Abstract
Hypoalbuminemia predicts disability and mortality in patients with various illnesses and in the elderly. The association between serum albumin concentration at the time of listing for lung transplantation and the rate of death after lung transplantation is unknown. We examined 6808 adults who underwent lung transplantation in the United States between 2000 and 2008. We used Cox proportional hazard models and generalized additive models to examine multivariable-adjusted associations between serum albumin and the rate of death after transplantation. The median follow-up time was 2.7 years. Those with severe (0.5–2.9 g/dL) and mild hypoalbuminemia (3.0–3.6 g/dL) had posttransplant adjusted mortality rate ratios of 1.35 (95% CI: 1.12–1.62) and 1.15 (95% CI: 1.04–1.27), respectively. For each 0.5 g/dL decrease in serum albumin concentration the 1-year and overall mortality rate ratios were 1.48 (95% CI: 1.21–1.81) and 1.26 (95% CI: 1.11–1.43), respectively. The association between hypoalbuminemia and posttransplant mortality was strongest in recipients with cystic fibrosis and interstitial lung disease. Hypoalbuminemia is an independent risk factor for death after lung transplantation.
Keywords: Cystic fibrosis, hypoalbuminemia, interstitial lung disease, lung transplantation, prognosis
Introduction
Lung transplantation is an effective therapy for advanced lung diseases, such as cystic fibrosis (CF), the interstitial lung diseases (ILD) and chronic obstructive pulmonary disease (COPD) (1,2). However, a scarce donor supply restricts lung transplantation to only a fraction of potentially eligible patients. Therefore, each transplant center attempts to “achieve the best use of donated organs” (3) by carefully selecting candidates with acceptably low predicted postoperative risks of death. While the Lung Allocation Score (LAS) system has improved organ allocation in the United States (4,5), recent data suggest that lung transplant recipients are sicker at the time of transplant than in prior years (6), and that the posttransplant survival measure of the LAS is in fact a poor predictor of post-transplant survival (7). Novel predictors of posttransplant survival time may improve allocation strategies and foster investigations of risk-reducing interventions.
Hypoalbuminemia (serum albumin < 3.5 mg/dL) (8,9) is a marker of poor overall health with influences from protein energy malnutrition (10), systemic inflammation (11,12) and hepatic and renal disease (13,14). Studies have shown that hypoalbuminemia has strong predictive validity for mortality across a number of pulmonary (15-17) and non-pulmonary diseases (18-20) and in healthy older adults (21-24). However, the association between pretransplant hypoalbuminemia and the rate of death after lung transplantation has not been examined to our knowledge.
We therefore examined the association of the serum albumin level at the time of listing for lung transplantation with the rate of death in a nationwide sample of lung transplant recipients in the United States. We hypothesized that a lower serum albumin level would be associated with an increased rate of death early after transplantation after accounting for potential confounders.
Methods
Data sources and participants
The Institutional Review Board on Human Research at the Columbia University Medical Center approved this study (approved protocol number: AAAB5142). All data were supplied by the United Network for Organ Sharing (UNOS) as a standard transplant analysis and research file supplemented with a coded center identifier based on Organ Procurement and Transplantation Network (OPTN) data as of March 1, 2010, as previously described (25).
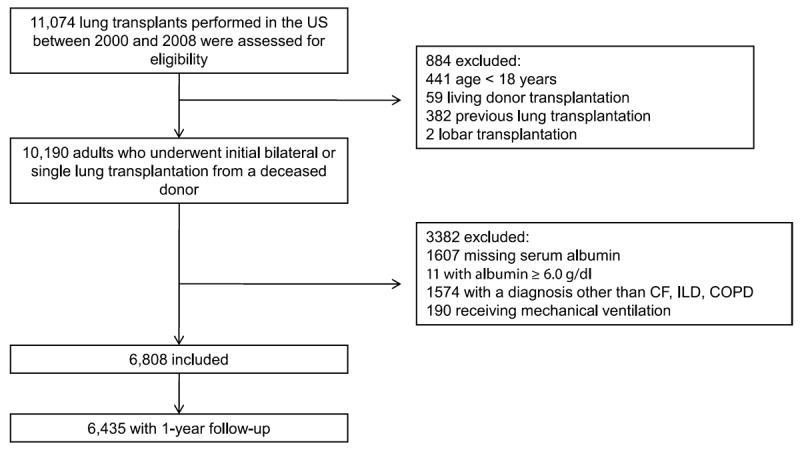
There were 11 074 lung transplants performed in the United States between January 1, 2000, and December 31, 2008. We excluded pediatric recipients (age < 18; n = 441) and anyone who had a living donor transplantation (n = 59), lobar transplantation (n = 2) or previous lung transplantation (n = 382). We further restricted the cohort to patients with ILD, COPD or CF (which account for more than 80% of lung transplant procedures in the United States) to minimize confounding by diagnosis. We sought to exclude patients with albumin < 0.5 g/dL (there were none) or albumin levels ≥ 6.0 g/dL (n = 11) because the standard assay for serum albumin, the bromcresol green (BCG) method (26), is inaccurate outside this range (9). Recipients with missing serum albumin were also excluded (n = 1607). We excluded 190 recipients receiving mechanical ventilation at the time of transplantation in the primary analysis because we believed it would be a key confounder of the association between serum albumin and the risk of death after lung transplantation, but we included and adjusted for mechanical ventilation in post hoc analyses. After all exclusions, 6808 recipients were included in the primary analysis, of whom, 6435 had 1-year follow-up (Figure 1).
Figure 1. Study participants.
Variables and statistical analyses
We categorized serum albumin concentration into five groups. We chose an albumin concentration of 4.0–5.0 g/dL as the reference range because this is the reported normal range for multiple commercial BCG assays (standard assay to measure albumin concentration), it includes the 3.5–5.0 g/dL normal range previously reported in the literature (9), and because prior studies have indicated substantial mortality risk in older persons falling below thresholds of 3.8 and 4.3 g/dL (23,24).
Severe hypoalbuminemia was defined as less than the 5th percentile of serum albumin in the cohort (0.5–2.9 g/dL). Mild hypoalbuminemia was defined as the 5th to 25th percentile (3.0–3.6 g/dL), and low-normal albumin was defined as the 25th to the 50th percentile (3.7–3.9 g/dL). Elevated albumin was defined as an albumin greater than 5.0 g/dL.
A generalized additive model (GAM) with loess smoothing functions for continuous variables was used to examine the linearity of the association between serum albumin and the odds of death after lung transplantation at 1 year after transplantation (27). The GAM allows for the flexible specification of the relationship between serum albumin and the risk of death and helps minimize misspecification of potential confounding variables (27).
