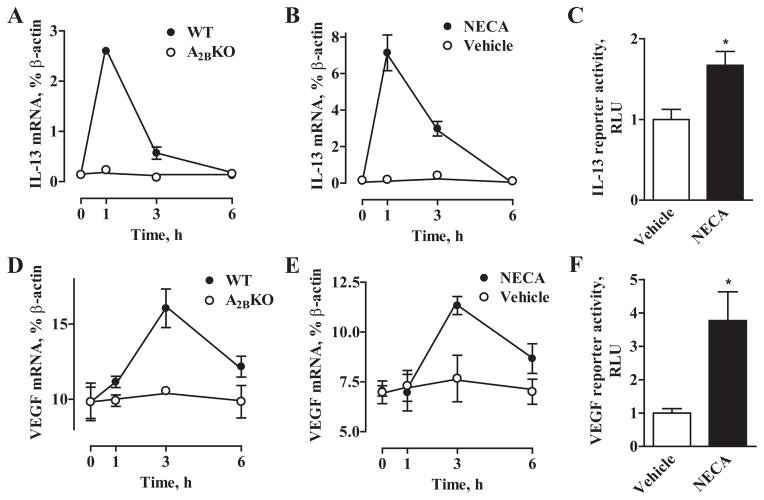
NECA induced a rapid transient elevation of IL-13 mRNA in WT BMMCs but not in A2BKO BMMCs, with levels increasing by 21.2 ± 0.4-fold at 1 h and returning to near basal levels at 6 h (Fig. 6A). As seen in Fig. 6B, changes in IL-13 mRNA levels in HMC-1 cells followed the same time course as in WT BMMCs. In contrast, VEGF mRNA elevations were delayed in WT BMMCs peaking only at 3 h after stimulation with NECA (Fig. 6D). Yet again, NECA-induced changes in VEGF mRNA levels in HMC-1 followed exactly the same time course as in WT BMMCs (Fig. 6E). Stimulation of reporter activities with 10 μM NECA (Fig. 6, C and F) suggests that regulation of IL-13 and VEGF mRNA levels via A2B adenosine receptors occurs at least in part due to increase in their transcription.
FIGURE 6.
Regulation of IL-13 and VEGF mRNA levels in BMMCs and HMC-1 cells. Time course of the effects of 10 μM NECA on IL-13 (A) and VEGF (D) mRNA levels in WT(●) and A2BKO (○) BMMCs. Values are expressed as mean ± SEM of three separate cell preparations. Time course of the effects of 10 μM NECA F (●) or its vehicle (○) on IL-13 (B) and VEGF (E) mRNA levels in HMC-1 cells. Values are expressed as mean ± SEM of three experiments. Effect of 10 μM NECA on IL-13 (C) and VEGF (F) reporter activities in HMC-1 cells. Data are presented as relative luciferase units (RLU) over basal activity. Values are expressed as mean ± SEM of three experiments. *, p < 0.05, statistical difference of reporter activity in the presence of NECA from corresponding values obtained in the presence of vehicle determined by unpaired two-tail t test.
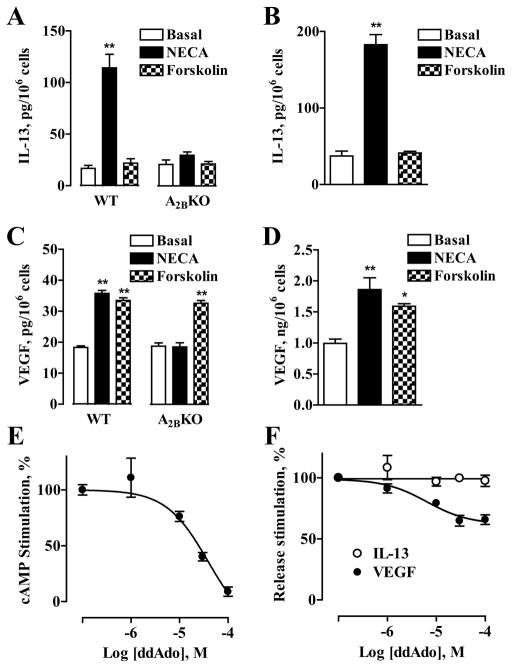
Because stimulation of A2B receptors in both BMMCs and HMC-1 cells increases intracellular cAMP (29) (Fig. 2), we next probed whether elevations of cAMP concentrations induced by forskolin could mimic the effects of NECA on IL-13 and VEGF secretion from these cells. We found that incubation of WT or A2BKO BMMCs with 100 μM forskolin had no effect on IL-13 secretion (Fig. 7A), but stimulated VEGF release (Fig. 7C). Similarly, forskolin stimulated VEGF secretion, but not IL-13 secretion in HMC-1 cells (Fig. 7, B and D). However, inhibition of cAMP production in HMC-1 cells with 100 μM 2′,5′-dideoxya-denosine (Fig. 7E) only partially suppressed NECA-induced VEGF secretion (Fig. 7F), suggesting that cAMP-dependent modulation of VEGF release cannot be solely responsible for the effects of NECA.
