Abstract
Adaptive humoral immune responses in the airways are mediated by B cells and plasma cells that express highly evolved and specific receptors and produce immunoglobulins of most isotypes. In some cases, such as autoimmune diseases or inflammatory diseases caused by excessive exposure to foreign antigens, these same immune cells can cause disease by virtue of overly vigorous responses. This review discusses the generation, differentiation, signaling, activation and recruitment pathways of B cells and plasma cells, with special emphasis on unique characteristics of subsets of these cells functioning within the respiratory system. The primary sensitization events that generate B cells responsible for effector responses throughout the airways usually occur in the upper airways, in tonsils and adenoid structures that make up Waldeyer’s Ring. Upon secondary exposure to antigen in the airways, antigen-processing dendritic cells migrate into secondary lymphoid organs such as lymph nodes that drain the upper and lower airways and further B cell expansion takes place at those sites. Antigen exposure in the upper or lower airways can also drive expansion of B lineage cells in the airway mucosal tissue and lead to the formation of inducible lymphoid follicles or aggregates that can mediate local immunity or disease.
Keywords: B cells, plasma cells, plasmablasts, respiratory diseases
Introduction
Along with the skin and the gastrointestinal tract, the respiratory tract is a large surface that interacts extensively with the environment outside the body. The exposed area of the respiratory tract is huge; in humans it has a surface area of 500m2, roughly the size of a tennis court.1 Since large volumes of air are moved through the respiratory system rapidly and constantly, there is considerable exposure to airborne organisms that might cause pathology. The nose performs a filtering role, and many bacteria, viruses and fungi are deposited there. Innate immune responses include passive mechanisms such as formation of a mucus blanket, mucociliary removal and swallowing of particles and constitutive expression of host defense molecules by airway epithelium and submucosal glands. Also at play are active innate responses, such as receptor mediated activation of release of host defense molecules by epithelial cells, alveolar macrophages and other cells, activation of glandular secretion, recruitment of phagocytic cells to the airways and exudation of vascular fluids; these responses provide a robust defense against all but the most aggressive of potential pathogens. Frequently, innate immune responses fail to deter microorganisms and adaptive immune responses must be martialed to maintain the integrity of airway function and survival of the host. Adaptive immune responses in the airways are mediated by B cells and T cells that express highly evolved and specific receptors. In some cases, such as autoimmune diseases or inflammatory diseases caused by excessive exposure to self- or foreign antigens, these same immune cells can cause disease by virtue of overly vigorous responses.
The purpose of this review is to discuss the cells of the B cell lineage and their role in disease and immunity in the respiratory system. We discuss the generation, differentiation, signaling, activation and recruitment pathways of B cells and plasma cells with special emphasis on unique characteristics of subsets of these cells functioning within the respiratory system. There are some important differences between humans and mice, the most studied species, with respect to the organization of B cell containing tissues and responses (see Table 1). Nonetheless, much of the best information on the molecular and cellular pathways that B cells employ has been derived in the mouse. Likewise, there is considerable information on the natural history of B lineage cells in the gastrointestinal tract, probably owing to the fact that the vast majority of the total bodily antibody production occurs in the gut. Although our greatest interest is in the role of B cells and plasma cells in human airways disease, we have frequently incorporated findings and interpretations that arise from study of the mouse and/or the gastrointestinal tract, although we have tried not to burden the review by qualifying all such references.
Table 1.
Comparative differences between murine and human airway anatomy and B cell biology
| Mouse | Human |
|---|---|
| Molecular | |
| Express only IgA | Express IgA1 and IgA2 |
| IgD only found co-expressed with IgM on immature B cells | Monomeric IgD found on memory B cells |
| Do not express FcalphaR | Express FcalphaR |
| Express pIgR in liver, gut and lung | Do not express pIgR in liver |
| Naïve B cells express TLR4 and respond to LPS | Naïve B cells don’t express TLR4 or respond to LPS |
| BTK knockout – partial B cell defect | BTK knockout – virtual B cell defect |
| Cellular | |
| Have distinct B1 and B2 cells | B1-like B cells express CD43 |
| CD27 not unique to memory B cells | CD27 is a marker for memory B cells |
| Anatomic | |
| Rudimentary paranasal sinuses in connected with nasal passage | Have multiple enclosed paranasal sinuses separated from nasal passage by relatively small ostia |
| Diffuse olfactory mucosa on extensively branched ethmoturbinals with limited respiratory mucosa | Focal olfactory mucosa on rudimentary ethmoturbinals comprised predominantly of respiratory mucosa |
| Have well developed constitutive NALT and variable constitutive BALT | Morphologically different but constitutive Waldeyer’s ring in the upper airway but lack BALT in the absence of disease |
| No bronchial circulation | Bronchial circulation for potential movement of B cell lineages |
Another important point to make is that B cells have been associated with immunoglobulin responses since their discovery and distinction from T cells in the middle of the 20th century. While immunoglobulin production remains as the most recognized, most studied, and probably most important function of B cells, we would be remiss if we did not point out that impressive recent studies have demonstrated many important roles of B cells that are independent of immunoglobulin production, e.g. in antigen presentation and as regulatory cells akin to Treg cells. Very little is known about either of these functions in the respiratory system. We have mentioned these activities where information is available but have focused primarily on immunoglobulin responses. Finally, although there have been a few very valuable reviews of B cells and the respiratory system published, it has become abundantly clear that the study of B cell biology in the respiratory system, especially in humans, is an exceedingly important subject matter that yearns for significantly more investigation.2–4
Overview of the Adaptive Immune Response and Immunoglobulin Production
B Cell Lineages
The majority of B cells develop from lymphoid progenitor cells in the specialized microenvironment of the bone marrow. Early stages of this process are dependent on contact between the developing B cell precursors and bone marrow stromal cells, but do not require antigen. These developmental steps revolve around functional rearrangement of the B cell receptor (BCR), are tightly regulated by positive and negative selection steps, and result in the formation of immature B cells that are licensed to traffic to peripheral lymphoid tissues. A detailed discussion of this process is beyond the scope of this review, but has recently been elegantly presented elsewhere.5 An overview of the important structural organization of immune tissues and events that occur in the selection and expansion of B lineage cells in the airways is provided in Figures 1–3. Once in lymphoid tissues in the periphery, immature B cells rapidly pass through two transitional stages, mainly in the spleen, before committing to either follicular or marginal zone (MZ) B cell fates. The majority of immature B cells will become follicular IgM+ IgD+ mature naïve cells that recirculate through peripheral lymphoid tissues until they encounter their cognate antigen6, after which they can further differentiate into long-lived memory B cells or antibody-secreting plasma cells (discussed below). The remaining immature B cells become marginal zone B cells and localize primarily in the marginal sinus of the spleen, which positions them to rapidly initiate immune responses against blood borne pathogens, especially encapsulated bacteria.6, 7 The decision to commit to one of these two fates is dependent on the integration of signals from the BCR, Notch2, and BAFF.6 Relatively strong BCR signaling in the presence of BAFF will funnel developing B cells toward the follicular fate, while weak BCR signaling in combination with BAFF and Notch2 signals will favor the development of MZ B cells.6 It is important to note, however, that while human marginal zone B cells share some features with their murine counterparts, they also have distinct characteristics, including the ability to recirculate through peripheral lymphoid organs and undergo somatic mutations. Based on these observations, it is thought that human marginal zone B cells represent a subpopulation of IgM+ memory cells rather than a distinct naïve B cell lineage.7
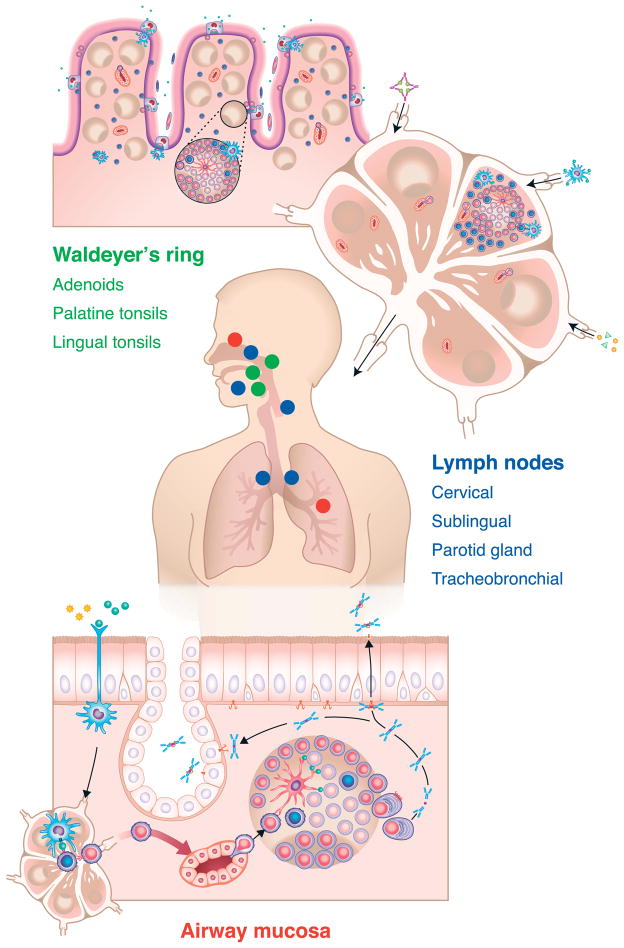
Figure 1.
Overview of the organization of tissues involved in the induction, elicitation and effector responses of B cells and plasma cells in the airways of humans. Shown in green text are the components of Waldeyer’s ring, including the adenoid(s) and palatine and lingual tonsils (shown in green dots on the transverse model of a human). The blue text and blue dots in the model indicate lymph nodes that are centrally involved in sensitization and elicitation of airways responses. These include cervical, sublingual and parotid nodes, as well as tracheobronchial nodes. The figure at the bottom displays an effector response in the airways (for details, see Figure 3A), which could occur either in the lower airways or the upper airways and sinuses, as indicated by the red dots in the transverse section of the model. An important point is that the primary sensitization events that generate B cells responsible for effector responses throughout the airways often occur in the upper airways. For further discussion, please see the main text of the review.
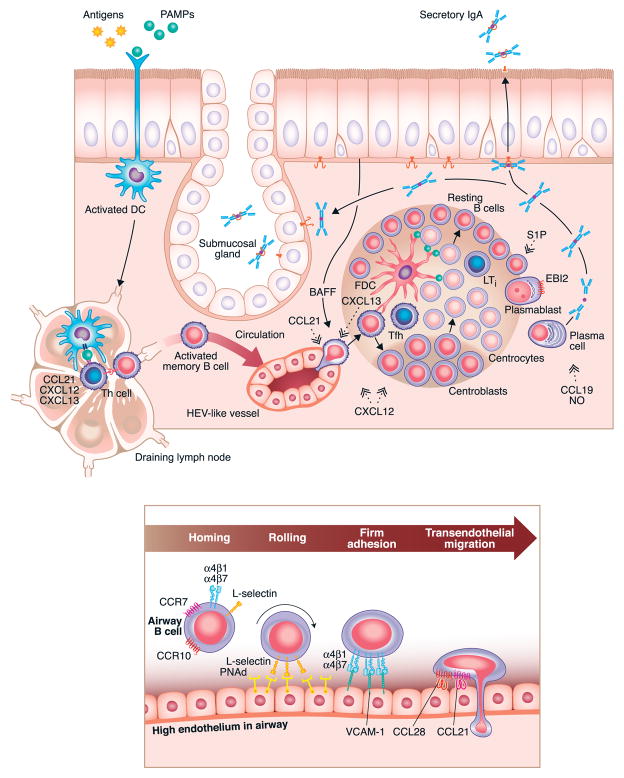
Figure 3.
Representative effector response within airway tissue (3A – top) in which antigen exposure leads to local recruitment of memory (and perhaps naïve) B cells (3B – bottom) and subsequent local expansion of B cells and plasma cells within the airway tissue. The chemokines that drive the recruitment of B cells from the circulation include CCL21, and those involved in localization within quasi-organized inducible lymphoid tissue include CXCL12 and CXCL13 as well as CCL19 and CCL21. The expansion of B cells within the tissue bears a resemblance to the initial induction and expansion of B cells discussed in Figure 1 and in the text. Recruitment of circulating B cells occurs partly via transendothelial migration across endothelium that resembles HEV found in lymph nodes. Some of the molecular participants in the rolling, firm adhesion and transendothelial migration of B cells into the tissue are described in Figure 3B.
In mice, a separate subset of B cells, the B1 B cells, develop in the fetal liver from a precursor cell that is most likely distinct from the one that gives rise to conventional B cells.8 These self-renewing cells can be divided into two subsets, B1a and B1b, based on their expression of CD5, and are part of a family of innate-like B cells that includes MZ B cells.9 B1 B cells express high levels of CXCR5 and traffic to the peritoneal and pleural cavities in response to CXCL13, where they represent the majority of the B cell subpopulations at these sites.9, 10 These cells can also be found at mucosal sites, including the gut and respiratory tract, as well as in lymph nodes and spleen.9 B1 B cells can secrete large amounts of natural IgM and IgA, and produce the majority of the natural antibodies that are important for early pathogen recognition and maintenance of tissue homeostasis.9 Natural antibodies are those that are primarily encoded in the germ line, are not generated by somatic hypermutation, and have antigen specificity for a number of naturally occurring epitopes on the surface of microorganisms, including phosphorylcholine, lysophosphatidylcholine, phosphatidylcholine, and LPS.11 These natural antibodies also often have self reactivity and can contribute to autoimmune responses, as is the case for rheumatoid factor, which contributes to the formation of immune complexes and pathogenesis in rheumatoid arthritis.12 Intriguingly, although BCR signaling is critical for B1 B cell development, it does not provide sufficient signal strength to activate most B1 B cells. However, they can be readily activated by other signals, including IL-5, IL-10 and TLR agonists, which trigger their migration to lymph nodes and mucosal sites where they differentiate into IgM or IgA secreting cells.9 Exposure to TLR agonists such as LPS can mobilize these cells from the peritoneum to peripheral tissues. It is not known whether the population of B1 B cells residing in the pleural cavity includes cells set to selectively migrate to the lung, but this question is worthy of study. However, it has been demonstrated that natural IgM secreted by B1 B cells in the draining lymph nodes of the respiratory tract accounts for the majority of the protective anti-viral IgM responses generated during an influenza infection13, indicating that B1 B cells can home to the airways during viral infections. Despite the accumulating evidence of the importance of B1 B cells in mice, an equivalent subset in humans has not been definitively characterized, in part because the classical marker of murine B1 B cells, CD5, is expressed on a variety of human B cells.14 Recently however, intriguing new work identified two subsets of B1-like B cells in humans that express CD43 and have distinct surface phenotypes, but share key functional characteristics with murine B1 B cells.15 The majority of these human B1 B cells do not express CD11b and spontaneously secrete IgM, while a smaller fraction of human B1 B cells express CD11b, have potent T cell modulatory properties and are elevated in patients with Lupus.14, 16, 17 Whether a similar population of cells plays a significant role in early antibody production and pathogen clearance in the respiratory system or elsewhere in humans remains to be determined.
B Cell Activation and Differentiation in Scondary Lymphoid Organs
It is generally thought that mature naïve B cells circulate mainly through secondary lymphoid organs (SLOs) in the periphery until they are activated by an encounter with their cognate antigen, although new evidence has emerged suggesting that these cells can also traffic through non-lymphoid tissues.18 Secondary lymphoid organs are immune induction sites that are developmentally pre-programmed and include lymph nodes (LNs), spleen and a variety of mucosal-associated lymphoid tissues (MALT)19 (Figures 1–3). Peripheral SLOs share many common organizational features, including follicular B cell zones with defined germinal centers (GC – where somatic hypermutation is believed to primarily occur) that help to ensure optimal cell-cell interactions that favor efficient B cell activation and production of high affinity antigen-specific antibodies while providing controls to limit survival of autoreactive clones.19 Prior to antigen encounter, naïve mature B cells express high levels of the chemokine receptor CXCR5 that allows them to respond to CXCL13 produced by follicular stromal cells and dendritic cells (fDCs) and home to the B cell-rich follicles of SLOs.20, 21 However, the helper T (Th) cells that are critical for full B cell activation and production of high affinity antibodies are located in a distinct area adjacent to the follicles, due to their expression of the chemokine receptor CCR7. Cognate antigen encounter is the first step toward bringing B cells and Th cells together for the induction of an optimal antibody response. Upon entry into a follicle, B cells can be exposed to their cognate antigen by a variety of different cell types. BCR signaling via antigen induces upregulation of CCR7 on follicular B cells, and the chemokine CCL21, which is produced by fibroblastic reticular cells and high endothelial venules (HEVs) in the T cell zone, stimulates them to traffic to the border of the T and B cell zones and interact with cognate Th cells.21 Importantly, proper localization of B cells to this border region requires integration of signals from both CCR7 and CXCR5.20 The interaction between T and B cells at this border is highly dynamic (Figure 2B), as visualized by intravital two photon imaging of the process showing active movement of both T and B cell participants.22 B cell antigen encounter not only stimulates their traffic towards CCL21 in this area, but also provides the signals for B cells to upregulate their own antigen presentation and co-stimulation capacities. As such, while T cells provide help to B cells via CD40-CD40L interaction and cytokine production, B cells provide peptide-MHC class II complexes in the context of co-stimulatory molecules to T cells which facilitates their survival and cytokine production.23 CD11chi dendritic cells (DCs) are also found in the border region between the B and T cell zones, and can contribute to B cell survival through presentation of antigen and production of BAFF and APRIL.24, 25 These initial antigen-specific interactions also play an important role in determining the specific functional and developmental fate to which the B cells will commit.
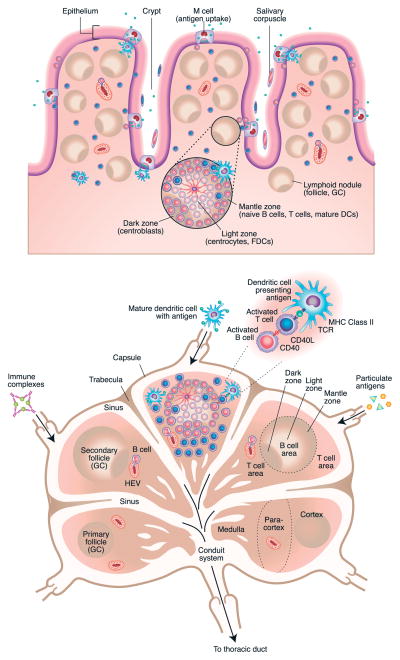
Figure 2.
Detailed overview of primary inductive lymphoid tissues contained in tonsils (2A – top) and lymph nodes (2B – bottom). Tonsils and adenoid tissue contain M cells that mediate antigen uptake into the tissue that is rich with lymphoid follicles in which the primary expansion of naïve B cells occurs, followed by the subsequent generation of memory B cells that populate other lymphoid tissues, especially lymph nodes. Dark and light zones, containing centroblasts and centrocytes, are shown, and the mantle zone, in which dendritic cells, B cells and T cells collaborate for B cell activation, are illustrated in the magnified inset. A similar expansion of memory (and naïve) B cells can occur with secondary exposure in the lymph nodes that are draining the airways (2B). Detailed discussion of the cellular dynamics in these primary and secondary expansions of antigen specific B cells is found within the text.
