Abstract
Neuroimaging research in adults has consistently found that differential perception of race is associated with increased amygdala activity. We hypothesized that such neural biases unlikely reflect innate processes, but instead emerge over development. In the current study, we used functional magnetic resonance imaging (fMRI) to examine the neurodevelopmental trajectory of the amygdala in response to race across childhood and adolescence ranging from 4–16 years. Thirty-two youths viewed African-American and European-American faces during a functional brain scan. Results suggest that differential amygdala response to African-American faces does not emerge until adolescence, reflecting the increasing salience of race across development. In addition, greater peer diversity was associated with attenuated amygdala response to African-American faces, suggesting that intergroup racial contact may reduce the salience of race.
Keywords: fMRI, race, amygdala, development
Although explicit cultural norms in the United States may endorse egalitarian values and nonprejudiced attitudes, African-Americans continue to be evaluated differently than other racial/ethnic groups (Dovodio Kawakami, Johnson, Johnson, & Howard, 1997; Plant & Devine, 1998; Rosette, Leonardelli, & Phillips, 2008). For example, African-American faces are detected more quickly in visual search tasks (Levin, 2000) and produce an attentional bias during a dot-probe task (Richeson & Trawalter, 2008; Trawalter, Todd, Baird, & Richeson, 2008), suggesting that African-American faces hold increased saliency in adulthood. Neuroimaging research in adults has consistently found that this differential perception is, in part, associated with increased amygdala activity. European-American adults show increased amygdala activity, even in the absence of conscious awareness, in response to African-American (AA) relative to European-American (EA) faces (Cunningham, Johnson, Raye, Gatenby, Gore, & Banaji, 2004). Moreover, EA adults who harbor implicit negative attitudes towards AAs show greater amygdala activation while viewing AA relative to EA faces (Phelps, O’Connor, Cunningham, Funayma, Gatenby, Gore, & Banaji, 2000). Interestingly, heightened amygdala response to AA faces is found for both EA and AA adults (Lieberman, Hariri, Jarcho, Eisenberger, & Bookheimer, 2005). This heightened amygdala response is thought to be involved in automatic, subconscious responses to race, reflecting the learned cultural knowledge that AAs are treated differently, and such cultural knowledge is shared across individuals from diverse backgrounds (Phelps et al., 2000, Lieberman et al., 2005). Given that the value placed on racial groups is socially constructed (Eberhardt, 2005), we hypothesized that such biases unlikely reflect innate processes, but instead emerge over developmental time through learning. In the current study, we used functional magnetic resonance imaging (fMRI) to examine the neurodevelopmental trajectory of the amygdala response to race across childhood and adolescence.
Cultural norms and biases about race develop over the course of childhood and adolescence. When social groups are treated or labeled differently in children’s environment, children learn that certain categories are salient (e.g., race), whereas others are not (e.g., handedness; Bigler & Liben, 2007). At a very young age, children learn that individuals can be sorted into social categories, such as race. For example, infants as young as 3–6 months can perceptually discriminate between racial groups (Bar-Haim, Ziv, Lamy, Hodes, 2006), and preschool-aged children can accurately identify others’ racial-group membership (Aboud, 2003). By 6 years some children demonstrate implicit biases about race (Baron & Banaji, 2006), and by 10 years, children internalize the social and moral norms of their culture, demonstrating increased knowledge regarding racial stereotypes and cultural norms (Apfelbaum, Pauker, Ambady, Norton & Sommers, 2008).
The amygdala is involved in processing of stimuli that have an acquired emotional significance based on previous experience and plays a role in sensitivity to the salience of environmental cues (Cunningham & Brosch, 2012; Fitzgerald, Angstadt, Jelsone, Nathan, & Phan, 2006; Fudge & Emiliano, 2003; Santos, Mier, Kirsch, & Meyer-Lindenberg., 2011; Whalen, Shin, McInerney, Fischer, Wright, Rauch, 2001). Whereas brain regions such as the cerebellum respond to visual and perceptual differences in ones environment, such as shades of color (Claeys, Orbam Dupont, Sunaert, Van Hecke, & De Schutter, 2003), the amygdala responds to emotionally salient stimuli (Cunningham & Brisch, 2012; Whalen et al., 2001). The amygdala responds to both negatively and positively valenced stimuli (Breiter et al., 1996; Hennenlotter et al., 2005), highlighting its role in learning about the emotional significance of the environment in general. Therefore, the amygdala is well positioned to acquire affective associations learned in the social environment, such as those associated with race. In addition to responding to emotionally salient stimuli based on experience, the amygdala is involved in fear-related learning, detecting and responding to threats, and encoding the hedonic value of learned and unlearned stimuli (Fanselow & Gale, 2003; LeDoux, 2003).
Both human and animal work shows that the amygdala is an early developing brain structure (Payne, Machado, Bliwise, & Bachevalier, 2010). Structurally, the amygdala undergoes rapid development early in life (Tottenham, Hare, & Casey, 2009). In fact, the basic neuroanatomical architecture of the human amygdala is present by birth (Humphrey, 1968; Ul g, Setzer, & Bohl, 2003). Although structurally mature by early childhood, the amygdala undergoes massive changes in functional processing during adolescence, increasing in responsiveness to social stimuli (Moore, Pfeifer, Masten, Iacoboni, Mazziotta, & Dapretto, 2012; Guyer, Monk, McClure-Tone, Nelson, Roberson-Nay, Adler, et al., 2008; Nelson, Leibenluft, McClure, & Pine, 2005). For example, the onset of puberty is associated with enhanced amygdala activation to facial stimuli (Moore et al., 2012). Adolescence is also a time when race becomes increasingly salient. For example, adolescents enter high school where ethnic clubs and coalitions form, and youth begin to explore the meaning and importance of ethnicity and race (Roberts, Phinney, Masse, Chen, Roberts, & Romero, 1999). Moreover, the transition to adolescence is marked by a greater awareness of racial stereotypes and norms (Apfelbaum, et al., 2008). The social-reorientation of the amygdala (Nelson et al., 2005), coupled with more mature cognitive skills (Bigler & Liben, 2007), as well as an increasing salience of race, renders the early adolescent years particularly amenable to enhanced amygdala response to race.
