Abstract
Purpose
To examine similarities and differences in the process that parents and adolescents use to make decisions concerning participation in an asthma clinical trial. We hypothesized that a single conceptual model, tested through structural equations modeling, could explain adolescent assent and parent consent for adolescent research participation.
Methods
109 adolescents enrolled with at least one parent and received an asthma evaluation from a pediatric asthma specialist and then evaluated a hypothetical asthma research protocol. Family members independently evaluated the protocol and made research participation decisions.
Results
Perceived risk, benefit and compensation were direct predictors of participation decisions for parents and adolescents. Adolescents perceived direct study benefit from the relationship with the physician, however parents did not. Parent decisions were most strongly associated with perceived risk, and parents associated discomfort with risk more strongly than did adolescents. Protocol procedures contributed to perceptions of benefit and discomfort for parents and adolescents.
Conclusions
Parent and adolescent research participation decisions are influenced by protocol variables in similar ways, although there are differences that account for disagreements within families. Findings may help investigators develop protocols that appeal to parents and adolescents and highlight issues of particular importance to address during the process of informed consent.
Keywords: asthma, biomedical-research-ethics, informed-consent, adolescent-assent, research-participation-decision-making, adolescent, child, research-support
Advancements in biomedical and behavioral science research are inextricably linked to the willingness of individuals to participate in research. For the past 25 years researchers have been studying the informed consent and assent process to better understand how biomedical research participants can be ethically recruited and enrolled. This issue is important since there are significant problems in recruitment for clinical trials (1-3). This recruitment problem is particularly salient in biomedical research involving minors. Unique complications occur because of minors’ protected population status and because they provide assent to biomedical research while their parents are required to provide permission (1).
A variety of important variables have been identified through empirical processes or because of their theoretical and ethical implications for research participants’ enrollment decisions. Some reasons for participation are based on protocol specific variables such as perceived risk, benefit or importance of the study (2-5). Other variables that are important to the participation decision-making relate to the informed consent/assent process. These variables refer to the manner in which informed consent is obtained, (e.g., the skill, status, and professional relationship of the person seeking consent and assent or the manner in which the study is presented) (6-8). A final category of factors pertains to participant attributes, which encompass patient demographics, individual values, idiosyncratic experience with prior research, or the cognitive capacities and affective state of the parent or adolescent (4, 5, 9-11).
Recent studies have also focused on decision-making processes within families, including parental influence and family dynamics in the assent process and several important themes have been identified. For example, research has revealed that parents and adolescents often disagree about research participation (12, 13), that both parents and adolescents claim decision-making responsibility for biomedical research participation decisions, yet when pressed to make a joint decision, adolescents tend to defer to parents (14). Other studies have indicated there are different family decision-making strategies. These strategies vary from family decision-making processes in which parents assume complete control to ones in which the family decision-making processes are open and inclusive (15). Regardless of these stylistic differences, prior research suggests that both children and parents tend to endorse collaborative decision-making as desirable (16).
These findings, derived from qualitative, descriptive, and quasi-experimental studies, have begun to identify potentially important variables associated with research participation decisions, although there are significant methodological limitations qualifying these findings (1, 17). Moreover, what’s lacking from this piecemeal empirical approach is an understanding of how these variables relate to one another and, whether individually or in combination, they augment or deter participation decision-making. Furthermore, it’s unclear whether there are important differences between parents and adolescents on the variables related to decision-making. To address these limitations, the current study utilizes structural equations modeling (SEM) (18) to examine similarities and differences in the process that parents and adolescents use to make decisions concerning participation in an asthma clinical trial. SEM provides a methodology to assess the independent and interdependent relationships among a set of concepts that are hypothesized to explain an outcome such as the willingness to enroll in research. The SEM procedures extend Multiple Regression Analyses by examining the interdependent mediational associations among explanatory constructs. The SEM technique is particularly useful in assessing whether one construct serves as a mediating mechanism for the influence of another construct.
We developed a single conceptual model of research participation decisions that could be tested with SEM and includes important variables identified in previous studies. Two tests of this conceptual model are presented: The first examines adolescent assent for research participation, the second examines parent consent for adolescent research participation. We hypothesized that perceptions of risk and benefit are important meditational processes in the decision to participate in research (13, 19).We included other variables which we hypothesized would explain the variability among potential research participants in their perceptions of risk and benefit. These additional explanatory variables included perceptions of the research protocol procedures (2-5, 20), perceived discomfort of the protocol (12), the patient’s relationship with (i.e. confidence in and comfort in interacting with) the physician conducting the research (6-8, 14), and the proposed compensation for research participation (21-25).