We estimated hazard ratios for albumin as a continuous and categorical predictor of 1-year and overall mortality after transplantation (ignoring retransplantation) using multivariable Cox proportional hazard models. Follow-up was administratively right censored on March 1, 2010. We selected covariates available in the OPTN dataset for inclusion into the multivariable models that were either mechanically plausible confounders of the relationship between serum albumin and the rate of death (i.e. possibly linked to serum albumin and the risk of death) or solely associated with the risk of death (i.e. precision variables). Variables were retained regardless of their statistical significance in multivariable models. Implausible recipient and donor BMI values (<10 kg/m2 or >45 kg/m2) were replaced with missing values. The proportionality assumptions of the Cox model were verified by examining log(-log(survival time)) plots and by regressing the Schoenfeld residuals against time to test for independence between residuals and time.
We used multiple imputation with a Markov Chain Monte Carlo method to account for missing covariate values in our multivariable analyses, as previously described (25,28,29). Predicted survival curves and plots of continuous associations of albumin and the risk of death were generated from models using the missing indicator method (30) since these analyses cannot be performed on multiply imputed datasets.
We created mixed-effects multivariable Cox models with transplant center modeled as a random effect. We employed a hierarchical modeling approach as follows: model 1 was adjusted for recipient factors (serum albumin concentration at the time of transplant listing, age, sex, diagnosis percent predicted forced expiratory volume in 1 s (FEV1), diabetes mellitus, pretransplant steroid use, hospitalization at the time of transplant and BMI (categorized using the World Health Organization (WHO) classification as previously described (25)). Model 2 included model 1 covariates as well as donor factors (age, sex, height, BMI, presence of clinical or culture-confirmed bronchopulmonary infection noted on the OPTN Organ Procurement Form, smoking history of > 20 pack-years and cause of death. Model 3 included covariates from models 1 and 2 as well as transplantation procedure characteristics (single versus double lung transplant, allograft ischemic time and transplant era [dichotomized at May 4, 2005]).
The LAS was instituted on May 4, 2005 and hence missing LAS values depended on the date of transplantation and were not random. We therefore did not multiply impute LAS score and instead evaluated the association between serum albumin and mortality after transplantation adjusting for the LAS only in those with an available LAS score. We performed post hoc analyses adjusting for donor–recipient cytomegalovirus (CMV) exposure mismatch, use of mechanical ventilation at the time of lung transplantation, oxygen use (liters per minute), mean pulmonary artery pressure (mm Hg) and distance (feet) walked during a 6-min walk test at listing.
The population attributable fraction (PAF) is the proportion of deaths related to an exposure of interest (and unmeasured and poorly measured confounders) and represents the greatest possible proportional reduction in the number of deaths if the exposure of interest were eliminated from the population (31). The PAF for all recipients at 1 year was estimated using the fully adjusted multivariable Cox proportional hazard model (model 3).
We examined interactions between serum albumin level and both diagnosis and BMI using likelihood ratio tests. Statistical significance was defined as two-tailed p values less than 0.05. Analyses were performed with Stata 11.0 (Stata Corp LP, College Station, TX, USA) and the GAM function in R 2.8.1 (R Foundation, Vienna, Austria) (32).
Results
Of the 11 074 lung transplant procedures performed in the United States between 2000 and 2008, 6808 patients met our inclusion criteria and were included in analyses (Figure 1). There were 3323 single-lung transplant recipients (1991 with COPD, 1328 with ILD and 4 with CF), and 3485 double-lung transplant recipients (1475 with COPD, 970 with ILD and 1040 with CF). The median age was 57 years (interquartile range, 49–62 years), and 57% were men. The median BMI (interquartile range) for CF, COPD and ILD patients was 19.0 (17.7–21.0), 24.0 (21.1–27.1) and 27.4 (24.4–30.0) kg/m2, respectively. Among the 3408 who underwent lung transplantation under the LAS and had an LAS score, the median LAS score was 36.8 (interquartile range, 33.5–43.7). There was a median of 5 months (interquartile range, 1.5–13.5 months) between lung transplant listing and transplantation. Of the 6808 participants, 288 (4.2%) had severe hypoalbuminemia (0.5–2.9 g/dL), 1274 (19%) had mild hypoalbuminemia (3.0–3.6 g/d) and 1421 (21%) had a low-normal serum albumin concentration (3.7–3.9 g/dL) at the time of listing. Participants with hypoalbuminemia tended to be younger and were more likely to be female, have CF and diabetes, be hospitalized at the time of transplant, and undergo bilateral lung transplantation (Table 1).
Table 1.
Recipient, donor and procedure characteristics at the time of transplantation
| Number of patients with data available | Severe hypoalbuminemia 0.5–2.9 (g/dL) | Mild hypoalbuminemia 3.0–3.6 (g/dL) | Low-normal albumin 3.7–3.9 (g/dL) | Normal albumin 4.0–5.0 (g/dL) | Elevated albumin 5.1–5.9 (g/dL) | |
|---|---|---|---|---|---|---|