After the initial interaction with Th cells, B cells can follow one of three alternative fates by differentiating into either extrafollicular plasma cells, germinal center (GC) B cells, or early memory B cells that have the capacity to recirculate.23, 26 Of these cell types, extrafollicular plasma cells and early memory B cells are formed independently of the GC reaction and therefore likely do not undergo somatic hypermutation (SHM), and are similar to the B cells activated during T cell-independent responses. The cells destined to become GC B cells reenter the follicle to participate in the GC reaction. While the factors that favor the development of extrafollicular plasma cells or GC B cells are fairly well understood, those that favor the development of early memory B cells are less clearly defined.27 BCR antigen recognition strength and differential expression of chemoattractant receptors are key factors in determining the fate of particular B cells.23, 28 Sensibly, cells with BCRs that are strongly activated by antigen, either through relatively high affinity or density, will become extrafollicular plasma cells, while those that are less strongly activated will enter the GC reaction.28 Those cells destined to become extrafollicular plasma cells will also maintain expression of Epstein Barr Virus-induced protein 2 (EBI2) and upregulate CXCR4, while those destined to become GC B cells will not express EBI2, and upregulate CXCR5.29–31 Recently, a novel ligand for EBI2 was identified as 7α,25-dihydroxycholesterol, and was shown to be critical for the migration of EBI2+ cells to extrafollicular sites.32, 33 Differential expression of specific transcription factors also regulates this fate decision. Whereas high expression of Blimp-1 and IRF4 (interferon regulatory factor 4) are required for plasma cell development, high expression of Bcl-6 is required for GC differentiation, and Blimp-1 and Bcl-6 repress each other (see below).23 Extrafollicular plasma cells are short-lived (3–5 days), but they provide an important source of relatively high affinity, germline-encoded antigen-specific IgM or IgG antibodies early in the immune response.28 However, in situations of persistent antigen exposure, such as during chronic infections, or with self antigens, extafollicular plasma cells have been shown to persist for much longer.34 These cells down regulate CXCR5, while upregulating CXCR4 and maintaining expression of EBI2 (Epstein-Barr virus-induced molecule 2), a G protein-coupled receptor that binds to ligands derived from cholesterol hydroxylation, allowing them to traffic to splenic bridging channels or lymph node medullary cords where they will receive survival signals such as BAFF and APRIL from CD11chi DCs.5, 23, 32, 33 Conversely, GC B cells maintain expression of CXCR5, do not express EBI2, and traffic back to the follicle in response to CXCL13.
The GC reaction is characterized by large amounts of rapid clonal expansion and is generally polarized into two distinct zones. The dark zone is proximal to the T cell zone and contains proliferating B cells, called centroblasts, while the light zone is distal to the T cell zone and contains noncycling B cells (centrocytes).35 This compartmentalization is shown in Figures 2B and 3A and is maintained by differential expression and localization of CXCL12 and CXCL13. Centroblasts express higher levels of CXCR4, and therefore traffic to the CXCL12-rich areas of the dark zone, while centrocytes express CXCR5, but low levels of CXCR4, and traffic to the CXCL13-rich areas of the light zone.36 Classically, the GC reaction is thought to proceed in a cyclical fashion wherein centroblasts downregulate surface BCR and undergo SHM while proliferating in the dark zone. They then exit the cell cycle, re-express their newly mutated BCR, and migrate to the light zone as centrocytes. Here, B cells with mutated BCRs that have the highest affinity have a competitive advantage in the presence of antigen, and are positively selected by interactions with follicular DCs (fDCs) and T follicular helper cells (Tfh). Positively selected cells can then exit the GC reaction to become long-lived memory B cells or plasma cells, or re-enter the cell cycle and migrate back to the dark zone for another round of proliferation, SHM, and selection.37 An alternative view to the above has been suggested in which B cell trafficking within the GC is predominantly unidirectional, rather than cyclical, because it was found that B cell re-entry to the dark zone from the light zone was a rare event.38 While recent studies have demonstrated that S1P and its receptor, S1P2, play a role in restricting localization of B cells in the bone marrow and within the germinal center by blocking the action of some of the chemokines that otherwise cause them to relocate33, 35, the signals that determine whether a particular cell will remain in the GC, leave as a memory B cell, or leave as a plasma cell are unclear.37 However, accumulating evidence suggests that GC output may be regulated temporally so that memory B cells emerge early and plasma cells emerge late during the GC reaction.34 In addition, intriguing new studies have demonstrated that during proliferation GC B cells assymetrically divide resulting in preferential expression of key molecules, such as Bcl6 and IL-21R, in one daughter cell.39 While it is still unclear what the functional consequences may be of this assymetrical division, it is tempting to speculate that this may provide cues that could influence the fate of respective daughter cells. Once they leave the GC, memory B cells recirculate through SLOs, are poised to rapidly produce high affinity antibodies upon antigen re-exposure, and can be found months after initial immunization events occur.36, 37 In contrast, GC-derived plasma cells typically home to the bone marrow or spleen, where they can continually secrete high affinity antibodies into the systemic circulation for decades.37 Tfh cells are critically important to several aspects of GC formation and maintenance, positive selection of high affinity B cell clones, and regulation of GC B cell commitment to either memory B or plasma cell fates.36, 37, 40, 41 While it has been demonstrated that Tfh cells can potentially be derived from other Th lineages, including FoxP3+ cells in the Peyer’s patch42, it has recently become evident that Tfh cells likely represent a separate, distinct lineage of Th cells.40 Tfh cells require Bcl6 expression for their differentiation, specialize in providing help to developing B cells, and require B cell interactions for their survival.40 An excellent comprehensive review of this newly identified Th cell subtype and its role in the GC reaction has recently been published elsewhere.40
B Cell Homing and Activation in the Airway
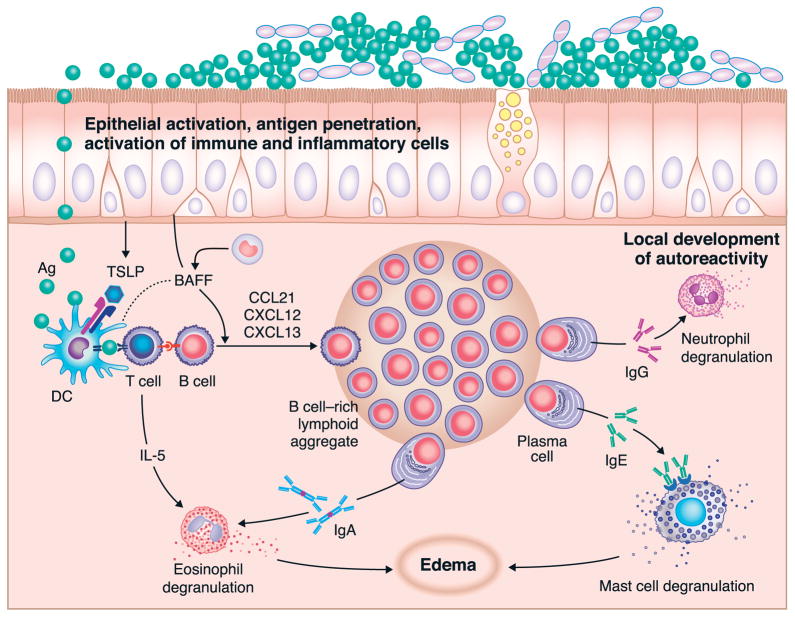
Classically, it was thought that primary immune responses could only be generated in SLOs, as described above.43 However, studies in lymphotoxin (LT)α-deficient mice that lack LNs and Peyer’s patches, and have a disorganized spleen, have demonstrated that antigen-specific B and T cells can be primed in non-lymphoid tissues.44, 45 In fact, many studies have revealed the presence of organized ectopic or tertiary lymphoid-like structures in tissues affected by chronic inflammation, including the airways.46 Unlike the well-organized and defined SLOs, ectopic lymphoid structures are relatively less well organized, can develop in a wide variety of tissues and sites, and arise quickly and spontaneously in response to infection and/or chronic inflammation.19, 46 Interestingly however, animal models have demonstrated that the factors required to generate ectopic lymphoid structures are similar to those required for development of SLOs, and include LT signaling and localized expression of CXCL12 and CXCL13, among others.46 Some of the best-described ectopic lymphoid structures are so-called isolated lymphoid follicles (ILFs) in the gut. These structures require microbial stimulation for their formation, and have been shown to contain large numbers of naïve lymphocytes.47 This suggests that these non-lymph node sites where memory B cells can readily be re-activated can also facilitate naïve B cell activation and maturation.47 While ectopic lymphoid structures do generally contain all the necessary cellular and biochemical components to facilitate B cell activation and production of high affinity antibodies as outlined above, and probably evolved as a local mechanism to control infection, their apparent lack of organization has prompted speculation that they may also facilitate abnormal activation of T and/or B cells that could lead to autoimmunity or amplification of chronic inflammation.46 Formation of ILFs is seen in the lungs and the upper airways in many normal airways immune responses as well as in many pathological conditions. Their presence and potential roles in airways disease are discussed further below.
Class switch recombination (CSR) is the process by which the heavy chain composition of a specific antibody gene is altered in a single B cell expressing that antibody, and plays a critical role in dictating the overall specificity and affinity of the resulting antibodies. This process can occur in a T cell-dependent manner via CD40-CD40L interactions, or in a T cell-independent manner via BAFF and APRIL (which are CD40L-related members of the TNF family).48 Similar to lymphocyte activation, CSR was thought to only occur in SLO, and yield circulating memory B cells or effector B cells that express isotypes such as IgG and IgM, and, of particular importance in the airways, IgE and IgA. However, significant data has accumulated to demonstrate that CSR can also occur in the airways. Studies by several groups of investigators have demonstrated this in allergic inflammation, showing that biopsy tissue from the lungs or the nasal cavity of patients with asthma or hayfever express the molecular fingerprint of ongoing CSR and are able to express IgE antibodies ex vivo.49, 50 Others have also implicated DC-derived iNOS as a centrally important enzyme in IgA CSR.51 iNOS regulates expression of the TGFβ receptor, as well as production of APRIL and BAFF, which all play important roles in IgA class switching.51 Since very large quantities of NO are produced in the respiratory system, especially in the sinuses and in the vicinity of NALT, this might be particularly important in the nasal cavity.
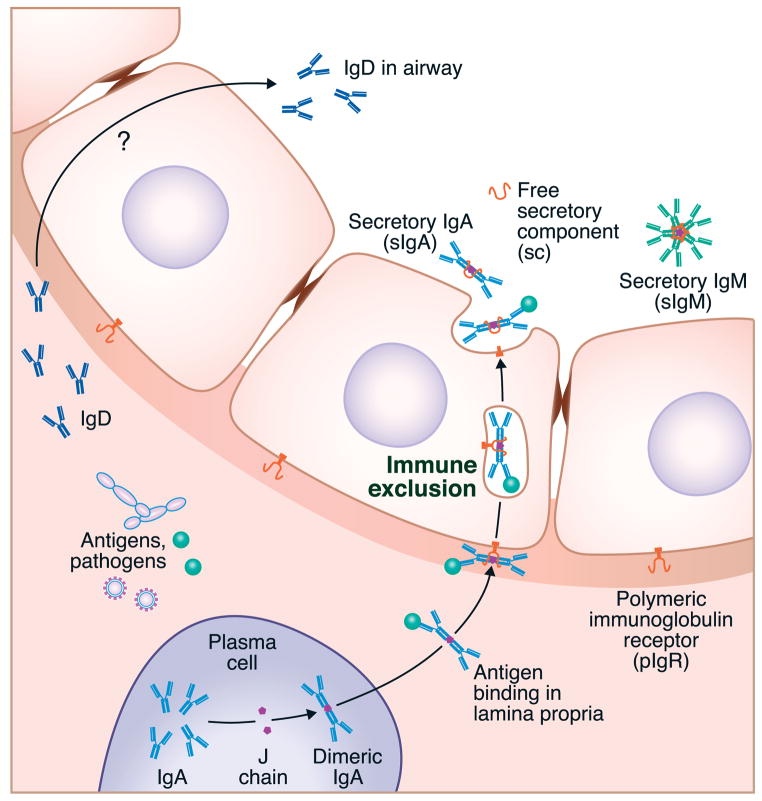
One of the primary functions of B cells in the airways is the production of immunoglobulins, both within the parenchyma as well as for export to the mucosal surface of the airway. In particular, airway B cells, like those in the gut, produce polymeric forms of IgA and IgM that are structurally maintained by the joining or J chain. Early studies determined that these polymeric immunoglobulins, when detected within the lumen of the airways or gut, also contained a secretory component (SC). This SC is added by the action of the polymeric immunoglobulin receptor, pIgR, which binds polymeric IgA or IgM at the basal surface of mucosal or glandular epithelium and transports the immunoglobulin across the epithelial cell.52–55(see Figure 4) In humans lacking IgA (selective IgA deficiency – see below), there is a relatively modest compromise in respiratory immunity because IgM production, and possibly IgD, is thought to compensate in the absence of IgA.56 The absence of pIgR causes a profound increase in respiratory infections however, as both of these secretory antibodies are lacking in the mucosal surface in such patients.57, 58 The combined action of secretory IgA (sIgA) and sIgM constitutes a process referred to as “immune exclusion”, in which antigens, and even entire organisms, can be carried from the lamina propria across the epithelium and expelled into the lumen of the airways (or gut) (Figure 4).
Figure 4.
The principle of immune exclusion. Local expression of immunoglobulins of the IgA and IgM isotype can be leveraged for the removal of antigens, allergens and pathogens via the polymeric immunoglobulin receptor (PIgR). PIgR binds to multimeric forms of IgA and IgM containing the J chain and moves them across the epithelium by a vesicular transport mechanism that is robust enough to transport whole organisms bound to the immunoglobulin.
Early studies in humans showed that after the oral ingestion of a bacterial vaccine, specific IgA positive cells appeared in the blood and could be associated with the production of sIgA in saliva, tears and other sites, leading to the proposal that there exists a common mucosal immune system, one in which B cell responses in the major mucosal sites would be unified or shared.59, 60 However, the preponderance of the evidence suggests that B cell homing is very specific within the airways of humans, based on a number of studies utilizing tonsillectomy of children as an opportunity to study this question. These studies are elegantly reviewed by Brandtzaeg and others.4, 61, 62 Studies compared intratonsillar injection of a vaccine with peroral administration, intranasal administration and parenteral injection and found that peroral and parenteral administration yielded little or no antibody secreting cells (ASC) in the tonsils, whereas intratonsillar injection not only led to very large local responses, but these responses were primarily restricted to the injected tonsil.63 The tonsils have been referred to as functional analogs of the intestinal Peyer’s patch for the “aerodigestive” tract, (i.e. the combined proximal locations where the respiratory and digestive systems are unified) insofar as tonsils are a major site of induction from which B cells that exert effector responses elsewhere in the respiratory system emanate. An early study by Ogra showed that tonsillectomy is associated with a profound and long lasting blunting of secretory IgA production in the nasopharynx.64 It is now well established that the specific location of antigen encounter and B cell activation can influence the type of immune response that is initiated, as well as the destination to which the activated cells will preferentially home. As such, the context of the initial activation of a B cell can have lasting implications on the type and location of the resulting immune response. Mucosal surfaces, including the intestines and airways, are continually exposed to foreign microbes and particles, and are therefore likely places for B cells to encounter antigens and become activated. Activation of B cells and their subsequent maturation and homing preferences have been extensively studied in the gut, and to a lesser extent in the airways. The gut mucosa contains a variety of immune induction sites, including secondary (PPs and draining LNs), and ectopic (ILFs) lymphoid organs. Analogous structures can be found in the airways and include SLOs (nasopharynx-associated lymphoid tissue (NALT) and draining LNs) and ectopic lymphoid structures (inducible bronchus-associated lymphoid tissue (iBALT))(Figure 1).62 Extensive studies in the gut have shown that B cell activation in PPs results in the formation of IgA-secreting plasma cells that preferentially home back to the gastro-intestinal tract.52 Furthermore, it has been clearly demonstrated that dendritic cells within the PPs influence both isotype switching and homing preferences by production of retinoic acid that enhances IgA switching (induced by TGF-β, BAFF, and APRIL) and expression of α4β7 integrin and CCR9.52 α4β7 integrin binds to MAdCAM-1, which is expressed mainly on intestinal endothelial cells and facilitates critical steps of lymphocyte rolling and entry into the tissue, while the ligand for CCR9, CCL25, is expressed by intestinal epithelial cells and further supports intestinal homing.52 Similarly, in the airway, it has been shown that B cells activated in the NALT become plasma cells that preferentially switch to IgA and home back to the airway, as well as other mucosal sites.62 While a specific airway homing molecule, similar to α4β7 integrin in the gut, has not been definitively identified, in some cases, homing to the airways may be dependent on α4β1 integrin and CCR10, whose ligands, VCAM-1 and CCL28, are expressed in the respiratory tract (Figure 3B).62 Supporting this, a unique IgD+IgM− mature B cell subset that is almost exclusively formed in the NALT has been shown to express CCR10 and home specifically to the airway mucosa, but not the gut.48, 56 Moreover, Cerutti and colleagues have demonstrated that IgD from these cells binds to mast cells and basophils and recognizes antigens from common respiratory pathogens, suggesting an important role for these unique B cells in immune responses within the airways.48, 65 Furthermore, based on the critical role of intestinal dendritic cells in influencing class switching and homing properties, it is reasonable to expect that airway dendritic cells would have a similar function. It seems unlikely that retinoic acid would be responsible for induction of site-specific homing molecules in both the intestines and airway, but there are many factors associated with IgA class switching (TGF-β, IL-4, IL-6, IL-10, iNOS) that could play an analogous role in the airway. As mentioned above, naïve B cells are known to continually traffic through SLOs in search of antigen, but there is mounting evidence that naïve cells can traffic through non-lymphoid tissues, and can become activated within ectopic lymphoid structures there.18, 47, 66 As in ILFs, it has been shown that iBALT can support activation of naïve B cells, and production of long lived antibody responses in models of allergic airway inflammation as well as during influenza infection.67–70
It seems likely that during any given immune response, B cells are activated in a variety of locations including draining LNs, and within the affected mucosal tissue itself. Because B cells activated at distinct sites will have unique properties and homing abilities, this insures maximal protection against regional re-exposure to a similar antigen. Activation within the mucosa will generate mainly IgA-secreting plasma cells that home back to the tissue and provide long lasting local immunity, while activation in LNs will generate mainly IgG-secreting plasma cells that home preferentially to the bone marrow and provide systemic protection.71 In addition, regardless of the location of initial activation, long lived memory B cells will also be generated that have similar homing patterns to naïve cells, and can be rapidly activated upon antigen re-encounter.
Proper resolution of any immune response is critical to prevent chronic inflammation and tissue damage. When these control measures fail, chronic inflammatory and autoimmune diseases can develop. These diseases are associated with increased production of pro-inflammatory mediators that perpetuate the cycle of inflammation. As mentioned above, formation of ectopic lymphoid structures has been associated with a variety of inflammatory diseases. In line with this, overexpression of pro-inflammatory cytokines in the lungs (IL-6 and the IL-6R, TNF, or IL-5) has also been shown to induce formation of iBALT in the absence of antigen.72–74 While these studies did not specifically address B cell activation within iBALT, they did note a large increase in gene transcription of several immunoglobulin genes in response to TNF overexpression73, as well as the presence of B cells in the follicle-like structures.72 These studies highlight the point that factors involved in chronic inflammation can favor induction, or perhaps the maintenance, of ectopic lymphoid structures in the airway, which may prolong or exacerbate inflammation. Moreover, while BAFF and APRIL play critical roles in B cell survival and CSR during normal immune responses, their presence in chronically inflamed tissues may promote the survival of local PCs.52 Depending on the disease, these PCs could exacerbate inflammation by producing antibodies that activate effector cells such as mast cells or eosinophils, or provide an uncontrolled source of autoantibodies. Studies aimed at the disruption of chronic local B cell activation or accumulation in the airway (see below) could provide valuable new targets for the development of improved therapeutic agents.
