Abstract
Ballooned hepatocytes distinguish nonalcoholic steatohepatitis (NASH) from steatosis. Such cells contain dilated endoplasmic reticulum and ubiquitin aggregates, characteristics of endoplasmic reticulum stress. Hepatocyte ballooning increases risk for fibrosis in NASH, suggesting ballooned hepatocytes release pro-fibrogenic factors. Hedgehog ligands function as pro-fibrogenic factors in liver diseases, but mechanisms for Hedgehog ligand production remain poorly understood. We evaluated the hypothesis that endoplasmic reticulum stress induces hepatocyte production of hedgehog ligands that provide paracrine pro-fibrogenic signals to neighboring cells. In livers from NASH patients, keratin 8/18 and ubiquitin staining demonstrated enlarged, keratin 8/18-negative/ubiquitin-positive hepatocytes (ballooned hepatocytes) that were positive for Sonic hedgehog. In order to model endoplasmic reticulum stress in vitro, primary mouse hepatocytes were treated with tunicamycin. Compared to vehicle, tunicamycin significantly increased Sonic hedgehog and Indian hedgehog expression. Furthermore, conditioned medium from tunicamycin-treated hepatocytes increased Gli-luciferase reporter activity 14-fold more than conditioned medium from vehicle-treated hepatocytes. Cyclopamine (hedgehog signaling inhibitor) abrogated the effect of conditioned medium from tunicamycin-treated hepatocytes, verifying that soluble hepatocyte-derived factors activate hedgehog signaling. Ballooned hepatocytes in NASH patients did not express the hedgehog target gene, Gli2, α-smooth muscle actin or vimentin but were surrounded by Gli2-positive stromal cells expressing these myofibroblast markers. Trichrome staining demonstrated accumulation of ballooned hepatocytes in areas of matrix deposition, and numbers of Sonic hedgehog-positive, hepatocytes correlated with degree of ballooning and fibrosis stage. Hepatocytes undergoing endoplasmic reticiulum stress generate hedgehog ligands which act as paracrine pro-fibrogenic factors for hedgehog-responsive stromal cells. These results help to explain why fibrosis stage correlates with hepatocyte ballooning in NASH.
Keywords: nonalcoholic steatohepatitis, liver fibrosis, endoplasmic reticulum stress, myofibroblasts
Introduction
Nonalcoholic fatty liver disease (NAFLD) refers to a spectrum of liver injury, ranging from a relatively benign condition, nonalcoholic fatty liver (NAFL), to a progressive form of injury, non-alcoholic steatohepatitis (NASH). Although steatosis is a hallmark of NAFL and NASH, inflammation and fibrosis are rare in the former but easily demonstrated in the latter. NASH has a higher likelihood of progressing to cirrhosis and hepatocellular carcinoma than NAFL.[1] While activation of the unfolded protein response (UPR) is seen in both NAFLD and NASH[2, 3], outcome disparities likely reflect differences in severity of hepatocyte injury because apoptotic bodies and degenerating hepatocytes are more numerous in NASH than NAFL.[3] Indeed, injury-related cellular enlargement of hepatocytes (“ballooned hepatocytes”) and accumulation of ubiquinated cytokeratins (Mallory Denk bodies) are histologic hallmarks of steatohepatitis and among the diagnostic criteria for NASH.[4-6]
Hedgehog (Hh) signaling is activated in the acute or chronically injured liver when liver reconstruction is required. Hh ligands support liver progenitor cell growth [7, 8] and function as pro-fibrogenic factors in many liver diseases [9, 10], including fibrotic NASH [11]. In both hepatitis C infection and NASH, the level of Hh pathway activity has been shown to significantly correlate with fibrosis stage.[12, 13] Liver cells produce and release Hh ligands into their microenvironment[14], activating neighboring Hh-responsive cells involved in tissue remodeling.[15] Recent studies have demonstrated that hepatocyte apoptosis promotes Hh ligand production[15], thus providing a mechanism that couples hepatocyte death with fibrogenic liver repair. However, factors inducing Hh ligand production remain poorly understood.
Ballooning degeneration, the presence of Mallory-Denk bodies, and hepatocyte apoptosis all independently predict severity of liver fibrosis in NASH[16, 17] and indicate greater risk of disease progression.[18] Evidence suggests that hepatocyte endoplasmic reticulum (ER) stress initially induces the compensatory overexpression of viability factors, including keratins 8 and 18 (K8/18), but ultimately results in accumulation of misfolded proteins.[19] Ballooned hepatocytes exhibit endoplasmic reticulum stress and represent an extreme morphologic manifestation of abnormal protein turnover. This is supported by immunohistochemical characterization of these cells which reveals loss of cytosolic K8/18 expression and accumulation of ubiquitinated aggregates of K8/18 proteins.[20] Based upon these observations, we hypothesize that ER stress induces hepatocyte production of Hh ligands which, in turn, provide paracrine pro-fibrogenic signals to neighboring cells.
Methods
Mice
C57Bl/6 mice were obtained from Jackson Laboratory (Bar Harbor, ME). 5-8 month old mice were housed with 12-hour light-dark cycle and given water/standard chow ad libitum. Animal studies were approved by the Institutional Animal Care and Use Committee as governed by the National Institute of Health's “Guide for the Care and Use of Laboratory Animals”.