| Number transplanted | 6808 | 288 (4.2%) | 1274 (19%) | 1421 (21%) | 3752 (55%) | 73 (1.1%) |
| Recipient characteristics | ||||||
| Age, years | 6808 | 48 (28–60) | 55 (35–61) | 57 (48–62) | 58 (52–62) | 59 (55–62) |
| Male | 6583 | 160 (56%) | 672 (53%) | 793 (56%) | 2241 (60%) | 46 (63%) |
| Height, cm | 6613 | 167 (162–175) | 168 (161–175) | 170 (163–178) | 170 (163–178) | 173 (165–178) |
| Weight, kg | 6733 | 63.6 (52.6–77.0) | 66.1 (53.7–80.3) | 70.7 (58.1–82.9) | 71.7 (60.3–83.4) | 65.3 (55.5–80.2) |
| BMI, kg/m2 | 6608 | 22.2 (18.8–26.6) | 23.4 (19.6–27.7) | 24.4 (20.8–28.0) | 24.9 (21.5–28.1) | 22.6 (20.7–26.1) |
| FEV1,% predicted | 6403 | 27 (19–45) | 28 (20–46) | 28 (19–48) | 25 (18–48) | 21 (16–29) |
| FVC,% predicted | 6410 | 44 (33–55) | 43 (34–56) | 47 (37–70) | 49 (39–61) | 50 (38–63) |
| LAS1 | 3408 | 41.2 (36.0–53.2) | 39.4 (34.9–48) | 37.9 (33.8–45.8) | 35.5 (32.9–41.1) | 33.6 (32.4–34.9) |
| Hospitalized | 6808 | 61 (21%) | 139 (11%) | 102 (7.2%) | 215 (5.7%) | 3 (4.1%) |
| Corticosteroid use | 6455 | 130 (48%) | 662 (55%) | 706 (50%) | 1617 (45%) | 32 (46%) |
| Diabetes | 6750 | 78 (28%) | 286 (23%) | 192 (14%) | 364 (9.7%) | 6 (8.2%) |
| Pre-LAS | 2561 | 71 (25%) | 504 (40%) | 587 (41%) | 1373 (37%) | 26 (36%) |
| Post-LAS | 4247 | 217 (75%) | 770 (60%) | 834 (59%) | 2379 (63%) | 47 (64%) |
| Recipient diagnosis | ||||||
| ILD | 2298 | 92 (32%) | 479 (38%) | 535 (38%) | 1184 (32%) | 8 (11%) |
| COPD | 3466 | 71 (25%) | 393 (31%) | 645 (45%) | 2295 (62%) | 62 (85%) |
| CF | 1044 | 125 (43%) | 402 (32%) | 241 (17%) | 273 (73%) | 3 (4.1%) |
| Donor characteristics | ||||||
| Age, years | 6808 | 29 (29–44) | 30 (20–44) | 28 (20–43) | 30 (20–45) | 27 (19–38) |
| Male | 6808 | 174 (60%) | 731 (57%) | 897 (63%) | 2388 (64%) | 54 (7%) |
| Height, cm | 6793 | 173 (165–180) | 170 (165–178) | 173 (166–180) | 173 (167–180) | 175 (168–180) |
| Weight, kg | 6808 | 72.9 (62.1–84) | 72.6 (61.2–83) | 73 (63.6–83.9) | 73 (64–84.8) | 73 (60–82.8) |
| BMI, kg/m2 | 6779 | 24.4 (21.6–27.5) | 24.4 (21.6–27.6) | 24.2 (21.6–27.6) | 24.3 (21.7–27.2) | 24.2 (21.7–27.4) |
| Smoked (>20 pack-years) | 6768 | 50 (17%) | 271 (21%) | 269(19%) | 722 (19%) | 13 (18%) |
| Bronchopulm. infection | 6808 | 72 (25%) | 313 (25%) | 366 (26%) | 947 (25%) | 21 (29%) |
| Recipient cause of death | ||||||
| Head trauma | 3644 | 157 (56%) | 672 (54%) | 782 (56%) | 1980 (54%) | 53 (74%) |
| Cerebrovascular | 2387 | 97 (35%) | 449 (36%) | 473 (34%) | 1354 (37%) | 14 (19%) |
| Anoxic brain injury | 569 | 22 (7.8%) | 114 (9.2%) | 117 (8.4%) | 311 (8.5%) | 5 (6.9%) |
| CNS tumor | 61 | 5 (1.8%) | 6 (0.5%) | 18 (1.3%) | 32 (0.9%) | 0 (0%) |
| Missing | 147 | 7 (2.4%) | 33 (2.6%) | 31 (2.2%) | 75 (2.0%) | 1 (1.4%) |
| Procedure characteristics | ||||||
| Bilateral transplant | 6808 | 202 (70%) | 765 (60%) | 710 (50%) | 1773 (47%) | 35 (48%) |
| Ischemic time, hours | 6031 | 5.0 (4.0–6.1) | 5.0 (3.8–6.1) | 4.6 (3.6–5.8) | 4.5 (3.5–5.6) | 4.5 (3.3–5.3) |
Only recipients transplanted after May 4, 2005, have a Lung Allocation Score (LAS).
Percentages indicate the percentage of patients with a characteristic in a given category of serum albumin.
For the entire cohort, the median survival time was 5.0 years (interquartile range, 1.8–8.8 years) with 84% (95% CI: 83– 85%) surviving at least 1 year. Figure 2 shows unadjusted and adjusted survival curves for categories of serum albumin (p < 0.001 for trend in both models). Compared to those with normal albumin levels, the overall multivariable-adjusted mortality rate ratios for severe and mild hypoalbuminemia were 1.35 (95% CI: 1.12–1.62) and 1.15 (95% CI: 1.04–1.27), respectively (model 3 in Table 2). A low-normal serum albumin concentration (3.7–3.9 g/dL) was associated with a multivariable-adjusted mortality rate ratio of 1.10 (95% CI: 1.00–1.21) compared to those with normal serum albumin. For each 0.5 g/dL decrease in serum albumin the multivariable-adjusted mortality rate ratio was 1.26 (95% CI: 1.11–1.43). Elevated serum albumin concentrations were detected in 73 (1.1%), and while the multivariable adjusted 1 year and overall mortality rate ratios were 1.50 (95% CI: 0.90–2.49) and 1.14 (95% CI: 0.81–1.61), respectively, neither association was statistically significant. When the percentage predicted forced vital capacity (FVC) at lung transplant listing was substituted for FEV1 as a covariate, there was no meaningful change in the effect estimates for serum albumin specified as either a categorical or continuous variable.
Figure 2. Unadjusted and multivariable-adjusted survival curves for the entire cohort (n = 6808) for severe hypoalbuminemia (2.9–3.6 g/dL), mild hypoalbuminemia (3.0–3.6 g/dL), low-normal albumin (3.7–3.9 g/dL), normal albumin(4.0–5.0 g/dL) and elevated albumin (5.1–5.9 g/dL) serum concentrations at lung transplant listing.
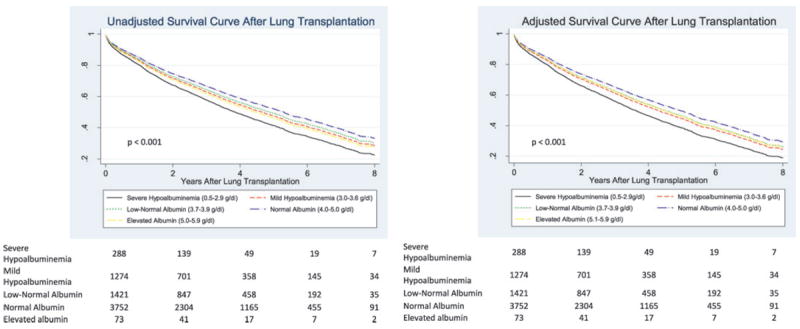
Adjusted survival estimates are adjusted for recipient covariates (age, sex, FEV1, WHO BMI category, diagnosis, diabetes, use of steroids before transplant and hospitalization at transplant), donor covariates (age, sex, BMI, height, smoking >20 pack years, pulmonary infection and donor cause of death), and procedure covariates (single vs. double lung transplant, transplant year (dichotomized at May 4, 2005, the date the LAS score was implemented), and graft ischemic time). From Table 2, unadjusted and adjusted p < 0.001 for trend in both models. Bottom: numbers indicate the number of surviving lung transplant recipients at each time point.
Table 2.