Signaling in B cells and plasma cells
IgA is the most abundant immunoglobulin of the healthy respiratory tract and is thought to be the most important immunoglobulin for lung defense. In addition, other immunoglobulins, IgM, IgD, IgG and IgE, also contribute to the health and disease of the lung. B cells and plasma cells are the sole producers of immunoglobulins, representing a major component of adaptive immunity. The different isotypes of immunoglobulins have very different functional characteristics, such as ability to activate complement, resistance to protease degradation, half-life within tissues and the blood etc. Antibodies also bind to specific receptors expressed on immune cells including macrophages, neutrophils, eosinophils, basophils and mast cells. There are 6 IgG receptors, one IgA receptor and 2 IgE receptors in humans that bind to and are activated (or inactivated) by immunoglobulins. Various IgG receptors can trigger phagocytosis and antigen presentation or can inhibit the function of several different cell types. Among the 6 known IgG receptors, FcγRIIB is an inhibitory receptor and is the only FcγR which is expressed on B cells and plasma cells. The cross-linking of FcγRIIB and BCR via immune complexes induces the termination of antibody production on B cells. The cross-linking of FcγRIIB in the absence of BCR can induce apoptosis of B cells and plasma cells.75 Receptors for IgE can activate mast cells and basophils to degranulate and cause profound local or systemic anaphylactic inflammation. IgA receptors can play a role in immune exclusion and tolerance mechanisms as well as activation of inflammatory cells such as neutrophils and eosinophils. As a consequence of this diversity, differentiation and isotype switching in B cells is exceedingly important and highly regulated. As discussed above, T cell- and protein antigen-dependent activation in GC leads to formation and expansion of B cells expressing high affinity antibodies. In addition, B cell expansion and functions are controlled by T cell-independent activation from DC or cytokines, inducing generation of low affinity antibodies, especially IgM and IgA, often against non-protein antigens including polysaccharides and glycolipids. In this section, we discuss the molecular pathways of differentiation and activation of B cells and plasma cells, as well as discuss potential therapeutic targets of each pathway relevant to diseases of the respiratory system.
Survival and differentiation of B cells/PC
Activation, differentiation, maintenance of survival and regulation of programmed cell death in B cell lineage cells involves a number of cell surface molecules and transcription factors (see Table 2). The network of transcription factors involved in the differentiation of B cells and plasma cells has been elucidated through the generation of gene knockout and transgenic mice and microarray analyses in isolated cells. Pax5 and Blimp-1 are well-recognized key transcription factors controlling the differentiation of B cells and plasma cells, respectively.23, 76, 77 Pax5 is a critical factor for B cell development and maintenance that activates genes associated with B cell function including activation-induced cytidine deaminase (AID) and BCR signaling molecules such as CD19, CD79a and BLINK.77 AID is an essential component of the machinery that mediates class switch recombination, a process in which the diverse variable (V) regions of the immunoglobulin molecule are transferred from one set of constant (C) regions to another. At the same time, Pax5 represses genes associated with plasma cell development and function, including immunoglobulin genes (IgH, IgL, J chain) and the transcription factor Xbp1, which induces formation of the secretory apparatus necessary for the production of large amounts of antibody.77 In contrast, Blimp-1 is required for plasma cell differentiation and for maintenance of long-lived plasma cells.76, 77 Blimp-1 controls genes involving in immunoglobulin secretion including IgH, IgL, J chain and Xbp1. Blimp-1 also induces thesuppression of mature B cell genes including Pax5, SpiB, CTIIA, AID and Bcl-6.76, 77 On balance, the reciprocal inhibitory effects of Pax5 and Blimp-1 suggest that these two factors are central to the molecular events that occur when the B cell and plasma cell lineages diverge. IRF-4 is induced during the plasma cell differentiation stage and it controls induction of Blimp-1.23, 77 Therefore it seems likely that IRF-4 also plays a critical role in the initiation of plasma cell differentiation. Although it has been reported that Pax5 is induced by IL-7 via the activation of STAT5 and Blimp-1 is induced by IL-21 via the activation of STAT3 and IRF-4, it is still not quite clear how activation of Pax5 in B cells and Blimp-1 in plasma cells is controlled.78, 79
Table 2.
Molecules involved in B lymphocyte lineage cell responses
| Trigger | Receptor | Signaling pathway | Transcriptional factor | B cell function |
|---|---|---|---|---|
| CD40L (TNFSF5) | CD40 (TNFRSF5) | Classical NF-κB | NF-κB1 (/AID) | Survival, Maturation, Proliferation, CSR |
| Alternative NF-κB | NF-κB2 | Survival, Maturation, Proliferation | ||
| JNK/p38 | JUN/ATF2/MEF2C? | Survival, Proliferation | ||
| ? | Pax5/IRF-4 | CSR, GC formation | ||
|
| ||||
| Ag | BCR | Classical NF-κB | NF-κB1 (/AID) | Survival, Maturation, Proliferation, CSR |
| ERK | Elk1/CREB | Survival, Proliferation | ||
| JNK | JUN/ATF2 | Survival, Proliferation | ||
| p38 | MEF2C | Proliferation | ||
| PI3K | AKT | Survival, Proliferation | ||
| Calcineurin | NFAT | Maturation, Proliferation | ||
|
| ||||
| BAFF (TNFSF13B) | BAFFR (TNFRSF13C) | Alternative NF-κB | NF-κB2 | Survival, Maturation, Proliferation |
| Classical NF-κB (weak) | NF-κB1 (/AID) | Survival, Maturation, Proliferation, CSR | ||
| PI3K | AKT | Survival, Proliferation | ||
|
| ||||
| BAFF (TNFSF13B), APRIL (TNFSF13) | TACI (TNFRSF13B) | MyD88/Classical NF-κB | NF-κB1 (/AID) | Plasma cell differentiation, CSR to IgA |
| Negative regulation of Alternative NF-κB? | Inhibits NF-κB2? | Negative regulation to B cells | ||
|
| ||||
| BAFF(lo), APRIL (hi) | BCMA (TNFRSF17) | Classical NF-κB | NF-κB1 | Later stage of B cell differentiation, survival of plasmablasts and plasma cells |
|
| ||||
| Pathogens, (ssRNA/CpG-DNA) | TLR7/9 (endosomal TLR) | MyD88/Classical NF-κB | NF-κB1, AP-1, IRF-7 | Survival, Maturation, Proliferation, CSR |
|
| ||||
| IL-4 | IL-4Rα/γc | Jak1/3 | STAT6 | CSR to IgG4 and IgE |
|
| ||||
| IL-4, IL-13 | IL-4Rα/IL-13Rα1 | Jak1, Tyk2 | STAT6/STAT3 | CSR to IgG4 and IgE |
|
| ||||
| IL-6 | IL-6Rα/gp130 | Jak1/2, Tyk2 | STAT3 | Proliferation |
|
| ||||
| IL-7 | IL-7Rα/γc | JAK1/3 | STAT5/Pax5 | Development, Survival, Differentiation |
|
| ||||
| IL-9 | IL-9Rα/γc | JAK1/3 | STAT3/STAT5 | Enhance IL-4 dependent CSR |
|
| ||||
| IL-10 | IL-10R1/IL-10R2 | Jak1, Tyk2 | STAT3 | CSR to IgG1, IgG3, IgG4 and IgA |
|
| ||||
| IL-21 | IL-21Rα/γc | JAK1/3 | STAT3/IRF-4 | Induction of Blimp-1, Differentiation to plasma cells, CSR to IgG1, IgG3 and IgA |
|
| ||||
| IL-24 | IL-20R1/IL-20R2 and IL-22R1/IL-20R2 | Jak1, Tyk2 | STAT1/STAT3 | Inhibit plasma cell differentiation |
|
| ||||
| TGF-β | TGFBR1/TGF BR2 | SMAD/RUNX | CSR to IgA | |
|
| ||||
| Type 1 IFN | IFNAR1/IFNA R2 | Jak1, Tyk2 | STAT1/STAT2 | Expand short-lived plasma cells |
|
| ||||
| Chemokines, Oxysterols | 7TMRs (CCRs, CXCRs, EBI2) | B cell and plasma cell migration | ||
Activation – molecular pathways and regulators
The airway is the place that is first contacted by inhaled allergen. Airway epithelial cells provide a first line of defense against exposure to potentially harmful inhaled allergens including particulate substances and microbial pathogens.80 Local and infiltrating DCs in the airway have a vital role in the initiation of adaptive immune responses to inhaled foreign antigens and airway epithelial cells control the recruitment of DC to epithelium by releasing chemokines including CCL20.80 Studies using tracer molecules including FITC-dextran and FITC-OVA clearly show that lung DCs take up antigen in the epithelium and then migrate into draining lymph nodes in a CCR7-dependent manner to present antigen to T cells.81 B cells that are simultaneously activated via BCR and TLR down-regulate CXCR5 and induce CCR7 and then migrate into the T cell zone as described above. In this area, B cells are activated by T cells via the interaction of CD40-CD40L and the production of cytokines, resulting in the initiation of proliferation, differentiation and early immunoglobulin class switch recombination (CSR). B cell activation and CSR also occur locally in airways via both T cell-dependent and T cell-independent mechanisms.2 T cell-independent mechanisms are mainly triggered by the TNF family cytokines, BAFF and APRIL.25, 82 In this section, we describe the molecular pathways of B cell activation.
BCR
BCR is a complex receptor consisting of membrane bound IgM (or IgD), Igα (CD79a) and Igβ (CD79b).83 Since membrane bound IgM has a short cytoplasmic tail, BCR mediated signals are transduced by CD79a and CD79b.83, 84 Cross-linking of membrane bound IgM induces the phosphorylation of ITAM domains on CD79a and CD79b by Src family kinases including Lyn, and it leads to the recruitment and activation of the tyrosine kinase Syk.82–84 Activation of Syk is a critical event in BCR signaling leading to the formation of a plasma membrane-associated signaling complex that contains adaptor molecules (including BLNK) and multiple tyrosine kinases (including Lyn and BTK). Phosphorylation of BLNK induces the activation of Ras, phospholipase Cγ2 (PLCγ2) and BTK contributing to the activation of distinct signaling pathways. Activation of Ras leads ultimately to phosphorylation of ERK, which in turn activates transcription factors, Elk1 and CREB that induce cell proliferation and inhibit expression and function of apoptotic proteins.84, 85 Activation of PLCγ2 induces the production of inositol-1,4,5-triphosphate (IP3) which is required for the subsequent release of cytosolic Ca2+, and diacylglycerol (DAG) which in turn is required for the activation of PKCβ 84, 86 Intracellular Ca2+ release and activation of PKCβ induce phosphorylation of MAPKs, ERK, JNK and p38, and then lead to the activation of transcription factors, Elk1, CREB, MEF2C, JUN and ATF2. Increased cytosolic Ca2+ also induces the activation of the transcription factor NFAT via the calmodulin/calcineurin pathway.82, 86 The activation of PKCβ also induces phosphorylation of IκB kinase (IKK) leading to the activation of the transcription factor NF-κB1 through the induction of the classical NF-κB pathway. 82 NF-κB1 induces the expression of anti-apoptotic genes and AID (see below).
Complement activation is an essential process of the early response to infection. The complement cleavage product C3d enhances adaptive immunity via binding to complement receptor 2 (CD21) that is a part of the B cell co-receptor complex.87 Activation of the B cell co-receptor complex consisting of CD21, CD19 and CD81 enhances the BCR-dependent reaction.82, 87, 88 Phosphorylation of CD19 results in the recruitment of Lyn that can efficiently amplify BCR signaling by enhancing the phosphorylation of CD79a/b.89 Phosphorylated CD19 also activates PI3K which in turn further induces the production of the lipid phosphatidylinositol-3,4,5-triphosphate (PIP3).88 PIP3 recruits a number of BCR signaling components including PLCγ2 and BTK to the plasma membrane and activates them. PIP3 also recruits and activates the serine/threonine kinase AKT that in turn activates the NF-κB pathway and inhibits GSK-3, which is a negative regulator of MYC and D-type cyclins. AKT also inactivates the forkhead box class O 1 (FOXO1) transcription factor that targets cell cycle arrest genes such as cyclin G2 and Rbl2.90
BCR-related signaling is critical for early B cell development and mutations of BCR-related genes result in primary B cell deficiencies. Mutations in BTK, CD79a, CD79b, IgLL1 and BLNK account for approximately 90% of patients with X-linked or autosomal-recessive agammaglobulinemia, characterized by defects in early B cell development, and 85% of these result from mutations of the BTK gene.91 Mutations in CD19 or CD81 result in hypogammaglobulinemia.91–93 As discussed below, these B cell deficiency diseases often result in frequent and severe upper and lower respiratory infections, due to the importance of an intact immunoglobulin response to immunity in the respiratory system.
BCR-related signaling molecules are one of the key groups of therapeutic targets for B cell-related diseases. Several small compound inhibitors for kinases including Syk (R788), BTK (PCI-32765 andGDC-0834), PKCβ (enzastaurin), Bcr-Abl/Lyn (bafetinib), p38 MAPK (pamapimod, BMS-582949, SB-681323 and SD0006), MEK/ERK (RO5068760, GSK1120212, AZD6244 and CH4987655) and Akt (MK2206, GSK2141795, PBI-05204 and SR13668) are currently in clinical trials for B cell-related diseases including rheumatoid arthritis, systemic lupus erythematosus (SLE), B cell lymphoma andleukemia. 94–104 Bortezomib is the first therapeutic proteasome inhibitor that suppressesNF-κB signaling in the treatment of human cancer.105 Although none of these inhibitors except SB-681323 are currently under study specifically for respiratory disease, they will likely become therapeutic options for B cell-related airway diseases as discussed below (Table 3).
Table 3.
Drugs targeting B lymphocyte lineage cell-related molecules
| Target | Drug | Diseases | Ref |
|---|---|---|---|
| BCR-related | |||
|
| |||
| Syk | Fostamatinib (R788) | RA (Phase III), lymphoma (Phase II) | 95 |
| BTK | PCI-32765 | Lymphoma, leukemia (Phase II/III) | 94 |
| GDC-0834 | RA (Phase I) | 100 | |
| PKCβ | Enzastaurin | Non-Hodgkin’s lymphoma (Phase III), Cancer (Phase II) | 104 |
| Bcr-Abl/Lyn | Bafetinib | Leukemia (Phase II) | 97 |
| p38 MAPK | Pamapimod | RA (Phase II) | 95 |
| BMS-582949 | RA, psoriasis (Phase II) | 95 | |
| SB-681323 | RA, ARDS, COPD (Phase II) | 95, 103 | |
| SD0006 | RA (Phase I) | 103 | |
| MEK/ERK | Trametinib (GSK1120212) | Cancer (Phase II/III) | 96 |
| Selumetinib (AZD6244) | Cancer, MM (Phase II) | 102 | |
| RO5068760 | Cancer (Phase I) | 98 | |
| CH4987655 | Cancer (Phase I) | 99 | |
| Akt | MK2206 | Cancer (Phase II) | 101 |
| GSK2141795 | Cancer (Phase I) | 101 | |
| SR13668 | Cancer (Phase I) | 101 | |
| PBI-05204 | Cancer (Phase I) | 101 | |
| NF-κB | Bortezomib | Cancer (Approved) | 105 |
|
| |||
| Cytokines | |||
|
| |||
| BAFF | Belimumab | SLE (Approved) | 122 |
| BAFF | Blisibimod (A-623) | SLE (Phase II/III) | 123 |
| BAFF | Tabalumab (LY2127399) | SLE, RA, MM (Phase III) | 123 |
| BAFF | Briobacept (BAFFR-Ig) | RA (Phase I) | 123 |
| BAFF/APRIL | Atacicept (TACI-Ig) | RA, SLE (Phase II/III), MS, RA (Phase II) | 95, 123 |
| IL-6 | Siltuximab | Cancer (Phase II) | 145 |
| IL-6R | Tocilizumab | RA (Market) | 142 |
| IL-9 | MEDI-528 | Asthma (Phase II) | 142 |
| IL-13 | Lebrikizumab | Asthma (Phase III) | 139, 141 |
| Tralokinumab | Asthma, ulcerative colitis (Phase II) | 139 | |
| IL-13/IL-4 | Pitrakinra (mutated IL-4) | Asthma, atopic eczema (Phase II) | 140 |
| IL-21 | NNC114-0005 (fully human anti-IL-21 mAb) | RA (Phase I) | 148 |
| IFNα | Rontalizumab | SLE (Phase II) | 153 |
| MEDI-545 | SLE (Phase II) | 153 | |
|
| |||
| Other | |||
|
| |||
| CD20 | Rituximab | RA, non-Hodgkin’s lymphoma and B-CLL (Approved) SLE (Phase III) |
153 |
| Ofatumumab | Chronic lymphocytic leukemia (Approved) RA (Phase III), MS (Phase II) |
153 | |
| Ocrelizumab | RA, SLE, MS (Phase III) | 153 | |
| Veltuzumab | RA, lymphoma (Phase II), | 153 | |
| TrU015 | RA (Phase II), SLE (Phase I) | 153 | |
| CD22 | Epratuzumab | SLE (Phase III) | 153 |
| IgE | Omalizumab | Asthma (Approved) | 138 |
| MEMP1972A (anti-M1 prime) | Asthma (Phase II), AR (Phase I) | 139 | |
CD40
T cell-mediated activation of B cells is generally controlled by CD40-CD40 ligand (CD40L) interactions. CD40 is a member of the TNF family (TNFRSF5) and is constitutively expressed on B cells. The engagement of CD40 by CD40L, which is expressed on activated helper T cells, promotes the recruitment of TNF receptor-associated factor (TRAF) family molecules leading to the activation of multiple signaling pathways, including both classical and alternative NF-κB pathways, MAPKs, PI3K and PLCγ pathways.23, 106, 107 One of the key gene products of the CD40 pathway is activation-induced cytidine deaminase (AID), a critical factor for somatic hypermutation and immunoglobulin class switch recombination (CSR).108 Knockout of either the CD40 or CD40L gene in mice results in profound defects in antibody production and CSR, and mutations of the CD40L gene in humans result in the disease called X-linked hyper IgM-syndrome, in which excess IgM is produced due to a defect in subsequent loss from the IgM+ cell population as CSR occurs.106, 109 CD40 signaling in B cells also promotes GC formation, and differentiation to memory B cells and long-lived plasma cells.107
Interference with the CD40-CD40L interaction is a potential target either to augment CD40 signaling for patients with immunodeficiency or to block signaling in chronic inflammatory diseases.110 Blockade of CD40 signaling with monoclonal antibodies has been shown to prevent or improve inflammatory diseases in several animal models. Antibodies against CD40L are also known to induce tolerance in mouse airway graft models.111 Various monoclonal antibodies against CD40 and CD40L are undergoing clinical trials to test their safety and potential clinical benefits in patients.110
BAFF/APRIL
B cell-activating factor of the TNF family (BAFF; also known as BLyS and TNFSF13B) and a proliferation-inducing ligand (APRIL; also known as TNFSF13) play centrally important roles in B cell function. Studies of deficient mice indicate that BAFF is an essential factor for B cell maturation and survival and APRIL is an important factor for CSR to IgA.25, 82, 112 BAFF and APRIL share two receptors: transmembrane activator and CAML interactor (TACI) and B cell maturation antigen (BCMA). TACI binds to BAFF and APRIL with moderate affinity, whereas BCMA has high affinity for APRIL and low affinity for BAFF. In addition, BAFF also binds to the high affinity receptor BAFF-R. BAFF-R is a potent regulator of mature B cell survival and also controls T cell-independent immunoglobulin production. Loss-of-function mutations of BAFF-R result in the impairment of B cell development, T cell-independent responses and immunoglobulin production except for IgA responses in humans.93, 113 Binding of BAFF to BAFF-R activates two major pathways, the alternative NF-κB pathway and the PI3K/AKT pathway. Activation of BAFF-R induces the recruitment of TRAF3/NF-κB-induced kinase (NIK) complex and degrades TRAF3 in a TRAF2-dependent manner. Degradation of TRAF3 leads the stabilization of free NIK and the activation of NF-κB2, which in turn induces genes involved in B cell survival. The PI3K/AKT pathway promotes B cell survival as well as B cell growth. In contrast to BAFF-R, the downstream signaling pathways of TACI and BCMA are poorly understood. TACI is an inhibitory receptor for the general B cell population, although the mechanism of this negative effect remains to be elucidated. TACI interacts with several TRAFs including TRAF3 that acts as a negative regulator of the alternative NF-κB pathway by maintaining low levels of cellular NIK.114 This might be a pathway of TACI-mediated negative regulation. TACI also interacts with MyD88 leading to the activation of the classical NF-κB pathway to promote plasma cell differentiation and immunoglobulin CSR, especially to IgA. 115 TACI mutations are significantly associated with common variable immunodeficiency (CVID) although they are likely to require additional genetic contributions as genetic mutation alone does not necessarily lead to a CVID phenotype. 116 BCMA strongly activates the classical NF-κB pathway and promotes later stages of B cell differentiation and the survival of plasmablasts and long lived plasma cells, although the details of the signaling pathways involved remain unclear.117
The crosstalk between BCR and BAFF-R signaling pathways plays a key role in B cell survival.82, 84 The BCR is the main activator of the classical NF-κB pathway while the BAFF-R primarily activates the alternative NF-κB pathway. Since it is now known that both of these NF-κB pathways are required for B cell development and survival, the participation of both the BCR and BAFF-R is believed to be key. Consequently, disruptions or genetic abnormalities that affect either the BCR, the production of ligands for the BAFF-R or the signaling of either receptor can have profound effects on B cells in homeostasis or disease.