Human Samples
Adult patients (≥18 years) with biopsy-proven NASH (n=7) and alpha-1-antitrypsin deficiency (n=3) enrolled in the Duke NALFD Clinical Database and Biorepository were identified. NAFLD diagnosis was established by: 1) liver histology, 2) no significant alcohol consumption, 3) absence of other chronic liver disease. The database, biorepository, and this study were approved by the Institutional Review Board. Tissue was studied in accordance with NIH and Institutional guidelines for human subject research. Liver biopsies were stained with hematoxylin-eosin, Masson's trichrome and PAS-diastase and scored by a liver pathologist (CG) according to the NASH CRN scoring system.[6] Cases were scored for steatosis (0-3), lobular inflammation (0-3), hepatocyte ballooning (0-2) and fibrosis (0-4) based on validated criteria.[6] Non-alcoholic steatohepatitis activity score (NAS) was calculated. Fibrosis stage 1a, 1b, and 1c were combined as stage 1.
Immunohistochemistry
Formalin fixed, paraffin embedded liver biopsies were cut into 4μm sections, dewaxed, and rehydrated. Slides were incubated in 3% hydrogen peroxide/methanol. Antigen retrieval performed by heating in 10mM sodium citrate or 0.25% pepsin (K8/18; ubiquitin). Sections were blocked (Dako Envision, Carpinteria, CA) and incubated with primary antibodies: Sonic hedgehog (1843-1,1:7500, Epitomics, Burlingame, CA), K 8/18 (Leica Microsystems, Bannockburn, IL, reagent 1:2), BiP (C50B12, 1:500, Cell Signaling, Danvers, MA), Vimentin (Vim 3B4, 1:750, Dako), α-SMA (1A4,1:500, Dako), Gli2 (26056, 1:4500; Abcam, Cambridge, MA) at 4°C overnight. Horseradish peroxidase (HRP)-conjugated (K4001 or K4003; Dako) secondary antibodies were used. 3,3′-Diaminobenzidine (DAB) was used for detection. Omitting primary antibodies from reactions eliminated staining, demonstrating specificity.
K8/18-ubiquitin double staining: tissue sections were incubated (1hour, 25°C) in an antibody cocktail of mouse K8/18 and rabbit ubiquitin (1:500, Dako). A negative control of mouse IgG/rabbit IgG were run in parallel. Mach 2, kit 2 (Biocare Medical, Concord CA) containing HRP-anti-mouse and alkaline phosphatase-anti-rabbit secondary antibodies, was applied. DAB used to demonstrate K8/18 followed by Vulcan Fast Red (Biocare Medical) to demonstrate ubiquitin. Hematoxylin counter stain applied.
To quantify Shh-positive hepatocytes, serial sections were stained for Shh. Number of Shh-positive hepatocytes were counted in contiguous 20x fields (field of view diameter 0.78 mm) down length of the biopsy specimen. Number of Shh-positive hepatocytes was normalized to length of biopsy specimen.
Cell Isolation and Culture
Mouse hepatocytes were isolated from C57Bl/6 mice.[21] Viability/purity assessed by Trypan blue exclusion. Hepatocytes were seeded onto plastic culture dishes coated with type 1 collagen (Sigma, St. Louis, MO) in DMEM/F12 (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS), ITS (1:200, Invitrogen) and dexamethasone (1 mM, Sigma). 24 hours after seeding, cells were transferred to DMEM/F12 + 2% FBS and treated with 12.5μg/ml or 25μg/ml of tunicamycin (Sigma) or equivalent volume of DMSO for 1, 6, or 24 hours. Cells and conditioned medium were collected. HepG2 cell line was purchased from ATCC (Manassas, VA) and cultured per directions.
Quantitative Real Time PCR
Total RNA was extracted with TRIzol (Invitrogen), reverse-transcribed using Superscript reverse transcriptase (Invitrogen). cDNAs were used for quantitative reverse-transcription PCR using iQ-SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA) as previously described.[22] ER stress primers: GADD153 (5′CTGGAAGCCTGGTATGAGGAT 3′; 5′CAGGGTCAAGAGTAGTGAAGGT3′); XBP-1 (5′GAGTCCGCAGCAGGTG 3′; 5′GTGTCA GAGTCCATGGGA3′). Samples analyzed in triplicate according to ΔΔCt method.
Western Blot Analysis
Total protein was extracted from hepatocytes into RIPA buffer (Sigma, St. Louis, MO) supplemented with protease inhibitors (Roche, Indianapolis, IN). Western blots were performed [23] and probed with primary antibodies: BiP (Cell Signaling, C5B012), Keratin 8/18 (Invitrogen, 18-0213), Ubiquitin (Abcam, Ab7780), Shh (sc-9024; Santa Cruz Biotechnology, Santa Cruz, CA), Ihh (Abcam, ab39634), Lamin B1 (Abcam, Ab16048), P-Akt (Cell Signaling, D9E), Akt (Cell Signaling). Densitometry performed using Adobe Photoshop (San Jose, CA).