In the current study we sought to understand how experience alters race-related processing in the amygdala. First, we examined age-related differences in amygdala response to race to test whether the pattern of amygdala response to AA and EA faces that is observed in adulthood is present in early childhood or whether it emerges across development. We examined amygdala sensitivity to race across a wide developmental age range, spanning 4 to 16 years. In addition, we examined how neural responses to race may differ across ethnically diverse youth. Children from diverse ethnic and racial backgrounds living in similar geographical areas are exposed to similar messages about race throughout their environment (Averhart & Bigler, 1997). Therefore, we expected that children from both EA and AA backgrounds will show a similar neurodevelopmental increase to AA faces, similar to the findings of Lieberman and colleagues (2005), who found that both AA and EA adults showed heightened amygdala response to AA faces.
Second, we examined whether children’s social environment modulates the amygdala response to race. Prior work has highlighted the importance of diverse social environments, such as neighborhood and school diversity, in shaping perceptions of race. For example, 3-month-old infants exhibit a preference for faces from their own racial group (i.e., in-group bias), but this bias is only present for infants living in racially homogeneous neighborhoods; infants living in a heterogeneous environment do not exhibit an in-group bias (Bar-Haim et al., 2006). In addition, children from racially mixed schools are less likely to develop race-related favorable in-group biases and negative out-group biases (Rutland, Cameron, Milne, & McGeorge, 2005). Contact between individuals from diverse backgrounds may reduce the salience of intergroup boundaries, producing more individuated and personalized relationships (Dovodio & Gaertner, 1999). In the current study, we examined the independent contribution of children’s neighborhood and peer diversity on their amygdala response to race to examine whether more racially heterogeneous contexts would decrease the amygdala’s response to AA faces. We expected that heightened amygdala response to AA faces would only be present among children and adolescents in racially homogenous contexts, due to the increased salience of race for these youth.
Methods
Participants
Participants included 32 healthy children and adolescents (20 males), ages 4–16.5 years (mean age (SD)=11.3(3.95)). Age was evenly distributed among males and females (females=5–16 years; males =4–16 years). Participants were predominantly from African- (N=11) and European- (N=11) American backgrounds, with the remaining from Asian- (N=6) and Latin-(N=4) American backgrounds. Participants from African- and European-American backgrounds were similar in age (AA: mean age(SD)=12.18(3.69), age range=4.6–16.5; EA: mean age(SD)=11.75(4.08), age range=5.3–16.5). All children were physically and psychiatrically healthy, which was confirmed by a telephone screening. Children’s IQs were within the normal range (mean(SD)=110.7(16.8)) as estimated via two subtests from the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). All participants were right handed.
Procedures
Individual difference measures
Parents completed several measures about their child.
Age
Parents indicated their child’s date of birth. Children’s age at the time of the scan was measured by taking the difference in months between the child’s birth date and the date of the scan.
Peer and Neighborhood Diversity
Parents indicated the racial diversity of their child’s peers by answering two questions, “Are your child’s friends…” and “Are the other children in your child’s current school…” 1=all his/her race, 2=mostly his/her race, 3=mixed, 4=some his her/race, 5=not at all his/her race. These two items were averaged to create one index of peer diversity where lower scores indicated greater homogeneity of peers. Using the same 5-point scale, parents indicated their child’s neighborhood diversity with the following item: “Is the neighborhood your child grows up in…”. Five parents did not provide Peer and Neighborhood Diversity scores, including 3 European-American, 1 African-American, and 1 Latin-American participant.
fMRI task
During the fMRI scan, participants completed two functional runs of the Emotional Matching Task, adapted from Hariri and colleagues(Hariri, Mattay, Tessitore, Kolachana, Fera, Goldman, et al., 2002) and Lieberman and colleagues(2005). During each run, two blocks of emotional faces were interleaved with 2 blocks of a sensorimotor control task (shapes). For the face blocks, children were presented with a trio of faces and were instructed to make a button response to indicate which of the two faces at the bottom was expressing the same emotion, or felt the same, as the face on top. The faces were displaying one of three emotions: Angry, Happy, or Neutral, and all were taken from the NimStim Set of Facial Expressions (Tottenham, Tanaka, Leon, McCarry, Nurse, Hare, et al., 2009). For the shapes blocks, children were presented with a trio of shapes and selected one of the two shapes at the bottom that was identical to the shape on top. Each block consisted of 6 faces or shapes, which were each presented for 5 seconds. Participants completed two runs of the Emotional Matching Task. Similar to the paradigm used by Lieberman and colleagues (2005), Participants played one run in which all the faces were EA and one run in which all the faces were AA. Run order was counterbalanced across participants. Participants were never instructed to attend to race.
fMRI data acquisition
Participants were scanned on a Siemens Trio 3.0 Tesla MRI scanner. For each participant, an initial 2D spin echo image (TR=4000ms, TE=40ms, matrix size 256×256, 4mm thick, 0mm gap) in the oblique plane was acquired to enable prescription of slices obtained in the structural and functional scans. A whole brain, high resolution, T1*weighted anatomical scan (MP-RAGE; 192 X 192 inplane resolution, 250 mm FOV; 176mm × 1mm sagittal slices) was acquired for each subject for registration and localization of functional data into Talairach space5. The Emotional Matching Task was presented on a computer screen through MR-compatible goggles. The task was completed during two functional scans. Ninety-nine T2*weighted echoplanar images were collected (TR=2000, TE=30ms, flip angle =90 degrees, matrix size 64×64, 34 slices, 4mm voxel, skip 0mm) at an oblique angle of approximately 30 degrees.
