Abstract
During an infection the antigen non-specific memory CD8 T cell compartment is not simply an inert pool of cells, but becomes activated and cytotoxic. It is unknown how these cells contribute to the clearance of an infection. We measured the strength of T-cell receptor (TCR) signals that bystander activated, cytotoxic CD8 T-cells (BA-CTLs) receive in vivo and found evidence of limited TCR signaling. Given this marginal contribution of the TCR, we asked how BA-CTLs identify infected target cells. We show that target cells express NKG2D ligands following bacterial infection and demonstrate that BA-CTLs directly eliminate these target cells in an innate-like, NKG2D-dependent manner. Selective inhibition of BA-CTL mediated killing led to a significant defect in pathogen clearance. Together these data suggest a previously unappreciated, innate role for memory CD8 T-cells in the early immune response before the onset of a de-novo generated, antigen-specific CD8 T-cell response.
Introduction
Antigen non-specific memory CD8 T cells are activated in an inflammation-dependent bystander fashion during the course of an infection (Berg et al., 2003; Doisne et al., 2004; Kohlmeier et al., 2010; Marshall et al., 2010; Odumade et al., 2012; Soudja et al., 2012; Su et al., 2005; Tough et al., 1996). This holds true in clinical studies as well as in mouse model systems: Cytomegalovirus (CMV)-, Epstein Barr virus (EBV)- and influenza-specific CD8 memory T cells are bystander-activated during a primary HIV infection (Doisne et al., 2004) and influenza- and CMV-specific cells are bystander-activated during primary EBV infection (Odumade et al., 2012). Data from animal studies show that CD8 memory cells can be bystander-activated to produce IFNγ in the absence of cognate antigen, which can be beneficial for the host (Berg et al., 2003; Kastenmuller et al., 2012; Kohlmeier et al., 2010; Kurepa et al., 2003; Marshall et al., 2010; Su et al., 2005). More recently it has been shown that monocytes and DCs both contribute in inducing this bystander activation of CD8 T cells leading to IFNγ and granzyme B production (Soudja et al., 2012).
Although it has been demonstrated that bystander activation occurs in a number of infections, the biological relevance and actual contribution of these cells to resolving an infection are poorly defined. The observation that bystander-activated memory cells can make granzyme B was attributed to potential cross-reactivity between the cognate antigen recognized by the memory T cell and new pathogen epitopes (Marshall et al., 2010) and killing was restricted to target cells presenting cognate antigen (Kohlmeier et al., 2010). These data raise the possibility that acquisition of cytotoxic potential would only be beneficial in some but not all new infections, depending on the extent of antigen cross-reactivity. T cell cross-reactivity against pathogens has been suggested to be fairly extensive, even if the pathogens are unrelated (Welsh et al., 2010). This raises the possibility that strong, agonist-like TCR signals play an essential role in the absence of cognate antigen and are involved in the process of bystander activation. Similarly to infection, bystander-activation of CD8 memory T cells has been reported in instances of autoimmunity. In case of celiac disease these CD8 T cells differentiate in response to pathologically high levels of IL-15 and acquire an NK cell phenotype including expression of NK cell specific markers (Meresse et al., 2004). It is unknown if these cells are autoreactive effector cells or truly bystander activated, but it has been demonstrated that these differentiated cells can attack target cells in an NKG2D-dependent manner. Regardless of the immunological context of bystander activation, the role of TCR signals for bystander activation and function has not been fully addressed. Thus, it is unclear if there is at least a partial role for a TCR stimulus, which would point to an integral role for the TCR in controlling T cell function at all times. The likelihood of antigen cross-reactivity needs to be especially considered in light of the frequent expression of more than one TCRα chain that can pair with the TCRβ chain in polyclonal and TCR-transgenic T cell pools (Heath et al., 1995).
We decided to use the recently described Nur77-GFP reporter mice to measure the role of TCR signals during bystander activation. These mice express GFP from the Nur77 locus and induction of GFP expression is strictly dependent on a TCR stimulus and cannot be induced by cytokines (Moran et al., 2011). We found that CD8 memory cells could acquire BA-CTL function without the involvement of a strong, agonist TCR signal. Brief exposure to IL-12, IL-15 and IL-18 acting directly on the T cells was sufficient to induce cytotoxic potential. Given this minimal role of the TCR, we hypothesized that a TCR-independent way of recognizing and killing infected target cells could be in place. We describe here that during an infection memory CD8 T cells temporarily acquire innate-like killing potential and kill target cells in an NKG2D-dependent manner. Most importantly, BA-CTL function was necessary to mediate early pathogen clearance during Listeria monocytogenes (LM) infection. Thus, BA-CTLs are an essential component of the immune response against a pathogen. We propose that the early response of BA-CTL serves to control pathogen replication until the primary immune response springs into action. Our experiments provide insight into the possible therapeutic potential of these cells and the risk of causing pathologies.