METHODS
The study sample was drawn from a statewide recruitment of adolescents with asthma and their parents. Families were recruited using brochures, advertisements, mailings and referrals from a variety of sources including, schools, the general pediatrics clinic of the local university health sciences center, private practice pediatrician offices and managed care organizations. Adolescents were eligible to enroll if they had a prior diagnosis of asthma, spoke English, and were between 11-17 years of age. The study was approved by the Oregon Research Institute IRB, the University of New Mexico Human Research Review Committee and the Presbyterian Health Systems IRB, and all data collection occurred at a single site. Informed consent/assent was obtained from parents and adolescents prior to initiating study procedures.
Adolescents, in the company of their parents, received an extensive asthma examination by a pediatric asthma specialist. At the completion of the clinical examination, adolescents and their parents came to the research laboratory (separate from the medical clinic) where they were shown a videotaped description of a hypothetical asthma clinical trial. By random assignment, half of the families saw a research presentation made by the same specialist who performed the asthma examination, the other half saw a presentation from an unknown asthma specialist. The videotape was used to standardize the explanation of the research protocol across families, and to incorporate the physician-investigator relationship variable into the conceptual model. The presentation included all information that would typically be provided in an informed consent meeting. Participants also received written materials summarizing the study procedures and research assistants followed-up the video presentation by responding to questions and providing additional details about the hypothetical asthma clinical trial procedures. Family members were instructed not to discuss their reactions to the videotaped vignette during the presentation. Parent(s) and adolescent privately completed the Vignette Evaluation Form assessing their views about the hypothetical trial. Adolescents were asked about their willingness to participate in the trial, and parents were asked if they would volunteer their child for the research. All family members were asked to indicate the factors most responsible for their participation decisions. Once private views had been obtained, the family discussed the research protocol during a 10 minute videotaped interaction and then made a final decision about whether they would be willing to participate in the hypothetical trial. Participants were compensated $25.00 per person. In addition, families traveling from rural areas of the state to participate received overnight accommodations and mileage reimbursement.
These data report on the private research participation decisions of adolescents and parents which were collected as part of a larger study examining family and physician influences on voluntary assent (14). The primary measure used for this analysis was the 60-item Vignette Evaluation Questionnaire which the authors developed specifically for this study. This questionnaire evaluated parent and adolescent private assessments of the specific research vignette. The first set of eight questions asked adolescents and parents to indicate the likelihood they would be willing to enroll in the study described in the vignette. They also indicated, via a 7-point Likert scale, the extent to which they believed they would influence the actual decision. The second set of 34 questions, detailed key aspects of this particular study (e.g. “allergy skin testing” or “possibility of a placebo”), and asked participants to indicate how much each item influenced their participation decision. The remaining 18 items asked participants to either provide general evaluations of the research vignette (e.g. “How beneficial is this study overall to you (your child),” or to indicate their level of agreement with statements about the vignette (e.g., “This doctor explained things so I could remember them.)”.
Vignette Development
The video vignette described a previously conducted asthma clinical trial selected from those in a previous report (13). The hypothetical trial vignette was adapted directly from the original protocol. It explained the purpose of the trial was “to see whether it is safe and effective for adolescents with asthma to take asthma medicine only when they are having symptoms, or whether it is better for them to take medicine all of the time.” Participants were told the trial would last 26 weeks and procedures would include: randomization (to Albuterol or placebo), being provided with rescue medication, 12 physical exams, spirometry at each clinic visit, methacholine challenge testing 7 times, allergy skin testing, EKG, pregnancy testing (for females), daily diary cards (including peak flow monitoring), and completion of a quality of life questionnaire. Risks associated with study participation were described and participants were informed that they could be temporarily taken off the study protocol if their symptoms worsened or required additional treatment. Compensation for this hypothetical trial was described as $1,000 for study completion.
Statistical Analysis
Data analyses proceeded in several steps. First, the investigators statistically validated the a priori latent variables in the SEM model to ensure they represented conceptually distinct constructs. This was accomplished through factor analyses and internal consistency reliability analyses (coefficient alpha) of the Vignette Evaluation Questionnaire. Latent variables included: participants’ perceptions of protocol procedures, risk, benefit, discomfort, compensation, relationship with the investigator, and the research participation decision outcome. Parent and adolescent data were verified separately. Preliminary analyses determined that randomization on the physician relationship variable did not influence the private research participation decisions made by parents and adolescents, therefore the data for parents and for adolescents were respectively combined for purposes of the SEM analyses.