Associations between serum albumin concentration at lung transplant listing and mortality after lung transplant
| Total | Severe hypoalbuminemia 0.5–2.9 (g/dL) | Mild hypoalbuminemia 3.0–3.6 (g/dL) | Low-normal albumin 3.7–3.9 (g/dL) | Normal albumin 4.0–5.0 (g/dL) | Elevated albumin 5.1–5.9 (g/dL) | p-for trend | Albumin HR for every 0.5 g/dL decrease | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Number transplanted (%) | 6808 | 288 | 1274 | 1421 | 3752 | 73 | |||
| Median follow-up time years (IQR) | 2.69 (1.06–4.51) | 1.98 (0.99–3.56) | 2.18 (1.00–4.18) | 2.77 (1.07–4.76) | 2.85 (1.14–4.70) | 2.26 (0.93–3.96) | |||
| Number with 1-year follow-up | 6435 | 269 | 1188 | 1352 | 3558 | 68 | |||
| % Survived 1-year (95% CI) | 84 (83–85) | 79 (73–83) | 81(79–83) | 83 (81–85) | 85 (84–87) | 77 (66–85) | |||
| Person years | 20 495 | 688 | 3652 | 4369 | 11 580 | 206 | |||
| Overall mortality | |||||||||
| Mortality rate per year (95% CI) | 0.15 (0.14–0.15) | 0.19 (0.16–0.23) | 0.16 (0.15–0.17) | 0.15 (0.14–0.16) | 0.14 (0.13–0.15) | 0.17 (0.12–0.23) | <0.001 | 0.13 (0.11–0.14) | <0.001 |
| Model 1: HR for death (95% CI) | 1.35 (1.12–1.62) | 1.15 (1.04–1.27) | 1.10 (1.00–1.21) | 1 | 1.14 (0.81–1.73) | <0.001 | 1.28 (1.11–1.46) | 0.001 | |
| Model 2: HR for death (95% CI) | 1.35 (1.12–1.62) | 1.15 (1.03–1.27) | 1.10 (1.00–1.21) | 1 | 1.14 (0.81–1.61) | <0.001 | 1.26 (1.11–1.43) | 0.001 | |
| Model 3: HR for death (95% CI) | 1.35 (1.12–1.62) | 1.15 (1.04–1.27) | 1.10 (1.00–1.21) | 1 | 1.14 (0.81–1.61) | <0.001 | 1.26 (1.11–1.43) | 0.001 | |
| 1-year mortality | |||||||||
| Mortality rate per year (95% CI) | 0.18 (0.17–0.19) | 0.24 (0.19–0.31) | 0.21 (0.19–0.24) | 0.19 (0.16–0.21) | 0.16 (0.14–0.17) | 0.26 (0.16–0.42) | <0.001 | 0.15 (0.12–0.18) | <0.001 |
| Model 1: HR for death at 1-year (95% CI) | 1.52 (1.15–2.01) | 1.35 (1.14–1.60) | 1.19 (1.01–1.39) | 1 | 1.49 (0.90–2.47) | <0.001 | 1.48 (1.21–1.81) | <0.001 | |
| Model 2: HR for death at 1-year (95% CI) | 1.52 (1.15–2.01) | 1.34 (1.14–1.58) | 1.20 (1.02–1.40) | 1 | 1.50 (0.90–2.48) | <0.001 | 1.47 (1.21–1.80) | <0.001 | |
| Model 3: HR for death at 1-year (95% CI) | 1.55 (1.17–2.05) | 1.33 (1.13–1.57) | 1.19 (1.01–1.39) | 1 | 1.50 (0.90–2.49) | <0.001 | 1.48 (1.21–1.81) | <0.001 |
All Cox models include the random effect of transplant center as a shared frailty component.
Model 1: Adjusted for recipient characteristics (age, age2, sex, diagnosis, FEV1, diabetes, steroid use, hospitalized at the time of transplant, and BMI categorized using the World Health Organization Classification [WHO] scheme as previously described [24]).
Model 2: Model 1 + donor characteristics (age, sex, height, BMI, pulmonary infection, smoking > 20 pack years, donor cause of death).
Model 3: Model 2 + procedure characteristics (single vs. double lung transplant, graft ischemic time, graft ischemic time2 and year of transplant (dichotomized at May 4, 2005, the date the LAS score was implemented).
Hypoalbuminemia was strongly associated with early (1-year) mortality. Compared to those with normal albumin levels, the multivariable-adjusted 1-year mortality rate ratios for severe and mild hypoalbuminemic recipients were 1.55 (95% CI: 1.17–2.05) and 1.33 (95% CI: 1.13–1.57), respectively (model 3 in Table 2). Those with a low-normal serum albumin concentration had a multivariable-adjusted 1-year mortality rate ratio of 1.19 (95% CI: 1.01–1.39) compared to those with normal serum albumin. For each 0.5 g/dL decrease in serum albumin concentration the multivariable-adjusted 1-year mortality rate ratio was 1.48 (95% CI: 1.21–1.81) (Table 2).
The multivariable adjusted GAM-fitted model showed a linear inverse association between serum albumin and the risk of death at 1 year for albumin values between 2 g/dL and 5 g/dL, but a weaker relationships below 2 g/dL (Figure 3). The significance of this subtle nonlinear relationship is uncertain given that only 59 (0.9%) participants had albumin levels below 2, and linear p for trend tests across categories of albumin were significant (Table 2). The multivariable-adjusted GAMs showed that continuous variables for recipient and donor BMI (p = 0.23 and 0.73, respectively), FEV1 (p = 0.48), recipient oxygen use (L/min) at the time of listing (p = 0.30) and recipient mean pulmonary artery pressure (mm Hg) at the time of listing (p = 0.59) all had a linear relationship with the rate of death at 1 year, and age (p = 0.01) and graft ischemic time (p = 0.04) did not. Addition of quadratic terms for age and graft ischemic time corrected for nonlinearity in the GAMs (age (p = 0.70), age2 (p = 0.71), ischemic time (p = 0.25), ischemic time2 (p = 0.17). These quadratic terms were therefore used in the Cox proportional hazards models.
Figure 3. Multivariable-adjusted generalized additive model fitted continuous relationship of serum albumin concentration (g/dL) at lung transplant listing for the risk of death at 1 year for the entire cohort (n = 6808).