FIGURE 7.
Effect of modulation of cAMP levels in BMMCs and HMC-1 cells on IL-13 and VEGF secretion. Effects of 10 μM NECA and 100 μM forskolin on IL-13 (A) and VEGF (C) secretion from WT and A2BKO BMMCs. Values are expressed as mean ± SEM of three separate cell preparations. **, p < 0.01, significant difference from basal shown by one-way ANOVA with Dunnett’s posttest. Effects of 10 μM NECA and 100 μM forskolin on IL-13 (B) and VEGF (D) secretion from HMC-1 cells. Values are expressed as mean ± SEM of three experiments. *, p < 0.05 and **, p < 0.01, significant differences from basal determined by one-way ANOVA with Dunnett’s posttest. Effect of adenylate cyclase inhibitor 2′,5′-dideoxyadenosine (ddAdo) on cAMP accumulation (E) in HMC-1 cells stimulated with 10 μM NECA. In the absence of 2′,5′-dideoxyade-nosine, 10 μM NECA increased cAMP levels from 3.2 ± 0.6 to 19.4 ± 2.9 pmol/106 cells. Values are presented as mean percentage ± SEM of NECA-stimulated response in three experiments. F, Effect of 2′,5′-dideoxyadenosine on IL-13 (○) and VEGF (●) secretion from HMC-1 cells stimulated with 10 μM NECA. Values are presented as mean percentage ± SEM of NECA-stimulated response for three experiments.
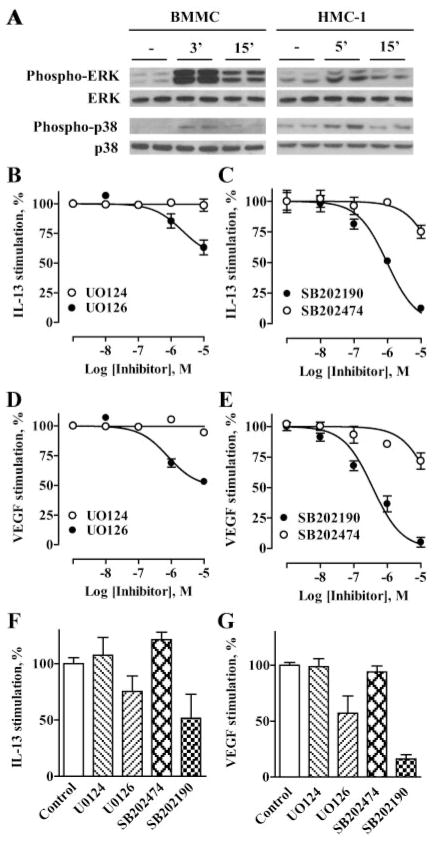
We have previously shown that ERK and p38 MAPKs, but not cAMP-dependent pathways play an important role in regulation of A2B receptor-mediated IL-8 production in HMC-1 cells (30). Similarly to HMC-1 cells, stimulation of BMMCs with 10 μM NECA induced a rapid transient phosphorylation of ERK and p38 MAPKs (Fig. 8A). We determined that the inhibitors of ERK and p38 MAPK pathways UO126 and SB202190, but not their inactive analogs UO124 and SB202474, respectively, effectively suppressed NECA-stimulated IL-13 and VEGF release from HMC-1 cells in a concentration-dependent manner (Fig. 8, B–E). We found that these inhibitors of MAPK pathways, but not their inactive analogs used at the same concentrations (10 μM) also attenuated NECA-stimulated IL-13 and VEGF release from BMMCs (Fig. 8, F and G). Taken together, our results demonstrate similarity in cytokine regulation in both mast cells and suggest that HMC-1 cells are an appropriate model for delineation of A2B receptor linked intracellular signaling pathways leading to secretion of Th2 cytokines and angiogenic factors.
FIGURE 8.
Effect of inhibitors of MAPK pathways on NECA-dependent IL-13 and VEGF secretion from BMMCs and HMC-1 cells. A, Western blot analysis of phosphorylation of ERK and p38 MAPK (phospho-ERK and phospho-p38, respectively) in BMMCs and HMC-1 cells that were either not stimulated (−) or stimulated with 10 μM NECA for the indicated time period. Equal loading of proteins was verified by reprobing the same blot with total ERK and p38 MAPK Abs (ERK and p38, respectively). Blots are representative of three experiments. Effects of MEK inhibitor UO126 (●) and its inactive analog UO124 (○) on IL-13 (B) or VEGF (D) secretion from HMC-1 cells stimulated with 10 μM NECA. Values are presented as mean percentage ± SEM of NECA-stimulated response from three experiments. Effects of p38 inhibitor SB202190 (●) and its inactive analog SB202474 (○) on IL-13 (C) or VEGF (E) secretion from HMC-1 cells stimulated with 10 μM NECA. Values are presented as mean percentage ± SEM of NECA-stimulated response from three experiments. F, Effects of MEK inhibitor UO126 (10 μM), p38 inhibitor SB202190 (10 μM), and their inactive analogs UO124 (10 μM) and SB202474 (10 μM), respectively, on IL-13 secretion from BMMCs stimulated with 10 μM NECA. Significant difference (p < 0.05) was determined by one-way ANOVA. Data are expressed as the percentage of NECA-stimulated response and presented as mean ± SEM of three separate cell preparations. G, Effects of MEK inhibitor UO124 (10 μM), p38 inhibitor SB202190 (10 μM), and their inactive analogs UO126 (10 μM) and SB202474 (10 μM) on VEGF secretion from BMMCs stimulated with 10 μM NECA. Significant difference (p < 0.001) was determined by one-way ANOVA. Data are expressed as the percentage of NECA-stimulated response and presented as mean ± SEM of three separate cell preparations.