Unreliability of measurement can substantially limit the assessment of mediational relationships (18). An important premise of SEM is that estimates from the latent constructs are more reliable and valid than can be obtained from any single indicator variables. For the SEM analyses, two to six indicator items were selected for each latent construct. Preliminary exploratory factor analyses and standardized coefficient alphas were computed, with the results shown in Table 1. LISREL software (26) provided an evaluation of the parent and adolescent models using full information likelihood procedures to account for missing data (0.4% of all scores). Goodness of fit indices (i.e., Root Mean Square Error of Approximation: RMSEA; Full Information Likelihood Goodness of Fit: Chi-square) provide an estimate of whether the data are actually consistent with the conceptual model. RMSEA provides an overall estimate for the goodness of fit of the hypothesized model to the data (27). RMSEA represents the average discrepancy per degree of freedom of the estimated model. The range of possible values is 0.0 to 1.00; values smaller than 0.10 represent a good fit, while values below 0.05 are an excellent fit. The goodness of fit indices provides additional statistical evidence for the reliability and validity of the factors used in the model; data with poor reliability and validity will not demonstrate an adequate fit. Path coefficients for the SEM analyses are shown in Figures 1 and 2. Parent and adolescent data were independently evaluated against the conceptual model but, due to the limited sample size, were not evaluated against each other.
Table 1.
Factor analyses and standardized coefficient alphas of the latent construct indices.
| Latent Construct and Items | M (SD) | Factor Loading | M (SD) | Factor Loading |
|---|---|---|---|---|
|
| ||||
| Adolescent Scores | Parent Scores | |||
|
| ||||
| Physician-Investigator Relationship | ||||
| 1. This doctor really seems to know what she/he is talking about. | 5.9 (1.4) | .81 | 5.9 (1.3) | .85 |
| 2. This doctor would give me the opportunity to ask questions. | 6.0 (1.2) | .85 | 6.0 (1.1) | .85 |
| 3. This doctor cares about me. | 5.4 (1.6) | .82 | 5.2 (1.4) | .81 |
| 4. I would be able to get along well with this doctor. | 5.3 (1.5) | .74 | 5.6 (1.3) | .80 |
| 5. This doctor explained things so I could remember them. | 5.5 (1.6) | .77 | 6.0 (1.1) | .74 |
| 6. This doctor appeared confident in this study she/he told me about? 1 | 5.7 (1.5) | .87 | 6.2 (1.0) | .74 |
| Std. α= .90 | Std. α = .89 | |||
|
| ||||
| Study Procedures | ||||
| 1. How much did the number of visits (12) influence your decision? | 3.9 (1.6) | .85 | 4.0 (1.7) | .88 |
| 2. How much did the time it takes to have medical tests influence your decision? | 4.1 (1.5) | .78 | 4.0 (1.4) | .86 |
| 3. How much did the length of the study (26wks) influence your decision? | 3.7 (1.6) | .82 | 3.8 (1.6) | .89 |
| 4. How much did taking study medication 4 times a day influence your decision? 1 | 4.0 (1.6) | .77 | 3.9 (1.5) | .74 |
| 5. How much did filling out diary cards influence your decision? 5 | 3.6 (1.5) | .78 | 4.3 (1.4) | .75 |
| Std. α = .86 | Std. α = .88 | |||
|
| ||||
| Risk | ||||
| 1. How risky do you think this study is for you (your child)? 2,3 | 3.3 (1.7) | .78 | 3.6 (1.9) | .79 |
| 2. How uncomfortable/stressful would taking a placebo be for you (your child)? 2,4 | 3.6 (2.1) | .76 | 3.7 (2.0) | .83 |
| 3. How risky would stopping regular medication be for you (your child)? 4 | 3.5 (2.0) | .89 | 3.8 (2.0) | .92 |
| Std. α = .74 | Std. α = .80 | |||
|
| ||||
| Benefit | ||||
| 1. How much did the potential to help your asthma influence you? | 5.9 (1.4) | .84 | 6.5 (1.0) | .90 |
| 2. How much did the regular monitoring of your asthma influence you? 5 | 5.1 (1.6) | .82 | 6.2 (1.0) | .81 |
| 3. How much did the scientific value of the study influence you? | 5.3 (1.4) | .84 | 6.2 (1.0) | .86 |
| Std. α = .78 | Std. α = .82 | |||
|
| ||||
| Compensation | ||||
| 1. How influenced would your mother (your child) be to participate because of the amount of compensation? | 4.7 (1.7) | .88 | 5.6 (1.6) | .60 |
| 2. How influenced would your father (child’s other parent) be to participate because of the amount of compensation? | 4.4 (1.9) | .87 | 3.3 (2.0) | .80 |
| 3. How influenced would you be to participate because of the amount of compensation? | 5.4 (1.6) | .84 | 3.7 (1.9) | .88 |
| Std. α = .83 | Std. α = .64 | |||
|
| ||||
| Discomfort | ||||
| 1. How physically unpleasant do you feel the study is for you (your child)? | 3.4 (1.5) | .78 | 3.9 (1.8) | .86 |
| 2. How stressful do you think this study is for you (your child)? 3 | 3.2 (1.5) | .83 | 3.7 (1.8) | .92 |
| 3. How much of an inconvenience is this study for you (your child)? | 3.4 (1.7) | .74 | 4.2 (1.7) | .83 |
| Std. α = .68 | Std. α = .84 | |||
|
| ||||
| Participation Decision | ||||
| 1. How likely would you be to enroll in this study? | 5.3 (1.4) | .90 | 5.2 (1.7) | .94 |
| 2. If you had to say for sure? | 0.9 (.39) | .90 | 0.7 (.45) | .94 |
| Std. α = .77 | Std. α = .86 | |||
Note: Std. α = Standardized Alpha. Items that shared a common superscript had significant residual (error) correlations included in the model. Superscripts 1-3 were for the adolescent model and 4-5 were for the parent model.