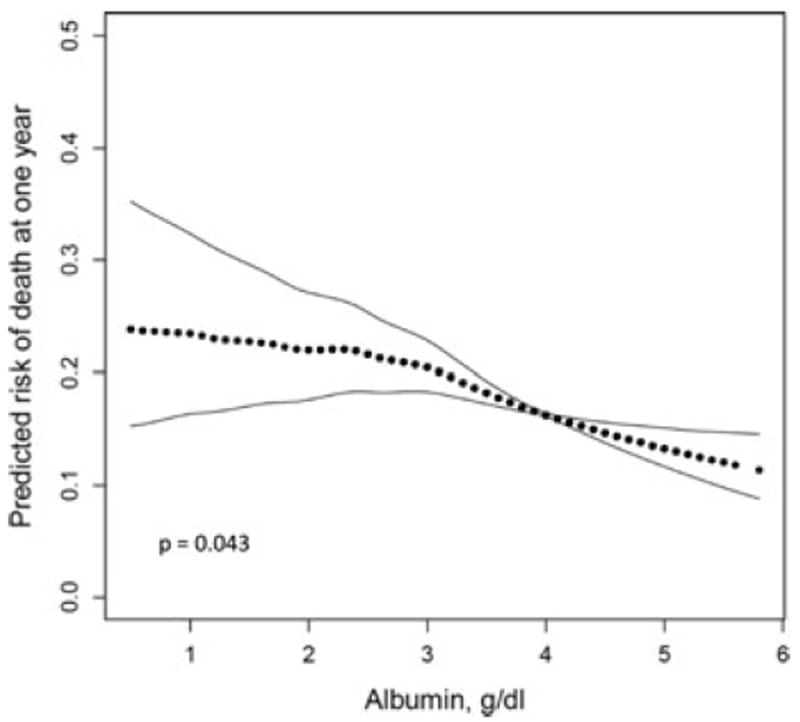
Estimates are adjusted for covariates listed in model 3 in the footnote to Figure 2. The significant p-value (0.043) for the smoothed curve for 1-year survival suggests a subtle nonlinear threshold of risk below serum albumin concentrations of 2 g/dL and above 5 g/dL. There is a linear inverse relationship between serum albumin concentrations of 2 and 5 g/dL with overall higher 1-year mortality for lower serum albumin. The significance of this subtle nonlinear relationship is uncertain given that only 59 (0.9%) participants had albumin levels below 2, and linear p for trend tests across categories of albumin were significant (Table 2).
In the subgroup of 4247 participants transplanted on or after May 4, 2005 (the day the LAS system was implemented), an LAS score was available for 3408 (80%). The median LAS score was highest among participants with severe hypoalbuminemia and decreased across categories of higher serum albumin (Table 1). After adjusting for LAS and all other model 3 covariates in the subset with an available LAS score (n = 3408), the 1-year mortality rate ratios for severe and mild hypoalbuminemia were 1.37 (95% CI: 0.95–1.97) and 1.28 (95% CI: 1.01–1.62), respectively, compared to normal serum albumin (Table S1). For each 0.5 g/dL decrease in serum albumin, the 1-year mortality rate ratio was 1.36 (95% CI: 1.04–1.78).
We performed post hoc analyses to determine whether mechanical ventilation at the time of transplant, donor–recipient CMV mismatch, mean pulmonary artery pressure (mm Hg) at listing, oxygen use (L/min) at listing, or 6-min walk distance (feet) at listing would confounded the association between serum albumin and posttransplant mortality. Adjustment for these factors did not substantially change our findings that lower serum albumin was associated with a higher rate of death in the first year after transplantation (Table S2).
The association between serum albumin concentration and the rate of death did not appear to vary by BMI (p for interaction = 0.20), but the association did appear to vary by diagnosis (p for interaction = 0.02). Figure 4 shows multivariable-adjusted survival curves for categories of serum albumin concentration stratified by diagnosis. Both multivariable-adjusted 1-year and overall survival varied significantly across albumin categories in both CF (p = 0.013 and 0.015) and ILD (p = 0.019 and 0.008) patients, but not in COPD patients (p = 0.144 and 0.140). Hypoalbuminemia was strongly associated with early mortality in CF and ILD recipients: for every 0.5 g/dL decrease in serum albumin the adjusted 1-year mortality rate ratio was 2.28 (95% CI: 1.32–3.96) and 1.40 (95% CI: 1.03–1.90), respectively. In COPD recipients, there was no association found between serum albumin concentration and the overall mortality rate, but for each 0.5 g/dL decrease in serum albumin, the adjusted 1-year mortality rate ratio increased 1.38 (95% CI: 1.01–1.90) (Table 3).
Figure 4. Multivariable-adjusted survival curves for categories of serum albumin concentration at lung transplant listing.
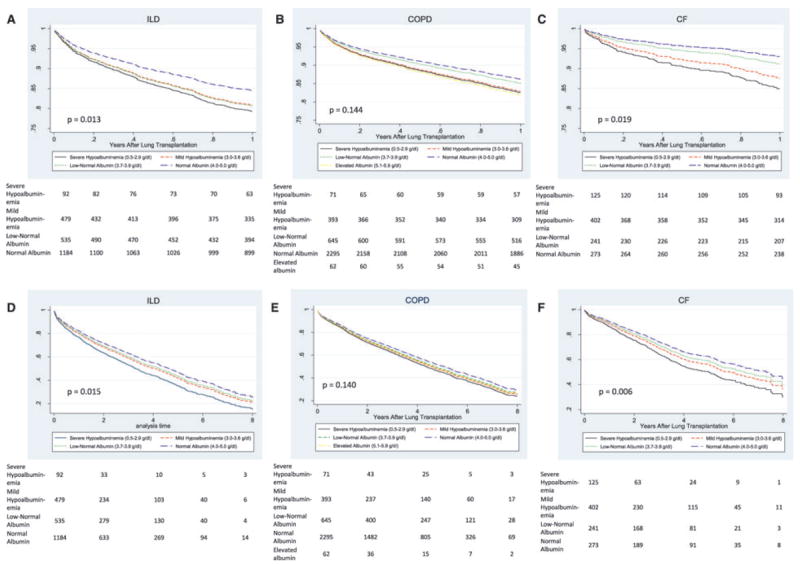
Survival censored at 1-year (A) and overall survival (D) for patients who had ILD (n = 2290); survival censored at 1-year (B) and overall survival (E) for patients who had COPD (n = 3466); and survival censored at 1-year (C) and overall survival (F) for patients who had CF (n = 1041). Survival estimates are derived from the stratified Cox model described in Table 3. p-Values are for trend tests across albumin categories from Table 3. Bottom: numbers indicate the number of surviving lung transplant recipients at each time point.
Table 3.