Results and Discussion
Acquisition of innate-like effector function in the absence of strong TCR signals
We first used an experimental system with defined TCR specificity to define BA-CTL phenotype and function. We generated memory T cells by transferring low numbers of congenically marked, naïve OT-I T cells into B6 mice. OT-I cells recognize the SIINFEKL (N4) epitope of chicken ovalbumin (OVA) (Hogquist et al., 1994). Mice were infected with a recombinant strain of vesicular stomatitis virus (VSV-OVA) to prime OT-I T cells. 30 days after the VSV-OVA infection, we infected mice with wild-type LM (WT-LM), which does not express any antigen with agonist properties for OT-I T cells (i.e. does not prime naïve OT-I T cells) to induce bystander-activation. In an effort to identify a possible TCR-independent killing mechanism used by bystander-activated cytotoxic CD8 memory cells (BA-CTLs), we phenotyped BA-CTLs for expression of receptors associated with NK cell activation. NK cells have been shown to have similarities with CD8 memory T cells in phenotype, function and gene expression patterns (Bezman et al., 2012; Sun and Lanier, 2011) and expression of inhibitory and activating NK cell receptors has been reported on activated and memory CD8 T cells (Coles et al., 2000; McMahon et al., 2002; Slifka et al., 2000).
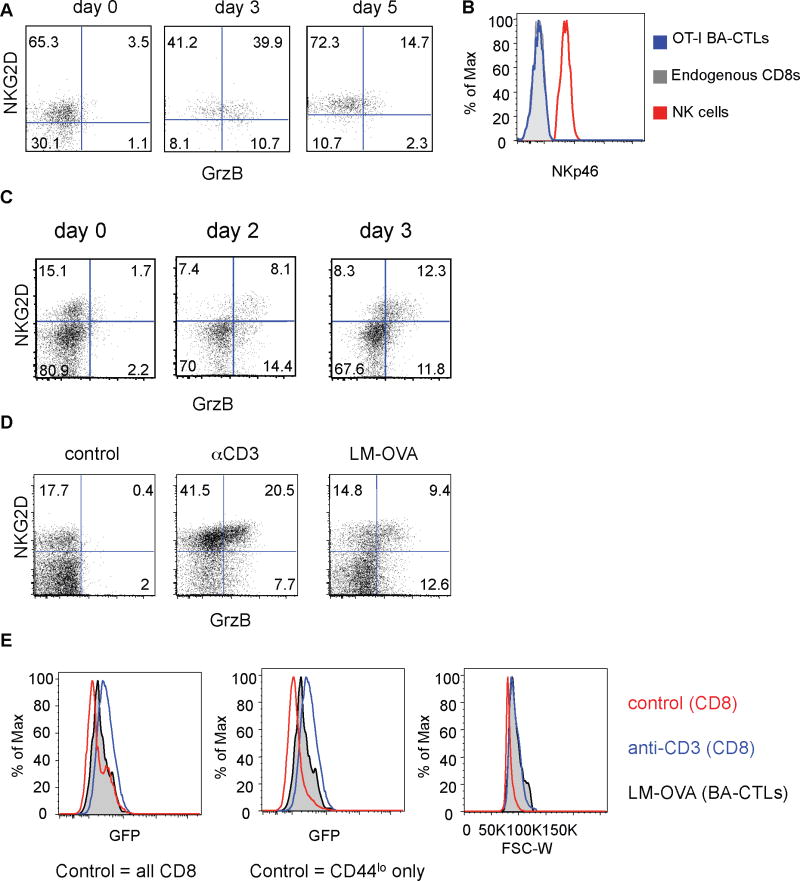
The majority of resting memory OT-I T cells expressed NKG2D, but not granzyme B before the WT-LM challenge (day 0, Fig. 1A – this time point is 30 days after the VSV-OVA infection). About half of the OT-I memory cells expressed granzyme B on day 3 after the WT-LM infection thus displaying a BA-CTL phenotype (Fig. 1A), but this percentage decreased on day 5 post WT-LM infection. One potential explanation for this observation is that BA-CTL function is temporary and wanes by the time the primary effector response becomes robust. There was no significant change in the frequency of OT-I T cells (data not shown). BA-CTLs did not express the NK-specific receptor NKp46 at any given time (Fig. 1B and data not shown) arguing that BA-CTLs are not differentiating into NK-like cells as reported during pathologic exposure to high levels of IL-15 (Meresse et al., 2004). We next determined if BA-CTL could be generated in a polyclonal memory-like (CD44hi) CD8 population following infection with recombinant LM expressing the model antigen ovalbumin (LM-OVA) to exclude the possibility of studying a potential TCR transgenic artifact. Within 48 hours of infection a significant fraction of memory-like CD44hi CD8 T cells expressed granzyme B and NKG2D (Fig. 1C). The overall percentage of granzyme B+ cells remained fairly constant in the first three days. Thus, similar to memory OT-I cells, a polyclonal memory phenotype population responds rapidly to a new infection. We next wanted to determine if these polyclonal memory T cells are truly activated in a cytokine-dependent and TCR-independent manner.
Figure 1. The BA-CTL phenotype is acquired early after infection in the presence of weak TCR signals.