Discussion
The low-affinity A2B receptor is known to mediate proinflammatory effects of adenosine by up-regulating production of cytokines and growth factors. This view has been supported by a large body of evidence provided by pharmacological analysis of adenosine-dependent cytokine and growth factor secretion in various cells, tissues, and organs (8, 11, 13, 18, 31–38). Pharmacological inhibition of A2B receptors significantly reduced elevations in proinflammatory cytokines as well as mediators of airway remodeling induced by high adenosine levels in the lungs of ADA-deficient mice (8). In the ragweed allergic mouse model, A2B antagonism plays an important role in inhibition of airway reactivity and inflammation (39, 40). In agreement with data obtained in these animal models of pulmonary inflammation, stimulation of A2B receptors in the human mast cell line HMC-1 was shown to induce secretion of proinflammatory Th2 cytokines IL-4 and IL-13 (11, 12), as well as angiogenic factors IL-8 and VEGF (13, 14).
A recent report by Hua et al. (15) disputed the proinflammatory role of A2B receptors in mast cells. Based on the finding in this study of an enhanced Ag-induced mast cell degranulation in mice lacking the A2B receptor, the authors inferred that this receptor subtype normally limits the magnitude of responsiveness toward Ag in WT mast cells. The authors postulated that A2B receptors are constitutively active in mast cells and speculated that A2B antagonists with inverse agonist activity would lower cAMP levels within the mast cell, potentially enhancing Ag-induced degranulation and proinflammatory cytokine production. However, no pharmacological data were presented to support this claim. In the current study, we combined pharmacological and genetic approaches to revisit these conclusions using exactly the same experimental model of mouse BMMCs obtained from WT and A2BKO mice backcrossed onto the same C57BL/6 genetic background.
Our data confirmed that genetic A2B receptor ablation in BMMCs does not change the surface expression of FcεRI or c-kit receptors, nor does it affect the expression of mRNA encoding A2A and A3 adenosine receptor subtypes present in these cells. In agreement with the report by Hua et al. (15), we observed an enhanced stimulation of Ag-induced degranulation in A2BKO BMMCs compared with WT cells. Notwithstanding the potential importance of this phenomenon, we thought that characterization of A2BKO BMMCs would be incomplete without actual testing whether adenosine agonist-induced signaling has been changed in these cells.
Adenosine is known to potentiate Ag-induced degranulation of mast cells. This effect of adenosine on preformed mediator release is mediated by A3 adenosine receptors in rodents (23, 41– 43), but perhaps not in human (44) or canine (45) mast cells. We found that an enhanced Ag-induced degranulation in A2BKO BMMCs was observed both in the absence and presence of the adenosine receptor agonist NECA, but the degree of potentiation mediated via A3 receptors was essentially the same as in WT cells. Thus, we conclude that genetic ablation of A2B receptors not only has no significant effect on the expression of A3 receptors, but also on their function in potentiating Ag-induced degranulation of BMMCs.
In addition to release of preformed mediators from mast cell granules, stimulation of mast cells with Ag results in de novo synthesis and secretion of proinflamatory cytokines and angiogenic factors (46). These events are regulated via distinct pathways that can be activated independently (24, 47–50). Adenosine can exert differential effects on some of these processes via distinct adenosine receptor subtypes or may have no effect at all. In agreement with previous reports in BMMCs (24) and in human mast cells (11), we found no effect of stimulation of adenosine receptors with NECA on IL-6 secretion in WT BMMCs. Consequentially, there was no significant effect of A2B receptor ablation on IL-6 secretion either in resting or Ag-stimulated BMMCs. However, stimulation of A2B receptors in human mast cell line HMC-1 was previously shown to induce secretion of other proinflammatory factors, and particularly Th2 cytokines and angiogenic factors (11–14). Even though it has been argued that the proinflammatory functions of A2B receptors described in HMC-1 expressing low levels of functional IgE receptors could be irrelevant to adenosine signaling in BMMCs (15), in this study we show for the first time that A2B receptors stimulate secretion of these factors in BMMCs as well. The adenosine agonist NECA stimulated IL-13 and VEGF secretion only in WT BMMCs but not in A2BKO BMMCs. Contrary to adenosine action on degranulation, which is only apparent in Ag-stimulated BMMCs, these effects did not require activation of FcεRI because they were evident even in the absence of Ag. We also found that A2B receptor-dependent cytokine regulation was identical in BMMCs and HMC-1 cells, based on effects of cAMP modulators and inhibitors of MAPK signaling pathways on IL-13 and VEGF production. Thus, our data in mouse mast cells expressing high levels of functional FcεRI confirmed our previous finding in human mast cell line HMC-1 that stimulation of A2B adenosine receptors results in secretion of Th2 cytokines and angiogenic factors. These observations suggest that HMC-1 cells are appropriate as a model for delineation of A2B receptor-linked intracellular signaling pathways.