fMRI data analysis
Functional imaging data were preprocessed and analyzed with the Analysis of Functional NeuroImages (AFNI) software package (Cox 1996). All data were free of movement greater than 2.5 mm in any direction. Preprocessing for each participant’s images included slice time correction to adjust for temporal differences in slice acquisition within each volume, spatial realignment to correct for head motion, registration to the first volume of each run, spatial smoothing using anisotropic 6mm Gaussian kernel, full width at half maximum to increase the signal to noise ratio, and transformation into the standard coordinate space of Talairach and Tournoux (Talairach & Tournoux, 1988)with parameters obtained from the transformation of each subject’s high-resolution anatomical scan. Talairached transformed images had a resampled resolution of 3mm3. Time series were normalized to percent signal change to allow comparisons across runs and individuals by dividing signal intensity at each time point by the mean intensity for that voxel and multiplying the result by 100.
The functional runs were concatenated before creating each participant’s individual-level model, which included three regressors for each of the stimulus types (AA faces, EA faces, and shapes) by convolving the stimulus timing files with canonical hemodynamic response function. Six motion parameters were included as separate regressors for a total of 9 regressors. General linear modeling (GLM) was performed to fit the percent signal change time courses to each regressor. Linear and quadratic trends were modeled in each voxel time course to control for correlated drift.
Next, the individual level regression coefficients were submitted to random-effects, group level analyses. We conducted regression analyses using the 3dRegAna program within AFNI to explore how neural responses to AA and EA faces changed as a function of age and diversity. Age and diversity scores were each entered as regressors. Correction for multiple comparisons was applied at the cluster level following Monte Carlo simulations conducted in the AlphaSim program within AFNI. This method controls for type I errors, offering a reasonable correction for multiple tests during group level analyses in regions of interest (ROIs). Results of the AlphaSim indicated a voxel-wise threshold of p<.05 combined with a minimum cluster size of 8 voxels for the bilateral amygdala (Phan, Fitzgerald, Nathan, P.J., & Tancer, 2006), corresponding to p<.05, False Discovery Rate (FDR) corrected. Non a priori regions outside of the amygdala were corrected for multiple comparisons within the whole brain at p<.01 with a minimum cluster size of 56 voxels. All analyses controlled for participants’ own race.
Results
Behavioral Performance on the Emotional Matching Task
Separate repeated measures ANOVAs were performed using the within subjects factor of Condition (AA faces, EA faces, Shapes) and the between subjects factor of age on the dependent measures of mean reaction time and percent accuracy. We found a significant main effect for condition on reaction time (F=81.03, p<.001), such that participants were faster at matching shapes than either face condition (see Table 1). There was no main effect of Age or interaction of Condition × Age. There was also a main effect for condition on accuracy. Participants made more errors when matching shapes than either face condition (F=8.68, p<.05). There was no main effect of Age or interaction of Condition × Age. These findings show that younger children and older adolescents’ performance is similar on the task, with high performance levels across age, suggesting that it is a developmentally appropriate paradigm. The behavioral data suggest that the shapes condition was experienced quantitatively differently than the face conditions, and therefore, we used the implicit baseline (crosshair fixation) rather than shapes to contrast with the faces in the fMRI analyses.
Table 1.
Behavioral Responses on the Emotional Matching Task
| Condition | Mean Reaction time (SD) | Mean Accuracy (%)(SD) |
|---|---|---|
| AA Faces | ||
| Children (4–9 years) | 1850.85 (536.62) | 91.7 (7.8) |
| Early Adolescents (10–13 years) | 1606.69 (474.40) | 92.5 (6.1) |
| Late Adolescents (14–16.5 years) | 1359.89 (333.85) | 95.8 (5.6) |
| EA Faces | ||
| Children (4–9 years) | 1876.21 (515.04) | 93.4 (7.7) |
| Early Adolescents (10–13 years) | 1597.56 (410.75) | 93.4 (10.2) |
| Late Adolescents (14–16.5 years) | 1506.06 (408.01) | 93.7 (7.2) |
| Shapes | ||
| Children (4–9 years) | 1284.60 (534.54) | 89.8 (16.4) |
| Early Adolescents (10–13 years) | 1040.78 (337.94) | 86.7 (18.0) |
| Late Adolescents (14–16.5 years) | 919.09 (202.71) | 96.3 (3.8) |
Note. For descriptive purposes only, participants were broken up into 3 age groups: children (N=10), early adolescence (N=10), and late adolescence (N=12). Statistical analyses treated age as a continuous variable. AA=African American; EA=European American; SD=standard deviation.
Amygdala Response to Race Across Development
Our first analyses examined whether the amygdala in our child and adolescent sample coincides with the adult template used for registration. We created an average anatomical from all participants in the study. As shown in Figure 1, the anatomical average from our developmental population shows that the amygdala region coincides with the adult template.
Figure 1.