(A) 1×104 OT-I T cells were adoptively transferred into congenic C57BL/6 mice followed by infection with VSV-OVA. 30 days later, mice were infected i.v. with 2000 cfu LM. Mice were bled before (day 0) and on day 3 and day 5 after LM infection and analyzed for phenotype and function (B) OT-I BA-CTLs did not express NKp46 on any of these time-points (histogram shows staining from day 3) (C) C57BL/6 mice were infected with 2000 cfu LM-OVA and euthanized for analysis on day 0, 2 and 3 after infection. Data shown are gated on CD8+ CD44+ T cells. (D) Nur77-GFP mice were infected with 2000 cfu LM-OVA, or injected with 50ug αCD3 antibody or left untreated (control). Mice were euthanized for analysis on day 2 after infection. (E) GFP expression levels of BA-CTLs (CD44hi NKG2D+ granzyme B+, black semi-transparent histogram) and CD8 T cells from αCD3 treated animals (blue histogram) were compared to GFP expression levels of control (CD44lo+hi) CD8 T cells (red histogram, left panel) and CD44lo naïve CD8 (red histogram, middle panel). We compared the cell size of control (CD44lo+hi) CD8 T cells, BA-CTLs and CD8 T cells from αCD3 treated animals (right panel). Data shown are representative of at least 3 independent experiments.
We considered that generation of BA-CTLs could (a) be completely independent of TCR signals, (b) need at least a basal signal (TCR tickling) or (c) depend on stronger than basal signals, possibly provided by cross-reactivity of the activated T cell to available antigen at any point of the activation process. BA-CTLs are not necessarily a homogeneous population and could be composed of some cells that are purely inflammation-activated and others that have cross-reactivity and are activated by TCR signals. To address these possibilities we used the recently described Nur77-GFP reporter mice. Hogquist and colleagues reported that in Nur77-GFP transgenic mice the GFP expression level in T cells is directly proportional to the strength of the TCR stimulus a T cell receives and, importantly, independent of inflammatory signals. This was specifically shown in the context of an LM infection (Moran et al., 2011). Most importantly, the Nur77-GFP system is sensitive enough that it reports even very weak TCR signals. Nur77-GFP OT-I T cells that are primed with LM-V4 (SIIVFEKL), an antigen that is about 700-fold less potent than SIINFEKL (Zehn et al., 2009), still express high levels of GFP (Moran et al., 2011). We thus believe that the Nur77-GFP reporter mouse is ideal to address the role of TCR signals in BA-CTL generation. To establish the baseline and maximum GFP signal, one group of mice remained untreated and another group received an injection of anti-CD3 antibody as a positive control for TCR signaling (Fig. 1D). We directly infected naïve Nur77-GFP mice with LM-OVA and determined GFP expression levels in the CD44hi NKG2D+ granzyme B+ population 48 hours after the infection (Fig. 1D). CD44hi NKG2D+ granzyme B+ CD8 T cells in the LM-OVA infected group displayed GFP expression levels above the baseline of naïve CD44lo CD8 T cells (Fig. 1E, middle panel), but congruent with the expression levels of the overall CD44lo+hi CD8 control population (Fig. 1E, left panel). BA-CTLs with the brightest GFP signal did not reach the level of GFP expression in the anti-CD3 treated group (Fig. 1E). It is noteworthy that BA-CTLs and CD8 T cells from the anti-CD3 treated group were blasting and significantly bigger in size than the baseline control population (Fig. 1E, right panel). Although BA-CTLs do receive a TCR signal above the naïve CD44lo baseline as indicated by the overall shift in GFP expression, our data also show that BA-CTLs do not receive TCR stimuli above the threshold of CD44hi CD8 T cells in an uninfected animal. Together these data suggest that BA-CTLs do receive TCR signals of limited strength during activation. We next wanted to address which signals can directly control granzyme and NKG2D expression.
Cytotoxic function and recognition of target cells
Previous studies have demonstrated a role for IL-15 in regulating granzyme expression in CD8 T cells (Marshall et al., 2010; Meresse et al., 2004; Soudja et al., 2012). It is currently unclear which factors regulate NKG2D expression on memory cells, although IL-4 has been shown to negatively regulate NKG2D expression in memory CD8 T cells (Ventre et al., 2012). Murine CD8 T cells start expressing NKG2D during the effector stage and 60-90% of VSV-OVA primed OT-I memory cells express NKG2D 30 days post-infection (day 0, Fig. 1A). OT-I cells that acquired a memory phenotype by homeostatic expansion expressed NKG2D as well, albeit at a reduced frequency (15-35%, data not shown).