Our study has clearly demonstrated that genetic ablation of A2B receptors has two different effects on BMMCs, one is the loss of adenosine signaling mediated by this receptor that regulates secretion of IL-13 and VEGF, whereas the other is the adenosine-independent phenomenon of enhanced Ag-induced degranulation previously described by Hua et al. (15). The latter phenomenon is similar to an enhanced production of TNF-α observed in macrophages obtained from A2BKO mice (51, 52). We have recently demonstrated that this enhanced basal and LPS-stimulated TNF-α secretion in A2BKO macrophages cannot be explained by the loss of adenosine signaling via A2B receptor (52). Likewise, our data in the current study indicate that the enhanced Ag-induced degranulation in BMMCs obtained from A2BKO mice cannot be explained by the loss of tonic stimulation of A2B receptors by endogenous adenosine. Because our experiments were conducted in the presence of ADA, it is unlikely that endogenous adenosine could accumulate in concentrations sufficient to stimulate this low-affinity receptor.
Acknowledging the implausibility of tonic stimulation of the low-affinity A2B receptor by endogenous adenosine, Hua et al. (15) proposed the alternative explanation that A2B receptors are constitutively active in WT BMMCs even in the absence of an agonist. However, we determined that A2B receptors expressed in WT BMMCs display no constitutive activity in regulation of cAMP. If these receptors were indeed constitutively active in BMMCs, then they are expected to activate Gs proteins in the absence of agonist stimulation. Because the increased levels of free Gsα subunits are known to enhance the responses to forskolin, this agent is often used to verify constitutive activity of Gs protein-coupled receptors (53). However, not only did we not find differences in basal cAMP levels between BMMCs obtained from A2BKO and WT mice, but there was no difference in forskolin-induced accumulation of cAMP between mast cells expressing and lacking A2B receptors. In contrast, cAMP accumulation stimulated with the nonselective adenosine receptor agonist NECA, but not with the selective A2A receptor agonist CGS21680, was significantly lower in A2BKO BMMCs compared with WT cells, reflecting the loss of adenosine signaling via A2B receptors. Finally, the lack of constitutive A2B receptor activity in BMMCs is supported by our experiments with inverse agonists, agents that bind to the same receptor binding site as agonists but exert the opposite pharmacological effect. If these receptors were indeed constitutively active, then inverse agonists are expected to repress their spontaneous activity (for a review, see Ref. 54). Recent studies have identified adenosine antagonists ZM241385, DPCPX, and MRS1706 as inverse agonists at A2B receptors (27). We chose two structurally unrelated compounds, the selective A2B antagonist MRS1706 (55) and the A1/A2B antagonist DPCPX (56), to study A2B receptor signaling in BMMCs. Given the fact that these cells do not express A1 receptors (Fig. 1C), we used DPCPX as an inverse agonist at A2B receptors complementary to MRS1706. We have demonstrated that both MRS1706 and DPCPX inhibited cAMP accumulation mediated by A2B receptor stimulation, but they had no significant effect on basal cAMP levels, further proving the lack of constitutive A2B receptor activity in BMMCs. Contrary to the hypothesis of Hua et al. (15), these compounds had no effect on Ag-induced mast cell degranulation, but they inhibited A2B-mediated IL-13 secretion in BMMCs.
Our results, therefore, confirm the proinflammatory effects resulting from activation of A2B receptors. They also confirm the phenomenon described by Hua et al. (15) of exaggerated Ag-induced degranulation in BMMCs deficient of A2B receptors. This phenomenon, however, is unrelated to the adenosine signaling function of A2B receptors. An alternative and more likely explanation of this phenomenon could be that the A2B receptor protein interacts with other signaling pathways unrelated to adenosine. Recent evidence suggests that adenosine receptors play a role in assembly of multiprotein signaling complexes (57– 61). Rearrangement of proteins normally coupled to the A2B receptor as a result of the A2BKO may affect FcεRI-dependent regulation of degranulation. Future studies that define the full makeup of A2B receptor complexes with associated signaling components are needed to validate this hypothesis.