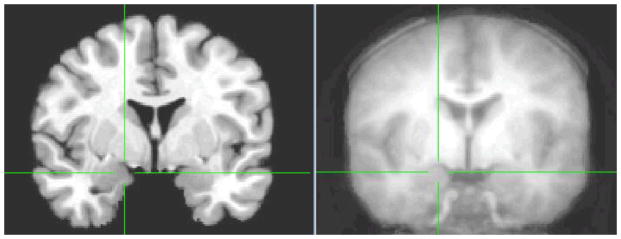
The amygdala in the adult template (left) corresponds to the amygdala in the average anatomical template from the developmental population in the current study (right). xyz coordinates at the crosshairs are 18, −3, −9
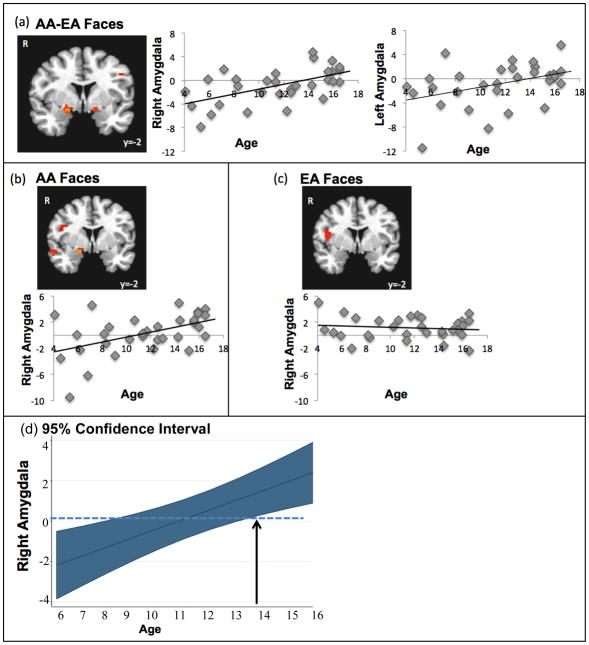
Our first primary goal was to examine whether there were neurodevelopmental changes to AA faces relative to EA faces. In whole brain regression analyses, we correlated age with neural activation to AA-EA faces. As shown in Figure 2a, with age, children showed increased bilateral amygdala activation to AA-EA faces (right: xyz=16 −2 −8, t(30)=3.67, p<.05, corrected; left: xyz=−14 −2 −7, t(30)=2.37, p<.05, corrected).
Figure 2.
(a) The bilateral amygdala to AA-EA faces correlated positively with age. This neurodevelopmental amygdala increase is specific to AA faces such that (b) the right amygdala response to AA faces relative to baseline correlated positively with age, whereas (c) the amygdala does not show a developmental increase in response to EA faces. (d) the age effect with the 95% confidence interval. Where the confidence interval does not include 0 on the y-axis (depicted with an arrow), participants are showing a significant differential response to AA faces
Next, we examined whether this neurodevelopmental increase in amygdala response to AA-EA faces is specific to AA faces, EA faces, or both. We correlated age with neural activation in the contrast of AA faces-baseline and EA faces-baseline separately in whole brain analyses. Developmental increases in the amygdala were specific to AA faces. Whereas activation in the right amygdala significantly increased to AA faces across development (t(31)=3.41, p<.05, corrected; Figure 2b), age did not correlate with amygdala activation to EA faces (Figure 2c). A repeated measures ANOVA using the within subject factor Race (AA and EA) and the between subject factor of Age on the dependent measure of percent BOLD signal change in the amygdala, revealed a significant Race × Age interaction, F(1,30)= 14.6, p<.001. Given this developmental increase that is specific to AAs faces, we explored at what age the amygdala responds differentially to AA faces. We ran follow-up analyses using the margins function in STATA11 (StataCorp, College Station, Texas). Figure 2d displays the age effect with the 95% confidence interval. Where the confidence interval does not include 0 on the y-axis, the participants are showing a significant differential response to Black faces. The margin becomes significant around age 14 (z=2.51, p=0.01, 95% CI= .32 to 2.66). Together, these findings indicate that there are age-related changes in the processing of AA but not EA faces, such that amygdala sensitivity to AAs is not present in early childhood but emerges during adolescence. For other significant regions that correlated with age to AA and EA faces, see Table 2a-c.
Table 2.
Whole Brain Significant Activations for AA and EA Faces that Correlated Positively with Age and Peer Diversity
| Anatomical Region | BA | x | y | z | t | k | |
|---|---|---|---|---|---|---|---|
| (a) AA>EA faces and Age | |||||||
| VLPFC | 45 | R | 48 | 27 | 2 | 5.78 | 1879 |
| Fusiform gyrus | 19 | R | 23 | −61 | −10 | 3.82 | 1161 |
| (b) AA faces (relative to baseline) and Age | |||||||
| Fusiform gyrus | 19 | R | 24 | −61 | −10 | 4.58 | 1723 |
| VLPFC | 47 | L | −34 | 20 | −19 | 3.34 | 64 |
| Middle Occipital Gyrus | 19 | L | −29 | −85 | 8 | 4.40 | 129 |
| Middle Occipital Gyrus | 18/19 | R | 29 | −88 | 5 | 4.91 | 76 |
| Culmen | 0 | −64 | −1 | 2.80 | 94 | ||
| (c) EA faces (relative to baseline) and Age | |||||||
| VLPFC | 47 | L | −45 | 25 | 4 | −3.37 | 1020 |
| Anterior Cingulate | 32 | L | −10 | 28 | 19 | −3.04 | 56 |
| Insula | L | −33 | 5 | −7 | −3.68 | 127 | |
Note. BA refers to putative Brodmann’s Area; L and R refer to left and right hemispheres; x, y, and z refer to Talairach coordinates; t refers to the t-score at those coordinates (local maxima); k refers to the number of voxels in each significant cluster. The following abbreviations are used for the names of specific regions: dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC). Non a priori regions outside of the amygdala were corrected for multiple comparisons within the whole brain at p<.05 with a minimum cluster size of 146 voxels.
Our next goal was to examine whether AA and EA participants showed similar neurodevelopmental trajectories to AA and EA faces. We extracted parameter estimates from the right amygdala to EA faces and AA faces and ran separate regression analyses in SPSS for each ethnic group, examining how age related to amygdala response to EA and AA faces separately. Both European-American (B=.79, SE=.20, β=.80, p<.005) and African-American (B=.42, SE=.11, β=.80, p<.005) participants showed increased right amygdala activation to AA faces with age, but neither group showed increased amygdala response to EA faces with age. These findings suggest that the amygdala becomes increasingly sensitive to AA faces with development, and this neurodevelopmental trajectory is similar for individuals from AA and EA backgrounds.