Figure 1.
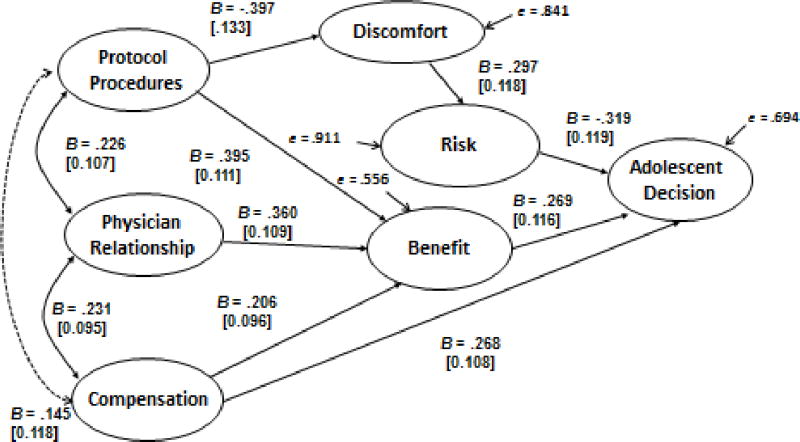
Unstandardized structural model of adolescent participation decision. Numbers in brackets are standard errors, B’s are unstandardized path coefficients, solid lines represent significant pathways and the dotted line represents a non significant pathway. Overall goodness of fit χ2(216) = 313.13, p < .001, RMSEA = .064, test of close fit (RMSEA < .05), p < .07. The model includes three correlated residuals (see Table 1).
Figure 2.
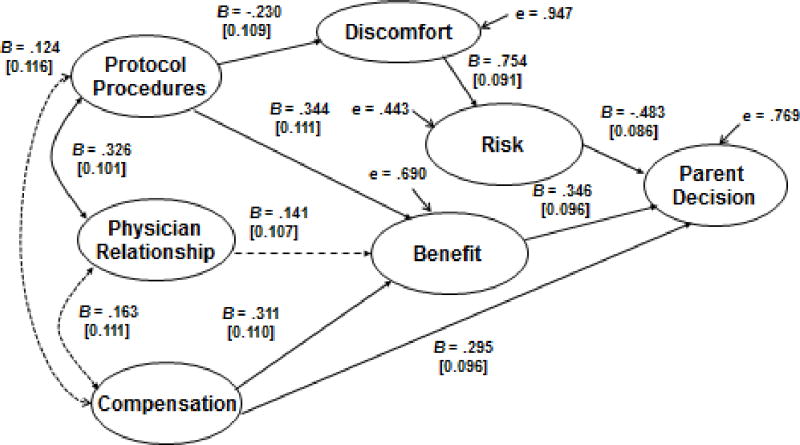
Unstandardized structural model of parent participation decision. Numbers in brackets are standard errors, B’s are unstandardized path coefficients, solid lines represent significant pathways, and dotted lines represent non significant pathways. Overall goodness of fit χ2(217) = 305.22, p < .001, RMSEA = .061, test of close fit (RMSEA < .05), p < .13. The model includes two correlated residuals (see Table 1).
RESULTS
One hundred and fifty six families responded to recruitment efforts by scheduling research appointments. Of those, 45 families cancelled or no-showed and either declined or could not be reached to reschedule their initial appointment and were not consent. Two families consented but did not complete all study procedures. The final sample consisted of 109 families. Participants were primarily mother-adolescent dyads. Table 2 provides detailed demographic information. All adolescents had a prior diagnosis of asthma. Using the National Heart, Lung, and Blood Institute’s guidelines, classification of adolescent’s asthma severity by the pediatric asthma specialist for this study (while on current therapy or treatment) was assessed as 73% mild-intermittent, 19% mild-persistent, and 8% moderate- persistent asthma (28). To maintain consistency in the analyses, the mother’s data was used to represent parental responses when both parents were present. Parents shared the same view of the research participation decision in all but five of the 32 families with two participating parents.