Adenovirus Infection
AdGFP (GFP driven by the cytomegalovirus promoter) was used as a control virus. The AddnAkt virus expresses the dominant negative form of Akt. HepG2 cells were infected with a multiplicity of infection of 25 for 24 hours. Virus-containing medium was aspirated and replaced with fresh medium for additional 24 hours. 100% of cells expressed GFP.[21, 24]
Cell Viability Assays
Cell viability was measured with the Cell Counting Kit-8 (Dojindo Molecular Technologies, Gaithersburg, MD).[22]
Luciferase Reporter Assay
Shh-LightII cells (ATCC, Manassas, VA) were cultured in triplicate and treated with dilutions of conditioned medium. Treated cells were cultured for 2 days. Firefly and renilla luciferase activities were determined via dual luciferase kit (Promega, Madison, WI). Other conditions utilized include standard hepatocyte growth medium (negative control). Sonic hedgehog-conditioned medium (positive control) was derived from HEK293 cells transfected with Shh-N plasmid. Conditioned medium was collected 24 hours post-transfection and tested in Shh-LightII cells. The fold-induction of Gli1 luciferase with 10% Shh-conditioned medium was equivalent to treatment with 50ng/ml purified Shh. The tunicamycin condition was standard growth medium supplemented with 25μg/ml tunicamycin. 3μM cyclopamine (Toronto Research Chemicals, Ontario, Canada) was added concurrently with conditioned medium. Fold Gli1 induction was normalized to the corresponding control medium. p-values were determined by Student's t-test relative to control medium treated cells.
Statistics
Results were expressed as mean±SD. Significance was established using the Student's t-test, with significance at p<0.05.
Results
Clinical characteristics and histology findings of 7 patients with NASH
We evaluated liver biopsies from 7 patients with biopsy-proven NASH. Clinical and histological information, including NASH risk factors (age, body mass index and presence/absence of diabetes mellitus) is presented in Supplementary Table 1. The mean age and BMI were 47.9 ± 6.4 years and 37.0 ± 5.5 kg/m2, respectively.
Increased expression of Hedgehog ligand by ballooned hepatocytes in patients with NASH
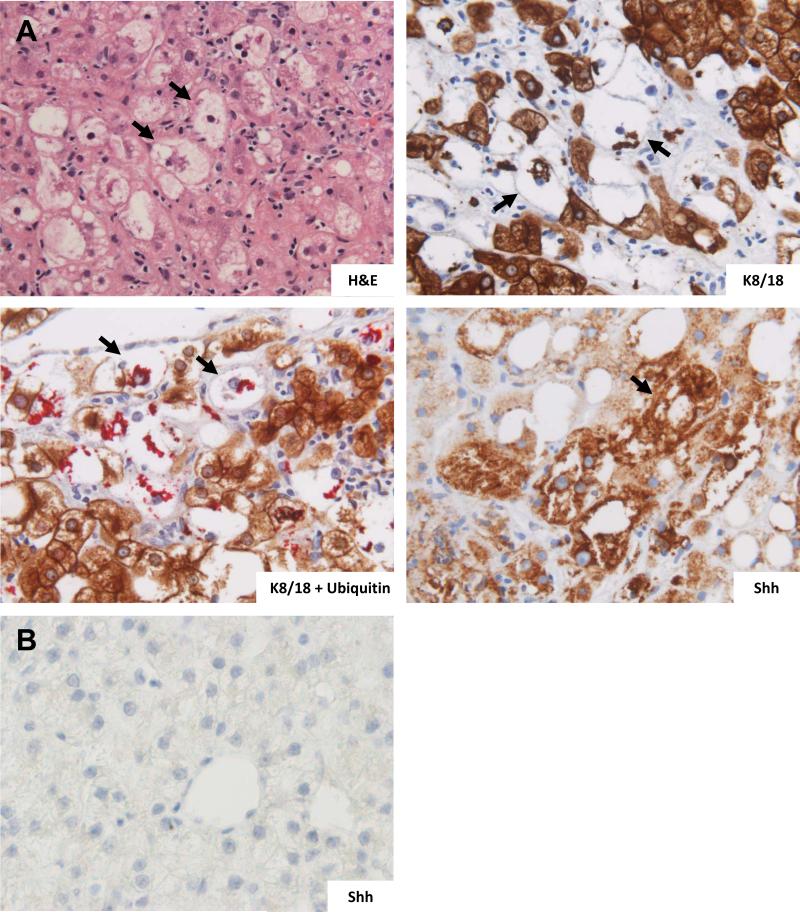
We recently demonstrated that immunohistochemical staining for K8/18 plus ubiquitin in NAFLD facilitates identification of ballooned hepatocytes (Guy et al., submitted). Thus we performed immunostaining for K8/18, and K8/18-ubiquitin double staining on the liver biopsies. H&E staining demonstrated characteristic ballooned hepatocytes (Figure 1A, H&E). These cells were K8/18-negative and ubiquitin-positive, consistent with ongoing, ER stress-related injury (Figure 1A, K8/18; K18 + Ubiquitin). Such cells were noted to be strongly and consistently positive for Sonic hedgehog (Shh) (Figure 1A, Shh). No Shh-positive hepatocytes were identified in healthy controls (Figure 1B).
Figure 1.
Increased expression of Sonic hedgehog (Shh) ligand by ballooned hepatocytes in patients with nonalcoholic steatohepatitis (NASH, n=7). (A) Hematoxylin and eosin (H&E) stained section of NASH shows enlarged ballooned hepatocytes (arrows); immunohistochemical (IHC) stained section for keratin 8/18 (K8/18) shows ballooned hepatocytes with loss of the normal, homogeneous, dark, cytoplasmic keratin staining (arrows); K8/18 plus ubiquitin double IHC stained section shows ballooned hepatocytes which are negative for the normal brown-chromagen tagged keratin staining but positive for perinuclear red-chromagen tagged ubiquitinated protein aggregates (Mallory-Denk bodies); and the IHC stained section of sonic hedgehog (Shh) shows ballooned hepatocytes with positive Shh staining . (B) Normal human liver tissue section with IHC staining for Shh shows normal appearing hepatocytes with lack of Shh staining. 40x magnification for all images.