Finally, as a control to ensure that it is possible to get amygdala response in our younger children, we examined whether all age groups show differential amygdala response to emotional faces (angry). Because we found evidence of a developmental increase in the amygdala to AA faces, we examined the contrast of EA angry faces>baseline, as we anticipated that angry faces would produce a stable signal across all age groups. For descriptive purposes, we divided our sample into 3 age groups, children (ages 4–9, N=10), early adolescents (ages 10–13, N=9), and adolescents (ages 14–16.5, N=12). We observed differential amygdala response to EA angry faces>baseline in each age group in the amygdala (children: right amygdala: xyz=−25 −1 −20, t(9)=3.88, p<.05, left amygdala: xyz= 22 10 −3, t(9)=2.95, p<.05; early adolescents: right amygdala: xyz=22 1 −18, t(8)=3.34, p<.05; adolescents: right amygdala: xyz= −28 2 −13, t(11)=3.77, p<.05, left amygdala: xyz= 20 9 −10, t(11)=3.28, p<.05). Moreover, in a whole brain regression analysis, correlating age with brain activation to EA angry faces, we do not find an age-related increase or decrease in the amygdala. Therefore, across the ages tested, we obtained a stable amygdala response.
Amygdala Response to Race as a Function of Neighborhood and Peer Diversity
Next, we tested whether racially diverse contexts would modulate the amygdala response to race. Prior work has highlighted the importance of diverse social environments, such as neighborhood and school diversity, in reducing racial in- and out-group biases (Bar-Haim et al., 2006; Rutland et al., 2005). Given the specificity of the amygdala to AA faces, we examined whether racial diversity of children’s neighborhood and peers would modulate this amygdala response. In separate whole brain analyses, we correlated neighborhood and peer diversity with neural activation to AA faces (relative to baseline), controlling for participants’ own race. Whereas neighborhood diversity was not related to amygdala response to AA faces, greater peer diversity was associated with attenuated right amygdala response to AA faces (xyz=16 −2 −8, t(25)=−3.27, p<.05, corrected; Fig 3)1, suggesting that more racially homogenous peer groups (regardless of racial composition), relate to greater amygdala response to AA faces. These findings suggest that children’s peer environment can shape how race is processed in the brain. No other brain regions correlated with racial diversity.
Figure 3.
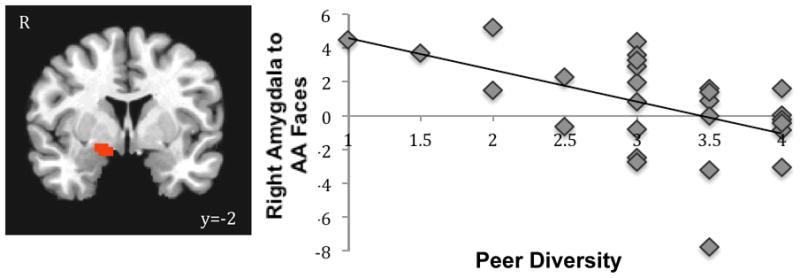
Children with more diverse peers show dampened activation to AA faces.
Finally, given that the amygdala cluster found for peer diversity was in the same region as that found for age, we conducted regression analyses in which we simultaneously entered peer diversity and age to predict amygdala response to AA faces, controlling for participants’ own race. Results show that age and peer diversity each independently predicted amygdala activation to AA faces (Age: B=.29, SE=.11, β=.42, p<.05; Peer Diversity: B=−1.38, SE=.55, β=−.41, p<.05). Age accounted for 35.9% of the variance, and peer diversity accounted for an additional 11.3%. Together, age and peer diversity explained nearly half (47.2%) of the amygdala response to AA faces.
Neural and Behavioral Response to Race
To examine whether the amygdala response to race was related to children’s behavior, we conducted multiple regression analyses in which we examined how the amygdala response to AA relative to EA faces predicted participants’ mean reaction time when matching the emotion of AA relative to EA faces. The behavioral bias was calculated by subtracting the standardized mean reaction time to EA faces from the standardized mean reaction time to AA faces. Negative scores indicate faster response times to AA faces and positive scores indicate faster response times to EA faces. We controlled for age and participants’ race. As shown in Figure 4, participants who showed greater activation to AA relative EA faces in the left amygdala were also faster at matching AA relative to EA faces. These behavioral data suggest that amygdala response to AA faces was associated with a decrease in speed in behavioral responding to AA faces.
Figure 4.
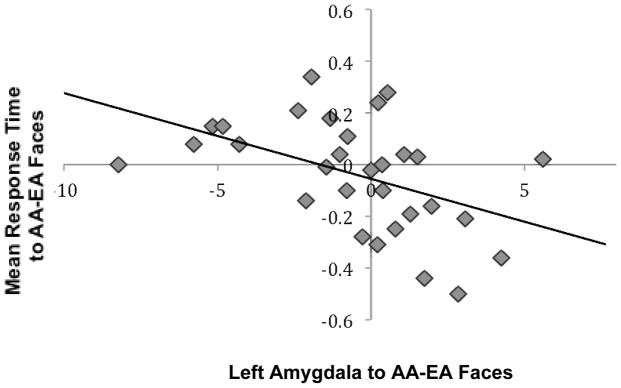
The left amygdala to AA relative to EA faces correlated negatively with mean reaction time to AA relative to EA faces. Adolescents who matched AA faces more quickly than EA faces showed enhanced amygdala activation to AA relative to EA faces.