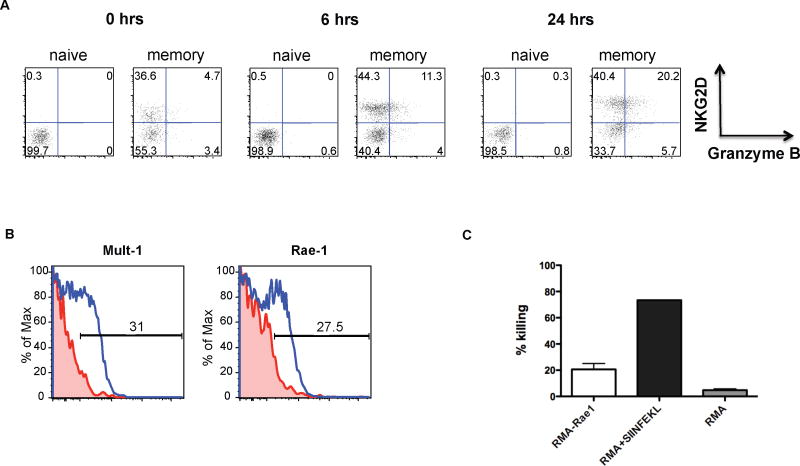
We tested if exposure to IL-12, IL-15 and IL-18 could directly induce granzyme expression and affect NKG2D expression in naïve and memory phenotype OT-I T cells. These cytokines have been previously shown to activate memory T cells (Smeltz, 2007). We found granzyme expression in the memory phenotype population (CD44high), but not naïve CD8 T cell population after 6 hours of incubation with IL-12, -15 and -18 (Fig. 2A). Expression of granzyme by memory phenotype CD8 T cells in such a short time-frame suggests that this occurs in the absence of cell proliferation. The fraction of NKG2D expressing memory CD8 T cells increased slightly over the course of 24 hours, but naïve CD8 T cells remained NKG2D and granzyme B negative. These data suggest that IL-12, IL-15 and IL-18 can directly act on memory CD8 T cells and are sufficient to induce BA-CTL function, but are not the primary regulators of NKG2D expression.
Figure 2. Expression of NKG2D ligands on target cells following infection and direct killing by BA-CTLs.
(A) Spleen OT-I T cells were enriched for naïve or memory phenotype cells and cultured in vitro with IL-12, IL-15 and IL-18 (each cytokine at 2ng/ml). NKG2D and granzyme B expression were measure before (0 hrs) and after 6 hours and 24 hours of culturing. (B) Mice were infected with 107 cfu ActA- LM-OVA (used only in this set of experiments to increase the number of initially infected cells). Expression of Mult-1 and Rae-1 was determined 24 hours post infection on I-Ab+ CD11c+ cells. NKG2D ligand expression levels on I-Ab+ CD11c+ cells from infected mice are in blue and from uninfected control mice in red. (C) RMA control cells were labeled with a low dose (0.2μM) of Cell Trace Violet (CTV) and RMA-Rae1 expressing target cells were labeled with a higher dose of CTV (5μM). The cells were mixed at a 1:1 ratio and incubated with OT-I BA-CTLs for 5 hours in vitro at an E:T ratio of 6:1. The CTV low to high ratio of the remaining live cells was determined by FACS analysis. Dead cells were excluded by propidium iodide staining. We included 1μM SIINFEKL pulsed RMA cells as a positive control group and an RMA+RMA group as a negative control group. FACS-purified OT-I BA-CTLs (generated as described in Fig. 1A) were isolated on day 3 after LM infection. Data shown in Fig. 3 are representative of at least 3 independent experiments.
Monocytes express NKG2D ligands (the Rae-1 family, H60 and Mult-1 in the mouse and MICA, MICB and the ULBP family in humans (Zafirova et al., 2011)) in response to TLR agonists such as LPS and WT-LM in vitro (Hamerman et al., 2004). We infected mice with 1×107 cfu actA- LM-OVA and determined Rae-1 and Mult-1 expression 24 hours later to identify possible BA-CTL target cells. The actA- Listeria strain was used to increase the number of initially infected cells. We found substantial upregulation of both ligands on CD11c+ MHC class II+ cells in the spleen (Fig. 2B), which are the predominantly infected cells early after infection with Listeria (Neuenhahn et al., 2006). To determine if BA-CTLs can directly lyse NKG2D ligand expressing target cells we used the VITAL in vitro killing assay (Hermans et al., 2004). We found that OT-I BA-CTLs could kill RMA-Rae1 expressing cells (but not RMA cells), albeit less efficiently than SIINFEKL pulsed RMA cells (Fig. 2C). These data suggest that BA-CTLs can directly kill target cells in an NKG2D-dependent manner. We consistently observed higher killing efficiency when the TCR was engaged. Whether this is due to differences in cytotoxic granule release per se or in formation of synapses that direct the granules to the target cells (Anikeeva and Sykulev, 2011) remains to be determined. It is also important to keep in mind that these differences are observed in a system that is based on expression of only one NKG2D ligand (Rae-1) on the target cells and a high dose of strong agonist peptide in the positive control. Future efforts will show how these killing mechanisms compare in in vivo in terms of efficiency.