In conclusion, we report that adenosine receptors mediate differential regulation of murine mast cell functions depending on the receptor subtype involved. Adenosine signaling that regulates BMMC degranulation previously shown being mediated through A3 receptors has no effect in resting cells, but potentiates the Ag-induced degranulation. In contrast, adenosine signaling through A2B receptors stimulates secretion of Th2 cytokines and angiogenic factors even in the absence of Ag. In this regard, our study in BMMCs obtained from A2BKO and WT mice has confirmed the concept drawn from previous pharmacological data that stimulation of A2B receptors with adenosine leads to proinflammatory events based on the observation that A2B receptors mediate adenosine-dependent secretion of IL-13 and VEGF. Our data argue against a constitutive activity of A2B receptors or their tonic activation by endogenous adenosine in BMMCs purportedly limiting Ag-stimulated mast cell degranulation. An enhancement in FcεRI-mediated degranulation observed in A2BKO BMMCs is not directly related to adenosine signaling through the A2B receptor, but may represent a loss of other yet unidentified functions of this protein, or reflect a developmental adaptation to the global A2B receptor gene ablation in mice.
Footnotes
This work was supported by Grants R01 HL070952 and R01 HL076306 from the National Institutes of Health.
Abbreviations used in this paper: ADA, adenosine deaminase; KO, knockout; BMMC, bone marrow-derived mast cell; HSA, human serum albumin; VEGF, vascular endothelial growth factor; WT, wild type.
Disclosures
Drs. I. Biaggioni and I. Feoktistov are inventors named on U.S. Patent No. 6,815,446 for “Selective antagonists of A2B adenosine receptors,” which was licensed through the Vanderbilt University Office of Technology Transfer and Enterprise Development (Nashville, TN) to CV Therapeutics (Palo Alto, CA) for the development of antiasthmatic drugs. Drs. I. Biaggioni, I. Feoktistov, and M. R. Blackburn have been recipients of research funding from CV Therapeutics. The remaining authors have no financial conflict of interest.
References
- 1.Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 2.Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001;61:443– 448. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 3.Driver AG, Kukoly CA, Ali S, Mustafa SJ. Adenosine in bronchoalveolar lavage fluid in asthma. Am Rev Respir Dis. 1993;148:91–97. doi: 10.1164/ajrccm/148.1.91. [DOI] [PubMed] [Google Scholar]
- 4.Huszar E, Vass G, Vizi E, Csoma Z, Barat E, Molnar VG, Herjavecz I, Horvath I. Adenosine in exhaled breath condensate in healthy volunteers and in patients with asthma. Eur Respir J. 2002;20:1393–1398. doi: 10.1183/09031936.02.00005002. [DOI] [PubMed] [Google Scholar]
- 5.Blackburn MR, Volmer JB, Thrasher JL, Zhong H, Crosby JR, Lee JJ, Kellems RE. Metabolic consequences of adenosine deaminase deficiency in mice are associated with defects in alveogenesis, pulmonary inflammation, and airway obstruction. J Exp Med. 2000;192:159–170. doi: 10.1084/jem.192.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackburn MR. Too much of a good thing: adenosine overload in adenosine-deaminase-deficient mice. Trends Pharmacol Sci. 2003;24:66–70. doi: 10.1016/S0165-6147(02)00045-7. [DOI] [PubMed] [Google Scholar]
- 7.Blackburn MR, Lee CG, Young HW, Zhu Z, Chunn JL, Kang MJ, Banerjee SK, Elias JA. Adenosine mediates IL-13-induced inflammation and remodeling in the lung and interacts in an IL-13-adenosine amplification pathway. J Clin Invest. 2003;112:332–344. doi: 10.1172/JCI16815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, Belardinelli L, Zeng D, Blackburn MR. Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J Clin Invest. 2006;116:2173–2182. doi: 10.1172/JCI27303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradding P, Walls AF, Holgate ST. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol. 2006;117:1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 10.Rorke S, Holgate ST. Targeting adenosine receptors: novel therapeutic targets in asthma and chronic obstructive pulmonary disease. Am J Respir Med. 2002;1:99–105. doi: 10.1007/BF03256599. [DOI] [PubMed] [Google Scholar]