Discussion
The social environment plays a large role in shaping affective perceptions of race (Bar-Haim et al., 2006). The amygdala is involved in nonconscious processing of stimuli that have an acquired emotional significance based on previous experience, and plays a role in sensitivity to the salience of environmental cues (Cunningham & Brisch, 2012; Fitzgerald et al., 2006; Fudge & Emiliano, 2003; Santos et al., 2011; Whalen et al., 2001). Thus, the amygdala is particularly amenable to learning about socially constructed values placed on social groups, such as those about race. We find that the amygdala becomes increasingly sensitive to AA faces across development, with activation to AA faces only becoming significant around 14 years of age. The heightened amygdala activity to AA faces previously reported in adults (Cunningham et al., 2004; Phelps et al., 2000, Lieberman et al., 2005) is not present during early childhood and only becomes evident during adolescence. Thus, amygdala responsivity to race is likely the result of a developmental process in which the amygdala acquires emotional knowledge learned over development, becoming more sensitive to AA faces. This heightened amygdala response to AA faces may reflect learned cultural knowledge, such as implicit and explicit stereotypes. Across development, youth internalize cultural biases and norms in their environment (Apfelbaum, et al., 2008). Additionally, this response may reflect the increasing salience of race that occurs during adolescence that is not associated with bias, such as adolescents’ ethnic identity explorations. For example, adolescents enter high school where ethnic clubs and coalitions may form and youth begin to explore their ethnic identity (Roberts et al., 1999). Therefore, the amygdala response may reflect increased learning, exploration, and awareness of race. Future research should explore whether cultural biases, awareness or endorsement of stereotypes, or ethnic identity exploratopm explain the age effect to race found in the current study. Alternatively, the increasing amygdala response to race may be driven by intrinsic factors of the child, such as puberty, rather than exposure to cultural messages. Indeed, prior research has found that puberty is associated with increased amygdala response to emotional stimuli (Moore et al., 2012), and pubertal hormones may partly drive the social-reorientation to of the amygdala during adolescence (Nelson et al., 2005). Future research should examine how pubertal hormones relate to the neural processing of race.
Children from both EA and AA backgrounds showed a similar neurodevelopmental increase in the amygdala to AA faces, consistent with behavioral research showing that AA youth internalize socially constructed views held by the dominant culture (Averhart & Bigler, 1997; Spencer & Marstrom-Addams, 1990)and neuroimaging research among AA adults showing heightened amygdala response to AA faces (Lieberman et al., 2005). Individuals from diverse ethnic and racial groups are exposed to similar cultural messages, and with age youth may internalize these messages, attaining the cultural knowledge that AA individuals are treated differently. Alternatively, the amygdala response in our AA and EA samples may be tapping different processes. For the AA participants, the heightened amygdala response may be following a developmental path parallel to AA youths’ explorations of their ethnic identity, which increases during high school more so than for EA youth (Phinney, 1996). For the EA participants, the heightened amygdala response may reflect the development of cultural biases. Thus, race may be salient for each ethnic group but for different reasons, and so the same neurodevelopmental activations may be reflecting different underlying processes. Future research should attempt to understand the mechanisms driving the amygdala response in different ethnic populations.
The amygdala is involved in the detection of motivationally relevant and salient aspects of ones environment (Cunningham & Brisch, 2012; Fitzgerald et al., 2006; Fudge & Emiliano, 2003; Santos et al., 2011; Whalen et al., 2001). When the amygdala detects salience, via substantial projections to primary and high-order sensory and motor areas of the brain, it guides further neural processing to appropriately respond, potentially impacting behavior (Cunningham & Brisch, 2012; Davis & Whalen, 2001). We observed that children who showed a stronger amygdala response to AA faces relative to EA faces were also faster at matching AA faces, suggesting that the heightened amygdala response to AA faces resulted in faster reaction times. This finding provides support that the amygdala is involved in detecting salience of the stimulus which may be part of the process whereby learning affective properties of social stimuli occurs. If the amygdala were responding to negativity, one might expect this to influence children’s behavior through avoidance (i.e., slower reaction times to matching AA faces). Thus, within this experimental context, the behavioral data suggest that the amygdala response may be signaling the increasing saliency of AA that accompanies age. Indeed, on its own, heightened salience of social groups increases negative out-group behaviors and positive in-group behaviors (Tajfel, 1978; Tajfel & Turner, 1979; Bigler, Brown, & Markell, 2001; Patterson & Bigler, 2006 Claeys) and may be the basis for the high correlation between negative appraisals of AAs and amygdala response that have been observed in adulthood (Phelps et al., 2000),
In addition to the amygdala, the fusiform gyrus (FG) and ventrolateral prefrontal cortex (VLPFC) were specifically recruited to AA faces as children got older. The FG is a brain region involved in face perception (Haxby, Hoffman, & Gobbini., 1994) and visual expertise (Kanwisher, McDermott, & Chun, 1997). Therefore, as children get older, they may have more experience and exposure to AA individuals, thus developing greater expertise and recruiting the FG to AA faces. The VLPFC is thought to mediate evaluative and regulatory processes and may modulate amygdala reactivity (Passarotti, Sweeny, & Pavuluri, 2009). Moreover, with age individuals are better able to regulate affective responses (Yurgelun-Todd, 2007). Therefore, as children get older, AA individuals may become more emotionally salient, as evidenced by the amygdala response, and the VLPFC may come online.
Our second goal was to understand how children’s social environment may alter the amygdala response to race by examining children’s peer and neighborhood contexts. The salience of social categories, such as race, varies according to social contexts (Turner, Hogg, Oakes, Reicher, & Wetherell, 1987). Increased racial diversity may reduce the salience of AA faces. Our results revealed that when children had more cross-race friends and schoolmates, they were less likely to exhibit a neural bias to AA faces, consistent with a body of work highlighting the benefit of racially diverse schools for decreasing in-group biases(Rutland et al., 2005; Juvonen, Nishina, & Graham 2006). This attenuation of amygdala response suggests that intergroup racial contact may reduce the salience of race. Contact between members of different racial groups may expose children to more diverse views, producing more individuated and personalized relationships across racial groups (Dovodio & Gaertner, 1999). Even for AAs themselves, contact between individuals from diverse backgrounds may reduce the salience of intergroup boundaries (Dovodio & Gaertner, 1999). Thus, interventions designed to reduce the development of racial biases could focus on providing children with opportunities to interact with individuals from diverse backgrounds, thereby potentially decreasing the salience of race. Interestingly, children’s neighborhood diversity was not related to their neural processing of race. Perhaps neighborhood diversity results in fewer opportunities to interact with individuals of different racial backgrounds compared to diversity in schools, which provides hourly interactions with one’s peers.