Associations between serum albumin concentration at lung transplant listing and the mortality after lung transplantation, stratified by diagnosis
| Total | Severe hypoalbuminemia 0.5–2.9 (g/dL) | Mild hypoalbuminemia 3.0–3.6 (g/dL) | Low-normal albumin 3.7–3.9 (g/dL) | Normal albumin 4.0–5.0 (g/dL) | Elevated albumin 5.1–5.9 (g/dL) | p-Value for trend | Albumin HR for every 0.5 g/dL decrease | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| ILD | |||||||||
| Number transplanted (%) | 2298 | 92 | 479 | 535 | 1184 | 8 | |||
| Cox proportional hazard model | |||||||||
| Mortality rate per year (95% CI) | 0.17 (0.16–0.18) | 0.25 (0.19–0.34) | 0.19 (0.16–0.21) | 0.18 (0.15–0.20) | 0.16 (0.15–0.20) | 0.006 | |||
| Model 3: HR for death (95% CI) | 1.37 (1.01–1.85) | 1.15 (0.97–1.35) | 1.10 (0.94–1.29) | 1 | 0.015 | 1.32 (1.07–1.62) | 0.009 | ||
| Censored at 1-year | |||||||||
| Mortality rate per year (95% CI) | 0.22 (0.20–0.24) | 0.33 (0.25–0.49) | 0.25 (0.21–0.31) | 0.24 (0.20–0.30) | 0.20 (0.16–0.22) | 0.001 | |||
| Model 3: HR for death at 1-year (95% CI) | 1.48 (0.96–2.27) | 1.27 (0.99–1.63) | 1.25 (0.99–1.59) | 1 | 0.013 | 1.40 (1.03–1.90) | 0.031 | ||
| COPD | |||||||||
| Number transplanted (%) | 3466 | 71 | 393 | 645 | 2295 | 62 | |||
| Cox proportional hazard models | |||||||||
| Mortality rate per year (95% CI) | 0.14 (0.13–0.15) | 0.17 (0.12–0.24) | 0.13 (0.15–0.18) | 0.14 (0.13–0.16) | 0.13 (0.13–0.14) | 0.16 (0.11–0.23) | 0.113 | ||
| Model 3: HR for death (95% CI) | 1.18 (0.82–1.69) | 1.13 (0.96–1.33) | 1.08 (0.95–1.24) | 1 | 1.15 (0.79–1.67) | 0.140 | 1.14 (0.94–1.39) | 0.180 | |
| Censored at 1-year | |||||||||
| Mortality rate per year (95% CI) | 0.16 (0.15–0.17) | 0.20 (0.11–0.36) | 0.20 (0.16–0.25) | 0.16 (0.13–0.20) | 0.15 (0.13–0.17) | 0.22 (0.12–0.40) | 0.103 | ||
| Model 3: HR for death at 1-year (95%) | 1.27 (0.70–2.30) | 1.30 (0.99–1.71) | 1.11 (0.88–1.41) | 1 | 1.34 (0.75–2.41) | 0.144 | 1.38 (1.01–1.90) | 0.043 | |
| CF | |||||||||
| Number transplanted (%) | 1044 | 125 | 402 | 241 | 273 | 3 | |||
| Cox proportional hazard model | |||||||||
| Mortality rate per year (95% CI) | 0.13 (0.12–0.14) | 0.17 (0.13–0.22) | 0.15 (0.12–0.16) | 0.13 (0.10–0.15) | 0.10 (0.09–0.13) | 0.003 | |||
| Model 3 HR for death | 1.51 (1.05–2.19) | 1.29 (0.98–1.70) | 1.20 (0.89–1.60) | 1 | 0.019 | 1.55 (1.09–2.20) | 0.016 | ||
| Censored at 1-year | |||||||||
| Mortality rate per year (95% CI) | 0.14 (0.12–0.17) | 0.21 (0.14–0.31) | 0.17 (0.13–0.22) | 0.13 (0.90–0.19) | 0.97 (0.66–0.14) | 0.005 | |||
| Model 3 HR for death at 1-year | 2.17 (1.19–3.96) | 1.73 (1.06–2.84) | 1.32 (0.76–2.29) | 1 | 0.006 | 2.28 (1.32–3.96) | 0.003 |
Too few observations to calculate meaningful effect estimates and were excluded from the associated models.
Estimates are adjusted for covariates listed in model 3 in the footnote to Figure 2.
The PAF of hypoalbuminemia was 10.7%, suggesting that hypoalbuminemia (and related unmeasured and poorly measured confounders) contributed to up to 10.7% of deaths in the first year after transplantation (Table 4). Among those with CF and ILD, below normal serum albumin concentrations contributed up to 32.2% and 12.2% of deaths in the first year after transplantation, respectively.
Table 4.
Multivariable-adjusted population attributable fractions stratified by diagnosis
| Number transplanted | PAF at 1 year (%) | |
|---|---|---|
| All recipients | 6808 | 10.7 |
| Interstitial lung disease | 2233 | 12.2 |
| Chronic obstructive Pulmonary disease | 3360 | 5.5 |
| Cystic fibrosis | 990 | 32.2 |
PAF= population attributable fraction.
The PAF for all recipients at 1 year was estimated using the multi-variable Cox proportional hazard model 3 from Table 2. The PAF for specific diagnoses was estimated using the multivariable Cox proportional hazard model 3 from Table 3. The PAF is the proportional reduction in the number of deaths if the exposure of interestwere eliminated from the population. For example, if all lung transplant recipients had a normal albumin, up to 10.7% of deaths might be prevented in the first year after transplantation.
Discussion
We have shown that hypoalbuminemia at the time of listing for lung transplantation is independently associated with a higher rate of death after lung transplantation. The risk appeared to be highest early after transplantation and varied by disease, with the greatest risk for CF patients and the least risk for COPD patients. Our findings support the hypothesis that extrapulmonary measures of overall health are important determinants of outcomes after lung transplantation.
While it is possible that hypoalbuminemia might directly contribute to poor outcomes after lung transplantation by promoting oxidative injury or platelet aggregation (33,34), it is more likely that hypoalbuminemia and early posttransplant mortality share common antecedent causes. The concentration of albumin in serum is determined by the balance between its synthesis in the liver and its catabolism by the vascular endothelium (11,14). Hypoalbuminemia can therefore be a result of reduced synthesis due to liver disease and protein malnutrition, enhanced catabolism due to inflammation or to renal loss (11,14,19). Since liver and kidney disease are rare among waitlisted lung transplant candidates, malnutrition and systemic inflammation are likely responsible for hypoalbuminemia in this population, and might lead to a higher risk of death by predisposing to infection and lung inflammation, or perhaps by contributing to a state of reduced physiologic reserve (frailty). Systemic inflammation is a primary cause of age-related muscle loss (sarcopenia), which is associated with hypoalbuminemia (35,36). Sarcopenia and its related clinical phenotype of frailty have been shown to predict complications after general surgery and mortality after liver transplantation (37,38). Future investigations that focus on identifying other measures of sarcopenia and frailty in patients listed for lung transplantation have promise to improve lung allocation and outcomes after lung transplantation.
We found that hypoalbuminemia had the strongest association with early posttransplant mortality in recipients with CF. Serum albumin levels parallel the decline in nutritional status caused by recurrent respiratory infections in CF (39). Supplemental nocturnal gastrostomy tube feeding has been shown to augment height and weight gain, and decrease infection rates and lung function decline in children with CF (40,41). While hypoalbuminemia in CF patients might be preventable with aggressive nutritional interventions, such as nocturnal gastrostomy feeding or orally ingested nutritional supplements, our findings should not be interpreted as supporting this practice, since we did not examine the effect of nutritional intervention on lung transplant outcomes.