Increased expression of Hedgehog ligand by hepatocytes in patients with α1-antitrypsin deficiency
To probe the relationship between ER stress and Hh ligand production in vivo, we examined livers of patients with α1-antitrypsin (A1AT) deficiency. In this condition, mutant α1-antitrypsin proteins are misfolded within the hepatocyte.[25, 26] The protein aggregates are retained in the ER, triggering ER stress that activates the UPR, resulting in progressive liver injury and fibrogenesis.[25, 26]. Immunostaining of liver biopsy specimens from three patients demonstrated the periodic acid-Schiff-positive, diastase-resistant globules characteristic of A1AT deficiency (Figure 2, H&E, PAS). Periportal hepatocytes expressed the ER stress marker, BiP (Figure 2, BiP). Increased hepatocyte expression of Shh ligand was noted in the same periportal regions on serial sections. Shh staining was more robust immediately adjacent to fibrotic tracts and was lost in more distant hepatocytes, mirroring the distribution of the α1-antitrypsin globules (Figure 2, Shh). K8/18-ubiquitin double staining of the A1AT samples demonstrated hepatocytes that were K8/18-negative and ubiquitin-positive, suggestive of ongoing ER stress-related cellular injury in the absence of ballooning (Figure 2, K8/18+ ubiquitin). Taken together, these data demonstrate that in two types of liver disease, NASH and A1AT deficiency, Hh ligand expression is increased in hepatocytes with evidence of ongoing ER stress and injury.
Figure 2.
Increased expression of Sonic hedgehog (Shh) ligand and BiP, a marker of endoplasmic reticulum (ER) stress, by hepatocytes in patients with α1-antitrypsin deficiency (A1AT). Hematoxylin and eosin (H&E) and periodic acid Schiff-diastase (PAS) staining was performed on liver tissue from 3 patients with A1AT. Hepatocytes with characteristic A1AT inclusions (arrows) were present. Immunohistochemical staining for Sonic hedgehog (Shh; arrow) and BiP (arrows) showed positive brown cytoplasmic staining in the affected hepatocytes. K8/18 plus ubiquitin (K8/18 + ubiquitin) double IHC stained section shows ballooned hepatocytes which are negative for the normal brown-chromagen tagged keratin staining but positive for perinuclear red-chromagen tagged ubiquitinated protein aggregates (arrows; Mallory-Denk bodies). 40x magnification for all images.
Increased production and release of Hedgehog ligands in an in vitro model of hepatocyte ER stress
To investigate a causative role for ER stress and Hh ligand production, we modeled ER stress in vitro by culturing wildtype mouse hepatocytes in the presence of tunicamycin (TM), a known ER stress-inducing agent, or DMSO control (D). Phase contrast microscopy images of tunicamycin treated hepatocytes demonstrated effects of TM on hepatocyte morphology (Supplementary Figure 1). TM treatment significantly upregulated BiP (Figure 3A), Gadd153 and XBP-1 (Supplementary Figure 2) in comparison to vehicle-treated controls, confirming the induction of ER stress. Western blot analysis revealed that TM-treated hepatocytes had decreased K8/18 and increased ubiquitin expression, recapitulating some of the key hepatocyte responses to ER stress and injury that were observed in the human liver samples. Tunicamycin treatment also resulted in a dose-dependent increase in Shh and Indian hedgehog (Ihh) mRNA transcripts as assessed by quantitative real time PCR (16.1-fold increase for Shh, 5.4-fold increase for Ihh; p <0.5, compared to vehicle) (Figure 3B). Similarly, expression of Shh and Ihh proteins was induced by tunicamycin treatment, again in a dose- and time-dependent fashion (Figure 3C, 3D).
Figure 3.
Increased production and release of Hedgehog ligands in an in vitro model of hepatocyte endoplasmic reticulum stress. Primary hepatocytes were isolated from wildtype mice and cultured in the presence of 12.5μg/ml or 25μg/ml Tunicamycin (TM) or vehicle control (DMSO) for 6 hours or 24 hours as indicated. (A) Representative western blot analysis for BiP, Keratin 8/18 (K8/18), ubiquitin, and lamin (loading control). (B) Sonic hedgehog (Shh) and Indian hedgehog ligand (Ihh) expression was evaluated by quantitative reverse transcription–PCR analysis. Cells were plated in triplicate for each experiment. The results of three experiments were averaged and mean±SD results are graphed. (*p<0.05, vs. DMSO-treated cells). (C) Representative western blot analysis of Shh, Ihh or lamin (loading control). (D) Cumulative densitometric analyses of Shh and Ihh western blots results (as normalized to lamin loading control) are displayed as the mean ±SD (**p<0.05, vs. DMSO-treated cells) (E) Hepatocytes were isolated from wildtype mice and cultured in the presence of 25μg/ml Tunicamycin or DMSO for 24 hours. Hepatocyte conditioned medium was collected. Shh-LightII cells were stably co-transfected with a Gli-responsive Firefly luciferase reporter and a pRL-TK constitutive Renilla luciferase reporter, then incubated in the presence of control medium, Shh positive control medium, hepatocyte conditioned medium (derived from hepatocytes treated with DMSO or 25μg/ml Tunicamycin), or tunicamycin (25μg/ml) +/- 3μM cyclopamine for 48hours. Cells were plated in triplicate for each experiment and values averaged. The results of 2-6 experiments are averaged and mean±SD results are graphed. p-values are calculated using the Student's t-test.