BAFF and APRIL may be involved in the pathogenesis of airway inflammatory diseases. It has been reported that BAFF is elevated in asthma, COPD and chronic rhinosinusitis with nasal polyps (CRSwNP).118–120 In a murine model of asthma, treatment with TACI-Ig that blocks both BAFF- and APRIL-dependent signals prevents airway inflammation and is more effective than treatment with anti-IgE in reducing airway hyperresponsiveness to inhaled antigen.121 Importantly, anti-BAFF (belimumab/Benlysta) has been recently approved for the treatment of SLE, and TACI-Ig (atacicept) is under clinical trials for B cell-related diseases including rheumatoid arthritis, SLE andB-cell lymphoma. 95, 122, 123 These and other new drugs may become therapeutic options for BAFF or B cell driven respiratory diseases in the future (Table 3).
TLR
The innate immune response is the first line of host defense and is responsible for immediate recognition and control of microbial invasion. One of the primary families of molecules responsible for innate immunity in humans is called the Toll-like receptors (TLR), homologues of Toll in Drosophila, which recognize pathogen-associated molecular patterns of microbial organisms.124 To date, 10 human TLRs have been identified. Among them, TLR1, 2, 6, 7, 9 and 10 are expressed on human B cells and TLR1–9 are expressed on human plasma cells.125, 126 Although lipopolysaccharide (LPS), which is a TLR4 ligand, is well known to induce immunoglobulin production in mouse B cells, TLR4 is absent on human B cells, except malignant B cells, suggesting that LPS does not directly activate human B cells. In addition, the expression of TLR2 in naïve B cells is low. In general, bacterial cell wall components that mainly activate cell surface TLRs (TLR1/2, TLR2/6 and TLR4), do not strongly activate human B cells.125 In contrast, endosomal TLRs such as TLR7, a receptor for single-stranded RNA, and TLR9, a receptor for unmethylated CpG-motifs containing DNA, are highly expressed in B cells.124–126 The activation of TLR7 and TLR9 by RNA and DNA initiates signaling via recruitment of the adaptor molecule MyD88 and the sequential activation of NF-κB, MAPKs, AP-1 and IRF-7, resulting in B cell proliferation and immunoglobulin production.124–126 However, many vaccine adjuvants contain ligands for surface TLR, including monophosphoryl lipid A, suggesting that TLR activation on other important cells such as DC, tissue structural cells or T cells can promote robust B cell responses.124, 125 Interestingly, a recent study showed that B cells respond rapidly during sepsis and play an important role in a rapid innate immune response via an interferon activation pathway downstream of TLR activation.127
Immune complexes (IC) that are formed from the multivalent binding of an autoantibody to an autoantigen are known to be pathogenic in autoimmune diseases. DNA- or RNA-binding IC are potent IFNα inducers in plasmacytoid DC via the activation of FcγRIIa and TLR7/9.128 This is now recognized to be one of the important pathogenic mechanisms for autoimmune diseases including systemic lupus erythematosus (SLE) and systemic sclerosis.128 In addition, DNA or RNA binding IC also potently activate autoreactive B cells via sequential engagement of BCR and TLR7/9, and play a key role in autoantibody responses in SLE.129–131 SLE is considered to be a multisystem disease. The pulmonary system in SLE is vulnerable to injury, in part mediated by IC.132 IC are also known to initiate acute lung injury in patients with hypersensitivity pneumonitis.133 Although mechanisms of IC-related inflammation in these diseases are well studied in regard to FcγR-related and complement-mediated reactions, BCR and TLR7/9 may also be involved in IC-mediated inflammation.
Cytokines
The expression of antibodies and cytokines is involved in homeostasis and disease in the lung. Although CSR is highly regulated by the CD40, BAFF/APRIL and TLR pathways, cytokines control immunoglobulin isotype switching. Interleukin-4 and IL-13 induce CSR to IgG4 and IgE by the activation of STAT6.134 Interleukin-9 enhances IL-4 dependent IgE production in B cells via the activation of STAT3 and STAT5, although IL-9 alone does not have this effect.135 Transforming growth factor-β induces CSR to IgA by the activation of Smad and Runx.134 Interleukin-10 is known to induce CSR to IgG1, IgG3, IgG4 and IgA.134 Interleukin-21 induces CSR to IgG1, IgG3 and IgA and also enhances IL-4-dependent CSR to IgE via the activation of STAT3.136, 137 Elevations of IL-4 and IL-13, and the subsequent induction of IgE from B cells in the lung, are well known factors in the initiation and exacerbation of bronchial asthma. An anti-IgE antibody, omalizumab, is approved for the therapeutic treatment of allergic asthma, emphasizing the importance of IgE producing B cells in this disease.138 Selective inhibition of IL-4 for the treatment of asthma has thus far not been effective, however. Currently, targeting of IL-13 alone or in the combination of IL-13 and IL-4 is being pursued using a humanized anti-IL-13 antibody (Lebrikizumab), a human anti-IL-13 antibody (Tralokinumab) and a mutated IL-4 (pitrakinra).139 Pitrakinra blocks the effect of both IL-4 and IL-13. Pitrakinra and Lebrikizumab showed promising effects in the treatment of asthma in clinical trials.140, 141 Immunoglobulin E-producing B cells will be targeted in a phase II trial by anti-M1 prime, an antibody that binds to the unique extracellular domain of IgE found on the cell surface form.139 Interleukin-9 is a classical Th2 cytokine and now is known to be produced by a novel T cell subtype called Th9. In addition to its effects on B cells, IL-9 is known to be involved in the growth and differentiation of mast cells, playing a role in allergic airway inflammation and fibrosis.142 An anti-IL-9 humanized antibody (MEDI-528) is in clinical trials for the treatment of asthma.142
Several cytokines promote B cell function. IL-6 is an important factor in the proliferation of B cells and plasma cells via the activation of STAT3.143 IL-6 is elevated in several airway inflammatory diseases including asthma, COPD and CRSwNP.142, 144 A humanized anti-IL-6 receptor antibody, tocilizumab, is approved for the therapeutic treatment of rheumatoid arthritis, and has been proposed for clinical trials in asthma and COPD.142 An anti-IL-6 antibody (Siltuximab) is in clinical trials for cancer.145 Although IL-7 is absolutely required for human T cell development, IL-7 also promotes B cell development via the activation of STAT5 and Pax5.146 Interleukin-21 is an important regulator of the proliferation and survival of B cells.147 In addition, IL-21 regulates differentiation of B cells to plasma cells. A fully human anti-IL-21 antibody is in clinical trials for rheumatoid arthritis. 148 Interleukin-17 producing T cells have been demonstrated to be potent activators of B cell help, and approaches to blocking IL-17 may have value in treatment of autoimmune diseases that involve B cells, such as SLE.149, 150 IL-24 inhibits plasma cell differentiation, and strategies to emulate this effect may have some promise.151 Type I IFNs promote expansion of short-lived plasma cells and is are known to play a pathogenic role in SLE.152 A humanized anti-IFNα antibody (Rontalizumab) and a fully human anti-IFNα antibody (MEDI-545) are in clinical trials for SLE.153 Earlier in the pipeline are compounds being developed to inhibit the receptor EBI2, which appears to be nearly essential for early plasmablast development.
Although we do not discuss the details of the mechanisms, several B cell depletion therapies had been approved or are in clinical trials. A chimeric anti-CD20 antibody, rituximab (Rituxan), is approved for the therapeutic treatment of rheumatoid arthritis, non-Hodgkin’s lymphoma andchronic lymphocytic leukemia. 153, 154 Other humanized and fully human anti-CD20 antibodies are in clinical trials for rheumatoid arthritis and SLE. CD22 is an inhibitory co-receptor of the BCR that is exclusively expressed on B cells. It plays a key role in setting the threshold of BCR responses.153 A humanized anti-CD22 causes partial B cell depletion and is currently in clinical trials of SLE.153 Importantly, the treatment of patients with Churg-Strauss syndrome with rituximab has resulted in a rapid and substantial reduction of disease activity.155 Neutralization of BAFF by a soluble receptor (Atacicept) or specific antibody (Benlysta) may also have some promise in the treatment of asthma (see above). The progress in the field suggests that B cell depletion or B cell targeting therapies may become therapeutic options for respiratory diseases in the future (Table 3).
Organization and structure of B-lymphocytes in the human airway
The organization and density of B-lymphocytes in the normal physiologic state exhibits significant variation across species and age. In healthy humans, classic nasal-associated lymphoid tissue (NALT), consisting of lymphoid follicles associated with lymphoepithelium, is frequently found in infants but, unlike in mice, is not found in adults.156 In the upper airway of humans, most mucosal-associated lymphoid aggregates are found in the Waldeyer’s ring that consists of the adenoid, tubal, palatine and lingual tonsils157 (Figure 1). While limited experimental evidence suggests that these sites are functional analogs of the NALT found in mice and other rodents, rigorous comparisons between the functional immunologic characteristics of these structures are still lacking. 158 Interestingly, the structure and tight junctions utilized by the overlying lymphoepithelium are uniquely specialized in each site, with pseudostratified ciliated columnar epithelium found in the adenoid and stratified squamous epithelium predominating in tonsillar tissue.159 While not as clearly delineated as in the gut, epithelial cells with some immunohistological and electron microscopic characteristics of M-cells are reported in human tonsils and adenoid159, 160, although experimental evidence for their role as antigen transporting cells in the nasopharynx has only been described in mice.161 Furthermore, it appears that the classic immunohistologic markers for M cells in the gut, namely vimentin and cytokeratins 8 and 18, are not specific markers for M cells in the upper airway, thus complicating efforts to study the antigen transporting characteristics of these cells.162 Other lymphoid aggregates are also found in the human larynx epithelium (termed laryngeal associate lymphoid tissue LALT) and have been shown to persist into adulthood.163
In the lower airway, bronchial-associated lymphoid tissue (BALT) is found rarely in the lungs of human fetuses without the presence of intrauterine infection164, 165 or healthy adults without a history of pulmonary disease.166, 167 This situation is unlike that in rats where BALT is found in germ-free pups as early as four days after birth along bifurcations of the upper bronchi. BALT in humans was frequently found homogenously distributed in the lungs of children (36–44%) who died of sudden infant death syndrome or trauma, suggesting that development of BALT in humans results from strong antigenic stimulation.168 It was also found in adults following infection in the setting of chronic inflammation from smoking166 or occlusion of the airway by tumor.169 Regardless of the differences in organization and density of NALT and BALT in humans, palatine tonsils, and possibly the adenoid, are thought to be highly efficient sites of antigen uptake and inductive sites for airway specific humoral immune responses. A previous study compared intratonsillar vaccination and intranasal vaccination of patients who were about to undergo a tonsillectomy with peroral and parenteral vaccination with tetanus and cholera toxin. They demonstrated that the intratonsillar and intranasal routes were both efficient inducers of systemic specific antibodies but mucosal immunity was conferred only to the upper airway, possibly due to preferential use of adhesion molecules and chemokines among upper airway mucosal B cells. Activation and induction of NALT and BALT is also prominently found in inflammatory airway disease and is further discussed in the following sections.2, 167
Immunoglobulins in the human airway and humoral immunodeficiencies
In humans, IgA is the major immunoglobulin of the healthy upper and lower respiratory tract, but, unlike the lower gastrointestinal tract, IgD and IgG represent approximately 25% of nasal immunoglobulins.170 Humans, unlike mice, express two forms of IgA- in airway secretions; the IgA1 subclass is predominant at both sites, but nasal airway secretions typically have less IgA2 than bronchial secretions.48 In humans, IgD comprises approximately 10% of nasally produced immunoglobulins, although secretion into the small intestine is minimal.170 While first discovered over 50 years ago, IgD was until recently recognized largely for its presence on naïve B cells. However, recent findings demonstrate that secreted IgD is produced largely by IgD+IgM− B cells with a plasmablast phenotype found in the human nasal mucosa and tonsils.65 Interestingly, IgM-to-IgD class switching occurs via a non-canonical mechanism and can occur both in a T-dependent or T-independent mechanism requiring AID. The secreted IgD antibodies are frequently polyreactive and recognize respiratory bacteria such as Moraxella catarrhalis and Haemophilus influenza, but, surprisingly, have a high rate of autoreactivity.171 Furthermore, IgD has interesting properties that enable it to interface between the adaptive and innate immune responses as it binds to peripheral basophils and tonsillar mast cells via an as yet undescribed receptor and is capable of triggering degranulation upon IgD crosslinking.65
The isotypes, quantity and transport of immunoglobulins into the airway each play a critical role in maintaining the equilibrium between immunity and inflammation. Patients with selective IgA deficiency do suffer from marginally increased rates of infection but have a relatively modest compromise in respiratory immunity, possibly because IgM, IgG and IgD compensate in the absence of IgA.56 In common variable immune deficiency (CVID), affected individuals have mutations in one of several genes including BAFFR and TACI and consequently have decreased levels of IgA and IgG but often normal serum levels of IgM.172 Despite an intact transepithelial transport of IgM by pIgR, affected individuals have recurrent pneumonia, otitis media, sinusitis and septicemia with particularly impaired immunity against encapsulated bacteria such as Haemophilus influenzae, Streptococcus pneumoniae and Staphylococcus aureus.173, 174 Paradoxically, these patients also exhibit higher rates of autoimmune diseases such as immune thrombocytic purpura and autoimmune hemolytic anemia. More profound demonstrations of the critical role immunoglobulins play in airway immunity can be found in the clinical manifestations of X-linked agammaglobulinemia (XLA) where a nearly complete absence of immunoglobulins causes a profound increase in respiratory infections beginning in infants once the effects of maternally derived IgG fade.91
Conversely, conditions resulting in the over production of specific isotypes of immunoglobulins also have a significant impact on airway health. The X-linked hyper-IgM (HIGM) syndrome is caused by a defect in the CD40L gene that results in normal to increased levels of serum IgM and low to undetectable IgG, IgD, IgA and IgE.175 Affected individuals have increased susceptibility to upper respiratory tract infections and autoimmune manifestations in addition to opportunistic pulmonary infections by Pneumocystis carinii, which are likely secondary to the effects of CD40L deficiency on T-cell function. A different pattern of disease is found in the hyper-IgE (HIGE) syndrome, which results from mutations in STAT3, wherein affected individuals have eczema, mucocutaneous candidiasis, recurrent staphylococcal abscesses of the skin, lungs and viscera along with elevated serum IgE concentrations.174, 176 These immunodeficiencies likely culminate from the critical role of STAT3 signaling in the differentiation and generation of memory T and B cells.177–179 Finally, in the hyper-IgD (HIGD) syndromes, patients have lifelong recurrent episodes of systemic inflammation and periodic attacks of aphthous ulcers and pharyngitis in some subsets of HIGD. Recent insights into the role of IgD in upper airway secretions demonstrated that patients with HIGD have increased numbers of IgD secreting B cells and increased numbers of “IgD-armed” basophils suggesting possible triggers for the periodic inflammatory episodes associated with HIGD.65
B-lymphocytes in chronic diseases of the lower airway
While classically associated with antibody production, B lymphocytes serve additional roles as antigen-presenting cells and sources of both inflammatory and regulatory cytokines180 - perhaps illustrative of the pleiotropic roles of B cells as effectors and regulators of the humoral immune response. B cell responses and airway-produced antibodies are also associated with pathology in a number of inflammatory diseases of the lower airway such as asthma, hypersensitivity pneumonitis, idiopathic fibrosing alveolitis, chronic obstructive pulmonary disease (COPD), sarcoidosis, autoimmune diseases and lung transplant rejection. (Table 4)
Table 4.
Evidence for B cell infiltrates and morbidity-associated specific antibodies in select airway disease
| Disease | Presence of B cell clusters | Morbidity associated antibody specifities | Ref. |
|---|---|---|---|
| Lower Airways | |||
| Asthma | Inconsistent | Allergen specific IgE Anti-collagen V |
182, 184, 247, 248 |
| Chronic Obstructive Pulmonary Disease | Yes, in adventitia of small airways | Anti-pulmonary epithelial cell, Anti-elastin, Anti-nuclear antibody | 192, 198, 201, 202, 249 |
| Chronic Transplant Rejection | Yes, peribronchiolar | Anti-HLA class I Anti-collagen V |
214, 250, 251 |
| Hypersensitivity Pneumonitis | Yes, peribronchiolar | Anti-avian (pigeon fancier’s disease) Rheumatoid factor |
187, 189, 190 |
| Idiopathic Pulmonary Fibrosis | Yes, around alveoli | Anti-epithelial Anti-annexin |
207, 209, 252 |
| Rheumatoid Arthritis | Yes, parenchymal | Locally anti-CCP in BAL | 186 |
| Sjogren’s disease | Yes, parenchymal | Anti-Ro/SSA, anti-La/SSB Anti-M3 muscarinic |
186, 206, 253 |
| Upper Airways | |||
| Allergic Rhinitis | Rare | Allergen specific IgE | 227, 254 |
| Nasal Polyposis | Yes, in nasal polyps | Allergen specific Ig, Staphylococcus aureus specific IgE Local Autoantibodies against dsDNA, BP180 and other antigens |
234, 240, 244 |
In mice sensitized by intratracheal OVA, ectopic germinal centers are found within the parenchyma of the inflamed lungs and OVA-specific immunoglobulin producing cells can be detected in the pulmonary tissue.67 These features are observed along with eosinophilia and epithelial basement membrane fibrosis classically found in asthma models.181 A recent study examined OVA-sensitized mice following aerosolized antigen challenge and found that pulmonary OVA exposure resulted in increases in the numbers of specific IgG and IgE producing pulmonary plasma cells.182 The plasma cells failed to persist following cessation of antigen exposure. In human asthma, reports on the prevalence of organized BALT and induction in lung tissue are inconsistent, although isolated clusters of B cells are frequently found in the lung biopsies of severe asthmatics.2, 183 It is unclear whether the majority of B cells found in the lungs of patients with asthma are sensitized within the secondary lymphatic organs such as bronchial lymph nodes and then traffic to the lungs, or if local activation, expansion and class switching occurs. Evidence for local class switch recombination and production of IgE is inferred by the detection of ε-circle transcripts, mRNA encoding the heavy chain of IgE and activation-induced cytidine deaminase (AID) in asthmatics compared to normal controls.184 In contrast to the IgE mediated responses in asthmatic disease, B lymphocyte responses resulting from chronic exposure to organic antigens, such as avian antigens in pigeon fanciers disease, can trigger hypersensitivity pneumonitis (HP).185 In HP, organized BALT containing B cell predominant follicles surrounded by a parafollicular T cell zone are frequently found in lung biopsies.186, 187 Bronchoalveolar lavage (BAL) from HP patients also demonstrates increased numbers of plasma cells that are temporally related to antigen exposure.188 Interestingly, while both patients with pigeon HP and asymptomatic pigeon breeders demonstrate elevated airway levels of anti-avian IgG and IgA antibodies189, patients with HP simultaneously express elevated levels of class-switched antibodies against self-IgG (or Rheumatoid Factor-RF). The levels of serum RF are similar to those found in rheumatoid arthritis190 and may be locally-produced in the lungs since titers were higher in BAL than serum.191 Together, these findings suggest that pulmonary challenge from inhaled antigen is capable of triggering a robust humoral response within the lung. However, the initiation of a pathogenic inflammatory response appears to depend on intrinsic factors of the antigen and yet unknown host susceptibility or secondary triggers. In addition, the persistence of the response, at least in HP, appears to require the continued presence of antigen.