Table 2.
Demographic Characteristics of participants (N=111)
| Demographic Variables | M (SD) | n | % |
|---|---|---|---|
| Adolescent age | |||
| Age (years) | 13.6 (1.7) | ||
| Range (years) | 10–17 | ||
| Adolescent gender | |||
| Male | 64 | 58 | |
| Female | 47 | 42 | |
| Adolescent ethnicity | |||
| Non-Hispanic, White | 52 | 47 | |
| Hispanic | 38 | 34 | |
| African American | 8 | 7 | |
| Asian | 1 | 1 | |
| American Indian | 4 | 4 | |
| Pacific Islander | 1 | 4 | |
| Other | 7 | 6 | |
| Parent age | |||
| Age (years) | 41.9 (6.8) | ||
| Range (years) | 29-59 | ||
| Highest parental educational level | |||
| Some school | 8 | 5 | |
| High school diploma | 49 | 34 | |
| Associates/Vocational degree | 35 | 24 | |
| Bachelors degree | 21 | 15 | |
| Post-graduate degree | 27 | 20 | |
| Missing | 3 | 2 | |
| Yearly household income | |||
| $20,001 - $40,000 | 23 | 21 | |
| $20,000 or less | 33 | 29 | |
| $40,001 -$60,000 | 18 | 16 | |
| >$60,001 | 36 | 32 | |
| Missing | 1 | 2 |
Note: M = mean; SD = standard deviation
Statistical Evaluation of the Vignette Evaluation Questionnaire
Factor analyses and coefficient alpha computations of the latent constructs for parent and adolescent data confirmed both the internal consistency reliability and construct validity of each construct. As detailed in Table 1, standardized alphas ranged from .68 to .90 for the adolescent data and from .64 to .89 for the parent data. Factor loadings for items on their respective constructs were also strong for both the parent and adolescent data, indicating good conceptual development of the constructs.
Comparisons of research participation decisions
Overall, 67% of the parents and adolescents privately agreed and 33% privately disagreed on their initial research participation decisions. Details on both the initial and final participation decisions, and how family discordance was ultimately resolved are available in an earlier publication (14).
Predicting Adolescent Research Participation Decisions
The first hypothesis tested was that the conceptual model would fit the adolescent data. Figure 1 presents the hypothesized SEM and the statistically significant paths that predicted the adolescent research participation decision. We used LISREL to estimate all parameters. The overall full information likelihood goodness of fit indices (RMSEA = .064) suggested that the proposed model provided a good, but not excellent fit to the data (27). Other indices suggest that further improvements can be achieved, χ2 (216) = 313.13, p < .001. All of the hypothesized pathways were statistically significant, confirming the hypothesized relationships among the variables in the conceptual model. However, modification indices suggested that correlations existed between three pairs of residuals. These correlations are identified in Table 1. Overall, these results indicate the latent construct representing the adolescent’s research participation decision was strongly predicted by the other latent constructs in the model. Specifically, the latent constructs representing risk, benefit and compensation all had significant direct effects on the adolescent’s research participation decision. Perceptions of benefits are multiply determined in the adolescent model. The two strongest predictors are perceptions of the protocol procedures and anticipated positive relationship with the research physician. Financial compensation was also perceived as a benefit, although its contribution to perceived benefit was weaker than the other two predictors. In addition to its association with perceived benefit, protocol procedures directly predicted perceived discomfort, and indirectly influenced perceptions of study risk.
Predicting Parent Research Consent Decisions
To examine the extent to which these hypothesized relationships similarly predict parent research consent decisions, the same conceptual model was applied to the parents’ independent evaluations of the research vignette, see Figure 2. The LISREL overall goodness of fit indices suggested that the proposed measurement model provided a good fit to the parent data. The RMSEA = 0.061 and this value was not significantly larger (p = .132) than the threshold value of 0.05, which represents an excellent fit of the model to the data. All but two of the predicted pathways were significant. The hypothesized connection between the exogenous measures of compensation and physician relationship was not significant. Additionally, counter to our hypothesis, there was no association between parental attitudes about the physician investigator and their views of study benefit. The full information likelihood goodness of fit [χ2 (218) = 324.48, p < .001] was significant and implies the model can be improved. Two correlations between pairs of residuals were estimated and this association is indicated in Table 1. The parameter coefficients shown in the Figure 2 path diagram demonstrate that perceived risk was the strongest direct predictor of the parent research consent decision, although perceived benefit and compensation were also significant predictors. One key difference from the adolescent model was that physician relationship was not a significant predictor of research benefit. In the parent model, the perception of risk was heavily dependent upon the perception of discomfort.