Providing early protection – a crucial role for BA-CTLs
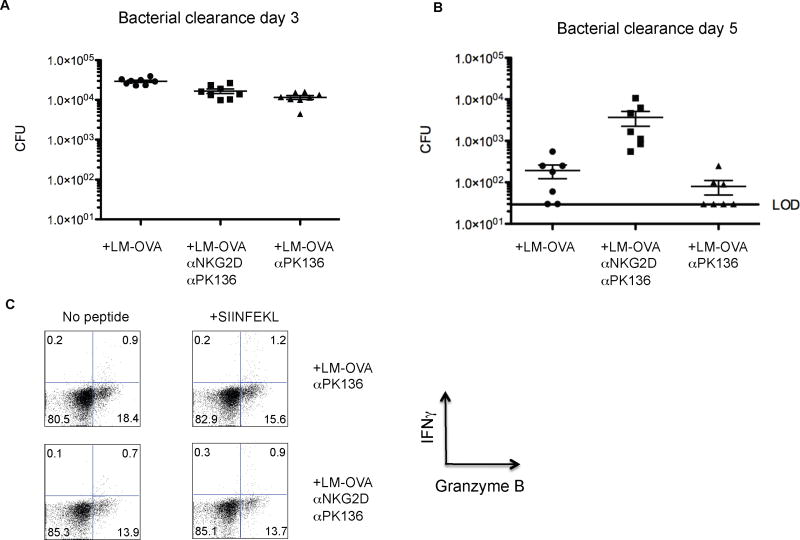
Our data so far suggest that expression of NKG2D ligands after infection and the ability of BA-CTLs to directly recognize induced NKG2D-ligands are a potential mechanism to eliminate infected cells. We next asked if BA-CTL-mediated, innate-like killing does indeed occur in vivo and, most importantly, whether it affects the course of an infection by blocking NKG2D on BA-CTLs. We administered an NKG2D-blocking antibody (HMG2D) (Ito et al., 2008) 2 days before and after infecting mice with LM-OVA. Mice were also injected with depleting αNK1.1 antibody (PK136) to eliminate potential skewing of the results by NK and NKT cells, which can also express NKG2D. There was no significant difference in bacterial counts on day 3 post-infection (Fig. 3A) excluding an effect on very early, innate immunity, but a >1log increase in bacterial counts on day 5 after infection in αNKG2D antibody treated mice (Fig. 3B). We also excluded a potential effect of γδ T cells in LM clearance in follow-up experiments (data not shown).
Figure 3. BA-CTLs limit early pathogen replication.
Mice were left untreated, injected with an NK-depleting antibody (PK136) and an NKG2D blocking antibody (HMG2D), or PK136 alone two days before and after an infection with 2000 cfu LM-OVA. Data are pooled from three independent experiments. Bacterial load in the spleen was determined on (A) day 3 and (B) day 5 and is shown as mean with SEM. The limit of detection (LOD) is indicated by a black bar. Mice that did not show any detectable bacterial load are marked as being at the LOD. (C) Granzyme B and IFNγ expression by CD8 T cells were measured on day 5 in all experimental groups (“no antibody” group is not shown) following in vitro stimulation with OVA peptide for 4 hours.
BA-CTLs in the αNKG2D/PK136 antibody treated group still expressed granzyme B (Fig. 3C) indicating that NKG2D-mediated signals are not required for BA-CTL/effector acquisition (as also indicated in Fig. 2A), but are critical for identifying target cells and possibly for granule exocytosis in the absence of a TCR signal. IFNγ production by newly induced effectors after SIINFEKL stimulation was small as expected on day 5, but equivalent in all three groups (no Ab group is not shown) and above the no restimulation background suggesting that treatment with the NKG2D blocking antibody did not affect the antigen specific response (Fig 3C). Thus, we propose that NKG2D-dependent, BA-CTL-mediated cytotoxicity plays a crucial, albeit not necessarily exclusive role in controlling early bacterial replication.
To further ensure that the increase in bacterial load was not due to an effect of the NKG2D-blocking antibody on the primary CD8 T cell response, we determined size, phenotype and function of the primary antigen-specific CD8 T cells in follow-up experiments. We compared the response of adoptively transferred OT-I T cells in the presence and absence of NKG2D-blocking antibody and found that αNKG2D antibody treatment did not affect the size (Fig. 4A) or function (Fig. 4B) of the effector OT-I T cell response on day 5 after infection with LM-OVA. These data along with published reports (Rajasekaran et al., 2010; Zafirova et al., 2011) strongly suggest that NKG2D is dispensable for generating a primary CD8 T cell response. Importantly, the αNKG2D antibody treated animals again had significantly higher bacterial cfu than animals from the two control groups (Fig. 4C; middle panel). These data show that a 100-fold increase in the precursor frequency (from ~100 endogenous CD8 responders to 1×104 OT-I T cells) and the ensuing large pool of effector cells did lower the bacterial burden in all three groups compared to untransferred, infected B6 mice (compare Fig. 3B right hand panel and Fig. 4C), indicating proper effector function and further supporting our conclusion that the primary T cell response and effector function are not affected by αNKG2D antibody treatment. Importantly, a significant impact on bacterial clearance by BA-CTLs was still observed. This further underlines the biological significance of the BA-CTL population as an important contributor of early immunity as even artificially increasing the antigen specific T cell pool at an early time point by transferring OT-I T cells could not compensate for BA-CTL mediated bacterial clearance.
Figure 4. The primary CD8 T cell response occurs independently of NKG2D signaling and cannot compensate for the loss of BA-CTL activity.