Similar to findings in HP, smokers appear to generate a dichotomous response to inhaled tobacco combustion products in which patients with COPD have a vigorous inflammatory response in contrast with non-afflicted at-risk smokers.192 Unlike pigeon HP, in which humoral immune responses to the inhaled antigen are thought to be a dominant pathogenic event, COPD is thought to result from the proteolytic destruction of the lung extracellular matrix by products of neutrophils and macrophages.193, 194 While a pathogenic role for B cells in COPD is not yet established, analysis of the inflammatory response demonstrates dramatic increases, particularly in the numbers of B cells and lymphoid follicles found in the adventitia of small airways.195 The organization of these follicles has also been proposed to be mediated by the expression of CXCR3 by B cells and T cells along with its cognate ligands IP-10/CXCL10, Mig/CXCL9 and I-TAC/CXCL11, which are expressed by epithelial cells, endothelial cells and CD68+ macrophages surrounding these follicles.196 Comparing lung biopsies from late GOLD stage (Stage 2–4) to those from early GOLD (Stage 0–1) subjects, Hogg et al. showed five to seven fold higher numbers of B cells and lymphoid follicles192 that correlated with the airway obstruction and pulmonary parenchymal destruction associated with this disease. Van der Strate et al. characterized the B cells found in the follicles in COPD patients and found that the majority of B cells were post-germinal center CD27+, and further utilized laser-capture microdissection to demonstrate evidence for a oligoclonal, antigen specific response similar to their findings in smoking mice.197 A subsequent study examined the bronchial biopsies of 114 COPD patients and found increased numbers of subepithelial CD20+ cells in COPD patients compared to controls without COPD. There was also a correlation between more advanced GOLD stage disease and increased subepithelial CD20+ cells.198 In contrast to the study by Hogg, this study did not find a follicular organization of these B lymphocytes, although the caliber of airways examined, and the severity of lung disease in these two studies were not directly comparable. Similarly, increased numbers of subepithelial and periglandular plasma cells were found in the lung biopsies of smokers with chronic bronchitis compared with asymptomatic smokers. Interestingly, 69% of periglandular plasma cells expressed IL-4 and this was correlated with the number of PAS+ mucous glands, suggesting that gland associated plasma cells may play a role in the mucin hyper-secretion associated with chronic bronchitis.199 While the previously discussed evidence suggests an excess of B cells and plasma cells surrounding the airway, a recent study found that sIgA was actually decreased in the lungs of patients with COPD – particularly those with the most severe GOLD staging. In this study, the decrease in sIgA correlated with increased airway remodeling and decreased pIgR expression, suggesting that defective sIgA transport may be a factor in the defective mucosal immunity observed in COPD.200 Further evidence for a pathogenic role for airway B cells is found in the discovery of elevated levels of autoantibodies against elastin201 and pulmonary epithelial cells202 found in patients with COPD. ELIspot analysis of single cells isolated from the peripheral lung tissue of COPD patients confirmed the local production of anti-elastin antibodies within lung tissue, and these findings were associated with a decrease in the numbers of CD4+CD25+CD62L+ Treg cells in patients with emphysema.201 These results are intriguing in light of the known pathogenic role for proteolytic enzymes from macrophages and neutrophils in COPD.193 Together, these studies suggest that the proteolytic breakdown of the extracellular matrix secondary to cigarette-smoke activation may generate self epitopes for a pathogenic autoimmune humoral response. It is also likely in COPD that repeated bacterial colonization of the lower airways drives a profound antibody response and formation of tertiary lymphoid tissue in this disease. COPD is not the only chronic airway disease in which this scenario appears to play out (see below).
The autoimmune connective tissue diseases (CTD) are a heterogeneous group of systemic inflammatory disorders including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), systemic sclerosis (SSc) and Sjögren’s syndrome. Pulmonary inflammation in CTD has been well described, although the role of B cells in the pathogenesis of this type of inflammation is still largely unexplored.203 RA and Sjögren’s syndrome are frequently associated with the development of ectopic lymphoid tissue that is thought to be a site where high-affinity autoreactive B cell clones are generated.183 Interstitial lung disease is the most common pulmonary manifestation in RA, and lung biopsies from these patients consistently demonstrated the presence of BALT.186 Since BALT is not constitutively found in the normal human airway, Randall and colleagues describe these as inducible forms of BALT (iBALT). While iBALT exhibited the presence of HEV and characteristics of B cell follicles, including CXCL13 and BCL-6 expression (see above), the authors did not mention the presence of specialized lymphoepithelium or M cells in conjunction with iBALT. The presence of iBALT also correlated with anti-cyclic citrullinated peptide (anti-CCP) antibodies, but not rheumatoid factor, in bronchial alveolar lavage. Similarly, other studies examining the interstitial lung disease of SSc and Sjögren’s syndrome have demonstrated increasedBALT.204, 205 Studies of the follicular structures associated with Sjögren’s syndrome demonstrate B cell enriched follicles with increased expression of CXCL13 and CCL21. Immunohistochemistry with biotinylated recombinant Ro- and La- proteins also demonstrated increased numbers of autoantibodies reactive to these antigens being produced in these follicles.205 Other studies have reported the presence of salivary IgA anti-M3 muscarinic acetylcholine receptors in Sjögren’s syndrome patients with sicca symptoms.206 These salivary antibodies may provide a novel pathway by which secretory glandular dysfunction is propagated in the upper and lower airways.
Idiopathic pulmonary fibrosis (IPF), also called cryptogenic fibrosing alveolitis, is a chronic diffuse interstitial lung disease of unknown etiology characterized by enhanced collagen deposition in the lung parenchyma.207 While the etiology of this disease continues to be unknown, consistently implicated factors include inhaled irritants and toxins from cigarette smoke and viruses. DNA from Epstein Barr virus and herpes viruses is frequently detected in the lungs of patients with IPF, although its role in the pathogenesis, progression or even exacerbation in this disease remains unclear.208 Regardless of etiology, loosely organized B cell aggregates interacting with T cells and follicular dendritic cells are one of the more consistent histological aspects of this disease.209–211 However, it is still unclear whether these B cells are responsible for the generation of the numerous autoantibodies, both circulating and in BAL, which target multiple pulmonary epithelial autoantigens in IPF.207 That a pathogenic link between these autoantibodies and the fibrosis observed in IPF exists may be inferred by studies showing enhanced TGF-β and tenascin production by epithelial cells stimulated in vitro with isolated autoantibodies.212
Recent studies have implicated an increasing role for alloimmune stimulation in breaking self-tolerance leading to autoimmunity to self-antigens. Obliterative bronchiolitis (OB), a chronic inflammatory and fibroproliferative condition of the small airways, is a frequent manifestation of chronic rejection following lung transplantation. Sato et al. examined lung biopsies of patients who had received a lung transplant and found lymphoid neogenesis in lungs affected by chronic rejection.213 The lymphoid clusters had T and B cells, along with PNAd positive high endothelial venules. The authors then expanded on these findings in an animal model of OB in which they transplanted tracheal isograft or allografts into lung tissue and demonstrated that both iso- and allo- grafts triggered an intense lymphocytic infiltrate in the host lung seven days following transplantation. By day 28 however, the lymphocytic aggregates in the lung had largely resolved in those receiving isografts but persisted in those receiving allografts. The grafted tracheal epithelium of isografts remained intact while those of the allograft showed complete loss of epithelium and obliterative airway fibrosis that replicates some of the features of OB. Downstream effects of the humoral immune activation in lung transplantation appear to result in a loss of self tolerance and the generation of autoantibodies. Indeed, the presence of anti-HLA class I and collagen V autoantibodies seems to correlate with the development of OB.214
Reviewing the spectrum of B cell inflammatory responses in pulmonary disease reveals several intriguing similarities. In these diseases, pulmonary exposure to an antigen, whether inhaled as in asthma and HP, possibly viral in IPF, generated via proteolytic breakdown in COPD, introduced in OB from chronic transplant rejection, or a self antigen as seen in CTD, results in an exuberant B cell response. The B cells present in these conditions apparently undergo local activation with evidence for local class switching in asthma, or localized oligoclonal antigen-specific activation as seen in HP, COPD and CTD. These localized responses generate elevated systemic levels of antigen-specific antibodies associated with the loss of tolerance to self-antigens and the generation of autoantibodies in the most severe manifestations of these diseases. Despite these similarities, there remain several key aspects of the variable susceptibility to these pathogenic processes that remain unclear. For example, the responsible factors by which an inhaled antigen triggers asthma instead of HP and the underlying host factors or comorbid illness that cause certain pigeon fanciers and some smokers to develop iBALT even though unaffected breeders and unaffected smokers are exposed to similar antigenic challenge remain yet to be determined.
B-lymphocytes in chronic diseases of the upper airway
While studies of B cell mediated inflammation of the upper airways are rare compared to studies of the gut, and even the lower airway, evidence suggests that B cells play a prominent role in both the normal immune homeostasis and the inflammatory diseases of the nose and paranasal sinuses. Studies in the 1970’s by Brostoff, Gleich and others demonstrated that IgA- and IgE-expressing B cells are found in nasal airways and that these cells produce IgE and IgA specific to known inhaled antigens.215–217 Several studies have shown that levels of aeroallergen-specific IgE are much higher in the airways than in the serum when normalized to total IgE or albumin.216–222 It is not uncommon for individuals with allergic rhinitis or chronic rhinosinusitis (CRS) to manifest antigen-specific IgE in nasal secretions or nasal tissue with no apparent specific IgE in the serum for the same antigen, and documented cases exist in which nasal antigen challenge responses are elicited in individuals that lack skin test sensitivity.215, 217, 218, 220 A survey study found that 19% of patients with rhinitis and polyposis had specific IgE in the nose but not the serum.223 More recent studies, that used time resolved fluorescence immunosorbent assays to determine the relative proportions of total and specific immunoglobulins in the airways and circulation, concluded that the majority of the total body aeroallergen-specific antibodies of the IgE and IgA isotypes are produced in the airways, and that systemic sensitization largely reflects spillover of immunoglobulins from the mucosal site of their production into the circulation.224 However, early studies compared the efficacy of systemically administered live-attenuated versus inactivated measles vaccines in generating both systemic and nasal specific immune responses. They found that both types of vaccine efficiently generated a systemic IgG response and the attenuated virus almost universally generated nasal IgA antibodies, the inactivated vaccine only generated measurable nasal anti-measles antibodies in three of seven cases.225 Together, these studies suggest the nasal antibody armamentarium does not passively reflect that of the systemic circulation. Possible explanations for superior local responses with local vaccination include SLO-independent localized B cell class switching within nasal mucosa such as the Sδ-Sμ switch circles found constitutively in nasal mucosa. Moreover, elevated levels of ε-germline transcripts, ε-circle transcripts, and mRNA for AID are found in the nasal mucosa of symptomatic allergic patients.49 Other possible explanations include the preferential use of homing molecules in mucosal B cells such as CCR7 and lack of α4β7 as discussed earlier. A separate study by Cameron et al. showed that antigen exposure of allergic nasal mucosa resulted in a significant increase in local IL-4 production and increased IgE production as evidenced by expression of the Iε germline promoter site and ε-circle transcripts that was not seen in control mucosa from non-allergic individuals.226 These responses could be blocked by pre-treatment with the glucocorticoid fluticasone propionate. Indeed, examination of nasal tissue from patients with allergic rhinitis does demonstrate the presence of small B cell clusters in addition to elevated levels of B cells and IgE positive plasma cells, suggesting the presence of ectopic germinal centers where localized activation may occur.49, 227 Recently, it has been shown that eosinophils in the bone marrow are nearly indispensable for the generation of plasma cells in the bone marrow, a finding that begs the question of whether highly eosinophilic airways diseases such as hay fever, asthma or CRS (see below) sustain local plasma cell presence by a similar eosinophil mediated process.228
Several laboratories, including our own, have had an interest in the study of B lymphocytes in the context of CRS with nasal polyps (CRSwNP). In this disease, patients manifest vigorous chronic inflammatory responses in the nose and paranasal sinuses that result in obstructive polyp formation and olfactory loss. Multiple etiologies including triggering by staphylococcus superantigens, fungal antigens and, more recently, an epithelial barrier defect, have been proposed as possible inciting mechanisms.229, 230 Clinically, over half of patients with CRSwNP are multiply sensitized to inhaled allergens231 and a large proportion (19%) of patients with CRS demonstrate the presence of local IgE against aeroallergens without evidence of circulating IgE against the same antigens.223 Patients with CRSwNP also have significantly higher rates of asthma232, most clearly manifested in the most severe forms of CRSwNP, which include a triad with concurrent asthma and aspirin sensitivity. Published reports have demonstrated the occasional presence of germinal-center like follicles, high numbers of B cells and plasma cells, increased CXCL12 and CXCL13 and increased levels of IgA in nasal polyp tissue.2, 233, 234 An overview of the potential role of B lineage cells in CRS is presented in Figure 5. One possible mechanism driving the formation of ectopic lymphoid aggregates is the presence of high levels of BAFF mRNA and protein in nasal polyp tissue compared with control tissue.118 Furthermore, the expression of BAFF correlated with the expression of CD20 in nasal polyp tissue. It has also been demonstrated that BAFF expression is highly induced by the TLR3 ligand dsRNA and is synergistically enhanced by TLR3- and IFN-β-dependent signaling in airway epithelial cells.119, 235 In fact, levels of BAFF expression from airway epithelial cells are similar to those produced by dendritic cells and other myeloid cells,235, 236 suggesting that BAFF synthesis by epithelial cells may contribute to local accumulation, activation, CSR and immunoglobulin synthesis by B cells in the airways. Following activation and expansion by BAFF, another potential factor that may drive B cells to undergo plasma cell differentiation in sinonasal tissues is IL-6, which is also found to be elevated within nasal polyp tissue.144, 237 Recent studies have demonstrated that the number of plasma cells in nasal polyps actually exceeds the number of B cells by an order of magnitude, and these plasma cells are frequently of the CD38+ plasma blast phenotype.238 Another study examined the immunoglobulin isotypes expressed by nasal polyp plasma cells and found the locally present plasma cells expressed predominantly IgA and IgG, but IgE.239 Whether this profound plasma cell response is mechanistically driven by BAFF and IL-6 has not been established, but patients with nasal polyps do demonstrate an exuberant nasal antibody response. Locally elevated levels of IgA and IgE antibodies to antigens commonly encountered in the nose, such as staphylococcus aureus, dust mite and fungal elements240, 241 have been reported in nasal polyp tissue. Since BAFF is a potent stimulator of B cell proliferation and class switching in B cells242, and since mice engineered to overexpress this cytokine manifest intense autoimmunity243, Tan et al. recently evaluated sinonasal tissue from CRSwNP patients for the presence of autoantibodies. These studies revealed that elevated levels of several class-switched autoantibodies, particularly anti-dsDNA, are present within nasal polyp tissue.244 Echoing the findings in the lower airway discussed earlier, the most highly elevated levels of anti-dsDNA autoantibodies were observed in the nasal polyps obtained from patients undergoing repeated nasal surgery for recurrent and obstructive nasal polyposis. In these studies, the presence of elevated levels of specific antibody within nasal polyps was not paralleled by elevations of the same antibodies in the systemic circulation. These findings suggest that class switching and antibody production in the nasal airway may be uniquely compartmentalized from systemic immunity. Indeed, studies of nasal biopsies from patients with allergic rhinitis that were exposed to allergen ex vivo demonstrated local induction of IL-4 and IL-13, as well as elevated levels of sterile transcripts of the IgE and IgG isotype, indicating local CSR to form IgG and IgE-producing B cells. Together, these studies drive home the importance of localized, extra-lymphatic humoral responses found in the nasal airway.
Figure 5.
Overview of the role of B cells in chronic rhinosinusitis. Studies of the pathogenesis of the polypoid form of CRS have implicated B cells and several isotypes of immunoglobulins in the pathogenesis of this important and common disease. Elevated levels of B cells and plasma cells are established in CRS and are accompanied by elevated levels of the chemokines that attract them (including CXCL12 and CXCL13) as well as TSLP and BAFF. Formation of organized follicular structures occurs in some cases of CRS. Although the specificity of the IgG, IgA and IgE antibodies made in nasal polyp tissue is not completely known, recent evidence shows the presence of autoantibodies of the IgA and IgG isotypes. Cellular targets of immunoglobulins that are enriched in nasal polyps include eosinophils (which respond briskly to IgA activation), mast cells (which respond to IgE receptor crosslinking) and neutrophils (which respond to IgG immune complexes). Release of mediators by these cell types is likely to be important in the pathogenesis of the disease, including formation of nasal polyps.
Clinical implications
The structural variability, antigenic specificity, adaptable affinity and functional characteristics of immunoglobulins make them superb effectors of immune responses, both within tissues and at the body’s surfaces, as attested to by the enormous clinical and commercial success of immunoglobulins in therapy. The airways make an almost irresistible surface for the growth of bacteria and fungi, containing ample nutrients, an ideal temperature for growth and a moist substrate. Were it not for the coordinated action of innate and adaptive immune responses, our respiratory system would be defenseless, and rapidly overrun by these organisms. B lineage cells in the airway exhibit a remarkable diversity in function serving roles ranging from efficient immunoglobulin producing plasma cells to regulatory B cells. Conversely, they can be agents of disease when a susceptible host is exposed to excessive antigen or when inappropriate sensitization occurs to self or to innocuous material. In such cases, B cells and immunoglobulins can exert their same awe-inspiring toxicity toward the host with devastating results.
The research and principles discussed in this review highlight the need for an improved understanding of the mechanisms of recruitment, activation, differentiation and regulation of B lineage cells in health and disease in the airways. Numerous important questions are still left unanswered about these important cells and their involvement in airways diseases (Box 1). Are there specific molecular pathways by which B lineage cells are recruited to the sinuses, the upper airways or the lower airways, as opposed to the gastrointestinal tract or urogenital tract? Do naïve B cells regularly traffic through upper or lower airways as part of their life cycle? Are plasmablasts or plasma cells activated within the airways and then subsequently recruited to remote locations such as the bone marrow to be long lived antibody producers? Although there are indeed many unanswered questions, increased awareness of pathological B cell responses elevates the recognition of a need to develop strategies to prevent, diagnose, treat and manage B cell mediated chronic inflammatory airway diseases. Consequently, numerous drugs designed to target elements of the B cell lineage are presently available or under development (Table 3).
Box 1.
What we do know
Airway B cells are capable of activation and differentiation independent of secondary lymphoid organs.
The presence of B cells in airway inflammatory infiltrates correlates with disease severity in many chronic airways diseases.
B-cells in the airways produce antibodies against extrinsic antigens as well as autoantigens.
The presence of autoantibodies in several airways diseases correlates with poorer prognosis or more severe disease.
What we don’t know
Existence of airway specific B cell trafficking mechanisms.
The mechanisms driving class switch recombination of airway IgD+IgM- B cells.
The mechanisms of appearance of IgD in the airways.
The role that migration and local activation of naïve B cells plays in airways disease.
The mechanisms that drive IgG subclass class switch recombination in humans.
As in most areas of medicine, the role of genetic or epigenetic variation in B cell lineage pathways in disease susceptibility in the airways is unknown and likely to be important. In this framework, individuals at high risk for developing airway disease such as pigeon fanciers at risk for HP may be found to have certain susceptibility genetic polymorphisms or epigenetic marks. In our own studies, African Americans with the minor “A” allele of the rs17564816 BAFF SNP were implicated to be at increased risk for asthma exacerbations.245, It seems likely that pharmaceutical agents targeted at suppressing B cell activation will be found to be influenced by genetic and epigenetic variability. The success of Rituximab in treating refractory RA demonstrates the potential for B cell specific therapies in treating inflammatory conditions affecting the airways, but its rare potential for progressive multifocal leukoencephalopathy from the JC virus (fatal in 90% of affected individuals) is cautionary, particularly for airway diseases or disease stages where attributable mortality is low.246 It is self evident that any therapeutic strategies designed to prevent pathological B cell responses should do so without undermining their essential roles in immunity. In this context, and given the critical role of B cells in tolerance and immunologic memory, long-term management of chronic inflammatory airway diseases by mechanisms inducing immune tolerance to specific extrinsic antigens is a worthy goal along with direct pharmacologic suppression of autoimmune responses.