- 11.Ryzhov S, Goldstein AE, Matafonov A, Zeng D, Biaggioni I, Feoktistov I. Adenosine-activated mast cells induce IgE synthesis by B lymphocytes: an A2B-mediated process involving Th2 cytokines IL-4 and IL-13 with implications for asthma. J Immunol. 2004;172:7726–7733. doi: 10.4049/jimmunol.172.12.7726. [DOI] [PubMed] [Google Scholar]
- 12.Ryzhov S, Goldstein AE, Biaggioni I, Feoktistov I. Cross-talk between Gs- and Gq-coupled pathways in regulation of interleukin-4 by A2B adenosine receptors in human mast cells. Mol Pharmacol. 2006;70:727–735. doi: 10.1124/mol.106.022780. [DOI] [PubMed] [Google Scholar]
- 13.Feoktistov I, Biaggioni I. Adenosine A2B receptors evoke interleukin-8 secretion in human mast cells: an enprofylline-sensitive mechanism with implications for asthma. J Clin Invest. 1995;96:1979–1986. doi: 10.1172/JCI118245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feoktistov I, Ryzhov S, Goldstein AE, Biaggioni I. Mast cell-mediated stimulation of angiogenesis: cooperative interaction between A2B and A3 adenosine receptors. Circ Res. 2003;92:485– 492. doi: 10.1161/01.RES.0000061572.10929.2D. [DOI] [PubMed] [Google Scholar]
- 15.Hua X, Kovarova M, Chason KD, Nguyen M, Koller BH, Tilley SL. Enhanced mast cell activation in mice deficient in the A2b adenosine receptor. J Exp Med. 2007;204:117–128. doi: 10.1084/jem.20061372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scoka B, Nemeth ZH, Virag L, Gergely P, Leibovich SJ, Pacher P, Sun CX, Blackburn MR, Vizi ES, Deitch EA, Hasko G. A2A adenosine receptors and C/EBPβ are crucially required for IL-10 production by macrophages exposed to E. coli. Blood. 2007;110:2685–2695. doi: 10.1182/blood-2007-01-065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryzhov S, McCaleb JL, Goldstein AE, Biaggioni I, Feoktistov I. Role of adenosine receptors in the regulation of angiogenic factors and neovascularization in hypoxia. J Pharmacol Exp Ther. 2007;382:565–572. doi: 10.1124/jpet.106.114850. [DOI] [PubMed] [Google Scholar]
- 18.Feoktistov I, Goldstein AE, Ryzhov S, Zeng D, Belardinelli L, Voyno-Yasenetskaya T, Biaggioni I. Differential expression of adenosine receptors in human endothelial cells: role of A2B receptors in angiogenic factor regulation. Circ Res. 2002;90:531–538. doi: 10.1161/01.res.0000012203.21416.14. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz LB, Austen KF, Wasserman SI. Immunologic release of β-hexosaminidase and β-glucoronidase from peritoneal rat serosal mast cells. J Immunol. 1979;330:393– 411. [PubMed] [Google Scholar]
- 20.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604– 4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung HK, Young HA, Goon PK, Heidecker G, Princler GL, Shimozato O, Taylor GP, Bangham CR, Derse D. Activation of interleukin-13 expression in T cells from HTLV-1-infected individuals and in chronically infected cell lines. Blood. 2003;102:4130– 4136. doi: 10.1182/blood-2003-04-1043. [DOI] [PubMed] [Google Scholar]
- 22.Marquardt DL, Walker LL, Wasserman SI. Adenosine receptors on mouse bone marrow-derived mast cells: functional significance and regulation by aminophylline. J Immunol. 1984;133:932–937. [PubMed] [Google Scholar]
- 23.Salvatore CA, Tilley SL, Latour AM, Fletcher DS, Koller BH, Jacobson MA. Disruption of the A3 adenosine receptor gene in mice and its effect on stimulated inflammatory cells. J Biol Chem. 2000;275:4429– 4434. doi: 10.1074/jbc.275.6.4429. [DOI] [PubMed] [Google Scholar]
- 24.Marquardt DL, Alongi JL, Walker LL. The phosphatidylinositol 3-kinase inhibitor wortmannin blocks mast cell exocytosis but not IL-6 production. J Immunol. 1996;156:1942–1945. [PubMed] [Google Scholar]
- 25.Burd PR, Thompson WC, Max EE, Mills FC. Activated mast cells produce interleukin 13. J Exp Med. 1995;181:1373–1380. doi: 10.1084/jem.181.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boesiger J, Tsai M, Maurer M, Yamaguchi M, Brown LF, Claffey KP, Dvorak HF, Galli SJ. Mast cells can secrete vascular permeability factor/vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of Fcε receptor I expression. J Exp Med. 1998;188:1135–1145. doi: 10.1084/jem.188.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, Ye K, Blad CC, den Dulk H, Brouwer J, Ijzerman AP, Beukers MW. ZM241385, DPCPX, MRS1706 are inverse agonists with different relative intrinsic efficacies on constitutively active mutants of the human adenosine A2B receptor. J Pharmacol Exp Ther. 2007;320:637– 645. doi: 10.1124/jpet.106.111203. [DOI] [PubMed] [Google Scholar]