DISCUSSION
This study examined the extent to which research assent and permission decisions by adolescents and parents could be reliably predicted. The variables examined in the SEM model included perceptions of the physician investigator, study procedures, financial compensation, discomfort, risk, and benefit. Reliable interrelationships among protocol-related factors were identified. Furthermore, the data indicate that parent and adolescent research participation decisions were influenced by these variables in similar ways, although important differences also emerged.
In both models, a positive perception of the relationship with the physician-investigator was associated with a positive view of the protocol procedures. Furthermore, a positive assessment of the protocol procedures contributed to the perception the study would be beneficial, and not uncomfortable. However, only the adolescents perceived a significant direct study benefit from the relationship with the physician. We speculate that adolescents perceive a direct benefit to study participation based on their positive perceptions of the physician-investigator because they are the ones who would undergo the trial procedures and be cared for by the physician-investigator. Adolescents often have ambivalent relationships with authority figures, especially those (such as physicians) with whom the relationship may be one of dependency. This may also have contributed to the variable being more important to adolescent decision-making than to parents’ decisions.
In the adolescent model, a positive perception of the physician-investigator also enhanced the extent to which financial compensation was perceived as a motivator to participate. However, this was not true of the parent model. Much has been written in the literature on research ethics about potentially coercive effects of financial compensation on research participation decisions (21, 23, 24, 29), however we did not systematically vary compensation in our study so it was not possible to examine the nuanced impact that differences in compensation may exert on the adolescents’ decisions, or on the other latent constructs. In these analyses it contributed to research participation decisions of in two ways; indirectly through an enhanced perception of study benefit and directly as a predictor of the participation decision.
The adolescent and parent models diverged in the connection between perceptions of study discomfort and research risk. Although a positive association between discomfort and risk exists in both models, the relationship between these variables is more than twice as strong for parents (B =.297 for adolescents as compared to B = .754 for parents). Moreover for parents, a lack of perceived research risk was the strongest direct predictor of decisions to permit adolescent research participation. By contrast, the adolescents’ perceptions of research risk contributed about as much to their assent decisions as did their perceptions of study benefit and compensation. This set of findings suggests that parents interpret research risk more broadly than do adolescents, and parent perceptions of risk ultimately outweigh their perceptions of benefit and compensation when making research participation decisions. Prior research has demonstrated that parents are less willing than adolescents to enroll in above-minimal risk studies and this broader view of research risk may be one mechanism for these more conservative decisions (13).
Perceptions of risk, benefit, and compensation were the three direct predictors of research participation decisions in both models. Thus, we can conclude that parents and adolescents generally attend to the same factors in making research participation decisions. However, differences in the strength of association among these variables may explain divergence of research participation decisions. For example, the parent model would suggest that a protocol with strong benefits and compensation might not outweigh parental concerns if the perceived level of research risk was high. By contrast, adolescents would not likely associate discomfort as strongly with perceived risk and, might be more inclined to enroll in the same study. If the protocol contained a variety of medical procedures that were not seen as uncomfortable, this would strengthen the perceived benefit for parents while simultaneously lowering perceived risk. Overall, these findings establish the relative importance of key factors affecting parent and adolescent research participation decisions in the context of asthma treatment trials. Further research will help establish the generalizability of the model to other research settings.
Study Limitations
These SEM models of research participation decisions were evaluated based on data from a single hypothetical asthma research protocol with a relatively modest sample size. A larger sample might have resulted in a more precise estimate of the correlations and improved the fit. While we believe the factors examined in these models are robust and would be important in a broad range of research, it is possible that the paths and relative strengths of association could change in other settings, or that a different model could provide a better fit to the data. For example, some research has suggested a connection between compensation and perception of research risk (30). This hypothesized relationship may prove to be important in some research protocols, (although we did not find evidence of its association with protocol procedures in our study). Other factors not included in this model, such as demographic variables, or level of participant comprehension of the protocol could also contribute to research participation decisions. Furthermore, the data were collected using a hypothetical research scenario so that we could more reliably control key variables associated with the larger study on voluntary assent. Families making actual research participation decisions may weigh variables differently.
CONCLUSION
These findings demonstrate the complex manner in which protocol features such as risk, benefit, and compensation mediate the research participation decisions of adolescents and their parents. This knowledge can help investigators provide information to parents and adolescents in a way that meets their respective interests, augments protocol understanding and enhances ethical enrollment. For example, one inference of these findings is that investigators should clarify distinctions between the discomfort and risk of asthma research procedures for parents. Another inference is that adolescents should be given the opportunity to develop rapport with the physician-investigator responsible for their care. Each of these steps is likely to result in a more favorable attitude toward research participation. Furthermore, given the importance of compensation as a variable in adolescent research participation decision-making, it is important for ethical reasons to use caution in discussing compensation and how it is disbursed, and to emphasize the relative importance of altruism.