We hope that this review has illustrated the importance of the B lineage cell system in both host defense and disease within the respiratory system and that it will serve as a catalyst for the further study of B cells and plasma cells in the human respiratory system.
Acknowledgments
Funding: This research was supported in part by NIH grants, K23DC012067, R01 HL078860, R01 AI072570 and R37 HL068546 and by a grant from the Ernest S. Bazley Trust.
Abbreviations
- AID
Activation-induced cytidine deaminase
- APRIL
A proliferation-inducing ligand
- BAFF
B cell-activating factor of the TNF family
- BAL
Bronchoalveolar lavage
- BCMA
B cell maturation antigen
- BCR
B cell receptor
- CRS
Chronic rhinosinusitis
- CRSwNP
CRS with nasal polyps
- CSR
Class switch recombination
- CTD
Autoimmune connective tissue diseases
- CVID
Common variable immune deficiency
- EBI2
Epstein-Barr virus-induced molecule 2
- GC
Germinal centers
- HEV
High endothelial venules
- HP
Hypersensitivity pneumonitis
- iBALT
Inducible bronchus-associated lymphoid tissue
- IC
Immune complexes
- ILF
Isolated lymphoid follicles
- LN
Lymph nodes
- IPF
Idiopathic pulmonary fibrosis
- IRF4
Interferon regulatory factor 4
- LALT
Laryngeal associate lymphoid tissue
- MALT
Mucosal-associated lymphoid tissues
- MZ
Marginal zone
- NALT
Nasopharynx-associated lymphoid tissue
- NIK
NF-κB-induced kinase
- OB
Obliterative bronchiolitis
- SC
Secretory component
- SHM
Somatic hypermutation
- SLE
Systemic lupus erythematosus
- SLO
Secondary lymphoid organs
- TACI
Transmembrane activator and CAML interactor
- Tfh
Follicular helper cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Salvi S, Holgate ST. Could the airway epithelium play an important role in mucosal immunoglobulin A production? Clin Exp Allergy. 1999;29:1597–605. doi: 10.1046/j.1365-2222.1999.00644.x. [DOI] [PubMed] [Google Scholar]
- 2.Drolet JP, Frangie H, Guay J, Hajoui O, Hamid Q, Mazer BD. B lymphocytes in inflammatory airway diseases. Clin Exp Allergy. 2010;40:841–9. doi: 10.1111/j.1365-2222.2010.03512.x. [DOI] [PubMed] [Google Scholar]
- 3.Wilkes DS, Twigg HL., 3rd B-lymphocytes in the lung: a topic to be revisited. Sarcoidosis Vasc Diffuse Lung Dis. 2001;18:34–49. [PubMed] [Google Scholar]
- 4.Brandtzaeg P. Potential of nasopharynx-associated lymphoid tissue for vaccine responses in the airways. Am J Respir Crit Care Med. 2011;183:1595–604. doi: 10.1164/rccm.201011-1783OC. [DOI] [PubMed] [Google Scholar]
- 5.Kurosaki T, Shinohara H, Baba Y. B cell signaling and fate decision. Annu Rev Immunol. 2010;28:21–55. doi: 10.1146/annurev.immunol.021908.132541. [DOI] [PubMed] [Google Scholar]
- 6.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat Rev Immunol. 2009;9:767–77. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- 7.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–85. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 8.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 9.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 10.Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002;16:67–76. doi: 10.1016/s1074-7613(01)00257-6. [DOI] [PubMed] [Google Scholar]
- 11.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 12.Dorner T, Kinnman N, Tak PP. Targeting B cells in immune-mediated inflammatory disease: a comprehensive review of mechanisms of action and identification of biomarkers. Pharmacol Ther. 2010;125:464–75. doi: 10.1016/j.pharmthera.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Choi YS, Baumgarth N. Dual role for B-1a cells in immunity to influenza virus infection. J Exp Med. 2008;205:3053–64. doi: 10.1084/jem.20080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin DO, Rothstein TL. Human b1 cell frequency: isolation and analysis of human b1 cells. Front Immunol. 2012;3:122. doi: 10.3389/fimmu.2012.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffin DO, Rothstein TL. A small CD11b(+) human B1 cell subpopulation stimulates T cells and is expanded in lupus. J Exp Med. 2011;208:2591–8. doi: 10.1084/jem.20110978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin DO, Rothstein TL. Human “Orchestrator” CD11b(+) B1 Cells Spontaneously Secrete Interleukin-10 and Regulate T-Cell Activity. Mol Med. 2012;18:1003–8. doi: 10.2119/molmed.2012.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inman CF, Murray TZ, Bailey M, Cose S. Most B cells in non-lymphoid tissues are naive. Immunol Cell Biol. 2011 doi: 10.1038/icb.2011.35. [DOI] [PubMed] [Google Scholar]
- 19.Carragher DM, Rangel-Moreno J, Randall TD. Ectopic lymphoid tissues and local immunity. Semin Immunol. 2008;20:26–42. doi: 10.1016/j.smim.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reif K, Ekland EH, Ohl L, Nakano H, Lipp M, Forster R, et al. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 2002;416:94–9. doi: 10.1038/416094a. [DOI] [PubMed] [Google Scholar]
- 21.Cyster JG. B cell follicles and antigen encounters of the third kind. Nat Immunol. 2010;11:989–96. doi: 10.1038/ni.1946. [DOI] [PubMed] [Google Scholar]
- 22.Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, et al. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. Control systems and decision making for antibody production. Nat Immunol. 2010;11:681–8. doi: 10.1038/ni.1900. [DOI] [PubMed] [Google Scholar]
- 24.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–14. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 26.Chan TD, Gatto D, Wood K, Camidge T, Basten A, Brink R. Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J Immunol. 2009;183:3139–49. doi: 10.4049/jimmunol.0901690. [DOI] [PubMed] [Google Scholar]
- 27.Inamine A, Takahashi Y, Baba N, Miyake K, Tokuhisa T, Takemori T, et al. Two waves of memory B-cell generation in the primary immune response. Int Immunol. 2005;17:581–9. doi: 10.1093/intimm/dxh241. [DOI] [PubMed] [Google Scholar]
- 28.Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med. 2006;203:1081–91. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan TD, Gardam S, Gatto D, Turner VM, Silke J, Brink R. In vivo control of B-cell survival and antigen-specific B-cell responses. Immunol Rev. 2010;237:90–103. doi: 10.1111/j.1600-065X.2010.00942.x. [DOI] [PubMed] [Google Scholar]
- 30.Gatto D, Wood K, Brink R. EBI2 operates independently of but in cooperation with CXCR5 and CCR7 to direct B cell migration and organization in follicles and the germinal center. J Immunol. 2011;187:4621–8. doi: 10.4049/jimmunol.1101542. [DOI] [PubMed] [Google Scholar]
- 31.Pereira JP, Kelly LM, Cyster JG. Finding the right niche: B-cell migration in the early phases of T-dependent antibody responses. Int Immunol. 2010;22:413–9. doi: 10.1093/intimm/dxq047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hannedouche S, Zhang J, Yi T, Shen W, Nguyen D, Pereira JP, et al. Oxysterols direct immune cell migration via EBI2. Nature. 2011;475:524–7. doi: 10.1038/nature10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu C, Yang XV, Wu J, Kuei C, Mani NS, Zhang L, et al. Oxysterols direct B-cell migration through EBI2. Nature. 2011;475:519–23. doi: 10.1038/nature10226. [DOI] [PubMed] [Google Scholar]
- 34.Shlomchik MJ, Weisel F. Germinal center selection and the development of memory B and plasma cells. Immunol Rev. 2012;247:52–63. doi: 10.1111/j.1600-065X.2012.01124.x. [DOI] [PubMed] [Google Scholar]
- 35.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 36.Gatto D, Brink R. The germinal center reaction. J Allergy Clin Immunol. 2010;126:898–907. doi: 10.1016/j.jaci.2010.09.007. quiz 8–9. [DOI] [PubMed] [Google Scholar]
- 37.Vinuesa CG, Linterman MA, Goodnow CC, Randall KL. T cells and follicular dendritic cells in germinal center B-cell formation and selection. Immunol Rev. 2010;237:72–89. doi: 10.1111/j.1600-065X.2010.00937.x. [DOI] [PubMed] [Google Scholar]
- 38.Beltman JB, Allen CD, Cyster JG, de Boer RJ. B cells within germinal centers migrate preferentially from dark to light zone. Proc Natl Acad Sci U S A. 2011;108:8755–60. doi: 10.1073/pnas.1101554108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnett BE, Ciocca ML, Goenka R, Barnett LG, Wu J, Laufer TM, et al. Asymmetric B cell division in the germinal center reaction. Science. 2012;335:342–4. doi: 10.1126/science.1213495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–63. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 41.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–92. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 43.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–34. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 44.Lund FE, Partida-Sanchez S, Lee BO, Kusser KL, Hartson L, Hogan RJ, et al. Lymphotoxin-alpha-deficient mice make delayed, but effective, T and B cell responses to influenza. J Immunol. 2002;169:5236–43. doi: 10.4049/jimmunol.169.9.5236. [DOI] [PubMed] [Google Scholar]
- 45.Lee BJ, Santee S, Von Gesjen S, Ware CF, Sarawar SR. Lymphotoxin-alpha-deficient mice can clear a productive infection with murine gammaherpesvirus 68 but fail to develop splenomegaly or lymphocytosis. J Virol. 2000;74:2786–92. doi: 10.1128/jvi.74.6.2786-2792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–17. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 47.McDonald KG, McDonough JS, Newberry RD. Adaptive immune responses are dispensable for isolated lymphoid follicle formation: antigen-naive, lymphotoxin- sufficient B lymphocytes drive the formation of mature isolated lymphoid follicles. J Immunol. 2005;174:5720–8. doi: 10.4049/jimmunol.174.9.5720. [DOI] [PubMed] [Google Scholar]
- 48.Cerutti A, Chen K, Chorny A. Immunoglobulin responses at the mucosal interface. Annu Rev Immunol. 2011;29:273–93. doi: 10.1146/annurev-immunol-031210-101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cameron L, Hamid Q, Wright E, Nakamura Y, Christodoulopoulos P, Muro S, et al. Local synthesis of epsilon germline gene transcripts, IL-4, and IL-13 in allergic nasal mucosa after ex vivo allergen exposure. J Allergy Clin Immunol. 2000;106:46–52. doi: 10.1067/mai.2000.107398. [DOI] [PubMed] [Google Scholar]
- 50.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–17. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 51.Tezuka H, Abe Y, Iwata M, Takeuchi H, Ishikawa H, Matsushita M, et al. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–33. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- 52.Brandtzaeg P. Mucosal immunity: induction, dissemination, and effector functions. Scand J Immunol. 2009;70:505–15. doi: 10.1111/j.1365-3083.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- 53.Corthesy B. Role of secretory immunoglobulin A and secretory component in the protection of mucosal surfaces. Future Microbiol. 2010;5:817–29. doi: 10.2217/fmb.10.39. [DOI] [PubMed] [Google Scholar]
- 54.Kaetzel CS, Robinson JK, Chintalacharuvu KR, Vaerman JP, Lamm ME. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function for IgA. Proc Natl Acad Sci U S A. 1991;88:8796–800. doi: 10.1073/pnas.88.19.8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tomasi TB, Jr, Tan EM, Solomon A, Prendergast RA. Characteristics of an Immune System Common to Certain External Secretions. J Exp Med. 1965;121:101–24. doi: 10.1084/jem.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johansen FE, Baekkevold ES, Carlsen HS, Farstad IN, Soler D, Brandtzaeg P. Regional induction of adhesion molecules and chemokine receptors explains disparate homing of human B cells to systemic and mucosal effector sites: dispersion from tonsils. Blood. 2005;106:593–600. doi: 10.1182/blood-2004-12-4630. [DOI] [PubMed] [Google Scholar]
- 57.Sun K, Johansen FE, Eckmann L, Metzger DW. An important role for polymeric Ig receptor-mediated transport of IgA in protection against Streptococcus pneumoniae nasopharyngeal carriage. J Immunol. 2004;173:4576–81. doi: 10.4049/jimmunol.173.7.4576. [DOI] [PubMed] [Google Scholar]
- 58.Tjarnlund A, Rodriguez A, Cardona PJ, Guirado E, Ivanyi J, Singh M, et al. Polymeric IgR knockout mice are more susceptible to mycobacterial infections in the respiratory tract than wild-type mice. Int Immunol. 2006;18:807–16. doi: 10.1093/intimm/dxl017. [DOI] [PubMed] [Google Scholar]
- 59.Crago SS, Prince SJ, Kulhavy R, Mestecky J. Molecular-cellular interactions in the secretory IgA system. Adv Exp Med Biol. 1978;107:209–17. doi: 10.1007/978-1-4684-3369-2_25. [DOI] [PubMed] [Google Scholar]
- 60.Czerkinsky C, Prince SJ, Michalek SM, Jackson S, Russell MW, Moldoveanu Z, et al. IgA antibody-producing cells in peripheral blood after antigen ingestion: evidence for a common mucosal immune system in humans. Proc Natl Acad Sci U S A. 1987;84:2449–53. doi: 10.1073/pnas.84.8.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brandtzaeg P, Kiyono H, Pabst R, Russell MW. Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 2008;1:31–7. doi: 10.1038/mi.2007.9. [DOI] [PubMed] [Google Scholar]
- 62.Kiyono H, Fukuyama S. NALT- versus Peyer’s-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quiding-Jarbrink M, Granstrom G, Nordstrom I, Holmgren J, Czerkinsky C. Induction of compartmentalized B-cell responses in human tonsils. Infect Immun. 1995;63:853–7. doi: 10.1128/iai.63.3.853-857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ogra PL. Effect of tonsillectomy and adenoidectomy on nasopharyngeal antibody response to poliovirus. N Engl J Med. 1971;284:59–64. doi: 10.1056/NEJM197101142840201. [DOI] [PubMed] [Google Scholar]
- 65.Chen K, Xu W, Wilson M, He B, Miller NW, Bengten E, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol. 2009;10:889–98. doi: 10.1038/ni.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Westermann J, Pabst R. How organ-specific is the migration of ‘naive’ and ‘memory’ T cells? Immunol Today. 1996;17:278–82. doi: 10.1016/0167-5699(96)80545-7. [DOI] [PubMed] [Google Scholar]
- 67.Chvatchko Y, Kosco-Vilbois MH, Herren S, Lefort J, Bonnefoy JY. Germinal center formation and local immunoglobulin E (IgE) production in the lung after an airway antigenic challenge. J Exp Med. 1996;184:2353–60. doi: 10.1084/jem.184.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moyron-Quiroz JE, Rangel-Moreno J, Hartson L, Kusser K, Tighe MP, Klonowski KD, et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 2006;25:643–54. doi: 10.1016/j.immuni.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 69.GeurtsvanKessel CH, Willart MA, Bergen IM, van Rijt LS, Muskens F, Elewaut D, et al. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med. 2009;206:2339–49. doi: 10.1084/jem.20090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rangel-Moreno J, Moyron-Quiroz JE, Hartson L, Kusser K, Randall TD. Pulmonary expression of CXC chemokine ligand 13, CC chemokine ligand 19, and CC chemokine ligand 21 is essential for local immunity to influenza. Proc Natl Acad Sci U S A. 2007;104:10577–82. doi: 10.1073/pnas.0700591104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cyster JG. Homing of antibody secreting cells. Immunol Rev. 2003;194:48–60. doi: 10.1034/j.1600-065x.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 72.Goya S, Matsuoka H, Mori M, Morishita H, Kida H, Kobashi Y, et al. Sustained interleukin-6 signalling leads to the development of lymphoid organ-like structures in the lung. J Pathol. 2003;200:82–7. doi: 10.1002/path.1321. [DOI] [PubMed] [Google Scholar]
- 73.Vuillemenot BR, Rodriguez JF, Hoyle GW. Lymphoid tissue and emphysema in the lungs of transgenic mice inducibly expressing tumor necrosis factor-alpha. Am J Respir Cell Mol Biol. 2004;30:438–48. doi: 10.1165/rcmb.2003-0062OC. [DOI] [PubMed] [Google Scholar]
- 74.Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–56. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith KG, Clatworthy MR. FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat Rev Immunol. 2010;10:328–43. doi: 10.1038/nri2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fairfax KA, Kallies A, Nutt SL, Tarlinton DM. Plasma cell development: from B-cell subsets to long-term survival niches. Semin Immunol. 2008;20:49–58. doi: 10.1016/j.smim.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 77.Oracki SA, Walker JA, Hibbs ML, Corcoran LM, Tarlinton DM. Plasma cell development and survival. Immunol Rev. 2010;237:140–59. doi: 10.1111/j.1600-065X.2010.00940.x. [DOI] [PubMed] [Google Scholar]
- 78.Hirokawa S, Sato H, Kato I, Kudo A. EBF-regulating Pax5 transcription is enhanced by STAT5 in the early stage of B cells. Eur J Immunol. 2003;33:1824–9. doi: 10.1002/eji.200323974. [DOI] [PubMed] [Google Scholar]
- 79.Kwon H, Thierry-Mieg D, Thierry-Mieg J, Kim HP, Oh J, Tunyaplin C, et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009;31:941–52. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol. 2007;19:711–20. doi: 10.1016/j.coi.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31:412–24. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 82.Mackay F, Figgett WA, Saulep D, Lepage M, Hibbs ML. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev. 2010;237:205–25. doi: 10.1111/j.1600-065X.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- 83.Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell. 2004;117:787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 84.Cancro MP. Signalling crosstalk in B cells: managing worth and need. Nat Rev Immunol. 2009;9:657–61. doi: 10.1038/nri2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lazarus AH, Kawauchi K, Rapoport MJ, Delovitch TL. Antigen-induced B lymphocyte activation involves the p21ras and ras. GAP signaling pathway. J Exp Med. 1993;178:1765–9. doi: 10.1084/jem.178.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.King LB, Freedman BD. B-lymphocyte calcium influx. Immunol Rev. 2009;231:265–77. doi: 10.1111/j.1600-065X.2009.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rickert RC. Regulation of B lymphocyte activation by complement C3 and the B cell coreceptor complex. Curr Opin Immunol. 2005;17:237–43. doi: 10.1016/j.coi.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 88.Harwood NE, Batista FD. Early events in B cell activation. Annu Rev Immunol. 2010;28:185–210. doi: 10.1146/annurev-immunol-030409-101216. [DOI] [PubMed] [Google Scholar]
- 89.Kurosaki T. Regulation of B-cell signal transduction by adaptor proteins. Nat Rev Immunol. 2002;2:354–63. doi: 10.1038/nri801. [DOI] [PubMed] [Google Scholar]
- 90.Yusuf I, Zhu X, Kharas MG, Chen J, Fruman DA. Optimal B-cell proliferation requires phosphoinositide 3-kinase-dependent inactivation of FOXO transcription factors. Blood. 2004;104:784–7. doi: 10.1182/blood-2003-09-3071. [DOI] [PubMed] [Google Scholar]
- 91.Conley ME, Dobbs AK, Farmer DM, Kilic S, Paris K, Grigoriadou S, et al. Primary B cell immunodeficiencies: comparisons and contrasts. Annu Rev Immunol. 2009;27:199–227. doi: 10.1146/annurev.immunol.021908.132649. [DOI] [PubMed] [Google Scholar]