Hh ligands are secreted in exosomes, signaling both in autocrine and paracrine manners by engaging the Hh receptor, Patch (PTCH), on the surface of Hh-responsive cells. In the absence of Hh ligands, PTCH inhibits Smoothened (SMO), preventing nuclear localization of the Glioblastoma (Gli) family transcription factors that regulate Hh-target gene expression. Binding of the Hh ligand to PTCH de-represses SMO and allows for signal propogation, leading to Hh-target gene transcription. Despite upregulating Hh ligands, tunicamycin treated hepatocytes did not express Gli1 or Gli2 transcripts (data not shown), consistent with evidence that mature hepatocytes are incapable of activating Hh signaling in an autocrine fashion.[8] Hence, we investigated the possibility that these hepatocytes released Hh ligands, signaling via a paracrine mechanism. Cultured hepatocytes were treated with tunicamycin (25μg/ml) or DMSO for 24 hours and conditioned medium was collected. The ability of the conditioned medium to activate Gli-luciferase reporter activity in Shh-LightII cells was assessed. Conditioned medium from TM-treated cells induced 14.7-fold more reporter activity than conditioned medium from DMSO-treated control cells (p< .0001; Figure 3E). Conditioned medium from TM-treated cells up-regulated levels of reporter activity to the same extent as conditioned medium from HEK293 cells expressing recombinant Shh (Shh-positive control). While direct treatment of Shh-LightII cells with TM alone increased luciferase activity, the effect of the conditioned medium from TM-treated hepatocytes was significantly more robust (p < 0.0001). Furthermore, treating target cells with cyclopamine (a specific Hh signaling inhibitor) abrogated the effect of conditioned medium from TM-treated hepatocytes, reducing it down to the level seen with TM alone (14.7 vs. 4.8 fold, p < 0.0001). Taken together, these data verify that ER-stressed hepatocytes secrete soluble Hh ligands that can initiate Hh signaling via a paracrine fashion in surrounding Hh-responsive cells.
Shh-positive ballooned hepatocytes are surrounded by Gli2-positive stromal cells that express myofibroblast markers
Hh ligands function as pro-fibrogenic factors in many liver diseases, including viral hepatitis[12] and alcoholic liver disease[9], and accumulate in fibrotic NASH[11, 27]. Trichrome staining of the human NASH samples demonstrated that ballooned hepatocytes were adjacent to and intermixed with areas of fibrosis (Figure 4A, trichrome). While the ballooned hepatocytes themselves were not positive for vimentin or α-smooth muscle actin (α-SMA), the cells in the surrounding matrix expressed these myofibroblast markers (Figure 4A, vimentin, α-SMA). Given that stressed hepatocytes are capable of producing and releasing Hh ligands, this suggested that the ballooned hepatocytes might provide a paracrine pro-fibrogenic signal to neighboring cells. To evaluate this possibility, serial sections were stained for the Hh-target gene, Gli2. As predicted by our cell culture findings, the ballooned hepatocytes were negative for Gli2 but were surrounded by Gli2-positive stromal cells (Figure 4A, Gli2). Furthermore, quantitation of Shh-positive hepatocytes in the 7 NASH samples demonstrated that 97% of such cells were adjacent to areas of fibrosis, and number of Shh-positive hepatocytes within a given biopsy correlated significantly with both fibrosis stage (Figure 4B) and degree of ballooning (Figure 4C). These human liver biopsy data complement the cell culture results, and together support the concept that the ballooned hepatocytes generate Hh ligands which act as paracrine pro-fibrogenic factors for Hh-responsive stromal cells.
Figure 4.
Liver biopsies of NASH demonstrating Shh positive ballooned hepatocytes which are surrounded by gli-2 positive myofibroblasts. (A) Masson trichrome stained section showing ballooned hepatocytes (arrows) encircled by dense fibrous matrix; vimentin and α-SMA immunohistochemistry highlighting stromal cells positive for these myofibroblastic markers with adjacent ballooned hepatocytes (arrows); and gli-2 immunohistochemistry showing positive nuclear staining in myofibroblasts with adjacent ballooned hepatocytes (arrows). (B and C) The number of Shh positive hepatocytes were counted in each biopsy specimen and normalized to biopsy length. The normalized number of Shh positive hepatocytes were plotted against fibrosis stage (B) and degree of ballooning (C). The mean ±SD results are graphed (*p<0.05, **p<0.001). 40x magnification for all images.
Akt activation in Hh ligand producing hepatocytes
In hepatic stellate cells, activation of the PI3K-Akt pathway is required for Hh ligand production.[28] Thus, we were interested in determining if Akt activation occurred in ER-stressed hepatocytes. Cultured hepatocytes were treated with 25μg/ml TM or DMSO for 1, 6 or 24 hours. Western blot analysis of p-Akt and Akt in lysates from TM-treated hepatocytes demonstrated an early increase in p-Akt, followed by an eventual decline (Figure 5A and 5B). Parallel assessment of cell viability demonstrated that hepatocyte viability was preserved for the initial 6 hours of tunicamycin treatment but decreased by >60% at 24 hours (Figure 5C). Furthermore, adenoviral transfer of dominant negative Akt (dnAkt) significantly inhibited Hh ligand expression in comparison to adenoviral vector (GFP) (Figure 5D). These findings suggest that hepatocytes exposed to ER stressors rapidly induce cytoprotective mechanisms, such as Akt activation. In turn, Akt signaling may enhance tissue repair in a non-cell autonomous fashion by promoting hepatocyte production of paracrine factors such as Hh ligands.