The constructs identified in this study and the SEM methodology for studying research participation decision-making can be employed across a broad range of research protocols and populations. This information will enable investigators to develop research protocols that appeal to parents and adolescents alike, with the promise of increasing research participation and furthering science.
Implications and Contribution.
These findings demonstrate the complex manner in which protocol features influence the research participation decisions of adolescents and their parents. This knowledge can help investigators provide information to parents and adolescents in a way that meets their respective interests, augments protocol understanding and enhances ethical enrollment.
Acknowledgments
We would like to thank the University of New Mexico Health Sciences’ Clinical and Translational Science Center (CTSC) for their assistance with recruitment efforts for this study.
This study was supported by funding from the National Heart, Lung, and Blood Institute of the National Institutes of Health, RO1 HL64677.
Footnotes
The authors have no conflicts of interest that could impact the conduct or presentation of this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Janet L. Brody, Center for Family and Adolescent Research, Oregon Research Institute
Charles Turner, Center for Family and Adolescent Research, Oregon Research Institute
Robert D. Annett, University of New Mexico Health Sciences Center
David G. Scherer, Department of Psychology, University of Massachusetts
Jeanne Dalen, Center for Family and Adolescent Research, Oregon Research Institute
Reference List
- 1.Miller VA, Drotar D, Kodish E. Children’s competence for assent and consent: a review of empirical findings. Ethics Behav. 2004;14(3):255–95. doi: 10.1207/s15327019eb1403_3. [DOI] [PubMed] [Google Scholar]
- 2.Rothmier JD, Lasley MV, Shapiro GG. Factors influencing parental consent in pediatric clinical research. Pediatrics. 2003 May;111(5 Pt 1):1037–41. doi: 10.1542/peds.111.5.1037. [DOI] [PubMed] [Google Scholar]
- 3.Caldwell PH, Murphy SB, Butow PN, Craig JC. Clinical trials in children. Lancet. 2004 Aug 28;364(9436):803–11. doi: 10.1016/S0140-6736(04)16942-0. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds WW, Nelson RM. Risk perception and decision processes underlying informed consent to research participation. Soc Sci Med. 2007 Nov;65(10):2105–15. doi: 10.1016/j.socscimed.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Wolthers OD. A questionnaire on factors influencing children’s assent and dissent to non-therapeutic research. J Med Ethics. 2006 May;32(5):292–7. doi: 10.1136/jme.2004.010579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tait AR, Voepel-Lewis T, Malviya S. Factors that influence parents’ assessments of the risks and benefits of research involving their children. Pediatrics. 2004 Apr;113(4):727–32. doi: 10.1542/peds.113.4.727. [DOI] [PubMed] [Google Scholar]
- 7.Hayman RM, Taylor BJ, Peart NS, Galland BC, Sayers RM. Participation in research: informed consent, motivation and influence. J Paediatr Child Health. 2001 Feb;37(1):51–4. doi: 10.1046/j.1440-1754.2001.00612.x. [DOI] [PubMed] [Google Scholar]
- 8.Tercyak KP, Jr, Johnson SB, Kirkpatrick KA, Silverstein JH. Offering a randomized trial of intensive therapy for IDDM to adolescents. Reasons for refusal, patient characteristics, and recruiter effects. Diabetes Care. 1998 Feb;21(2):213–5. doi: 10.2337/diacare.21.2.213. [DOI] [PubMed] [Google Scholar]
- 9.Tait AR, Voepel-Lewis T, Malviya S. Participation of children in clinical research: factors that influence a parent’s decision to consent. Anesthesiology. 2003 Oct;99(4):819–25. doi: 10.1097/00000542-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Tait AR, Voepel-Lewis T, Siewert M, Malviya S. Factors that influence parents’ decisions to consent to their child’s participation in clinical anesthesia research. Pediatric Anesthesia. 1998;86(50):53. doi: 10.1097/00000539-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Cherrill J, Hudson H, Cocking C, Unsworth V, Franck L, McIntyre J, et al. Clinical trials: the viewpoint of children. Arch Dis Child. 2007 Aug;92(8):712–3. doi: 10.1136/adc.2006.114207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brody JL, Scherer DG, Annett RD, Pearson-Bish M. Voluntary assent in biomedical research with adolescents: a comparison of parent and adolescent views. Ethics Behav. 2003;13(1):79–95. doi: 10.1207/S15327019EB1301_10. [DOI] [PubMed] [Google Scholar]