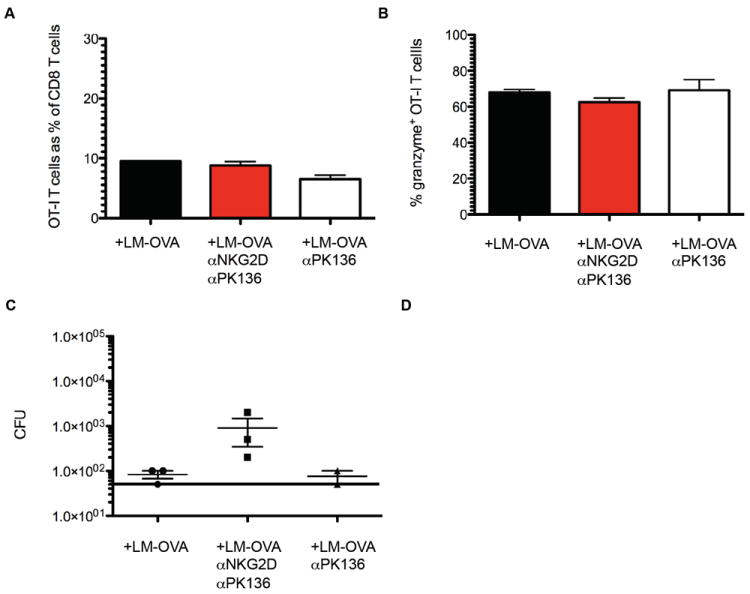
Mice were injected with depleting and/or blocking antibody and infected with LM-OVA as described in Figure 3. Mice received 104 naïve OT-I T cells on the day of infection. (A) The size of the OT-I T cell population on day 5 is shown as a % of the total CD8 T cell pool. (B) The percentage of granzyme B expressing OT-I T cells in the three experimental groups. (C) The bacterial load in the spleen on day 5 post infection. Data shown are representative of two independent experiments.
In summary, our data show that BA-CTLs play a crucial role in limiting pathogen replication at the early stages of an infection pointing to a novel and so far unappreciated role of memory CD8 T cells. BA-CTLs limit pathogen replication prior to the onset of the de novo, antigen-specific CD8 T cell response, thus preceding TCR-dependent target cell elimination. We provide evidence that this is done by recognizing target cells in an NKG2D-dependent manner. Why would BA-CTLs essentially mimic NK cells in their ability to kill NKG2D-ligand expressing target cells? We propose that this functional redundancy could provide the host the following potential advantages: (1) signals that could inhibit NK cell function (e.g. viral proteins mimicking MHC class I) likely do not affect BA-CTLs, since only a small fraction expresses inhibitory Ly49 receptors (data not shown), (2) given the considerable size of the CD8 memory compartment, BA-CTLs can be a very large population, outnumbering NK cells and are hence capable of effectively limiting viral or bacterial spreading.
Although NKG2D ligand expression may not occur in all infections, we think that the fact that TLR stimulation induces NKG2D ligand expression in macrophages (Hamerman et al., 2004) is a very good indicator that this is a broadly applicable, rather than specific mechanism. Safe activation of memory cells to temporarily transform them into BA-CTLs could provide the potential to help clear infections (e.g. after bone marrow transplantation when antigen-specific cells are scarce) and NKG2D ligand-expressing tumors. Our data suggest that both bona fide TCR transgenic antigen-experienced memory T cells and polyclonal memory phenotype cells can be bystander-activated. The ability of memory CD8 T cells to turn into BA-CTLs may be situation dependent: exhausted and senescent memory T cells may differ in their ability to become BA-CTLs. Understanding the potential of homeostatically expanded memory phenotype T cells to become BA-CTLs in patients that had myeloablative treatment and are susceptible to opportunistic infections will be of great interest.
Material and Methods
Mice
C57BL/6 and C57BL/6 Thy1.1+ mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and housed in specific pathogen-free conditions in the animal facilities at the University of Washington (Seattle, WA), the Fred Hutchinson Cancer Research Center (Seattle, WA). OT-I TCR transgenic mice congenic for CD45.1 or Thy1.1 were bred and maintained in the same facilities. Nur77-GFP mice were housed at the La Jolla Institute for Allergy and Immunology (La Jolla, CA).
Infections and BA-CTL generation
LM, LM-OVA and actA- LM-OVA were grown as previously described (Zehn et al., 2009). For primary infections C57BL/6 mice were injected i.v. with 2×103 cfu WT-LM or LM-OVA. Bacterial counts were measured by homogenizing a fraction of the spleen in 0.1% IGEPAL and plating on BHI plates. To determine NKG2D ligand expression mice were infected with 107 cfu actA- LM-OVA (i.v.). To generate BA-CTL OT-I T cells mice received 1×104 naïve (CD44lo) OT-I T cells and were subsequently infected (i.v.) with 2×106 pfu of a recombinant VSV strain expressing the SIINFEKL epitope (Kim et al., 1998) and >30 days after VSV-OVA infection mice were infected with 2000 cfu WT-LM.
In vitro BA-CTL induction
Memory phenotype cells were isolated from OT-I spleens by depleting CD62Lhi bead-labeled OT-I T cells along with other cell populations in a magnetic column (Miltenyi). Naïve OT-I T cells were isolated by depleting CD44hi expressing OT-I T cells in the same manner.