Figure 5.
Akt activation in Hh ligand producing hepatocytes. Primary hepatocytes were isolated from wildtype mice and cultured in the presence of 12.5μg/ml or 25μg/ml Tunicamycin or vehicle control (DMSO) for 1, 6 or 24 hours. (A) Representative western blot analysis for p-Akt, Akt, and lamin (loading control). (B) Cumulative densitometric analyses of p-Akt western blots results (as normalized to lamin expression) are displayed as the mean ±SD. (C) Cell numbers were measured by CCK-8 assay following incubation in 25μg/ml Tunicamycin. Cells were plated in triplicate for each experiment. The results of two experiments were averaged and mean±SD results are graphed. (*p<0.05, 24 hour vs. 1 hour-treated cells). (D) HepG2 cells, a hepatocytic cell line demonstrating basal expression of Ihh, were infected with an adenovirus containing dominant Akt (dnAkt; multiplicity of infection 25) or GFP (multiplicity of infection 25) for 24 h (to inhibit Akt). Cells were harvested at 48 hours and quantitative real-time PCR performed for Ihh. Graphed is one representative experiment; results were reproduced four times. Results were normalized to GFP and graphed as mean ± S.D. of triplicate plates. (**p = 0.003)
Discussion
We demonstrate that in livers from NASH patients, K8/18-negative/ubiquitin-positive ballooned hepatocytes stain strongly for Shh, and localize within fibrotic areas. Similarly A1AT-deficient hepatocytes exhibit expression of the ER stress marker, BiP, and demonstrate robust Shh expression in the absence of cellular ballooning. To elucidate the causes and consequences of Hh ligand production by these stressed, degenerating hepatocytes, these cells were modeled in vitro by treating mouse hepatocytes with TM at doses that induced ER stress and eventually killed a significant number of cells. Similar to ballooned hepatocytes in NASH livers, the TM-treated hepatocytes lost K8/18 expression, upregulated ubiquitin, and actively expressed Hh ligands. Although ER stress induced in vitro and in vivo Hh ligand production, the stressed hepatocytes were Gli2-negative, indicating that they were not Hh-responsive. The hepatocytes, however, released Hh ligands, enriching the hepatic microenvironment with these factors, thereby stimulating the outgrowth of Hh-responsive stromal cells such as myofibroblasts that promote fibrogenic repair. This finding explains why fibrosis stage correlates with the extent of hepatocyte apoptosis and ballooning in NASH.
Recent ultrastructural studies have demonstrated that hepatocellular ballooning in NASH is associated with accumulation of fat droplets, ER dilation, and cytsoskeletal injury.[29] The ballooned hepatocytes are depleted of cytoplasmic K8/18 which are protective to epithelial cells. Mutations or abnormal turnover of these proteins renders the hepatocyte vulnerable to cell stress and apoptosis.[5, 30] Hh ligand induction by metabolic stress has not been previously described in hepatocytes. However, we reported that hepatitis B and hepatitis C virus infection of hepatocytes results in the robust production of Hh ligands.[12] Given the results of the current study and other evidence that viral infection promotes ER stress [31, 32], increased production of Hh ligands by virally-infected hepatocytes may also be mediated via ER stress. Future studies are planned to clarify this issue. The possibility that injury-related induction of Hh ligand production might be a general regenerative mechanism is further supported by a recent report that oxidative stress triggers Hh ligand production in cortical neurons.[33]
Growing evidence suggests that multiple cell types that accumulate in injured livers, including ballooned hepatocytes, myofibroblastic stellate cells (MF-HSCs), and reactive ductular cells, are capable of producing pro-fibrogenic Hh ligands.[14] In healthy human livers, quiescent hepatic stellate cells (Q-HSCs) silence Hh signaling via production of Hh-interacting protein (Hip), a Hh antagonist. During liver injury, however, Hip production falls and Hh ligands become abundant, resulting in the transition of Q-HSCs into MF-HSCs.[23] Hh ligands also enhance the viability and proliferative activity of MF-HSCs, thereby perpetuating fibrogenic repair.[28] Mature Hh ligands diffuse away from the producing cells and variably modulate gene expression in nearby and distant Hh-responsive cells in a time- and concentration-dependent manner. Ballooned hepatocytes enrich the hepatic microenvironment by locally releasing Hh ligand, and signaling to adjacent myofibroblasts; accordingly, early stages of NASH fibrosis are characterized by deposition of fibrous matrix around hepatocytes (often ballooned) and along sinusoids.[34] Exosomes containing active Hh ligand have been purified from the blood and bile of rodents subjected to liver injury, providing a mechanism for distant transport of Hh ligand throughout the liver lobule and extrahepatically.[14]
Prior work demonstrated that ballooned hepatocytes are histological markers of liver disease and provide prognostic information regarding disease progression. However, to date it has remained unclear whether these degenerating cells were merely innocent bystanders. Our novel findings suggest that the ballooned hepatocytes are not an epiphenomenon of injury but instead play a central role in the pathogenesis of fibrotic liver disease.
Supplementary Material
Supplementary Figure 1: Representative phase contrast images (20x) of wildtype mouse hepatocytes treated with 25μg/ml Tunicamycin or vehicle control (DMSO) for 6 hours or 24 hours as indicated.