Among participants with COPD, we found that pretransplant hypoalbuminemia was only weakly associated with 1-year mortality and was not associated with overall mortality. While the hazard ratios for early mortality among those with severe hypoalbuminemia were notably lower for participants with COPD compared to those with ILD (1.27 and 1.48, respectively), the hazard ratios for albumin as a continuous variable were almost identical for these two groups (1.38 and 1.40, respectively) and were statistically significant. The reasons for this discrepancy is not clear, but may be a result of the smaller number of COPD participants with severe hypoalbuminemia (n = 71), the arbitrary thresholds separating albumin categories, and/or differences in the influences of systemic inflammation and nutritional status on albumin levels between participants with and without COPD.
Elevated serum albumin concentrations (5.1–5.9 g/dL) were detected in 73 (1.1%) recipients and multivariable adjusted mortality rate ratios ranged from 1.14 to 1.50, but neither association was statistically significant. It is likely that serum albumin was misclassified in some of these cases. For example, total protein may have been recorded instead of albumin. Accordingly, patients with low total protein concentrations between 5.1 g/dL and 5.9 g/dL will likely also be hypoalbuminemic and therefore have a higher posttransplant mortality. Elevated albumin is most often associated with severe dehydration, which is unlikely at the time of transplant listing because patients are already receiving care from many healthcare providers. Severe vitamin A deficiency is associated with elevated serum albumin (42), and CF patients are at risk for fat-soluble vitamin deficiencies due to fat malabsorption. However, only three CF recipients had elevated serum albumin at the time of transplant listing, and such severe vitamin A deficiency seems unlikely given these patients are receiving medical care.
Our study has several limitations. First, we retrospectively ascertained serum albumin concentrations as reported by transplant center personnel. Interlaboratory variation in the measurement of serum albumin concentration is unlikely, since a standard assay, the BCG method (9,26), is used for the measurement of serum albumin. Second, serum albumin was recorded at the time of listing for lung transplantation rather than at the time of transplantation. It is likely that the serum albumin at transplantation differed from that at the time of listing, since these events were a median of 5 months apart, thereby misclassifying albumin for many study participants. Since it is likely that this error is independent of the risk of death after transplantation, we would expect such misclassification to bias toward the null. If so, our results may underestimate the true association between serum albumin and the risk of death after transplantation. Prospective studies with attention to the timing of albumin measurement may provide additional information about the predictive validity of serum albumin in this setting. Third, inclusion of imprecisely measured confounders and failure to include unmeasured confounders in our models could have contributed to some or all of the associations we observed. Our findings, however, were independent of important potential confounders such as diagnosis, BMI, diabetes, corticosteroid use, donor characteristics and procedural characteristics. Nevertheless, residual confounding by other factors cannot be entirely excluded.
In summary, we found that hypoalbuminemia at lung transplant listing was independently associated with a higher risk of death in a nationwide cohort of lung transplant recipients, and accounted for up to 11% deaths in the first year after lung transplantation. Our findings should prompt investigations of extrapulmonary factors, such as systemic inflammation, sarcopenia and frailty that might contribute to early complications of lung transplantation.
Supplementary Material
Table S1: The association between serum albumin at lung transplant listing and mortality after lung transplant adjusting for the lung allocation score
Table S2: Post hoc analyses of serum albumin at lung transplant listing and mortality after lung transplant
Acknowledgments
This work was supported by the National Institutes of Health Grants K23 HL086714 and UL1 RR024156, the Robert Wood Johnson Physician Faculty Scholars Program, the Herbert and Florence Irving Scholar Award, for D.J.L. This work was supported in part by HRSA contract 231-00-0115.
Disclosure
The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Abbreviations
- CF
cystic fibrosis
- COPD
chronic obstructive pulmonary disease
- CMV
cytomegalovirus
- FEV1
forced expiratory volume in 1 s
- FVC
forced vital capacity
- GAM
generalized additive model
- ILD
interstitial lung disease
- LAS
lung allocation system
- OPTN
Organ Procurement and Transplantation Network
- PAF
population attributable fraction
- UNOS
United Network for Organ Sharing
- WHO
World Health Organization
Footnotes
Supporting Information
Additional supporting information may be found in the online version of this article:
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Hosenpud JD, Bennett LE, Keck BM, Edwards EB, Novick RJ. Effect of diagnosis on survival benefit of lung transplantation for end-stage lung disease. Lancet. 1998;351:24–27. doi: 10.1016/S0140-6736(97)06405-2. [DOI] [PubMed] [Google Scholar]
- 2.Titman A, Rogers CA, Bonser RS, Banner NR, Sharples LD. Disease-specific survival benefit of lung transplantation in adults: A national cohort study. Am J Transplant. 2009;9:1640–1649. doi: 10.1111/j.1600-6143.2009.02613.x. [DOI] [PubMed] [Google Scholar]
- 3.Organ Procurement and Transplantation Network. Title 42 Code of Federal Regulations. Pt 121.8(a) 2007;2 [Google Scholar]
- 4.Lingaraju R, Blumenthal NP, Kotloff RM, et al. Effects of lung allocation score on waiting list rankings and transplant procedures. J Heart Lung Transplant. 2006;25:1167–1170. doi: 10.1016/j.healun.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Kozower BD, Meyers BF, Smith MA, et al. The impact of the lung allocation score on short-term transplantation outcomes: A multicenter study. J Thorac Cardiovasc Surg. 2008;135:166–171. doi: 10.1016/j.jtcvs.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 6.Iribarne A, Russo MJ, Davies RR, et al. Despite decreased wait-list times for lung transplantation, lung allocation scores continue to increase. Chest. 2009;135:923–928. doi: 10.1378/chest.08-2052. [DOI] [PubMed] [Google Scholar]
- 7.Gries CJ, Rue TC, Heagerty PJ, Edelman JD, Mulligan MS, Goss CH. Development of a predictive model for long-term survival after lung transplantation and implications for the lung allocation score. J Heart Lung Transplant. 2010;29:731–738. doi: 10.1016/j.healun.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heymsfield SBWP. Nutritional assessment by clinical and biochemical methods. In: Shils Me, Young VR., editors. Modem Nutrition in Health and Disease. Philadelphia: Lea & Febiger; 1998. pp. 830–848. [Google Scholar]