- 92.van Zelm MC, Smet J, Adams B, Mascart F, Schandene L, Janssen F, et al. CD81 gene defect in humans disrupts CD19 complex formation and leads to antibody deficiency. J Clin Invest. 2010;120:1265–74. doi: 10.1172/JCI39748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eibel H, Salzer U, Warnatz K. Common variable immunodeficiency at the end of a prospering decade: towards novel gene defects and beyond. Curr Opin Allergy Clin Immunol. 2010;10:526–33. doi: 10.1097/ACI.0b013e32833fea1c. [DOI] [PubMed] [Google Scholar]
- 94.Alinari L, Christian B, Baiocchi RA. Novel targeted therapies for mantle cell lymphoma. Oncotarget. 2012;3:203–11. doi: 10.18632/oncotarget.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Buch MH, Emery P. New therapies in the management of rheumatoid arthritis. Curr Opin Rheumatol. 2011;23:245–51. doi: 10.1097/BOR.0b013e3283454124. [DOI] [PubMed] [Google Scholar]
- 96.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kantarjian H, le Coutre P, Cortes J, Pinilla-Ibarz J, Nagler A, Hochhaus A, et al. Phase 1 study of INNO-406, a dual Abl/Lyn kinase inhibitor, in Philadelphia chromosome-positive leukemias after imatinib resistance or intolerance. Cancer. 2010;116:2665–72. doi: 10.1002/cncr.25079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee L, Niu H, Goelzer P, Rueger R, Deutsch J, Busse-Reid R, et al. The safety, tolerability, pharmacokinetics, and pharmacodynamics of single oral doses of RO5068760, an MEK inhibitor, in healthy volunteers: assessment of target suppression. J Clin Pharmacol. 2010;50:1397–405. doi: 10.1177/0091270010361254. [DOI] [PubMed] [Google Scholar]
- 99.Leijen S, Middleton MR, Tresca P, Kraeber-Bodere F, Dieras V, Scheulen ME, et al. Phase I dose-escalation study of the safety, pharmacokinetics, and pharmacodynamics of the MEK inhibitor RO4987655 (CH4987655) in patients with advanced solid tumors. Clin Cancer Res. 2012;18:4794–805. doi: 10.1158/1078-0432.CCR-12-0868. [DOI] [PubMed] [Google Scholar]
- 100.Liu L, Halladay JS, Shin Y, Wong S, Coraggio M, La H, et al. Significant species difference in amide hydrolysis of GDC-0834, a novel potent and selective Bruton’s tyrosine kinase inhibitor. Drug Metab Dispos. 2011;39:1840–9. doi: 10.1124/dmd.111.040840. [DOI] [PubMed] [Google Scholar]
- 101.Pal SK, Reckamp K, Yu H, Figlin RA. Akt inhibitors in clinical development for the treatment of cancer. Expert Opin Investig Drugs. 2010;19:1355–66. doi: 10.1517/13543784.2010.520701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Patel SP, Lazar AJ, Papadopoulos NE, Liu P, Infante JR, Glass MR, et al. Clinical responses to selumetinib (AZD6244; ARRY-142886)-based combination therapy stratified by gene mutations in patients with metastatic melanoma. Cancer. 2012 doi: 10.1002/cncr.27790. [DOI] [PubMed] [Google Scholar]
- 103.Singh D, Smyth L, Borrill Z, Sweeney L, Tal-Singer R. A randomized, placebo-controlled study of the effects of the p38 MAPK inhibitor SB-681323 on blood biomarkers of inflammation in COPD patients. J Clin Pharmacol. 2010;50:94–100. doi: 10.1177/0091270009347873. [DOI] [PubMed] [Google Scholar]
- 104.Vansteenkiste J, Ramlau R, von Pawel J, San Antonio B, Eschbach C, Szczesna A, et al. A phase II randomized study of cisplatin-pemetrexed plus either enzastaurin or placebo in chemonaive patients with advanced non-small cell lung cancer. Oncology. 2012;82:25–9. doi: 10.1159/000335268. [DOI] [PubMed] [Google Scholar]
- 105.Chen D, Frezza M, Schmitt S, Kanwar J, Dou QP. Bortezomib as the first proteasome inhibitor anticancer drug: current status and future perspectives. Curr Cancer Drug Targets. 2011;11:239–53. doi: 10.2174/156800911794519752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–72. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grammer AC, Lipsky PE. CD154-CD40 interactions mediate differentiation to plasma cells in healthy individuals and persons with systemic lupus erythematosus. Arthritis Rheum. 2002;46:1417–29. doi: 10.1002/art.10287. [DOI] [PubMed] [Google Scholar]
- 108.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–63. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 109.Vale AM, Schroeder HW., Jr Clinical consequences of defects in B-cell development. J Allergy Clin Immunol. 2010;125:778–87. doi: 10.1016/j.jaci.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chatzigeorgiou A, Lyberi M, Chatzilymperis G, Nezos A, Kamper E. CD40/CD40L signaling and its implication in health and disease. Biofactors. 2009;35:474–83. doi: 10.1002/biof.62. [DOI] [PubMed] [Google Scholar]
- 111.Chalermskulrat W, McKinnon KP, Brickey WJ, Neuringer IP, Park RC, Sterka DG, et al. Combined donor specific transfusion and anti-CD154 therapy achieves airway allograft tolerance. Thorax. 2006;61:61–7. doi: 10.1136/thx.2005.047316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khan WN. B cell receptor and BAFF receptor signaling regulation of B cell homeostasis. J Immunol. 2009;183:3561–7. doi: 10.4049/jimmunol.0800933. [DOI] [PubMed] [Google Scholar]
- 113.Warnatz K, Salzer U, Rizzi M, Fischer B, Gutenberger S, Bohm J, et al. B-cell activating factor receptor deficiency is associated with an adult-onset antibody deficiency syndrome in humans. Proc Natl Acad Sci U S A. 2009;106:13945–50. doi: 10.1073/pnas.0903543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mackay F, Schneider P. TACI, an enigmatic BAFF/APRIL receptor, with new unappreciated biochemical and biological properties. Cytokine Growth Factor Rev. 2008;19:263–76. doi: 10.1016/j.cytogfr.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 115.He B, Santamaria R, Xu W, Cols M, Chen K, Puga I, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol. 2010;11:836–45. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yong PF, Thaventhiran JE, Grimbacher B. “A rose is a rose is a rose,” but CVID is Not CVID common variable immune deficiency (CVID), what do we know in 2011? Adv Immunol. 2011;111:47–107. doi: 10.1016/B978-0-12-385991-4.00002-7. [DOI] [PubMed] [Google Scholar]
- 117.Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev. 2011;244:115–33. doi: 10.1111/j.1600-065X.2011.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2008;121:1385–92. 92, e1–2. doi: 10.1016/j.jaci.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kato A, Xiao H, Chustz RT, Liu MC, Schleimer RP. Local release of B cell- activating factor of the TNF family after segmental allergen challenge of allergic subjects. J Allergy Clin Immunol. 2009;123:369–75. doi: 10.1016/j.jaci.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Polverino F, Baraldo S, Bazzan E, Agostini S, Turato G, Lunardi F, et al. A novel insight into adaptive immunity in chronic obstructive pulmonary disease: B cell activating factor belonging to the tumor necrosis factor family. Am J Respir Crit Care Med. 2010;182:1011–9. doi: 10.1164/rccm.200911-1700OC. [DOI] [PubMed] [Google Scholar]
- 121.Bilsborough J, Chadwick E, Mudri S, Ye X, Henderson WR, Jr, Waggie K, et al. TACI-Ig prevents the development of airway hyperresponsiveness in a murine model of asthma. Clin Exp Allergy. 2008;38:1959–68. doi: 10.1111/j.1365-2222.2008.03099.x. [DOI] [PubMed] [Google Scholar]
- 122.Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–31. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 123.Stohl W. Biologic differences between various inhibitors of the BLyS/BAFF pathway: should we expect differences between belimumab and other inhibitors in development? Curr Rheumatol Rep. 2012;14:303–9. doi: 10.1007/s11926-012-0254-6. [DOI] [PubMed] [Google Scholar]
- 124.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 125.Chiron D, Bekeredjian-Ding I, Pellat-Deceunynck C, Bataille R, Jego G. Toll-like receptors: lessons to learn from normal and malignant human B cells. Blood. 2008;112:2205–13. doi: 10.1182/blood-2008-02-140673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dorner M, Brandt S, Tinguely M, Zucol F, Bourquin JP, Zauner L, et al. Plasma cell toll-like receptor (TLR) expression differs from that of B cells, and plasma cell TLR triggering enhances immunoglobulin production. Immunology. 2009;128:573–9. doi: 10.1111/j.1365-2567.2009.03143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kelly-Scumpia KM, Scumpia PO, Weinstein JS, Delano MJ, Cuenca AG, Nacionales DC, et al. B cells enhance early innate immune responses during bacterial sepsis. J Exp Med. 2011;208:1673–82. doi: 10.1084/jem.20101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–51. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 129.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–7. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 130.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–7. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Marshak-Rothstein A, Rifkin IR. Immunologically active autoantigens: the role of toll-like receptors in the development of chronic inflammatory disease. Annu Rev Immunol. 2007;25:419–41. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- 132.Swigris JJ, Fischer A, Gillis J, Meehan RT, Brown KK. Pulmonary and thrombotic manifestations of systemic lupus erythematosus. Chest. 2008;133:271–80. doi: 10.1378/chest.07-0079. [DOI] [PubMed] [Google Scholar]
- 133.Gao H, Neff T, Ward PA. Regulation of lung inflammation in the model of IgG immune-complex injury. Annu Rev Pathol. 2006;1:215–42. doi: 10.1146/annurev.pathol.1.110304.100155. [DOI] [PubMed] [Google Scholar]
- 134.Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008;26:261–92. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fawaz LM, Sharif-Askari E, Hajoui O, Soussi-Gounni A, Hamid Q, Mazer BD. Expression of IL-9 receptor alpha chain on human germinal center B cells modulates IgE secretion. J Allergy Clin Immunol. 2007;120:1208–15. doi: 10.1016/j.jaci.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 136.Avery DT, Bryant VL, Ma CS, de Waal Malefyt R, Tangye SG. IL-21-induced isotype switching to IgG and IgA by human naive B cells is differentially regulated by IL-4. J Immunol. 2008;181:1767–79. doi: 10.4049/jimmunol.181.3.1767. [DOI] [PubMed] [Google Scholar]
- 137.Avery DT, Ma CS, Bryant VL, Santner-Nanan B, Nanan R, Wong M, et al. STAT3 is required for IL-21-induced secretion of IgE from human naive B cells. Blood. 2008;112:1784–93. doi: 10.1182/blood-2008-02-142745. [DOI] [PubMed] [Google Scholar]
- 138.Kopp MV. Omalizumab: Anti-IgE therapy in allergy. Curr Allergy Asthma Rep. 2011;11:101–6. doi: 10.1007/s11882-010-0173-4. [DOI] [PubMed] [Google Scholar]
- 139.Webb S. Attacks on asthma. Nat Biotechnol. 2011;29:860–3. doi: 10.1038/nbt.1994. [DOI] [PubMed] [Google Scholar]
- 140.Levine SJ, Wenzel SE. Narrative review: the role of Th2 immune pathway modulation in the treatment of severe asthma and its phenotypes. Ann Intern Med. 2010;152:232–7. doi: 10.1059/0003-4819-152-4-201002160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–98. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 142.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118:3546–56. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kishimoto T. Interleukin-6: from basic science to medicine--40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 144.Peters AT, Kato A, Zhang N, Conley DB, Suh L, Tancowny B, et al. Evidence for altered activity of the IL-6 pathway in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2010;125:397–403. e10. doi: 10.1016/j.jaci.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fizazi K, De Bono JS, Flechon A, Heidenreich A, Voog E, Davis NB, et al. Randomised phase II study of siltuximab (CNTO 328), an anti-IL-6 monoclonal antibody, in combination with mitoxantrone/prednisone versus mitoxantrone/prednisone alone in metastatic castration-resistant prostate cancer. Eur J Cancer. 2012;48:85–93. doi: 10.1016/j.ejca.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 146.Malin S, McManus S, Busslinger M. STAT5 in B cell development and leukemia. Curr Opin Immunol. 2010;22:168–76. doi: 10.1016/j.coi.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 147.Konforte D, Simard N, Paige CJ. IL-21: an executor of B cell fate. J Immunol. 2009;182:1781–7. doi: 10.4049/jimmunol.0803009. [DOI] [PubMed] [Google Scholar]
- 148.Maurer MF, Garrigues U, Jaspers SR, Meengs B, Rixon MW, Stevens BL, et al. Generation and characterization of human anti-human IL-21 neutralizing monoclonal antibodies. MAbs. 2012;4:69–83. doi: 10.4161/mabs.4.1.18713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 150.Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A. 2010;107:14292–7. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Maarof G, Bouchet-Delbos L, Gary-Gouy H, Durand-Gasselin I, Krzysiek R, Dalloul A. Interleukin-24 inhibits the plasma cell differentiation program in human germinal center B cells. Blood. 2010;115:1718–26. doi: 10.1182/blood-2009-05-220251. [DOI] [PubMed] [Google Scholar]
- 152.Liu Z, Zou Y, Davidson A. Plasma cells in systemic lupus erythematosus: the long and short of it all. Eur J Immunol. 2011;41:588–91. doi: 10.1002/eji.201041354. [DOI] [PubMed] [Google Scholar]
- 153.Dorner T, Radbruch A, Burmester GR. B-cell-directed therapies for autoimmune disease. Nat Rev Rheumatol. 2009;5:433–41. doi: 10.1038/nrrheum.2009.141. [DOI] [PubMed] [Google Scholar]
- 154.Amoroso A, Hafsi S, Militello L, Russo AE, Soua Z, Mazzarino MC, et al. Understanding rituximab function and resistance: implications for tailored therapy. Front Biosci. 2011;16:770–82. doi: 10.2741/3719. [DOI] [PubMed] [Google Scholar]
- 155.Donvik KK, Omdal R. Churg-Strauss syndrome successfully treated with rituximab. Rheumatol Int. 2011;31:89–91. doi: 10.1007/s00296-009-1146-6. [DOI] [PubMed] [Google Scholar]
- 156.Debertin AS, Tschernig T, Tonjes H, Kleemann WJ, Troger HD, Pabst R. Nasal-associated lymphoid tissue (NALT): frequency and localization in young children. Clin Exp Immunol. 2003;134:503–7. doi: 10.1111/j.1365-2249.2003.02311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bienenstock J, McDermott MR. Bronchus- and nasal-associated lymphoid tissues. Immunol Rev. 2005;206:22–31. doi: 10.1111/j.0105-2896.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- 158.Park HS, Francis KP, Yu J, Cleary PP. Membranous cells in nasal-associated lymphoid tissue: a portal of entry for the respiratory mucosal pathogen group A streptococcus. J Immunol. 2003;171:2532–7. doi: 10.4049/jimmunol.171.5.2532. [DOI] [PubMed] [Google Scholar]
- 159.Ogasawara N, Kojima T, Go M, Takano K, Kamekura R, Ohkuni T, et al. Epithelial barrier and antigen uptake in lymphoepithelium of human adenoids. Acta Otolaryngol. 2011;131:116–23. doi: 10.3109/00016489.2010.520022. [DOI] [PubMed] [Google Scholar]
- 160.Verbrugghe P, Kujala P, Waelput W, Peters PJ, Cuvelier CA. Clusterin in human gut-associated lymphoid tissue, tonsils, and adenoids: localization to M cells and follicular dendritic cells. Histochem Cell Biol. 2008;129:311–20. doi: 10.1007/s00418-007-0369-4. [DOI] [PubMed] [Google Scholar]
- 161.Kim DY, Sato A, Fukuyama S, Sagara H, Nagatake T, Kong IG, et al. The airway antigen sampling system: respiratory M cells as an alternative gateway for inhaled antigens. J Immunol. 2011;186:4253–62. doi: 10.4049/jimmunol.0903794. [DOI] [PubMed] [Google Scholar]
- 162.Koshi R, Mustafa Y, Perry ME. Vimentin, cytokeratin 8 and cytokeratin 18 are not specific markers for M-cells in human palatine tonsils. J Anat. 2001;199:663–74. doi: 10.1046/j.1469-7580.2001.19960663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hiller AS, Tschernig T, Kleemann WJ, Pabst R. Bronchus-associated lymphoid tissue (BALT) and larynx-associated lymphoid tissue (LALT) are found at different frequencies in children, adolescents and adults. Scand J Immunol. 1998;47:159–62. doi: 10.1046/j.1365-3083.1998.00276.x. [DOI] [PubMed] [Google Scholar]
- 164.Gould SJ, Isaacson PG. Bronchus-associated lymphoid tissue (BALT) in human fetal and infant lung. J Pathol. 1993;169:229–34. doi: 10.1002/path.1711690209. [DOI] [PubMed] [Google Scholar]
- 165.Heier I, Malmstrom K, Sajantila A, Lohi J, Makela M, Jahnsen FL. Characterisation of bronchus-associated lymphoid tissue and antigen-presenting cells in central airway mucosa of children. Thorax. 2010;66:151–6. doi: 10.1136/thx.2010.149591. [DOI] [PubMed] [Google Scholar]
- 166.Richmond I, Pritchard GE, Ashcroft T, Avery A, Corris PA, Walters EH. Bronchus associated lymphoid tissue (BALT) in human lung: its distribution in smokers and non-smokers. Thorax. 1993;48:1130–4. doi: 10.1136/thx.48.11.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tschernig T, Pabst R. Bronchus-associated lymphoid tissue (BALT) is not present in the normal adult lung but in different diseases. Pathobiology. 2000;68:1–8. doi: 10.1159/000028109. [DOI] [PubMed] [Google Scholar]
- 168.Tschernig T, Kleemann WJ, Pabst R. Bronchus-associated lymphoid tissue (BALT) in the lungs of children who had died from sudden infant death syndrome and other causes. Thorax. 1995;50:658–60. doi: 10.1136/thx.50.6.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Delventhal S, Brandis A, Ostertag H, Pabst R. Low incidence of bronchus-associated lymphoid tissue (BALT) in chronically inflamed human lungs. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;62:271–4. doi: 10.1007/BF02899692. [DOI] [PubMed] [Google Scholar]
- 170.Brandtzaeg P, Farstad IN, Johansen FE, Morton HC, Norderhaug IN, Yamanaka T. The B-cell system of human mucosae and exocrine glands. Immunol Rev. 1999;171:45–87. doi: 10.1111/j.1600-065X.1999.tb01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zheng NY, Wilson K, Wang X, Boston A, Kolar G, Jackson SM, et al. Human immunoglobulin selection associated with class switch and possible tolerogenic origins for C delta class-switched B cells. J Clin Invest. 2004;113:1188–201. doi: 10.1172/JCI20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cunningham-Rundles C, Ponda PP. Molecular defects in T- and B-cell primary immunodeficiency diseases. Nat Rev Immunol. 2005;5:880–92. doi: 10.1038/nri1713. [DOI] [PubMed] [Google Scholar]
- 173.Cunningham-Rundles C. Lung disease, antibodies and other unresolved issues in immune globulin therapy for antibody deficiency. Clin Exp Immunol. 2009;157 (Suppl 1):12–6. doi: 10.1111/j.1365-2249.2009.03952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Buckley RH. Pulmonary complications of primary immunodeficiencies. Paediatr Respir Rev. 2004;5 (Suppl A):S225–33. doi: 10.1016/s1526-0542(04)90043-7. [DOI] [PubMed] [Google Scholar]
- 175.Jesus AA, Duarte AJ, Oliveira JB. Autoimmunity in hyper-IgM syndrome. J Clin Immunol. 2008;28 (Suppl 1):S62–6. doi: 10.1007/s10875-008-9171-x. [DOI] [PubMed] [Google Scholar]