- 13.Brody JL, Annett RD, Scherer DG, Perryman ML, Cofrin KM. Comparisons of adolescent and parent willingness to participate in minimal and above-minimal risk pediatric asthma research protocols. J Adolesc Health. 2005 Sep;37(3):229–35. doi: 10.1016/j.jadohealth.2004.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brody JL, Annett RD, Scherer DG, Turner C, Dalen J. Enrolling adolescents in asthma research: adolescent, parent, and physician influence in the decision-making process. J Asthma. 2009 Jun;46(5):492–7. doi: 10.1080/02770900902866768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snethen JA, Broome ME, Knafl K, Deatrick JA, Angst DB. Family patterns of decision-making in pediatric clinical trials. Res Nurs Health. 2006 Jun;29(3):223–32. doi: 10.1002/nur.20130. [DOI] [PubMed] [Google Scholar]
- 16.Miller VA, Reynolds WW, Nelson RM. Parent–Child Roles in Decision Making About Medical Research. Ethics Behav. 2008;18(2-3):161–81. [Google Scholar]
- 17.Sachs GA, Hougham GW, Sugarman J, Agre P, Broome M, et al. Conducting Empirical Research on Informed Consent: Challenges and Questions. IRB: Ethics and Human Research. 2011;25(5):S4–S10. [PubMed] [Google Scholar]
- 18.MacKinnon DP. Introduction to statistical mediation analysis. New York: Psychology Press; 2008. [Google Scholar]
- 19.Annett RD, Brody JL, Scherer DG, Perkett EA. Perception of risk associated with asthma research procedures among adolescents, parents, and pediatricians. J Allergy Clin Immunol. 2004 Nov;114(5):1138–45. doi: 10.1016/j.jaci.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 20.Drotar D. Ethics of Treatment and Intervention Research With Children and Adolescents With Behavioral and Mental Disorders: Recommendations for a Future Research Agenda. Ethics Behav. 2008;18(2-3):307–13. [Google Scholar]
- 21.Halpern SD, Karlawish JH, Casarett D, Berlin JA, Asch DA. Empirical assessment of whether moderate payments are undue or unjust inducements for participation in clinical trials. Arch Intern Med. 2004 Apr 12;164(7):801–3. doi: 10.1001/archinte.164.7.801. [DOI] [PubMed] [Google Scholar]
- 22.Bentley JP, Thacker PG. The influence of risk and monetary payment on the research participation decision making process. J Med Ethics. 2004 Jun;30(3):293–8. doi: 10.1136/jme.2002.001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scherer DG, Brody JL, Annett RD, Hetter J, Roberts LW, Cofrin KM. Financial compensation to adolescents for participation in biomedical research: adolescent and parent perspectives in seven studies. J Pediatr. 2005 Apr;146(4):552–8. doi: 10.1016/j.jpeds.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 24.Kimberly MB, Hoehn KS, Feudtner C, Nelson RM, Schreiner M. Variation in standards of research compensation and child assent practices: a comparison of 69 institutional review board-approved informed permission and assent forms for 3 multicenter pediatric clinical trials. Pediatrics. 2006 May;117(5):1706–11. doi: 10.1542/peds.2005-1233. [DOI] [PubMed] [Google Scholar]
- 25.Bagley SJ, Reynolds WW, Nelson RM. Is a “Wage-Payment” Model for Research Participation Appropriate for Children? Pediatrics. 2007;119:46–51. doi: 10.1542/peds.2006-1813. [DOI] [PubMed] [Google Scholar]
- 26.Joreskog KG, Sorbom D. LISREL 8: User’s reference guide. Chicago: Scientific Software International; 1996. [Google Scholar]
- 27.Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing Structural Equation Models. Beverly Hills, CA: Sage; 1993. pp. 136–62. [Google Scholar]
- 28.Lippincott Health Promot Lett. 7. Vol. 2. National Heart, Lung and Blood Institute; 1997. Aug, New NHLBI guidelines for the diagnosis and management of asthma; pp. 1, 8–1, 9. [PubMed] [Google Scholar]
- 29.Rice M, Broome ME. Incentives for children in research. J Nurs Scholarsh. 2004;36(2):167–72. doi: 10.1111/j.1547-5069.2004.04030.x. [DOI] [PubMed] [Google Scholar]
- 30.Cryder CE, John LA, Volpp KG, Loewenstein G. Informative inducement: study payment as a signal of risk. Soc Sci Med. 2010 Feb;70(3):455–64. doi: 10.1016/j.socscimed.2009.10.047. [DOI] [PubMed] [Google Scholar]