Supplementary Figure 2: Wildtype hepatocytes treated with tunicamycin have evidence of ER stress. Primary hepatocytes were isolated from wildtype mice and cultured in the presence of 25μg/ml Tunicamycin or vehicle control (DMSO) for 1 hour, 6 hours or 24 hours as indicated. (A) Gadd153 and (B) XBP-1 expression were evaluated by quantitative reverse transcription–PCR analysis. PCR reactions were performed in triplicate and results were normalized to DMSO control. The results of two experiments were averaged and mean±SD results are graphed. (*p<0.05, **p<0.01, *** p<0.001 , vs. DMSO-treated cells).
Supplementary Table 1: Clinical characteristics and liver histology findings of 7 patients with non-alcoholic steatohepatitis (NASH). Clinical data including age, sex, body-mass index, and presence of diabetes mellitus are reported. The liver biopsy samples were reviewed by one pathologist and scored for steatosis, lobular inflammation, ballooning and fibrosis by standard criteria. NAS score was calculated.
Acknowledgements
This work was supported, in part, by The National Institutes of Health, USA (Grant Nos R01 DK077794 and R01 AA010154, to AMD); and Duke Oncology training grant (Nos T32 302-0202, to FR).
List of Abbreviations (in order of appearance)
- NASH
nonalcoholic steatohepatitis
- NAFLD
Nonalcoholic fatty liver disease
- NAFL
nonalcoholic fatty liver
- UPR
unfolded protein response
- Hh
Hedgehog
- ER
endoplasmic reticulum
- K8/18
keratin 8 and 18
- NAS score
nonalcoholic fatty liver disease activity score
- HRP
horseradish peroxidase
- DAB
Diaminobenzidine
- FBS
fetal bovine serum
- Shh
Sonic Hedgehog
- A1AT
α1-antitrypsin deficiency
- TM
tunicamycin
- Ihh
Indian Hedgehog
- PTCH
Patch
- SMO
Smoothened
- Gli
Glioblastoma
- α-SMA
α-smooth muscle actin
- MF-HSCs
myofibroblastic stellate cells
- Q-HSCs
quiescent hepatic stellate cells
- Hip
Hedgehog-interacting protein
Footnotes
Disclosures: No conflicts of interest exist
Author Contribution:
Fatima Rangwala: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; statistical analysis
Cynthia D. Guy: concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Jiuyi Lu: acquisition of data; analysis and interpretation of data
James L. Burchette: acquisition of data; analysis and interpretation of data
Ayako Suzuki: acquisition of data; analysis and interpretation of data, statistical analysis
Manal F. Abdelmalek: acquisition of data; critical revision of the manuscript for important intellectual content
Wei Chen: critical revision of the manuscript for important intellectual content, obtained funding
Anna Mae Diehl: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; obtained funding
All authors were involved in writing the paper and had final approval of the submitted and published versions.
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Kapoor A, Sanyal AJ. Endoplasmic reticulum stress and the unfolded protein response. Clin Liver Dis. 2009;13:581–590. doi: 10.1016/j.cld.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Puri P, Mirshahi F, Cheung O, et al. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134:568–576. doi: 10.1053/j.gastro.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Strnad P, Zatloukal K, Stumptner C, et al. Mallory-Denk-bodies: lessons from keratin-containing hepatic inclusion bodies. Biochim Biophys Acta. 2008;1782:764–774. doi: 10.1016/j.bbadis.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Omary MB, Ku NO, Strnad P, et al. Toward unraveling the complexity of simple epithelial keratins in human disease. J Clin Invest. 2009;119:1794–1805. doi: 10.1172/JCI37762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 7.Ochoa B, Syn WK, Delgado I, et al. Hedgehog signaling is critical for normal liver regeneration after partial hepatectomy in mice. Hepatology. 2010;51:1712–1723. doi: 10.1002/hep.23525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sicklick JK, Li YX, Melhem A, et al. Hedgehog signaling maintains resident hepatic progenitors throughout life. Am J Physiol Gastrointest Liver Physiol. 2006;290:G859–870. doi: 10.1152/ajpgi.00456.2005. [DOI] [PubMed] [Google Scholar]
- 9.Jung Y, Brown KD, Witek RP, et al. Accumulation of hedgehog-responsive progenitors parallels alcoholic liver disease severity in mice and humans. Gastroenterology. 2008;134:1532–1543. doi: 10.1053/j.gastro.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Omenetti A, Diehl AM. The adventures of sonic hedgehog in development and repair. II. Sonic hedgehog and liver development, inflammation, and cancer. Am J Physiol Gastrointest Liver Physiol. 2008;294:G595–598. doi: 10.1152/ajpgi.00543.2007. [DOI] [PubMed] [Google Scholar]
- 11.Fleig SV, Choi SS, Yang L, et al. Hepatic accumulation of Hedgehog-reactive progenitors increases with severity of fatty liver damage in mice. Lab Invest. 2007;87:1227–1239. doi: 10.1038/labinvest.3700689. [DOI] [PubMed] [Google Scholar]