Because our participants spanned a broad age range from 4 to 16 years, it was important to demonstrate that warping to the adult template did not bias the results towards less amygdala activation in younger individuals, thereby driving our race-related developmental effects. We addressed this issue in two ways. First, we created an anatomical average of our developmental participants and overlay it on the adult template. The anatomical average from our developmental population shows that the amygdala region coincides with the adult template. Second, we examined neural activation in the amygdala to angry faces across age and show that we get differential amygdala response in the youngest participants in response to emotional stimuli. In fact, there are no age-related changes in amygdala response to emotional faces; children, young adolescents, and older adolescents all show enhanced activation to angry faces. Moreover, results from our primary analysis show that children across our entire age range evidence stable amygdala activation to EA faces. Together, this suggests that warping the child brains to the adult template did not bias the results towards less amygdala activation in younger children. Recent advances in developmental neuroscience have shown that pediatric and adult neuroimaging data can be analyzed in the same strerotactic space. For instance, Burgund and colleagues (2002) and Kang and colleagues (2003) found that atlas-transformed brain morphology, BOLD responses, and locations of functional activation foci are consistent between 7- and 8-year-old children and adults.
In conclusion, the findings in the current study demonstrate the continuous functional maturation of the amygdala in response to social groups across development spanning a large age range of children from 4 to 16 years. The differential response of the amygdala to AA faces does not emerge until adolescence, suggesting that the increasing salience of race across development may shape the functional architecture of the amygdala. Importantly, these findings suggest that neural biases to race are not innate and that race is a social construction, learned over time.
Footnotes
The N in each racial group is too small to warrant a formal separate analysis. However, for descriptive purposes we present the findings for European- (N=8) and African- (N=10) American participants. Although the relationship between peer diversity and amygdala response is not significant for either group alone, both European American (B=−1.12, SE=1.15, β=−.37) and African American (B=−1.98, SE=1.04, β=−.56) participants show similar decreases in amygdala response to AA faces with more diverse peers.
References
- Aboud FE. The formation of ingroup favoritism and outgroup prejudice in young children. Developmental Psychology. 2003;39:48–60. doi: 10.1037//0012-1649.39.1.48. [DOI] [PubMed] [Google Scholar]
- Apfelbaum EP, Pauker K, Ambady N, Sommers SR, Norton MI. Learning (not) to talk about race: When older children underperform in social categorization. Developmental Psychology. 2008;44:1513–1518. doi: 10.1037/a0012835. [DOI] [PubMed] [Google Scholar]
- Averhart CJ, Bigler RS. Shades of meaning: Skin tone, racial attitudes, and constructive memory in African American children. Journal of Experimental Child Psychology. 1997;67:363–388. doi: 10.1006/jecp.1997.2413. [DOI] [PubMed] [Google Scholar]
- Baron AS, Banaji MR. The development of implicit attitudes: Evidence of race evaluations from ages 6 and 10 and adulthood. Psychological Science. 2006;17:53–58. doi: 10.1111/j.1467-9280.2005.01664.x. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Ziv T, Lamy D, Hodes RM. Nature and nurture in own-race face processing. Psychological Science. 2006;17:159–163. doi: 10.1111/j.1467-9280.2006.01679.x. [DOI] [PubMed] [Google Scholar]
- Bergund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, Schlaggar BL. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. NeuroImage. 2002;17:184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Bigler RS, Liben LS. Developmental intergroup theory: Explaining and reducing children’s social stereotyping and prejudice. Current Directions in Psychological Science. 2007;16:162–166. [Google Scholar]
- Bigler RS, Brown CS, Markell M. When groups are not created equal: Effects of group status on the formation of intergroup attitudes in children. Child Development. 2001;72:1151–1162. doi: 10.1111/1467-8624.00339. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Claeys KG, Orbam GA, Dupont P, Sunaert S, Van Hecke P, De Schutter E. Involvement of multiple functionally distinct cerebellar regions in visual discrimination: A human functional imaging study. NeuroImage. 2003;20:840–854. doi: 10.1016/s1053-8119(03)00366-5. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 2006;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Brosch T. Motivational salience. Amygdala tuning from traits, needs, values, and goals. Current Directions in Psychological Science. 2012;21:54–59. [Google Scholar]
- Cunningham WA, Johnson MK, Raye CL, Gatenby JC, Gore JC, Banaji MR. Separable neural components in the processing of Black and White Faces. Psychological Science. 2004;15:806–813. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: Vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dovidio JF, Kawakami K, Johnson C, Johnson B, Howard A. The nature of prejudice: Automatic and controlled processes. Journal of Experimental Social Psychology. 1997;33:510–540. [Google Scholar]
- Dovodio JF, Gaertner SL. Reducing prejudice: Combating intergroup biases. Current Directions in Psychological Science. 1999;8:101–105. [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: Amygdala reactivity across multiple expressions of facial affect. NeuroImage. 2006;30:1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Gale GD. The amygdala, fear, and memory. Annals of the New York Academy of Sciences. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Emiliano AB. The extended amygdala and the dopamine system: Another piece of the dopamine puzzle. The Journal of Neuropsychiatry and Clinical Neuroscience. 2003;15:306–316. doi: 10.1176/jnp.15.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, Fromm S, Leibenluft E, Pine DS, Ernst M. A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Haxby J, Hoffman EA, Gobbini MI. The effect of face inversion on activity in human neural systems for face and object perception. Neuron. 1999;22:189–199. doi: 10.1016/s0896-6273(00)80690-x. [DOI] [PubMed] [Google Scholar]