VITAL assay
CD45.1+ OT-I BA-CTLs for the VITAL assay were first enriched from the spleens of day 3 infected B6 mice for CD8 T cells by depleting bead-labeled CD4, CD19 and I-Ab expressing cells using a magnetic column (Miltenyi), followed by sorting on a FACSAria (BD) on the CD8+ and CD45.2- cells. We did not directly stain BA-CTLs with CD45.1 to avoid complications in the VITAL assay. RMA control cells were labeled with a low dose (0.2μM) of CTV (Invitrogen) and RMA-Rae1e expressing target cells were labeled with a higher dose of CTV (5μM). The cells were mixed at a 1:1 ratio and incubated with OT-I BA-CTLs for 6 hours in vitro at an E:T ratio of 6:1. The CTV low to high ratio of the remaining live cells was determined by FACS analysis. Dead cells were excluded by propidium iodide staining.
Antibody blocking and depletion experiments
anti-NK1.1 (PK136) and/or anti-NKG2D (HMG2D) antibody was injected i.p. (100-200μg) two days before and after infection with LM. NK cell depletion was confirmed by FACS analysis using an anti-NKp46 antibody.
Flow Cytometry
Mice were sacrificed at the time points indicated. For intracellular staining, cells were prepared with the Cytofix/Cytoperm kit (BD). IFNγ assays were performed in the presence of brefeldin A (BD) and incubated with or without 100nM SIINFEKL peptide for 4-5 hours in complete RP10. Cells were stained with LIVE/DEAD cell stain kit (Invitrogen) prior to FACS analysis. Overall Rae-1 expression was measured using a pan-Rae-1 antibody (R&D Systems) all other antibodies were purchased from eBioscience, BioLegend, Invitrogen (granzyme B) and BD Pharmingen. Cells were analyzed using an LSRII (BD) and analyzed using FlowJo (TreeStar) software.
memory CD8 T cells become cytotoxic when activated by inflammation (BA-CTLs)
bystander activation of memory CD8 T cells occurs with minimal TCR signaling
BA-CTLs eliminate target cells in an innate-like, NKG2D-dependent manner
BA-CTLs are necessary to limit pathogen replication early after an infection
Acknowledgments
This work was supported by NIH grants AI 079159 (to M.P.), AI 019335 and the Howard Hughes Medical Institute (to M.J.B.) and ProDoc grant (PDFMP3_137128), project grant (310030_130512) from the Swiss National Science Foundation and funds from the “Swiss Vaccine Research Institute” to DZ. We thank Kris Hogquist and Mitch Kronenberg for their critical input and generous support in performing the Nur77-GFP experiments. We also thank David Raulet and Lewis Lanier for providing NKG2D-ligand expressing cell lines and the matching control cell lines.
Footnotes
Publisher's Disclaimer: This is a PDF le of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its nal citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anikeeva N, Sykulev Y. Mechanisms controlling granule-mediated cytolytic activity of cytotoxic T lymphocytes. Immunologic research. 2011;51:183–194. doi: 10.1007/s12026-011-8252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. 2003;198:1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezman NA, Kim CC, Sun JC, Min-Oo G, Hendricks DW, Kamimura Y, Best JA, Goldrath AW, Lanier LL, Gautier EL, et al. Molecular definition of the identity and activation of natural killer cells. Nat Immunol. 2012;13:1000–1009. doi: 10.1038/ni.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles MC, McMahon CW, Takizawa H, Raulet DH. Memory CD8 T lymphocytes express inhibitory MHC-specific Ly49 receptors. Eur J Immunol. 2000;30:236–244. doi: 10.1002/1521-4141(200001)30:1<236::AID-IMMU236>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Doisne JM, Urrutia A, Lacabaratz-Porret C, Goujard C, Meyer L, Chaix ML, Sinet M, Venet A. CD8+ T cells specific for EBV, cytomegalovirus, and influenza virus are activated during primary HIV infection. J Immunol. 2004;173:2410–2418. doi: 10.4049/jimmunol.173.4.2410. [DOI] [PubMed] [Google Scholar]
- Hamerman JA, Ogasawara K, Lanier LL. Cutting edge: Toll-like receptor signaling in macrophages induces ligands for the NKG2D receptor. J Immunol. 2004;172:2001–2005. doi: 10.4049/jimmunol.172.4.2001. [DOI] [PubMed] [Google Scholar]
- Heath WR, Carbone FR, Bertolino P, Kelly J, Cose S, Miller JF. Expression of two T cell receptor alpha chains on the surface of normal murine T cells. Eur J Immunol. 1995;25:1617–1623. doi: 10.1002/eji.1830250622. [DOI] [PubMed] [Google Scholar]