- 28.Butterfield JH, Weiler D, Dewald G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leukemia Res. 1988;12:345–355. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- 29.Feoktistov I, Biaggioni I. Pharmacological characterization of adenosine A2B receptors: studies in human mast cells co-expressing A2A an A2B adenosine receptor subtypes. Biochem Pharmacol. 1998;55:627– 633. doi: 10.1016/s0006-2952(97)00512-1. [DOI] [PubMed] [Google Scholar]
- 30.Feoktistov I, Goldstein AE, Biaggioni I. Role of p38 mitogen-activated protein kinase and extracellular signal-regulated protein kinase kinase in adenosine A2B receptor-mediated interleukin-8 production in human mast cells. Mol Pharmacol. 1999;55:726–735. [PubMed] [Google Scholar]
- 31.Fiebich BL, Biber K, Guyfko K, Berger M, Bauer J, van Calker D. Adenosine A2b receptors mediate an increase in interleukin (IL)-6 mRNA and IL-6 protein synthesis in human astroglioma cells. J Neurochem. 1996;66:1426–1431. doi: 10.1046/j.1471-4159.1996.66041426.x. [DOI] [PubMed] [Google Scholar]
- 32.Schwaninger M, Neher M, Viegas E, Schneider A, Spranger M. Stimulation of interleukin-6 secretion and gene transcription in primary astrocytes by adenosine. J Neurochem. 1997;69:1145–1150. doi: 10.1046/j.1471-4159.1997.69031145.x. [DOI] [PubMed] [Google Scholar]
- 33.Zeng D, Maa T, Wang U, Feoktistov I, Biaggioni I, Belardinelli L. Expression and function of A2B adenosine receptors in the U87MG tumor cells. Drug Dev Res. 2003;58:405– 411. [Google Scholar]
- 34.Rees DA, Lewis BM, Lewis MD, Francis K, Scanlon MF, Ham J. Adenosine-induced IL-6 expression in pituitary folliculostellate cells is mediated via A2b adenosine receptors coupled to PKC and p38 MAPK. Br J Pharmacol. 2003;140:764–772. doi: 10.1038/sj.bjp.0705488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong H, Belardinelli L, Maa T, Feoktistov I, Biaggioni I, Zeng D. A2B adenosine receptors increase cytokine release by bronchial smooth muscle cells. Am J Resp Cell Mol Biol. 2004;30:118–125. doi: 10.1165/rcmb.2003-0118OC. [DOI] [PubMed] [Google Scholar]
- 36.Fiebich BL, Akundi RS, Biber K, Hamke M, Schmidt C, Butcher RD, van Calker D, Willmroth F. IL-6 expression induced by adenosine A2b receptor stimulation in U373 MG cells depends on p38 mitogen activated kinase and protein kinase C. Neurochem Int. 2005;46:501–512. doi: 10.1016/j.neuint.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Zhong H, Belardinelli L, Maa T, Zeng D. Synergy between A2B adenosine receptors and hypoxia in activating human lung fibroblasts. Am J Resp Cell Mol Biol. 2005;32:2– 8. doi: 10.1165/rcmb.2004-0103OC. [DOI] [PubMed] [Google Scholar]
- 38.Evans BA, Elford C, Pexa A, Francis K, Hughes AC, Deussen A, Ham J. Human osteoblast precursors produce extracellular adenosine, which modulates their secretion of IL-6 and osteoprotegerin. J Bone Miner Res. 2006;21:228–236. doi: 10.1359/JBMR.051021. [DOI] [PubMed] [Google Scholar]
- 39.Fan M, Qin W, Mustafa SJ. Characterization of adenosine receptor(s) involved in adenosine-induced bronchoconstriction in an allergic mouse model. Am J Physiol. 2003;284:L1012–L1019. doi: 10.1152/ajplung.00353.2002. [DOI] [PubMed] [Google Scholar]
- 40.Mustafa SJ, Nadeem A, Fan M, Zhong H, Belardinelli L, Zeng D. Effect of a specific and selective A2B adenosine receptor antagonist on adenosine agonist AMP and allergen-induced airway responsiveness and cellular influx in a mouse model of asthma. J Pharmacol Exp Ther. 2007;320:1246–1251. doi: 10.1124/jpet.106.112250. [DOI] [PubMed] [Google Scholar]
- 41.Ramkumar V, Stiles GL, Beaven MA, Ali H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J Biol Chem. 1993;268:16887–16890. [PubMed] [Google Scholar]
- 42.Reeves JJ, Jones CA, Sheehan MJ, Vardey CJ, Whelan CJ. Adenosine A3 receptors promote degranulation of rat mast cells both in vitro and in vivo. Inflamm Res. 1997;46:180–184. doi: 10.1007/s000110050169. [DOI] [PubMed] [Google Scholar]
- 43.Zhong H, Shlykov SG, Molina JG, Sanborn BM, Jacobson MA, Tilley SL, Blackburn MR. Activation of murine lung mast cells by the adenosine A3 receptor. J Immunol. 2003;171:338–345. doi: 10.4049/jimmunol.171.1.338. [DOI] [PubMed] [Google Scholar]
- 44.Walker BA, Jacobson MA, Knight DA, Salvatore CA, Weir T, Zhou D, Bai TR. Adenosine A3 receptor expression and function in eosinophils. Am J Resp Cell Mol Biol. 1997;16:531–537. doi: 10.1165/ajrcmb.16.5.9160835. [DOI] [PubMed] [Google Scholar]