- Hennenlotter A, Schroeder U, Erhard P, Castrop F, Haslinger B, Stoecker D, Lange KW, Ceballos-Baumann AO. A common neural basis for receptive and expressive communication of pleasant facial affect. Neuroimage. 2005;26:581–591. doi: 10.1016/j.neuroimage.2005.01.057. [DOI] [PubMed] [Google Scholar]
- Humphrey T. The development of the human amygdala during early embryonic life. Journal of Comparative Neurology. 1968;132:135–165. doi: 10.1002/cne.901320108. [DOI] [PubMed] [Google Scholar]
- Juvonen J, Nishina A, Graham S. Ethnic diversity and perceptions of safety in urban middle schools. Psychological Science. 2006;17:393–400. doi: 10.1111/j.1467-9280.2006.01718.x. [DOI] [PubMed] [Google Scholar]
- Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL. Comparison of functional activation foci in children and adults using a common sterotactic space. NeuroImage. 2003;19:16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. The Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. Race as a visual feature: Using visual search and perceptual discrimination tasks to understand face categories and the cross-race recognition deficit. Journal of Experimental Psychology. 2000;129:559–574. doi: 10.1037//0096-3445.129.4.559. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Hariri A, Jarcho JM, Eisenberger NI, Bookheimer SY. An fMRI investigation of race-related amygdala activity in African-American and Caucasian-American individuals. Nature Neuroscience. 2005;8:720–722. doi: 10.1038/nn1465. [DOI] [PubMed] [Google Scholar]
- Moore WE, Pfeifer JH, Masten CL, Iacoboni M, Mazziotta JC, Dapretto M. Facing puberty: Associations between pubertal development and neural responses to affective facial displays. Social Cognitive and Affective Neuroscience. 2012;7:35–43. doi: 10.1093/scan/nsr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:163–74. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Passarotti AM, Sweeny JA, Pavuluri MN. Neural correlates of incidental and directed facial emotion processing in adolescents and adults. Social Cognitive and Affective Neuroscience. 2009;4:387–398. doi: 10.1093/scan/nsp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson MM, Bigler RS. Preschool children’s attention to environmental messages about groups: Social categorization and the origins of intergroup bias. Child Development. 2006;77:847–860. doi: 10.1111/j.1467-8624.2006.00906.x. [DOI] [PubMed] [Google Scholar]
- Payne C, Machado CJ, Bliwise NG, Bachevalier J. Maturation of the hippocampal formation and amygdala in Macaca mulatta: a volumetric magnetic resonance imaging study. Hippocampus. 2010;20:922–935. doi: 10.1002/hipo.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety symptoms in generalized social phobia. Biological Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Cunningham WA, Funayma ES, Gatenby JC, Gore JC, Banaji MR. Performance on indirect measures of race evaluation predicts amygdala activity. Journal of Cognitive Neuroscience. 2000;12:1–10. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- Phinney JS. Understanding ethnic diversity: The role of ethnic identity. American Behavioral Scientist. 1996;40:143–152. [Google Scholar]
- Plant EA, Devine PG. Internal ad external motivation to respond without prejudice. Journal of Personality and Social Psychology. 1998;75:811–832. [Google Scholar]
- Richeson JA, Trawalter S. The threat of appearing prejudiced and race-based attentional bias. Psychological Science. 2008;19:98–102. doi: 10.1111/j.1467-9280.2008.02052.x. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Phinney JS, Masee LC, Chen R, Roberts CR, Romero A. The structure of ethnic identity of young adolescents from diverse ethnocultural groups. The Journal of Early Adolescence. 1999;19:301–322. [Google Scholar]
- Rosette AS, Leonardelli GJ, Phillips KW. The White standard: Racial bias in leader categorization. Journal of Applied Psychology. 2008;93:758–777. doi: 10.1037/0021-9010.93.4.758. [DOI] [PubMed] [Google Scholar]
- Rutland A, Cameron L, Milne A, McGeorge P. Social norms and self presentation: Children’s implicit and explicit intergroup attitudes. Child Development. 2005;76:451–466. doi: 10.1111/j.1467-8624.2005.00856.x. [DOI] [PubMed] [Google Scholar]
- Santos A, Mier D, Kirsch P, Meyer-Lindenberg A. Evidence for a general face salience signal in human amygdala. NeuroImage. 2011;54:3111–3116. doi: 10.1016/j.neuroimage.2010.11.024. [DOI] [PubMed] [Google Scholar]
- Spencer MB, Markstrom-Adams C. Identity processes among racial and ethnic minority children in America. Child Development. 1990;61:290–310. [Google Scholar]
- Tajfel H, editor. Differentiation between social groups. London: Academic Press; 1978. [Google Scholar]
- Tajfel H, Turner JC. An integrative theory of intergroup conflict. In: Austin WG, Worschel S, editors. The social psychology of intergroup relations. Monterey, CA: Brooks/Cole; 1979. pp. 77–98. [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotactic Atlas of the Human Brain. Thieme; Stuttgart/New York: 1988. [Google Scholar]
- Tottenham N, Tanaka J, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson CA. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Casey BJ. A developmental perspective on human amygdala function. In: Phelps E, Whalen P, editors. The human amygdala. New York: Guilford Press; 2009. pp. 107–117. [Google Scholar]
- Trawalter S, Todd AR, Baird AA, Richeson JA. Attending to threat: Race-based patterns of selective attention. Journal of Experimental Social Psychology. 2008;44:1322–1327. doi: 10.1016/j.jesp.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JC, Hogg MA, Oakes PJ, Reicher S, Wetherell MS. Rediscovering the social group: A self-categorization theory. Oxford: Basil Blackwell; 1987. [Google Scholar]
- Ulfig N, Setzer M, Bohl J. Ontogeny of the human amygdala. Annals of the New York Academy of Sciences. 2003;985:22–33. doi: 10.1111/j.1749-6632.2003.tb07068.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Current Opinions in Neurobiology. 2007;17:251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]