- Hermans IF, Silk JD, Yang J, Palmowski MJ, Gileadi U, McCarthy C, Salio M, Ronchese F, Cerundolo V. The VITAL assay: a versatile fluorometric technique for assessing CTL- and NKT-mediated cytotoxicity against multiple targets in vitro and in vivo. J Immunol Methods. 2004;285:25–40. doi: 10.1016/j.jim.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Ito A, Shimura H, Nitahara A, Tomiyama K, Ito M, Kanekura T, Okumura K, Yagita H, Kawai K. NK cells contribute to the skin graft rejection promoted by CD4+ T cells activated through the indirect allorecognition pathway. Int Immunol. 2008;20:1343–1349. doi: 10.1093/intimm/dxn092. [DOI] [PubMed] [Google Scholar]
- Kastenmuller W, Torabi-Parizi P, Subramanian N, Lammermann T, Germain RN. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell. 2012;150:1235–1248. doi: 10.1016/j.cell.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Reed DS, Olson S, Schnell MJ, Rose JK, Morton PA, Lefrancois L. Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals. Proc Natl Acad Sci U S A. 1998;95:10814–10819. doi: 10.1073/pnas.95.18.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmeier JE, Cookenham T, Roberts AD, Miller SC, Woodland DL. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity. 2010;33:96–105. doi: 10.1016/j.immuni.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurepa Z, Su J, Forman J. Memory phenotype of CD8+ T cells in MHC class Iadeficient mice. J Immunol. 2003;170:5414–5420. doi: 10.4049/jimmunol.170.11.5414. [DOI] [PubMed] [Google Scholar]
- Marshall HD, Prince AL, Berg LJ, Welsh RM. IFN-alpha beta and self-MHC divert CD8 T cells into a distinct differentiation pathway characterized by rapid acquisition of effector functions. J Immunol. 2010;185:1419–1428. doi: 10.4049/jimmunol.1001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon CW, Zajac AJ, Jamieson AM, Corral L, Hammer GE, Ahmed R, Raulet DH. Viral and bacterial infections induce expression of multiple NK cell receptors in responding CD8(+) T cells. J Immunol. 2002;169:1444–1452. doi: 10.4049/jimmunol.169.3.1444. [DOI] [PubMed] [Google Scholar]
- Meresse B, Chen Z, Ciszewski C, Tretiakova M, Bhagat G, Krausz TN, Raulet DH, Lanier LL, Groh V, Spies T, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–366. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuenhahn M, Kerksiek KM, Nauerth M, Suhre MH, Schiemann M, Gebhardt FE, Stemberger C, Panthel K, Schroder S, Chakraborty T, et al. CD8alpha+ dendritic cells are required for efficient entry of Listeria monocytogenes into the spleen. Immunity. 2006;25:619–630. doi: 10.1016/j.immuni.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Odumade OA, Knight JA, Schmeling DO, Masopust D, Balfour HH, Jr, Hogquist KA. Primary Epstein-Barr virus infection does not erode preexisting CD8(+) T cell memory in humans. J Exp Med. 2012;209:471–478. doi: 10.1084/jem.20112401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran K, Xiong V, Fong L, Gorski J, Malarkannan S. Functional dichotomy between NKG2D and CD28-mediated co-stimulation in human CD8+ T cells. PloS one. 2010;5 doi: 10.1371/journal.pone.0012635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifka MK, Pagarigan RR, Whitton JL. NK markers are expressed on a high percentage of virus-specific CD8+ and CD4+ T cells. J Immunol. 2000;164:2009–2015. doi: 10.4049/jimmunol.164.4.2009. [DOI] [PubMed] [Google Scholar]
- Smeltz RB. Profound enhancement of the IL-12/IL-18 pathway of IFN-gamma secretion in human CD8+ memory T cell subsets via IL-15. J Immunol. 2007;178:4786–4792. doi: 10.4049/jimmunol.178.8.4786. [DOI] [PubMed] [Google Scholar]
- Soudja SM, Ruiz AL, Marie JC, Lauvau G. Inflammatory Monocytes Activate Memory CD8(+) T and Innate NK Lymphocytes Independent of Cognate Antigen during Microbial Pathogen Invasion. Immunity. 2012;37:549–562. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Berg RE, Murray S, Forman J. Thymus-dependent memory phenotype CD8 T cells in naive B6.H-2Kb-/-Db-/- animals mediate an antigen-specific response against Listeria monocytogenes. J Immunol. 2005;175:6450–6457. doi: 10.4049/jimmunol.175.10.6450. [DOI] [PubMed] [Google Scholar]
- Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8 T cells. Nat Rev Immunol. 2011;11:645–657. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- Ventre E, Brinza L, Schicklin S, Mafille J, Coupet CA, Marcais A, Djebali S, Jubin V, Walzer T, Marvel J. Negative Regulation of NKG2D Expression by IL-4 in Memory CD8 T Cells. J Immunol. 2012;189:3480–3489. doi: 10.4049/jimmunol.1102954. [DOI] [PubMed] [Google Scholar]
- Welsh RM, Che JW, Brehm MA, Selin LK. Heterologous immunity between viruses. Immunol Rev. 2010;235:244–266. doi: 10.1111/j.0105-2896.2010.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafirova B, Wensveen FM, Gulin M, Polic B. Regulation of immune cell function and differentiation by the NKG2D receptor. Cell Mol Life Sci. 2011;68:3519–3529. doi: 10.1007/s00018-011-0797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]