- 9.Doumas BT, Peters T., Jr Serum and urine albumin: A progress report on their measurement and clinical significance. Clin Chim Acta. 1997;258:3–20. doi: 10.1016/s0009-8981(96)06446-7. [DOI] [PubMed] [Google Scholar]
- 10.Kirsch R, Frith L, Black E, Hoffenberg R. Regulation of albumin synthesis and catabolism by alteration of dietary protein. Nature. 1968;217:578–579. doi: 10.1038/217578a0. [DOI] [PubMed] [Google Scholar]
- 11.Don BR, Kaysen G. Serum albumin: Relationship to inflammation and nutrition. Semin Dial. 2004;17:432–437. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
- 12.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. Hypoalbuminemia, cardiac morbidity, and mortality in end-stage renal disease. J Am Soc Nephrol. 1996;7:728–736. doi: 10.1681/ASN.V75728. [DOI] [PubMed] [Google Scholar]
- 14.Rothschild MA, Oratz M, Schreiber SS. Serum albumin. Hepatology. 1988;8:385–401. doi: 10.1002/hep.1840080234. [DOI] [PubMed] [Google Scholar]
- 15.Zisman DA, Kawut SM, Lederer DJ, et al. Serum albumin concentration and waiting list mortality in idiopathic interstitial pneumonia. Chest. 2009;135:929–935. doi: 10.1378/chest.08-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawut SM, Horn EM, Berekashvili KK, et al. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol. 2005;95:199–203. doi: 10.1016/j.amjcard.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Chan JC, Tsui EL, Wong VC. Prognostication in severe acute respiratory syndrome: A retrospective time-course analysis of 1312 laboratory-confirmed patients in Hong Kong. Respirology. 2007;12:531–542. doi: 10.1111/j.1440-1843.2007.01102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boonpipattanapong T, Chewatanakornkul S. Preoperative carcinoembryonic antigen and albumin in predicting survival in patients with colon and rectal carcinomas. J Clin Gastroenterol. 2006;40:592–595. doi: 10.1097/00004836-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Kovesdy CP, George SM, Anderson JE, Kalantar-Zadeh K. Outcome predictability of biomarkers of protein-energy wasting and inflammation in moderate and advanced chronic kidney disease. Am J Clin Nutr. 2009;90:407–414. doi: 10.3945/ajcn.2008.27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menon V, Greene T, Wang X, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68:766–772. doi: 10.1111/j.1523-1755.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- 21.Reuben DB, Cheh AI, Harris TB, et al. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc. 2002;50:638–644. doi: 10.1046/j.1532-5415.2002.50157.x. [DOI] [PubMed] [Google Scholar]
- 22.Reuben DB, Ferrucci L, Wallace R, et al. The prognostic value of serum albumin in healthy older persons with low and high serum interleukin-6 (IL-6) levels. J Am Geriatr Soc. 2000;48:1404–1407. doi: 10.1111/j.1532-5415.2000.tb02629.x. [DOI] [PubMed] [Google Scholar]
- 23.Reuben DB, Ix JH, Greendale GA, Seeman TE. The predictive value of combined hypoalbuminemia and hypocholesterolemia in high functioning community-dwelling older persons: MacArthur Studies of Successful Aging. J Am Geriatr Soc. 1999;47:402–406. doi: 10.1111/j.1532-5415.1999.tb07230.x. [DOI] [PubMed] [Google Scholar]
- 24.Corti MC, Guralnik JM, Salive ME, Sorkin JD. Serum albumin level and physical disability as predictors of mortality in older persons. JAMA. 1994;272:1036–1042. [PubMed] [Google Scholar]
- 25.Lederer DJ, Wilt JS, D’Ovidio F, et al. Obesity and underweight are associated with an increased risk of death after lung transplantation. Am J Respir Crit Care Med. 2009;180:887–895. doi: 10.1164/rccm.200903-0425OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blagg CR, Liedtke RJ, Batjer JD, et al. Serum albumin concentration-related Health Care Financing Administration quality assurance criterion is method-dependent: Revision is necessary. Am J Kidney Dis. 1993;21:138–144. doi: 10.1016/s0272-6386(12)81084-5. [DOI] [PubMed] [Google Scholar]
- 27.Hastie TJTR. Generalized Additive Models. London: Chapman & Hall; 1990. [Google Scholar]
- 28.Lederer DJ, Benn EK, Barr RG, et al. Racial differences in waiting list outcomes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:450–454. doi: 10.1164/rccm.200708-1260OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thabut G, Ravaud P, Christie JD, et al. Determinants of the survival benefit of lung transplantation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:1156–1163. doi: 10.1164/rccm.200708-1283OC. [DOI] [PubMed] [Google Scholar]
- 30.Mietten O. Theoretical Epidemiology: Principles of Occurrence Research in Medicine. New York, NY: John Wiley and Sons, Inc.; 1985. [Google Scholar]
- 31.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88:15–19. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Team RDC. R: A language and environment for statistical computing. [January 17, 2012];2009 Available at: http://www.R-project.org.
- 33.Roy S, Sen CK, Kobuchi H, Packer L. Antioxidant regulation of phorbol ester-induced adhesion of human Jurkat T-cells to endothelial cells. Free Radic Biol Med. 1998;25:229–241. doi: 10.1016/s0891-5849(98)00062-8. [DOI] [PubMed] [Google Scholar]
- 34.Joles JA, Willekes-Koolschijn N, Koomans HA. Hypoalbuminemia causes high blood viscosity by increasing red cell lysophosphatidylcholine. Kidney Int. 1997;52:761–770. doi: 10.1038/ki.1997.393. [DOI] [PubMed] [Google Scholar]
- 35.Baumgartner RN, Koehler KM, Romero L, Garry PJ. Serum albumin is associated with skeletal muscle in elderly men and women. Am J Clin Nutr. 1996;64:552–558. doi: 10.1093/ajcn/64.4.552. [DOI] [PubMed] [Google Scholar]
- 36.Visser M, Kritchevsky SB, Newman AB, et al. Lower serum albumin concentration and change in muscle mass: The health, aging and body composition study. Am J Clin Nutr. 2005;82:531–537. doi: 10.1093/ajcn.82.3.531. [DOI] [PubMed] [Google Scholar]
- 37.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 38.Englesbe MJ, Patel SP, He K, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211:271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solomons NW, Wagonfeld JB, Rieger C, et al. Some biochemical indices of nutrition in treated cystic fibrosis patients. Am J Clin Nutr. 1981;34:462–474. doi: 10.1093/ajcn/34.4.462. [DOI] [PubMed] [Google Scholar]
- 40.Shepherd RW, Holt TL, Thomas BJ, et al. Nutritional rehabilitation in cystic fibrosis: Controlled studies of effects on nutritioal growth retardation, body protein turnover, and course of pulmonary disease. J Pediatr. 1986;109:788–794. doi: 10.1016/s0022-3476(86)80695-3. [DOI] [PubMed] [Google Scholar]
- 41.Moore MC, Greene HL, Donald WD, Dunn GD. Enteral-tube feeding as adjunct therapy in malnourished patients with cystic fibrosis: A clinical study and literature review. Am J Clin Nutr. 1986;44:33–41. doi: 10.1093/ajcn/44.1.33. [DOI] [PubMed] [Google Scholar]
- 42.Pasantes-Morales H, Wright CE, Gaull GE. Protective effect of taurine, zinc and tocopherol on retinol-induced damage in human lymphoblastoid cells. J Nutr. 1984;114:2256–2261. doi: 10.1093/jn/114.12.2256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: The association between serum albumin at lung transplant listing and mortality after lung transplant adjusting for the lung allocation score
Table S2: Post hoc analyses of serum albumin at lung transplant listing and mortality after lung transplant