- 45.Auchampach JA, Jin X, Wan TC, Caughey GH, Linden J. Canine mast cell adenosine receptors: Cloning and expression of the A3 receptors and evidence that degranulation is mediated by the A2B receptor. Mol Pharmacol. 1997;52:846– 860. doi: 10.1124/mol.52.5.846. [DOI] [PubMed] [Google Scholar]
- 46.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 47.Arzubiaga C, Morrow J, Roberts LJ, Biaggioni I. Neuropeptide Y, a putative cotransmitter in noradrenergic neurons, induces mast cell degranulation but not prostaglandin D2 release. J Allergy Clin Immunol. 1991;87:88–93. doi: 10.1016/0091-6749(91)90216-b. [DOI] [PubMed] [Google Scholar]
- 48.Abdel-Majid RM, Marshall JS. Prostaglandin E2 induces degranulation-independent production of vascular endothelial growth factor by human mast cells. J Immunol. 2004;172:1227–1236. doi: 10.4049/jimmunol.172.2.1227. [DOI] [PubMed] [Google Scholar]
- 49.Nakayama T, Mutsuga N, Yao L, Tosato G. Prostaglandin E2 promotes degranulation-independent release of MCP-1 from mast cells. J Leukocyte Biol. 2006;79:95–104. doi: 10.1189/jlb.0405226. [DOI] [PubMed] [Google Scholar]
- 50.Qiao H, Andrade MV, Lisboa FA, Morgan K, Beaven MA. FcεR1 and Toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood. 2006;107:610– 618. doi: 10.1182/blood-2005-06-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, StHilaire C, Seldin DC, Toselli P, et al. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006;116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Blackburn MR, Biaggioni I, Feoktistov I. Effect of A2B adenosine receptor gene ablation on adenosine-dependent regulation of pro-inflammatory cytokines. J Pharmacol Exp Ther. 2008;324:694–700. doi: 10.1124/jpet.107.131540. [DOI] [PubMed] [Google Scholar]
- 53.Alewijnse AE, Smit MJ, Rodriguez Pena MS, Verzijl D, Timmerman H, Leurs R. Modulation of forskolin-mediated adenylyl cyclase activation by constitutively active GS-coupled receptors. FEBS Lett. 1997;419:171–174. doi: 10.1016/s0014-5793(97)01440-3. [DOI] [PubMed] [Google Scholar]
- 54.Costa T, Cotecchia S. Historical review: negative efficacy and the constitutive activity of G-protein-coupled receptors. Trends Pharmacol Sci. 2005;26:618– 624. doi: 10.1016/j.tips.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 55.Kim YC, Ji X, Melman N, Linden J, Jacobson KA. Anilide derivatives of an 8-phenylxanthine carboxylic congener are highly potent and selective antagonists at human A2B adenosine receptors. J Med Chem. 2000;43:1165–1172. doi: 10.1021/jm990421v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;317:172–180. doi: 10.1124/jpet.105.096016. [DOI] [PubMed] [Google Scholar]
- 57.Gsandtner I, Charalambous C, Stefan E, Ogris E, Freissmuth M, Zezula J. Heterotrimeric G protein-independent signaling of a G protein-coupled receptor: direct binding of ARNO/cytohesin-2 to the carboxyl terminus of the A2A adenosine receptor is necessary for sustained activation of the ERK/MAP kinase pathway. J Biol Chem. 2005;280:31898–31905. doi: 10.1074/jbc.M506515200. [DOI] [PubMed] [Google Scholar]
- 58.Milojevic T, Reiterer V, Stefan E, Korkhov VM, Dorostkar MM, Ducza E, Ogris E, Boehm S, Freissmuth MS, Nanoff C. The ubiquitin-specific protease Usp4 regulates the cell surface level of the A2A receptor. Mol Pharmacol. 2006;69:1083–1094. doi: 10.1124/mol.105.015818. [DOI] [PubMed] [Google Scholar]
- 59.Sun CN, Cheng HC, Chou JL, Lee SY, Lin YW, Lai HL, Chen HM, Chern Y. Rescue of p53 blockage by the A2A adenosine receptor via a novel interacting protein, translin-associated protein X. Mol Pharmacol. 2006;70:454– 466. doi: 10.1124/mol.105.021261. [DOI] [PubMed] [Google Scholar]
- 60.Sitaraman SV, Wang L, Wong M, Bruewer M, Hobert M, Yun CH, Merlin D, Madara JL. The adenosine 2b receptor is recruited to the plasma membrane and associates with E3KARP and Ezrin upon agonist stimulation. J Biol Chem. 2002;277:33188–33195. doi: 10.1074/jbc.M202522200. [DOI] [PubMed] [Google Scholar]
- 61.Pacheco R, Martinez-Navio JM, Lejeune M, Climent N, Oliva H, Gatell JM, Gallart T, Mallol J, Lluis C, Franco R. CD26, adenosine deaminase, and adenosine receptors mediate costimulatory signals in the immunological synapse. Proc Natl Acad Sci USA. 2005;102:9583–9588. doi: 10.1073/pnas.0501050102. [DOI] [PMC free article] [PubMed] [Google Scholar]