- 176.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357:1608–19. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 177.Meyer-Bahlburg A, Renner ED, Rylaarsdam S, Reichenbach J, Schimke LF, Marks A, et al. Heterozygous signal transducer and activator of transcription 3 mutations in hyper-IgE syndrome result in altered B-cell maturation. J Allergy Clin Immunol. 2012;129:559–62. 62, e1–2. doi: 10.1016/j.jaci.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 178.Siegel AM, Heimall J, Freeman AF, Hsu AP, Brittain E, Brenchley JM, et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity. 2011;35:806–18. doi: 10.1016/j.immuni.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Speckmann C, Enders A, Woellner C, Thiel D, Rensing-Ehl A, Schlesier M, et al. Reduced memory B cells in patients with hyper IgE syndrome. Clin Immunol. 2008;129:448–54. doi: 10.1016/j.clim.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 180.Cunningham-Rundles C. Autoimmune manifestations in common variable immunodeficiency. J Clin Immunol. 2008;28 (Suppl 1):S42–5. doi: 10.1007/s10875-008-9182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Blyth DI, Pedrick MS, Savage TJ, Hessel EM, Fattah D. Lung inflammation and epithelial changes in a murine model of atopic asthma. Am J Respir Cell Mol Biol. 1996;14:425–38. doi: 10.1165/ajrcmb.14.5.8624247. [DOI] [PubMed] [Google Scholar]
- 182.Luger EO, Fokuhl V, Wegmann M, Abram M, Tillack K, Achatz G, et al. Induction of long-lived allergen-specific plasma cells by mucosal allergen challenge. J Allergy Clin Immunol. 2009;124:819–26. e4. doi: 10.1016/j.jaci.2009.06.047. [DOI] [PubMed] [Google Scholar]
- 183.Randall TD. Bronchus-associated lymphoid tissue (BALT) structure and function. Adv Immunol. 2010;107:187–241. doi: 10.1016/B978-0-12-381300-8.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Takhar P, Corrigan CJ, Smurthwaite L, O’Connor BJ, Durham SR, Lee TH, et al. Class switch recombination to IgE in the bronchial mucosa of atopic and nonatopic patients with asthma. J Allergy Clin Immunol. 2007;119:213–8. doi: 10.1016/j.jaci.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 185.McSharry C. B lymphocytes in allergic alveolitis. Clin Exp Allergy. 2003;33:159–62. doi: 10.1046/j.1365-2222.2003.01603.x. [DOI] [PubMed] [Google Scholar]
- 186.Rangel-Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, Randall TD. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest. 2006;116:3183–94. doi: 10.1172/JCI28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Suda T, Chida K, Hayakawa H, Imokawa S, Iwata M, Nakamura H, et al. Development of bronchus-associated lymphoid tissue in chronic hypersensitivity pneumonitis. Chest. 1999;115:357–63. doi: 10.1378/chest.115.2.357. [DOI] [PubMed] [Google Scholar]
- 188.Drent M, van Velzen-Blad H, Diamant M, Wagenaar SS, Donckerwolck-Bogaert M, van den Bosch JM. Differential diagnostic value of plasma cells in bronchoalveolar lavage fluid. Chest. 1993;103:172–4. doi: 10.1378/chest.103.6.1720. [DOI] [PubMed] [Google Scholar]
- 189.Aguilar Leon DE, Novelo Retana V, Martinez-Cordero E. Anti-avian antibodies and rheumatoid factor in pigeon hypersensitivity pneumonitis. Clin Exp Allergy. 2003;33:22–32. doi: 10.1046/j.1365-2222.2003.01526.x. [DOI] [PubMed] [Google Scholar]
- 190.Araiza MT, Aguilar Leon DE, Retana VN, Martinez-Cordero E. IgM, IgG, and IgA rheumatoid factors in pigeon hypersensitivity pneumonitis. J Clin Lab Anal. 2007;21:315–21. doi: 10.1002/jcla.20188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Martinez-Cordero E, Negrete-Garcia MC, Mendoza A. Rheumatoid factor activity in serum and bronchoalveolar lavage fluid from patients with acute hypersensitivity pneumonitis. J Investig Allergol Clin Immunol. 1992;2:254–7. [PubMed] [Google Scholar]
- 192.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–53. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 193.Churg A, Zay K, Shay S, Xie C, Shapiro SD, Hendricks R, et al. Acute cigarette smoke-induced connective tissue breakdown requires both neutrophils and macrophage metalloelastase in mice. Am J Respir Cell Mol Biol. 2002;27:368–74. doi: 10.1165/rcmb.4791. [DOI] [PubMed] [Google Scholar]
- 194.Saetta M, Turato G, Facchini FM, Corbino L, Lucchini RE, Casoni G, et al. Inflammatory cells in the bronchial glands of smokers with chronic bronchitis. Am J Respir Crit Care Med. 1997;156:1633–9. doi: 10.1164/ajrccm.156.5.9701081. [DOI] [PubMed] [Google Scholar]
- 195.Bosken CH, Hards J, Gatter K, Hogg JC. Characterization of the inflammatory reaction in the peripheral airways of cigarette smokers using immunocytochemistry. Am Rev Respir Dis. 1992;145:911–7. doi: 10.1164/ajrccm/145.4_Pt_1.911. [DOI] [PubMed] [Google Scholar]
- 196.Kelsen SG, Aksoy MO, Georgy M, Hershman R, Ji R, Li X, et al. Lymphoid follicle cells in chronic obstructive pulmonary disease overexpress the chemokine receptor CXCR3. Am J Respir Crit Care Med. 2009;179:799–805. doi: 10.1164/rccm.200807-1089OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.van der Strate BW, Postma DS, Brandsma CA, Melgert BN, Luinge MA, Geerlings M, et al. Cigarette smoke-induced emphysema: A role for the B cell? Am J Respir Crit Care Med. 2006;173:751–8. doi: 10.1164/rccm.200504-594OC. [DOI] [PubMed] [Google Scholar]
- 198.Gosman MM, Willemse BW, Jansen DF, Lapperre TS, van Schadewijk A, Hiemstra PS, et al. Increased number of B-cells in bronchial biopsies in COPD. Eur Respir J. 2006;27:60–4. doi: 10.1183/09031936.06.00007005. [DOI] [PubMed] [Google Scholar]
- 199.Zhu J, Qiu Y, Valobra M, Qiu S, Majumdar S, Matin D, et al. Plasma cells and IL-4 in chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:1125–33. doi: 10.1164/rccm.200602-161OC. [DOI] [PubMed] [Google Scholar]
- 200.Polosukhin VV, Cates JM, Lawson WE, Zaynagetdinov R, Milstone AP, Massion PP, et al. Bronchial secretory immunoglobulin a deficiency correlates with airway inflammation and progression of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;184:317–27. doi: 10.1164/rccm.201010-1629OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13:567–9. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 202.Feghali-Bostwick CA, Gadgil AS, Otterbein LE, Pilewski JM, Stoner MW, Csizmadia E, et al. Autoantibodies in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:156–63. doi: 10.1164/rccm.200701-014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Crestani B. The respiratory system in connective tissue disorders. Allergy. 2005;60:715–34. doi: 10.1111/j.1398-9995.2005.00761.x. [DOI] [PubMed] [Google Scholar]
- 204.Lafyatis R, O’Hara C, Feghali-Bostwick CA, Matteson E. B cell infiltration in systemic sclerosis-associated interstitial lung disease. Arthritis Rheum. 2007;56:3167–8. doi: 10.1002/art.22847. [DOI] [PubMed] [Google Scholar]
- 205.Salomonsson S, Jonsson MV, Skarstein K, Brokstad KA, Hjelmstrom P, Wahren-Herlenius M, et al. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjogren’s syndrome. Arthritis Rheum. 2003;48:3187–201. doi: 10.1002/art.11311. [DOI] [PubMed] [Google Scholar]
- 206.Berra A, Sterin-Borda L, Bacman S, Borda E. Role of salivary IgA in the pathogenesis of Sjogren syndrome. Clin Immunol. 2002;104:49–57. doi: 10.1006/clim.2002.5228. [DOI] [PubMed] [Google Scholar]
- 207.Borchers AT, Chang C, Keen CL, Gershwin ME. Idiopathic pulmonary fibrosis-an epidemiological and pathological review. Clin Rev Allergy Immunol. 2011;40:117–34. doi: 10.1007/s12016-010-8211-5. [DOI] [PubMed] [Google Scholar]
- 208.Pulkkinen V, Salmenkivi K, Kinnula VL, Sutinen E, Halme M, Hodgson U, et al. A novel screening method detects herpesviral DNA in the idiopathic pulmonary fibrosis lung. Annals of medicine. 2011 doi: 10.3109/07853890.2010.532151. [DOI] [PubMed] [Google Scholar]
- 209.Wallace WA, Howie SE, Krajewski AS, Lamb D. The immunological architecture of B-lymphocyte aggregates in cryptogenic fibrosing alveolitis. J Pathol. 1996;178:323–9. doi: 10.1002/(SICI)1096-9896(199603)178:3<323::AID-PATH467>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 210.Campbell DA, Poulter LW, Janossy G, du Bois RM. Immunohistological analysis of lung tissue from patients with cryptogenic fibrosing alveolitis suggesting local expression of immune hypersensitivity. Thorax. 1985;40:405–11. doi: 10.1136/thx.40.6.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Marchal-Somme J, Uzunhan Y, Marchand-Adam S, Valeyre D, Soumelis V, Crestani B, et al. Cutting edge: nonproliferating mature immune cells form a novel type of organized lymphoid structure in idiopathic pulmonary fibrosis. J Immunol. 2006;176:5735–9. doi: 10.4049/jimmunol.176.10.5735. [DOI] [PubMed] [Google Scholar]
- 212.Wallace WA, Howie SE. Upregulation of tenascin and TGFbeta production in a type II alveolar epithelial cell line by antibody against a pulmonary auto-antigen. J Pathol. 2001;195:251–6. doi: 10.1002/path.916. [DOI] [PubMed] [Google Scholar]
- 213.Sato A, Chida K, Iwata M, Hayakawa H. Study of bronchus-associated lymphoid tissue in patients with diffuse panbronchiolitis. Am Rev Respir Dis. 1992;146:473–8. doi: 10.1164/ajrccm/146.2.473. [DOI] [PubMed] [Google Scholar]
- 214.Seetharam A, Tiriveedhi V, Mohanakumar T. Alloimmunity and autoimmunity in chronic rejection. Curr Opin Organ Transplant. 2010;15:531–6. doi: 10.1097/MOT.0b013e32833b31f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Huggins KG, Brostoff J. Local production of specific IgE antibodies in allergic-rhinitis patients with negative skin tests. Lancet. 1975;2:148–50. doi: 10.1016/s0140-6736(75)90056-2. [DOI] [PubMed] [Google Scholar]
- 216.Nakajima S, Gillespie DN, Gleich GJ. Differences between IgA and IgE as secretory proteins. Clin Exp Immunol. 1975;21:306–17. [PMC free article] [PubMed] [Google Scholar]
- 217.Merrett TG, Houri M, Mayer AL, Merrett J. Measurement of specific IgE antibodies in nasal secretion-evidence for local production. Clin Allergy. 1976;6:69–73. doi: 10.1111/j.1365-2222.1976.tb01413.x. [DOI] [PubMed] [Google Scholar]
- 218.Deuschl H, Johansson SG. Specific IgE antibodies in nasal secretion from patients with allergic rhinitis and with negative or weakly positive RAST on the serum. Clin Allergy. 1977;7:195–202. doi: 10.1111/j.1365-2222.1977.tb01442.x. [DOI] [PubMed] [Google Scholar]
- 219.Platts-Mills TA. Local production of IgG, IgA and IgE antibodies in grass pollen hay fever. J Immunol. 1979;122:2218–25. [PubMed] [Google Scholar]
- 220.Small P, Barrett D, Frenkiel S, Rochon L, Cohen C, Black M. Local specific IgE production in nasal polyps associated with negative skin tests and serum RAST. Ann Allergy. 1985;55:736–9. [PubMed] [Google Scholar]
- 221.Jones E, Frenkiel S, Small P, Rochon L. Immunopathological characteristics of nasal polyps. J Otolaryngol. 1987;16:19–22. [PubMed] [Google Scholar]
- 222.Hoffmann-Sommergruber K, Ferreira ED, Ebner C, Barisani T, Korninger L, Kraft D, et al. Detection of allergen-specific IgE in tears of grass pollen-allergic patients with allergic rhinoconjunctivitis. Clin Exp Allergy. 1996;26:79–87. doi: 10.1111/j.1365-2222.1996.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 223.Shatkin JS, Delsupehe KG, Thisted RA, Corey JP. Mucosal allergy in the absence of systemic allergy in nasal polyposis and rhinitis: a meta-analysis. Otolaryngol Head Neck Surg. 1994;111:553–6. doi: 10.1177/019459989411100503. [DOI] [PubMed] [Google Scholar]
- 224.Yoshida T, Usui A, Kusumi T, Inafuku S, Sugiyama T, Koide N, et al. A quantitative analysis of cedar pollen-specific immunoglobulins in nasal lavage supported the local production of specific IgE, not of specific IgG. Microbiol Immunol. 2005;49:529–34. doi: 10.1111/j.1348-0421.2005.tb03758.x. [DOI] [PubMed] [Google Scholar]
- 225.Bellanti JA, Sanga RL, Klutinis B, Brandt B, Artenstein MS. Antibody responses in serum and nasal secretions of children immunized with inactivated and attenuated measles-virus vaccines. N Engl J Med. 1969;280:628–33. doi: 10.1056/NEJM196903202801202. [DOI] [PubMed] [Google Scholar]
- 226.Cameron LA, Durham SR, Jacobson MR, Masuyama K, Juliusson S, Gould HJ, et al. Expression of IL-4, Cepsilon RNA, and Iepsilon RNA in the nasal mucosa of patients with seasonal rhinitis: effect of topical corticosteroids. J Allergy Clin Immunol. 1998;101:330–6. doi: 10.1016/s0091-6749(98)70244-1. [DOI] [PubMed] [Google Scholar]
- 227.KleinJan A, Vinke JG, Severijnen LW, Fokkens WJ. Local production and detection of (specific) IgE in nasal B-cells and plasma cells of allergic rhinitis patients. Eur Respir J. 2000;15:491–7. doi: 10.1034/j.1399-3003.2000.15.11.x. [DOI] [PubMed] [Google Scholar]
- 228.Chu VT, Frohlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol. 2011;12:151–9. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- 229.Tan BK, Schleimer RP, Kern RC. Perspectives on the etiology of chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2010;18:21–6. doi: 10.1097/MOO.0b013e3283350053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Tieu DD, Kern RC, Schleimer RP. Alterations in epithelial barrier function and host defense responses in chronic rhinosinusitis. J Allergy Clin Immunol. 2009;124:37–42. doi: 10.1016/j.jaci.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tan BK, Zirkle W, Chandra RK, Lin D, Conley DB, Peters AT, et al. Atopic profile of patients failing medical therapy for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2011;1:88–94. doi: 10.1002/alr.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Settipane GA, Chafee FH. Nasal polyps in asthma and rhinitis. A review of 6,037 patients. J Allergy Clin Immunol. 1977;59:17–21. doi: 10.1016/0091-6749(77)90171-3. [DOI] [PubMed] [Google Scholar]
- 233.Patadia M, Dixon J, Conley D, Chandra R, Peters A, Suh LA, et al. Evaluation of the presence of B-cell attractant chemokines in chronic rhinosinusitis. Am J Rhinol Allergy. 2009 doi: 10.2500/ajra.2010.24.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Van Zele T, Gevaert P, Holtappels G, van Cauwenberge P, Bachert C. Local immunoglobulin production in nasal polyposis is modulated by superantigens. Clin Exp Allergy. 2007;37:1840–7. doi: 10.1111/j.1365-2222.2007.02838.x. [DOI] [PubMed] [Google Scholar]
- 235.Kato A, Truong-Tran AQ, Scott AL, Matsumoto K, Schleimer RP. Airway epithelial cells produce B cell-activating factor of TNF family by an IFN-beta-dependent mechanism. J Immunol. 2006;177:7164–72. doi: 10.4049/jimmunol.177.10.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–9. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ghaffar O, Lavigne F, Kamil A, Renzi P, Hamid Q. Interleukin-6 expression in chronic sinusitis: colocalization of gene transcripts to eosinophils, macrophages, T lymphocytes, and mast cells. Otolaryngol Head Neck Surg. 1998;118:504–11. doi: 10.1016/s0194-5998(98)70209-8. [DOI] [PubMed] [Google Scholar]
- 238.Hulse KE, Norton J, Harris K, Conley D, Chandra R, Kern R, et al. A New Method For Cell Isolation Reveals Elevated Numbers of T and B Lymphocytes In Sinonasal Tissue From Patients With Chronic Rhinosinusitis. J Allergy Clin Immunol. 2011;127:Ab121-Ab. [Google Scholar]
- 239.Sanchez-Segura A, Brieva JA, Rodriguez C. Regulation of immunoglobulin secretion by plasma cells infiltrating nasal polyps. Laryngoscope. 2000;110:1183–8. doi: 10.1097/00005537-200007000-00022. [DOI] [PubMed] [Google Scholar]
- 240.Sabirov A, Hamilton RG, Jacobs JB, Hillman DE, Lebowitz RA, Watts JD. Role of local immunoglobulin E specific for Alternaria alternata in the pathogenesis of nasal polyposis. Laryngoscope. 2008;118:4–9. doi: 10.1097/MLG.0b013e3181567a7a. [DOI] [PubMed] [Google Scholar]
- 241.Van Zele T, Gevaert P, Watelet JB, Claeys G, Holtappels G, Claeys C, et al. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol. 2004;114:981–3. doi: 10.1016/j.jaci.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 242.Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–9. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:1198–206. e1. doi: 10.1016/j.jaci.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kumar R, Williams LK, Kato A, Peterson EL, Favoreto S, Jr, Hulse K, et al. Genetic variation in B cell-activating factor of the TNF family (BAFF) and asthma exacerbations among African American subjects. J Allergy Clin Immunol. 2012;130:996–9. e6. doi: 10.1016/j.jaci.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Carson KR, Evens AM, Richey EA, Habermann TM, Focosi D, Seymour JF, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113:4834–40. doi: 10.1182/blood-2008-10-186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Liu M, Subramanian V, Christie C, Castro M, Mohanakumar T. Immune responses to self-antigens in asthma patients: clinical and immunopathological implications. Hum Immunol. 2012;73:511–6. doi: 10.1016/j.humimm.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Simpson BM, Custovic A, Simpson A, Hallam CL, Walsh D, Marolia H, et al. NAC Manchester Asthma and Allergy Study (NACMAAS): risk factors for asthma and allergic disorders in adults. Clin Exp Allergy. 2001;31:391–9. doi: 10.1046/j.1365-2222.2001.01050.x. [DOI] [PubMed] [Google Scholar]
- 249.Nunez B, Sauleda J, Anto JM, Julia MR, Orozco M, Monso E, et al. Anti-tissue antibodies are related to lung function in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183:1025–31. doi: 10.1164/rccm.201001-0029OC. [DOI] [PubMed] [Google Scholar]
- 250.Iwata T, Philipovskiy A, Fisher AJ, Presson RG, Jr, Chiyo M, Lee J, et al. Antitype V collagen humoral immunity in lung transplant primary graft dysfunction. J Immunol. 2008;181:5738–47. doi: 10.4049/jimmunol.181.8.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Sato M, Hirayama S, Hwang DM, Lara-Guerra H, Wagnetz D, Waddell TK, et al. The role of intrapulmonary de novo lymphoid tissue in obliterative bronchiolitis after lung transplantation. J Immunol. 2009;182:7307–16. doi: 10.4049/jimmunol.0803606. [DOI] [PubMed] [Google Scholar]
- 252.Kurosu K, Takiguchi Y, Okada O, Yumoto N, Sakao S, Tada Y, et al. Identification of annexin 1 as a novel autoantigen in acute exacerbation of idiopathic pulmonary fibrosis. J Immunol. 2008;181:756–67. doi: 10.4049/jimmunol.181.1.756. [DOI] [PubMed] [Google Scholar]
- 253.Salomonsson S, Wahren-Herlenius M. Local production of Ro/SSA and La/SSB autoantibodies in the target organ coincides with high levels of circulating antibodies in sera of patients with Sjogren’s syndrome. Scand J Rheumatol. 2003;32:79–82. doi: 10.1080/03009740310000076. [DOI] [PubMed] [Google Scholar]
- 254.Smurthwaite L, Durham SR. Local IgE synthesis in allergic rhinitis and asthma. Curr Allergy Asthma Rep. 2002;2:231–8. doi: 10.1007/s11882-002-0024-z. [DOI] [PubMed] [Google Scholar]