- 12.Pereira TA, Witek RP, Syn WK, et al. Viral factors induce Hedgehog pathway activation in humans with viral hepatitis, cirrhosis, and hepatocellular carcinoma. Lab Invest. 2010 doi: 10.1038/labinvest.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Syn WK, Jung Y, Omenetti A, et al. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009;137:1478–1488. e1478. doi: 10.1053/j.gastro.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witek RP, Yang L, Liu R, et al. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology. 2009;136:320–330. e322. doi: 10.1053/j.gastro.2008.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung Y, Witek RP, Syn WK, et al. Signals from dying hepatocytes trigger growth of liver progenitors. Gut. 2010;59:655–665. doi: 10.1136/gut.2009.204354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldstein AE, Canbay A, Angulo P, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 17.Gramlich T, Kleiner DE, McCullough AJ, et al. Pathologic features associated with fibrosis in nonalcoholic fatty liver disease. Hum Pathol. 2004;35:196–199. doi: 10.1016/j.humpath.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 19.Zatloukal K, French SW, Stumptner C, et al. From Mallory to Mallory-Denk bodies: what, how and why? Exp Cell Res. 2007;313:2033–2049. doi: 10.1016/j.yexcr.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 20.Lackner C, Gogg-Kamerer M, Zatloukal K, et al. Ballooned hepatocytes in steatohepatitis: the value of keratin immunohistochemistry for diagnosis. J Hepatol. 2008;48:821–828. doi: 10.1016/j.jhep.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 21.Yang S, Koteish A, Lin H, et al. Oval cells compensate for damage and replicative senescence of mature hepatocytes in mice with fatty liver disease. Hepatology. 2004;39:403–411. doi: 10.1002/hep.20082. [DOI] [PubMed] [Google Scholar]
- 22.Omenetti A, Yang L, Li YX, et al. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest. 2007;87:499–514. doi: 10.1038/labinvest.3700537. [DOI] [PubMed] [Google Scholar]
- 23.Choi SS, Omenetti A, Witek RP, et al. Hedgehog pathway activation and epithelial-tomesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1093–1106. doi: 10.1152/ajpgi.00292.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi SS, Syn WK, Karaca GF, et al. Leptin promotes the myofibroblastic phenotype in hepatic stellate cells by activating the hedgehog pathway. J Biol Chem. 2010;285:36551–36560. doi: 10.1074/jbc.M110.168542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrell RW, Lomas DA. Alpha1-antitrypsin deficiency--a model for conformational diseases. N Engl J Med. 2002;346:45–53. doi: 10.1056/NEJMra010772. [DOI] [PubMed] [Google Scholar]
- 26.Fairbanks KD, Tavill AS. Liver disease in alpha 1-antitrypsin deficiency: a review. Am J Gastroenterol. 2008;103:2136–2141. doi: 10.1111/j.1572-0241.2008.01955.x. quiz 2142. [DOI] [PubMed] [Google Scholar]
- 27.Jung Y, Diehl AM. Non-alcoholic steatohepatitis pathogenesis: role of repair in regulating the disease progression. Dig Dis. 2010;28:225–228. doi: 10.1159/000282092. [DOI] [PubMed] [Google Scholar]
- 28.Yang L, Wang Y, Mao H, et al. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol. 2008;48:98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caldwell S, Ikura Y, Dias D, et al. Hepatocellular ballooning in NASH. J Hepatol. 2010;53:719–723. doi: 10.1016/j.jhep.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ku NO, Toivola DM, Strnad P, et al. Cytoskeletal keratin glycosylation protects epithelial tissue from injury. Nat Cell Biol. 2010;12:876–885. doi: 10.1038/ncb2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von dem Bussche A, Machida R, Li K, et al. Hepatitis C virus NS2 protein triggers endoplasmic reticulum stress and suppresses its own viral replication. J Hepatol. 2010;53:797–804. doi: 10.1016/j.jhep.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asselah T, Bieche I, Mansouri A, et al. In vivo hepatic endoplasmic reticulum stress in patients with chronic hepatitis C. J Pathol. 2010;221:264–274. doi: 10.1002/path.2703. [DOI] [PubMed] [Google Scholar]
- 33.Dai RL, Zhu SY, Xia YP, et al. Sonic Hedgehog Protects Cortical Neurons Against Oxidative Stress. Neurochem Res. 2010;36:67–75. doi: 10.1007/s11064-010-0264-6. [DOI] [PubMed] [Google Scholar]
- 34.Brunt EM. Histopathology of non-alcoholic fatty liver disease. Clin Liver Dis. 2009;13:533–544. doi: 10.1016/j.cld.2009.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Representative phase contrast images (20x) of wildtype mouse hepatocytes treated with 25μg/ml Tunicamycin or vehicle control (DMSO) for 6 hours or 24 hours as indicated.
Supplementary Figure 2: Wildtype hepatocytes treated with tunicamycin have evidence of ER stress. Primary hepatocytes were isolated from wildtype mice and cultured in the presence of 25μg/ml Tunicamycin or vehicle control (DMSO) for 1 hour, 6 hours or 24 hours as indicated. (A) Gadd153 and (B) XBP-1 expression were evaluated by quantitative reverse transcription–PCR analysis. PCR reactions were performed in triplicate and results were normalized to DMSO control. The results of two experiments were averaged and mean±SD results are graphed. (*p<0.05, **p<0.01, *** p<0.001 , vs. DMSO-treated cells).
Supplementary Table 1: Clinical characteristics and liver histology findings of 7 patients with non-alcoholic steatohepatitis (NASH). Clinical data including age, sex, body-mass index, and presence of diabetes mellitus are reported. The liver biopsy samples were reviewed by one pathologist and scored for steatosis, lobular inflammation, ballooning and fibrosis by standard criteria. NAS score was calculated.